Abstract
It is well known that few weeks of high fat (HF) diet may induce metabolic disturbances and mitochondrial dysfunction in skeletal muscle. However, little is known about the effects of long-term HF exposure and effects on brain mitochondria are unknown. Wistar rats were fed either chow (13E% fat) or HF diet (60E% fat) for 1 year. The HF animals developed obesity, dyslipidemia, insulin resistance, and dysfunction of isolated skeletal muscle mitochondria: state 3 and state 4 were 30% to 50% increased (P<0.058) with palmitoyl carnitine (PC), while there was no effect with pyruvate as substrate. Adding also succinate in state 3 resulted in a higher substrate control ratio (SCR) with PC, but a lower SCR with pyruvate (P<0.05). The P/O2 ratio was lower with PC (P<0.004). However, similar tests on isolated brain mitochondria from the same animal showed no changes with the substrates relevant for brain (pyruvate and 3-hydroxybutyrate). Thus, long-term HF diet was associated with obesity, dyslipidemia, insulin resistance, and significantly altered mitochondrial function in skeletal muscle. Yet, brain mitochondria were unaffected. We suggest that the relative isolation of the brain due to the blood-brain barrier may play a role in this strikingly different phenotype of mitochondria from the two tissues of the same animal.
Keywords: brain, high fat diet, isolated mitochondria, long term, skeletal muscle
Introduction
Insulin resistance is associated with obesity and type 2 diabetes, and the prevalence of these conditions is increasing worldwide. A high fat (HF) diet has been used for decades to model obesity and insulin resistance in rodents.1, 2, 3 More recently, an association between insulin resistance and various types of mitochondrial dysfunctions has been observed in a range of tissues including skeletal muscle.4, 5 Furthermore, studies point to mitochondrial respiratory dysfunction as a central event in HF diet-induced insulin resistance.6, 7 However, findings are ambiguous in particular with skeletal muscle, with some reports indicating decreased,8 unchanged or increased mitochondrial respiratory capacity.9, 10, 11 Moreover, some studies report a time dependence of the effects of the HF feeding on mitochondrial function. Accordingly, it has been shown that skeletal muscle mitochondria seems at first to adapt to the oversupply of lipids and only upon prolonged exposure (beyond 1 month) to develop mitochondrial dysfunction.8, 12 Conversely, others concluded that long-term (20 weeks) HF feeding led to increased fatty acid oxidative capacity.11 However, only very few studies have evaluated the effects on skeletal muscle mitochondria after exposure to HF diet for 1 year ( ~1/3 of the life time of a rat),13 and no studies have tested the respiratory function in muscle, as well as in brain, after 1 year of extreme dietary fat (60E%). Thus, the involvement of mitochondrial respiratory dysfunction in HF diet-induced insulin resistance is unclear.
Probably due to the long-held belief that free fatty acids does not cross the blood–brain barrier, few studies concerning brain metabolism have been performed manipulating dietary fat levels. However, there is now evidence that free fatty acids does cross the blood–brain barrier,14, 15 and it has been reported that brain free fatty acid uptake is increased in obese, insulin-resistant subjects.16 Furthermore, recent observations suggest that the brain may also respond with insulin resistance to a HF diet,17, 18 and there may be a link between such an effect and cognitive impairment.19, 20
The investigations of HF diet-induced mitochondrial abnormalities has mainly been focused on non-central nervous system tissues such as muscle and liver; however, such abnormalities may also occur in the brain and thereby have functional implications for the brain. Few studies have observed brain mitochondrial alterations after HF diet exposure;18, 21, 22 however, the possible effects of chronic HF feeding on mitochondrial respiratory function in the brain are unknown, and at present, it is unclear whether brain mitochondria respond similarly as skeletal muscle mitochondria to consumption of a HF diet.
Therefore, the present study compared the effects of 1-year HF diet (60E%) in rats on mitochondrial respiratory function in skeletal muscle and in brain from the same animals to test whether mitochondria from these two tissues respond differently to this extreme dietary challenge.
Materials and methods
Animals, Diets, and Study Design
Male Wistar rats (220 g, 7 to 8 weeks old) were purchased from Taconic (Ejby, Denmark) and were individually housed with a 12-hour light/dark cycle. The total number of rats was 21. After a period of acclimatization (7 days), the rats were randomized into a control group (12 rats) fed a standard chow (C) (Diet no. 1319 FORTI, Altromin, Germany) and a HF group (9 rats) fed ad libitum on a HF diet for a period of 1 year (Diet no. D12492, Research Diets, New Brunswick, NJ, USA). The calorie composition of the diets is given in Table 1. Food intake and animal weight were monitored once or twice weekly (weight was not measured between weeks 36 and 50). Energy efficiency was calculated as change (Δ) in body weight per kcal food intake (i.e., grams/kcal for the interval week 0 to week 50). Measurements of adiposity and lean body mass were performed at 1.5, 6, 39, and 50 weeks on unanesthetized animals using a whole body composition analyzer (EchoMRITM scanner, Echo Medical Systems, Houston, TX, USA). Blood sampling (app. 100 μL) at 10 and 51 weeks (after a 16- to 18-hour overnight fast) was obtained from the tail vein using EDTA-dipotassium-coated tubes (Microvette CB 300, Sarstedt, Nümbrecht, Germany), which were spun down at 3,000 g for 15 min at 4°C for collection of plasma.
Table 1. Composition of the experimental diets.
| Diet component | Chow | High fat | ||
|---|---|---|---|---|
| Protein supply | ||||
| Casein (g/kg) | − | 258.5 | ||
| Prot. from wheat, corn, soybean (g/kg) | 225 | − | ||
| Carbohydrate supply | ||||
| Starch (g/kg) | 330 | − | ||
| Mono- and oligosaccharides (g/kg) | 170 | 250.4 | ||
| Cellulose (g/kg) | 45 | 64.6 | ||
| Fat supply | ||||
| Lard (g/kg) | − | 316.6 | ||
| Soybean oil (g/kg) | 27 | 32.3 | ||
| Fat from grain (g/kg) | 23 | − | ||
| Micronutrients | ||||
| Folic acid (mg/kg) | 2 | 2.6 | ||
| Vitamin B6 (mg/kg) | 9 | 9.1 | ||
| Vitamin B12 (μg/kg) | 24 | 12.9 | ||
| Added Choline Bitartrate/Cl (g/kg) | 0.6 | 2.6 | ||
| Added L-Cystine (g/kg) | − | 3.9 | ||
| Total Cystine+Methionine (g/kg) | 7 | 12 | ||
| Energy density (kcal/g) | 2.988 | 5.240 | ||
| Main diet components | Gram% | Kcal% | Gram% | Kcal% |
| Protein | 22.5 | 27 | 23.1 | 20 |
| Carbohydrate | 50.5 | 60 | 25 | 20 |
| Fat | 5 | 13 | 34.9 | 60 |
After 1 year on the diet (subsequent to a 24-hour fast), retro orbital blood, quadriceps muscle, as well as liver biopsies were sampled under anesthesia with sodium pentobarbital intraperitoneally (50 mg/kg body weight). The animal was killed by decapitation and the brain (only cerebrum) was taken out and placed in ice-cold saline within 30 seconds. Tissue samples from quadriceps muscle and brain from six animals in each group were used immediately for preparation of mitochondria as described below. Sample of muscle, brain, and liver were frozen in liquid nitrogen and stored at −80°C for later determination of citrate synthase activity and triglyceride (TG) content.
All experimental procedures complied with guidelines laid down by The Danish Animal Experiments Inspectorate (permit 2013-15-2934-00904) and by the local animal facility at the University of Copenhagen, Denmark and were performed according to the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines.
Plasma Analyses
Fasting blood glucose levels were measured in tail vein blood using two glucometers (Accu-Chek Compact Plus, Roche, Mannheim, Germany); thus, all measurements were performed in duplicate.
Insulin levels were assessed in 10 μL plasma using the Ultrasensitive Rat Insulin Elisa enzyme immunoassay according to the manufacturer's instructions (Mercodia, Uppsala, Sweden). Tissue insulin sensitivity was evaluated by the homeostasis model assessment (HOMA), which was previously validated in Wistar rats,23 using the HOMA index of insulin resistance (HOMA-IR)=fasting insulin (mU/L) × (fasting glucose (mmol/L))/22.5.24 Plasma nonesterified fatty acids (NEFAs) were measured at 540 nm at 37°C using the NEFA-HR(2) Kit (Wako Chemicals GmbH, Neuss, Germany) based on an enzymatic colorimetric method according to instructions from the manufacturer (Wako Chemicals GmbH, Neuss, Germany). Plasma levels of cholesterol, albumin, and alanine aminotransferase (ALAT) were measured on a Roche Modular P chemistry analyzer using Roche assays as described by the manufacturer (Cholesterol: CHOL #11491458, albumin: ALB plus #11929631 and #11970917, ALAT: ALAT IFCC #11876805 (Roche Diagnostics GmbH, Mannheim, Germany).
Oral Glucose Tolerance Test
Oral glucose tolerance test (OGTT) was performed after 40 weeks on the HF diet. After an overnight fast, animals were weighed and baseline blood glucose levels (time point −30 minutes) were determined as described above. The animals were given an oral bolus of glucose by gavage (2.93 g/kg lean body weight from a 45% w/v glucose solution) and glucose levels were measured in tail vein blood using two glucometers (duplicate measurements) at −30 minutes, 0 minutes, 30 minutes, 60 minutes, 90 minutes, 120 minutes, 180 minutes, and 220 minutes after the glucose administration. The area under the curve of the glucose tolerance test was calculated between 0 minute and 180 minutes.
Plasma and Tissue Triglycerides
Triglycerides were measured in plasma (10 μL), liver tissue (~30 mg), quadriceps muscle (~50 mg), and brain tissue (~50 mg) homogenized in 1 mol/L KOH/85% ethanol with a 5-mm steel bead using a Qiagen Tissuelyzer (Qiagen, Retsch, Germany). All homogenates were suspended in 1 mol/L KOH/85% ethanol and hydrolyzed at 60°C for 30 minutes.25 After cooling, MgCl2 was added to 0.1 mol/L and samples were spun down at 16,000 × g for 20 minutes at 4°C. Glycerol was measured spectrophotometrically using a coupled enzymatic assay in which the conversion of NADH into NAD+ was measured at 340 nm at 37°C as described previously.26
Isolation of Skeletal Muscle and Brain Mitochondria
Skeletal muscle mitochondria
Mitochondria were prepared essentially as described previously.27 In brief: A quadriceps muscle biopsy (app. 2 g) was taken from the hind leg of the 24-hour fasted rats while anesthetized. The biopsy was kept in KCl-buffer (100 mmol/L KCl, 50 mmol/L Tris-Base, 5 mmol/L MgSO4·7H2O, 1 mmol/L EDTA, pH 7.4) on ice and cut into small pieces. After decanting the buffer, 20 mL of proteinase/ATP-buffer (1 mmol/L ATP, 0.5% BSA (fatty acid free) in KCl-buffer with 1 mg/mL Subtilisin A proteinase) was added and the tissue was incubated for 10 minutes on ice with occasional stirring. The tissue was then washed three times in the ATP-buffer before homogenization for 8 minutes in an ice-cooled glass-Teflon Potter-Elvehjem homogenizer. The homogenate was centrifuged at 380 × g for 5 minutes to remove connective tissue. The supernatant was centrifuged at 5,400 × g for 10 minutes and the pellet was carefully resuspended in 8 mL KCl-buffer and further centrifuged at 6,700 × g for 10 minutes. All centrifugations were at 4°C. The final mitochondrial pellet was resuspended in 1 mL of MSTPi-medium (225 mmol/L mannitol, 75 mmol/L sucrose, 20 mmol/L Tris-Base, 10 mmol/L KH2PO4, 0.5 mmol/L EDTA, pH 7.0). This suspension was used for respiratory measurements and protein determination.
Brain mitochondria
Mitochondria were isolated from brain (without the cerebellum) using a procedure adapted from Rosenthal et al.28 with a discontinuous Percoll gradient centrifugation.29 After sedation as described above, the 24-hour fasted rats were killed by quickly cutting off the head with a pair of scissors and the brain was extracted, followed by removal of cerebellum. The brain tissue (app. 1 g) was immediately placed in 10 volume ice-cold buffer A (225 mmol/L mannitol, 75 mmol/L sucrose, 5 mmol/L HEPES, 1 mmol/L EGTA, 1 mmol/L ATP, pH 7.4). The tissue was minced with a pair of scissors and homogenized using a Potter-Elvehjem homogeniser with a loose fitting Teflon pistil (10 to 15 strokes at 200 r.p.m.). The volume of the homogenate was brought to 20 mL and centrifuged at 1,300 × g for 3 minutes. The supernatant was further centrifuged for 10 minutes at 20,000xg and the pellet was resuspended in 2.5 mL 15% Percoll (v/v in buffer A) and transferred to a 15-mL centrifuge tube. Using a syringe, 2.5 mL 23% Percoll was added to the bottom, followed by 2.5 mL 40% Percoll, and the tube was centrifuged at 30,700 × g for 10 minutes. (with centrifuge brakes off). The lower fraction containing the mitochondria was carefully removed using a syringe and transferred to a new 15 mL centrifuge tube, to which buffer A was added to a total volume of 10 mL. The tube was then centrifuged at 16,600 × g for 10 minutes and the supernatant gently removed. The pellet was resuspended in 10 mL buffer A and centrifuged at 6,300 × g for 10 minutes. The resulting pellet was resuspended in 600 μl buffer B (225 mmol/L mannitol, 75 mmol/L sucrose, 5 mmol/L HEPES, pH 7.4). A 25-μL sample for measuring protein content and citrate synthase activity was frozen at −80°C until analysis. The rest of the mitochondrial suspension was kept on ice for high-resolution mitochondrial respirometry. All centrifugations for the preparation of brain mitochondria were at 4°C.
Protein Concentration and Citrate Synthase Activity
Protein concentrations were determined using Lowry's method30 with bovine serum albumin as a standard. Citrate synthase activity was determined in isolated mitochondria and whole tissue as described.31
Mitochondrial Respiration
Mitochondrial oxygen consumption was measured using Oroboros Oxygraph-2 K instruments (Oroboros Inc., Innsbruck, Austria) as previously described,32 operating 10 oxygraph chambers in parallel at 25°C. In brief, 10 μL muscle mitochondrial suspension or 15 μL brain mitochondrial suspension was added to 2 mL of MSTPi-medium with 0.1% BSA or incubation medium (125 mmol/L KCl, 20 mmol/L HEPES, 2 mmol/L K2HPO4, 1 mmol/L MgCl2, 0.1 mmol/L EGTA, 0.025% BSA, pH 7.0 at room temperature), respectively, in each chamber. Stirrer speed was 600 r.p.m. Approximately 5 min after the mitochondria were introduced into the chambers, malate and pyruvate, palmitoyl carnitine (PC) (skeletal muscle mitochondria only) or 3-hydroxybutyric acid (3HBA) (brain mitochondria only) were added to concentrations of 1 mmol/L, 0.5 mmol/L, 10 μmol/L, and 3 mmol/L, respectively. Once a steady state 4 respiration was observed, ADP was added to a concentration of 0.2 mmol/L to measure the P/O2 ratio. When the state 4 respiration rate was reached again, ADP was added to a concentration of 2 mmol/L to again obtain a state 3 respiration, now followed by succinate addition to a concentration of 5 mmol/L (the step with 0.2 mmol/L ADP for measurement of P/O2 ratio was not performed with 3HBA). The respiratory control ratio (RCR) was calculated as the state 3/state 4 respiration ratio and the substrate control ratio (SCR) was calculated as the state 3 respiration with succinate added relative to the state 3 respiration without succinate added. All measurements were performed in duplicate and between experiments the chambers were washed twice with ethanol and water. Due to technical problems, the number of measurements of PC respiration in skeletal muscle was two in the chow-fed group. However, since these experiments were similar to our previously published values of PC respiration in muscle mitochondria from 16-week-old chow-fed Wistar rats,32 the latter data were included in the present study to save on animal experiments (see Table 4).
Statistical Analyses
Data are presented as means±s.d. The number of rats included in the study was estimated based on our previous experience with preparations of skeletal muscle—as well as brain mitochondria.27, 32, 33 From these data, we could infer that a sample size of, e.g., 6 per group would provide an actual power of >0.95 with a 15% difference between groups (G*Power version 3.1.9.2) (www.gpower.hhu.de). Exclusion criteria for mitochondrial experiments were based on RCR values <3 and an increase in oxygen consumption of >10% upon addition of 20 μmol/L cytochrome c.
Blinding of the experiments and the analyses was for practical reasons not done. However, in case of the mitochondrial analyses it is not possible to judge the outcome until the final computerized analysis of the total data complement is performed.
Statistical calculations were performed using GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA) and SAS 9.3 (The SAS Institute, Cary, NC, USA).
Significance was assessed by paired or unpaired Student's t-test. The paired test was used for comparison of skeletal muscle and brain mitochondria from the same animals. For measures over time, a mixed model ANOVA to analyze effects of diet, time and interaction (diet × time) was performed with least squares post hoc tests with Bonferroni adjustment. The level of significance was set at P<0.05. Plasma insulin concentrations and HOMA-IR indexes were log-transformed to obtain a normal distribution before statistical analyses.
Results
Effect of the High Fat Diet on Body Weight, Adiposity, Food Intake, and Energy Efficiency
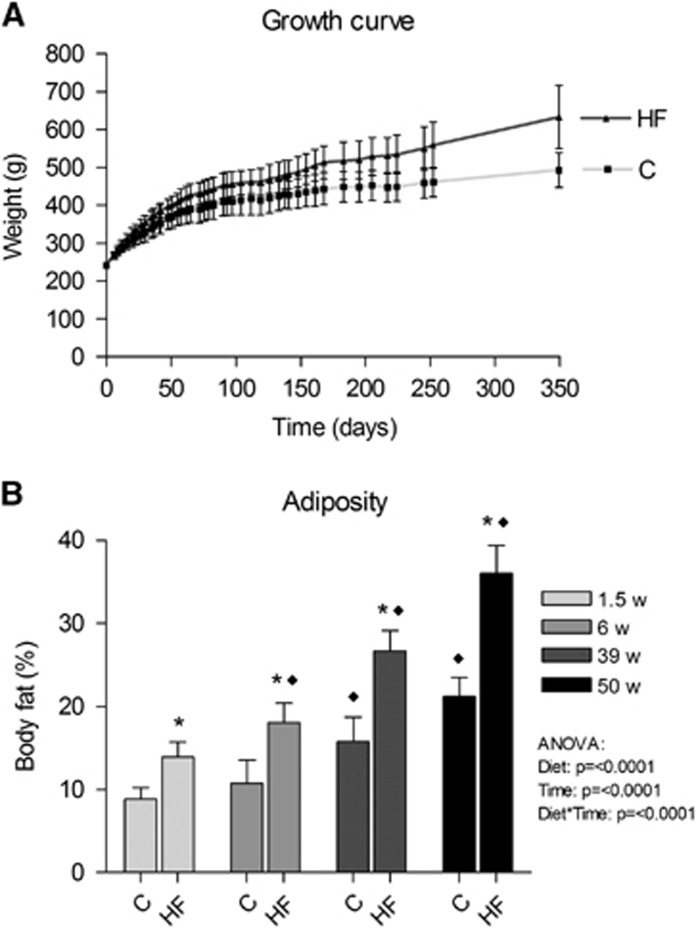
The animals consuming a HF diet increased their accumulated calorie intake and gained significantly more weight and were on average 28% heavier than controls after 1 year (P=0.008, Figure 1A and Table 2). As the rise in lean body weight was similar among the groups, the increased body weight was attributable to the increased fat accumulation (Table 2). Already 1.5 weeks after the introduction of the HF diet, there was significantly increased adiposity, which was further increased after 6, 39, and 50 weeks with a final body fat percent of 36±3% compared with 21±2% for the controls (Figure 1B). In addition, energy efficiency was increased with the HF diet over the time course of the experiment (Table 2).
Figure 1.
Effect of HF feeding on body weight and adiposity in male Wistar rats. Rats were fed a HF diet (60E% fat) or a standard chow (13E% fat) for a period of 1 year. (A) Growth curve and (B) adiposity measured by magnetic resonance (MR) scanning. HF, high fat; C, Chow. Presented data are means±s.d., n=9 HF and 10 to 12 C, * and ♦ denote a diet effect and a time effect, respectively, P<0.05.
Table 2. Physiologic data.
| Chow | High fat | P-value | |
|---|---|---|---|
| Δ Body weight (g) | 252±44 | 389±81 | 2.0 × 10−4 |
| Δ Lean weight (g) | 100.9±22 | 119.5±26 | 0.112 |
| Δ Fat weight (g) | 78.8±15 | 189.4±50 | 4.3 × 10−6 |
| Accumulated calorie intake (kcal) | 19,278±1,856 | 22,445±2,660 | 0.008 |
| Energy efficiency (mg/kcal) | 13.0±1.5 | 17.2±1.6 | 2.1 × 10−5 |
Abbreviation: HF, high fat. Numbers are means±s.d., n=9 HF and 10 to 12 C. Δ Body weight, accumulated calorie intake, and energy efficiency were calculated from week 0 to 50, whereas Δ lean weight and Δ fat weight were calculated from week 1.5 to 50.
Effect of the High Fat Diet on Peripheral Insulin Resistance, Plasma Lipid Profile, and Tissue Lipid Accumulation
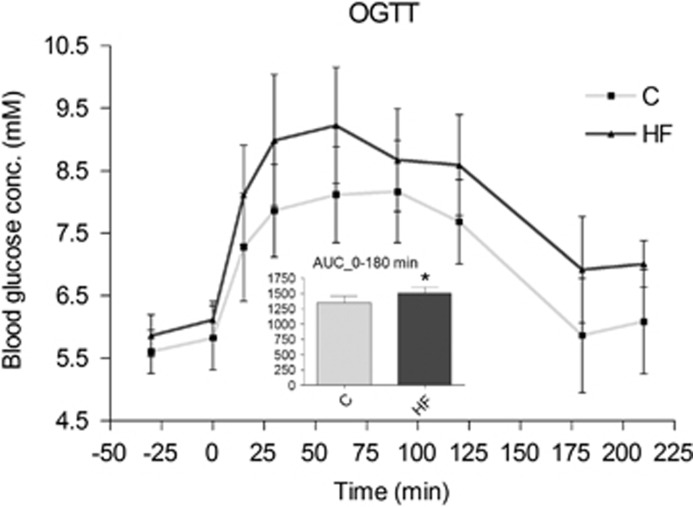
We measured blood glucose and plasma insulin, calculated the HOMA-IR index and performed an OGTT to monitor the development of insulin resistance. Fasting samples showed that the HF animals were hyperinsulinemic with a progressing increase in HOMA-IR index (Table 3). There was a mild, overall increase in fasting blood glucose levels in the HF animals (P=0.043), but no significant difference between the groups in blood glucose values obtained in the fed state (P=0.096, Table 3). The OGTT at 40 weeks confirmed the development of insulin resistance in the HF animals (Figure 2).
Table 3. Plasma parameters after 10 weeks and 1 year of diet intervention.
|
10 weeks
|
1 year
|
P-value |
|||||
| Chow | High fat | Chow | High fat | Diet | Time | Diet × Time | |
| Fasting glucose (mmol/L) | 5.6±0.4 | 5.9±0.3 | 5.3±0.5 | 5.5±0.3 | 0.043 | 0.005 | 0.754 |
| Nonfasting glucose (mmol/L) | − | − | 6.4±0.4 | 6.8±0.5 | 0.096 | − | − |
| Fasting insulin (pmol/L) | 97.4±47.0 | 181.0±45.2 | 154.9±43.5 | 476.8±241.9 | <0.0001 | <0.0001 | 0.040 |
| HOMA-IR index (arbitrary units) | 4.19±2.18 | 7.90±1.95 | 5.94±1.44 | 19.39±9.21 | <0.0001 | <0.0001 | 0.031 |
| Fasting NEFA (mmol/L) | 1.28±0.19 | 0.92±0.15 | 1.18±0.14 | 0.85±0.15 | <0.0001 | 0.042 | 0.654 |
| Nonfasting NEFA (mmol/L) | − | − | 0.31±0.09 | 0.41±0.07 | 0.021 | − | − |
| Fasting TG (mmol/L) | − | − | 1.58±0.48 | 1.38±0.27 | 0.296 | − | − |
| Nonfasting TG (mmol/L) | − | − | 2.08±0.59 | 2.44±0.98 | 0.339 | − | − |
| Fasting total cholesterol (mmol/L) | − | − | 1.98±0.29 | 1.68±0.31 | 0.041 | − | − |
| Fasting albumin (g/L) | − | − | 35.4±2.98 | 36.0±6.29 | 0.783 | − | − |
| Fasting ALAT (units/L) | − | − | 39.5±15.2 | 45.1±19.5 | 0.492 | − | − |
Abbreviations: ALAT, alanine aminotransferase; HF, high fat; HOMA-IR, homeostasis model assessment of insulin resistance; NEFA, nonesterified fatty acids; TG, triglyceride. Numbers are means±s.d., n=9 HF and 10 to 12 C.
Figure 2.
Oral glucose tolerance test (OGTT) after 40 weeks of HF feeding. AUC, area under the curve, calculated from 0 minute to 180 minutes after glucose administration. HF, high fat; C, Chow. Presented data are means±s.d., n=9 HF and 12 C. *Denotes a significant difference from control, P<0.05.
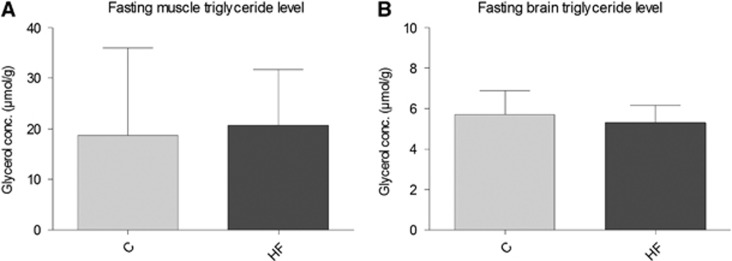
Additionally, the plasma lipid profile was evaluated (Table 3). Fasting plasma NEFA was significantly lower in the HF group (1.28±0.19 versus 0.92±0.15 after 10 weeks, 1.18±0.14 versus 0.85±0.15 after 1 year, P<0.0001), whereas nonfasting NEFA levels were higher (0.31±0.09 versus 0.41±0.07, P=0.021) after 1 year with the HF diet. Fasting plasma total cholesterol was slightly lower in the HF animals, while plasma TG, albumin, and ALAT were unaffected by the 1-year HF diet (Table 3). Moreover, the fasting level of TGs in skeletal muscle and brain was similar in the two groups (Figure 3). While fasting liver TG content was 3- to 4-fold higher in the HF diet fed than in the chow-fed animals (121±43 versus 33±11 μmol glycerol/g, P<0.05).
Figure 3.
Fasting triglyceride (TG) content in skeletal muscle and brain. (A and B) TG content in skeletal muscle and brain, respectively, measured as glycerol concentration in 24-hour fasted rats after 1 year of HF diet consumption. Presented data are means±s.d., n=9 HF and 10 C for skeletal muscle measurements and n=6 for brain. C, Chow; HF, High fat.
Changed Skeletal Muscle-But Unaffected Brain-Mitochondrial Respiration after High Fat Exposure
We tested whether mitochondria isolated from skeletal muscle and brain were affected similarly by the 1-year long HF feeding. The results are shown in Tables 4 and 5, respectively. In skeletal muscle mitochondria with pyruvate as a substrate, there was no difference between the control and the HF group, while addition of succinate in state 3 resulted in a smaller increase in respiration in the HF group with an SCR of 1.42±0.02 compared to 1.47±0.03 (P=0.016) (~17% smaller increase in oxygen consumption in the HF group, P=0.009) (Table 4). With a fatty acid substrate (PC), however, state 4 respiration was ~50% higher (P=0.001), there was a trend of increased state 3 respiration of ~30% (P=0.058) and the increase in respiration after succinate also tended to be higher in the HF group (P=0.057), as also reflected in the higher SCR in the HF group (1.51±0.04 versus 1.39±0.08, P=0.009) (Table 4). The phosphorylation efficiency (P/O2 ratio) was unaffected by the HF diet with pyruvate as mitochondrial substrate, but with PC we observed a significant decrease (~10%, P=0.004) (Table 4).
Table 4. Oxygen consumption in isolated skeletal muscle mitochondria.
| Condition | Substrates | Chow | High fat | P-value |
|---|---|---|---|---|
| State 4 respiration | Malate+pyruvate | 5.63±1.0 | 4.83±1.3 | 0.266 |
| Malate+PC | 7.59±1.5 | 11.3±1.6 | 0.001 | |
| State 3 respiration | Malate+pyruvate+ADP | 158±15 | 145±13 | 0.150 |
| Malate+pyruvate+ADP+succinate | 231±21 | 206±19 | 0.052 | |
| Succinate contribution | 73.6±7.1 | 60.9±6.3 | 0.009 | |
| Malate+PC+ADP | 78.4±13 | 104±22 | 0.058 | |
| Malate+PC+ADP+succinate | 109±20 | 158±41 | 0.090 | |
| Succinate contribution | 30.9±8.4 | 53.7±16 | 0.057 | |
| SCR | Malate+pyruvate | 1.47±0.03 | 1.42±0.02 | 0.016 |
| Malate+PC | 1.39±0.08 | 1.51±0.04 | 0.009 | |
| RCR | Malate+pyruvate | 28.5±4.1 | 31.2±6.4 | 0.414 |
| Malate+PC | 11.0±3.1 | 9.73±2.8 | 0.484 | |
| P/O2 ratio | Malate+pyruvate+ADP (0.4 μmol) | 5.20±0.34 | 5.01±0.15 | 0.293 |
| Malate+PC+ADP (0.4 μmol) | 5.04±0.21 | 4.60±0.19 | 0.004 |
Abbreviations: HF, high fat; PC, palmitoyl carnitine; P/O2 ratio, phosphorylation efficiency given as moles ATP produced per consumed mole of oxygen; RCR, respiratory control ratio calculated as state 3/state 4 respiration; SCR, substrate control ratio calculated as state 3 respiration with succinate addition/state 3 respiration without succinate addition. Mitochondria from quadriceps muscle were isolated from male Wistar rats fed a HF or a control chow diet for 1 year. Oxygen consumption is expressed per mg mitochondrial protein (nmol/min mg). Numbers are means±s.d., n=4 to 7. Due to technical problems, the number of animals in the PC respiration measurements was only two in the control chow-fed group. We have therefore included data from a similar control group from a previous study.32
Table 5. Oxygen consumption in isolated brain mitochondria.
| Condition | Substrates | Chow | High fat | P-value |
|---|---|---|---|---|
| State 4 respiration | Malate+pyruvate | 9.69±2.0 | 10.1±1.0 | 0.658 |
| Malate+3HBA | 8.62±1.3 | 8.85±0.85 | 0.727 | |
| State 3 respiration | Malate+pyruvate +ADP | 178±14 | 187±13 | 0.287 |
| Malate+pyruvate+ADP+succinate | 259±18 | 270±14 | 0.232 | |
| Succinate contribution | 80.7±5.8 | 83.7±5.0 | 0.352 | |
| Malate+3HBA+ADP | 36.7±3.7 | 38.6±2.3 | 0.327 | |
| Malate+3HBA+ADP+succinate | 107±14 | 97.9±18 | 0.345 | |
| Succinate contribution | 70.5±11 | 59.3±21 | 0.324 | |
| SCR | Malate+pyruvate | 1.45±0.03 | 1.45±0.04 | 0.852 |
| Malate+3HBA | 2.92±0.25 | 2.55±0.57 | 0.230 | |
| RCR | Malate+pyruvate | 19.1±4.1 | 18.6±2.2 | 0.821 |
| Malate+3HBA | 4.33±0.7 | 4.34±0.5 | 0.370 | |
| P/O2 ratio | Malate+pyruvate+ADP (0.4 μmol) | 4.60±0.26 | 4.43±0.43 | 0.436 |
Abbreviations: 3HBA, 3-hydroxybutyric acid; P/O2 ratio, phosphorylation efficiency given as moles ATP produced per consumed mole of oxygen; RCR, respiratory control ratio calculated as state 3/state 4 respiration; SCR, substrate control ratio calculated as state 3 respiration with succinate addition/state 3 respiration without succinate addition. Mitochondria from brain were isolated from male Wistar rats fed a high fat or a control chow diet for 1 year. Oxygen consumption is expressed per mg mitochondrial protein (nmol/min mg). Numbers are means±s.d., n=6.
In isolated brain mitochondria from the same animal, however, the long-term HF feeding was without effect on all tested substrate conditions (Table 5). We observed an ~5-fold lower state 3 respiration with 3HBA than with pyruvate in both groups, indicating that 3HBA oxidation, rather than the respiratory capacity of brain mitochondria, was limiting respiration. In this case, typical traces of oxygen consumption of isolated skeletal muscle and brain mitochondria are given in Supplementary Figure S1.
Citrate synthase activity was similar in HF and control animals in isolated skeletal muscle mitochondria (2.04±0.15 units/mg protein in HF versus 2.02±0.21 in controls, P=0.809), isolated brain mitochondria (3.44±0.18 versus 3.47±0.12, P=0.694), skeletal muscle tissue (0.21±0.09 versus 0.25±0.09, P=0.391) as well as in brain tissue (0.60±0.02 versus 0.62±0.02, P=0.146).
Discussion
Whole-Body Effects of 1 Year of High Fat Feeding
Here we have tested the effects of long-term (1 year, corresponding to ~1/3 of the life span of a rat) HF diet (60E% fat compared with 13E% fat in the control diet). At the whole-body level, the HF diet resulted in obesity and peripheral insulin resistance, in agreement with numerous previous studies using comparable diets, but with shorter HF diet exposure time.12, 34, 35 In the present study, calorie intake, energy efficiency, and fat mass were increased in the HF-fed animals, whereas lean body weight was similar in the two groups. These findings are comparable to those found in another study evaluating the effects of a similar diet on Wistar rats for 8 weeks.36 Yet, these authors reported that the animals spontaneously adjusted their calorie intake to match that of the controls.36 The apparent increased energy efficiency observed in our study occurs in spite of an ~4% to 9% decreased phosphorylation efficiency in skeletal muscle (Table 4) and may therefore result from a decreased physical activity as indeed reported by So et al.36
The HF diet resulted in a prediabetic phenotype: we observed a 3-fold increase in fasting insulin level after 1 year in the HF animals and insulin resistance was demonstrated as a 3- to 4-fold increased HOMA-IR index combined with a decreased glucose clearance during an OGTT as well as fasting blood glucose increase (Table 3 and Figure 2). This is in line with several previous reports.2, 18, 34, 35
Obesity and insulin resistance have been associated with dyslipidemia including increased fasting plasma NEFA and TG.37 However, the plasma lipid profile associated with obesity and insulin resistance in the present study, diverged from this profile. We found decreased fasting plasma NEFA throughout the study, but increased NEFA level in the fed state, and unchanged fasting and nonfasting TG levels in the HF animals compared with controls. Nevertheless, decreased fasting plasma NEFA after HF diet consumption is an observation also reported by others in both human and animal studies3, 38 and it has recently been questioned whether increased plasma NEFA concentration is related to insulin resistance.39 The cause of decreased fasting plasma NEFA after 1 year in the present study could be suppression of lipolysis in adipose tissue mediated by the elevated insulin level or an increased flux of NEFA to the tissues. In support of the latter possibility, we observed a 3- to 4-fold increase in liver TGs in HF-fed animals.
Effects of Long-Term High Fat Exposure on Mitochondrial Function in Skeletal Muscle and Brain
The present study set out to compare the susceptibility of skeletal muscle mitochondria and brain mitochondria to chronic consumption of a HF diet. The overall result was that mitochondrial respiration was affected in skeletal muscle, but not in brain with the substrates tested. Citrate synthase activity was similar in the two groups in skeletal muscle as well as in brain tissue, suggesting that mitochondrial content was unchanged by HF feeding.40
In skeletal muscle mitochondria, state 4 and state 3 oxygen consumption with pyruvate as substrate was similar in chow- and HF-fed animals (Table 4), as has been found also by others after 25 weeks of HF diet,41 indicating an unaffected pyruvate dehydrogenase and/or complex I activity. Addition of the complex II substrate succinate caused an average increase in oxygen consumption of 47% in the chow-fed animals, indicating that the total capacity of complexes III, IV, and V is at least this much higher than complex I (and/or pyruvate dehydrogenase) in the skeletal muscle mitochondria of these animals. In the HF group, however, this respiration increase with succinate addition was significantly lower than in the controls. Whether this reflects a lower succinate dehydrogenase (SDH) activity compared with the chow group cannot be inferred from the oxygen consumption measurements alone. The effect of HF diet on SDH activity seems to vary depending on study design, as previous studies assessing SDH activity in skeletal muscle after HF diet regimes have reported decreased,42 unchanged10, 35 as well as increased41 activity, and recently, Yuzefovych et al.43 found reduced SDH protein in C57Bl/6 J mice after 16 weeks of receiving the same diet as used in our study.
Only few studies have evaluated the effect on oxygen consumption in skeletal muscle of adding succinate in addition to a NAD-linked substrate in HF-fed animals. In a study with permeabilized soleus muscle fibers from rats fed a diet enriched in calories and fat for 6 weeks, Chanséaume et al.44 found an unchanged contribution of succinate to state 3 respiration in contrast to the present study. However, further studies are needed to elucidate the mechanisms of the smaller SCR seen with succinate addition in the HF group (Table 4), since SDH activity is known also to be strongly influenced by the concentration of oxaloacetate,45 which in turn may be modulated by other citric acid cycle intermediates.46 Thus, the rate of the succinate dehydrogenase reaction (SDH flux) may well differ in respiring mitochondria with different substrates.
With the fatty acid PC as substrate, the state 4 rate of oxygen consumption was ~50% higher (P=0.001) and tended to be higher by ~30% (P=0.058) in state 3 in mitochondria from the HF animals. This indicates that the HF diet caused an increased β-oxidation flux, yet the actual oxygen consumption rate under these conditions was still within the respiratory capacity seen with pyruvate. In support of this, others have reported increased activity of enzymes involved in β-oxidation after HF diet feeding.11, 47 The increased respiration seen when adding also succinate to the state 3 respiration was greater in the HF group as reflected in the SCR (P=0.009). Furthermore, the actual increase in oxygen consumption in the HF animals was similar to that observed with pyruvate as substrate (53.7±16 nmol/min mg versus 60.9±6.3 nmol/min mg with pyruvate), suggesting that there was little or no interaction between β-oxidation-derived and SDH-derived FADH2, contrary to what seemed to be the case in controls (30.9±8.4 nmol/min mg versus 73.6±7.1 nmol/min mg). This further suggests that the SDH flux was in fact not limiting the oxygen consumption under the conditions of PC+succinate oxidation in the HF animals.
We also observed lower phosphorylation efficiency (P/O2 ratio) in HF animals with PC as substrate. It therefore could be speculated that this effect is caused by increased expression of uncoupling proteins (UCPs) as previously shown (as a higher UCP3 expression) in skeletal muscle of rats fed a HF diet,11 and, rather surprisingly, in brain mitochondria (as a higher UCP5 expression) after gestational fructose load.33 However, the lack of change in P/O2 with malate+pyruvate as substrate does not seem to support this explanation.
As in the present study, others have found increased respiration in isolated skeletal muscle mitochondria with a fatty acid substrate after 20 to 25 weeks of HF diet.11, 41 Yet, a decrease in both palmitate and glucose oxidation has also been reported in isolated single muscle fibers of mice given a HF diet for 8 weeks.35 However, our data support the notion of an augmented rather than diminished mitochondrial respiratory capacity to oxidize a fatty acid. This could be interpreted as a compensatory response to cope with the increased level of NEFA seen in the HF group in the fed state (Table 3).
The Possible Effects of High Fat on Brain Function Are Not Caused by Mitochondrial Dysfunction
It has long been recognized that a HF diet is associated with peripheral insulin resistance as well as altered mitochondrial function in skeletal muscle. This association has, however, only recently been observed also for the brain, linked to a decline in cognitive function,21, 48 albeit insulin resistance in the brain is not a well-defined phenomenon. Yet, insulin receptor function in the brain has been shown to be important for peripheral glucose and fat metabolism.49
It has been shown that obese, insulin-resistant individuals have increased fatty acid transport into the brain.16 Thus, it could be speculated that a prolonged HF diet with development of obesity and insulin resistance would lead to an overload of free fatty acids also in the brain as seen in other tissues during HF exposure, especially as neurons have low capacity for β-oxidation and primarily astrocytes oxidize fatty acids.50 This would be expected to cause similar changes in brain mitochondria as seen in skeletal muscle mitochondria.
However, the present study clearly disproves such a hypothesis since brain mitochondria are unaffected by the long-term HF exposure. Whether this actually reflects an insignificant fatty acid transport into brain needs further study. Conversely, if brain mitochondria actually are exposed to an increased fatty acid level, our results would seem to indicate mechanisms other than increased fatty acid availability as the cause of the observed effects on skeletal muscle mitochondria.
Acknowledgments
Ib Therkelsen and Lillian Helene Lund Hansen are thanked for their technical assistance and Svend Høime Hansen for assisting with the measurements of plasma concentrations of cholesterol, albumin, and ALAT.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research was supported by The Danish Strategic Research Council (# 09-067124 AND # 09-059921; Center for Fetal Programming).
Supplementary Material
References
- 1Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol 1986; 251: E576–E583. [DOI] [PubMed] [Google Scholar]
- 2Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004; 53: S215–S219. [DOI] [PubMed] [Google Scholar]
- 3Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW. Long-term high-fat feeding leads to severe insulin resistance but not diabetes in Wistar rats. Am J Physiol Endocrinol Metab 2002; 282: E1231–E1238. [DOI] [PubMed] [Google Scholar]
- 4Chow L, From A, Seaquist E. Skeletal muscle insulin resistance: the interplay of local lipid excess and mitochondrial dysfunction. Metabolism 2010; 59: 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Lionetti L, Mollica MP, Crescenzo R, D'Andrea E, Ferraro M, Bianco F et al. Skeletal muscle subsarcolemmal mitochondrial dysfunction in high-fat fed rats exhibiting impaired glucose homeostasis. Int J Obes 2005 2007; 31: 1596–1604. [DOI] [PubMed] [Google Scholar]
- 6Morris EM, Jackman MR, Meers GME, Johnson GC, Lopez JL, Maclean PS et al. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1α overexpression. Am J Physiol Gastrointest Liver Physiol 2013; 305: G868–G880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Yokota T, Kinugawa S, Hirabayashi K, Matsushima S, Inoue N, Ohta Y et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 2009; 297: H1069–H1077. [DOI] [PubMed] [Google Scholar]
- 8Chanséaume E, Tardy A-L, Salles J, Giraudet C, Rousset P, Tissandier A et al. Chronological approach of diet-induced alterations in muscle mitochondrial functions in rats. Obesity (Silver Spring, Md) 2007; 15: 50–59. [DOI] [PubMed] [Google Scholar]
- 9Català-Niell A, Estrany ME, Proenza AM, Gianotti M, Lladó I. Skeletal muscle and liver oxidative metabolism in response to a voluntary isocaloric intake of a high fat diet in male and female rats. Cell Physiol Biochem 2008; 22: 327–336. [DOI] [PubMed] [Google Scholar]
- 10Iossa S, Mollica MP, Lionetti L, Crescenzo R, Botta M, Liverini G. Skeletal muscle oxidative capacity in rats fed high-fat diet. Int J Obes Relat Metab Disord 2002; 26: 65–72. [DOI] [PubMed] [Google Scholar]
- 11Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007; 56: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 12Laurent D, Yerby B, Deacon R, Gao J. Diet-induced modulation of mitochondrial activity in rat muscle. Am J Physiol Endocrinol Metab 2007; 293: E1169–E1177. [DOI] [PubMed] [Google Scholar]
- 13Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O et al. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 2009; 284: 22840–22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Hamilton JA, Brunaldi K. A model for fatty acid transport into the brain. J Mol Neurosci 2007; 33: 12–17. [DOI] [PubMed] [Google Scholar]
- 15Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res 2001; 42: 678–685. [PubMed] [Google Scholar]
- 16Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes 2010; 59: 2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Oh H, Boghossian S, York DA, Park-York M. The effect of high fat diet and saturated fatty acids on insulin signaling in the amygdala and hypothalamus of rats. Brain Res 2013; 1537: 191–200. [DOI] [PubMed] [Google Scholar]
- 18Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci 2013; 37: 839–849. [DOI] [PubMed] [Google Scholar]
- 19Emmanuel Y, Cochlin LE, Tyler DJ, de Jager CA, David Smith A, Clarke K. Human hippocampal energy metabolism is impaired during cognitive activity in a lipid infusion model of insulin resistance. Brain Behav 2013; 3: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Greenwood CE, Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiol Learn Mem 2001; 75: 179–189. [DOI] [PubMed] [Google Scholar]
- 21Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 2012; 153: 329–338. [DOI] [PubMed] [Google Scholar]
- 22Ho L, Varghese M, Wang J, Zhao W, Chen F, Knable LA et al. Dietary supplementation with decaffeinated green coffee improves diet-induced insulin resistance and brain energy metabolism in mice. Nutr Neurosci 2012; 15: 37–45. [DOI] [PubMed] [Google Scholar]
- 23Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab 2008; 295: E1269–E1276. [DOI] [PubMed] [Google Scholar]
- 24Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 25Kates M. Techniques of Lipidology. Elsevier: Amsterdam. 1986. [Google Scholar]
- 26Wieland OH. Methods of Enzymatic Analysis. 3rd ed. Verlag Chemie: Weinheim. 1984. [Google Scholar]
- 27Fritzen AJ, Grunnet N, Quistorff B. Flux control analysis of mitochondrial oxidative phosphorylation in rat skeletal muscle: pyruvate and palmitoyl-carnitine as substrates give different control patterns. Eur J Appl Physiol 2007; 101: 679–689. [DOI] [PubMed] [Google Scholar]
- 28Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab 1987; 7: 752–758. [DOI] [PubMed] [Google Scholar]
- 29Tretter L, Takacs K, Hegedus V, Adam-Vizi V. Characteristics of alpha-glycerophosphate-evoked H2O2 generation in brain mitochondria. J Neurochem 2007; 100: 650–663. [DOI] [PubMed] [Google Scholar]
- 30Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275. [PubMed] [Google Scholar]
- 31Passonneau JV, Lowry OH. Enzymatic Analysis: A Practical Guide V. II. Humana Press Inc.: New Jersey. 1993. [Google Scholar]
- 32Jørgensen W, Jelnes P, Rud KA, Hansen LL, Grunnet N, Quistorff B. Progression of type 2 diabetes in GK rats affects muscle and liver mitochondria differently: pronounced reduction of complex II flux is observed in liver only. Am J Physiol Endocrinol Metab 2012; 303: E515–E523. [DOI] [PubMed] [Google Scholar]
- 33Mortensen OH, Larsen LH, Ørstrup LKH, Hansen LHL, Grunnet N, Quistorff B. Developmental programming by high fructose decreases phosphorylation efficiency in aging offspring brain mitochondria, correlating with enhanced UCP5 expression. J Cereb Blood Flow Metab 2014; 34: 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006; 36: 485–501. [DOI] [PubMed] [Google Scholar]
- 35Shortreed KE, Krause MP, Huang JH, Dhanani D, Moradi J, Ceddia RB et al. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS ONE 2009; 4: e7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36So M, Gaidhu MP, Maghdoori B, Ceddia RB. Analysis of time-dependent adaptations in whole-body energy balance in obesity induced by high-fat diet in rats. Lipids Health Dis 2011; 10: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013; 5: 1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Brøns C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 2009; 587: 2387–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011; 60: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 2012; 590: 3349–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Van den Broek NMA, Ciapaite J, De Feyter HMML, Houten SM, Wanders RJA, Jeneson JAL et al. Increased mitochondrial content rescues in vivo muscle oxidative capacity in long-term high-fat-diet-fed rats. FASEB J 2010; 24: 1354–1364. [DOI] [PubMed] [Google Scholar]
- 42Nagatomo F, Fujino H, Kondo H, Takeda I, Tsuda K, Ishihara A. High-fat diet-induced reduction of peroxisome proliferator-activated receptor-γ coactivator-1α messenger RNA levels and oxidative capacity in the soleus muscle of rats with metabolic syndrome. Nutr Res 2012; 32: 144–151. [DOI] [PubMed] [Google Scholar]
- 43Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI, Mitochondrial DNA. damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE 2013; 8: e54059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Chanséaume E, Malpuech-Brugère C, Patrac V, Bielicki G, Rousset P, Couturier K et al. Diets high in sugar, fat, and energy induce muscle type-specific adaptations in mitochondrial functions in rats. J Nutr 2006; 136: 2194–2200. [DOI] [PubMed] [Google Scholar]
- 45Zeylemaker WP, Klaasse AD, Slater EC. Studies on succinate dehydrogenase. V. Inhibition by oxaloacetate. Biochim Biophys Acta 1969; 191: 229–238. [DOI] [PubMed] [Google Scholar]
- 46Tyler DB. Effect of citric acid-cycle intermediates on oxaloacetate utilization and succinate oxidation. Biochem J 1960; 76: 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Nemeth PM, Rosser BW, Choksi RM, Norris BJ, Baker KM. Metabolic response to a high-fat diet in neonatal and adult rat muscle. Am J Physiol 1992; 262: C282–C286. [DOI] [PubMed] [Google Scholar]
- 48Pintana H, Apaijai N, Chattipakorn N, Chattipakorn SC. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J Endocrinol 2013; 218: 1–11. [DOI] [PubMed] [Google Scholar]
- 49Williamson R, McNeilly A, Sutherland C. Insulin resistance in the brain: an old-age or new-age problem? Biochem Pharmacol 2012; 84: 737–745. [DOI] [PubMed] [Google Scholar]
- 50Schönfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab 2013; 33: 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.