Abstract
Deficiency of Sirtuin 6 (SIRT6), a chromatin-related deacetylase, in mice reveals severe premature aging phenotypes including osteopenia. However, the underlying molecular mechanisms of SIRT6 in bone metabolism are unknown. Here we show that SIRT6 deficiency in mice produces low-turnover osteopenia caused by impaired bone formation and bone resorption, which are mechanisms similar to those of age-related bone loss. Mechanistically, SIRT6 interacts with runt-related transcription factor 2 (Runx2) and osterix (Osx), which are the two key transcriptional regulators of osteoblastogenesis, and deacetylates histone H3 at Lysine 9 (H3K9) at their promoters. Hence, excessively elevated Runx2 and Osx in SIRT6−/− osteoblasts lead to impaired osteoblastogenesis. In addition, SIRT6 deficiency produces hyperacetylation of H3K9 in the promoter of dickkopf-related protein 1 (Dkk1), a potent negative regulator of osteoblastogenesis, and osteoprotegerin, an inhibitor of osteoclastogenesis. Therefore, the resulting up-regulation of Dkk1 and osteoprotegerin levels contribute to impaired bone remodeling, leading to osteopenia with a low bone turnover in SIRT6-deficient mice. These results establish a new link between SIRT6 and bone remodeling that positively regulates osteoblastogenesis and osteoclastogenesis.
Keywords: SIRT6, osteopenia, Runx2, Osx, osteoblast, osteoclast
Introduction
Bone mass is maintained throughout life by the coordinated activities of various bone cells. Osteoblasts are the cells responsible for bone formation and osteoclasts are the primary cells involved in bone resorption. To achieve a balance of bone mass, these cells produce factors that stimulate intercellular signaling and strictly regulate bone formation and resorption (1). Age-related bone loss is associated with aging in both genders, and it is characterized by a greater decrease in bone formation by osteoblasts than in bone resorption by osteoclasts (1). Thereby, age-related bone loss is accompanied by low-turnover bone remodeling that produces osteopenia (1).
Sirtuins (SIRTs) are nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase enzymes and are classified into the Class III histone deacetylases (HDACs) family (2). There are seven mammalian SIRTs, known as SIRT1-SIRT7 (2). SIRT6 has been found in the nucleus and triggers chromatin silencing by NAD+-dependent histone deacetylation that leads to proper chromatin function in controlling telomeric chromatin, genome stabilization, DNA repair, and gene expression programs (2). One of the important physiological roles of SIRT6 is transcriptional repression (3). SIRT6 interacts with nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and hypoxia-inducible transcription factor (Hif-1α), which are transcription factors that play critical roles in regulating aging (2), and is recruited to their target gene promoters, leading to suppression of these target genes (2). Hence, SIRT6 deficiency is known to lead to histone H3 lysine 9 (H3K9) hyperacetylation on the target gene promoters, increasing the expression of these genes (3). To date, only a few transcription factors are implicated in the regulation of gene expression programs by SIRT6. However, given that hundreds of genes are differentially expressed in SIRT6-null cells compared to control cells (3), SIRT6 may be implicated in the regulation of numerous gene expression programs. SIRT6-deficient (SIRT6−/−) mice have revealed a progeroid degenerative syndrome including acute loss of subcutaneous fat, lordokyphosis, colitis, severe lymphopenia, and osteopenia (4). In addition, some metabolic defects are observed, such as a decrease in circulating insulin-like growth factor (IGF-I) and glucose levels, and SIRT6−/− mice eventually die between 3 and 4 weeks of age after birth (4). Reportedly, SIRT6−/− mice show 30% reduction in bone mineral density compared to control mice (4). Moreover, SIRT6 positively regulates proliferation and differentiation of chondrocytes (5). However, the underlying molecular mechanisms by which SIRT6 regulate the bone formation and bone resorption have been remained unclear. In this study, we found that loss of SIRT6 in osteoblasts may cause low-turnover osteopenia due to hyperacetylation of Runx2 and osterix along with the increased production of Dkk1, a potent negative regulator of osteoblastic development, and osteoprotegerin (Opg), an inhibitor of osteoclastic development. Thus, loss of SIRT6 in osteoblasts may trigger the onset of age-related bone loss
Materials and methods
Experimental animals and analysis of the skeletal phenotype
The breeding pair of heterozygous SIRT6 (129/SvJ) mice was purchased from the Jackson Laboratory to produce homozygous SIRT6 (129/SvJ) mice. We used male mice in all studies. For micro-CT (μCT) analysis, the bones (3-week-old) were scanned using μCT 40 Scanner (Scanco Medical). Trabecular bone evaluation of distal femur metaphysis were performed using the indicated parameters in Fig. 1F. Bone histomorphometric analysis was conducted at the University of Kentucky. All bone samples were dehydrated, and embedded in methylmethacrylate. Serial sections of 4~7-μm thicknesses were cut with a microm microtome and the sections were stained with the modified Masson-Goldner trichrome stain. Histomorphometric parameters of bone were evaluated at standardized sites in cancellous bone using the semiautomatic method (Osteoplan II, Kontron, Germany) (6). Dynamic bone histomorphometric analysis was performed using calcein labeling. Calcein (Sigma) was injected 2 times on day 1 and day 5, and then the mice were sacrificed on day 7. All animals were housed under pathogen-free conditions according to the guidelines of the Division of Comparative Medicine, Washington University School of Medicine. The animal ethics committee approved all experiments.
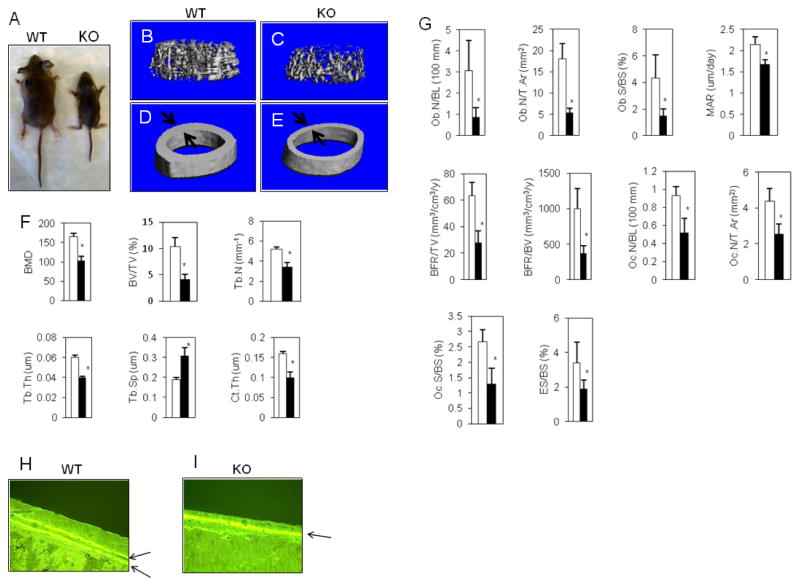
Fig. 1. SIRT6−/− mice reveals osteopenia.
(A) The picture of 3-week-old male mice. (B and C) Representative uCT images of the femur trabecular. (D and E) Representative uCT images of the femoral mid shaft. The arrow indicates the cortical bone thickness. (F) The indicated parameters were measured by uCT analysis in the distal femurs. The data were expressed as the means ± S.D. of six mice of each genotype. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (G) The indicated parameters were measured by bone histomorphometric analysis. The data were expressed as the means ± S.D. of six mice of each genotype. Ob.N/BL: osteoblast number per bone length, Ob.N/T.Ar: osteoblast number per tissue area, Ob.S/BS: osteoblast surface per bone surface, Oc.N/BL: osteoclast number per bone length, Oc.N/T.Ar: osteoclast number per tissue area, Oc.S/BS: osteoclast surface per bone surface, MAR: mineral apposition rate, BFR/TV: bone formation rate per tissue volume, BFR/BV: bone formation rate per bone volume, ES/BS: erosion surface per bone surface. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (H and I) Cortical mineral apposition rate was determined by calcein labeling (arrows).
Cell cultures
Calvarias were digested from neonatal mice (1-to-3-day-old) and sequentially by 2 mg/ml of collagenease (Roche) and the cells were employed for assays. Alkaline phosphatase (ALP) activity was measured using the TRACP & ALP assay kit (Takara) according to the manufacturer’s instructions. Alizarin red staining and its quantitative analysis were performed using the osteogenesis assay kit (Millipore) according to the manufacturer’s instructions. Colony-forming unit assays were performed using bone marrow cells from the long bone tissues of SIRT6−/− mice and control littermates. The cells were seeded at a density of 1.5 × 106 cells/well in a 6-well plate and cultured in osteoblastic differentiation media including 50 uM ascorbic acid plus 5 mM β-glycerophosphate (OB-media). To determine ALP-positive colony forming units, cells were stained for ALP after 10 days in culture. Cell colonies containing at least 20 cells were designated ALP-positive colony forming units. Osteoclasts were induced from splenocytes isolated from the spleen of SIRT6−/− mice and control littermates at 1-week-old in response to 50 ng/ml receptor activator of nuclear factor kappa-B ligand (RANKL) (Peprotech) and 50 ng/ml macrophage colony-stimulating factor (M-CSF) (Peprotech) for 5 days as described (7). In a coculture system, splenocytes were cocultured with calvarial osteoblasts in the presence of 10−8 M 1,25-dihydroxyvitamin D3 and 10−6 M prostaglandin E2 for 10 days. Tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells (TRAP+ MNCs, >3 nuclei) were counted under microscopic examination.
End-point PCR and real-time PCR (Q-PCR)
Total RNA was isolated using the mirVana™ miRNA isolation kit (Life technologies) according to the manufacturer’s instructions. For end-point PCR and real-time PCR, cDNA was generated using the 1st strand cDNA synthesis system kit (OriGene) following the manufacturer’s suggestion. The PCR analysis was performed using the GeneAmp fast PCR master mix (Life technologies) as per manufacturer’s protocol. All primers were synthesized by Integrated DNA Technologies. The primer for mouse SIRT6 was forward (5-AGA GGA ATG TCC CAA GTG TAA G-3) and reverse (5-AAA GAC ACC AGG GTG AGA TAT G-3). Q-PCR analysis was performed using the OriGene qSTAR SYBR green kit following the manufacturer’s suggestion. The relative expression for osteocalcin (Ocn) mRNA was estimated from triplicate Q-PCR reactions following normalization to the β2-microglobulin. All primers for Q-PCR assay were purchased from OriGene.
Western blotting
Whole-cell lysates were prepared using the RIPA buffer (Thermo Scientific) according to the manufacturer’s instructions. For the detection of β-catenin, nuclear extracts were employed using the nuclear and cytoplasmic extraction reagent (Pierce) according to the manufacturer’s suggestions. The proteins were separated on a 8–12 % SDS-PAGE and electrotransformed to PVDF membrane (Millipore). Immunoblots were performed by using antibodies against SIRT6 (1:500 OriGene), Runx2 (1:1000 Cell signaling), Osx (1:500 Santa Cruz), Dkk1 (1:200 Santa Cruz), Opg (1:500 OriGene), cathepsin K (1:1000 Cell signaling), integrin β3 (1:1000 Cell signaling), NFATc1 (nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1) (1:1000 Santa Cruz), acetyl-histone H3 (Lys9) (1:1000 Cell signaling), β-catenin (1:1000 Cell signaling), or α-tubulin (1:1000 Santa Cruz). Blots were developed by chemiluminescence (Thermo Scientific). We used a standard exposure time of 1 min for all of the western blot data, and loaded 20ug of proteins.
Chromatin Immunoprecipitation assays (ChIP assay)
The cells were treated with or without bone morphogenetic protein-2 (BMP-2) stimulation. ChIP assay was performed with the simpleChIP® enzymatic chromatin IP kit (Cell Signaling) according to the manufacturer’s suggestions using antibodies against Runx2 (1:100 Cell Signaling), Osx (1:100 Abcam), SIRT6 (1:100 Abcam), Smad4 (1:50 Cell signaling), or acetyl-histone H3 (Lys9) (1:50 Cell signaling). The purified DNA was analyzed by PCR using primers that detect sequences containing the mouse Runx2 promoter (forward: 5-TGG TAG GCA GTC CCA CTT TAC-3, and reverse: 5-GGC TGG TAG TGA CCT GCA GAG-3), the mouse Osx promoter (forward: 5-CTC ATT GGA TCC GGA GTC TTC T-3, reverse: 5-TGT CTG TAG GGA TCC ACC CTC TA-3), the mouse ALP promoter (forward: 5-GGC TGG GAC AGA CAG AAT GT-3, reverse: 5- CTG CAA CAG GCA GGG TAA C-3), the mouse Ocn promoter (forward: 5-CTG AAC TGG GCA AAT GAG GAC A-3, reverse: 5-AGG GGA TGC TGC CAG GAC TAA T-3), the mouse Dkk1 promoter (5-CTA GTG CTC TAG TGA CCC ACA CTC-3, reverse: 5-TGG ACT GCG GAA CCT CAA CTT C-3), and the mouse Opg promoter (forward: 5-TTA GGG AAT ACC TCA GGA AAA TAC A-3, reverse: 5- TTG TAG GAG CAC GAG GTG AA-3). In addition, the indicated promoter fragments were quantified by Q-PCR using the OriGene qSTAR SYBR green kit as per manufacturer’s protocol. All primers were synthesized by Integrated DNA Technologies. Total amounts of binding by a specific antibody were determined by expressing the amount of DNA obtained for each immunoprecipitation (IP) sample as a percentage of total DNA (8)
Retroviral infection and gene transfection
SIRT6 inserted pCMV6-entry vectors were purchased from OriGene. SIRT6 insert was shuttled into the pBluescript II KS(+) vectors (Strategene), which are the pBlu-SIRT6 vectors, digested with BamHl and EcoRl restriction enzymes (Thermo Scientific). The inserted SIRT6 was again shuttled into the pMX-IRES-IB retrovirus vectors (Cell Biolabs) from the pBlu-SIRT6 vectors digested with BamHl and Xhol restriction enzymes (Thermo Scientific). Retroviral infection was performed as described (7). Flag-tagged Runx2 vectors (OriGene) or Flag-tagged Osx vectors (OriGene) were transiently transfected into NIH3T3 or MC3T3-E1 cells by FuGENE (Roche) as per manufacturer’s protocol.
IP assay
IP assay was conducted following the IP protocol utilizing magnetic separation which provided by Cell Signaling using antibodies against flag (1:200 OriGene), SIRT6 (1:200 Cell Signaling), or acetylated-lysine (1:100 Cell Signaling). The samples were boiled in 5 X SDS-PAGE sample buffer and subjected to immunoblot analysis
ELISA assay
WT or SIRT6−/− osteoblasts were cultured with BMP-2 treatment and the culture media was harvested. Serum was collected from SIRT6−/− mice and control littermates at 3-week-old. ELISA assay was performed using the mouse Dkk1 or Opg PicoKine ELISA kit (Boster) according to the manufacturer’s instructions.
Conditioned media preparation
SIRT6−/− osteoblasts were cultured and these cells were rinsed three times with PBS and incubated for 24h in serum free αMEM (Sigma). Serum free conditioned media were harvested and concentrated 10–50 fold (v/v) using Amicon Centricon Plus70 centrifugation concentrators.
Statistical evaluations
All the numeral data in the results were presented as mean values ± SEMs or ± SDs. Statistical analysis was performed based on Student’s t-test. Fig. 1F,G and Fig. 6A: The data were expressed as the means ± S.D. of five or six mice of each genotype. Fig. 2A,C,D,F,I and Fig. 3F–I and Fig. 4C and Fig. 5B and E: Data represented means ± S.E. of three experiments in triplicate.
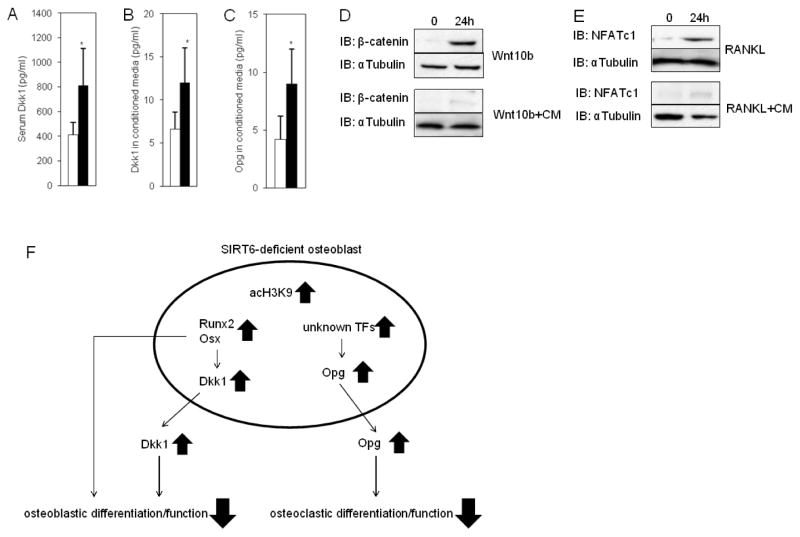
Fig. 6. Elevated Dkk1 and Opg levels in SIRT6−/− Osteoblasts.
(A) Elevated serum Dkk1 levels in SIRT6−/− mice. The data were expressed as the means ± S.D. of five mice of each genotype. *, p < 0.01. Open bar: WT, closed bar: SIRT6 deficiency. (B and C) Elevated Dkk1 or Opg levels in culture media of SIRT6−/− osteoblasts. Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (D) Western blots showing the nuclear accumulation of β-catenin in osteoblasts in response to Wnt10b with or without conditioned media (CM) harvested from SIRT6−/− osteoblast culture media. αTubulin was used as a loading control. (E) Western blots showing NFATc1 expression in splenocytes in response to RANKL with or without conditioned media (CM) harvested from SIRT6−/− osteoblast culture media. αTubulin was used as a loading control. (F) A model of a novel mechanism for controlling Dkk1 and Opg levels by SIRT6 in osteoblasts. Hyperacetylation of histone H3K9 on the promoters of Runx2 and Osx in SIRT6-deficient osteoblasts produces increased Runx2 and Osx levels so that Dkk1 levels are up-regulated that result in impaired osteoblastic differentiation and function. Opg levels are also increased by unknown mechanisms in SIRT6-deficient osteoblasts so that osteoclastic differentiation and function are diminished. This novel mechanism may trigger age-related bone loss. acH3K9: acetylated H3K9. TFs: transcription factors.
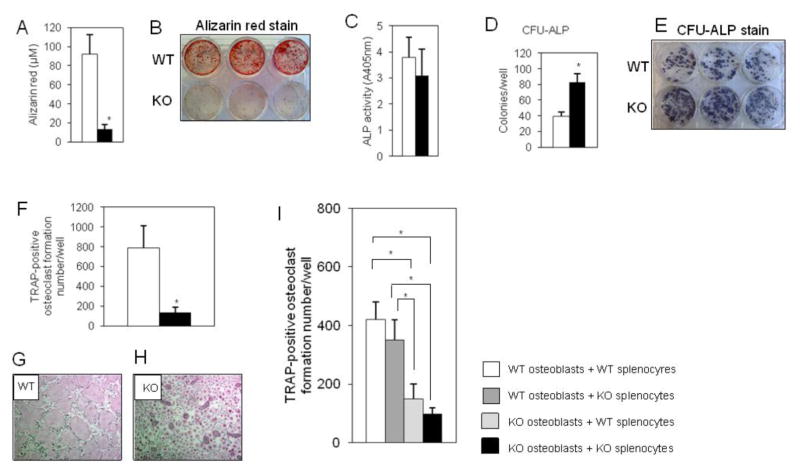
Fig. 2. SIRT6 deficiency causes impaired osteoblast and osteoclast differentiation in culture.
(A) Mineralization assay. WT and SIRT6−/−osteoblasts were cultured in OB-media for 21 days. Data represented means ± S.E. of alizarin red levels in three experiments in triplicate. *, p < 0.01. Open bar: WT, closed bar: SIRT6 deficiency. (B) Representative alizarin red staining. (C) ALP activity. WT and SIRT6−/−osteoblasts were cultured in OB-media for 7 days. Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. (D) Colony forming units-ALP assay. Bone marrow cells from WT and SIRT6−/− mice were cultured in OB-media for 10 days. Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (E) Representative CFU-ALP staining. (F) Osteoclast formation assay by osteoblast free system. Splenocytes from WT and SIRT6−/− mice were cultured by RANKL for 5 days. Data represented means ± S.E. of three experiments in triplicate. *, p < 0.01. Open bar: WT, closed bar: SIRT6 deficiency. (G and H) Representative TRAP-positive osteoclasts. (I) Osteoclast formation assay by coculture system. Indicated cells were cocultured in response to 10−8 M 1,25-dihydroxyvitamin D3 and 10−6 M prostaglandin E2 (10 days). Data represented means ± S.E. of three experiments in triplicate. *, p < 0.01.
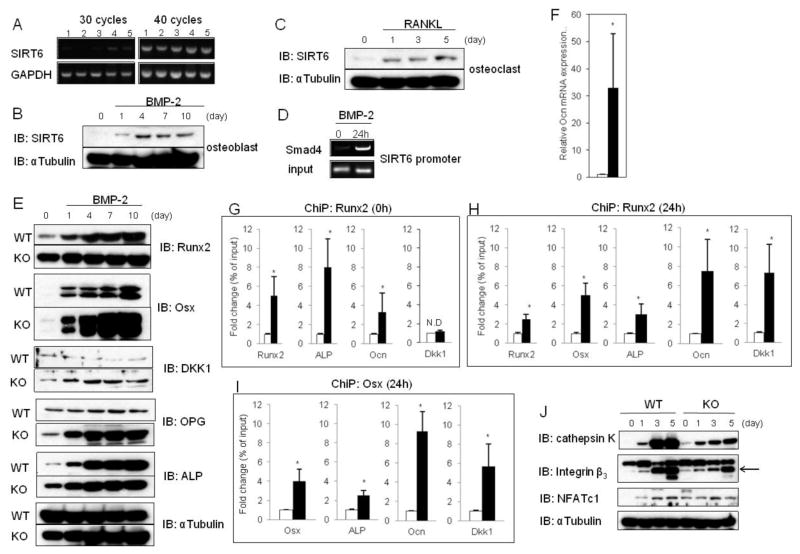
Fig. 3. Elevated Runx2 and Osx levels in SIRT6−/− osteoblasts.
(A) PCR analysis showing SIRT6 expression in various types of cells. Lane 1: osteoblasts, lane 2: bone marrow macrophages, lane 3: splenocytes, lane 4: bone marrow stromal cell-line ST2 cells, lane 5: osteoblast precursor cell-line MC3T3-E1 cells. (B) Western blot showing SIRT6 expression during osteoblastogenesis with BMP-2 stimulation. αTubulin was used as a loading control. (C) Western blot showing SIRT6 expression during osteoclastogenesis with RANKL stimulation. αTubulin was used as a loading control. (D) ChiP analysis to detect Smad4 at the promoter of SIRT6. (E) Western blots showing expression of the indicated osteoblastic markers during osteoblastogenesis with BMP-2 stimulation. αTubulin was used as a loading control. (F) Q-PCR analysis showing Ocn expression in WT and SIRT6−/−osteoblasts with BMP-2 treatment (10 days). Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (G) ChiP analysis to detect Runx2 at the indicated promoters in WT and SIRT6−/−osteoblasts without BMP-2 treatment (day 0). Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (H and I) ChiP analysis to detect Runx2 or Osx at the indicated promoters in WT and SIRT6−/−osteoblasts with BMP-2 treatment (24h). Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01. (J) Western blot showing expression of the indicated osteoclastic markers during osteoclastogenesis with RANKL stimulation. αTubulin was used as a loading control.
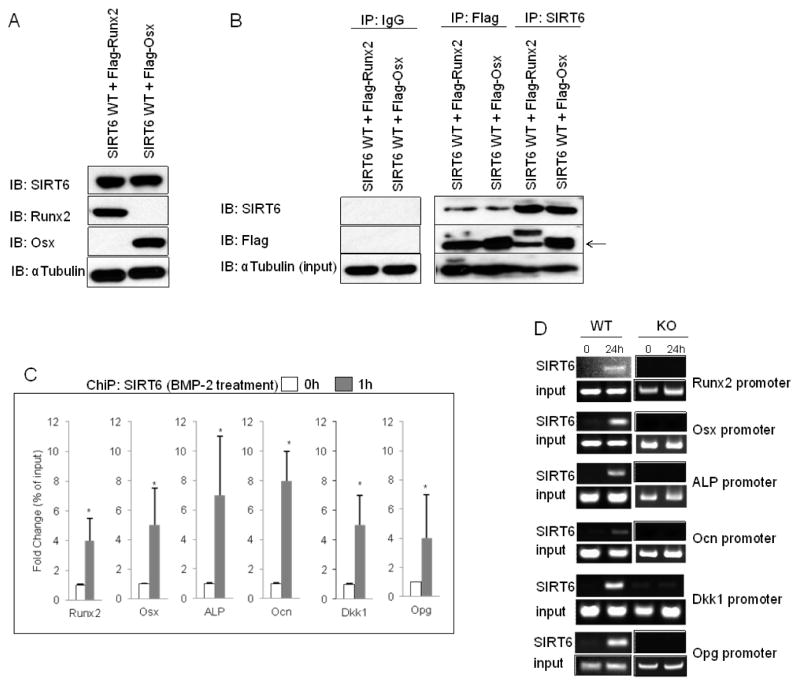
Fig. 4. SIRT6 interacts physically with Runx2/Osx and is recruited to their target promoters.
(A) Western blots showing expression of SIRT6, Runx2, and Osx in NIH3T3 cells with retroviral infection of SIRT6 and transient transfection of Runx2 or Osx. αTubulin was used as a loading control. (B) SIRT6 binding to Runx2 and Osx, as determined by western blots of SIRT6 or Flag antibody immunoprecipitates from SIRT6 WT overexpressing cells with Flag-Runx2 or Flag-Osx. IgG antibody was used as a negative control for IP assay. The arrow indicates Runx2 and Osx expression. (C) ChiP analysis to detect SIRT6 at the indicated promoters in SIRT6 overexpressing MC3T3-E1 cells with or without BMP-2 treatment. Data represented means ± S.E. of three experiments in triplicate. *, p < 0.01. (D). ChiP analysis to detect endogenous SIRT6 at the indicated promoters in osteoblasts with BMP-2 stimulation (24h).
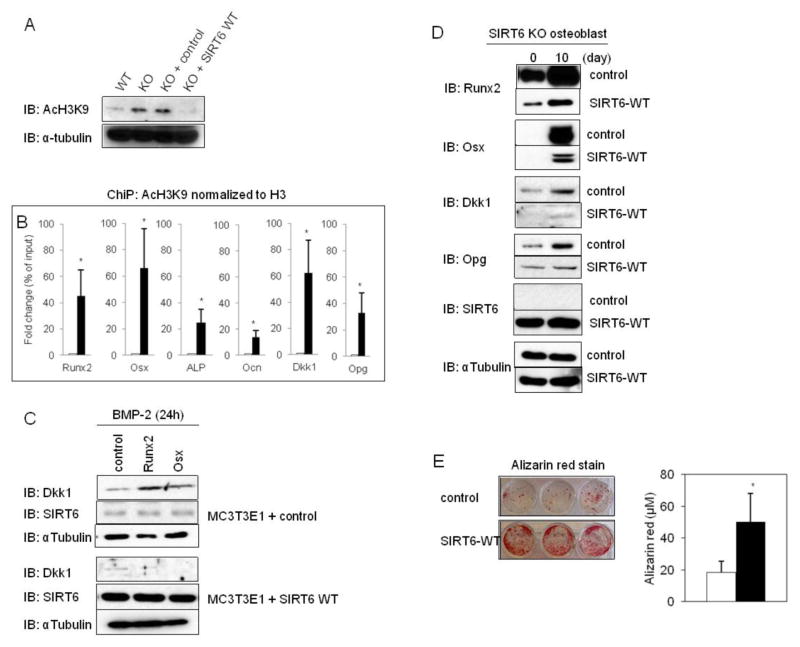
Fig. 5. Hyperacetylation of the target gene promoters of Runx2 and Osx in SIRT6−/− osteoblasts.
(A) SIRT6 overexpression diminished global H3K9 hyperacetylation (AcH3K9) in SIRT6−/− osteoblasts. (B) ChiP analysis to detect AcH3K9 at the indicated promoters in WT and SIRT6−/−osteoblasts with BMP-2 treatment (3h). Antibodies to AcH3K9 and to histone 3 (H3) were used, and AcH3H9 levels are revealed relative to total H3 levels. Data represented means ± S.E. of three experiments in triplicate. *, p < 0.01. Open bar: WT, closed bar: SIRT6 deficiency. (C) Western blots showing Dkk1 expression in Runx2 or Osx overexpressing cells with or without SIRT6 WT overexpression. αTubulin was used as a loading control. (D) Western blots showing expression of the indicated osteoblastic markers in SIRT6−/−osteoblasts with or without SIRT6 WT overexpression. αTubulin was used as a loading control. (E) SIRT6−/−osteoblasts infected with control or SIRT6 WT were cultured in OB-media for 21 days. Representative alizarin red staining (Left). Mineralization assay (Right). Data represented means ± S.E. of three experiments in triplicate. Open bar: WT, closed bar: SIRT6 deficiency. *, p < 0.01.
Results
Impaired bone remodeling in SIRT6-deficient mice
SIRT6−/− mice harbor skeletal abnormalities, including growth retardation and osteopenia (Fig. 1A–F) in agreement with a previous report (4). Also, consistent with a previous report (5), we observed that the size difference between SIRT6−/− and wild-type (WT) mice at birth (data not shown), was continued throughout their growth (Fig. 1A). Analysis of SIRT6−/− mice femurs was performed using μCT and bone histomorphometry. μCT analysis of SIRT6−/− mice revealed significantly reduced trabecular bone mass (Fig. 1B,C) and cortical bone thickness (Fig. 1 D,E) and F; Ct.Th: cortical thickness), Trabecular bone parameters of bone mineral density (BMD), trabecular bone volume per tissue volume (BV/TV), trabecular number (Tb.N) and trabecular thickness (Tb.Th), were decreased with concomitant increase in trabecular spacing (Tb.Sp) (Fig. 1F). Trabecular bone histomorphometry revealed reduced number of osteoblasts (Ob.N/BL, Ob.N/T.Ar, Ob.S/BS) and osteoclasts (Oc.N/BL, Oc.N/T.Ar, Oc.S/BS) and decreased osteoblastic bone formation (MAR, BFR/TV, BFR/BV) and osteoclastic bone resorption (ES/BS) (Fig. 1G). As shown in Fig. 1D and E, the cortical bone thickness of SIRT6−/− mice was significantly decreased, due in part, to reduced cortical endosteal mineral apposition rate (MAR) (Fig. 1H,I). These findings indicate that decreased number of osteoblasts and osteoclasts and attenuated ability of osteoblastic bone formation and osteoclastic bone resorption caused low-turnover osteopenia similar to the features of age-related bone loss.
Impaired osteoblastogenesis and osteoclastogenesis in SIRT6 deficiency in culture
Consistent with in vivo findings, culture of SIRT6−/− osteoblasts in OB-media for 21 days revealed drastically reduced osteoblastic mineralization as shown by a quantitative alizarin red stain assay (Fig. 2A,B). However, ALP production, a marker of osteoblastic activity (9), was not different between SIRT6−/− osteoblasts and control cells cultured in OB-media for 7 days (Fig. 2C). Similar to the cell culture findings, western blots of cell homogenates showed that in culture ALP levels induced by BMP-2 were not different between SIRT6−/− osteoblasts and control cells from day 4 to day 10.(Fig. 3E), although ALP levels were significantly elevated in SIRT6−/− osteoblasts by day 0 and day 1 (Fig. 3E). Surprisingly, the numbers of ALP-positive colony forming units, which have the osteoblastic precursor capability of bone marrow stromal cells, were significantly increased in SIRT6 deficiency compared to control cells (Fig. 2D,E). In addition, the cell proliferation was not different between control and SIRT6-null cells (data not shown), suggesting that SIRT6 deficiency leads to increased abundance of osteoblastic precursor cells which accumulate due to the block in early stages of osteoblastogenesis. Similar to impaired osteoblastogenesis, osteoclast formation assay with osteoblast free system revealed that the number of TRAP, a marker of osteoclasts (7), -positive osteoclasts were remarkably reduced in SIRT6 deficiency with RANKL stimulation for 5 days (Fig. 2F,G,H). Taken together, these findings indicate that SIRT6 positively regulates osteoblastogenesis and osteoclastogenesis as a decisive intrinsic factor.
Next, coculture assays were performed to make osteoclasts using both genotypes of osteoblasts isolated from calvaria and splenocytes isolated from the spleen. Osteoclastic development was not impaired when WT osteoblasts were cocultured with either genotype of splenocytes (Fig. 2I). Conversely, osteoclastic development was remarkably impaired when SIRT6−/− osteoblasts were cocultured with either genotypes of splenocytes (Fig. 2I), implying that factors secreted from SIRT6−/− osteoblasts may be implicated in impaired osteoclastogenesis.
Elevated Runx2 and Osx levels in SIRT6-null osteoblasts with BMP-2 stimulation
To investigate the mechanisms by which SIRT6 positively regulates osteoblastic development, we confirmed the RNA expression levels of SIRT6 in various types of cells isolated from mice (see figure legends). We failed to detect SIRT6 expression in all cells by 30 cycle PCR analysis except in a bone marrow stromal cell-line, ST2 cells (Fig. 3A, lane 4). However, using 40 cycle PCR analysis, SIRT6 expression was detectable in all cell types studied (Fig. 3A), suggesting that the expression levels of SIRT6 may was low in these cells including osteoblasts. Consistent with the PCR results, SIRT6 protein expression was barely detectable in osteoblasts (Fig. 3B) and splenocytes (Fig. 3C) at day 0, but, SIRT6 levels were elevated during osteoblastogenesis induced with BMP-2 (Fig. 3B) and during osteoclastogenesis induced with RANKL (Fig. 3C). SIRT6 may be a downstream target of the BMP-2/Smad pathway during osteoblastogenesis. BMP-2 activated Smads (phosphorylated-Smad1/5/8) form a complex with Smad4 and translocate into the nucleus where they interact with other transcription factors to trigger target gene expression (10). Using a ChIP assay, we found that Smad4 is recruited to the promoter of SIRT6 by BMP-2 stimulation after 24h in primary osteoblasts (Fig. 3D), indicating that SIRT6 is a direct target of the BMP-2/Smad pathway in osteoblasts. However, we failed to find the transcription factors that are recruited to the promoter of SIRT6 together with activated Smads. In addition, we failed to identify transcription factors that boost SIRT6 levels in osteoclasts. Reportedly, only c-Fos is able to up-regulate SIRT6 levels directly (11). However, despite c-Fos being highly expressed in splenocytes, SIRT6 protein was not highly expressed in the cells at day 0 (Fig. 3C). Thus, further studies are needed to identify the positive regulators for SIRT6 gene expression in osteoblasts and osteoclasts.
Next, we analyzed the expression of Runx2 and Osx during osteoblastogenesis in SIRT6 deficiency because Runx2 and Osx are key regulators for osteoblastogenesis (9). Surprisingly, Runx2 levels were strongly enhanced at day 0 and the levels were gradually up-regulated in time-dependent fashion in osteoblastogenesis in SIRT6 deficiency (Fig. 3E). Similarly, Osx levels were also remarkably elevated during osteoblastogenesis in SIRT6 deficiency (Fig. 3E) in spite of osteoblastogenesis in SIRT6 deficiency being impaired in vivo and in vitro (Fig. 1G and Fig. 2A,B). Runx2 and Osx positively regulate osteoblastic development at an early stage (12). However, they negatively control the differentiation at a late stage (12). In fact, transgenic mice overexpressing Runx2 or Osx have osteopenia caused by osteoblast dysfunctions (12). In addition, osteocalcin (Ocn), a negative regulator of osteoblastic bone mineralization in the late stage (13), was extremely elevated in SIRT6−/− osteoblasts by BMP-2 treatment after 10 days compared to control cells (Fig. 3F) probably due to Runx2 and Osx transactivation at the Ocn promoter which were remarkably stimulated in SIRT6−/− osteoblasts with BMP-2 stimulation (Fig. 3G,H,I). In Figure 3G,H and I, ChiP analysis used to detect Runx2 and Osx at the Runx2, Osx, ALP, Ocn, and Dkk1 promoters revealed marked increases in the promoters from SIRT6−/− osteoblasts. These results and previous reports support our observations that excessively elevated Runx2 and Osx in SIRT6−/− osteoblasts lead to impaired osteoblastic development and bone mineralization.
We next studied the expression levels of NFATc1 (14) during osteoclastogenesis with RANKL stimulation of SIRT6 deficient splenocytes. In control, NFATc1 was not detected at day 0 and the levels were time-dependently up-regulated (Fig. 3J). In SIRT6 deficient cells, NFATc1 was detected at day 0 and the expression reached the maximal levels at day 3. Consistent with in vivo data that SIRT6 deficiency decreased eroded bone surface by osteoclasts (Fig. 1G: ES/BS), the levels of cathepsin K and integrin β3, which are indispensable for osteoclastic bone resorption (15), were significantly diminished in SIRT6 deficiency (Fig. 3J), implying that SIRT6 deficiency may impact osteoclastic bone resorption.
Two extremely important findings discovered during BMP-2 induced osteoblastogenesis were strongly increased Dkk1 and Opg levels in SIRT6 deficiency (Fig. 3E). Wnt signaling has critical roles in regulating osteoblastogenesis which is blocked by Dkk1 (9), and RANKL signaling is critically involved in regulating osteoclastogenesis and the pathway is blocked by Opg (16). Hence, we speculated that these elevated protein factors may cause impaired osteoblastic and osteoclastic development and their functions so that SIRT6−/− mice may produce low-turnover osteopenia.
Runx2 and Osx are recruited to a variety of osteoblast-specific marker gene promoters, for instance, ALP and Ocn, for the formation and activity of osteoblasts (9). Therefore, we asked whether Runx2 or Osx are recruited to their target promoters in SIRT6−/− osteoblasts. ChiP assay showed that in SIRT6−/− osteoblasts, Runx2 and Osx were actively recruited to the Runx2, Osx, ALP, Ocn and Dkk1 promoters at day 0 and 24 h after BMP-2 stimulation compared to control (Fig. 3G,H,I). On the other hand, Runx2 and Osx had no binding to the Opg promoter (data not shown). These results indicate that the enhanced transcriptional activity of Runx2 and Osx in SIRT6−/− osteoblasts contribute to the increased Runx2, Osx, ALP, Ocn, and Dkk1 levels.
SIRT6 interacts physically with Runx2/Osx and is recruited to their target promoters
SIRT6 is known to interact with a few transcription factors, such as NFκB, Hif-1α and c-Jun, and deacetylates histone H3K9 at their target gene promoters (3,17). Therefore, we asked whether SIRT6 physically binds to Runx2 or Osx and is recruited to their target gene promoters. Flag-tagged Runx2 or Osx were transiently expressed in NIH3T3 cells infected with SIRT6 WT retrovirally and IP assay was performed with anti-Flag or anti-SIRT6 antibodies. We confirmed the protein expression levels of SIRT6, Runx2, and Osx in these cells (Fig. 4A). Next, western blot analysis of the immunoprecipitates revealed that SIRT6 associates with Runx2 and Osx (Fig. 4B). We examined whether SIRT6 is recruited to the promoters of Runx2 or Osx target genes. SIRT6 was actively recruited to the indicated promoters in SIRT6 WT overexpressing osteoblast precursor MC3T3-E1 cells at 1h after BMP-2 stimulation (Fig. 4C). Endogenous SIRT6 was also recruited to the indicated promoters by BMP-2 treatment after 24h in primary osteoblasts (Fig. 4D). Overexpressed-SIRT6 WT and endogenous SIRT6 were also recruited to the promoter of Opg, which is not a target for Runx2 and Osx, (Fig. 4C,D), suggesting that SIRT6 may be recruited to the Opg promoter with other transcription factors. Thus, SIRT6 is recruited to chromatin at the promoters of Runx2 and Osx target genes through its physical interaction with these transcription factors.
SIRT6 deficiency results in histone H3K9 hyperacetylation on the promoters of Runx2 and Osx target genes
SIRT6 is a specific histone H3K9 deacetylase on the promoters of NFκB, Hif1-α and c-Jun target genes (3,17). In the context of gene expression mechanisms, histone H3K9 acetylation has been implicated in transcriptional activation, whereas histone H3K9 deacetylation has been correlated with transcriptional repression (18). Therefore, we asked whether SIRT6 deficiency culminates in histone H3K9 hyperacetylation on the promoters of Runx2 or Osx target genes and Opg. First, we found strongly enhanced global histone H3K9 acetylation in SIRT6−/− osteoblasts compared to control cells and the effect was drastically diminished by forced expression of SIRT6 WT (Fig. 5A). Consistent with the finding, ChiP analysis revealed that SIRT6 deficiency produces the hyperacetylation of histone H3K9 on the indicated promoters at 3h after BMP-2 stimulation (Fig. 5B), suggesting that SIRT6 directly controls the expression levels of target genes of Runx2 and Osx by deacetylating histone H3K9 at their promoters.
We demonstrated that Dkk1 protein levels are strongly enhanced during osteoblastogenesis in SIRT6 deficiency in response to BMP-2 (Fig. 3E) because Dkk1 promoter is hyperacetylated (Fig. 5B) and potently increased Runx2 and Osx proteins strikingly boost Dkk1 levels (Fig. 3H,I). To further demonstrate the relationship between SIRT6 function and Dkk1 production, MC3T3-E1 cells with or without SIRT6 WT overexpression were transiently transfected with Runx2 or Osx and treated with BMP-2 for 24 h. As expected, Dkk1 levels were strongly stimulated in the cells transfected with Runx2 or Osx compared to control cells and the effects were completely inhibited by the forced expression of SIRT6 WT (Fig. 5C). Next, SIRT6−/− osteoblasts were retrovirally infected with SIRT6 WT, and we asked whether SIRT6 WT overexpression attenuates Dkk1 levels. Western blot analysis showed that SIRT6 WT overexpression in SIRT6−/− osteoblasts dramatically diminished Dkk1 protein levels in response to BMP-2 (Fig. 5D), indicating that SIRT6−/− osteoblast causes up-regulation of Dkk1 production that results in impaired osteoblastic development and mineralization. In addition, forced expression of SIRT6 WT dramatically diminished excessively expressed Runx2, Osx, and Opg protein levels in SIRT6−/− osteoblasts with BMP-2 stimulation (Fig. 5D). Moreover, SIRT6 overexpression in SIRT6−/− osteoblasts significantly rescued osteoblastic mineralization compared to control (Fig. 5E), indicating that SIRT6 plays a critical role in osteoblastogenesis as important intrinsic factor.
Elevated Dkk1 and Opg production in SIRT6−/− osteoblasts
We demonstrated excessively expressed Dkk1 and Opg protein levels during osteoblastogenesis in SIRT6 deficiency (Fig. 3E). Therefore, we measured circulating Dkk1 and Opg levels in mice and these levels in culture media. Serum Dkk1 levels were significantly elevated in SIRT6−/− mice (Fig. 6A); however, serum Opg levels were not different between control and SIRT6−/− mice (data not shown). In culture media, the levels of Dkk1 and Opg secreted from SIRT6−/− osteoblasts were significantly increased compared to control (Fig. 6B,C). Next, we asked whether the increased Dkk1 production levels observed in SIRT6−/− osteoblasts affect the ability of the cells to respond to Wnt signaling. Activated Wnt signaling stimulates the nuclear accumulation of β-catenin in osteoblasts (19). Therefore, we confirmed whether the nuclear accumulation of β-catenin is diminished by conditioned media harvested from SIRT6−/− osteoblasts culture media with BMP-2 treatment. Western blot analysis showed the nuclear shift of β-catenin stimulated by Wnt10b was stimulated in osteoblasts (Fig. 6D). However, the effect was blocked by conditioned media (Fig. 6D). Moreover, we conformed whether the conditioned media attenuates NFATc1 levels in splenocytes in response to RANKL. RANKL stimulated NFATc1 expression in the cells; however, the effect was inhibited by the conditioned media (Fig. 6E). These results indicate that locally increased Dkk1 and Opg secreted from SIRT6−/− osteoblasts may impact bone remodeling in SIRT6−/− mice.
Discussion
Our results are consistent with prior reports (4) that SIRT6−/− mice have a progeroid degenerative syndrome including skeletal osteopenia with a 30 % reduction in bone mineral density compared to control mice. Recently, growth plates of SIRT6−/− mice were investigated revealing impaired chondrocyte proliferation and differentiation. These data indicated that SIRT6 positively regulates cartilage development (5). However, histomorphometric analysis of bone and the bone cell biology of SIRT6−/− mice has not been performed. Therefore, we analyzed the bone tissues of SIRT6−/− mice in detail. We found that SIRT6−/− mice have low-turnover osteopenia caused by impaired osteoblastic and osteoclastic development and function (Fig. 1). The low-turnover osteopenia of SIRT6−/− mice is histomorphometrically similar to age-related bone loss. Thus, age-related reduction of SIRT6 levels or activity may be implicated in age-related osteopenia and/or osteoporosis.
It is well-known that the activities of specific transcription factors at distinct time points are indispensable in osteoblastic development (9), and the critical osteoblastogenesis-related transcription factors (OB-TFs) have been identified (9). In other words, the functions of OB-TFs recruited to specific cis-acting elements in their target promoters have been well-investigated. However, the role of HDACs, which directly impact chromatin structure and transcription factor activity during osteoblastogenesis remain poorly understood. Several HDACs have been implicated in regulating osteoblastogenesis (20). However, the role of HDAC III SIRT6 during osteoblastic development was unknown until the data presented here. SIRT6 physically interacts with Runx2/Osx, and SIRT6 directly regulates their expression levels during osteoblastogenesis through histone H3K9 deacetylation at chromatin. Runx2 and Osx protein levels were significantly boosted in SIRT6−/− osteoblasts (Fig. 3E) through SIRT6 deficiency producing hyperacetylation of histone H3K9 on the promoters of Runx2 and Osx (Fig. 5B). This suggested that SIRT6 is a specific histone H3K9 deacetylase of the promoters of Runx2 and Osx during osteoblastogenesis. Our results are in agreement with previous reports (12) that excessive expression of Runx2 or Osx are negative factors for osteoblastogenesis because SIRT6−/− osteoblasts had dramatically diminished osteoblastic mineralization (Fig. 2A.B). However, the impaired mineralization was significantly rescued by SIRT6 overexpression (Fig. 5E), suggesting that the moderate expression levels of Runx2 and Osx controlled by SIRT6 may be indispensable for osteoblastogenesis. Thus, SIRT6 regulates the expression levels of Runx2/Osx and their target genes during osteoblastogenesis.
Other important discoveries in our studies were that Dkk1 and Opg protein levels are remarkably elevated in SIRT6−/− osteoblasts (Fig. 3E) due to hyperacetylation of the Dkk1 and Opg promoters (Fig. 5B). The amount of Dkk1 and Opg secreted from SIRT6−/− osteoblasts were significantly elevated (Fig. 6B,C). On these grounds, elevated Dkk1 and Opg production (which are secreted factors) by SIRT6−/− osteoblasts synergize with Runx2 and Osx overexpression to produce low-turnover osteopenia caused by impaired osteoblastogenesis and osteoclastogenesis and decreased osteoblastic bone formation and osteoclastic bone resorption (Fig. 6F). These data raise the question whether SIRT6 is an intrinsic factor for osteoclastogenesis. Osteoclastogenesis in SIRT6 deficient splenocytes treated with RANKL was remarkably impaired in this osteoblast free system (Fig. 2F,G,H), which is to say that SIRT6 seems to act as an intrinsic factor for osteoclastogenesis. However, forced expression of SIRT6 in SIRT6−/− splenocytes failed to rescue the impaired osteoclastogenesis in the osteoblast free system (data not shown). In addition, osteoclast formation was impaired when SIRT6−/− osteoblasts were used regardless of genotypes of splenocytes in coculture assay (Fig. 2I). Thus, these results imply that SIRT6−/− osteoblasts may negatively regulate osteoclastogenesis because of increased Opg secretion from SIRT6−/− osteoblasts in SIRT6−/− mice, although further studies are needed.
Another possibility to explaining osteopenia in SIRT6−/− mice is decreased serum IGF-1 levels (4), because it is well-known that IGF-1 plays critical roles for bone developmental growth, osteoblastic bone formation, and osteoclastic bone resorption (21). Since systemic IGF-1 is mainly secreted from the liver with stimulation of growth hormone (GH) secreted from the pituitary grand (22), we analyzed the expression of GH in the brain and IGF-1 in the liver at RNA levels and found decreased expression levels in SIRT6−/− mice (data not shown). Although global IGF-1-deficient (IGF-1−/−) mice reveal impaired bone growth (23) and osteoblast-specific IGF-1 receptor-deficient mice produce completely blocked osteoblastic bone formation (24), the liver-specific IGF-1−/− (liver-IGF-1−/−) mice show no significant impact on bone growth in spite of circulating IGF-1 levels, which are significantly decreased in liver-IGF-1−/− mice (25). These data suggested that the autocrine/paracrine functions of IGF-1 produced by osteoblasts or osteocytes in bone tissues may play a more critical role than systemic IGF-1 in bone development and metabolism. However, the expression of IGF-1 at RNA levels in bone tissues isolated from SIRT6−/− mice were significantly elevated compared to WT mice (data not shown). Moreover, the body size of SIRT6−/− mice was smaller than that of WT mice at birth in spite of systemic IGF-1 levels being reduced approximately 2 weeks after birth in SIRT6−/− mice (4), suggesting that decreased systemic IGF-1 levels and locally increased IGF-1 levels in bone tissues seem to not be the direct cause of osteopenia with a low bone turnover in SIRT6−/− mice. Thus, SIRT6 may play critical roles in bone metabolism as a critical intrinsic factor in bone cells. An essential question arises as to whether the aging-like phenotype of osteopenia in SIRT6−/− mice is caused by degenerative defects (aging) or bone developmental defects, as we have analyzed the bone tissues of SIRT6−/− mice at 3 weeks old and this age is still during the bone growth period in mice. Due to the short lifespan of SIRT6−/− mice, we were unable to conclusively answer this question, indicating that further studies are needed.
In this study, we showed that structural changes of chromatin through loss of SIRT6 leads to functional changes that are important determinants of gene expression related to osteoblastogenesis. These results provide an in depth molecular understanding of the regulation of bone remodeling-associated with osteoblast functions. In addition, since SIRT6−/− mice reveal characteristic features of low-turnover osteopenia in humans, the cellular and molecular mechanisms of SIRT6 in bone turnover could be potential therapeutic targets for age-related bone loss.
Highlights.
The underlying molecular mechanisms of SIRT6 in bone metabolism are unclear.
SIRT6 deficiency in mice produces low-turnover osteopenia.
SIRT6 interacts with Runx2 and Osx.
SIRT6-null osteoblasts produce the excessive expression of Dkk1 and Opg.
SIRT6 positively regulates osteoblastogenesis and osteoclastogenesis.
Acknowledgments
This work was supported by the National Institutes of Health grants DK070790 and DK089137 (K.A.H.).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author contribution
Contribution: The original hypothesis was formulated by T.S. and most of the experiments were performed by T.S. O.A. performed uCT analysis. H.H.M. conducted bone histomorphometric analysis. The manuscript was written by T.S. and K.A.H.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–45. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci. 2014;39(2):72–81. doi: 10.1016/j.tibs.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tennen RI, Chua KF. Chromatin regulation and genome maintenance by mammalian SIRT6. Trends Biochem Sci. 2011;36(1):39–46. doi: 10.1016/j.tibs.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–29. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Piao J, Tsuji K, Ochi H, Iwata M, Koga D, Okawa A, et al. Sirt6 regulates postnatal growth plate differentiation and proliferation via Ihh signaling. Sci Rep. 2013;23(3):3022. doi: 10.1038/srep03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malluche HH, Sherman D, Meyer W, Massry SG. A new semiautomatic method for quantitative static and dynamic bone histology. Calcif Tissue Int. 1982;34(5):439–48. doi: 10.1007/BF02411282. [DOI] [PubMed] [Google Scholar]
- 7.Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood. 2011;117(13):3648–57. doi: 10.1182/blood-2010-10-311415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbuto R, Mitchell J. Regulation of the osterix (Osx, Sp7) promoter by osterix and its inhibition by parathyroid hormone. Mol Endocrinol. 2013;51(1):99–108. doi: 10.1530/JME-12-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min L, Ji Y, Bakiri L, Qiu Z, Cen J, Chen X, et al. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14(11):1203–11. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida CA, Komori H, Maruyama Z, Miyazaki T, Kawasaki K, Furuichi T, et al. SP7 inhibits osteoblast differentiation at a late stage in mice. PLoS One. 2012;7(3):e32364. doi: 10.1371/journal.pone.0032364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 14.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 15.Ross FP, Teitelbaum SL. αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 16.Guerrini MM, Takayanagi H. The immune system, bone and RANKL. Arch Biochem Biophys. 2014;561:118–23. doi: 10.1016/j.abb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18(11):1643–50. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15(2):172–83. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 19.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 20.McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in skeletal development and bone mass maintenance. Gene. 2011;474(1–2):1–11. doi: 10.1016/j.gene.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crane JL, Cao X. Function of matrix IGF-1 in coupling bone resorption and formation. J Mol Med. 2014;92(2):107–15. doi: 10.1007/s00109-013-1084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. doi: 10.1016/j.exger.2014.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, et al. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144(3):929–36. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–12. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 25.Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96(12):7088–92. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]