Summary
Using a protein microarray, a broad spectrum of autoantibodies were demonstrated in patients with either Wiskott-Aldrich syndrome (WAS) or with X-linked thrombocytopenia (XLT), indicating that immune dysregulation is an integral component of both diseases.
Keywords: Wiskott-Aldrich syndrome, X-linked thrombocytopenia, autoantibodies, BAFF
To the Editor
Wiskott Aldrich syndrome (WAS) and X-linked thrombocytopenia (XLT) are allelic diseases, due to mutations of the WAS gene [1]. Autoimmune manifestations (especially cytopenias, inflammatory bowel disease, vasculitis, arthritis, and IgA nephropathy) affect between 24% and 72% of WAS patients in various series, with important implications on quality of life and survival [2]. Although patients with XLT do not suffer from autoimmune manifestations at diagnosis, some of them may develop autoimmunity over time [3].
In order to investigate in greater detail and compare the degree of immune dysregulation of WAS and XLT, we have studied 17 patients with WAS and 10 patients with XLT. The clinical and laboratory features of the patients are reported in Table 1.
Table 1.
Molecular, immunological, and clinical characteristics of patients
| Patient | Age | Mutation | IgG (mg/dL) (ref. range) | IgA (mg/dL) (ref. range) | IgM (mg/dL) (ref. range) | IgE (kU/L) (ref. range) | BAFF (pg/ml) (ref. range) | Autoimmunity | Autoantibodies (clinical testing) |
|---|---|---|---|---|---|---|---|---|---|
| XLT 18 | 19 yr | p. L39P | 664 (639–1344) | 241 (70–312) | 14 (34–210) | >5000 (0–200) | 2400 (469–1104) | -a,b | - |
| XLT 19 | 22 yr | p. L39P | 954 (639–1344) | 221 (70–312) | 15 (34–210) | 374 (0–200) | 3300 (469–1104) | -a | ASMA |
| XLT 22 | 19 yr | p. P58R | 741(639–1344) | 128 (70–312) | 66 (34–210) | 109 (0–200) | n.a. | - | - |
| XLT 23 | 16 yr | p. P58R | 1220 (639–1344) | 144 (70–312) | 149 (34–210) | 171 (0–200) | 2250 (469–1104) | - | - |
| XLT 33 | 8 yr | p.T45K | 914 (639–1344) | 266 (70–312) | 34 (34–210) | 31 (0–200) | 1550 (469–1104) | -a | ANA, ANCA, ASMA |
| XLT 37 | 15 yr | p. D77G | 1000 (639–1344) | 605 (70–312) | 20 (34–210) | 374 (0–200) | 1200 (469–1104) | - | ANA |
| XLT 38 | 11 yr | p. D77G | 950 (639–1344) | 211 (70–312) | 20 (34–210) | 83 (0–500) | 2100 (469–1104) | - | ANA |
| XLT 40 | 17 yr | p. I481N | 1360 (639–1344) | 396 (70–312) | 43 (34–210) | 13 (0–200) | n.a. | - | - |
| XLT 51 | 5 yr | p. V75M | 950 (600–1500) | 110 (50–150) | 40 (22–100) | 27 (0–200) | 450 (469–1104) | - | - |
| XLT 52 | 19 mo | p.V51L | 680 (400–1300) | 65 (20–230) | 57 (30–120) | 73 (0–30) | 2550 (469–1104) | - | - |
| WAS 3 | 4 mo | p. F74S | n.a. | n.a. | n.a. | n.a. | 9900 (469–1104) | n.a. | n.d. |
| WAS 9 | 2 yr | p. W64R | 1090 (400–1300) | 210 (20–230) | 119 (30–120) | n.a. | 2700 (469–1104) | - | ANA |
| WAS 17 | 5 yr | c.777+1g>c | 491 (600–1500) | 431 (50–150) | 44 (22–100) | 383 (0–200) | n.a. | - | - |
| WAS 21 | 1 mo | p.Q255Pfs*5 | 617 (700–1300) | 38 (6–50) | 20 (15–70) | 69 (0–30) | 750 (469–1104) | - | - |
| WAS 25 | 5 yr | p. P362Qfs*132 | 533 (600–1500) | 44 (50–150) | 70 (22–100) | 170 (0–200) | n.a. | - | Plt, TPO |
| WAS 27 | 3 yr | p. W64R | 1750 (600–1500) | 594 (50–150) | 28 (30–120) | 770 (0–200) | n.a. | AIHA, IBD | Coombs |
| WAS 28 | 5 yr | p. W64R | 246 (600–1500) | 17 (50–150) | 27 (22–100) | n.a. | n.a. | AIHA, IBD, vasculitis | Coombs |
| WAS 30 | 3 yr | p. V106Cfs*15 | 246 (600–1500) | 17 (50–150) | 27 (30–120) | n.a. | 6300 (469–1104) | vasculitis | PL, Plt |
| WAS 33 | 9yr | p. E67Efs*4 | 968 (639–1344) | 526 (70–312) | 20 (34–210) | 35 (0–200) | n.a. | IBD | - |
| WAS 35 | 20mo | p. D495Mfs*98 | 483 (400–1300) | 45 (20–230) | 5 (30–120) | n.a. | n.a. | - | Coombs, ANCA |
| WAS 37 | 8 mo | p. P110Lfs*13 | 924 (300–1500) | 71 (16–100) | 33 (25–115) | 128 (0–30) | 2200 (469–1104) | - | n.d. |
| WAS 38 | 1 yr | p. G424Afs*20 | 749 (300–1500) | 225 (16–100) | 28 (25–115) | n.a. | 5100 (469–1104) | n.a. | n.d. |
| WAS 40 | 10mo | p. R86H | n.a. | n.a. | n.a. | n.a. | 2600 (469–1104) | - | ANA, Coombs, Plt |
| WAS 42 | 11mo | p.P373Hfs*72 | 898 (300–1500) | 82 (16–100) | 59 (25–115) | 370 (0–30) | 1200 (469–1104) | - | - |
| WAS 43 | 2 yr | p. V473Gfs*14 | 1784 (400–1300) | 233 (20–230) | 111 (30–120) | n.a. | 10000 (469–1104) | - | n.d. |
| WAS 49 | 2 yr | c.559+5g>a | n.a. | n.a. | n.a. | n.a. | 2400 (469–1104) | - | TPO |
| WAS 50 | 3 yr | c.559+5g>a | n.a. | n.a. | n.a. | n.a. | 2000 (469–1104) | - | TPO |
| WAS 60 | 48 yr | c.559+5g>a | 1068 (639–1344) | 881 (70–312) | 15 (34–210) | 440 (0–200) | n.a. | - | PL |
n.a. not available; n.d.: not done.
This patient developed vasculitis subsequently in life
This patient developed arthritis later in life
Values highlighted in bold are outside of age-matched reference values (shown in parenthesis)
AIHA: autoimmune hemolytic anemia; ANA: anti-nuclear antibodies; ANCA: anti-neutrophil cytosplasmic antibodies; ASMA: anti-smooth muscle antibodies; IBD: inflammatory bowel disease; PL: anti-phospholipid antibodies; Plt: anti-platelet antibodies; TPO: anti-thyroid peroxidase antibodies.
To analyze the frequency, antigen specificity, and isotype composition of autoantibodies, plasma samples from WAS/XLT patients were diluted 1:100 in PBS, and 100μl of the dilution were incubated in duplicate with an autoantigen proteomic array (University of Texas Southwestern Medical Center, Genomic and Microarray Core Facility) [4], that includes 67 and 77 self-antigens, respectively. Plasma from six healthy control subjects and from five patients with Systemic Lupus Erythematous (SLE) served as negative and positive controls, respectively. To define the IgG or IgA isotype specificity of the autoantibodies, the arrays were then incubated with Cy3-labelled anti-human IgG and Cy5-labelled anti-human IgA antibodies, respectively. Tiff images were generated using Genepix 4000B scanner with laser wavelengths 532 (for Cy3) and 635 (for Cy5) and analyzed using Genepix Pro 6.0 software. Net fluorescence intensities (defined as the spot minus background fluorescence intensity) data obtained from duplicate spots were averaged. Data were normalized as follows: across all samples, the immunoglobulin positive controls (IgG or IgA) were averaged and the positive controls in each sample were divided by the averaged positive control generating a Normalization Factor (NF) for each sample. Each signal was than multiplied by the NF for each block (sample). For each antigen, values from healthy donor samples (at least 3) were averaged, and ratios were calculated between each sample and the average of healthy donors plus 2 standard deviations (s. d.) (Relative Autoantibody Reactivity, RAR). Values of RAR >1 were considered positive. A heat map of the ratio values was generated using Multi experiment viewer software (MeV, DFCI Boston, MA). Significant differences in autoantibody signal between groups were assessed using the significance analysis of microarray (SAM, Stanford University Lab) with a false discovery rate (FDR) <1%.
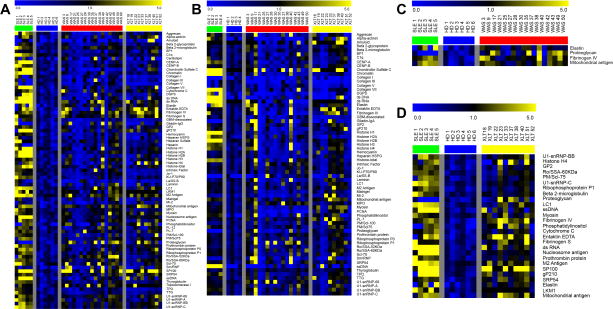
As shown in Fig. 1A and 1B, the presence of at least one positive IgG and IgA autoantibody was documented in the vast majority of WAS and XLT patients. Autoantibodies that were significantly increased in WAS and XLT individuals as compared to healthy donors are shown in Figure 1C and 1D.
Figure 1.
Heat-map of IgG (panel A) and IgA (panel B) autoantibodies in XLT and WAS patients. For each self-antigen, a colorimetric representation of relative autoantibody reactivity in each sample is shown according to the scale depicted on top. Panel C and D show autoantibodies with significantly increased reactivity in patients with WAS and XLT, respectively, as compared to healthy donors (HD).
Samples were considered multi-reactive if they contained autoantibodies to at least 20% of the self-antigens represented on the array. Multi-reactivity of IgG autoantibodies was observed in 16/27 patients (59.2%), specifically in 11 out of 17 samples from WAS, and in 5 out of 10 samples from XLT patients (Figure E1 in the Online Repository). Multireactivity of IgA autoantibodies was observed in 12/26 patients (46.1%), in particular 7/16 WAS samples and 5/10 XLT samples (Figure E1 in the Online Repository). Patients with autoantibody multireactivity had significantly higher levels of serum IgA as compared to patients that showed reactivity to less than 20% of the self-antigens tested, and a similar trend was observed for serum IgG levels (see: Figure E2 in the Online Repository). Self-antigens to which autoantibodies were demonstrated in more than 20% of WAS/XLT patients were defined as “common autoantigens”. The 25 most common IgG and IgA autoantibodies are reported in Figure E3 in the Online Repository. Of note, 9 of the 25 top most common autoantigens (36%) were the target of both IgG and IgA autoantibodies (mitochondrial antigen, fibrinogen IV, entaktin, M2 antigen, myosin, elastin, LC1, SRP54, sn-RNP-68, and Scl-70). Using relative autoantibody reactivity (RAR) for semiquantitative analysis of signal intensity, IgG autoantibodies to two common antigens (fibrinogen IV, mitochondrial antigen) were present at higher levels in WAS and XLT patients vs. healthy controls (Figure E3 in the Online Repository).
Multiple immunological abnormalities have been identified that may account for immune dysregulation in WAS [5], including impaired function of regulatory T (Treg) and regulatory B (Breg) cells, defective apoptosis, abnormalities of the distribution and diversity of T and B lymphocytes, and defective function of T and NK cells, resulting in impaired clearance of pathogens and persistent inflammation. Moreover, WASP-deficient plasmacytoid dendritic cells are hyper-responsive to TLR9 stimulation, and produce high amounts of type 1 interferon, which may also contribute to autoimmunity [6]. More recently, we and others have identified B cell autonomous effects of WASp deficiency that are likely to play a critical role in the autoimmunity of the disease [7–9]. These include: a) hyper-responsiveness of WASp-deficient B cells to stimulation via the B cell receptor (BCR) and Toll-like receptors (TLRs); b) accumulation of B lymphocytes with a characteristic phenotype (CD21low CD38low), indicative of type 1 interferon signature, and a marker of self-reactivity; c) preferential usage of immunoglobulin variable genes that are enriched in patients with autoimmune disease, and decreased somatic hypermutation; d) increased release of immature B cells from the bone marrow to the periphery; e) elevated BAFF serum levels; f) decreased function of regulatory B cells. In our series, elevated BAFF serum levels were found not only in patients with WAS, but also in those with XLT (Table 1).
To our knowledge, our study represents the first attempt at extensively analyzing the frequency and diversity of autoantibodies in patients with WAS vs. XLT. Our data indicate that biological signs of immune dysregulation are a characteristic feature of patients with loss-of-function mutations of the WAS gene, irrespective of the severity of the clinical phenotype. This biological signature of immune dysregulation may set the stage for progressive development of clinical manifestations of autoimmunity also in patients with XLT. Consistent with this, three of the XLT patients included in this study (XLT18, XLT19, XLT33) developed cutaneous vasculitis subsequently in the course of their disease, and one of them (XLT18) also suffered from arthritis, a pattern that has been reported in several other bona fide XLT patients [9]. These data strongly suggest that XLT should not be considered as a distinct disease entity, but rather as part of the clinical spectrum of WAS. Prospective longitudinal studies are needed to assess whether differences in the amount, diversity and avidity of autoantibodies produced are predictive of development of clinical manifestations of autoimmunity in patients with XLT/WAS.
Supplementary Material
Acknowledgments
Source of funding: This work was supported by a grant from the National Heart Lung and Blood Institute, National Institutes of Health (grant 5P01HL059561-13 to L.D.N.), by an educational grant (5T32AI007512) from the National Institute of Allergy and Infectious Diseases to Elena Crestani (Dr Raif S Geha, PI), and by a grant from the UNIL-CHUV (CGRB 29583 to F.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. The Journal of Allergy and Clinical Immunology. 2006;117(4):725–38. doi: 10.1016/j.jaci.2006.02.005. quiz 39. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111(5 Pt 1):e622–7. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 3.Albert MH, Bittner TC, Nonoyama S, Notarangelo LD, Burns S, Imai K, et al. X-linked thrombocytopenia (XLT) due to WAS mutations: clinical characteristics, long-term outcome, and treatment options. Blood. 2010;115(16):3231–8. doi: 10.1182/blood-2009-09-239087. [DOI] [PubMed] [Google Scholar]
- 4.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147(1):60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catucci M, Castiello MC, Pala F, Bosticardo M, Villa A. Autoimmunity in Wiskott-Aldrich syndrome: an unsolved enigma. Front Immunol. 2012;3:209. doi: 10.3389/fimmu.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prete F, Catucci M, Labrada M, Gobessi S, Castiello MC, Bonomi E, et al. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J Exp Med. 2013 Feb 11;210(2):355–74. doi: 10.1084/jem.20120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon KL, Anderson SM, Garabedian EK, Moratto D, Sokolic RA, Candotti F. Molecular and phenotypic abnormalities of B lymphocytes in patients with Wiskott-Aldrich syndrome. The Journal of Allergy and Clinical immunology. 2014;133(3):896–9. e4. doi: 10.1016/j.jaci.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castiello MC, Bosticardo M, Pala F, Catucci M, Chamberlain N, van Zelm MC, et al. Wiskott-Aldrich Syndrome protein deficiency perturbs the homeostasis of B-cell compartment in humans. J Autoimmun. 2014;50:42–50. doi: 10.1016/j.jaut.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du HQ, Zhang X, An YF, Ding Y, Zhao XD. Effects of Wiskott-Aldrich Syndrome Protein Deficiency on IL-10-Producing Regulatory B Cells in Humans and Mice. Scand J Immunol. 2015 Jun;81(6):483–93. doi: 10.1111/sji.12282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.