Abstract
Background
Allergy-related studies that include biological measurements of vitamin D preceding well-measured outcomes are needed.
Objective
Examine the associations between early life vitamin D levels and the development of allergy-related outcomes in the racially diverse WHEALS birth cohort.
Methods
25-hydroxyvitamin D [25(OH)D] was measured in stored blood samples from pregnancy, cord blood and age 2 years. Logistic regression models were used to calculate odds ratios (OR) with 95% confidence intervals (CI) for a 5 ng/ml increase in 25(OH)D level for the outcomes at age 2 years: eczema, skin prick tests (SPT), elevated allergen-specific IgE (sIgE≥0.35IU/ml), and doctor diagnosis of asthma (3–6 years).
Results
Prenatal 25(OH)D was inversely associated with eczema (OR=0.85, 95% CI 0.75, 0.96). The association was stronger in White children (White: OR=0.79, 95% CI 0.57, 1.09; Black: OR=0.96, 95% CI 0.82, 1.12), although this was not statistically significant. Cord blood 25(OH)D was inversely associated with having ≥1 positive SPT and aeroallergen sensitization. Both associations were statistically significant in White children (SPT: OR=0.50, 95% CI 0.32, 0.80; ≥1 aeroallergen: OR=0.50, 95% CI 0.28, 0.92) in contrast with Black children (SPT:OR=0.88, 95% CI 0.68, 1.14; aeroallergen: OR=0.85, 95% CI 0.65, 1.11). 25(OH)D measured concurrent with outcome assessment was inversely associated with aeroallergen sensitization (OR=0.79, 95% CI 0.66, 0.96) only among Black children (White children: OR=1.21, 95% CI 0.87, 1.69).
Conclusions
Prenatal and cord 25(OH)D were associated with some allergy-related outcomes with a general pattern indicating that children with higher 25(OH)D tend to have fewer allergy-related outcomes.
Keywords: vitamin D, allergy, eczema, asthma, IgE, racial differences
INTRODUCTION
Interest in vitamin D’s role in allergic disease incidence has grown tremendously in recent years, particularly since vitamin D supplementation is perceived as affordable, non-invasive and easily administered. Overall, the evidence regarding whether vitamin D affects the incidence of allergy-related outcomes is mixed due to reliance of many studies on the use of parental- or self-reported outcomes and food frequency questionnaires (FFQ). (1–3) FFQs approximate dietary vitamin D intake rather than reflect bioavailable levels, which are also influenced by sun exposure and supplement intake. The number of studies that include a direct measurement of vitamin D (25-hydroxyvitamin D [25(OH)D] or 25(OH)D2 or 25(OH)D3) that clearly preceded childhood allergic disease incidence are growing in number. (4–8) Other studies continue to measure vitamin D concurrent with the clinical outcomes.(9–12)
The mechanism by which vitamin D may alter immune development and immune related diseases has been proposed to be one in which vitamin D directly and indirectly affects the immune system.(13) In their review of the influences of gut microbiota, probiotics and vitamin D on allergies and asthma, Ly et al. suggested that vitamin D may be an important modifier of the association between intestinal flora and inflammatory disorders.(14) Recent discussion that low levels of vitamin D may be a risk factor for developing inflammatory bowel diseases such as Crohn’s disease suggest that vitamin D can affect gut inflammation.(15–17) This gut inflammation could, in turn, affect immune development and function which can subsequently manifest itself in the first readily observable inflammatory diseases – eczema and allergies.(18)
There were multiple objectives for this work. The first goal was to examine whether serum levels of 25(OH)D collected at various time points (during pregnancy, delivery and at the time of a clinical evaluation at approximately age 2 years) were related to the incidence of eczema, elevated allergen-specific IgE (sIgE) and skin prick testing (SPT) in the first two years of life or related to parental report of doctor diagnosis of asthma at age 3–6 years. We also sought to assess whether any associations differed between Black children and White children. Finally, we compared associations between 25(OH)D and the outcomes at different timepoints of 25(OH)D measurement in order to examine whether the associations varied with timing of sample collection (i.e., prenatal sample versus cord sample). We thought this sample timing variation could be contributing to the conflicting evidence in the literature investigating the role of vitamin D in allergic disease development.
METHODS
Study Population
“WHEALS” is an NIH and institutionally funded cohort study that enrolled pregnant women receiving care at Henry Ford Health System (HFHS) obstetrics clinics in the Detroit, Michigan, USA area for longitudinal study of their children through early childhood with the goal of examining early life exposures related to childhood allergies and asthma. These children and their mothers served as the source population for the analyses. Details of cohort creation have been published.(19–21) The children included in this study have information on at least one outcome at age 2 years (results from SPT, total IgE, sIgE, or physician evaluation of eczema) or participated in a health interview when the child was 3–6 years of age (mean age=4.2 years, SD=0.9). We are also including only those children who are African American and White/Non-Hispanic/Non-Middle Eastern to best examine racial differences. The subgroups of children who are White/Hispanic or White/Middle Eastern are too few in number to analyze as subgroups in this study.
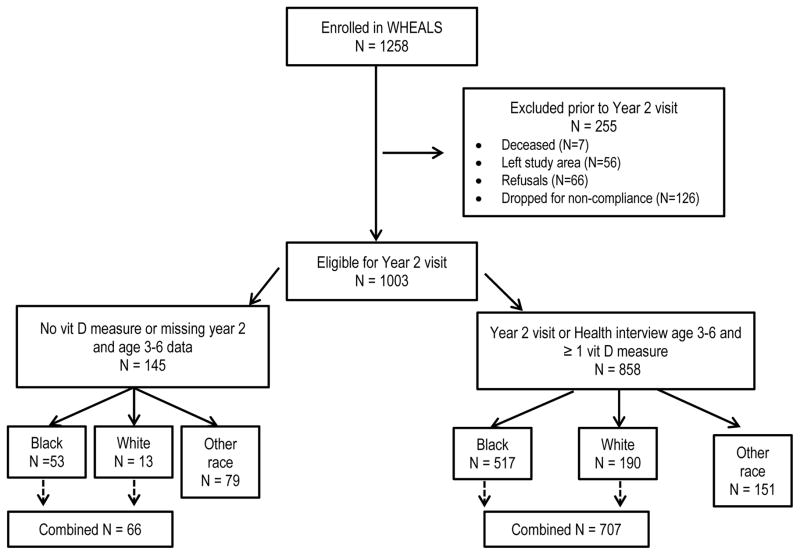
Figure 1 provides details describing the inclusion and exclusion of participants. Some children completed the 2 year clinic visit, but did not complete all aspects of the clinical protocol. For example, they may have had skin prick testing but did not have a blood sample with sufficient volume to permit analyses of sIgEs. If a child had a result for an outcome, they were included in the analyses.
Figure 1.
Details of the inclusion and exclusion of WHEALS study participants in the current analyses.
Interviews
Parents were interviewed about the health of their children (allergic diseases, symptoms/diagnoses), breastfeeding, and exposure to animals at 1, 6 and 12 month home visits and at 2 years of age (included data since the last contact). Health updates were obtained with parental interview at child age 3–6 years during which the parents were asked to report whether the child had ever received a diagnosis of asthma from a doctor.
Allergic Diseases
All children were invited for a standardized exam with skin prick testing and parental interview at age 2 years with a physician trained in the study protocol. The physicians discussed allergy symptoms and examined the child for any eczema. Skin prick testing for Dermatophagoides farinae, dog, cat, timothy grass, ragweed, Alternaria alternata, egg, peanut, milk, and German cockroach was performed. A positive skin test was defined as one producing a wheal with the longest diameter ≥3 mm greater than the negative control. Allergen-specific IgEs were also measured for the same allergens among those who provided a blood sample at age 2 years. sIgE levels in mothers’ prenatal blood samples were assessed for a subset of the allergens (Dermatophagoides farinae, dog, cat, timothy grass, ragweed, Alternaria alternata, egg and German cockroach) using the same methods. Atopy was defined as having at least one sIgE ≥0.35 IU/ml.
Vitamin D
25(OH)D, representing the sum of 25(OH)D2 (ergocalciferol which is diet related) and 25(OH)D3 (cholecalciferol which is sun related), was measured in frozen plasma samples (−80°C) in the laboratory of Dr. Neil Binkl ey at the University of Wisconsin. An HPLC method was used and has been used in previously published research.(22–28) 25(OH) is expressed in ng/ml. 25(OH)D was measured in the stored samples from pregnancy, delivery (cord blood) and at age 2 years. Vitamin D levels can be affected by the time of year (seasonal effects) and adjustment for this variation is possible. The seasonal variation of serum 25(OH)D was modeled with a sinusoidal model of the values (25(OH)D value) and time (month denoted as m) of collection:
Seasonally adjusted values (called deseasonalized by van der Mei et al.) were then calculated by taking each subject’s value and subtracting the predicted value and adding back in the overall mean.(29) The seasonally adjusted 25(OH)D levels were used in the analyses detailed below.
Statistical Analyses
Logistic regression models were calculated for a 5 ng/ml increase in 25(OH)D level for each analytical outcome (eczema, SPT, atopy, food atopy, inhalant allergen (aeroallergen) atopy, doctor diagnosis of asthma) for all children and separately for Black and White children. Means and standard deviations of the deseasonalized 25(OH)D levels were calculated and compared across groups using t-tests at the various collection timepoints. This was done for all children and separately for Black and White children. Additional logistic regression models were run to investigate potential heterogeneity of associations (i.e., effect measure modification)(30) between 25(OH)D and each allergic disease outcome by the following variables: maternal atopic status (yes/no), maternal prenatal use of antibiotics or vaginally applied antifungal medications (yes/no), mother lived with a dog during pregnancy (yes/no), child lived with a dog in the first year of life (yes/no), delivery mode (vaginal or not), firstborn status (yes/no), breast fed (yes/no), baby gender, home ownership (yes/no), maternal education (less than high school diploma/at least a high school diploma) and household income (<$40,000/≥$40,000). While sample size limited the precision of these estimates in these subgroups, we thought it important to consider possible effect modification as there is a paucity of evidence on the associations between vitamin D and allergy-related outcomes in subgroups. A likelihood ratio test was performed to compare 25(OH)D quintiles to the linear model to assess possible non-linearity. Finally, we reran our analyses including samples only from maternal/child pairs who contributed samples from both pregnancy and cord blood to evaluate whether the results for each time-point were similar.
Because our goal was to examine relationships between 25(OH)D levels and allergy-related outcomes, potential confounders were considered in evaluating these associations. The same factors that were examined for potential effect modification were assessed as potential confounders. We examined whether any of these factors were associated with any of the 25(OH)D measures and any of the outcomes. Only living with a dog during pregnancy and living with a dog in the 1st year were associated with both. When we adjusted the models for these factors, the associations were unchanged.
RESULTS
Characteristics of children included and not included in the analyses are presented in Table 1. The groups were quite similar with respect to rates of maternal sensitization, delivery type, race and firstborn status. Those included in the analyses were more likely to have had a mother who resided with an indoor dog during pregnancy.
Table 1.
Comparison of women from WHEALS whose children were included and excluded from the current analyses.*
| Women with a Child Included in the Analyses | Women with a Child Excluded from the Analyses | p value | |
|---|---|---|---|
| N | 707 | 66 | |
| Ever breastfed the child (n(%) yes) | 522 (77.4%) | 41 (69.5%) | 0.17 |
| Mother had an indoor dog during pregnancy | 201 (28.4%) | 10 (15.2%) | 0.021 |
| Child lived with an indoor dog in the first year of life | 183 (27.3%) | 10 (16.7%) | 0.074 |
| Mother sensitized to allergens | 400 (58.1%) | 34 (56.7%) | 0.82 |
| Child’s gender | |||
| Male | 357 (50.5%) | 34 (51.5%) | 0.87 |
| Female | 350 (49.5%) | 32 (48.5%) | |
| Race | |||
| Black | 517 (73.1%) | 53 (80.3%) | 0.21 |
| White | 190 (26.9%) | 13 (19.7%) | |
| Delivery type | |||
| Vaginal | 451 (63.9%) | 36 (54.6%) | 0.13 |
| C-section | 255 (36.1%) | 30 (45.4%) | |
| Child was firstborn | 273 (38.6%) | 21 (31.8%) | 0.28 |
There are some missing data. P-values are for a comparison of those included and those excluded.
Table 2 presents the means (standard deviations) of the deseasonalized 25(OH)D levels for all children included in the analyses, and separately for Black children and White children. White children tended to have higher levels of 25(OH) at each time point (prenatal, cord blood, 2 years) compared to the means of the Black children. The rates of each outcome overall and separately for White children and Black children are presented in Table 3. Our previous publication demonstrated the Black children have higher rates of each outcome in this birth cohort.(19) More than half of the Black children and more than a third of the White children were atopic. More than a quarter of the Black children had a history of eczema – more than double the rate of the White children. While only 9.9% of the White children had received a doctor diagnosis of asthma, 14.7% of the Black children had.
Table 2.
Mean (SD) for 25(OH)D at each time point (in ng/ml).*
| 25(OH)D | Black children | White children | All children | P value for Black versus White children* |
|---|---|---|---|---|
| Prenatal | 19.5 (9.5) N=380 |
33.1 (11.2) N=144 |
23.2 (11.7) N=524 |
<0.001 |
| Cord | 8.9 (6.3) N=315 |
16.7 (7.6) N=101 |
10.8 (7.4) N=416 |
<0.001 |
| 2 Years | 23.0 (7.8) N=283 |
26.3 (8.4) N=96 |
23.8 (8.1) N=379 |
<0.001 |
p-value for t-test; 25(OH)D levels have been deseasonalized
Table 3.
Rates of each outcome for those included in the current analyses.
| 25(OH)D | Eczema ever | SPT+ | ≥1 sIgE ≥ 0.35 IU/ml | ≥1 food allergen sIgE ≥ 0.35 IU/ml | ≥1 aeroallergen sIgE ≥ 0.35 IU/ml | Doctor diagnosis of asthma at 3–6 years |
|---|---|---|---|---|---|---|
| All Children | 116/516 (22.5%) | 123/506 (24.3%) | 226/454 (49.8%) | 188/468 (40.2%) | 106/450 (23.6%) | 85/635 (13.4%) |
| White Children | 18/153 (11.8%) | 25/148 (16.9%) | 46/117 (39.3%) | 36/125 (28.8%) | 19/115 (16.5%) | 17/172 (9.9%) |
| Black Children | 98/363 (27.0%) | 98/358 (27.4%) | 180/337 (53.4%) | 152/343 (44.3%) | 87/335 (26.0%) | 68/463 (14.7%) |
The associations between vitamin D and each allergy-related outcome were then examined using odd ratios (OR) and 95% confidence intervals (CI) for a 5 ng/ml increase in 25(OH)D. The associations are presented by each time-point for all children and separately for White children and Black children (Table 4). An increase in the prenatal level of 25(OH)D was associated with decreased odds of eczema (OR=0.85, 95% CI 0.75, 0.96), overall, and the association was stronger, although not statistically significantly, in the White children (OR=0.79, 95% CI 0.57, 1.09) compared with the Black children (OR=0.96, 95% CI 0.82, 1.12). Higher levels of cord blood 25(OH)D were also associated with decreased odds of having at least one positive skin prick test (OR=0.77, 95% CI 0.62, 0.94) and being sensitized to at least one aeroallergen (OR=0.74, 95% CI 0.58, 0.94). Both associations were statistically significant in the White children (SPT: OR=0.50, 95% CI 0.32, 0.80; ≥1 aeroallergen: OR=0.50, 95% CI 0.28, 0.92) but not the Black children (SPT: OR=0.88, 95% CI 0.68, 1.14; aeroallergen: OR=0.85, 95% CI 0.65, 1.11). Among Black children only, higher 25(OH)D at the time of the clinic visit was associated with decreased odds of being sensitized to at least one aeroallergen (OR=0.79, 95% CI 0.66, 0.96). This was not true for the White children (OR=1.21, 95% CI 0.87, 1.69). These results are similarly reflected by statistically significant differences in mean levels of 25(OH)D (Online Table 1).
Table 4.
Odds ratios (95% CI) associated with 5 ng/ml change in 25(OH)D.
| 25(OH)D | Eczema ever | SPT+ | ≥1 sIgE ≥ 0.35 IU/ml | ≥1 food allergen sIgE ≥ 0.35 IU/ml | ≥1 aeroallergen sIgE ≥ 0.35 IU/ml | Doctor diagnosis of asthma at 3–6 years |
|---|---|---|---|---|---|---|
| Prenatal | 0.85 (0.75–0.96) | 0.95 (0.85–1.06) | 0.97 (0.85–1.07) | 0.97 (0.88–1.07) | 0.91 (0.81–1.03) | 0.92 (0.82, 1.03) |
| Cord | 0.88 (0.72–1.08) | 0.77 (0.62–0.94) | 0.86 (0.73–1.02) | 0.91 (0.77–1.07) | 0.74 (0.58–0.94) | 0.82 (0.67, 1.02) |
| 2 Years | 0.88 (0.75–1.03) | 1.03 (0.87–1.20) | 0.97 (0.85–1.11) | 0.97 (0.85–1.10) | 0.87 (0.75–1.02) | 1.00 (0.82, 1.23) |
| White Children Only | ||||||
| Prenatal | 0.79 (0.57–1.09) | 0.86 (0.66–1.12) | 1.02 (0.83–1.25) | 1.12 (0.91–1.39) | 0.75 (0.54–1.04) | 0.98 (0.78, 1.25) |
| Cord | 0.64 (0.37–1.09) | 0.50 (0.32–0.80) | 1.20 (0.84–1.70) | 1.38 (0.93–2.02) | 0.50 (0.28–0.92) | 0.82 (0.53, 1.25) |
| 2 Years | 0.94 (0.63–1.40) | 1.16 (0.82–1.64) | 1.27 (0.96–1.68) | 1.14 (0.86–1.50) | 1.21 (0.87–1.69) | 1.42 (0.77, 2.62) |
| Black Children Only | ||||||
| Prenatal | 0.96 (0.82–1.12) | 1.12 (0.96–1.31) | 1.11 (0.97–1.28) | 1.07 (0.93–1.22) | 1.05 (0.90–1.21) | 0.91 (0.77, 1.06) |
| Cord | 1.08 (0.87–1.35) | 0.88 (0.68–1.14) | 0.82 (0.66–1.01) | 0.86 (0.70–1.07) | 0.85 (0.65–1.11) | 0.81 (0.61, 1.08) |
| 2 Years | 0.91 (0.76–1.08) | 1.02 (0.85–1.23) | 0.91 (0.78–1.07) | 0.95 (0.81–1.11) | 0.79 (0.66–0.96) | 1.02 (0.81, 1.29) |
Stratum-specific estimates were examined for any interaction term where p≤0.05 to investigate possible heterogeneity of associations. Higher prenatal 25(OH)D was associated with decreased odds of having any sensitization among children born to atopic mothers (atopic mother: OR=0.89, 95%CI 0.78, 1.02; mother not atopic: OR=1.10, 95%CI 0.95, 1.27) (Table 5). Prenatal 25(OH)D varied in its association with doctor diagnosis of asthma at age 3–6 years based on delivery mode: c-section, OR=1.16, 95%CI 0.91, 1.48; and vaginal delivery, OR=0.86, 95% CI 0.75, 0.98 (Table 5). Associations between prenatal 25(OH)D and eczema varied by maternal education (less than high school: OR=0.76, 95% CI 0.64, 0.90; at least high school: OR=1.06, 95% CI 0.86, 1.30) and the associations between prenatal 25(OH)D and having at least one elevated inhalant sIgE also varied by household income (≥$40,000: OR=0.77, 95% CI 0.60, 0.97; <$40,000: OR=1.03, 95% CI 0.88, 1.20).
Table 5.
Subgroup associations for statistically significant (p<0.05) interactions between prenatal 25(OH)D and other factors for each outcome. Odds ratios (95% CI) is for a 5 ng/ml increase in 25(OH)D
| PRENATAL 25(OH)D | Interaction p value | |
|---|---|---|
| ≥1 sIgE ≥ 0.35 IU/ml | ||
| Mom atopic | 0.04 | |
| - Yes | 0.89 (0.78, 1.02) | |
| - No | 1.10 (0.95, 1.27) | |
| Doctor diagnosis of asthma 3–6 years | ||
| C-section | ||
| - Yes | 1.16 (0.91, 1.48) | 0.03 |
| - No | 0.86 (0.75, 0.98) | |
| Maternal education | ||
| Eczema | 0.02 | |
| - Less than high school diploma | 0.76 (0.64, 0.90) | |
| - At least high school diploma | 1.06 (0.86, 1.30) | |
| Household income | ≥ 1 aeroallergen sIgE ≥ 0.35 IU/ml | |
| ≥$40,000 | 0.77 (0.60, 0.97) | 0.04 |
| < $40,000 | 1.03 (0.88, 1.20) | |
We reran the models using different approaches. First, we included season as a covariate with the deseasonalized variable and all results were unchanged. The results were also unchanged when the models were rerun using the actual 25(OH)D values and a covariate to adjust for season. No nonlinear associations were found using Likelihood Ratio Tests (all p>0.05).
Finally, a limited set of analyses were run on the subset of children who had both a prenatal and cord blood sample (n=304). The 25(OH)D levels in the prenatal and cord samples were more strongly correlated (Spearman r=0.74) compared with the associations between the prenatal levels and the cord levels with the levels at age 2 years (r=0.34 and r=0.33, respectively). Mean levels for each time-point are presented separately for Black and White children in Online Table 2. As expected, Black children tended to have lower levels than White children. Prenatal 25(OH)D was not statistically significantly associated with any of the outcomes; however, cord blood 25(OH)D level was inversely associated with being sensitized overall (OR=0.81, 95% CI 0.66, 0.99) and being sensitized to an aeroallergen (OR=0.71, 95% CI 0.53, 0.96). This was true when either the OR for a 5 unit change in 25(OH)D or the differences in means of those who were and were not sensitized were compared (Online Tables 3 and 4).
DISCUSSION
In this cohort study, 25(OH)D was associated with some allergy-related outcomes. The general pattern of the results indicates that children with higher 25(OH)D tended to have fewer allergy-related outcomes. More specifically, higher prenatal 25(OH)D was associated with lower odds of eczema, with stronger, albeit not statistically significantly different, associations in the White children compared to the Black children. Cord blood 25(OH)D was inversely associated with the odds of having at least one positive skin prick test and being sensitized to at least one aeroallergen. Both associations were statistically significant only in the White children. However, among only Black children, higher 25(OH)D at the time of the clinic visit was associated with decreased odds of being sensitized to at least one aeroallergen. Prenatal 25(OH)D was positively associated with the odds of a doctor diagnosis of asthma among children born via c-section, but inversely associated in children born vaginally. Additional subgroup analyses suggest that maternal atopic status may modify the associations between prenatal 25(OH)D and developing atopy. Furthermore, prenatal 25(OH)D was inversely associated with eczema in children with mothers who had less education and inversely associated with being sensitized to aeroallergens among children from families with higher income. The data suggest that maternal atopic status, education and household income and delivery mode may need to be incorporated into any vitamin D study’s design, and sample size, recruitment and analytical plans.
In children with both a prenatal and cord blood 25(OH)D sample, some of the associations between vitamin D and the outcomes differed by whether the sample was collected prenatally or at delivery (cord blood). Differences existed in both the estimates of the ORs and the statistical significance. These discrepancies have potential implications for both study design and interpretation of the literature. While not conclusive, these data support the need for further investigation.
While vitamin D has been considered a treatment for atopic dermatitis (AD),(31) there have been few investigations of the relationship between early life vitamin D levels and childhood AD risk. Children whose mothers had the highest quartiles of 25(OH)D during pregnancy had an increased risk of AD at age 9 months in a birth cohort of 440 English infants (OR=3.26, 95%CI 1.15, 9.29).(32) In the ALSPAC birth cohort, prenatal 25(OH)D levels were not associated with eczema assessed by questionnaire among 7–8 year old children.(7) In an Australian cohort (n=231) in which the children had at least 1 allergic parent, cord blood 25(OH)D3 was weakly inversely associated with eczema (as defined by clinical examination or parental report of doctor diagnosis) at 1 year of age (OR=2.66, 95% CI 1.24,5.72 for 25(OH)D3 <50 nmol/l versus ≥75 nmol/l).(8) Cord blood 25(OH)D levels were also inversely associated with AD (assessed by questionnaire) by age 5 years in children (n=239) in the EDEN birth cohort (adjusted OR=0.75, 95% CI 0.63, 0.88).(6) These inconsistent results could be due to the use of outcomes defined by questionnaire data rather than a clinical examination and that prenatal and cord blood may not be considered as interchangeable measures of early life vitamin D as suggested in the current analyses.
While higher cord 25(OH)D levels were associated with decreased odds of having a positive skin prick test in our study population, prenatal 25(OH)D levels were not associated with childhood skin prick test results in either the ALSPAC cohort (7) or the Southampton Women’s Survey (n=860)(33) nor were associations found between cord blood 25(OH)D3 and skin prick tests in the Perth cohort (8). Higher prenatal and cord blood 25(OH)D3 were associated with increased risk of food allergy (report of doctor diagnosis) at age 2 years in the German LINA cohort (n=272) (adjusted OR=4.65, 95% CI 1.50, 14.48)(34) and high cord blood 25(OH)D levels were associated with increased rates of having a positive skin prick test (OR=4.2, 95% CI 1.2, 2.7) while both high and low 25(OH)D levels were associated with higher levels of total IgE in the Tucson Infant Immune Study.(35)
The study in Southampton, UK, although limited by loss to follow-up (30% remained at age 9 years), found that higher 25(OH)D levels in late pregnancy were associated with increased frequency of asthma at age 9 based on maternal report (OR=5.40, 95% CI 1.09, 26.65).(32) Prenatal 25(OH)D level was not associated with lung function in the ALSPAC cohort(7) or with childhood asthma in the Southampton Women’s Study(33) and cord blood 25(OH)D was not associated with asthma at age 5 years as defined by parental report in the EDEN study(6) or New Zealand Allergy and Asthma cohort (n=922; parental report of doctor diagnosis of asthma).(36)
While the results of the present study do not suggest that vitamin D, represented by circulating 25(OH)D, is overwhelmingly associated with disease causation, the data do suggest that vitamin D may play a role with importance that varies depending on other risk factors present in an individual. Prescott cites speculation that vitamin D may actually be a proxy for UV exposure that can affect reduction in an individual’s propensity for inflammation(18). If this is the case, then it would be important to note that vitamin D supplementation would not overcome the effects of a UV deficiency.(18)
There are numerous strengths to this work. We investigated multiple time points for their association with outcomes that were established at a clinic visit and with report of doctor diagnoses rather than through the use of interview data to assess clinical and other allergy-related outcomes. Two of the time points analyzed clearly precede “disease” incidence. The analyses also include Black and White children from the same region who were not recruited based on allergic disease status or family history. Finally, the subgroup analyses add important information suggesting that the role of vitamin D may vary within subgroups.
Unfortunately, we do not have more frequent measures of 25(OH)D, which is a limitation. Additionally, the parental report of a doctor diagnosis of asthma may lack sensitivity as diagnosing asthma in young children is challenging; however, recent reports suggest the strong possibility of high specificity of this methods. Yang et al. recently reported that when compared to an asthma diagnosis based on claims data, parental report of a doctor diagnosis of asthma was not sensitive (59%) but was specific (95.9%).(37) A larger sample size would have improved the precision of the associations in the subgroups. Some of our associations could be spurious findings.
As previously suggested, direct measurement of vitamin D and specific measurement of the outcomes are critical to improving our understanding of any potential role that vitamin D might play in developing allergy-related health conditions.(13) We further add that consideration of the possibility that the role vitamin D may play in causation is one of relative importance that may depend on other factors present. Observational studies and clinical trials will only be able to uncover this role if the studies are adequately designed and powered to conduct the important subgroup investigations.
Clinical Implications (2–3 bullet points).
Prenatal 25(OH)D was inversely associated with eczema and cord blood 25(OH)D was inversely associated with SPT and aeroallergen sensitization. Strengths of the associations varied between Black children and White children.
The associations between vitamin D and some allergy-related outcomes may vary within subgroups and observational studies and clinical trials should account for these subgroups in the design and analyses.
Acknowledgments
This work was funded by the National Institutes of Health (HL113010, AI051598, AI089473), USA
Abbreviations
- sIgE
allergen-specific IgE
- SPT
Skin Prick Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007 Mar;85(3):788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maslova E, Hansen S, Jensen CB, Thorne-Lyman AL, Strom M, Olsen SF. Vitamin D intake in mid-pregnancy and child allergic disease - a prospective study in 44,825 Danish mother-child pairs. BMC Pregnancy Childbirth. 2013;13:199. doi: 10.1186/1471-2393-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, Abou El-Magd W, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007 Sep;62(9):773–9. doi: 10.1136/thx.2006.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anto JM, Pinart M, Akdis M, Auffray C, Bachert C, Basagana X, et al. Understanding the complexity of IgE-related phenotypes from childhood to young adulthood: a Mechanisms of the Development of Allergy (MeDALL) seminar. J Allergy Clin Immunol. 2012 Apr;129(4):943–54. e4. doi: 10.1016/j.jaci.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 5.Magnus MC, Stene LC, Haberg SE, Nafstad P, Stigum H, London SJ, et al. Prospective study of maternal mid-pregnancy 25-hydroxyvitamin D level and early childhood respiratory disorders. Paediatr Perinat Epidemiol. 2013 Nov;27(6):532–41. doi: 10.1111/ppe.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baiz N, Dargent-Molina P, Wark JD, Souberbielle JC, Annesi-Maesano I Group EM-CCS. Cord serum 25-hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. J Allergy Clin Immunol. 2014 Jan;133(1):147–53. doi: 10.1016/j.jaci.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Wills AK, Shaheen SO, Granell R, Henderson AJ, Fraser WD, Lawlor DA. Maternal 25-hydroxyvitamin D and its association with childhood atopic outcomes and lung function. Clin Exp Allergy. 2013 Oct;43(10):1180–8. doi: 10.1111/cea.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012 Nov;130(5):e1128–35. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 9.Noh S, Park CO, Bae JM, Lee J, Shin JU, Hong CS, et al. Lower vitamin D status is closely correlated with eczema of the head and neck. J Allergy Clin Immunol. 2014 Jun;133(6):1767–70. e6. doi: 10.1016/j.jaci.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Wang SS, Hon KL, Kong AP, Pong HN, Wong GW, Leung TF. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr Allergy Immunol. 2014 Feb;25(1):30–5. doi: 10.1111/pai.12167. [DOI] [PubMed] [Google Scholar]
- 11.Heimbeck I, Wjst M, Apfelbacher CJ. Low vitamin D serum level is inversely associated with eczema in children and adolescents in Germany. Allergy. 2013 Jul;68(7):906–10. doi: 10.1111/all.12167. [DOI] [PubMed] [Google Scholar]
- 12.Gergen PJ, Teach SJ, Mitchell HE, Freishtat RF, Calatroni A, Matsui E, et al. Lack of a relation between serum 25-hydroxyvitamin D concentrations and asthma in adolescents. Am J Clin Nutr. 2013 Jun;97(6):1228–34. doi: 10.3945/ajcn.112.046961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacharier LB. Vitamin D status at birth: an important and potentially modifiable determinant of atopic disease in childhood? J Allergy Clin Immunol. 2014 Jan;133(1):154–5. doi: 10.1016/j.jaci.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011 May;127(5):1087–94. doi: 10.1016/j.jaci.2011.02.015. quiz 95–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012 Mar;142(3):482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentschew L, Ferguson LR. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol Nutr Food Res. 2012 Apr;56(4):524–35. doi: 10.1002/mnfr.201100630. [DOI] [PubMed] [Google Scholar]
- 17.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 2014 Mar 25; doi: 10.1177/1535370214523890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. 2013 Jan;131(1):23–30. doi: 10.1016/j.jaci.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Wegienka G, Havstad S, Joseph CL, Zoratti E, Ownby D, Woodcroft K, et al. Racial disparities in allergic outcomes in African Americans emerge as early as age 2 years. Clin Exp Allergy. 2012 Jun;42(6):909–17. doi: 10.1111/j.1365-2222.2011.03946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegienka G, Joseph CL, Havstad S, Zoratti E, Ownby D, Johnson CC. Sensitization and allergic histories differ between black and white pregnant women. J Allergy Clin Immunol. 2012 Sep;130(3):657–62. e2. doi: 10.1016/j.jaci.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aichbhaumik N, Zoratti EM, Strickler R, Wegienka G, Ownby DR, Havstad S, et al. Prenatal exposure to household pets influences fetal immunoglobulin E production. Clin Exp Allergy. 2008 Nov;38(11):1787–94. doi: 10.1111/j.1365-2222.2008.03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008 Jun;87(6):1738–42. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 23.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007 Mar;103(3–5):631–4. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem. 2006 Jun;52(6):1120–6. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 25.Binkley N, Krueger D. Evaluation and correction of low vitamin D status. Curr Osteoporos Rep. 2008 Sep;6(3):95–9. doi: 10.1007/s11914-008-0017-5. [DOI] [PubMed] [Google Scholar]
- 26.Binkley N, Krueger D, Gemar D, Drezner MK. Correlation among 25-hydroxy-vitamin D assays. J Clin Endocrinol Metab. 2008 May;93(5):1804–8. doi: 10.1210/jc.2007-2340. [DOI] [PubMed] [Google Scholar]
- 27.Binkley N, Krueger D, Lensmeyer G. 25-hydroxyvitamin D measurement, 2009: a review for clinicians. J Clin Densitom. 2009 Oct-Dec;12(4):417–27. doi: 10.1016/j.jocd.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Binkley N, Krueger DC, Morgan S, Wiebe D. Current status of clinical 25-hydroxyvitamin D measurement: an assessment of between-laboratory agreement. Clin Chim Acta. 2010 Dec 14;411(23–24):1976–82. doi: 10.1016/j.cca.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007 May;254(5):581–90. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3. LWWW; 2010. [Google Scholar]
- 31.Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010 Aug;30(3):397–409. doi: 10.1016/j.iac.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008 Jan;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pike KC, Inskip HM, Robinson S, Lucas JS, Cooper C, Harvey NC, et al. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012 Nov;67(11):950–6. doi: 10.1136/thoraxjnl-2012-201888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013 Feb;68(2):220–8. doi: 10.1111/all.12081. [DOI] [PubMed] [Google Scholar]
- 35.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011 Nov;128(5):1093–9. e1–5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011 Jan;127(1):e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 37.Yang CL, To T, Foty RG, Stieb DM, Dell SD. Verifying a questionnaire diagnosis of asthma in children using health claims data. BMC Pulm Med. 2011;11:52. doi: 10.1186/1471-2466-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]