Abstract
Ischemic stroke can cause striatal dopamine efflux that contributes to cell death. Since Kv7 potassium channels regulate dopamine release, we investigated the effects of their pharmacological modulation on dopamine efflux, measured by fast cyclic voltammetry (FCV), and neurotoxicity, in Wistar rat caudate brain slices undergoing oxygen and glucose deprivation (OGD). The Kv7 activators retigabine and ICA27243 delayed the onset, and decreased the peak level of dopamine efflux induced by OGD; and also decreased OGD-induced damage measured by 2,3,5-triphenyltetrazolium chloride (TTC) staining. Retigabine also reduced OGD-induced necrotic cell death evaluated by lactate dehydrogenase activity assay. The Kv7 blocker linopirdine increased OGD-evoked dopamine efflux and OGD-induced damage, and attenuated the effects of retigabine. Quantitative-PCR experiments showed that OGD caused an ~6-fold decrease in Kv7.2 transcript, while levels of mRNAs encoding for other Kv7 subunits were unaffected; western blot experiments showed a parallel reduction in Kv7.2 protein levels. Retigabine also decreased the peak level of dopamine efflux induced by L-glutamate, and attenuated the loss of TTC staining induced by the excitotoxin. These results suggest a role for Kv7.2 in modulating ischemia-evoked caudate damage.
Keywords: dopamine, ischemia, Kv7 potassium channels, neuroprotection, oxygen–glucose deprivation, voltammetry
Introduction
Pharmacological treatments for ischemic stroke mostly rely on recombinant tissue plasminogen activator administration, often showing suboptimal efficiency.1 Therefore, new neuroprotective drugs, possibly targeting different mechanisms occurring during ischemic stroke, are needed. In stroke and hypoxia, ATP loss results in failure of membrane transporters and ion channels, with subsequent membrane depolarization and aberrant neurotransmitters efflux, thus amplifying injury.2 Increased neuronal excitability seems to be a pathogenic mechanism underlying different diseases, such as Alzheimer's disease and epilepsy; moreover, epidemiologic studies as well as experiments on animal models have shown a relationship between ischemia and seizures.3 Based on these observations, antiepileptic drugs have been also proposed for stroke treatment.4 Although glutamate release has been viewed as a main cause of neuronal damage, massive release of monoamines, such as dopamine, also occurring immediately after the onset of ischemia, can be neurotoxic and thus contribute to cell death.5 Moreover, it has been shown that chronic methamphetamine administration as well as striatal injection of dopamine caused a marked presynaptic and postsynaptic neuronal death in rodents.6 Dopamine release during ischemia is supposed to be a consequence of different mechanisms, such as cell depolarization, reversal of dopamine transporters, and ATP shortage.7 Among the various regulators of neurotransmitter release, voltage-gated potassium channels belonging to the Kv7 subfamily have emerged as possible targets for hyperexcitability central nervous system diseases. The Kv7 family comprises five members; Kv7.2 to Kv7.5 subunits underlie the M current (IKM), a potassium current that reduces neuronal excitability, limiting repetitive neuronal firing.8 In neurons, IKM is mainly determined by heterotetramers formed by Kv7.2 and Kv7.3 subunits; mutations in Kv7.2 and Kv7.3 are responsible for inherited and sporadic forms of neonatal-onset epilepsies with wide phenotypic heterogeneity, ranging from Benign Familial Neonatal Seizures to epileptic encephalopathy.9, 10, 11 Kv7.5 and Kv7.4 subunits also contribute to M-current heterogeneity in neurons, but their role is more dominant in nonneuronal tissue, such as inner ear (Kv7.4), smooth muscle (Kv7.4 and Kv7.5),12 and skeletal muscle (Kv7.4 and Kv7.5).8, 12 Pharmacological modulation of the M current modifies depolarization-induced neurotransmitter release in different brain areas.13 In particular, the Kv7 activator retigabine, a recently marketed antiepileptic drug used as an adjunctive treatment in patients with refractory epilepsy, was able to decrease dopamine release from isolated striatal nerve terminals exposed to high extracellular K+ concentrations.14 In addition, while some studies have shown that Kv7 activators reduced neuronal damage in hippocampal brain slices,15, 16 others found that M-current activation increased death in hippocampal cultured neurons.17
The aim of this study was to investigate the potential neuroprotective role of Kv7 channels in an in vitro model of ischemia, by evaluating the pharmacological modulation of Kv7 channels on dopamine efflux and on neurotoxicity in rat caudate, a region often damaged in ischemic stroke.18
Materials and methods
Animals and Brain Slices
Adult male Wistar rats (7 to 9 weeks old) were bought from Charles River (Oxford, UK), kept 4 per cage, and acclimated to the animal house for 1 week before use. Standard rat chow and water was freely available and rats were kept on a 12-hour L:D cycle, lights on at 0700 h. Rats were killed by cervical dislocation (1030 h), and were treated in accordance with UK Home Office legislation (Animals (Scientific Procedures) Act 1986) and the European Directive 2010/63/EU. Although not in vivo research we followed the ARRIVE guidelines as best we could in reporting this work. The brain was quickly dissected and coronal slices (400 μm thick) at the level of the striatum were cut and maintained in artificial cerebrospinal fluid (aCSF). The composition of aCSF was (mmol/L): NaCl (126.0), KCl (2.0), KH2PO4 (1.4), MgSO4 (2.0), NaHCO3 (26.0), CaCl2 (2.4), (+)-glucose (10.0), bubbled for at least 30 minutes with 95% O2/5% CO2. Once cut, slices were left to equilibrate for 1 to 4 hours at 21±1°C, to allow recovery from any trauma associated with slicing.7
Fast Cyclic Voltammetry
The fast cyclic voltammetry (FCV) recording apparatus comprised a slice chamber (~2 mL) with the superfusate heated via a thermostatically controlled circulating water bath (Grant, Cambridgeshire, UK). Brain slices were continuously superfused with aCSF, at 32.5±0.5°C, with a flow rate of 100 mL/h. We applied a triangular voltage waveform (−1 to +1.4 V) to the carbon fiber electrode (7 μm diameterx50 μm length), with a Ag/AgCl reference electrode;12 the electrochemical signal from carbon-fiber microelectrode, placed into the dorsolateral caudate, was sampled once per second, digitized, and recorded using Spike7 (CED, Cambridge, UK). An increase in the current at +600 mV (the oxidation peak, see Figure 1 inset) together with a corresponding reduction peak at −200 mV is indicative of dopamine.
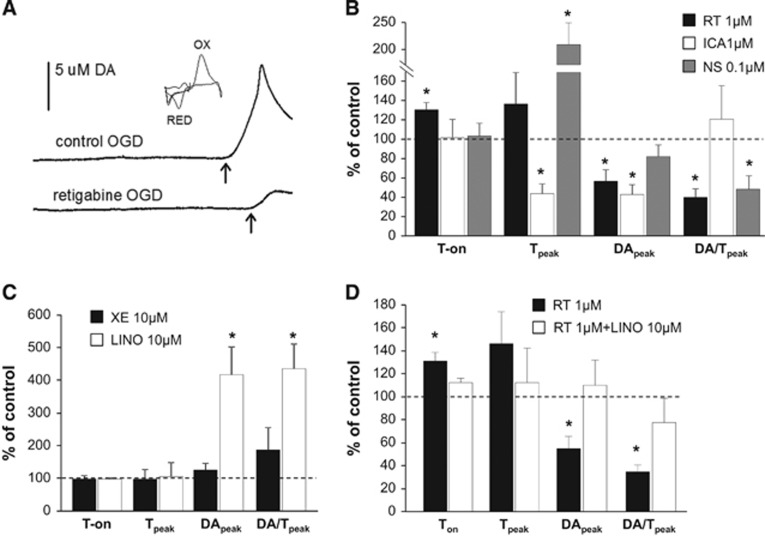
Figure 1.
Effects of Kv7-acting drugs on oxygen and glucose deprivation (OGD)-induced dopamine (DA) efflux. (A) Representative experiments showing dopamine efflux elicited by OGD in the presence of vehicle (top trace) and 1 μmol/L retigabine (bottom trace); the arrow indicates the onset of dopamine efflux. The inset shows the cyclic voltammogram obtained by plotting the faradaic current against the input voltage. Faradaic currents are obtained by subtracting the signal recorded at the carbon fiber electrode in the absence of dopamine from the signal in the presence of dopamine. Peaks due to the oxidation (OX) and reduction (RED) of dopamine are indicated. (B and C) Effects of Kv7 activators (B: retigabine, ICA27243 and NS15370) and blockers (C: XE-991 and linopirdine) on time to onset, time to peak, maximum extracellular dopamine concentration, and mean rate of dopamine efflux. (D) Effects of 1 μmol/L retigabine alone (black columns) or coincubated with 10 μmol/L linopirdine (white columns) on dopamine efflux parameters. Data are expressed as percentage of DMSO-treated slices (controls, represented by the dashed line). Values are mean±s.e.m. of 5 to 9 slices from 5 to 6 rats. *P<0.05.
Fast Cyclic Voltammetry Data Analysis
Analysis of data was performed offline using Spike7 (CED, Cambridge, UK). Four parameters were measured: (1) time to onset of dopamine release from the initiation of oxygen and glucose deprivation (OGD) superfusion (Ton); (2) time taken to reach maximum dopamine release after the onset of release (Tpeak); (3) maximum extracellular dopamine concentration (DApeak); and (4) mean rate of dopamine release (DA/Tpeak).
Experimental Protocol for Oxygen and Glucose Deprivation and L-Glutamate-Induced Toxicity
Slices were allowed to equilibrate for 15 minutes in normal aCSF, to assess that no spontaneous dopamine release occurred, indicating poor slice health,12 then incubated with aCSF containing 0.1% DMSO or drug for an additional 15 minutes before exposure to ischemic or excitotoxic insults. To this aim, slices were superfused with oxygen and glucose-deprived aCSF (OGD-aCSF, 2 mmol/L glucose, continuously bubbled with 95% N2/5% CO2) or with 10 mmol/L L-glutamate, respectively. These insults were delivered during continued presence of DMSO or test agent until dopamine release occurred (for FCV experiments) or for 15 minutes (for 2,3,5-triphenyltetrazolium chloride (TTC) staining and lactate dehydrogenase (LDH) assay). For qPCR, western blot, TTC staining experiments, and LDH assay, slices were also reperfused for 30 to 60 minutes with normal aCSF containing DMSO or drug after OGD, to mimic reperfusion and allow time for cell damage to occur.
2,3,5-Triphenyltetrazolium Chloride Staining
Separate slices were stained with TTC in aCSF (0.125% w/v) at 21±1°C for 30 minutes and then fixed in 4% paraformaldehyde; photographed and analyzed using the ImageJ software (http://rsb.info.nih.gov/ij/). Data analysis was performed independently by two researchers, one of which was blinded to experimental groups.
Real-Time PCR
Total RNA was extracted from brain slices using the RNeasy Mini kit (Qiagen, Manchester, UK) and reverse transcribed using Moloney Murine Leukemia Virus (M-MLV; Invitrogen, Life Technologies, Paisley, UK). Quantitative PCR analysis was performed with the CFX96 machine (Bio-Rad, Hemel Hempstead, UK), using SYBR green master mix and specific primers (PrimerDesign, Ltd., UK), followed by melt curve analysis. No template controls were run alongside all reactions to assess contamination. Cycle threshold (Ct) values were determined and normalized to GAPDH housekeeping gene. Relative abundance of each gene of interest was calculated using the 2−ΔΔCt formula.
Western Blot
Brain slices were homogenized in 100 μL lysis buffer (in mmol/L: 20 Tris base, 137 NaCl, 2 EDTA, 1% NP-40, 10% glycerol, pH 8, and 10 μL/mL protease inhibitor cocktail; Sigma-Aldrich, Gillingham, UK), denatured at 95°C for 5 minutes in the presence of sample buffer and reducing agent (Invitrogen) and loaded onto SDS-PAGE gels (4% to 12% Bis-Tris, Invitrogen), followed by transfer onto a polyvinylidene fluoride membrane (Amersham Biosciences, GE Healthcare, Hatfield, UK). The membrane was then probed with an anti-Kv7.2 antibody (dilution 1:500; Millipore, Watford, UK). Protein bands were visualized with enhanced chemiluminescence (Thermo Scientific, GE Healthcare, Hatfield, UK) and hyperfilm (Amersham Bioscience). The membranes were reprobed for β-actin (1:5,000; Sigma-Aldrich, A1978), used as a loading control. Bands were quantified with Image J.
Lactate Dehydrogenase Assay
Neuronal necrotic damage was assessed by measuring LDH activity in the incubation medium, using a specific kit and following the manufacturer's instructions (Abcam, Cambridge, UK). Briefly, slices were incubated in 24-well plates and exposed to normal- and OGD-aCSF, in the presence of 0.1% DMSO or drug, as described above. In all, 50 μL of incubation medium was taken at different time points (30 and 60 minutes of reoxygenation period) and mixed with reaction buffer, in the presence of a substrate for LDH. NADH generated by LDH activity interacted with a specific probe in a colorimetric reaction that can be read at 450 nm in a microplate reader. Each sample was assayed in duplicate in a 96-well plate. Absorbance was measured at time 0 and after 30 minutes incubation at 37°C. Lactate dehydrogenase activity (mU/mL) was calculated by comparing samples OD values with those obtained from known NADH standards. Values were then normalized to protein concentration and results are shown as percentage of control slices.
Drugs
Several drugs with specificity for different Kv7 channel subtypes were tested, in particular: (1) the pan-Kv7 activator retigabine, opening Kv7.2 and Kv7.3 channels at low concentration (<1 μmol/L); (2) ICA27243, a Kv7.2/Kv7.3-preferring activator, showing 20- to 100-fold lower potency on Kv7.4 and Kv7.5; (3) NS15370, a retigabine-analog able to activate all Kv7 subunits at submicromolar concentrations. All activators were a kind gift from Prof SP Olesen (Biomedical Sciences Institute, University of Copenhagen). The Kv7 blockers XE991 and linopirdine as well as the dopamine D1 receptor antagonist SCH23390 were from Tocris Bioscience (Cambridge, UK). The dopamine D2 receptor antagonist metoclopramide and L-glutamate were from Sigma-Aldrich.
Statistics
Data were compared by the use of parametric tests assuming normal distribution of data: Student's t-tests or one-way ANOVA, with Dunnett's post hoc correction for multiple comparisons. GraphPad Prism was used for statistical analysis. Significance was set at P<0.05. Values shown are mean±s.e.m. Authors were not blind to treatment groups but one author was blinded for the TTC image analysis and data compared to ensure objectivity and accuracy.
Results
OGD-Evoked Dopamine Efflux
Exposure of striatal slices to OGD evoked dopamine efflux after 715±36 seconds (time to onset, Ton). Peak dopamine efflux (DApeak) was 7.2±0.9 μmol/L and time to peak dopamine efflux from the onset of dopamine efflux (Tpeak) was 125±12 seconds. The average rate of dopamine efflux (DA/Tpeak) in control slices was 0.11±0.04 μmol/L per second (Figure 1A). The Kv7 activator retigabine (1 μmol/L) delayed the onset of dopamine efflux by about 30% and also reduced dopamine peak and the rate of change of dopamine efflux by about 40% and 60%, respectively (Figure 1B, black columns). ICA27243 (1 μmol/L), a Kv7.2/Kv7.3-preferring activator, reduced dopamine peak by ~60% but it did not significantly affect Ton. ICA27243 also reduced Tpeak by ~60%, although dopamine efflux rate was unaffected (Figure 1B, white columns). In contrast NS15370 (0.1 μmol/L), a more potent Kv7 opener with respect to retigabine doubled the time to reach dopamine peak, reduced the rate of dopamine efflux by ~55%, but did not significantly modify Ton or DApeak (Figure 1B, gray columns). We then tested two different pan-Kv7 blockers, XE991 and linopirdine (Figure 1C). While 10 μmol/L XE991 did not interfere with OGD-evoked dopamine efflux (Figure 1C, black columns), linopirdine at 10 μmol/L evoked a 4-fold increase in peak dopamine and rate of dopamine efflux when compared with controls (Figure 1C, white columns). Moreover, linopirdine prevented the changes in Ton, DApeak, and DA/Tpeak induced by retigabine after OGD superfusion (Figure 1D, white columns).
2,3,5-Triphenyltetrazolium Chloride Staining
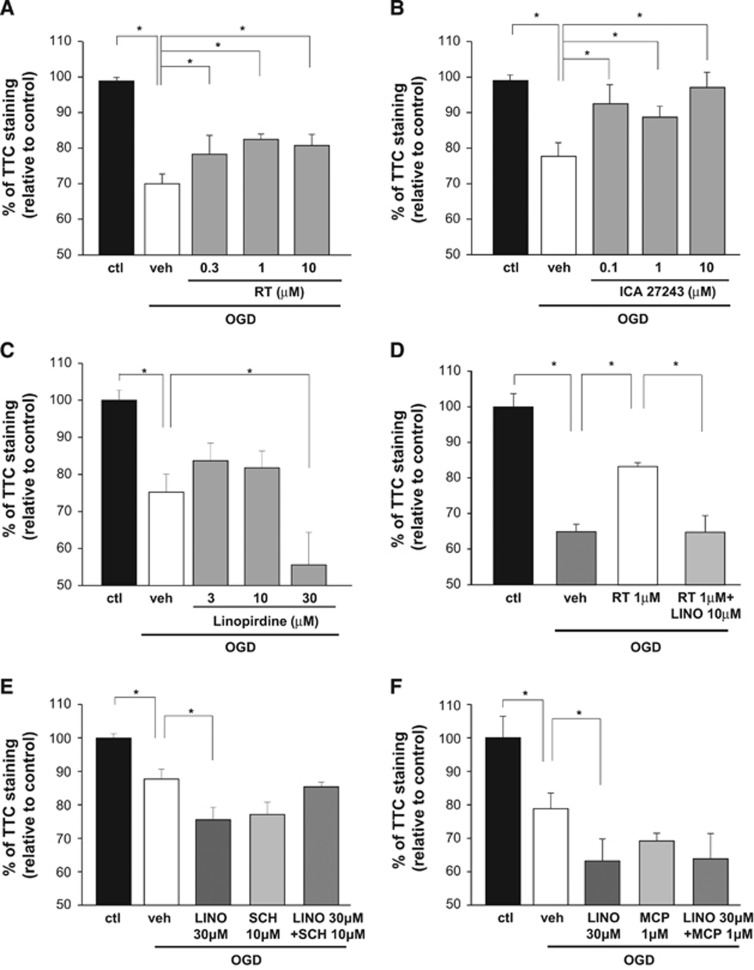
To further investigate whether Kv7 activators decreased OGD-induced damage, we performed TTC staining experiments on brain slices (Figure 2). Retigabine (0.3 to 10 μmol/L; Figure 2A) and ICA27243 (0.1 to 10 μmol/L; Figure 2B) attenuated the loss of TTC staining in slices exposed to OGD. In contrast, exposure of slices to linopirdine (30 μmol/L) enhanced OGD-induced loss of TTC staining (Figure 2C). Coincubation of linopirdine at 10 μmol/L reversed the reduction in the loss of TTC staining exerted by 1 μmol/L retigabine (Figure 2D).
Figure 2.
Effects of Kv7-acting drugs and dopamine receptor antagonists on oxygen and glucose deprivation (OGD)-induced toxicity measured by 2,3,5-triphenyltetrazolium chloride (TTC) staining. (A-C) Effects on TTC staining exerted by increasing concentrations of retigabine (A), ICA27243 (B), and linopirdine (C) in OGD-exposed brain slices, as indicated. Data from vehicle-treated slices are represented by white columns. (D) Effects on TTC staining of 1 μmol/L retigabine alone (white columns) or coincubated with 10 μmol/L linopirdine (light gray columns). (E and F) Effects on TTC staining in OGD-exposed slices of 10 μmol/L SCH23390 (E) and 1 μmol/L metoclopramide (F) used alone and coincubated with 30 μmol/L linopirdine. Data from vehicle-treated slices are represented by white columns. All data are expressed as percentage of normal artificial cerebrospinal fluid (aCSF)-incubated slices (control, black columns). Each bar represents the mean±s.e.m. of 4 to 7 slices from 4 to 5 rats. *P<0.05.
To evaluate whether dopamine exerts neurotoxic effects via overstimulation of dopamine receptors, we tested the effects of SCH23390 or metoclopramide, D1- and D2-receptor antagonists respectively, on OGD-induced damage. Neither SCH23390 (10 μmol/L) nor metoclopramide (1 μmol/L) prevented the loss of TTC staining in slices exposed to OGD, and these drugs failed to attenuate the potentiated neurotoxic effects evoked by linopirdine (30 μmol/L) (Figures 2E and 2F).
Lactate Dehydrogenase Activity
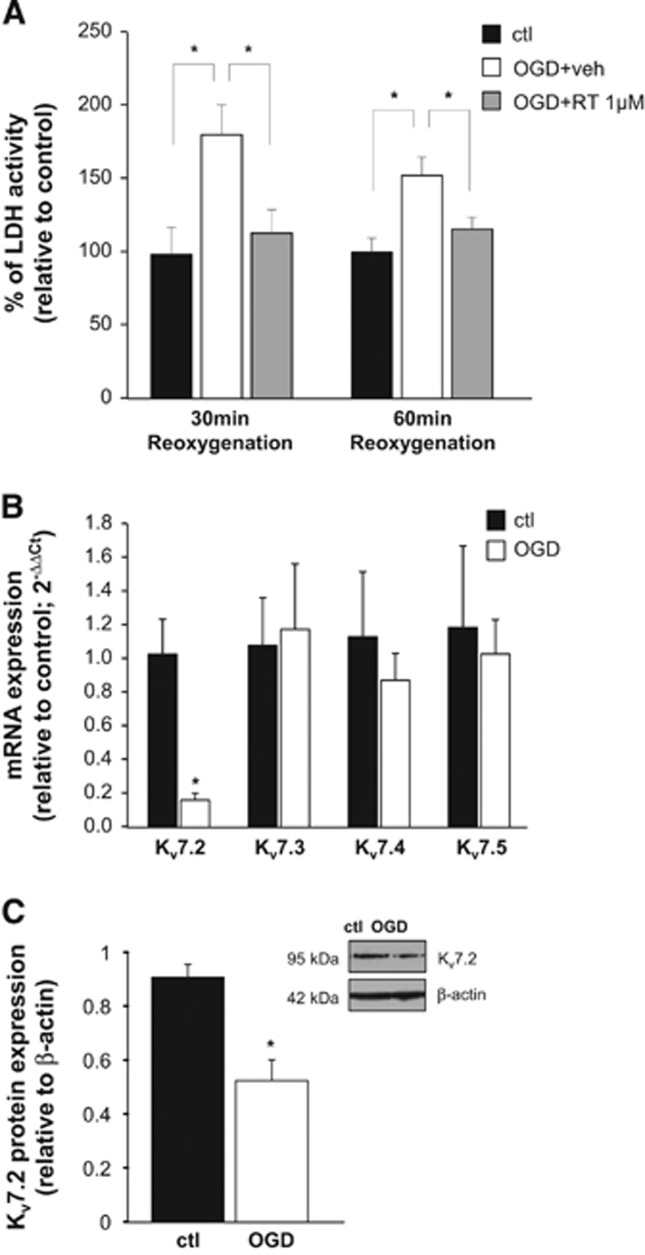
To evaluate the possible molecular mechanisms underlying retigabine-induced neuroprotection, we measured LDH activity in the incubation medium of slices exposed to OGD, as an index of cell necrosis. In OGD-exposed slices, LDH activity was increased by ~80% and ~50% compared with control slices after 30 and 60 minutes of reoxygenation, respectively (Figure 3A, white columns); retigabine (1 μmol/L) reduced OGD-induced increase of LDH activity at both time points (Figure 3A, gray columns).
Figure 3.
Effects of retigabine on oxygen and glucose deprivation (OGD)-induced necrosis and effects of OGD on Kv7 subunits expression. (A) Bar graph showing lactate dehydrogenase (LDH) activity measured in the incubation medium in brain slices exposed to normal artificial cerebrospinal fluid (aCSF) (control, black columns), to OGD+vehicle (white columns), or to OGD+1 μmol/L retigabine, after 30 and 60 minutes of reoxygenation. Data are expressed as percentage of respective control. Each bar represents the mean±s.e.m. of 8 to 10 slices from 4 to 5 rats. *P<0.05. (B) Q-PCR experiments showing mRNA levels encoding for Kv7 subunits in striatal slices exposed to normal aCSF (control) or OGD. Data are normalized to housekeeping gene gapdh and expressed as relative to the average of controls, using the 2−ΔΔCt formula. Values are mean±s.e.m. of 4 to 6 slices. *P<0.05. (C) Western blot analysis for Kv7.2 protein expression on total lysates from control- or OGD-treated slices. The inset shows a representative blot for Kv7.2 and β-actin. The approximate molecular mass for each of these proteins (expressed in kDa) is shown on the left. Bar graph shows the quantification of the averaged OD values for the Kv7.2 bands, normalized to the OD value for β-actin, in both normal aCSF (black columns) and OGD-exposed (white columns) slices. *P<0.05 vs respective control; n=5 for both control- and OGD-exposed slices.
Q-PCR and Western Blots
We next evaluated possible changes in Kv7 transcripts expression after OGD. Exposure of striatal slices to OGD followed by incubation in normal aCSF caused a 6-fold decrease in Kv7.2-transcripts measured by Q-PCR; instead, mRNAs encoding for other Kv7 subunits were unaffected (Figure 3B). Western blot experiments showed a reduction also in Kv7.2 protein levels (Figure 3C).
L-Glutamate Toxicity
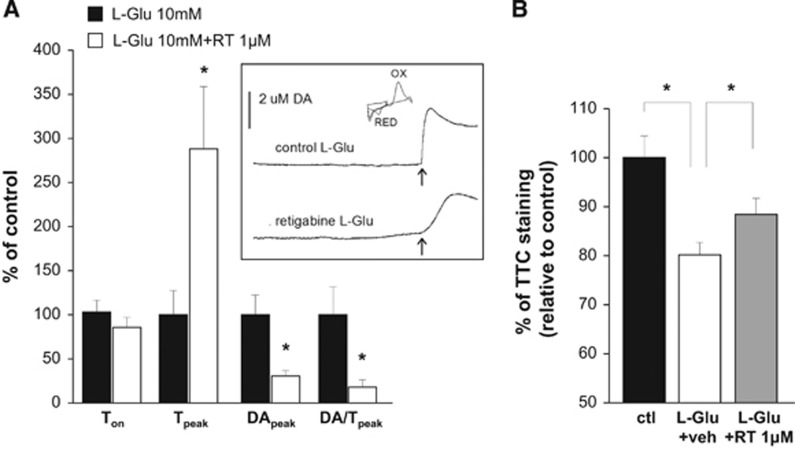
To assess whether Kv7 channel activation might exert neuroprotective actions in a different model of cytotoxicity, we evaluated the effects of retigabine on both dopamine release and cell damage after exposure of brain slices to high concentrations of L-glutamate. Incubation of slices with L-glutamate (10 mmol/L) caused dopamine efflux after 354±40 seconds, reaching peak dopamine efflux (6.6±1.4 μmol/L) after 44±13 seconds. Coincubation with retigabine (1 μmol/L) caused an ~3-fold increase in time to peak and reduced DApeak and DA/Tpeak by ~70% and ~80% respectively, while it did not significantly affect Ton (Figure 4A). Moreover, retigabine attenuated the loss of TTC staining in slices exposed to L-glutamate (Figure 4B).
Figure 4.
Effects of retigabine on L-glutamate-induced dopamine release and toxicity. (A) Fast cyclic voltammetry (FCV) experiments showing the effects of 1 μmol/L retigabine on time to onset, time to peak, maximum extracellular dopamine concentration, and mean rate of dopamine efflux in brain slices exposed to 10 mmol/L L-glutamate. Data are expressed as percentage of DMSO-treated slices (controls, black columns). Values are mean±s.e.m. of 5 to 9 slices from 5 to 6 rats. *P<0.05. The inset shows representative experiments of dopamine efflux elicited by oxygen and glucose deprivation (OGD) in the presence of vehicle (top trace) and 1 μmol/L retigabine (bottom trace); the arrow indicates the onset of dopamine peak. The cyclic voltammogram generated by plotting the faradaic current against the input voltage is also shown. Peaks due to the oxidation (OX) and reduction (RED) of dopamine are indicated. (B) Bar graph showing the effects of exposure to 10 mmol/L L-glutamate in the presence of DMSO (white column) or 1 μmol/L retigabine (gray column). Data are expressed as percentage of DMSO-treated slices (control with no L-glutamate, black column). Values are mean±s.e.m. of 6 to 8 slices from 3 to 4 rats. *P<0.05.
Discussion
Ionic homeostasis is fundamental for excitable cells such as neurons, since alterations in the concentrations of different ionic species are both causes and consequences of different pathologic processes (apoptosis, cell shrinkage, proteases activation) and diseases. In particular, K+ ion imbalance seems to be strictly linked to neuronal dysfunction, also for the pivotal role exerted by this ion species in controlling the resting membrane potential and cell excitability. Indeed, hyperexcitability is a common feature of many diseases such as epilepsy and ischemia; massive neurotransmitters release occurs during brain ischemia, possibly due to damage-induced depolarization and/or the rising of intracellular Ca2+ concentrations.2 Thus, modulation of K+ channel activity has been acknowledged as a possible target for neuroprotection. Among the different neurotransmitters, dopamine release has been shown to reach very high levels soon after an ischemic insult in the caudate, and also to contribute to neuronal damage;5 indeed, depletion of dopaminergic nigro-striatal projections has been shown to reduce ischemic damage.19 Dopamine and related compound such as L-3,4-dihydroxyphenylalanine and 6-OH-DOPA are considered as potent excitotoxic agents, possibly contributing to neurodegeneration in Parkinson's and Huntington's disease.20 Given the known effect of the antiepileptic drug retigabine in reducing dopamine release,14, 21 we have investigated the possibility that the pharmacological activation of Kv7-mediated currents could exert a neuroprotective effect in an in vitro model of ischemia. In particular, we focused on the striatum, a region frequently damaged in ischemic stroke in humans and rodent models of stroke, such as middle cerebral artery occlusion. In our experiments the Kv7 activator retigabine was able to reduce the peak dopamine efflux and to delay OGD-evoked dopamine efflux. These effects occurred at a retigabine concentration (1 μmol/L) close to the EC50 for activation of Kv7.2/Kv7.3 heteromers underlying M current,8 and to the plasma concentrations achieved in vivo in humans.22 Because these aberrant dopamine concentrations have been shown to be neurotoxic, reductions in the peak dopamine efflux or the rate of dopamine efflux can be considered neuroprotective, as also suggested by TTC staining experiments. This view was corroborated by the results of FCV and TTC experiments using ICA27243, a compound with higher selectivity at Kv7.2/Kv7.3 heterotetramers. In addition, NS15370 reduced the time to reach dopamine peak, but did not reproduce the same effects on OGD-evoked dopamine efflux obtained with retigabine and ICA27243. However, it should be mentioned that NS15370 has a very complex pharmacological profile, showing also Kv7 channel blockade at depolarized potentials,23 a characteristic that might in part explain the lack of effects on dopamine efflux. Blockade of Kv7 channels by linopirdine increased the amplitude and hastened the rate of OGD-evoked dopamine efflux; this result was paralleled by the worsening of OGD-induced brain damage caused by exposure to linopirdine, although at a concentration (30 μmol/L) higher than that able to increase OGD-evoked dopamine efflux. Moreover, linopirdine 10 μmol/L was able to reverse the changes in dopamine efflux induced by retigabine, as well as the reduction in TTC staining loss induced by the Kv7 activator. In particular, linopirdine prevented the effect on Ton exerted by retigabine, although it did not modify this parameter when incubated alone, thus arguing in favor of a specific involvement of the M current. By contrast, the linopirdine-analog XE-991 (10 μmol/L) did not modulate OGD-evoked dopamine efflux, although it was previously showed that it increased depolarization-induced dopamine efflux from isolated striatal synaptosomes14 and from striatal brain slices.21 Differences in the experimental models (isolated terminals/chopped slices vs brain slices) as well as in the experimental procedures (KCl- vs OGD-evoked release, preincubation with dopamine reuptake inhibitors) might explain this discrepancy.
Despite the well-known neurotoxic effects prompted by high levels of dopamine, the molecular mechanisms underlying these actions are not fully understood. Aberrant dopamine levels can induce damage through the formation of oxidative metabolites, derived from both spontaneous and enzyme-mediated dopamine oxidation.24, 25 In addition, receptor-mediated mechanisms have been proposed to explain dopamine-induced neurotoxicity and the possible neuroprotective effects of dopamine D1 and D2 receptor antagonists have been investigated in several studies.26 In our TTC staining experiments, both SCH23390 (D1 receptor antagonist) and metoclopramide (D2 receptor antagonist) failed to reduce OGD-induced damage and did not revert the enhanced toxicity caused by linopirdine, suggesting that, in our model, brain damage occurred in a dopamine receptor-independent manner. These results are not surprising, since conflicting evidence are present in the literature; indeed, while some studies failed to show neuroprotection by D1 and D2 receptor antagonists, others found that receptor stimulation (mainly the D2 subtype) reduced neuronal damage by blocking the apoptotic pathway (for a comprehensive review, see Bozzi and Borrelli27).
The neuroprotective action of Kv7 channel activation was confirmed by LDH assay showing that incubation with retigabine reduced necrotic death in brain slices exposed to OGD. We also investigated whether retigabine could affect the apoptotic pathway; unfortunately, in our experimental model, no clear programmed cell death (measured by the evaluation of caspase 3 activation) occurred after OGD, even after 2 hours of reoxygenation (data not shown). However, it should be mentioned that the degree of apoptosis can significantly vary depending on the experimental model and type of insult (duration of OGD and reoxygenation period), being often undetectable.28 Moreover, in our experimental model, it was not possible to assess cell damage after long incubation time, as viability of brain slice was significantly reduced also in control conditions.
Both qPCR and western blot data showed a selective decrease in Kv7.2 subunit expression after OGD. Although we do not know whether this reduction is only a minor consequence of anoxia, or a main pathogenic mechanism through which OGD exerts its neurotoxic effects, pharmacological data suggest that the activation of channels incorporating Kv7.2 subunits might counteract the changes prompted by ischemia and, at least in part, protect from brain damage. In addition, it has been shown that in striatal synaptosomes, selective Kv7.2 blockade with a subunit-specific antibody was able to completely abolish the effects of retigabine,14 suggesting a primary role of Kv7.2 subunits as the molecular determinant of M current in this brain area. Moreover, Kv7.2 and Kv7.3 subunit expression levels can be directly regulated by transcription factors such as Sp1 and REST (Repressor Element 1-Silencing Transcription factor).29 Neuronal expression of REST is upregulated in many neuronal pathologic conditions, such as cerebral ischemia;30 thus, the possibility exists that modulation of Kv7.2 expression is a primary mechanism to regulate neuronal behavior during pathologic CNS conditions characterized by hyperexcitability. Previous studies have shown that retigabine was neuroprotective in hippocampal slices, possibly because of its antioxidant activity.15, 16 Although different brain regions have been investigated, the efficacy of ICA27243, as well as the reversal of retigabine effects by linopirdine, suggests a neuroprotection mediated by M-current activation. The neuroprotective effects of retigabine are further corroborated by the results of our experiments using a different trigger for brain damage. Indeed, retigabine was able to decrease dopamine release and reduce damage in brain slices exposed to high concentrations of L-glutamate. Since massive release of glutamate occurs during stroke thus determining excitotoxicity, these results highlight Kv7 channels as an exciting target for stroke. Indeed, the M current activates in the range of resting membrane potential, maintaining it below the threshold for sodium current activation, thus limiting repetitive firing after sustained stimulation.8 Moreover, the M current can reduce subthreshold excitability, representing a ‘high-pass filter' for triggers arriving to the axon initial segment, a region particularly important for the integration of stimuli converging to the neuron; interestingly, Kv7.2 channels are robustly expressed in this region of the neuron.31 In this regard, the M current seems to be a major determinant of intrinsic neuronal firing characteristics32, 33 and its activation might exert beneficial effects not only at presynaptic level (reduction of neurotransmitter release) but also at postsynaptic and axonal level (decreased neuronal excitability). In this scenario, retigabine, by causing a leftward shift in the voltage dependence of Kv7 channels, facilitates channel opening at more depolarized potentials, thus impeding neuronal depolarization and preventing ischemia-induced hyperexcitability.
During ischemia, lack of oxygen leads the damaged tissue to switch to anaerobic processes, lactic acid production, and subsequent acidosis. A decrease in pH has been shown to reduce Kv7.2/Kv7.3-mediated currents;34 although we did not measure pH in the extracellular fluid or in the brain slices, in similar voltammetric experiments in rodent brain slice changes in pH due to local electrical stimulation of the brain slice have been observed.35 Nevertheless, the possibility exists that, due to the continuous perfusion system used, H+ has largely been washed away and the M current is artificially upregulated in our experiments. Lactate has been proposed as a neuroprotective agent, based on the ‘astrocyte-neuron lactate shuttle hypothesis' suggesting that astrocytes can supply lactate as an alternative, possibly the last, energetic substrate to neurons through monocarboxylate transporters during sustained neuronal activity.36 Moreover, blockade of the lactate transporter exacerbates damage induced by glutamate37 and exogenous lactate can reduce brain damage in in vitro and in vivo models of ischemia.38 These data are apparently in contradiction with our hypothesis, as lactate production and subsequent acidosis decrease Kv7 currents, thus suggesting that Kv7 activation should be neurotoxic. However, lactate administration does not seem to modify pH, even at high concentration, in contrast to lactic acid.38, 39 Moreover, endogenous lactate production can be limited by monocarboxylate transporter activity which, when saturated, can lead to the accumulation of lactic acid and consequent acidosis. Finally, the evidence concerning the neuroprotective effects of lactate are conflicting, as other studies have found that reducing lactate levels can be neuroprotective and that regional acidosis enhances brain damage.40 We do not know whether, given the complex functional changes produced by ischemia (involving not only acidosis but lactate production also, see below), the protective effects herein reported for Kv7 activators in an ex vivo slice model would be more or less pronounced in vivo. However, the idea that increasing Kv7 channels activity can be neuroprotective is further confirmed by research published while this manuscript was under review.41 The authors found that retigabine and two new Kv7 activators reduced infarct size and functional deficits in two in vivo models of stroke.
Taken together, our data suggest that the M-current activation counteracts the OGD- and glutamate-induced efflux of dopamine and subsequent neurotoxicity. This suggests that, in addition to increasing cerebral blood flow that follows cerebral arteries vasodilation,42 M-current openers might provide direct parenchymal protection. Such a dual mechanism of action might represent a new strategy for neuroprotection in stroke.
The authors declare no conflict of interest.
References
- 1Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010; 41: 2254–2258. [DOI] [PubMed] [Google Scholar]
- 2Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology 2008; 55: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Kadam SD, White AM, Staley KJ, Dudek FE. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci 2010; 30: 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Costa C, Martella G, Picconi B, Prosperetti C, Pisani A, Di Filippo M et al. Multiple mechanisms underlying the neuroprotective effects of antiepileptic drugs against in vitro ischemia. Stroke 2006; 37: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 5Globus MY-T, Busto R, Dietrich WD, Martinez E, Valdes I, Ginsberg MD1988Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and gamma-aminobutyric acid studied by intracerebral microdialysis. J Neurochem 51: 1455–1464. [DOI] [PubMed] [Google Scholar]
- 6Kita T, Saraya T, Konishi N, Matsunari Y, Shimada K, Nakamura M et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine pretreatment attenuates methamphetamine-induced dopamine toxicity. Pharmacol Toxicol 2003; 92: 71–80. [DOI] [PubMed] [Google Scholar]
- 7Toner CC, Stamford JA. Effects of metabolic alterations on dopamine release in an in vitro model of neostriatal ischaemia. Brain Res Bull 1999; 48: 395–399. [DOI] [PubMed] [Google Scholar]
- 8Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 2011; 26: 365–376. [DOI] [PubMed] [Google Scholar]
- 9Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ et al. A potassium channel mutation in neonatal human epilepsy. Science 1998; 279: 403–406. [DOI] [PubMed] [Google Scholar]
- 10Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 1998; 18: 53–55. [DOI] [PubMed] [Google Scholar]
- 11Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol 2012; 71: 15–25. [DOI] [PubMed] [Google Scholar]
- 12Stott JB, Jepps TA, Greenwood IA. K(V)7 potassium channels: a new therapeutic target in smooth muscle disorders. Drug Discov Today 2014; 19: 413–424. [DOI] [PubMed] [Google Scholar]
- 13Martire M, Castaldo P, D'Amico M, Preziosi P, Annunziato L, Taglialatela M. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci 2004; 24: 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Martire M, D'Amico M, Panza E, Miceli F, Viggiano D, Lavergata F et al. Involvement of KCNQ2 subunits in [3H]dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J Neurochem 2007; 102: 179–193. [DOI] [PubMed] [Google Scholar]
- 15Boscia F, Annunziato L, Taglialatela M. Retigabine and flupirtine exert neuroprotective actions in organotypic hippocampal cultures. Neuropharmacology 2006; 51: 283–294. [DOI] [PubMed] [Google Scholar]
- 16Gamper N, Zaika O, Li Y, Martin P, Hernandez CC, Perez MR et al. Oxidative modification of M-type K(+) channels as a mechanism of cytoprotective neuronal silencing. EMBO J 2006; 25: 4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Zhou X, Wei J, Song M, Francis K, Yu SP. Novel role of KCNQ2/3 channels in regulating neuronal cell viability. Cell Death Differ 2011; 18: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Nys GMS, van Zandvoort MJE, van der Worp HB, Kapelle LJ, de Haan EHF. Neuropsychological and neuroanatomical correlates of perseverative responses in subacute stroke. Brain 2006; 129: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 19Globus MY-T, Ginsberg MD, Dietrich WD, Busto R, Scheinberg P1987Substantia nigra lesion protects against ischemic damage in the striatum. Neurosci Lett 80: 251–256. [DOI] [PubMed] [Google Scholar]
- 20Olney JW, Zorumski CF, Stewart GR, Price MT, Wang GJ, Labruyere J. Excitotoxicity of L-dopa and 6-OH-dopa: implications for Parkinson's and Huntington's diseases. Exp Neurol 1990; 108: 269–272. [DOI] [PubMed] [Google Scholar]
- 21Jensen MM, Lange SC, Thomsen MS, Hansen HH, Mikkelsen JD. The pharmacological effect of positive KCNQ (Kv7) modulators on dopamine release from striatal slices. Basic Clin Pharmacol Toxicol 2011; 109: 339–342. [DOI] [PubMed] [Google Scholar]
- 22Large CH, Sokal DM, Nehlig A, Gunthorpe MJ, Sankar R, Crean CS et al. The spectrum of anticonvulsant efficacy of retigabine (ezogabine) in animal models: implications for clinical use. Epilepsia 2012; 53: 425–436. [DOI] [PubMed] [Google Scholar]
- 23Dalby-Brown W, Jessen C, Hougaard C, Jensen ML, Jacobsen TA, Nielsen KS et al. Characterization of a novel high-potency positive modulator of K(v)7 channels. Eur J Pharmacol 2013; 709: 52–63. [DOI] [PubMed] [Google Scholar]
- 24Graham DG, Tiffany SM, Bell WR, Jr, Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol 1978; 14: 644–653. [PubMed] [Google Scholar]
- 25Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem 1995; 64: 919–924. [DOI] [PubMed] [Google Scholar]
- 26Davis S, Brotchie J, Davies I. Protection of striatal neurons by joint blockade of D1 and D2 receptor subtypes in an in vitro model of cerebral hypoxia. Exp Neurol 2002; 176: 229–236. [DOI] [PubMed] [Google Scholar]
- 27Bozzi Y, Borrelli E. Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci 2006; 29: 167–174. [DOI] [PubMed] [Google Scholar]
- 28Jones PA, May GR, McLuckie JA, Iwashita A, Sharkey J. Apoptosis is not an invariable component of in vitro models of cortical cerebral ischaemia. Cell Res 2004; 14: 241–250. [DOI] [PubMed] [Google Scholar]
- 29Mucha M, Ooi L, Linley JE, Mordaka P, Dalle C, Robertson B et al. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J Neurosci 2010; 30: 13235–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y et al. Ischemic insults depress the gene silencer REST in neurons destined to die. J Neurosci 2003; 23: 2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci 2006; 26: 2599–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Prescott SA, Ratté S, De Koninck Y, Sejnowski TJ. Nonlinear interaction between shunting and adaptation controls a switch between integration and coincidence detection in pyramidal neurons. J Neurosci 2006; 26: 9084–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 2012; 53: 412–424. [DOI] [PubMed] [Google Scholar]
- 34Prole DL, Marrion NV. Ionic permeation and conduction properties of neuronal KCNQ2/KCNQ3 potassium channels. Biophys J 2004; 86: 1454–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast cyclic voltammetry. Analytical Chemistry 2004; 76: 5697–5704. [DOI] [PubMed] [Google Scholar]
- 36Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 1994; 91: 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Schurr A, Miller JJ, Payne RS, Rigor BM. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J Neurosci 1999; 19: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab 2009; 29: 1780–1789. [DOI] [PubMed] [Google Scholar]
- 39Morishima T, Aoyama M, Iida Y, Yamamoto N, Hirate H, Arima H et al. Lactic acid increases aquaporin 4 expression on the cell membrane of cultured rat astrocytes. Neurosci Res 2008; 61: 18–26. [DOI] [PubMed] [Google Scholar]
- 40Geng X, Sy CA, Kwiecien TD, Ji X, Peng C, Rastogi R et al. Reduced cerebral monocarboxylate transporters and lactate levels by ethanol and normobaric oxygen therapy in severe transient and permanent ischemic stroke. Brain Res 2015; 1603: 65–75. [DOI] [PubMed] [Google Scholar]
- 41Bierbower SM, Choveau FS, Lechleiter JD, Shapiro MS. Augmentation of M-Type (KCNQ) potassium channels as a novel strategy to reduce stroke-induced brain injury. J Neurosci 2015; 35: 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC et al. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol 2010; 588: 3277–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]