Abstract
Background Anthropogenic climate change (ACC) will influence all aspects of plant biology over coming decades. Many changes in wild species have already been well-documented as a result of increased atmospheric CO2 concentrations, warming climate and changing precipitation regimes. A wealth of available data has allowed the use of meta-analyses to examine plant–climate interactions on more sophisticated levels than before. These analyses have revealed major differences in plant response among groups, e.g. with respect to functional traits, taxonomy, life-history and provenance. Interestingly, these meta-analyses have also exposed unexpected mismatches between theory, experimental, and observational studies.
Scope We reviewed the literature on species’ responses to ACC, finding ∼42 % of 4000 species studied globally are plants (primarily terrestrial). We review impacts on phenology, distributions, ecophysiology, regeneration biology, plant–plant and plant–herbivore interactions, and the roles of plasticity and evolution. We focused on apparent deviations from expectation, and highlighted cases where more sophisticated analyses revealed that unexpected changes were, in fact, responses to ACC.
Conclusions We found that conventionally expected responses are generally well-understood, and that it is the aberrant responses that are now yielding greater insight into current and possible future impacts of ACC. We argue that inconclusive, unexpected, or counter-intuitive results should be embraced in order to understand apparent disconnects between theory, prediction, and observation. We highlight prime examples from the collection of papers in this Special Issue, as well as general literature. We found use of plant functional groupings/traits had mixed success, but that some underutilized approaches, such as Grime's C/S/R strategies, when incorporated, have improved understanding of observed responses. Despite inherent difficulties, we highlight the need for ecologists to conduct community-level experiments in systems that replicate multiple aspects of ACC. Specifically, we call for development of coordinating experiments across networks of field sites, both natural and man-made.
Keywords: Climate change, global change, phenology, distributions, range shifts, invasive species, assisted colonization, elevated CO2, plant functional groups, plant functional traits, plasticity, evolution.
INTRODUCTION
Climate change represents one of the greatest research challenges currently faced by plant biologists, agronomists and conservation biologists. With global greenhouse gas emissions set to continue to rise for the foreseeable future, the impact of elevated atmospheric CO2 (eCO2), and associated shifts in temperature and precipitation are all expected to impact plant ecophysiology, distribution and interactions with other organisms (Intergovernmental Panel on Climate Change (IPCC), 2014). Consequently, effects of anthropogenic climate change (ACC) on plant growth, reproduction, phenology, and distribution have already generated several thousand scientific articles. This wealth of literature has provided fodder for independent global meta-analyses synthesizing data from long-term observational records for some 4000 eukaryote species, about 42 % of which are plants (primarily in terrestrial systems), spread across the world (Table 1). Collectively, these meta-analyses document coherent patterns across the globe of poleward and upward range shifts and advancement of spring phenologies (Parmesan and Yohe, 2003; Root et al., 2003; Parmesan, 2007; Poloczanska et al., 2013).
Table 1.
Overview of five major global meta-analyses from long-term observational data on individual wild species with diverse distributions in terrestrial (T), marine (M) and freshwater (F) systems
| Study | Number of species and functional groups | Species in given system (% of all) |
Plants in each system (% of each system) |
Species showing significant long-term change in phenologies, distributions, abundances or morphology (% of all) | Changes consistent with local or regional climate change (% of species that showed change) | ||||
|---|---|---|---|---|---|---|---|---|---|
| T | M | F | T | M | F | ||||
| Parmesan and Yohe 2003 | 1598 | 85 | 13 | <2 | 63 | 0 | 0 | 59 | 841 |
| Root et al. 2003 | 1468 | 94 | 5 | <1 | 49 | <1 | <1 | 40 | 821 |
| Root et al. 2005 | 130 | 100 | 0 | 0 | 65 | 0 | 0 | 100 | 921 |
| Rosenzweig et al. 2008 | 55 studies | 65 | 13 | 22 | 44 | 14 | 42 | – | 902 |
| Poloczanska et al. 2013 | 857 | 0 | 100 | 0 | 0 | 16 | 0 | 76 | 831 |
Each study includes data from multiple continents and oceans, and together there is representation from every continent and every major ocean. Not all studies provided all metrics; missing information is indicated with a dash. Percentages are approximate and estimated for the studies as whole-individual analyses within the studies may differ. The specific metrics of climate change analysed for associations with biological change vary somewhat across studies, but most use changes in local or regional temperatures (e.g. mean monthly temperature or mean annual temperature), with some using precipitation metrics (e.g. total annual rainfall). Individual species were analysed by Rosenzweig et al. (2008) but data on species not provided in publication—percentages shown are based on numbers of studies. 1P < 10−15; 2P < 0·001 (from binomial test against random expectation of 50-50 chance of change in either direction—either consistent or not consistent with local or regional climate change).
These results fed into the latest IPCC report leading to the following statement in the Summary for Policy Makers:
‘In recent decades, changes in climate have caused impacts on natural and human systems on all continents and across the oceans. Evidence of climate-change impacts is strongest and most comprehensive for natural systems … . Many terrestrial, freshwater, and marine species have shifted their geographic ranges, seasonal activities, migration patterns, abundances, and species interactions in response to ongoing climate change (high confidence).’
Summary for Policy Makers (IPCC, 2014)
With specific reference to plants, Working Group II of the IPCC (Settele et al., 2014) concluded with ‘high confidence’ that anthropogenic climate change has had, and will continue to have, a strong effect on plant life cycles and species’ interactions.
These simple, expected patterns of response to ACC, which have less than one in a billion chance of occurring at random (Table 1), have had crucial input into policy, as witnessed by the Copenhagen Accord drawn up at COP15 which decreed:
‘To achieve the ultimate objective of the Convention to stabilize greenhouse gas concentration in the atmosphere at a level that would prevent dangerous anthropogenic interference with the climate system, we shall, recognizing the scientific view that the increase in global temperature should be below 2 degrees Celsius, … enhance our long-term cooperative action to combat climate change.’
(Report of the Conference of the Parties on its fifteenth session, Copenhagen December 2009, United Nations Framework Convention on Climate Change (UNFCC))
This focus on the ‘big picture’ overall impacts has meant that relatively little research has gone into understanding changes that are counter-intuitive or unexpected as responses to warming climate. Nonetheless, every biological community investigated in detail has contained a minority of species that appeared to be showing no response or even changing in a direction counter to expectation from local climate change. This has been true even for communities experiencing strong regional warming. Thus, while there is much that we do know about plant response to climate change, many aspects remain poorly studied, controversial, or simply confusing. This is particularly true for studies on plant population and community ecology where translation of experiments conducted in controlled environments into ‘real-world’ plant communities has proven to be notoriously difficult.
In a recent meta-analysis, Wolkovich et al. (2012) showed that phenological responses to experimental warming treatments failed to match long-term observational responses for many plants, even for the same species growing in the same regions. Most disturbingly, responses not only differed in magnitude, but sometimes differed in direction as well. It is not surprising that plant community responses to climate change in real-world environments are more complex than predictions from relatively simple experiments and models of past decades, but the question remains ‘what drives these differences from expectation?’.
With these issues in mind, Annals of Botany invited a number of experts to present their research at a sponsored symposium session titled Plants and Climate Change: Complexities and Surprises, held during the 99th Ecological Society of America (ESA) meeting in Sacramento, California, in August 2014. In this special issue, we bring together studies presented at ESA 2014 with additional manuscripts submitted by researchers from around the globe. We highlight ways in which increased ability to interrogate long-term data sets with sophisticated statistical and modelling techniques is generating evidence that many apparently counter-intuitive changes can indeed be understood in the light of climate change after accounting for the true complexity of species’ responses and species’ interactions.
Our aim in this Review article is to set the context for these new studies by summarizing current knowledge and then to suggest how future research might be targeted to better understand observed departures from straightforward expectations. An emergent theme from this synthesis is that in order to better forecast long-term consequences of climate change on plant community structure and function, ecologists must embrace and dissect apparent departures from theoretical predictions, rather than simply assume that a given study ‘got it wrong’.
We begin with the most data-rich topic—phenological shifts—for which a substantial body of research has shown that advancement of plant growth and flowering has been widely associated with spring warming (Parmesan and Yohe, 2003; Root et al., 2003, 2005; Parmesan, 2007; Poloczanska et al., 2013). There is also emerging evidence for differences in magnitude of responses across different trophic levels (Parmesan, 2007; Thackeray et al., 2010; Poloczanska et al., 2013). As usual, these generalities camouflage individual changes that run counter to the overall trend, and in this case these apparent idiosyncrasies have proven amenable to the ‘dissection’ approach that we recommend. For example, Cook et al. (2012, summarized in detail below) found that some three-quarters of ‘non-responding’ species actually were responding quite strongly to warming seasons, simply in more complex ways than previously recognized.
Next, we turn to the second most common topic in climate change impacts, that of changes in species’ distributions. The overall message from global meta-analyses of long-term observational datasets indicates that major shifts in species’ distributions have already occurred, with some species showing range contractions and others range expansions (Parmesan and Yohe, 2003; Root et al., 2003; Parmesan, 2006; Poloczanska et al., 2013). Species distribution models (SDMs) offer some insights into future biogeographies, but there are still large uncertainties. Both observational and modelling studies point to a series of issues for plant conservation. New challenges include the need for new conservation tools, including controversial approaches such as assisted colonization (Hoegh-Guldberg et al., 2008). Further, as species alter their distributions in attempts to track a shifting climate space, they move into novel geographic areas, opening the possibility for these exotic species to become invasive. Indeed, early concerns about climate change were that existing exotics would benefit over natives and become invasive, and that already invasive species could become even more damaging to native communities and ecosystems (Dukes and Mooney, 1999).
We then discuss what is known about the roles of plasticity and evolution in shaping species’ responses to anthropogenic climate change. While recognizing that studies on this issue are still relatively few, there are emerging signs of limited capacity for in-situ adaptive response (Parmesan, 2006). We then turn to some under-studied areas. Experiments on seed and seedling responses are relatively rare, and there is no comprehensive review of these early plant life history stages, although this critical phase often suffers the highest mortality. Although there is a long history of research on the impacts of eCO2 on plant physiology, growth and reproduction (Bolas and Henderson, 1928), few experiments couple CO2 treatments with expected climatic warming and/or shifts in precipitation regimes. Looking more broadly, there is a dearth of studies that incorporate ACC treatments (climate and eCO2) into experiments with other global change drivers (e.g. nutrient addition or land fragmentation). Once again, our need for better mechanistic understanding of plant responses, including physiological and life-history responses, in the context of simultaneous pressure from multiple environmental changes is illuminated by apparent inconsistencies between laboratory and field experiments, and between experimental results and long-term observational data.
Finally, we ask what, if anything, can be done to improve our ability to predict which plant species are likely to respond most to ‘climate change’ in the broadest sense, that is including the direct impact of eCO2 together with indirect effects on plants via changes in temperature and precipitation. More specifically, does incorporation of plant functional traits or functional groupings (based on shared life history traits) into analyses of experiments and long-term observations, as well as into theoretical models, offer any improvement in understanding and predicting plant responses?
PHENOLOGY: OTHER SEASONS, OTHER REASONS
Spring advancement: expected and counter-intuitive responses
The impact of a warming climate on spring plant phenology is beyond doubt (Menzel et al., 2006; Settele et al., 2014). Germination (Milbau et al., 2009; De Frenne et al., 2012), leaf emergence (Slayback et al., 2003; Jeong et al., 2011), flowering and fruiting (Fitter and Fitter, 2002; Cook et al., 2012; Xia and Wan, 2013), and general green-up of the northern hemisphere (Piao et al., 2015) have all advanced in concert with regional warming trends (Menzel et al., 2006; Parmesan, 2007; Poloczanska et al., 2013). Further, the way in which species respond to warming may itself be changing. In a study of 13 temperate trees from 1980–2012, Fu et al. (2015) found that the ‘heat requirement’ for leaf flushing had increased over time in every case, on average by almost 50 %—a striking result for which the mechanism was not understood.
While patterns of advancing spring events are the dominant response, in every study there have been some species showing no response (no change in timing) and even a few that have delayed spring events in places where regional climate has warmed. Cook et al. (2012) explored this diversity of response by re-analysing the long-term database of Fitter and Fitter (2002) from England, investigating sensitivities of individual plant species to temperatures throughout the year, not just in spring as in prior analyses.
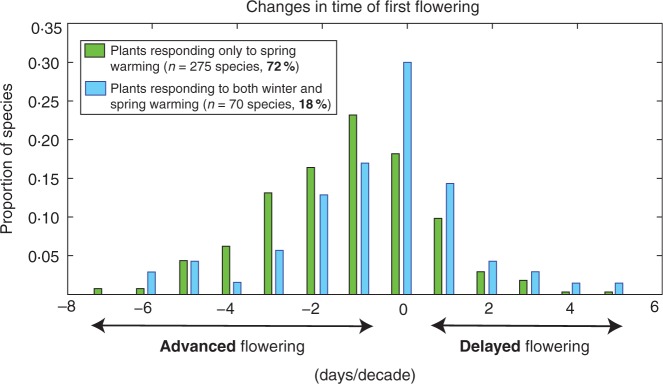
The majority of species in the Cook et al. (2012) study (72 %) were sensitive only to spring temperatures and responded to warmer springs by flowering earlier in the year, with a mode at 1 day/decade flowering advancement (Fig. 1, green bars). Most of the remaining plants—18 % of those in the study—were apparently unresponsive to warming, with a mode at ‘0’ change, or were changing counter to expectations, by delaying their flowering (Fig. 1, blue bars). By seeking separate responses to different time periods, Cook et al. found that these ‘non-responding’ species were indeed sensitive to climate, but in a manner consistent with a winter ‘vernalization’ requirement. These were species whose strategy to avoid initiating activity in midwinter (i.e. because of a brief winter warm period, called a ‘false spring’) was to require accumulated winter chilling before responding to spring warmth. In these species, spring activity was advanced by more intense winter chilling and retarded by the recent trend for warming autumns and winters. However, just as in the 72 % majority, warming spring temperatures still drove spring advancement.
Fig. 1.
Changes in flowering phenology (timing of first flowering) from 1954 to 2000 for plant species in northern England. Green bars are species that showed sensitivity only to spring temperatures; blue bars are species that showed sensitivity to both spring and autumn/winter temperatures. Modified from Cook et al. (2012).
Thus, it was the sum of two opposing drivers, spring retardation driven by winter warming and spring advancement driven by spring warming, that generated the observed long-term data in the minority of species that failed to show spring advancement over 50 years of local warming. Despite appearances, these ‘non-responders’ were indeed sensitive to climate change. Overall, 90 % of the plants in the Cook et al. (2012) study were responding to climate warming, with 72 % of species responding in the classically expected manner, 18 % showing the conflicting responses associated with vernalization, and only 10 % being genuinely climate insensitive.
Such complex responses suggest that we are likely underestimating the proportion of species sensitive to anthropogenic climate change. The Cook et al. (2012) study also implies that better knowledge of vernalization requirements would help predict plant responses in seasonal climates. However, despite the fact that vernalization is well established as a concept and well-studied for its practical importance to model species and to crop plants (Colton-Gagnon et al., 2014), few ecologists have explicitly examined this process in wild plants (but see Körner and Basler, 2010). Although there are already indications that reduced vernalization has affected many northern temperate species, research on this, and on follow-on impacts such as subsequent growth, reproduction, fecundity and germination, lags far behind other aspects of plant physiological response to climate change.
Autumn delay vs. advance: uncertain expectations
A recent review (Gallinat et al., 2015) titled ‘Autumn, the neglected season….’ laments continued emphasis on studies of spring rather than autumnal events, despite the diversity and biological importance of the latter, including fruit ripening, leaf senescence, carbon storage, and phenological synchrony of plant and herbivore preparation for winter. Specifically with respect to plant phenology, the relative paucity of autumnal studies and long-term databases limits our ability to test even simple predictions, such as whether warming autumns drive ‘autumn delay’ of leaf senescence. The largest meta-analysis of autumnal events, by Menzel et al. (2006) across Eurasia, found that, on average, fruit ripening was advanced and leaf senescence was delayed by warming temperatures, especially by warming in the month preceding the event. However, these generalities were much less clear than the phenological advancements associated with spring warming. For example, the effect of each 0·1 °C of warming on autumn delay of leaf colour was much less strong than its effect on spring advance (e.g. of budburst, leaf-out or flowering), and appeared to be region specific. In one geographic region, Eastern Europe/Russia, warming was associated with advancing rather than delayed leaf senescence, while in Germany, with extensive data, no effect at all was detected.
A recent study suggests a causal link between spring budburst and autumn senescence. Using satellite data plus detailed studies of three deciduous trees, Keenan and Richardson (2015), found that early spring budburst was associated with early autumn senescence, and that models including both spring budburst and autumn temperatures predicted timing of leaf senescence better than traditional models using combinations of temperature and photoperiod.
It is important to continue improving our understanding of autumn events, since they have the potential to impact plant growth and nutrient resorption, and thus ecosystem productivity and functioning (Richardson et al., 2010; Wu et al., 2013; Estiarte and Peñuelas, 2015). Moreover, delayed autumn, when combined with advanced springs, may lengthen the growing season for many plants that are in regions where summer growth is not limited by extremes of temperature or precipitation (Parmesan, 2007; Jeong et al., 2011). Marchin et al. (2015) imposed experimental warming to woody deciduous species in North Carolina and extended the growing season by as much as 20–29 days, with almost equal contributions from spring advance and autumn delay.
Changing trophic synchrony
Magnitude of phenological responses differs among trophic levels. In their global meta-analysis of marine systems, Poloczanska et al. (2013) found that predators (fish and zooplankton) had advanced significantly more than their potential food resources (phytoplankton). Similarly, in a meta-analysis of northern hemisphere data, herbivorous insects (butterflies) had advanced at rates three times faster than potential host plants (herbs) (Parmesan, 2007). Likewise, Thackeray et al. (2010) found a trend for UK primary consumers to advance more than producers, though this was not significant. In the same study, secondary consumers had advanced significantly less than all other trophic levels, at about half the rate of the plants and herbivores. These meta-analyses suggest increasing asynchrony between interacting trophic levels (predator–prey and insect–host).
An increasing asynchrony between flowers and their pollinators has already been detected. McKinney et al. (2012), for example, found that peak flowering has advanced more than the arrival dates for migratory hummingbirds, but only at the most northern breeding sites, indicating greater ACC induced stress on poleward rather than equatorial limits. This is contrary to expectations, in which climate amelioration at the northern range limit would be expected to provide less stressful breeding conditions, highlighting the importance of taking species interactions into account.
Interactions of plants and their pollinators may also be expressed through shifts in nectar allocation as well as shifts in timing of flowering. For example, Mu et al. (2015, this issue) looked at the effects of seasonal and diurnal warming patterns on nectar production in the Tibetan Saussurea nigrescens (Asteraceae). Although nectar concentrations remained unchanged by experimental warming treatments, there was a large-scale reduction in nectar volume. Remarkably few studies have examined how floral rewards are likely to be affected by climate warming or drought, yet as Mu et al. (2015) point out, the repercussions for interactions with pollinators could well be profound (see Scaven and Rafferty, 2013 for a review).
Future directions in phenological research
Overall, considerable uncertainty remains about the relative roles of seasonal changes in temperature, precipitation and photoperiod in driving phenological dynamics, thereby hindering our abilities to predict how yearly phenological events may, or may not, shift with changes in climate (Körner and Basler, 2010). More detailed analyses of geographical variation in plant response may help, and it is clear from Menzel et al. (2006) that strong regional differences exist. This variability opens up the possibilities of comparing responses of different species in different sites that differ in some systematic way (e.g. in resource limitation or other abiotic or biotic environments) that carries an a priori expectation of having specific differences in effects on phenologies under similar climatic change (from theory or empirical studies).
Two papers in this issue contribute to the development of this crucial area. Panchen et al. (2015, this issue) monitored leaf phenology of over 1300 deciduous woody species at six botanical gardens and arboreta in Asia, North America, and Europe. They report that although leaf senescence times varied markedly between species and location, they were not predictable according to taxonomic affiliation or plant growth form. Gill et al. (2015, this issue) conducted a meta-analysis of studies on the timing of autumn leaf senescence in the northern hemisphere and showed that warming could explain an overall 0·2 d per year delay in leaf senescence. They also report how senescence at high-latitude sites is more sensitive to photoperiod and at low-latitude sites it is more sensitive to temperature. These patterns contrasted markedly with leaf emergence times, suggesting that senescence is governed by a larger suite of local environmental factors than spring emergence. This makes understanding what governs autumn senescence more challenging and adds complexity if we wish to model how autumn delay might affect plant species, communities, and interactions with herbivores.
While remote-sensing techniques have been effective in discerning and analysing differences among years and regions in community metrics, such as ‘green-up’ (Fitchett et al., 2015; Piao et al., 2015), they cannot effectively distinguish between component species within ecosystems. However, many plant species of interest, such as shrubs and trees, are too large to transplant into common gardens. Primack et al. (2015, this issue) discuss a technique for comparing tree phenologies that involves clipping dormant twigs in the field for use in subsequent laboratory studies that focus on key phenology metrics like leaf mergence, frost sensitivity, flowering, and leaf senescence. They argue that this technique offers an opportunity to disentangle the drivers of plant phenology by permitting examination side-by-side of diverse species from distant regions. Conducting these comparisons in controlled and repeatable conditions should nicely complement the ever more detailed observations available from field and satellite studies.
As this series of studies show, climate fluctuations within, as well as among, years are vital to our understanding of plant phenology and suggest a pressing need to combine at least two approaches: experimental to examine plant ecophysiological response to changes in climate change via changes in phenologies, and modelling to determine how each phase of the life cycle responds to long-term climate trends. The latter has been done only rarely, partly because the lack of long-term data sets is a major hindrance. Plant responses that cover replicate climate events are needed to eliminate the stochastic from actual trends and this often requires five decades or more of continuous data gathering (see Cook et al., 2012).
DISTRIBUTIONS: RUNNING TO KEEP UP
Data on long-term distribution changes in plants are much less plentiful than those on phenological changes. Among the data that exist, there is, as expected, a general trend for poleward and upward (altitudinal) range shifts (Table 1). While there is no continental-global scale analysis of distributional changes devoted solely to plants, many regional meta-analyses of plant geographic changes exist (e.g. for areas in Antarctica, Le Roux and McGeogh, 2008; for plants in southern California, Kelly and Goulden, 2008; and the forests of Vermont, Beckage et al., 2008). These overall trends belie large differences among species, even among those within a given taxonomic group in a given region. In contrast to the phenological literature, where the overall signal of response is strong, the range shift literature contains multi-species studies where the signal is weak, non-significant (e.g. van Bogaert et al., 2011; Zhu et al., 2012), or counter to expectation from known regional warming (e.g. Crimmins et al., 2011).
This diversity among studies may simply stem from the greater impact of other global change drivers (e.g. land use change) on distributions than on phenology, making it more difficult to control for confounding factors in studies of the former. However, as we have seen with aberrant phenological changes, in some cases apparently counter-intuitive distributional changes can be understood in the light of climatic drivers other than the typical reference metric of mean annual temperature. For example, Crimmins et al. (2011) studied elevation data for 64 plant species gathered across a large portion of California in two census periods: the first in the 1930s, the second in the 2000s. Contrary to simple expectations, they documented downward shifts in about 72 % of species (mean shift = 88·2 m downhill). Subsequent analyses revealed that these changes in distribution mirror changes in water availability, and so species were actually tracking geographic shifts in their climate niche over time, but that niche was driven mainly by water deficit rather than temperature (Fig. 2).
Fig. 2.

ACC has driven an increase in precipitation in much of the more northerly latitudes (IPCC, 2014). This has, in turn, driven complex responses in wild plants. Mountain hemlock (Tsuga mertensiana) has shifted the centre of gravity of its distribution to lower elevations in California (a 255 -m downwards elevational shift), in spite of an increase in mean annual temperature of 2·85°C during the study period (1930s to 2000s). The observed downhill shift, while counter to expectations from warming, was in concert with a decline in water deficit of 134 mm over the past 70 years (Crimmins et al., 2011). Photo is courtesy of Charles Webber © California Academy of Sciences.
Camarero et al. (2015, this issue) document how an extreme cold event in 2001 caused large-scale mortality and die-back in Scots pine (Pinus sylvestris) close to its low-latitude range limit in Spain. The wider point they make is that traditional modelling approaches that rely solely on shifts in maximum and minimum monthly temperatures cannot predict how distributions respond to climate change. Rather, understanding impacts of climatic extremes is essential if we are to understand more fully how climate will affect plant distributions over coming decades. A second important contribution offered by this paper is that it focuses on a so-called ‘trailing’ range edge (i.e. equatorial range boundaries), rather than the more commonly studied leading range edge (i.e. poleward range boundaries) generated from the majority of data from northern temperate and boreal zones (Parmesan, 2006; Poloczanska et al., 2013).
Another possible factor limiting expected range expansions could be the physical difficulty of colonizing outside the existing range limit. To test this possibility, Mondoni et al. (2015, this issue), using experimental seed planting, showed that range expansion could occur, even into historically unvegetated areas exposed by retreating glaciers. Their results indicate that these alpine species may increase recruitment with moderate levels of warming (2·7 °C), due primarily to an extended snow-free season. Therefore, the absence of these same species in the wild at lower elevation sites that are naturally 2–3 °C warmer than current habitat suggests that establishment success may be dependent more on competitive interactions with other species in the plant community than on climate alone.
Range shifts driving exotics into novel areas
ACC, by driving shifts in species ranges, has directly caused the introduction of exotics into new geographic areas. Walther et al. (2009) reviewed studies of climate-mediated shifts of plants, invertebrates, fish and birds into novel lands. They found that these newly exotic species have varied impacts on their recipient communities. Some of this variation may stem from inherent differences between an exotic turning up in new areas as a native species expands its range locally, versus an exotic introduced by humans from a distant region (often another continent), expanding its local abundance and/or range from a point of introduction. These two situations are often not distinguished in the literature, and yet they are very different circumstances with different processes operating and different expectations of the impacts of range expansion. Moreover, studies often fail to distinguish between establishment of an exotic plant (i.e. having a reproducing population) versus an invasive plant (i.e. having measureable negative impacts on the native community).
Assisted colonization
Growing evidence that even the small level of warming associated with ACC (∼0·8 °C globally) has driven significant, and sometimes large, shifts in species’ distributions has led to calls for radical new approaches in conservation. One of the most controversial is to help species migrate across fractured, human-dominated landscapes through human-assisted transport of individuals and populations. This process is known as ‘assisted colonization’, ‘assisted migration’ or ‘assisted translocation’. It remains controversial, primarily because it involves introducing species into areas where they have not existed in recent history (or ever). Some conservation biologists worry about the risk that introduced species will become invasive in the recipient communities (e.g. Ricciardi and Simberloff, 2009), but a review of 50 peer-reviewed studies (Hewitt et al., 2011) found that 60 % were generally supportive of some form of assisted colonization. Cognizant of the inherent risks but also likely benefits of assisted colonization, some authors have developed frameworks to minimize risk and guide practitioners in deciding when assisted colonization may be both necessary and justified to prevent extinction of particular species (Hoegh-Guldberg et al., 2008; Richardson et al., 2009).
There is a clear need for basic research that would give us greater insight into what happens when novel communities are formed, whether from ACC or other anthropogenic activities (Lindenmayer et al., 2008; Hobbs et al., 2009). The goal is to have a predictive framework for anticipating when a particular species might become invasive when introduced into a novel community versus when it might simply increase local species richness without obvious harm.
After two decades of demographic monitoring, Gross and Mackay (2014) concluded that the persistence of translocated Olearia flocktonia in new habitats could only be ensured where active management facilitated seedling establishment through annual disturbance to the soil. Wadgymar et al. (2015) transplanted seeds from four populations of a North American annual legume, Chamaecrista fasciculata, into a common garden sited >130 km north of the species’ current distribution. Seeds from northern populations did well in slightly more northerly sites, but differences in flowering time between northern and southern populations at the transplant site, both in ambient and in artificially warmed treatments, ‘severely restricted mating opportunities’. While this study is generally supportive of the potential for success of assisted colonization outside the historic species’ range boundary, the observed persistent asynchrony in reproduction among population sources suggests that attempts to increase resilience to climate change through increasing local genetic diversity may be more difficult than previously thought. These studies also highlight the fact that active intervention may be needed even after plants have been relocated.
However, individual species are not alone in being impacted by ACC. Many projections, primarily using SDMs, show that entire ecosystems may lose climate space over coming decades, necessitating translocation of entire communities (Williams et al., 2007). This would likely require a level of ecosystem engineering that is not yet possible (Hoegh-Guldberg et al., 2008; Lindenmayer et al., 2008; Hobbs et al., 2009), but for which sound science is slowly emerging out of recent advances in basic research in restoration ecology (Perring et al., 2015). However, as Wiens and Hobbs (2015) caution, restoration goals often differ from conservation goals, and to protect biodiversity in the face of rapid climate change, these two disciplines require rapid transference of emerging knowledge and convergence of priorities.
DOES ACC PROMOTE INVASION?
Leaving aside the issue of ACC-driven range shifts driving arrival of exotic species into new areas, the question remains as to what extent ACC may aid exotic species already present to become invasive (so-called ‘sleeper weeds’), and encourage already invasive species to thrive even more. Owing in no small part to their higher phenotypic plasticity (Funk, 2008; van Kleunen et al., 2015), there is a widespread assumption that exotic species will increase their invasiveness as a result of global change. Even though there has been a concerted effort to understand what causes some established exotics to become invasive while others do not, the precise mechanisms that favour invasion are still obscure. Further, while there is some evidence that exotics tend to respond more favourably to anthropogenic disturbances than do natives (Dukes and Mooney, 1999; Bradley et al., 2010), it is unclear how anthropogenic climate change fits into this generalization.
Diez et al. (2012) lay out a detailed set of arguments as to why non-natives should, theoretically, thrive as extreme climate events increase in frequency and intensity due to ACC. However, empirical data lag far behind theory. Diez et al. point out that few experimental or long-term field studies have quantified the impacts of extreme climate events on natives versus non-natives, and the ones that do have not consistently supported theory. For example, experimental fire treatments conducted in Texas in 2011 were unusually hot due to that year suffering a combination of mega-drought and heat wave. These extreme fire events (on top of the underlying drought and heat stress) did not have much effect on plant performance of non-natives of any kind or on native grasses, but significantly increased species richness of native forbs (Twidwell et al., 2012).
There have been two major syntheses of native/non-native plant responses to anthropogenic climate change. Bradley et al. (2010), in a review of seven studies manipulating CO2, concluded that eCO2 tends to favour non-native species, and thereby could promote invasiveness. However, in this same review, Bradley et al. (2010) found 15 studies that examined the effects of increased temperatures and/or altered precipitation regimes on measures of performance of exotic compared with native plant species and concluded that ‘experiments have shown that increasing temperatures and changing precipitation do not consistently aid plant invasion…. These findings indicate that changing temperature and precipitation could help or hinder invasive plants depending on the species, location, magnitude and seasonality of change.’
A larger formal meta-analysis by Sorte et al. (2013) included 68 plant-based studies that incorporated experimental manipulations of 103 combinations of eCO2, altered precipitation (increased and decreased) and increased temperatures on some 249 native plants and 212 non-native plants (note: some species were replicated across multiple treatments). They found no overall quantitative differences between native and non-native terrestrial species (dominated by plants) in response to any of the treatments. Though some interesting (non-significant) trends did emerge, exotics did not always do better. Exotics responded most favourably to eCO2 and increased precipitation, while native species performed better under higher temperatures and decreased precipitation.
ROLES OF PLASTICITY AND EVOLUTION IN SHAPING RESPONSES
A high likelihood of being exposed to novel, potentially stressful conditions is shared by invading exotic species and by populations experiencing climate warming in situ. In both circumstances, plants may respond by a combination of plastic and genetic/epigenetic change. Some authors stress the importance of plasticity (Nicotra et al., 2010), while others protest that evolution is too frequently ignored in predicting responses to climate change (Hoffmann and Sgro, 2011; Anderson et al., 2012).
It is clearly important to understand both processes, for the purposes of planning conservation under climate change and for managing productivity of economically important plant populations (des Marais et al., 2013). In recognition of this need, a special issue of Evolutionary Applications, edited by Merilä and Hendry (2014), summarizes the relative roles of plasticity and evolution in climate change biology, with one paper (Franks et al., 2014) devoted exclusively to plants. The editors urged better and more standardized methodology to distil the processes involved as natural populations adapt, or fail to adapt, to current climate warming. They also caution against what they regard as a too frequent untested assumption that observed changes, whether plastic or genetic, are indeed adaptive.
Evolution and climate change
In stressing the importance of evolution, Hoffmann and Sgro (2011) argue that evolutionary adaptation ‘might be the only way that threatened species can persist if they are unable to disperse naturally or through human-mediated translocation to climatically suitable habitats’. They also point out that threatened species are not necessarily rare or ecologically insignificant, and that the ‘dominant conifers’ threatened by climate-augmented bark-beetle attack are keystone species in their communities. In the light of the their view that evolutionary responses will be important, Hoffman and Sgro (2011) lay out criteria for assessing the potential for evolutionary adaptation, and Sgro et al. (2011) recommended methods for assessing and promoting evolutionary resilience in threatened populations and communities.
Despite this potential importance of evolution, Merilä and Hendry (2014) conclude that ‘evidence for genetic adaptation to climate change has been found in some systems, but is still relatively scarce’. Moreover, Anderson et al. (2012) failed to find a single study of undisturbed plants that actually tracked allele changes over time. Their conclusion is apparently at odds with Franks et al. (2014), however, who found ‘at least some’ evidence of evolutionary response by plants in every study that sought it (n = 35 studies) as well as evidence of plastic response in every study that met Merilä and Hendry’s (2014) criteria for inclusion (n = 29 studies). However, the appearance of conflicting results is deceptive, since Franks et al. (2014) deduced evidence for evolution indirectly from a diversity of phenomena, including results from experiments, as well as using ‘space-for-time substitution’, which is essentially documentation of local population adaptation across a species’ range.
Plasticity and climate change
Overall, effects of plasticity have dominated the botanical literature (Ghalambor et al., 2007; Nicotra et al., 2010; Anderson et al., 2012). Anderson et al. (2012) concluded that the bulk of responses to experimental eCO2 have been physiologically based plastic responses and are not associated with genetic adaptation. They also found that long-term studies of individual populations tend to be dominated by phenotypically plastic responses rather than rapid evolution. Ghalambor et al. (2007) noted that adaptive plasticity facilitates persistence in novel environments, while non-adaptive plasticity in response to stress may trigger expression of cryptic genetic variation and thereby assist evolutionary adaptation to the novel environment. Plasticity could also be important in predicting population dynamics at range boundaries (Nicotra et al., 2010). Along the trailing edge, plasticity is an advantage as it allows a species to adapt to new conditions (Thuiller et al., 2008), but at the leading edge new ecological interactions also favour plastic response (Nicotra et al., 2010).
There are clear, positive, effects of plasticity on plant performance. Working with a long-term database in Massachusetts, USA, at a site originally surveyed by Henry David Thoreau in the mid-19th century, Willis et al. (2008, 2010) found that population growth was significantly more positive in exotic species that were tracking the warming trends by phenological shifts, compared with native species that were less plastic in their timing. Their conclusion that species with relatively fixed phenologies had reduced fitness under climate change compared with more flexible members of the community was replicated in a meta-analysis of warming experiments by Cleland et al. (2012). In these studies, the fitness consequences of warming climate were highly dependent upon the degree of phenological plasticity of the species.
Evidence for constraints
In what sense are plastic and evolutionary responses enabling plants to keep up with environmental change? In general, terrestrial species are shifting their distributions poleward and toward higher altitude, and marine species are shifting poleward and to greater depths. The consistency of these trends across multiple independent, regional and global meta-analyses implies that most species are relatively fixed in their ‘climate space’, the range of climates that they can tolerate and in which their populations can persist through generations. Likewise, the general trends in phenological response tend to maintain the climate spaces in which sensitive events occur. For example, earlier flowering with climate warming mitigates the change in temperature that would be experienced by flowers if they retained their original timing.
The conclusion we drew above from the generality of climate-change-driven range shifts and phenological shifts, namely that most species’ climate spaces are constrained, can be tested by asking how well plants perform in climate spaces that lie outside their recent experience. Comparison of climate spaces of species in their exotic and native ranges suggests loose constraints, since exotics can exist outside their modelled native climate spaces (Early and Sax, 2014).
In contrast, meta-analyses of transplant experiments beyond species’ ranges indicate strong constraints. Hargreaves et al. (2014) looked at performance measures of 93 species (88 of which were plants) in 42 studies. They found that 75 % of experiments documented declines in performance measures in transplants beyond the species’ range, with greater declines at greater geographical distances from the existing range boundary. The finding by Willis et al. (2008) of significant phylogenetic signal in the strength of phenological response to recent warming suggests that evolution of responsiveness is itself somehow constrained.
We present in this special issue two contrasting studies that illustrate limitations to the ability of trees to adapt to warming climate, either as individuals, through plasticity, or as populations, though rapid evolution. Šigut et al. (2015, this issue) report that photosynthetic capacity in two common European trees (the deciduous Fagus sylvatica and coniferous Picea abies) was unable to acclimate to higher leaf temperatures when plants were exposed to eCO2. While they do not rule out long-term acclimation, the authors argue that short-term heat waves might cause significant damage to primary photochemistry.
Using phylogeographic analyses across the species range of Eucalyptus wandoo in Western Australia, Dalmaris et al. (2015, this issue) report that the species’ historical range contraction from lower rainfall areas is consistent with contemporary observations of decline along the semi-arid margin of the current range. Together these observations suggest that E. wandoo has a low capacity to evolve tolerance of the reduced precipitation forecast for the region within the timeframe of ongoing change.
In contrast to these various implications of climatic constraint, Early and Sax (2014) compared ranges of 51 plant species in their native European and naturalized North American ranges, and found little concordance in climate space occupied, using climate/distribution models to define climate space. For 22 species, the majority of the introduced range lay outside the projected climate space of the native range, leading the authors to conclude that the native ranges were constrained by non-climatic factors. A similar conclusion was drawn by Bradley et al. (2015) from their study of geographic ranges of >13 000 plant species, in which potential ranges modelled from climate data were larger for exotics than for ‘comparable’ natives.
Although plasticity itself is well known to be variable among individuals and populations, there remains a dearth of studies on variation in plasticity across the geographic ranges of single species (Valladares et al., 2014), as well as among species within a given community (Nicotra et al., 2010). In order to understand how phenotypic plasticity might affect a given species’ response to ACC, and in turn how differences among species might affect community shifts, a common framework is needed (e.g. Nicotra et al., 2010).
Reciprocal transplant experiments are particularly useful in this regard. Gugger et al. (2015, this issue), in experimental reciprocal transplants of mid- and high-elevation plant species in Switzerland, found highly advanced reproduction and shortened phenophases at the lower (warmer) site for both mid- and high-elevation species, as expected. Manipulated drought stress amplified these responses and induced even further advances and shortening of phenophases, a response consistent with an ‘escape strategy’. An unexpected result was that high-elevation species were less capable of tracking warmer temperatures than mid-elevation species were of tracking colder temperatures. High-elevation species appeared more genetically constrained to their specific adaptations to an extreme environment (i.e. a short, cold growing season). These differences in plasticity revealed themselves in plant species that grew just a few hundred metres apart in elevation.
SEEDS AND SEEDLINGS: UNDERSTUDIED PHASES
Seed germination may be also be affected by ACC, as many species have specific patterns and thresholds of warm/cold and/or dry/wet periods that they must experience to break seed dormancy and trigger germination (reviewed by Donohue et al., 2010; Walck et al., 2011). However, ecologists have only recently begun to recognize how recent changes in climate patterns over the autumn and winter periods may impact subsequent germination and seedling establishment (Mondoni et al., 2012; Porceddu et al., 2013). For example, 20 years ago Fenner (1995) demonstrated that flowering and seed set of three northern temperate winter annual species were greatly increased following the imposition of pre-germination chilling. Remarkably, since this observation, there has been no systematic investigation of how winter seed stratification affects subsequent plant growth and reproductive performance in wild systems (but see Meyer et al., 2004).
Winter warming is not unique in affecting plant growth and reproduction. For example, a number of studies have shown that above-normal summer temperatures can enhance seed production, germination, and seedling establishment (Walck et al., 2011), although this response is by no means universal (Gruwez et al., 2014). Variation in seasonal precipitation regimes (with associated impacts on soil humidity) might also be expected to affect the timing and success of germination and seedling regeneration (see Walck et al., 2011 for a summary).
In their study of seed dormancy in Acacia saligna, Tozer and Ooi (2014) showed that shifts in humidity affect capacity for long-term dormancy in this Australian Mediterranean-climate species. Like many plants native to fire-prone ecosystems, seed dormancy in A. saligna ensures germination occurs only after a major disturbance event like fire clears established vegetation. In its native Western Australia, a likely increase in hot, dry conditions means that dormancy will be lengthened and the species perhaps remains able to tolerate future climate shifts. However, a similar response to the hotter drier conditions predicted for the South African Cape could further increase A. saligna’s regeneration capacity, exacerbating an already major invasive problem caused by this species in this region.
More generally, the impact of global change may be particularly severe for plants that rely on climate fluctuations to trigger regeneration. Recent evidence suggests that while global temperatures are increasing in tandem with greenhouse gas emissions, night-time temperatures have tended to increase more than daytime temperature (Easterling et al., 1997; Donat and Alexander, 2012). The germination of many species is triggered by variations in the diurnal temperature range (Thompson and Grime, 1983; Koutsovoulou et al., 2014), and for this reason their regeneration phenology may be particularly sensitive to changes in the difference between night-time minima and daytime maxima. Indeed species-specific germination response to fluctuating temperatures may contribute to plant species distributions and the maintenance of species richness in diverse plant communities (Liu et al., 2013). Climate fluctuations during a particular season may also be important; in cool temperate regions, an increased likelihood of ‘warm spells’ during winter (IPCC, 2013) might be expected to trigger germination, only for a return to ‘normal’ cold winter conditions to kill an entire cohort. It is for these reasons that an understanding of climate fluctuations is at least as important as long-term shifts in average temperature and precipitation, but as yet few studies have approached the subject from a climate change perspective.
ACC-driven increases in precipitation may pose further problems. Increased soil moisture can favour fungal pathogens and so increase pre-germination seed mortality (Walck et al., 2011), and presumably also affect post-germination susceptibility to disease and even perhaps their ability to form associations with symbiotic micro-organisms. Seedlings are also especially prone to herbivore attack (Barton and Hanley, 2013) and climate-linked fluctuation in seedling herbivore populations has been suggested as a natural filter that helps maintain plant species diversity (Hanley and Sykes, 2014). These hypothetical, yet likely impacts on plant regeneration also highlight the fact that we know comparatively little about how this key life history stage will actually respond to climate change.
COMPLEX INTERACTIONS AMONG ECOLOGICAL DRIVERS
Complex responses to rising CO2
As long ago as the 1880s experiments revealed significant plant responses to eCO2 (Kreusler, 1885), but it was not until a century later and the realization that CO2 in the Earth’s atmosphere was increasing through anthropogenic activities that plant biologists began to consider eCO2 as more than an abstract problem. A theoretical framework explaining why eCO2 affects plant ecophysiology and growth became quickly established, and many early greenhouse experiments confirmed predictions for short-term enhanced plant growth and reproduction in eCO2 (Hurd and Thornley, 1974; Polley et al., 1993; Jablonski et al., 2002). However, in subsequent longer-term experiments, including mixed microcosm and field experiments, most researchers have reported negligible impacts of eCO2 on individual species’ performance (Navas et al., 1995; Niklaus et al., 2001; Hanley et al., 2004; Smith et al., 2014). Indeed the most recent Working Group II report to the IPCC concluded that there is no clear signal that rising CO2 concentrations contribute directly to an observed increase in global primary production (Settele et al., 2014).
There are a number of likely reasons why this disparity occurs. Many of the strongest responses to eCO2 were reported initially from studies with crop species in controlled environments. Crop plants are selected for, and bred to enhance, ecophysiological traits linked to biomass accumulation and reproduction, and grow in an environment where resources are plentiful and enemies controlled. Further, they are often annuals, for which long-term trade-offs between survival and reproduction are non-existent. It should be no surprise, therefore, that crop species respond quickly to one of the few factors (CO2) that might limit productivity under conditions of plentiful nutrients and water availability. However, both crop and wild plants have shown diminished, or even no, benefits of increased CO2 when one or more nutrients are limiting (Leuzinger et al., 2011; Sardans et al., 2012).
One such example is Jin et al. (2015a, this issue) who showed that any benefits to plant performance (in field pea, Pisum sativum) arising from exposure to eCO2 were significantly reduced by soil nutrient deficiencies common in natural plant communities. In Jin et al.’s study, addition of phosphorus (P) to the eCO2 treatment enhanced water-use efficiency by a small but significant amount (+6 %), and increased the stress (drought) tolerance index quite substantially, by some 60 %. Thus, fertilization of phosphorus-deficient soils was necessary to gain maximum resistance to drought under high-CO2 conditions, as well as associated gains in deep rooting and carbon assimilation.
In their second contribution to this special issue, Jin et al. (2015b) review more deeply the implications of plant phosphorus nutrition under eCO2. They discuss possible mechanisms of how eCO2 might affect plant P demand and acquisition by changing root growth, root exudation, and other physiological processes and, in turn, how eCO2 affects the availability of P through changing biochemical processes in the rhizosphere. The studies encapsulated in Jin et al.’s review not only show that for crop and wild plant species soil fertility can influence plant response to eCO2, but also highlight that plant response to climate change is dictated as much by below-ground factors as it is by above-ground interactions.
We also know that eCO2 results in medium- to long-term progressive nitrogen limitation in the soil as increased decomposition of organic materials stimulates microbial drawing-down of soil nitrogen levels (Luo et al., 2004). Warming, on the other hand, tends to stimulate nitrogen mineralization rates (Rustad et al., 2001). Therefore, the counter-balancing effects of eCO2 and warming on soil nitrogen availability tend to cancel out any community-level effect, and together may even increase the potential for nitrogen limitation to occur (Dieleman et al., 2012).
Remarkably few studies consider the combined effects of eCO2 with experimental warming. In fact there are only about 10 operational multi-factorial FACE experiments worldwide and all these systems are located in temperate (and often relatively low-diversity) plant communities. Consequently, their capacity to inform global patterns of plant species or community response to climate change seems limited (Bond and Midgely, 2012).
In addition, only three FACE + warming designs also include water availability as an additional treatment (see Dieleman et al., 2012). Given that major changes in global and regional precipitation patterns are predicted (IPCC, 2013), that eCO2 and warming impact upon evapotranspiration and water use efficiency (Dieleman et al., 2012; Settele et al., 2014), and that in experimental water manipulations drought or additional moisture have marked effects on plant community productivity (Wu et al., 2011; Settele et al., 2014), the inclusion of precipitation in multifactorial manipulations would seem to be critical. But then one runs into a further problem; given the range of CO2, temperature, and rainfall scenarios, and the fact that the acute effects of climate extremes are probably more important than chronic changes in mean conditions, identification and application of realistic future conditions will always be challenging, and perhaps impossible (Kreyling and Beier, 2013).
Complex interactions across global change drivers
Expanding beyond climate change and eCO2, it is now clear that other anthropogenic changes are also acting in non-additive ways to alter natural systems. Tylianakis et al. (2008) synthesized data from 688 published studies on ‘the main drivers of global environmental change (CO2 enrichment, nitrogen deposition, climate change, biotic invasions and land use)’, and showed that ‘these drivers often alter competitive interactions among plants and animals, exert multi-trophic effects on the decomposer food web, increase intensity of pathogen infection, weaken mutualisms involving plants, and enhance herbivory while having variable effects on predation. A recurrent finding is that there is substantial variability among studies in both the magnitude and direction of effects of any given (global change) driver on any given type of biotic interaction.’
Similar results were found in two subsequent meta-analyses. Darling and Cote (2008) reviewed experiments with more than two treatments across 112 studies in freshwater, marine and terrestrial systems. They found that more than three-quarters of the experiments exhibited significant interaction among treatments. Crain et al. (2008), reviewing 171 experimental studies in marine systems, found that 74 % of studies showed significant, non-additive interaction effects among two or more stressors. These large syntheses support a strong conclusion that the impacts of multiple global change drivers, including ACC, do not act independently. Actual responses of wild populations, species, communities, or ecosystems are dependent upon the interactive effects among drivers operating simultaneously, and each species’ responses will differ among sites as each population experiences different combinations of drivers.
Experiments conducted under natural field conditions are helping to shed light on plant responses to multiple stressors, using highly heterogenous landscapes to mimic diverse environmental ‘treatments’. Eskelinen and Harrison (2015, this issue), working at a natural reserve in California composed of Mediterranean-climate grasslands, showed that plant responses to experimental watering treatments varied not only according to plant competition, but were also strongly influenced by soil fertility and structure. Consequently, they concluded that micro variation in soil properties can dictate plant community response at the local scale to climate-linked shifts in precipitation regimes. Without understanding this complex interplay, botanists might otherwise dismiss the variable results of studies conducted in natural conditions as ‘noise’.
Leuzinger et al. (2011) argued that ‘there might be a general trend for the magnitude of responses to decline with higher-order interactions, longer time periods and larger spatial scales. This means that on average, both positive and negative global change impacts on the biosphere might be dampened.’ Gilman et al. (2010) propose a framework for future study of species interactions under ACC.
The existence of strong interactions among global change drivers may have a silver lining. The ubiquity of this phenomenon was used by Parmesan et al. (2013) to argue for some hope in our ability to manage biodiversity conservation in the face of rapidly strengthening ACC. If a second stressor added to a climate warming treatment has been shown to increase, synergistically, the negative impacts of warming, then action to reduce that stressor in managed populations should reduce the overall negative impacts of climate change.
PLANT FUNCTIONAL TRAITS/GROUPINGS: USEFUL METRICS?
Inconsistent messages
Some authors have advocated using metrics of plant responses based on shared life history characteristics or ecophysiological traits to better understand variation in species’ responses to ACC (Lavorel et al., 1997; Chapin, 2003; Wullschleger et al., 2014). Plant functional traits (PFTs) or plant functional groups (PFGs) may aid our ability to identify characteristics most likely to exhibit plasticity in the face of environmental change (Nicotra et al., 2010; McLean et al., 2014). However, broad groupings based on a vague similarity in growth form often do not offer sufficient resolution to capture important ecophysiological characteristics. There is a need for candidate PFTs to go beyond simplistic comparisons of plant growth forms and instead capture essential ecophysiological characteristics and retain a ‘common currency’.
Despite 20 years of effort, no clear consensus about what PFTs or PFGs best predict climate response has emerged. In a large synthesis of the literature (29 studies, 6 of which were on plants; mean length of study 58 years), Buckley and Kingsolver (2012) found only a few traits significantly associated with responses, and those were inconsistent across studies. For plant distributions, only one study found a strong association, and that was for species’ growth rates to be positively associated with distributional change. Plant phenologies showed a greater number of associated traits, with phylogeny strongly associated with response in two studies, and growth rates and earlier seasonality showing strong association in a third study.
Some studies have suggested that ‘shrubs’ perform relatively well in water-stressed treatments, but closer inspection of the overall trend shows that responses tend to be confined to a limited number of species and/or result from shifts in the performance of one member of a different PFG (Grime et al., 2008; Prieto et al., 2009). Similarly Hanley et al. (2004) showed that while ‘forbs’ and ‘grasses’ both exhibited strong responses to eCO2 in chalk grassland microcosms (increasing and decreasing productivity, respectively), these changes were entirely driven by only one species in each group. This seems to be a general trend; Körner’s 2006 review noted that plant community responses to CO2 manipulations were dominated by only a few very responsive species. A meta-analysis by Poorter and Navas (2003) found no variation between ‘fast’ and ‘slow’ growing, nor between ‘monocot’ versus ‘dicot’ species, in their responses to eCO2. Interestingly, while Poorter and Navas (2003) found a difference between C3 and C4 plants, this was only seen when soil nutrients were abundant, harking back to the strong effects on interactions among drivers.
Use of broader notions for PFGs may provide additional insight. For example, focus on the community level has improved understanding of vegetation dynamics in alpine environments. Carlson et al. (2015, this issue), found a significant association between snowpack dynamics and species' taxonomic and functional diversity in the French Alps. In considering species’ mating systems, Hereford (2010) found that whether a plant was out-crossing versus selfing was not associated with patterns of local adaptation in general. Given this result, it is not surprising then that a review by Anderson et al. (2012) concluded that, in response to climate change, ‘whether outcrossers will evolve faster than selfers likely depends on a complex interplay between existing genetic variation, the source of new genetic variation, and effective population sizes’. Anderson et al. (2012) further note that ‘Seed longevity, seed dispersal, and generation time are complex functional traits that could also influence adaptive responses to changing environments’.
Back to basics: explanatory power in Grime’s C-S-R strategies
Given the lack of generalities that have emerged from the literature, does the use of PFTs and PFGs hold any predictive value? The answer may lie in how strictly one defines the term. Broad groupings based on vague similarities in growth form that often mirror taxonomic associations probably fail to offer sufficient resolution to capture important ecophysiological characteristics (Hanley et al., 2004). Yet while more sophisticated groupings based on strictly defined sets of readily quantifiable traits have been available for decades (Grime, 1979; Westoby, 1998), these seem to be applied infrequently to manipulative climate-change experiments. This is all the more remarkable given the fact that one of the first experiments to look at the effect of eCO2 on wild plants (Hunt et al., 1991), did so within the context of Grime’s C-S-R strategy scheme, in which plants are categorized as having one of three life history strategies: Competitive (able to maximize resource acquisition in productive environments), Stress-tolerant (able to survive in a poor environment), or Ruderal (able to exploit ephemeral/variable environments through rapid growth and generation time). Hunt et al. (1991) showed that the only plants to respond positively to eCO2 were those of the Competitive strategy (sensu Grime, 1979).
Further, stress strategies are beginning to emerge as driving common responses to climate change among otherwise unrelated species. For example, Zwicke et al. (2015) describe how the strategies for coping with drought tolerance in six upland grassland plants varied between species, and indeed note that such variation may even be crucial if plant communities are to remain resilient in the face of extreme drought events. Gugger et al. (2015, this issue), similarly found that high-elevation plants differed significantly from mid-elevation plants in their responses to both warming and drought, a result the authors attributed to high-elevation plants being better adapted to extreme climatic stress, which in turn drove a trade-off that compromised their ability to take advantage of an ameliorated climate.
The role of stress adaptation was also tackled by Harrison et al. (2015, this issue) who compared naturally nutrient-stressed plants of infertile serpentine soils in the Northwest USA to plant communities inhabiting nearby non-serpentine soils. They found that serpentine specialists were less sensitive to rainfall change than species on more fertile (non-serpentine) soils due to the prevalence of stress-tolerant (sensu Grime, 1979) PFTs in serpentine species. One particular trait (specific leaf area (SLA)) proved to be an excellent indicator of plant response to shifting rainfall patterns across six decades of climate change.
A future for PFTs and PFGs
Thus, in spite of failure to find associations between PFTs and impacts of ACC in the past, these new studies underscore the value of using PFTs as a ‘common currency’ in climate change studies. PFTs appear to be particularly relevant where target communities share few common plant species and for which phylogenetic controls (e.g. species pairing by genus) may be impossible (e.g. Gallagher et al., 2013; Soudzilovskaia et al., 2013).
CONCLUSIONS: COMPLEXITY AS A VIRTUE
We have dealt with only a few of the key issues facing contemporary climate change biology; in addition, myriad interactions between plants and their herbivores, symbionts and competitors are likely to be part, but not all, of the story. It is increasingly clear that variation in plant ecophysiological traits, their inherent adaptability (within and between individuals and entire populations) are vital, but attempts to treat these factors in isolation have confounded our ability to predict how any given species or community will respond to an increase in CO2, temperature, or rainfall. Nonetheless, the complexities of interactions among drivers must be better understood if we are to have any hope of predicting the effects of ACC on biological systems.
To address this need, some authors have suggested that coordinating experiments across a network of field sites (both natural and man-made) could overcome some of the problems associated with traditional manipulative experiments by allowing for application of identical manipulative treatments across a diverse set of environmental conditions as well as allowing appropriate replication of many treatment combinations by spreading different treatments across multiple experimental units (Tilman et al., 2003; Fraser et al., 2013; Parmesan et al., 2013). However, such experimental networks are only just beginning to emerge, and those that have begun are still in nascent stages with unknown outcomes. One major impediment is uncertain sources of large-scale, long-term funding needed for such networks to succeed. For example, NEON (the National Ecological Observation Network) is a set of 62 sites that encompass the major habitats ranging across the USA (including Alaska, the Hawaiian Islands and Puerto Rico). Infrastructure costs and considerable funds for monitoring were covered by a major grant from the US Congress, but funding for individual experiments must still come from traditional granting agencies, making it difficult to acquire a level of funding necessary for coordinated experimental treatments across multiple sites and habitat types.
Even without experimental manipulations, long-term coordinated observations for plant response to climate change across different regions and climates have already yielded important insights. An excellent example of this are results that have emerged from the European Phenological Gardens, which began as replicate clones of shrubs and trees planted in scientific gardens across Europe (Menzel and Fabian, 1999). This controlled database has subsequently been analysed alongside diverse data from long-term field studies at sites across Europe, ultimately numbering 542 plant species across 21 countries, providing a rich set of insights into plant responses to ACC (Menzel et al., 2006, 2008).
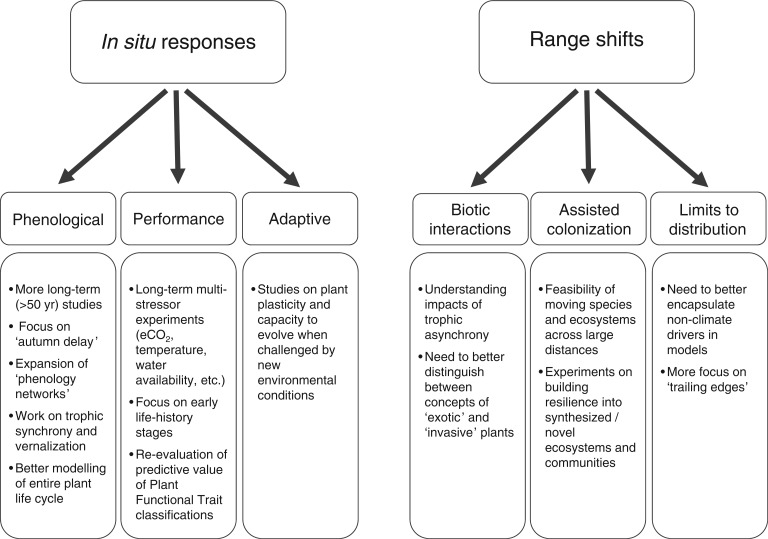
We have highlighted specific research needs for each of the topics in individual sections of our Review article (for a summary see Fig. 3). There is a plethora of other research and management recommendations in the literature, from the perspectives of ecological (Hobbs et al., 2009; Standish et al., 2014) and evolutionary resilience (Sgro et al., 2011) and conservation management and planning (Mawdsley et al., 2009; Pettorelli, 2012; Perring et al., 2015).
Fig. 3.
A summary of research priorities to enable plant scientists to better understand plant species and community response to various climate change drivers.
The papers compiled within this special issue highlight unusual, sophisticated, and even bizarre responses that plants have exhibited when challenged with complex changes in patterns of climate. We remind ourselves that the range of changes actually being experienced by wild plants is much larger than that encompassed by any individual study, and includes increases in frequencies and severities of extreme climate events associated with ACC. Plants are at least as likely to respond principally to complex interactions among elements of global change as they are to the more traditionally studied single drivers. We argue that the typically expected responses are generally well-understood, and that it is the aberrant responses that are now yielding greater insight into impacts of ACC, and therefore offer the greatest prospect for improving our ability to project plant impacts into a very uncertain future.
ACKNOWLEDGEMENTS
We thank Professor Michael C. Singer and Dr Trude Schwarzacher for their insightful comments on earlier drafts of this manuscript.
LITERATURE CITED
- Anderson JT, Panetta AM, Mitchell-Olds T. 2012. Evolutionary and ecological responses to anthropogenic climate change. Plant Physiology 160: 1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE, Hanley ME. 2013. Seedling–herbivore interactions: insights into plant defence and regeneration patterns. Annals of Botany 112: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckage B, Osborne B, Gavin DG, et al. 2008. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proceedings of the National Academy of Science of the USA 105: 4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolas B, Henderson F. 1928. Effect of increased carbon dioxide on the growth of plants. Annals of Botany 42: 509–523. [Google Scholar]
- Bond WJ, Midgley GF. 2012. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Philosophical Transactions of the Royal Society Series B Biological Sciences 367: 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH. 2010. Predicting plant invasions in an era of global change. Trends in Ecology and Evolution 25: 310–318. [DOI] [PubMed] [Google Scholar]
- Bradley BA, Early R, Sorte CJB. 2015. Space to invade? Comparative range infilling and potential range of invasive and native plants. Global Ecology and Biogeography 24: 348–359. [Google Scholar]
- Buckley LB, Kingsolver JG. 2012. Functional and phylogenetic approaches to forecasting species’ responses to climate change. Annual Review of Ecology, Evolution and Systematics 43: 205–226. [Google Scholar]
- Camarero JJ, Gazol A, Sancho-Benages S, Sangüesa-Barreda G. 2015. Know your limits? Climate extremes impact the range of Scots pine in unexpected places. Annals of Botany 116: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BZ, Choler P, Renaud J, Dedieu J-P, Thuiller W. 2015. Modelling snow cover duration improves predictions of functional and taxonomic diversity for alpine plant communities. Annals of Botany 116: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS. 2003. Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Annals of Botany 91: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland EE, Allen JM, Crimmins TM, et al. 2012. Phenological tracking enables positive species responses to climate change. Ecology 93: 1765–1771. [DOI] [PubMed] [Google Scholar]
- Colton-Gagnon K, Ali–Benali MA, Mayer BF, et al. 2014. Comparative analysis of the cold acclimation and freezing tolerance capacities of seven diploid Brachypodium distachyon accessions. Annals of Botany 113: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook BI, Wolkovich EM, Parmesan C. 2012. Divergent responses to spring and winter warming drive community level flowering trends. Proceedings of the National Academy of Sciences of the USA 109: 9000–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters 11: 1304–1315. [DOI] [PubMed] [Google Scholar]
- Crimmins SM, Dobrowski SZ, Greenberg JA, et al. 2011. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 331: 324–327. [DOI] [PubMed] [Google Scholar]
- Dalmaris E, Ramalho CE, Poot P, Veneklaas EJ, Byrne M. 2015. A climate change context for the decline of a foundation tree species in south-western Australia: insights from phylogeography and species distribution modelling. Annals of Botany 116: 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling ES, Cote IM. 2008. Quantifying the evidence for ecological synergies. Ecology Letters 11: 1278–1286. [DOI] [PubMed] [Google Scholar]
- De Frenne P, Graae BJ, Brunet J, et al. 2012. The response of forest plant regeneration to temperature variation along a latitudinal gradient. Annals of Botany 109: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais DL, Hernandez KM, Juenger TE. 2013. Genotype-by-environment interaction and plasticity: exploring genomic responses of plants to the abiotic environment. Annual Review of Ecology, Evolution and Systematics 44: 5–29. [Google Scholar]
- Dieleman WIJ, Vicca S, Dijkstra FA, et al. 2012. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Global Change Biology 18: 2681–2693. [DOI] [PubMed] [Google Scholar]
- Diez JM, D’Antonio CM, Dukes JS, et al. 2012. Will extreme climatic events facilitate biological invasions? Frontiers in Ecology and the Environment 10: 249–257. [Google Scholar]
- Donat MG, Alexander LV. 2012. The shifting probability of global daytime and night-time temperatures. Geophysical Research Letters 39: L14707. [Google Scholar]
- Donohue K, de Casas RR, Burghardt L, Kovach K, Willis CG. 2010. Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution and Systematics 41: 293–319. [Google Scholar]
- Dukes JS, Mooney HA. 1999. Does global change increase the success of biological invaders? Trends in Ecology and Evolution 14: 135–139. [DOI] [PubMed] [Google Scholar]
- Early R, Sax DF. 2014. Climatic niche shifts between species’ native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Global Ecology and Biogeography 23: 1356–1365. [Google Scholar]
- Easterling DR, Horton B, Jones PD, et al. 1997. Maximum and minimum temperature trends for the globe. Science 277: 364–367. [Google Scholar]
- Eskelinen A, Harrison S. 2015. Biotic context and soil properties modulate native plant responses to enhanced rainfall. Annals of Botany 116: 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estiarte M, Peñuelas J. 2015. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: effects on nutrient proficiency. Global Change Biology 21: 1005–1017. [DOI] [PubMed] [Google Scholar]
- Fenner M. 1995. The effect of pre-germination chilling on subsequent growth and flowering in three arable weeds. Weed Research 35: 489–493. [Google Scholar]
- Fitchett JM, Grab SW, Thompson DI. 2015. Plant phenology and climate change: progress in methodological approaches and application. Progress in Physical Geography 39: 460–482. [Google Scholar]
- Fitter AH, Fitter RSR. 2002. Rapid changes in flowering time in British plants. Science 296: 1689–1691. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Webber JJ, Aitken SN. 2014. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evolutionary Applications 7: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser LH, Henry HAL, Carlyle CN, et al. 2013. Coordinated distributed experiments: an emerging tool for testing global hypotheses in ecology and environmental science. Frontiers in Ecology and Environment 11: 147–155. [Google Scholar]
- Fu YSH, Piao S, Vitasse Y, et al. 2015. Increased heat requirement for leaf flushing in temperate woody species over 1980–2012: effects of chilling, precipitation and insolation. Global Change Biology 21: 2687–2697. [DOI] [PubMed] [Google Scholar]
- Funk JL. 2008. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology 96: 1162–1173. [Google Scholar]
- Gallinat AS, Primack RB, Wagner DL. 2015. Autumn, the neglected season in climate change research. Trends in Ecology and Evolution 30: 169–176. [DOI] [PubMed] [Google Scholar]
- Gallagher RV, Hughes L, Leishman MR. 2013. Species loss and gain in communities under future climate change: consequences for functional diversity. Ecography 36: 531–540. [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21: 394–407. [Google Scholar]
- Gill AL, Gallinat AS, Sanders-DeMott R, et al. 2015. Changes in autumn senescence in northern hemisphere deciduous trees: a meta-analysis of autumn phenology studies. Annals of Botany 116: 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends in Ecology and Evolution 25: 325–331. [DOI] [PubMed] [Google Scholar]
- Grime JP. 1979. Plant Strategies and Vegetation Processes. Chichester: Wiley. [Google Scholar]
- Grime JP, Fridley JD, Askew AP, et al. 2008. Long-term resistance to simulated climate change in an infertile grassland. Proceedings of the National Academy of Sciences of the USA 105: 10028–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CL, Mackay D, 2014. Two decades of demography reveals that seed and seedling transitions limit population persistence in a translocated shrub. Annals of Botany 114: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruwez R, De Frenne P, De Schrijver A, et al. 2014. Negative effects of temperature and atmospheric depositions on the seed viability of common juniper (Juniperus communis) Annals of Botany 113: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugger S, Kesselring H, Stöcklin J, Hamann E. 2015. Lower plasticity exhibited by high- versus mid-elevation species in their phenological responses to manipulated temperature and drought. Annals of Botany 116: 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. 2014. Seedling herbivory and the temporal niche. In: Kelly CK, Bowler MA, Fox GA. eds. Environmental fluctuation, temporal dynamics and ecological process. Cambridge University Press. [Google Scholar]
- Hanley ME, Trofimov S, Taylor G. 2004. Species-level effects determine community-level responses to elevated CO2: evidence from simulated turves. Functional Ecology 18: 304–313. [Google Scholar]
- Hargreaves AL, Samis KE, Eckert CG. 2014. Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. The American Naturalist 183: 157–173. [DOI] [PubMed] [Google Scholar]
- Harrison S, Damschen E, Fernandez-Going B, Eskelinen A, Copeland S. 2015. Plant communities on infertile soils are less sensitive to climate change. Annals of Botany 116: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford J. 2010. Does selfing or outcrossing promote local adaptation? American Journal of Botany 97: 298–302. [DOI] [PubMed] [Google Scholar]
- Hewitt HN, Klenk N, Smith AL, et al. , 2011. Taking stock of the assisted migration debate. Biological Conservation 144: 2560–2572. [Google Scholar]
- Hobbs RJ, Higgs E, Harris JA. 2009. Novel ecosystems: implications for conservation and restoration. Trends in Ecology and Evolution 24: 599–605. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Hughes L, McIntyre S, et al. 2008. Assisted colonization and rapid climate change. Science 321: 345–346. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Sgro CM. 2011. Climate change and evolutionary adaptation. Nature 470: 479–485. [DOI] [PubMed] [Google Scholar]
- Hunt R, Hand DW, Hannah MA, Neal AM. 1991. Response to CO2 enrichment in 27 herbaceous species. Functional Ecology 5: 410–421. [Google Scholar]
- Hurd RG, Thornley JHM. 1974. An analysis of the growth of young tomato plants in water culture at different light integrals and CO2 concentrations. I. Physiological aspects. Annals of Botany 38: 375–388. [Google Scholar]
- IPCC. 2013. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner G-K, et al. eds. Cambridge: Cambridge University Press. [Google Scholar]
- IPCC. 2014. Summary for policymakers. In: Field CB, Barros VR, Dokken DJ, et al. eds. Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 1–32. [Google Scholar]
- Jablonski LM, Wang X, Curtis PS. 2002. Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytologist 156: 9–26. [Google Scholar]
- Jeong SJ, Ho CH, Gim HJ, Brown ME. 2011. Phenology shifts at start vs end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Global Change Biology 17: 2385–2399. [Google Scholar]
- Jin J, Lauricella D, Armstrong R, Sale P, Tang C. 2015a Phosphorus application and elevated CO2 enhance drought tolerance in field pea grown in a phosphorus-deficient vertisol. Annals of Botany 116: 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Tang C, Sale P. 2015b The impact of elevated carbon dioxide on the phosphorus nutrition of plants: a review. Annals of Botany 116: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan TF, Richardson AD. 2015. The timing of autumn senescence is affected by the timing of spring phenology: implications for predictive models. Global Change Biology 21: 2634–2641. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Goulden ML. 2008. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences of the USA 105: 11823–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. 2006. Plant CO2 responses: an issue of definition, time and resource supply. New Phytologist 172: 393–411. [DOI] [PubMed] [Google Scholar]
- Körner C, Basler D. 2010. Phenology under global warming. Science 327: 1461–1462. [DOI] [PubMed] [Google Scholar]
- Koutsovoulou K, Daws MI, Thanos CA. 2014. Campanulaceae: a family with small seeds that require light for germination. Annals of Botany 113: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusler U. 1885. Uber eine methode zur beobachtung der assimilation und atmung der pflanzen und uber einige diese vorgange beeinflussende momente. Landwirtschaftliche Jahrbücher 14: 913–965. [Google Scholar]
- Kreyling J, Beier C. 2013. Complexity in climate change manipulation experiments. Bioscience 63: 763–767. [Google Scholar]
- Lavorel S, McIntyre S, Landsberg J, Forbes TDA. 1997. Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends in Ecology and Evolution 12: 474–478. [DOI] [PubMed] [Google Scholar]
- Le Roux PC, McGeogh MA. 2008. Rapid range expansion and community reorganization in response to warming. Global Change Biology 14: 2950–2962. [Google Scholar]
- Leuzinger S, Luo Y, Beier C, et al. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends in Ecology and Evolution 26: 236–241. [DOI] [PubMed] [Google Scholar]
- Lindenmayer DB, Fischer J, Felton A, et al. 2008. Novel ecosystems resulting from landscape transformation create dilemmas for modern conservation practice. Conservation Letters 1: 129–135 [Google Scholar]
- Liu K, Baskin JM, Baskin CC, et al. 2013. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the Eastern Tibet Plateau. PLOS One 8: e69364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Su B, Currie WS, et al. 2004. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience 54: 731–739. [Google Scholar]
- Marchin RM, Salk CF, Hoffmann WA, et al. 2015. Temperature alone does not explain phenological variation of diverse temperate plants under experimental warming. Global Change Biology 21: 3138–3151. [DOI] [PubMed] [Google Scholar]
- Mawdsley JR, O’Malley R, Ojima DS. 2009. A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conservation Biology 23: 1080–1089. [DOI] [PubMed] [Google Scholar]
- McKinney AM, CarraDonna PJ, Inouye DW, et al. 2012. Asynchronous changes in phenology of migrating broad-tailed hummingbirds and their early-season nectar resources. Ecology 93: 1987–1993. [DOI] [PubMed] [Google Scholar]
- McLean EH, Prober SM, Stock WD, et al. 2014. Plasticity of functional traits varies clinally along a rainfall gradient in Eucalyptus tricarpa . Plant, Cell and Environment 37: 1440–1451. [DOI] [PubMed] [Google Scholar]
- Menzel A, Fabian P. 1999. Growing season extended in Europe. Nature 397: 659. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- Menzel A, Estrella N, Schleip C. 2008. Impacts of climate variability, trends and NAO on 20th century European plant phenology. Advances in Global Change Research 33: 221–233. [Google Scholar]
- Merilä J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evolutionary Applications 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SE, Nelson DL, Carlson SL. 2004. Ecological genetics of vernalization response in Bromus tectorum L. (Poaceae). Annals of Botany 93: 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbau A, Graae BJ, Shevtsova A, Nijs I. 2009. Effects of a warmer climate on seed germination in the subarctic. Annals of Botany 104: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Rossi G, Orsenigo S, Probert RJ. 2012. Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany 110: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Pedrini S, Bernareggi G, et al. 2015. Climate warming could increase recruitment success in glacier foreland plants. Annals of Botany 116: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Peng Y, Xi X, et al. 2015. Artificial asymmetric warming reduces nectar yield in a Tibetan alpine species of Asteraceae. Annals of Botany 116: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas M, Guillerm J, Fabreguettes J, Roy J. 1995. The influence of elevated CO2 on community structure, biomass and carbon dioxide balance of Mediterranean old-field microcosms. Global Change Biology 1: 325–335. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 684–692. [DOI] [PubMed] [Google Scholar]
- Niklaus PA, Leadley PW, Schmid B, Körner C. 2001. A long-term field study on biodiversity – elevated CO2 interactions in grassland . Ecological Monographs 71: 341–356. [Google Scholar]
- Panchen ZA, Primack RB, Gallinat AS, et al. 2015. Substantial variation in leaf senescence times among 1360 temperate woody plant species: implications for phenology and ecosystem processes. Annals of Botany 116: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics 37: 637–669. [Google Scholar]
- Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Global Change Biology 13: 1860–1872. [Google Scholar]
- Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Duarte CM, Poloczanska ES. 2013. Beyond climate change attribution in ecology and conservation research. Ecology Letters 16: 58–71. [DOI] [PubMed] [Google Scholar]
- Perring MP, Standish RJ, Price JN, et al. 2015. Advances in restoration ecology: rising to the challenges of the coming decades. Ecosphere 6: doi:10:1890/ES15-00121.1. [Google Scholar]
- Pettorelli N. 2012. Climate change as a main driver of ecological research. Journal of Applied Ecology 49: 542–545. [Google Scholar]
- Piao S, Jianguang T, Anping C, et al. 2015. Leaf onset in the northern hemisphere triggered by daytime temperature. Nature Communication 6: 6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley HW, Johnson HB, Mayeux HS, Malone SR. 1993. Physiology and growth of wheat across a subambient carbon dioxide gradient. Annals of Botany 71: 347–356. [Google Scholar]
- Poloczanska ES, Brown CJ, Sydeman WJ, et al. 2013. Global imprint of climate change on marine life. Nature Climate Change 3: 919–925. [Google Scholar]
- Porceddu M, Mattana E, Pritchard HW, Bacchetta G. 2013. Thermal niche for in situ seed germination by Mediterranean mountain streams: model prediction and validation for Rhamnus persicifolia seeds. Annals of Botany 112: 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Navas M-L. 2003. Plant growth and competition and elevated CO2 on winners, losers and functional groups. New Phytologist 157: 175–198. [DOI] [PubMed] [Google Scholar]
- Prieto P, Penuelas J, Lloret F, Llorens L, Estiarte M. 2009. Experimental drought and warming decrease diversity and slow down post-fire succession in a Mediterranean shrubland. Ecography 32: 623–636. [Google Scholar]
- Primack RB, Laube J, Gallinat AS, Menzel A. 2015. From observations to experiments in phenology research: investigating climate change impacts on trees and shrubs using dormant twigs. Annals of Botany 116: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi A, Simberloff D. 2009. Assisted colonization: good intentions and dubious risk assessment. Trends in Ecology and Evolution 24: 476–477. [DOI] [PubMed] [Google Scholar]
- Richardson DM, Hellmann JJ, McLachlan J. 2009. Multidimensional evaluation of managed relocation. Proceedings of the National Academy of Sciences of the USA 106: 9721–9724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AD, Black TA, Ciais ND, et al. 2010. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philosophical Transactions of the Royal Society B Biological Sciences 365: 3227–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root TL, Price JT, Hall KR, et al. 2003. Fingerprints of global warming on wild animals and plants. Nature 421: 57–60. [DOI] [PubMed] [Google Scholar]
- Root TL, MacMynowski DP, Mastrandrea MD, Schneider SH. 2005. Human-modified temperatures induce species changes: joint attribution. Proceedings of the National Academy of Sciences of the USA 102: 7465–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig C, Karoly D, Vicarelli M, et al. 2008. Attributing physical and biological impacts to anthropogenic climate change. Nature 453: 353–358. [DOI] [PubMed] [Google Scholar]
- Rustad L, Campbell J, Marion G, et al. 2001. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126: 543–562. [DOI] [PubMed] [Google Scholar]
- Sardans J, Rivas-Ubach A, Penuelas J. 2012. The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspectives in Plant Ecology, Evolution and Systematics 14: 33–47. [Google Scholar]
- Scaven V, Rafferty NE. 2013. Physiological effects of climate warming on flowering plants and insect pollinator and potential consequences for their interaction. Current Zoology 59: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settele J, Scholes R, Betts R, et al. 2014. Terrestrial and inland water systems. In: Field CB, Barros VR, Dokken DJ, et al. eds. Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 271–359. [Google Scholar]
- Sgro CM, Lowe AJ, Hoffmann AA. 2011. Building evolutionary resilience for conserving biodiversity under climate change. Evolutionary Applications 4: 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šigut L, Holišová P, Klem K, et al. 2015. Does long-term cultivation of saplings under elevated CO2 concentration influence their photosynthetic response to temperature? Annals of Botany 116: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayback DA, Pinzon JE, Los SO, Tucker CJ. 2003. Northern hemisphere photosynthetic trends 1982–99. Global Change Biology 9: 1–15. [Google Scholar]
- Smith SD, Charlet TN, Ziter SF, Arbella SR, Vanier CH, Huxman TS. 2014. Long-term response of a Mojave desert winter annual plant community to a whole system-ecosystem atmospheric CO2 manipulations (FACE). Global Change Biology 20: 879–892. [DOI] [PubMed] [Google Scholar]
- Sorte CJB, Ibanez I, Blumenthal DM, et al. 2013. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species’ performance. Ecology Letters 16: 261–270. [DOI] [PubMed] [Google Scholar]
- Soudzilovskaia NA, Elumeeva TG, Onipchenko VG, et al. 2013. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proceedings of the National Academy of Sciences of the USA 110: 18180–18184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standish RJ, Hobbs RJ, Mayfield MM, et al. 2014. Resilience in ecology: abstraction, distraction, or where the action is? Biological Conservation 177: 43–51. [Google Scholar]
- Thackeray SJ, Sparks TH, Frederiksen M, et al. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Global Change Biology 16: 3304–3313. [Google Scholar]
- Thompson K, Grime JP. 1983. A comparative study of germination responses to diurnally fluctuating temperatures. Journal of Applied Ecology 20: 141–156. [Google Scholar]
- Thuiller W, Alberta C, Araújob MB, et al. 2008. Predicting global change impacts on plant species’ distributions: future challenges. Perspectives in Plant Ecology, Evolution and Systematics 9: 137–152. [Google Scholar]
- Tilman DG, Aber JD, Bergelson JM, et al. 2003. NEON: addressing the nation's environmental challenges. An assessment of the National Research Council of the National Academies, Committee on the National Ecological Observatory Network. Washington, DC: The National Academies Press. [Google Scholar]
- Tozer MG, Ooi MKJ. 2014. Humidity-regulated dormancy onset in the Fabaceae: a conceptual model and its ecological implications for the Australian wattle Acacia saligna . Annals of Botany 114: 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twidwell D, Rogers WE, McMahon EA, et al. 2012. Prescribed extreme fire effects on richness and invasion in coastal prairie. Invasive Plant Science and Management 5: 330–340. [Google Scholar]
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecology Letters 11: 1351–1363. [DOI] [PubMed] [Google Scholar]
- Valladares F, Matesanz S, Guilhaumon F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecology Letters 17: 1351–1364. [DOI] [PubMed] [Google Scholar]
- van Bogaert R, Haneca K, Hoogesteger J, et al. 2011. A century of tree line changes in sub-Arctic Sweden shows local and regional variability and only a minor influence of 20th century climate warming. Journal of Biogeography 38: 907–921. [Google Scholar]
- van Kleunen M, Dawson W, Maurel N. 2015. Characteristics of successful alien plants. Molecular Ecology 24: 1954–1968. [DOI] [PubMed] [Google Scholar]
- Wadgymar SM, Cumming MN, Weis AE. 2015. The success of assisted colonization and assisted gene flow depends on phenology. Global Change Biology 21: 3786–3799. [DOI] [PubMed] [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. 2011. Climate change and plant regeneration from seed. Global Change Biology 17: 2145–2161. [Google Scholar]
- Walther G-R, Roques A, Hulme PE. 2009. Alien species in a warmer world: risks and opportunities. Trends in Ecology and Evolution 24: 686–693. [DOI] [PubMed] [Google Scholar]
- Westoby M. 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and Soil 199: 213–227. [Google Scholar]
- Wiens JA, Hobbs RJ. 2015. Integrating conservation and restoration in a changing world. BioScience 65: 302–312. [Google Scholar]
- Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proceedings of the National Academy of Sciences of the USA 104: 5738–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CG, Ruhfel BR, Primack RB, et al. 2008. Phylogenetic patterns of species loss in Thoreau's woods are driven by climate change. Proceedings of the National Academy of Sciences of the USA 105: 17029–17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CG, Ruhfel BR, Primack RB, et al. 2010. Favorable climate change response explains non-native species’ success in Thoreau’s Woods. PLoS ONE 5: e8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkovich EM, Cook BI, Allen JM, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485: 494–497. [DOI] [PubMed] [Google Scholar]
- Wu CY, Gough CM, Chen JM, Gonsamo A. 2013. Evidence of autumn phenology control on annual net ecosystem productivity in two temperate deciduous forests. Ecological Engineering 60: 88–95. [Google Scholar]
- Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biology 17: 927–942. [Google Scholar]
- Wullschleger SD, Epstein HE, Box EO, et al. 2014. Plant functional types in Earth system models: past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Annals of Botany 114: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JY, Wan SQ. 2013. Independent effects of warming and nitrogen addition on plant phenology in the Inner Mongolian steppe. Annals of Botany 111: 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Woodall CW, Clark JS. 2012. Failure to migrate: lack of tree range expansion in response to climate change. Global Change Biology 18: 1042–1052. [Google Scholar]
- Zwicke M, Picon-Cochard C, Morvan-Bertrand A, Prud’homme M-P, Volaire F. 2015. What functional strategies drive drought survival and recovery of perennial species from upland grassland? Annals of Botany 116: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]