Abstract
Myeloproliferative neoplasms (MPNs) are clonal stem cell disorders characterized by chronic proliferation of hematopoietic progenitors. We studied the telomere length (TL) of 335 MPN patients and 93 gender- and age-matched controls using a quantitative PCR method (relative TL calculated as the ratio of the amount of telomere DNA vs single-copy DNA: T/S ratio). TL was markedly reduced in MPN patients compared with controls (T/S 0.561 vs 0.990, P<0.001). In JAK2V617F MPN patients, TL correlated inversely with allelic burden (P<0.001). Patients homozygous for the mutation (allelic burden 90–100%) had the shortest TL, even when compared with patients with lower allele burdens consistent with a dominant heterozygous population (allelic burden 55–65%) (T/S 0.367 vs 0.497, P = 0.037). This suggests that the high degree of proliferation of the MPN clone reduces TL and suggests the possibility that TL shortening may be indicative of progressive genomic instability during MPN progression. The TL of JAK2V617F-negative MPN patients was similar to JAK2V617F-positive counterparts (T/S 0.527 vs 0.507, P = 0.603), suggesting that the yet-to-be-discovered causative mutation(s) impact the mutated stem cell similarly to JAK2V617F, and that TL measurement may prove useful in the diagnostic workup of JAK2V617F-negative MPN.
Keywords: JAK2V617F, myeloproliferative neoplasms, telomere, clonality
Introduction
Telomeres are structures of variable length that cap the ends of chromosomes. They consist of thousands of repeated sequences (TTAGGG) ending in a terminal t-loop that protects the chromosome during cell division1 and prevents fusion events.2 Telomere length (TL) is longer in normal adult females than in their age-matched male counterparts, but progressively shortens with each cell division in both genders.3 For this reason, TL is considered a marker of aging and cellular turnover.4,5 When telomeres shorten to a critical length, normal cells can no longer divide, and they undergo senescence and apoptosis.5 The telomerase enzyme complex is the principal mechanism responsible for the maintenance of TL. Previous studies have documented telomerase activity in normal stem cells and germline cells.6 Telomerase activity has been shown to increase in human cancers,7–9 and this is thought to contribute to the ability of cancer cells to evade the mechanisms that limit the proliferation of normal cells.
Although the role of TL and telomerase activity has been extensively studied in a wide range of human malignancies, less is known about the biology of telomeres in myeloproliferative neoplasm (MPN). In chronic myelogenous leukemia (CML), a subtype of MPN, TL progressively shortens with disease progression and is shorter in cells harboring the causal BCR–ABL fusion protein compared with BCR–ABL-negative cells.10–15 Imatinib (CML treatment) restores polyclonal hematopoiesis and increases mean TL, probably by augmenting the pool of BCR–ABL-negative cells.16 Unlike CML patients, in which the BCR–ABL-positive clone predominates regardless of disease stage, MPN patients with polycythemia vera (PV), essential thrombocytosis (ET) or primary myelofibrosis present a variable state of chimerism between mutated and unmutated cells, which complicates molecular studies. Studies performed before the discovery of the JAK2V617F mutation had to rely on X-inactivation-based clonality determination as a surrogate marker of mutation and neoplasia. PV and ET patients with clonal hematopoiesis have shorter telomeres than normal controls,17 and PV patients have increased telomerase activity in their polymorphonucleated and mononucleated clonal cells.18 In this study, we (i) compare the TL of a well-characterized MPN cohort to that of healthy controls to document the extent of TL reduction in MPN, (ii) analyze the relationship between JAK2V617F mutational burden and TL and (iii) compare TL of JAK2V617F-positive and -negative MPN patients.
Materials and methods
Patients and controls
The patient cohort comprised of 401 MPN patients from The Harvard Myeloproliferative Disorders Study;19 of this cohort, sufficient DNA was available from 335 patients to perform JAK2V617F, clonality and TL analysis. The control cohort consisted of 93 women with normal complete blood counts (CBC) and no known hematological disorder recruited from the general community. All patients and controls provided informed consent. The study was approved by the Dana-Farber Cancer Institutional Review Board and by the Ethics Committee of the Maisonneuve-Rosemont Hospital (Montreal, Quebec).
Samples
Assays were performed on DNA extracted by standard methods from polymorphonucleated cells collected from the peripheral blood of patients and controls.
Real-time quantitative PCR assay for JAK2V617F
A quantitative real-time JAK2V617F Taqman SNP genotyping assay (Applied Biosystems, Foster City, USA) was used to measure JAK2V617F/JAK2total allelic ratio in genomic DNA, as described previously.20 All samples were measured in triplicate, and the mean ΔCt value was used to calculate allelic ratios.
X-inactivation-based clonality determination
Clonality was determined by HUMARA assay.21 X-chromosome inactivation ratios are expressed as the percentage of the predominant allele (p.p.a.) ranging from 51 to 100%. In accordance with previous studies,22–25 clonality was defined as a p.p.a. ≥75%, corresponding to at least 50% of cells clonally derived. Results presented herein correspond to the mean of triplicate.
Measurement of TL
Telomere length was measured using a quantitative PCR method described by Cawthon.26 This method is based on the determination of the telomere repeat number (T) relative to the copy number of a single copy number gene (36B4, S). A T/S ratio was obtained for each DNA sample and compared with the T/S ratio of a reference DNA sample (arbitrarily set as 1) to produce a relative T/S ratio. The reference DNA sample originated from a single individual, which was the same individual that was used to establish the standard curve. The relative T/S ratio of a given DNA sample represents its telomere copy number relative to the reference DNA sample. For example, a sample with a relative T/ S ratio of 0.5 signifies that its TL is 50% shorter than that of the reference DNA. Results for individual patients are the mean of triplicate. For cohort comparison, the mean of individuals is used. Samples were analyzed on an ABI SDS 7000 real-time PCR apparatus (Applied Biosystems).
Statistical analyses
Student’s t-test was used to compare TL of different cohorts: MPN patients with normal controls, clonal patients with polyclonal ones and JAK2V617F-positive individuals with JAK2V617F-negative individuals. As TL did not follow a normal distribution, it necessitated a square roots transformation before linear regression analysis. SAS 9.1.3 was used to evaluate the relationship between TL and JAK2V617F allelic burden by a general linear model controlling for age and sex (both of which influence TL).
Results
Subjects
Data on JAK2V617F mutational burden and TL were available for 335 MPN patients. MPN subtypes were as follows: 49% PV (n = 163), 37% ET (n = 125), 13% primary myelofibrosis (n = 43) and 1% uncharacterized (n = 4). Among the 335 MPN patients, 116 were men and 219 were women. Age was available for 207 MPN patients. The median age of these patients was 59 years for women (n = 148) and 61 years for men (n = 59). The normal (control) cohort consisted of 93 women from a large cohort of healthy females, each of which was perfectly aged-matched to a randomly selected MPN woman (total = 93).
TL correlates with gender and age in MPN patients
TL of MPN patients (n = 207) was inversely correlated with age (P = 0.001). TL was shorter in MPN men than in MPN women (T/ S 0.432±0.27 s.d. vs 0.553±0.30 s.d., P<0.001). Multivariate analysis confirmed that TL correlated with age (P<0.001) and gender (P = 0.006), but not with MPN subtype (P = 0.4). These results indicate that, although MPN confers a significant reduction of TL in both genders, some aspects of normal TL biology are preserved in MPN. Meaningful comparisons of TL can be made only by respecting gender and age.
TL is shortened in MPN patients
To investigate potential TL differences between MPN patients and normal individuals, we compared the TL of 93 MPN women with that of 93 aged-matched normal women. The TL of MPN women was markedly shorter than that of healthy women (T/S 0.561±0.31 s.d. vs 0.990±0.37 s.d., P<0.001). We could not directly compare the TL of MPN men with that of normal men because we did not have a male age-matched control group. However, given that the TL of normal men is known to be slightly shorter than that of age-matched normal women,27 and that we found the TL of MPN men to be slightly shorter than that of MPN women (T/S 0.432 vs 0.553), but much shorter than that of normal women (T/S 0.423 vs 0.990), this indirectly suggests that MPN men have reduced TL compared with normal men.
TL shortening correlates with clonality in MPN
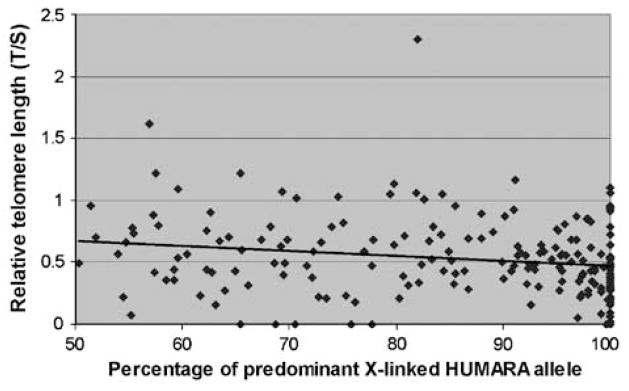
We have shown previously that in JAK2V617F-positive MPN, not all cells are clonally derived from the mutated progenitor cells,20 and that JAK2V617F-mutated cells are admixed with clonal cells and normal cells that are not part of the MPN clone. To investigate the link between TL and clonality in MPN, we compared the TL of clonal patients (that is, patients for which HUMARA assays yielded a p.p.a. ≥75%) with that of patients with a lower clonal burden (p.p.a. ≤74%). Data on TL and clonality were obtained from 190 MPN women heterozygous at the HUMARA locus, and analyzed regardless of their JAK2V617F status. The mean TL of clonal MPN women was shorter than that of polyclonal MPN women (T/S 0.519±DS vs 0.634±DS, P = 0.016). The link between clonality and TL was confirmed by linear regression analysis (P = 0.003, Figure 1).
Figure 1.
Relative telomere length (TL) according to the percentage of predominant HUMARA X-linked allele (clonality). TL was measured by PCR and compared with a control (relative TL) for female patients informative for the X-linked clonality assay HUMARA (n = 190). Clonality results are expressed as percentage of predominant allele, where 50% corresponds to balanced X-inactivation and 100% corresponds to complete skewing in favor of one of the two X-linked alleles. An increased degree of X-linked derived clonality correlated with shortening of TL (y = −0.0041x+0.8781; R2 = 0.037), indicating that clonal cells have shorter TL than polyclonal cells. Acquired skewing of X-inactivation, which is found in 40% of normal females, may explain the increased proportion of females at the right end of the clonality spectrum.
TL shortening correlates with JAK2V617F allelic burden
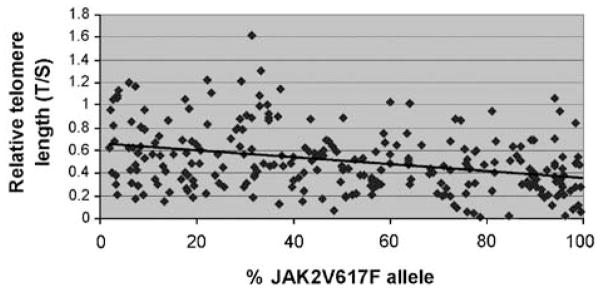
As JAK2V617F allelic burden is known to be linked with clonality,20 and clonality was shown to correlate with TL shortening in MPN, we hypothesized that JAK2V617F allelic burden and TL were correlated. To test this hypothesis, we analyzed the TL of 255 JAK2V617F-positive MPN patients and found a strong inverse correlation between JAK2V617F allelic burden and TL (Figure 2, P<0.0001). This correlation remained statistically significant after controlling for age and gender. Our data indicate a gradual decrease in TL across the entire spectrum of JAK2V617F allelic burden, including that in the context of increasing homozygosity (that is, when allelic burden is greater than 50%). We further evaluated the relationship between TL and increasing JAK2V617F homozygosity by analyzing the subcohort of JAK2V617F-positive MPN patients with an allelic burden of at least 55% (excluding patients with an inferior allelic burden to prevent sample contamination with non-mutated cells most likely to have longer telomeres). Within the JAK2617F-positive patients with an allelic burden of at least 55%, we compared the TL of those with an allelic ratio 55–65% (n = 24) with the TL of those with an allelic ratio 90–100% (n = 36). TL was found to be shorter in patients almost completely homozygous (T/S 0.367±0.23 s.d. vs 0.497±0.22 s.d., P = 0.037).
Figure 2.
Relative telomere length (TL) declines with an increased allelic burden of JAK2V167F alleles. TL was measured by PCR and compared with a control (relative TL). Relative TL was correlated with JAK2V617F allelic burden measured by quantitative PCR (y = −0.003x+0.6865; R2 = 0.1074). All MPD JAK2V617F-positive patients were included in this analysis (n = 255). This indicates that cells with the highest JAK2V617F allelic burden have the shorter telomeres.
MPNs have similar TL reduction regardless of JAK2V617F status
As a significant proportion of MPN do not harbor the JAK2V617F mutation, we compared the TL of patients with the JAK2V617F mutation (JAK2V617F ≥2%) with that of patients with wild-type JAK2 (JAK2V617F <2%). TL measurements were available for 255 JAK2V617F-positive and 80 JAK2V617F-negative MPN patients and were found to be similar in the two groups (T/S 0.527 and 0.507, P = 0.603). This indicates that MPN patients have similarly shortened TL irrespective of their JAK2V617F status.
Discussion
Shortening of telomeres has been described previously in cohorts of patients with various neoplasia,9 including in a small cohort of MPN patients.17 In this study, we analyzed a large cohort of MPN patients to further characterize variations of TL in relation to age, gender, clonality and JAK2V617F allelic burden. We showed that, similar to normal individuals, MPN patients exhibit decreased TL with age, and that there was shorter TL in males than females. We also showed that the TL of PV, ET and primary myelofibrosis patients is shorter than that of normal individuals, and that the TL of MPN patients is independent of MPN subtype and JAK2 mutational status. These data demonstrate that significant reductions in TL are a hallmark of the MPN clone, and that the MPN clone is able to continue to proliferate in the setting of short telomeres.
Although previous work has demonstrated reductions in TL in small numbers of patients with PV and ET,17 one of the limitations of previous studies of TL in MPN has been the inability to account for the mixed chimerism between mutated and nonmutated cells, which varies among MPN patients. However, with the advent of quantitative assays for JAK2617F mutational burden and for X-chromosome inactivation, we are able to assess the relationship between the size of the MPN clone and TL. Using such a strategy on female MPN patients in our cohort, we demonstrated a correlation between clonality and TL shortening, irrespective of JAK2V617F status, suggesting that the emergence of the MPN clone is responsible for the reduction in TL. We then used a quantitative assay for the JAK2V617F mutation to further investigate the relationship between TL and the causing mutation. As expected, we documented a strong reverse correlation between JAK2V617 allelic burden and TL. The correlation between TL shortening and JAK2V617F allelic burden was stronger than that between TL shortening and X-linked clonality, which may be due to the higher reliability of the JAK2V617F assay, but alternatively may be indicative of a biological link between JAK2V617F activation and TL. Clonality and cytogenetic data are consistent with the notion of a ‘pre-JAK2 allele’, in that the clonal burden in MPN patients, most commonly in ET, exceeds the JAK2V617F mutated burden.20 This is also supported by the recent demonstration that JAK2V617F negative acute myeloid leukemia commonly evolves in the setting of a preexisting JAK2V617F-positive MPN.28,29 The data from this study are consistent with the existence of somatic mutations that precede acquisition of JAK2V617F in MPN. However, the observation of a stronger correlation between TL and JAK2V617F allelic burden than between TL and clonality suggests that TL is primarily affected by acquisition of the JAK2V617F allele and not by these other mutations. We would predict, on the basis of these insights and on the observation that a single JAK2 allele predominates in these diseases, that these additional MPN alleles may initiate clonal hematopoiesis, but that JAK2V617F is the primary allele driving the excess proliferation, which is a hallmark of MPN. These ‘pre-JAK2’ alleles might have more subtle effects on proliferation, possibly through modulation of the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathway, which increases the selective advantage of cells within the MPN clone that acquire JAK2V617F.
To further investigate the influence of JAK2V617F allele burden on TL, we compared patients with different levels of mutated alleles (55–65%) with those nearly homozygous (90– 100% of mutated alleles) and observed an even greater reduction in TL with increases in allele burden beyond 50%. This observation is consistent either with reductions in TL with continued emergence of a dominant JAK2V617F homozygous clone already present in patients with lower allele burden or with reductions in TL associated with the conversion from hetero-zygosity to homozygosity for JAK2V617F. Either way, these data suggest that TL is shortest in cells that are homozygous for JAK2V617F. The very short TL seen in homozygous patients may increase genomic instability, as chromosome ends are more susceptible to replication errors (as reviewed by Lansdorp30), and this may confer a greater risk of leukemic transformation. This situation is reminiscent of the CML situation, where disease progression is associated with TL shortening, and shorter telomeres are associated with a more rapid progression from chronic phase to accelerated phase.10–15 Boultwood et al.11 reported that TL reduction in chronic-phase CML is associated with a less favorable prognosis, probably due to increased genetic instability at an individual cell level. Most importantly, these data suggest that there are important biological differences between cells that are heterozygous or homozygous for the JAK2V617F allele, including TL and that future studies of telomere dynamics in MPN must distinguish between JAK2V617F heterozygous and homozygous cells.
The subgroup of MPN patients that lack the JAK2V617F mutation poses a greater scientific and diagnostic challenge, as in most cases the causative mutation(s) has not yet been identified. We observed similar reductions in TL in JAK2V617F-positive and -negative MPN, which demonstrate that telomere dynamics are similar in patients with and without known mutations. This suggests that JAK2V617F-negative MPN have an unidentified mutation that affects the stem cell in an analogous manner as the JAK2V617F mutation, in terms of the number of cell divisions necessary to obtain the phenotype and the requirement for adaptive mechanisms to proliferate in the setting of marked reductions in TL. Our demonstration that JAK2V617F-negative patients have severe TL reduction may support the use of a TL assay to investigate patients with a potential JAK2V617F-negative MPN. However, because of the great variability in TL in normal individuals and in MPN patients, further validation studies are needed before incorporating TL measurement as a diagnostic test.
The marked reduction in TL in MPN is highly significant, in comparison with lack of a reduction in TL in normal blood donors with increased myeloid (and lymphoid) cellular turnover 31 and the modest reductions in TL seen in donor-derived cells in stem cell transplant patients, even after the replicative stress associated with mobilization, harvest and transplantation. 32 The marked reduction in TL seen in the MPN clone is particularly surprising in light of recent data demonstrating reductions in TL and mutations in the telomerase gene in patients with aplastic anemia.33,34 This suggests that unlike normal hematopoietic stem cells, which cannot maintain normal stem cell function in the setting of reductions in TL, the MPN clone has acquired mechanisms that allow continued proliferation in the setting of reduced TL. Whether this mechanism is the result of increased telomerase activity, or the alternative lengthening of telomere (ALT) pathway35,36 is not known, but is the subject of current investigation. Most importantly, if the mechanism by which the MPN stem cell adapts to critical reductions in TL can be inhibited pharmaco-logically, it would afford a novel, JAK2-indepdendent target for molecular therapy, which might synergize with current and future MPN therapies. Moreover, the progression of PV to both acute leukemia and myelofibrosis suggests that additional genetic lesions can contribute to MPN progression, and that genomic instability in the setting of marked reductions in TL might contribute to MPN progression.
Conclusion
In conclusion, we have demonstrated a relationship between TL and JAK2V617F burden in PV, ET and primary myelofibrosis, and have observed an analogous reduction in TL in the absence of the JAK2V617F allele. These data provide important insight into mechanisms of initiation and progression in these MPN and suggest that TL measurement may help to support the diagnosis of MPN in patients with an MPN phenotype in the absence of a known pathogenic allele.
Acknowledgments
LM is an FRSQ scholar. DGG is an Investigator of the Howard Hughes Medical Institute, and a Doris Duke Charitable Foundation Distinguished Clinical Scientist Award recipient. RLL is an American Society of Hematology Basic Research Fellow Award recipient, a Doris Duke Charitable Foundation Clinical Scientist Development Award recipient and is the Geoffrey Beene Junior Chair at Memorial Sloan Kettering Cancer Center.
Footnotes
Conflicts of interest
No conflicts of interest are declared.
References
- 1.Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 2.Hande MP, Samper E, Lansdorp P, Blasco MA. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J Cell Biol. 1999;144:589–601. doi: 10.1083/jcb.144.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verfaillie CM, Pera MF, Lansdorp PM. Stem cells: hype and reality. Hematol Am Soc Hematol Educ Program. 2002:369–391. doi: 10.1182/asheducation-2002.1.369. [DOI] [PubMed] [Google Scholar]
- 4.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 5.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 6.Drummond MW, Balabanov S, Holyoake TL, Brummendorf TH. Concise review: telomere biology in normal and leukemic hematopoietic stem cells. Stem Cells. 2007;25:1853–1861. doi: 10.1634/stemcells.2007-0057. [DOI] [PubMed] [Google Scholar]
- 7.Stewart SA, Weinberg RA. Telomeres: cancer to human aging. Annu Rev Cell Dev Biol. 2006;22:531–557. doi: 10.1146/annurev.cellbio.22.010305.104518. [DOI] [PubMed] [Google Scholar]
- 8.Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA. 1998;95:90–92. doi: 10.1073/pnas.95.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 10.Boultwood J, Fidler C, Shepherd P, Watkins F, Snowball J, Haynes S, et al. Telomere length shortening is associated with disease evolution in chronic myelogenous leukemia. Am J Hematol. 1999;61:5–9. doi: 10.1002/(sici)1096-8652(199905)61:1<5::aid-ajh2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Boultwood J, Peniket A, Watkins F, Shepherd P, McGale P, Richards S, et al. Telomere length shortening in chronic myelogenous leukemia is associated with reduced time to accelerated phase. Blood. 2000;96:358–361. [PubMed] [Google Scholar]
- 12.Brummendorf TH, Holyoake TL, Rufer N, Barnett MJ, Schulzer M, Eaves CJ, et al. Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood. 2000;95:1883–1890. [PubMed] [Google Scholar]
- 13.Engelhardt M, Mackenzie K, Drullinsky P, Silver RT, Moore MA. Telomerase activity and telomere length in acute and chronic leukemia, pre- and post-ex vivo culture. Cancer Res. 2000;60:610–617. [PubMed] [Google Scholar]
- 14.Ohyashiki K, Ohyashiki JH, Iwama H, Hayashi S, Shay JW, Toyama K. Telomerase activity and cytogenetic changes in chronic myeloid leukemia with disease progression. Leukemia. 1997;11:190–194. doi: 10.1038/sj.leu.2400560. [DOI] [PubMed] [Google Scholar]
- 15.Iwama H, Ohyashiki K, Ohyashiki JH, Hayashi S, Kawakubo K, Shay JW, et al. The relationship between telomere length and therapy-associated cytogenetic responses in patients with chronic myeloid leukemia. Cancer. 1997;79:1552–1560. doi: 10.1002/(sici)1097-0142(19970415)79:8<1552::aid-cncr17>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Brummendorf TH, Ersoz I, Hartmann U, Bartolovic K, Balabanov S, Wahl A, et al. Telomere length in peripheral blood granulocytes reflects response to treatment with imatinib in patients with chronic myeloid leukemia. Blood. 2003;101:375–376. doi: 10.1182/blood-2002-08-2557. [DOI] [PubMed] [Google Scholar]
- 17.Ferraris AM, Pujic N, Mangerini R, Rapezzi D, Gallamini A, Racchi O, et al. Clonal granulocytes in polycythaemia vera and essential thrombocythaemia have shortened telomeres. Br J Haematol. 2005;130:391–393. doi: 10.1111/j.1365-2141.2005.05618.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris AM, Mangerini R, Pujic N, Racchi O, Rapezzi D, Gallamini A, et al. High telomerase activity in granulocytes from clonal polycythemia vera and essential thrombocythemia. Blood. 2005;105:2138–2140. doi: 10.1182/blood-2004-06-2375. [DOI] [PubMed] [Google Scholar]
- 19.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Levine RL, Belisle C, Wadleigh M, Zahrieh D, Lee S, Chagnon P, et al. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood. 2006;107:4139–4141. doi: 10.1182/blood-2005-09-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- 22.Gale RE, Wheadon H, Linch DC. X-chromosome inactivation patterns using HPRT and PGK polymorphisms in haematologically normal and post-chemotherapy females. Br J Haematol. 1991;79:193–197. doi: 10.1111/j.1365-2141.1991.tb04521.x. [DOI] [PubMed] [Google Scholar]
- 23.Vogelstein B, Fearon ER, Hamilton SR, Preisinger AC, Willard HF, Michelson AM, et al. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987;47:4806–4813. [PubMed] [Google Scholar]
- 24.Busque L, Mio R, Mattioli J, Brais E, Blais N, Lalonde Y, et al. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood. 1996;88:59–65. [PubMed] [Google Scholar]
- 25.Allen RC, Nachtman RG, Rosenblatt HM, Belmont JW. Application of carrier testing to genetic counseling for X-linked agammaglobulinemia. Am J Hum Genet. 1994;54:25–35. [PMC free article] [PubMed] [Google Scholar]
- 26.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 28.Theocharides A, Boissinot M, Girodon F, Garand R, Teo SS, Lippert E, et al. Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood. 2007;110:375–379. doi: 10.1182/blood-2006-12-062125. [DOI] [PubMed] [Google Scholar]
- 29.Campbell PJ, Baxter EJ, Beer PA, Scott LM, Bench AJ, Huntly BJ, et al. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations, and role in leukemic transformation. Blood. 2006;108:3548–3555. doi: 10.1182/blood-2005-12-013748. [DOI] [PubMed] [Google Scholar]
- 30.Lansdorp PM. Telomeres, stem cells, and hematology. Blood. 2008;111:1759–1766. doi: 10.1182/blood-2007-09-084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheding S, Ersoz I, Hartmann U, Bartolvic K, Balabanov S, Salama A, et al. Peripheral blood cell telomere length measurements indicate that hematopoietic stem cell turnover is not significantly increased in whole blood and apheresis PLT donors. Transfusion. 2003;43:1089–1095. doi: 10.1046/j.1537-2995.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 32.Brummendorf TH, Rufer N, Baerlocher GM, Roosnek E, Lansdorp PM. Limited telomere shortening in hematopoietic stem cells after transplantation. Ann N Y Acad Sci. 2001;938:1–7. doi: 10.1111/j.1749-6632.2001.tb03568.x. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 33.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998;91:3582–3592. [PubMed] [Google Scholar]
- 34.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 35.Muntoni A, Reddel RR. The first molecular details of ALT in human tumor cells. Hum Mol Genet. 2005;14(Spec No 2):R191–R196. doi: 10.1093/hmg/ddi266. [DOI] [PubMed] [Google Scholar]
- 36.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]