Abstract
Diabetes prevalence is rising rapidly, and diabetes disproportionately affects Hispanics and other underserved groups. Chronic stress may contribute to diabetes risk, but few studies have examined this relationship in U.S. Hispanics. We examined associations of chronic stress with fasting glucose, glucose tolerance, and glycosylated hemoglobin (HbA1c) in Hispanics without diabetes, and also assessed indirect effects of stress through inflammation (CRP). Participants were 3923 men and women, aged 18-74, without diabetes, from the four U.S. field centers (Bronx, NY; Chicago, IL; Miami, FL; San Diego, CA) of the Hispanic Community Health Study/Study of Latinos (HCHS/SOL)-Sociocultural Ancillary study. Participants completed a measure of chronic life stress and a physical exam with oral glucose tolerance test. In a multivariate regression analysis with adjustment for demographic and health covariates, higher chronic stress was related to higher fasting glucose (standardized regression coefficient: β=.09, p<0.01), post load glucose (β=.07, p<0.05), and HbA1c levels (β=.08, p<0.01). However, there was no indirect effect of stress through inflammation. Findings suggest that higher chronic stress is associated with poorer glucose regulation in Hispanics, prior to the onset of a clinical diabetes diagnosis.
Keywords: Stress, glucose, insulin, Hispanic, inflammation
Diabetes mellitus is highly prevalent among Hispanics/Latinos (hereafter referred to as “Hispanics”) in the United States and prevalence has risen rapidly in recent years (Cowie et al., 2009; Maskarinec et al., 2009). The Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a community-based cohort study of 16,415 Hispanic/Latino adults in the U.S. of which the current study is an ancillary, found diabetes prevalence to be 16.9% (Schneiderman et al., 2014), higher than prevalence estimates in non-Hispanic whites. For example, in the 2005-2006 National Health and Nutrition Survey (NHANES), diabetes prevalence in non-Hispanic white adults was estimated at 12.9% (Cowie et al., 2009). Hispanics with type 2 diabetes have poorer glycemic control (Campbell, Walker, Smalls, & Egede, 2012), more frequent complications, and worse outcomes (s for Disease Control and Prevention (CDC), 2011; Lanting, Joung, Mackenback, Lamberts, & Bootsma, 2005) than non-Hispanic whites. Diabetes is a recognized to be a progressive disease, and β-cell impairment (i.e., impairment of the pancreatic cells that produce insulin) can be observed many years before the onset of clinical diabetes (Ferrannini et al., 2005; Tabak, Herder, Rathmann, Brunner, & Kivimaki, 2012) This has led to an interest in identifying and intervening at earlier stages, e.g., when individuals meet diagnostic thresholds for “prediabetes” (American Diabetes Association, 2014; Tabak, Herder, Rathmann, Brunner, & Kivimaki, 2012). Prediabetes represents an intermediate high-risk state for development of diabetes and its complications, and it affects an estimated 29% of Hispanics (according to 2005-2006 NHANES; Cowie et al., 2009). As the U.S. Hispanic population increased by 47.5% between 2000 and 2011, totaling over 51 million individuals (CDC, 2011), the rising prevalence of prediabetes and diabetes in this population is of great economic and public health concern. Identification of risk factors early in the pathophysiological trajectory of diabetes is crucial for guiding prevention and early intervention efforts and reducing diabetes prevalence and outcome disparities in this population.
Chronic psychological stress is believed to influence metabolic functioning and contribute to insulin resistance (CDC, 2011; Marcovecchio & Chiarelli, 2012; Matthews, Gump, & Owens, 2001). Stressful life events and work stress are associated with increased risk for metabolic syndrome and diabetes, though some findings indicate heightened risk for women only (Chandola, Brunner, & Marmot, 2006; Heraclides, Chandola, Witte, & Brunner, 2009; Norberg et al., 2007). Substantial evidence also links chronic stress with CVD, including the development of hypertension, atherosclerosis, and coronary events (Dimsdale, 2008; Rosengren et al., 2004; Steptoe & Kivimaki, 2013). The impact of stress on CVD risk has been shown to be comparable in magnitude to that of known biological and behavioral risk factors, such as cholesterol and smoking (Dimsdale, 2008; Richardson et al., 2012). Importantly, Hispanics may experience high rates of social and economic stressors compared to non-Hispanic whites, acculturation stress, low socioeconomic status (SES), and neighborhood-related stressors (National Research Council, 2006; Ornelas & Perreira, 2011).
Although little research to date has examined associations of stress with diabetes or CVD in Hispanics, a recent study in the HCHS/SOL cohort showed a positive association of chronic stress with CVD, hypertension and diabetes (Gallo et al., 2014) and obesity (Isasi et al., 2014). Other publications from the HCHS/SOL have reported prevalence of diabetes (Schneiderman et al., 2014), metabolic syndrome (Heiss et al., 2014), and CVD and CVD risk factors (Daviglus, Pirzada, & Talavera, 2014), but none have explored the relationship of stress and glucose regulation prior to diabetes diagnosis. The current study focuses on the subset of 5312 HCHS/SOL participants who completed the HCHS/SOL Sociocultural Ancillary Study, which included an in-depth sociocultural interview that was subsequently linked with clinical measures of cardiometabolic risk. This analysis expands the small body of literature on stress and diabetes in Hispanics by reporting on the association of chronic stress with indicators of glucose metabolism in a large cohort of individuals of diverse Hispanic heritages (i.e. Cuban, Dominican, Mexican, Puerto Rican, Central and South American).
Mechanisms by which stress interacts with glucose metabolism and insulin resistance to influence diabetes risk remain unclear and are likely to be multi-faceted. Chronic stress may influence the development of diabetes by contributing to risk-related behaviors, such as high dietary fat and sugar consumption, sedentary lifestyle, smoking, and alcohol use (Laugero, Falcon, & Tucker, 2011; Torres & Nowson, 2007) and increased negative emotions such as depression and anxiety (Hammen, 2005). Physiological response to stress, including activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (Vedder, Berry, Sabatier, & Sam, 2009) axis, may also have a role in the relationship between stress and diabetes. Though the acute stress response is adaptive, sustained activation of either axis in the context of prolonged stress can result in adverse effects on various organ systems and confer risk for the metabolic syndrome. HPA axis activation initiates production of cortisol and other glucocorticoids, which can lead to increased glucose production in liver cells, hyperglycemia, and inhibition of insulin secretion (Kyrou, Chrousos, & Tsigos, 2006). Activation of the SAM axis, alternately, releases catecholamines and leads to production of cytokines and acute phase proteins, including C-Reactive Protein (CRP), that induce a systemic inflammatory response (Kyrou & Tsigos, 2009). Proinflammatory cytokines are known to interact with insulin signaling and contribute to dyslipidemia and insulin resistance (Hotamisligil, 2006). Previous studies linking psychological stress with elevated levels of CRP and pro-inflammatory cytokines (Black, 2006; McDade, Hawkley, & Cacioppo, 2006), together with those linking elevated CRP with increased risk for type-2 diabetes mellitus (Bertoni et al., 2010; Wang et al., 2013), support the notion that chronic psychological stress may affect glucose regulation indirectly through inflammatory processes.
Fasting glucose, glucose tolerance (established by oral glucose tolerance test, OGTT), and glycosylated hemoglobin (HbA1c) are indicators of glucose metabolism that are used in diabetes diagnosis (American Diabetes Association, 2014) and are sensitive to changes in glucose regulation and progression of disease risk. All three indicators have been associated with incident diabetes and CVD, with the relationships generally being linear or continuous in nature (Bergman, 2010; Buysschaert & Bergman, 2011). The aim of the current study was to test whether self-reported chronic psychological stress is associated with fasting glucose, glucose tolerance, and glycemic regulation in U.S. Hispanics who do not meet criteria for diabetes, and whether inflammation measured by CRP is an indirect mechanism in these relationships. We hypothesized that higher chronic stress would be associated with glucose dysregulation, as indicated by higher fasting glucose, poorer glucose tolerance, and higher HbA1c levels, and that these associations would persist with control for demographic covariates and health factors. Further, we hypothesized that CRP would act as an indirect pathway through which chronic stress relates to glucose regulation. Since some research has identified demographic differences in the magnitude or consistency of associations of stress with physiological regulation or disease states (e.g., according to sex, age, SES)(Steptoe & Kivimaki, 2013), we also conducted sensitivity analyses to determine if these associations were consistent across demographic groups. The current study is the first to explore the relationship of chronic stress to glucose regulation in a diverse population of Hispanic adults without diabetes.
Method
Participants and Procedures
HCHS/SOL is a community-based epidemiological cohort study of risk and protective factors for chronic disease in 16,415 Hispanic/Latino adults aged 18-74 years. Participants self identified as Mexican, Cuban, Puerto Rican, Dominican, Central and South American, or Other/More than one Hispanic background and were recruited between 2008 and 2011 via a two-stage area household probability sampling design in four U.S. field centers (Bronx, NY; Chicago, IL; Miami, FL; San Diego, CA). The 45-74 year old age group was oversampled to facilitate the goal of understanding chronic disease incidence and prevalence. Full descriptions of the study design (Sorlie et al., 2010) and sampling design (Lavange et al., 2010) have been presented elsewhere. Participants underwent a baseline clinical examination that included comprehensive biological (e.g., anthropometrics, fasting blood draw, 2-hour OGTT), behavioral (e.g. dietary intake, physical activity assessment), and sociodemographic (e.g., socioeconomic status, migration history) assessments. The study was approved by the Institutional Review Boards at all HCHS/SOL sites and all participants provided informed written consent.
The HCHS/SOL-Sociocultural Ancillary study (SCAS) recruited 5,312 participants from the HCHS/SOL cohort (~1320 per field center) to thoroughly explore psychosocial and sociocultural factors related to health and chronic disease (Gallo et al., 2014). Between February 2010 and June 2011, these participants attended a study visit within 9 months of their HCHS/SOL baseline clinical exam to complete an in-person interview and self-report questionnaires, including the measure of chronic psychological stress used in the current analysis. A full description of the SCAS methods and sample has been published elsewhere (Gallo et al., 2014). The SCAS participants are a representative sub-sample of the HCHS/SOL parent study, except for slightly lower participation of individuals from higher SES groups (Gallo et al., 2014).
Participants were excluded from the current analysis if they met criteria for diabetes at the time of the baseline clinical assessment (N=1078), based on diagnostic criteria recommended by the American Diabetes Association [fasting plasma glucose >=126 mg/dL (7mmol/L), 2-hour post-load glucose (2-hr OGTT) >200 mg/dl (11.1mmol/L), or HbA1c >=6.5%] (American Diabetes Association, 2014 ) and/or by self-report of diabetes diagnosis from a physician, and/or prescribed glucose-lowering medication. Participants with CRP values above 10 mg/L (N=311) were also excluded from analyses as these values could indicate systemic or infectious illness (Yeh, 2003), leaving a final analytic sample of N= 3923 men and women.
Measures
Chronic stress
Chronic stress was measured with an 8-item self-report scale that assessed ongoing (past 6 months or more) exposure to psychological stress in several important life domains (i.e., work, relationship, finances, health problems for self or family)(Bromberger & Matthews, 1996). Each item measures whether a participant has ongoing problems in a particular domain (i.e., “Have you experienced ongoing financial strain?; “Have you had a serious ongoing health problem?”), chronicity of the problem (“Has this been a problem for six months or more?), and perceived stressfulness of the problem (“not very stressful,” “moderately stressful,” or “very stressful”). A summary score is generated that reflects a count of the total number of stressors with a minimum of six months’ duration that participants perceived to be moderately or very stressful. These 8 items are meant to represent a range of stress experiences that are likely to be applicable across diverse adult populations. As the total score (range 0 to 8) represents a count of stress in various life domains that may be unrelated, items are not intended to form a single factor or scale and thus coefficient alpha for this measure is not applicable. However, the scale has been used previously to measure chronic psychological stress in large, multi-ethnic epidemiological studies, including the Multi-Ethnic Study of Atherosclerosis (MESA) (Shivpuri, Gallo, Crouse, & Allison, 2012) and the Study of Women’s Health Across the Nation (SWAN) (Troxel, Matthews, Bromberger, & Sutton-Tyrrell, 2003) and scores on the measure have shown expected relationships between chronic stress and related psychosocial constructs such as depression and anxiety.
Glucose regulation
All glucose variables were obtained from a laboratory blood draw conducted during the HCHS/SOL baseline clinical exam. Fasting glucose and post-load glucose were measured in plasma on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation) using a hexokinase enzymatic method. Normal fasting glucose is defined as fasting glucose level < 100mg/mL and impaired fasting glucose as between 100 and 125 mg/dL (5.6 – 6.9 mmol/L; American Diabetes Association, 2014). Glucose tolerance was measured with a 2-hour oral glucose tolerance test (OGTT) given to all participants except those with fasting plasma glucose >150mg/dL or who had previously been diagnosed with diabetes. Normal glucose tolerance is defined as post 2-hour glucose load level < 140 mg/dL, while impaired glucose tolerance is 140-199 mg/dL (American Diabetes Association, 2014). Glycosylated hemoglobin (HbA1c) was measured by assay using a Tosoh G7 Automated HPLC Analyzer (Tosoh Bioscience, Inc., San Francisco, CA). HbA1c is an indicator of glucose regulation over the prior 2 to 3 months. Normal HbA1c is defined as levels < 5.7%, “pre-diabetes” or at-risk for diabetes as 5.7% to 6.4%, and levels 6.5% or above meet criteria for diabetes (American Diabetes Association, 2014). Normal and clinical ranges are provided here for reference only; glucose outcomes were analyzed continuously to preserve statistical variance and because clinical risk for diabetes associated with these indicators is continuous in nature.
Inflammation
Serum high sensitivity CRP (hs-CRP) was assessed via blood draw in the HCHS/SOL baseline clinical exam and assayed with a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation) using an immunoturbidimetric method (Roche Diagnostics, Indianapolis, IN 46250). Inter-assay coefficient of variation was <2.5% and intra-assay coefficient of variation was <4.7%. Hs-CRP was examined as a continuous variable in all analyses.
Sociodemographic and health covariates
Sociodemographic variables were obtained through in-person interview with trained assessors and included age, sex, education, income, Hispanic/Latino background group, nativity/immigration status (place of birth, length of time in the U.S.), and preferred interview language. Variables analyzed categorically include sex, education [<high school diploma/general education degree (GED), high school diploma/GED only, > HS diploma/GED], household yearly income (10 categories ranging from < $10,000 to > $100,000), Hispanic/Latino background group (Mexican, Cuban, Puerto Rican, Dominican, Central American, South American, Other/Multiple), nativity/immigration status (born in U.S. mainland, immigrated >10 years ago, immigrated < 10 years ago), and language of interview (Spanish or English).
Health and behavioral variables were included as additional covariates in some models. Diet quality was assessed using the Alternative Healthy Eating Index-2010 (AHEI-2010), a measure of consumption of foods and nutrients predictive of future chronic disease risk (Chiuve et al., 2012). In HCHS/SOL, the AHEI-2010 was calculated by the National Cancer Institute (National Research Council, 2006) method of using 24-hour dietary recall data with one or two recalls per participant to assess typical consumption across 11 dietary components (e.g., vegetables, whole fruits, whole grains, sugar sweetened beverages, trans fats, sodium). Scores on each dietary component range from 0 (worst) to 10 (best), culminated in total AHEI-2010 scores ranging from 0 to 110. Physical activity was measured in metabolic equivalent of task units (METs) of moderate and vigorous physical activity as self-reported on an adapted version of the World Health Organization (WHO) Global Physical Activity Questionnaire (Bull, Maslin, & Armstrong, 2009), a measure of physical activity in a typical week in three domains (work-related, transportation, and leisure/recreational). Body mass index (BMI) was calculated as kg/height2 and waist circumference was measured in centimeters. All of these variables were analyzed continuously.
Smoking status was categorized as never, former, or current based on participant self-report. Individuals who reported currently smoking on some or all days and having smoked at least 100 cigarettes in their lifetime were assessed as current smokers. Alcohol use status was determined to be one of four categories based on participant self-report of frequency and quantity of alcohol consumption: non-drinker (no alcohol consumed in the past year), former drinker (has stopped consumption of alcohol), low risk drinker (≤ 7 drinks per week for women and ≤ 14 for men) and at-risk drinker (> 7 drinks per week for women and >14 for men).
Statistical Analysis
Descriptive statistics were calculated in IBM SPSS Statistics 20.0 (SPSS, Inc., Chicago IL) using complex survey analyses. All other analyses were completed using the maximum likelihood robust (MLR) estimation procedure in Mplus (Muthén & Muthén, 2006). This procedure incorporates a full-information maximum likelihood (FIML) approach to missing outcome data whereby model parameters and standard errors are adjusted for multivariate non-normality and missing outcome data; thus, participant cases with missing fasting glucose, (n=5), post OGTT glucose (n=183), and HbA1c (n=9) were included in the analyses. The MLR procedure with FIML approach generates unbiased parameter estimates and standard errors in various missing data conditions (Enders, 2010). All analyses accounted for the HCHS/SOL sampling design and weights (Lavange et al., 2010). Assumptions regarding linearity and normality were examined and data transformations were conducted when necessary (specifically, GPAQ scores were log-transformed).
Multivariate linear regression was conducted to examine the relationship between chronic stress and glucose regulation. Associations of chronic stress with all three indicators of glucose regulation were tested simultaneously (a single model with three individual outcomes); this analytic approach accounted for correlations between the outcome variables. Model 1 specified a direct path from chronic stress to each outcome, adjusting for demographic covariates. Model 2 adjusted additionally for the health and behavioral variables defined above.
The indirect effect of inflammation was explored by path analysis, with stress relating to glucose regulation outcomes indirectly via CRP. A multivariable approach was used so that associations of stress and CRP with all indicators of glycemic regulation tested simultaneously. Again two models were tested, with Model 1 adjusting for demographic factors only and Model 2 adding adjustment for health-relevant and behavioral variables. MacKinnon’s asymmetric confidence interval was computed to determine statistical significance of the indirect effect (Tofighi & MacKinnon, 2011).
Finally, we tested whether observed relationships between stress and glucose variables were consistent across sex, age, Hispanic background, income, education level, nativity/immigration status and language of interview groups by including relevant interaction terms (e.g., sex by stress; age by stress) in models that included main effects of stress and demographic variables (Model 1).
Results
Demographic and Health Characteristics
Demographic characteristics and descriptive statistics are reported in Table 1. Participants in the sample were between 18 and 74 years of age (M = 44.46, SD = 13.56); 60.51% were women and 81.56% were born outside of the U.S. mainland. Individuals who identified as having Mexican background were the largest Hispanic heritage group (N = 1552; 39.55%). The majority of the sample had a household income under $30,000 (69.97%) and preferred to complete their interview in Spanish (79.96%). Participants in the sample reported 1.76 chronic stressors on average (SD = 1.60); 19.91% reported 2 chronic stressors and 27.28% reported 3 or more chronic stressors. Average fasting glucose was 92.68 mg/dL (SD = 8.17), 2-hour post load glucose was 113.85 mg/dL (SD = 31.38), and HbA1c was 5.47% (SD = 0.37).
Table 1.
Descriptive statistics for all study variables: HCHS/SOL Sociocultural Ancillary Study (N=3923)
| Characteristic | N | Sample Percent (%) | Weighted Percent (%) |
|---|---|---|---|
| Female | 2374 | 60.51 | 52.73 [50.70, 54.76] |
| Age group | |||
| 18-44 | 1763 | 44.94 | 63.45 [61.13, 65.71] |
| 45+ | 2160 | 55.06 | 36.55 [34.29, 38.87] |
| Hispanic/Latino Heritage | |||
| Central American | 416 | 10.61 | 7.74 [6.22, 9.60] |
| Cuban | 588 | 15.00 | 20.07 [16.22, 24.56] |
| Dominican | 405 | 10.33 | 11.55 [9.65, 13.77] |
| Mexican | 1552 | 39.58 | 38.12 [34.03, 42.39] |
| Puerto Rican | 570 | 14.54 | 14.18 [12.28, 16.32] |
| South American | 275 | 7.01 | 4.88 [3.96, 5.99] |
| More than one/Other | 115 | 2.93 | 3.46 [2.66, 4.49] |
| Household yearly income < $30K | 2519 | 69.97 | 68.17 [65.08, 71.11] |
| Education < HS diploma or GED | 1295 | 33.71 | 30.40 [28.15, 32.74] |
| Nativity/Immigration Status | |||
| Born in the US Mainland | 722 | 18.44 | 23.10 [20.70, 25.69] |
| Immigrated ≥ 10 years ago | 2177 | 55.61 | 47.38 [44.61, 50.17] |
| Immigrated ≤ 10 years ago | 1016 | 25.95 | 29.52 [26.58, 32.64] |
| Spanish Language Interview | 3137 | 79.96 | 74.94 [72.13, 77.56] |
| N | Unweighted M (SD) | Weighted | |
|
|
|||
| BMI (kg/m2) | 3917 | 28.86 (5.38) | 28.53 (7.87) |
| Waist Circumference (cm) | 3920 | 96.01 (13.00) | 95.39 (18.79) |
| Diet quality (AHEI Score, 0-110) | 3896 | 48.78 (7.55) | 47.41 (13.66) |
| Physical Activity (Total METS from GPAQ) | 3885 | 601.03 (887.93) | 653.29 (1624.67) |
| Chronic Stress (# of stressors, 0-8) | 3836 | 1.76 (1.60) | 1.68 (2.44) |
| Inflammation (hs-CRP, mg/L) | 3923 | 2.50 (2.18) | 2.45 (3.21) |
| Fasting Glucose (mg/dL) | 3918 | 92.68 (8.17) | 92.27 (11.08) |
| Glucose Tolerance (2-hr post OGTT, mg/dL) | 3740 | 113.85 (31.38) | 109.45 (44.03) |
| Glycosylated Hemoglobin (%) | 3914 | 5.47 (0.37) | 5.40 (0.53) |
HS = High School; GED = General Education Development Test; BMI = Body Mass Index; AHEI = Alternative Healthy Eating Index; METS = Metabolic Equivalent of Task units; GPAQ = Global Physical Activity Questionnaire; CRP = C-Reactive Protein; OGTT – Oral Glucose Tolerance Test.
Chronic Stress and Glucose Regulation
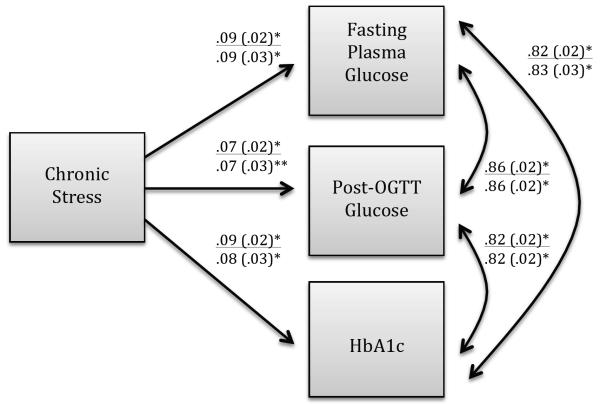
Multivariate linear regression analysis showed that after adjusting for demographic factors (Model 1), higher chronic stress was significantly related to higher levels of fasting glucose (β = .092, p < .01; R2 = .007, p < .01), post-load glucose (β = .073, p < .05; R2 = .002; p <.01), and HbA1c (β = .086, p < .01; R2 = .005; p < .01). When the model additionally adjusted for behavioral and health covariates (Model 2), the relationships were attenuated but remained statistically significant. Results for both models are shown in a path diagram in Figure 1.
Inflammation
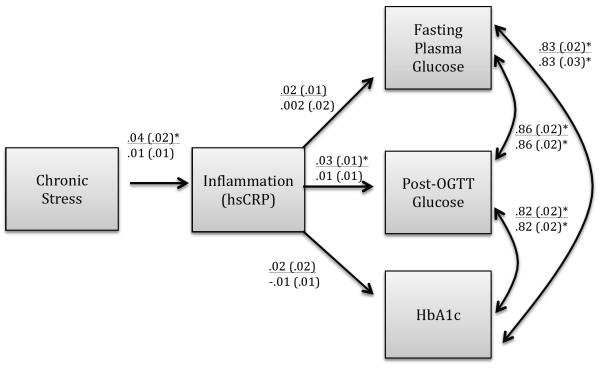
Inflammation measured by CRP was positively and significantly associated with chronic stress and with all three glucose regulation variables. Bivariate correlations of these variables with CRP were as follows: fasting glucose (r = .043, p < .01), post load glucose (r = .179, p < .001), HbA1c (r = .193, p < .001), and stress (r = .055, p < .01). A multivariate path analytic model was tested that specified an indirect relationship from chronic stress to glucose regulation variables via CRP adjusting for design effects, sample weights, and demographic factors. Results are shown in Figure 2. The pathway comprising the indirect effect found a significant relationship between chronic stress and inflammation (β=.05, p<0.05) and between inflammation and one glucose variable, glucose tolerance (β=.03, p<0.05); however, the asymmetric confidence interval showed that the compound pathway of the total indirect effect was not statistically significant. The indirect effect was also non-significant in the model that further adjusted for behavior and health covariates.
Effect Modification
Tests of effect modification showed that associations of chronic stress with glucose regulation variables were consistent across participant age, Hispanic heritage group, income, education level, nativity/immigration status, and interview language (p values for all interaction tests > .05). A sex by chronic stress interaction effect was observed for fasting glucose (β =.13, p<.01), but neither post-load glucose nor in HbA1c (p > .05). Sex stratified analyses showed that chronic stress was significantly associated with fasting glucose in men (β =.16, p<.001) but not women (β =.02, p > .05).
Discussion
The current analysis is the first to our knowledge to explore relationships between chronic psychological stress and glucose regulation in a large, diverse cohort of Hispanics without diabetes, and to do so in a study that included a thorough clinical exam and multiple indicators of glucose regulation. As hypothesized, higher chronic stress was related to higher levels of fasting blood glucose, 2-hour post-load glucose, and HbA1c - three primary glucose regulation endpoints used for diagnosis of diabetes and for indicating diabetes risk. These findings indicate that ongoing stress may interact with glucose homeostasis prior to occurrence of clinical diabetes in Hispanics and may contribute to impaired glucose regulation. Clinically, these findings suggest that chronic stress may be an important factor to consider when assessing total cardiometabolic risk. The magnitude of observed relationships between chronic stress and glucose regulation variables were small; however, they were present across all participants’ demographic groups and remained significant after controlling for multiple demographic and behavioral covariates. Furthermore, it is well established that even small changes in glucose regulation have significant implications for diabetes and CVD risk. A recent systematic review and meta-analysis found that the risk for incident diabetes increased markedly across the HbA1c range of 5.0 to 6.5% (Zhang et al., 2010). Other large epidemiological studies have shown increases in risk for incident CVD across this range (Selvin et al., 2010), with a 1 percentage point increase in HbA1c being associated with 20 - 30% increase in cardiovascular events and mortality for both men and women without diabetes (Khaw et al., 2004). In the current study, each 1 standard deviation increase in chronic stress was associated with .08% increase in HbA1c and .09 mmol/L increase in fasting glucose, which though small in magnitude, is likely to be a clinically meaningful change at the population level.
These relationships are consistent with the longstanding body of literature documenting detrimental health effects of prolonged exposure to stress (Cohen, Janicki-Deverts, & Miller, 2007; Schneiderman, Ironson, & Siegel, 2005). Chronic stress in work and other life domains is associated with increased risk for metabolic syndrome, diabetes, hypertension, and CVD, and is believed to have an impact on CVD risk that is comparable in magnitude to having established risk factors such as high cholesterol and smoking (Chandola et al., 2006; Dimsdale, 2008; Richardson et al., 2012).
As previously noted, psychological stress may contribute to glucose dysregulation directly, through physiological stress pathways (i.e., the HPA and SAM axes), but this relationship may also be mediated by the influence of stress on lifestyle risk factors, such as diet, physical activity, and smoking (Bergmann, Gyntelberg, & Faber, 2014; Torres & Nowson, 2007; Wardle, Chida, Gibson, Whitaker, & Steptoe, 2011). Although we did not test specific indirect pathways for behavioral risk factors in the current study, associations between chronic stress and glucose regulation variables remained significant and were essentially unchanged after adjusting for BMI, waist circumference, dietary quality, physical activity, smoking status, and alcohol use, indicating that there may be an independent effect of stress apart from adverse health behaviors. This direct effect may take place when unremitting experiences of psychological stress maintain stimulation of the HPA and SAM axes beyond the initially adaptive acute stress response, resulting in the continued taxation and depletion of multiple bodily systems over time, referred to as allostatic load (Juster, McEwen, & Lupien, 2010). Chronically increased glucocorticoid and catecholamine production by the HPA and SAM axes as part of this pathologically unbalanced or allostatic state may contribute to hyperglycemia and insulin resistance prior to the onset of the metabolic syndrome or type 2 diabetes (Kyrou & Tsigos, 2009; Wardle et al., 2011). The cross-sectional design of the current study prevents any assessment of causality, a significant limitation of the current analysis. However, previous prospective studies have shown psychological stress and depression to be associated with later development of abnormal glucose metabolism (CDC, 2011; Eriksson et al., 2008; Williams, Magliano, Tapp, Oldenburg, & Shaw, 2013). Although no prior study has specifically addressed this relationship in Hispanics, our findings provide initial support of a similar phenomenon in this population.
Contrary to our hypothesis, the indirect pathway specified between chronic stress and glucose regulation via inflammation was not significant for any of the glucose variables. Though a large base of evidence supports the link between obesity, recognized as a chronic low-grade inflammatory state, and pathogenesis of type 2 diabetes, the role of inflammation in glucose regulation in non-obese individuals is less clear (Hotamisligil, 2006; Kyrou & Tsigos, 2009). Furthermore, while CRP is a widely used, validated, and non age-dependent indicator of systemic inflammation (Crowson, 2009), measuring multiple inflammatory markers is generally preferable to any single test. Sampling of additional inflammatory markers, such as tumor necrosis factor-α (TNF-α), interleukin-7β (IL-7β) and interleukin-6 (IL-6) may have provided a more robust depiction of the relationship of inflammation and glucose regulation; TNF-α in particular has been shown to interfere with insulin signaling in prior research (Gaesser, Angadi, Ryan, & Johnston, 2011). Finally, relationships of inflammation and glucose regulation processes are likely reciprocal in nature, with each process contributing to the other differentially or in complex ways over time (Black, 2006; Kyrou & Tsigos, 2009). As our study measured inflammation and glucose regulation at only one point in time, we were unable to track any temporal patterning in their relationship.
In general, associations of chronic stress were found to be consistent across demographic groupings. An exception was the stronger association of chronic stress with fasting glucose in men than in women. Many previous studies have shown relationships to the contrary, with work-related and other forms of psychological stress contributing to risk of type 2 diabetes in women but not men (Chandola et al., 2006; Heraclides et al., 2009; Williams et al., 2013). The opposite pattern has also been observed, however. A large prospective cohort study in Sweden found psychological stress to be associated with increased risk for incidence of pre-diabetes and Type-2 diabetes in men but not women (Eriksson et al., 2008) and the Copenhagen City Heart Study found that men with higher stress were more than two times as likely as women to develop diabetes during a 10-year follow-up (Rod, Gronbaek, Schnohr, Prescott, & Kristensen, 2009). As the gender interaction effect was observed for only one of three glucose outcomes in our study, additional research is needed to clarify if the implications of stress for glucose regulation differ for Hispanic men and women.
A notable strength of this study is the examination of multiple indicators of glucose regulation. While fasting glucose, OGTT, and HbA1c are all considered to be reliable diagnostic markers for diabetes and prediabetes (American Diabetes Association, 2014), each test provides distinct information about metabolic processes related to diabetes risk. Fasting plasma glucose is a simple, point in time assessment of levels of circulating glucose after a fast of at least 8 hours. The OGTT, also administered following an 8 hour fast, provides information about how effectively glucose is cleared from the blood 2 hours after a standardized dose of oral glucose is ingested. While more time-consuming to administer, the OGTT can reveal impairments in glucose metabolism that are not indicated by a point estimate of fasting glucose alone (Hu et al., 2010). HbA1c, by comparison, is an indicator of longer-term glycemic regulation that gives information about glucose levels in the 2-3 months preceding the test. In individuals with diabetes, HbA1c is often used to measure glycemic control, but it also provides clinicians with an approach to detecting diabetes and prediabetes that does not require fasting (Gillett, 2009; Hu et al., 2010). By including all three glucose indicators in our analysis, we were able to detect relationships between chronic stress and variety of indicators of glucose regulation, adding to the current literature on specific physiological mechanisms by which stress contributes to diabetes development.
In addition to the cross-sectional study design, limitations of this study include lack of measurement of the neuroendocrine pathways that connect stress with metabolic functioning. In addition, the measure of chronic stress was brief and it did not include consideration of perceived coping ability or skills. The true magnitude of association between stress and glucose regulation may be underestimated due to limitations of the stress measurement approach. Future research should more comprehensively assess both stress and its context to more fully understand the association with glucose regulation. A further limitation of the study is the fact that the chronic stress and glucose measures were collected at different points in time. The allowable time window between each participant’s HCHS/SOL baseline clinical exam and Sociocultural Study interview was up to 9 months, although 72.6% of participants completed interviews within 4 months of their baseline exam (Gallo et al., 2014).
Despite these limitations, this study adds to the growing body of literature on psychosocial aspects of health in U.S. Hispanics, a critically important area of investigation given the rapid growth and unique risk profile of this population. Specifically, the findings present valuable information regarding a potential role of stress in the development of diabetes and cardiometabolic disorders that could be useful in identifying individuals at risk and preventing the occurrence of disease. Insulin resistance is a known contributor to risk for CVD in both Hispanic and non-Hispanic populations (Bonora et al., 2007; Resnick et al., 2003; Vella et al., 2013). Even among individuals free of diabetes, insulin resistance is related to incident CVD and myocardial infarction, and increased risk of new cardiovascular events and all-cause mortality in patients with manifest arterial disease (Vella et al., 2013; Verhagen et al., 2011). If chronic stress, a modifiable psychological variable, contributes to poorer glucose regulation and insulin resistance in Hispanics, then the development of interventions or intervention components that target stress reduction in this population could be an important goal of future research. Few stress reduction interventions have been implemented with Hispanics thus far, however, one recent peer-led pilot intervention for increasing coping skills in Hispanic/Latino immigrants was effective in reducing both stress and depression (Tran et al., 2014). Further research is needed to clarify the best approaches to monitoring and reducing both stress and diabetes risk in this population.
Acknowledgements
The Hispanic Community Health Study/Study of Latinos is a collaborative investigation supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices also contributed to funding the HCHS/SOL through the NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. The HCHS/SOL Sociocultural Ancillary Study was supported by MIH/NHLBI grant number RC2HL101649 (PIs Gallo/Penedo). The authors thank the staff and participants of HCHS/SOL and the HCHS/SOL Sociocultural Ancillary Study for their important contributions. A complete list of staff and investigators is available on the study website http://www.cscc.unc.edu/hchs.
Footnotes
Conflict of Interest Statement:
None of the authors have any financial or other conflicts of interest.
References
- American Diabetes Association Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- Bergman M. Inadequacies of absolute threshold levels for diagnosing prediabetes. Diabetes Metab Res Rev. 2010;26(1):3–6. doi: 10.1002/dmrr.1013. doi: 10.1002/dmrr.1013. [DOI] [PubMed] [Google Scholar]
- Bergmann NC, Gyntelberg F, Faber J. Chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect. 2014 doi: 10.1530/EC-14-0031. doi: 10.1530/EC-14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, Rotter JI. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010;33(4):804–810. doi: 10.2337/dc09-1679. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Obesity and Cortisol. Nutrition. 2000;16(10):924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67(4):879–891. doi: 10.1016/j.mehy.2006.04.008. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Muggeo M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30(2):318–324. doi: 10.2337/dc06-0919. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA. A Longitudinal Study of the Effects of Pessimism, Trait Anxiety, and Life Stress on Depressive Symptoms in Middle-Aged Women. Psychology and Aging. 1996;11(2):207–213. doi: 10.1037//0882-7974.11.2.207. [DOI] [PubMed] [Google Scholar]
- Bull FC, Maslin TS, Armstrong T. Global Physical Activity Questionnaire (GPAQ): Nine Country Reliability and Validity Study. Journal of Physical Activity and Health. 2009;6:790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- Buysschaert M, Bergman M. Definition of Prediabetes. The Medical Clinics of North America. 2011;95(2):289–297. doi: 10.1016/j.mcna.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Campbell JA, Walker RJ, Smalls BL, Egede LE. Glucose control in diabetes: the impact of racial differences on monitoring and outcomes. Endocrine. 2012;42(3):471–482. doi: 10.1007/s12020-012-9744-6. doi: 10.1007/s12020-012-9744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521–525. doi: 10.1136/bmj.38693.435301.80. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SS, Janicki-Deverts D, Miller GE. Psychological Stress and Disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowson CR, MU, Matteson EL. Which measure of inflammation to use? A comparison of erythrocyte sedimentation rate and C-reactive protein measurements from randomized clinical trials of golimumab in rheumatoid arthritis. Journal of Rheumatology. 2009;36(8):1606–1610. doi: 10.3899/jrheum.081188. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Pirzada A, Talavera G. Cardiovascular disease risk factors in the Hispanic/Latino population: Lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Progress in Cardiovascular Disease. 2014;57(3):230–236. doi: 10.1016/j.pcad.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237–1246. doi: 10.1016/j.jacc.2007.12.024. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C. Applied Missing Data Analysis. Guilford Press; New York: 2010. [Google Scholar]
- Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Ostenson CG. Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet Med. 2008;25(7):834–842. doi: 10.1111/j.1464-5491.2008.02463.x. doi: 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. B -Cell Function in Subjects Spanning the Range from Normal Glucose Tolerance to Overt Diabetes: A New Analysis. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- Gaesser GA, Angadi SS, Ryan DM, Johnston CS. Lifestyle Measures to Reduce Inflammation. American Journal of Lifestyle Medicine. 2011;6(1):4–13. doi: 10.1177/1559827611411646. [Google Scholar]
- Gallo LC, Roesch SC, Fortmann AL, Carnethon MR, Penedo FJ, Perreira KM, Isasi CR. Associations of chronic stress burden, perceived stress, and traumatic stress with cardiovascular disease prevalence and risk factors in the HCHS/SOL Sociocultural Ancillary Study. Psychosom Med. 2014;76(6):468–75. doi: 10.1097/PSY.0000000000000069. doi: 10.1097/PSY.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett AJ. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32(7):1237–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Heiss G, Snyder ML, Teng Y, Schneiderman N, Llabre MM, Cowie CC, Aviles-Santa LM. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: The Hispanic Community Health Study/Study of Latinos. Diabetes Care. 2014;37(8):2391–2399. doi: 10.2337/dc13-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heraclides A, Chandola T, Witte DR, Brunner EJ. Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. Diabetes Care. 2009;32(12):2230–2235. doi: 10.2337/dc09-0132. doi: 10.2337/dc09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu W, Chen Y, Zhang M, Wang L, Zhou H, Jia Y. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol. 2010;47(3):231–236. doi: 10.1007/s00592-009-0143-2. doi: 10.1007/s00592-009-0143-2. [DOI] [PubMed] [Google Scholar]
- Isasi CR, Parrinello CM, Jung MM, Carnethon MR, Birnbaum-Weitzman O, Espinoza RA, Gallo LC. Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Ann Epidemiol. 2014;25(2):84–9. doi: 10.1016/j.annepidem.2014.11.002. doi: 10.1016/j.annepidem.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Khaw K, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of Hemoglobin A1c with Cardiovascular Disease and Mortality in Adults: The European Prospective Investigation into Cancer in Norfolk. Annals of Internal Medicine. 2004;141:413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Ann N Y Acad Sci. 2006;1083:77–110. doi: 10.1196/annals.1367.008. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9(6):787–793. doi: 10.1016/j.coph.2009.08.007. doi: 10.1016/j.coph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Lanting LC, Joung IMA, Mackenback JP, Lamberts SWJ, Bootsma AH. Ethnic Differences in Mortality, End- Stage Complications, and Quality of Care Among Diabetic Patients. Diabetes Care. 2005;28(9):2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Falcon LM, Tucker KL. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite. 2011;56(1):194–204. doi: 10.1016/j.appet.2010.11.001. doi: 10.1016/j.appet.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Elder JP. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–649. doi: 10.1016/j.annepidem.2010.05.006. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovecchio ML, Chiarelli F. The effects of acute and chronic stress on diabetes control. Science signaling. 2012;5(247) doi: 10.1126/scisignal.2003508. Pt.10. doi: 10.1126/scisignal.2003508. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Owens JF. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery especially in men. Health Psychol. 2001;20:403–10. [PubMed] [Google Scholar]
- Maskarinec G, Grandinetti A, Matsuura G, Sharma S, Mau M, Henderson BE, Kolonel LN. Diabetes Prevalence and Body Mass Index Differ by Ethnicity: The Multiethnic Cohort. Ethnicity and Disease. 2009;19(1):49–55. [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68(3):376–381. doi: 10.1097/01.psy.0000221371.43607.64. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. Muthén & Muthén; Los Angeles: 2006. [Google Scholar]
- National Research Council . In: Hispanics and the Future of America. Tienda M, Mitchell F, editors. The National Academies Press; Washington, D. C.: 2006. [PubMed] [Google Scholar]
- Norberg M, Stenlund H, Lindahl B, Andersson C, Eriksson JW, Weinehall L. Work stress and low emotional support is associated with increased risk of future type 2 diabetes in women. Diabetes Res Clin Pract. 2007;76(3):368–377. doi: 10.1016/j.diabres.2006.09.002. doi: 10.1016/j.diabres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Ornelas IJ, Perreira KM. The role of migration in the development of depressive symptoms among Latino immigrant parents in the USA. Soc Sci Med. 2011;73(8):1169–1177. doi: 10.1016/j.socscimed.2011.07.002. doi: 10.1016/j.socscimed.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Hispanic Center How Many Hispanics? Comparing New Census Counts with the Latest Census Estimates. 2011 http://www.pewhispanic.org.
- Resnick HE, Henderson J, Jones K, Lu W, Routolo G, Howard BV, Jain AK. Insulin Resistance, the Metabolic Syndrome, and Risk of Incident Cardiovascular Disease in Nondiabetic American Indians. Pathophysiology/Complications. 2003;26(3):861–867. doi: 10.2337/diacare.26.3.861. [DOI] [PubMed] [Google Scholar]
- Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110(12):1711–1716. doi: 10.1016/j.amjcard.2012.08.004. doi: 10.1016/j.amjcard.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rod NH, Gronbaek M, Schnohr P, Prescott E, Kristensen TS. Perceived stress as a risk factor for changes in health behaviour and cardiac risk profile: a longitudinal study. J Intern Med. 2009;266(5):467–475. doi: 10.1111/j.1365-2796.2009.02124.x. doi: 10.1111/j.1365-2796.2009.02124.x. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N, Llabre MM, Cowie CC, Barnhart J, Carnethon MR, Gallo LC, Aviles-Santa LM. Prevalence of Diabetes among Hispanics/Latinos from Diverse Backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Diabetes Care. 2014;37(8):2233–9. doi: 10.2337/dc13-2939. doi: 10.2337/dc13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivpuri S, Gallo LC, Crouse JR, Allison MA. The association between chronic stress type and C-reactive protein in the multi-ethnic study of atherosclerosis: does gender make a difference? J Behav Med. 2012;35(1):74–85. doi: 10.1007/s10865-011-9345-5. doi: 10.1007/s10865-011-9345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Heiss G. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–641. doi: 10.1016/j.annepidem.2010.03.015. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–354. doi: 10.1146/annurev-publhealth-031912-114452. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: A high-risk state for developing diabetes. Lancet. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11-12):887–894. doi: 10.1016/j.nut.2007.08.008. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Tran AN, Ornelas IJ, Kim M, Perez G, Green M, Lyn MJ, Corbie-Smith G. Results from a pilot promotora program to reduce depression and stress among immigrant latinas. Health Promot Pract. 2014;15(3):365–372. doi: 10.1177/1524839913511635. doi: 10.1177/1524839913511635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychology. 2003;22(3):300–309. doi: 10.1037/0278-6133.22.3.300. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- Vedder P, Berry J, Sabatier C, Sam D. The intergenerational transmission of values in national and immigrant families: the role of Zeitgeist. J Youth Adolesc. 2009;38(5):642–653. doi: 10.1007/s10964-008-9375-7. doi: 10.1007/s10964-008-9375-7. [DOI] [PubMed] [Google Scholar]
- Vella CA, Burgos X, Ellis CJ, Zubia RY, Ontiveros D, Reyes H, Lozano C. Associations of insulin resistance with cardiovascular risk factors and inflammatory cytokines in normal-weight Hispanic women. Diabetes Care. 2013;36(5):1377–1383. doi: 10.2337/dc12-1550. doi: 10.2337/dc12-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen SN, Wassink AM, van der Graaf Y, Gorter PM, Visseren FL, Group SS. Insulin resistance increases the occurrence of new cardiovascular events in patients with manifest arterial disease without known diabetes. the SMART study. Cardiovasc Diabetol. 2011;10:100. doi: 10.1186/1475-2840-10-100. doi: 10.1186/1475-2840-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xiao X, Bao W, Shan Z, Liu J, Zhang Y, Liu L. Inflammatory Markers and Risk of Type 2 Diabetes. Diabetes Care. 2013;36:166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: a meta-analysis of longitudinal studies. Obesity (Silver Spring) 2011;19(4):771–778. doi: 10.1038/oby.2010.241. doi: 10.1038/oby.2010.241. [DOI] [PubMed] [Google Scholar]
- Williams ED, Magliano DJ, Tapp RJ, Oldenburg BF, Shaw JE. Psychosocial stress predicts abnormal glucose metabolism: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Ann Behav Med. 2013;46(1):62–72. doi: 10.1007/s12160-013-9473-y. doi: 10.1007/s12160-013-9473-y. [DOI] [PubMed] [Google Scholar]
- Yeh ETH. Coming of Age of C-Reactive Protein: Using Inflammation Markers in Cardiology. Circulation. 2003;107(3):370–371. doi: 10.1161/01.cir.0000053731.05365.5a. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, Albright AL. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33(7):1665–1673. doi: 10.2337/dc09-1939. doi: 10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]