Abstract
Connexin43 (Cx43) forms gap junction channels and hemichannels that allow the communication among osteocytes, osteoblasts, and osteoclasts. Cx43 carboxy-terminal (CT) domain regulates channel opening and intracellular signaling by acting as a scaffold for structural and signaling proteins. To determine the role of Cx43 CT domain in bone, mice in which one allele of full length Cx43 was replaced by a mutant lacking the CT domain (Cx43ΔCT/fl) were studied. Cx43ΔCT/fl mice exhibit lower cancellous bone volume but higher cortical thickness than Cx43fl/fl controls, indicating that the CT domain is involved in normal cancellous bone gain but opposes cortical bone acquisition. Further, Cx43ΔCT is able to exert the functions of full length osteocytic Cx43 on cortical bone geometry and mechanical properties, demonstrating that domains other than the CT are responsible for Cx43 function in cortical bone. In addition, parathyroid hormone (PTH) failed to increase endocortical bone formation or energy to failure, a mechanical property that indicates resistance to fracture, in cortical bone in Cx43ΔCT mice with or without osteocytic full length Cx43. On the other hand, bone mass and bone formation markers were increased by the hormone in all mouse models, regardless of whether full length or Cx43ΔCT were or not expressed. We conclude that Cx43 CT domain is involved in proper bone acquisition; and that Cx43 expression in osteocytes is dispensable for some but not all PTH anabolic actions.
Keywords: bone, osteocyte, connexin 43, carboxy-terminal domain, PTH
Introduction
Connexins are ubiquitously expressed proteins that form channels on cell membranes. These molecules are structurally conserved and exhibit four transmembrane domains and the amino- and the carboxy-terminal (CT) domains facing the cytoplasm. The transmembrane domains form the pore of the channel and dictate the size and charge of the molecules that can pass through [1]; the N-terminal domain is part of the pore and controls voltage-induced gating [2]; and the CT domain modulates channel opening through changes in its phosphorylation status [3]. Further, the CT domain interacts with intracellular structural and catalytic proteins and through this scaffolding function it regulates subcellular localization of newly synthesized connexin proteins and their rate of degradation [3] as well as cell behavior through changes in gene expression and modulation of pro-survival signaling pathways [4, 5]. Even though connexins exhibit high homology, the CT domains vary significantly among different members of the family, both in amino acid sequence and in length, giving distinct properties to each connexin [6].
Connexin 43 (Cx43), one of the most studied members of the connexin family, is highly expressed in bone cells [7–9]. Its expression is required for full differentiation and function of cells of both osteoblastic and osteoclastic lineage and for the maintenance of osteocyte viability [10]. In osteoblasts, deletion of Cx43 results in reduced levels of the transcription factor Runx2 and downstream osteoblastic genes including osteocalcin, and impaired mineralization in vitro and in vivo [11–15]. Another function of Cx43 in osteoblastic cells is protection from apoptosis, as demonstrated by accelerated spontaneous death of osteocytic cells silenced for Cx43 and high prevalence of osteocyte apoptosis in cortical bone of mice lacking Cx43 in osteoblasts and osteocytes or only in osteocytes [16–18]. Moreover, osteoblastic or osteocytic cells silenced for Cx43 are not protected by bisphosphonates or parathyroid hormone (PTH) from apoptosis induced by glucocorticoids or etoposide [16, 19]. In the case of bisphosphonates, this lack of protection from glucocorticoid-induced apoptosis also occurs in vivo, in mice lacking Cx43 in osteoblasts and osteocytes [16]. The requirement of Cx43 for these anti-apoptotic actions is conferred by the cytoplasmic CT domain of the protein, as demonstrated by the inability of bisphosphonates/PTH to prevent apoptosis in cells expressing a truncated Cx43 mutant lacking the CT domain [19, 20]. The fact that preservation of osteoblast viability contributes at least in part to the anabolic effect of PTH in cancellous bone [21] and that mice lacking Cx43 in 2.3kb-col1a1-expressing osteoblastic cells exhibit a deficient response to intermittent PTH administration [12] suggests that Cx43 might be a central player for PTH action. However, the role of Cx43 expression in particular in osteocytes as well as the CT domain for the anabolic effect of PTH on the skeleton has not been investigated.
Deletion of Cx43 from osteoblastic cells at different stages of differentiation leads to a reduction in cancellous bone, which is progressively less pronounced as the gene is deleted in more differentiated cells of the lineage, with no effect when the gene is deleted from osteocytes [10]. On the other hand, cortical bone is similarly affected by deleting Cx43 at any stage of osteoblast differentiation. The resulting shared phenotype is characterized by increased endocortical bone resorption and periosteal bone apposition leading to geometrical changes of the long bones, and reduced bone material strength. The fact that deletion of Cx43 from osteocytes recapitulates the phenotype and that expression of the gene in less mature cells is not sufficient to prevent it suggests that osteocytic Cx43 is the one required to achieve proper bone geometry and strength in cortical bone. However, the domain that mediates the functions of Cx43 is not known. Knock-in mice in which the full length Cx43 is replaced by a truncated Cx43 mutant lacking the CT domain (named Cx43ΔCT) have been generated [22]. Expression of the truncated Cx43 has been demonstrated in these mice by Western blotting in epidermis, brain, and isolated astrocytes, indicating that the truncated protein is not unstable [22, 23]. Homozygous mice expressing two alleles of Cx43ΔCT die by dehydration soon after birth due to increased skin permeability. In contrast, mice expressing only one allele of the truncated gene have a less pronounced phenotype and reach adulthood [24, 25]. However, their skeletal phenotype has not been investigated.
We addressed in the current study three outstanding questions regarding the role of the scaffolding function of Cx43 mediated by its CT domain in bone: (1) whether the Cx43 CT domain dictates the functions of the entire Cx43 protein in cancellous and cortical bone; (2) whether the truncated Cx43ΔCT mutant lacking the CT domain is able to exert the functions of the full length Cx43 in osteocytes; and (3) whether expression of either full length Cx43 or Cx43ΔCT in osteocytes is required for the effects of intermittent PTH administration in bone. We report that the Cx43 CT domain is involved in normal bone acquisition and bone formation in cancellous bone, but it is dispensable for cortical bone accrual. Moreover, truncated Cx43ΔCT is able to exert the functions of osteocytic full length Cx43 in cortical bone geometry, suggesting that the CT domain is not required. We also found that lack of full length osteocytic Cx43 or absence of the CT domain did not alter the actions of PTH on cancellous bone; however, the effects of PTH on cortical bone are impaired in the absence of Cx43 in osteocytes, and are not present even when the truncated Cx43 is expressed in osteocytes. These findings reveal that the scaffolding Cx43 CT domain mediates Cx43 action in cancellous bone and the response to PTH on the endocortical bone surface.
Materials and Methods
Animals and treatment
Knock-in mice in which one allele of the endogenous Cx43 was replaced by a mutant Cx43K258stop allele, which consists of Cx43 molecule truncated at amino acid 258 in the CT domain (abbreviated as Cx43ΔCT) in all tissues, were generated by K. Willecke (Universität Bonn, Bonn, Germany) [22] and crossed with mice harboring the “floxed” Cx43 full length (abbreviated as Cx43fl/fl) [26] in order to obtain Cx43ΔCT/fl mice. These mice were then mated with Cx43fl/fl mice expressing Cre recombinase under control of a DNA fragment containing 8 kb of the murine dentin matrix protein 1 promoter (abbreviated as Cx43fl/fl;DMP1-8kb-Cre), which lacks Cx43 in osteocytes [18]. From this breeding, 4 genotypes were generate, as follows: Cx43fl/fl, Cx43ΔCT/fl, Cx43fl/fl;DMP1-8kb-Cre, and Cx43ΔCT/fl;DMP1-8kb-Cre. A schematic representation of the mice indicating the expression of full length and truncated Cx43 is shown in Fig. 1A. All animal lines were developed in a C57BL/6 background and littermates were used as controls. Mice were fed a regular diet and water ad libitum and maintained on a 12 h light/dark cycle. Four-month-old female mice were injected daily for 14 days with either vehicle (0.9% saline, 10 μM β-mercaptoethanol and 0.01% acid acetic) or 100 ng/g of human PTH (1–34) (Bachem California Inc., Torrance, CA, USA) diluted in vehicle solution. For dynamic histomorphometric studies, mice received intraperitoneal injections of calcein (20 mg/kg, Sigma Chemical, St. Louis, MO, USA) and alizarin red (20 mg/kg, Sigma) 7 and 2 days before sacrifice, as published [27]. The protocols involving mice were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine.
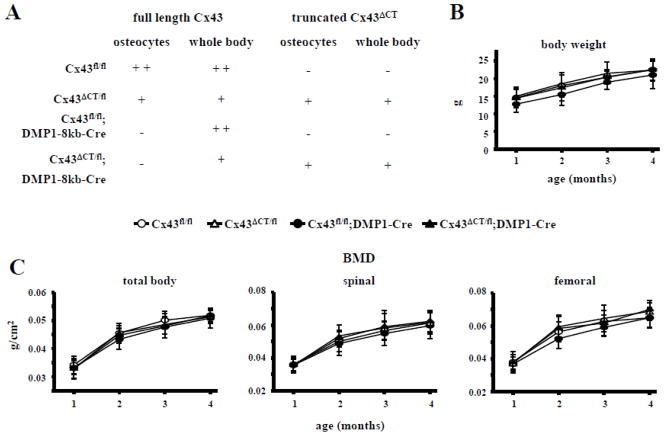
Fig. 1. Body weight and BMD are not affected by global absence of the Cx43 CT domain.
(A) Cx43ΔCT/fl were mated with Cx43fl/fl;DMP1-8kb-Cre mice and 4 different genotypes were obtained: 1) Cx43fl/fl mice have both alleles of Cx43 gene flanked by LoxP sites and are similar to wild-type mice, 2) Cx43ΔCT/fl mice have one allele of Cx43 replaced by a Cx43 truncated at amino acid 258, which can generate functional channel but lacks the scaffolding ability, and the other allele is the full length floxed Cx43, 3) Cx43fl/fl;DMP1-8kb-Cre mice have both alleles flanked by LoxP sites and are recognized and excised by the Cre recombinase under control of a DNA fragment containing 8kb of the murine dentin matrix protein 1 promoter (DMP1), thus lacking Cx43 protein in osteocytes, and 4) Cx43ΔCT/fl;DMP1-Cre mice, expressing ubiquitously Cx43ΔCT and lacking osteocytic full length Cx43. (B) Body weight from mice of all genotypes was measured monthly from 1 to 4 months of age. Symbols represent mean ± s.d., n=12–28. (C) Total body, spinal, and femoral BMD was measured monthly from 1 to 4 months of age by dual-DXA. Symbols represent mean ± s.d., n=12–28.
Bone mineral density and micro-computed tomography analysis
Bone mineral density (BMD) was measured monthly by dual x-ray absorptiometry (DXA) using a PIXImus densitometer (G.E. Medical Systems, Lunar Division, Madison, WI, USA). BMD measurements included total BMD (excluding the head and tail), entire femur (femoral BMD) and L1–L6 vertebra (spinal BMD) [27]. Calibration was performed before scanning with a standard phantom, as recommended by the manufacturer. For micro-computed tomography (μCT) analysis, femora and L4 vertebrae from mice at 4.5 months of age were dissected, cleaned of soft tissue and frozen at −20°C until imaging, at 6 μm pixel resolution on a Skyscan 1172 (SkyScan, Kontich, Belgium) following standard procedures [28].
Serum markers of bone turnover
Blood was collected by cheek bleeding after approximately 6 hours of fasting. Plasma was collected, aliquoted, and stored at −80°C until used. Plasma osteocalcin (OCN) (Biomedical Technologies Inc., Soughton, MA, USA) and C-telopeptide fragments (CTX) (RatLaps, Immunodiagnostic Systems Inc., Fountain Hill, AZ, USA) were measured as published [18, 27].
Bone histomorphometry
Vertebrae (L1–L3) were dissected and fixed in 10% neutral buffered formalin, and embedded in methyl methacrylate as published [27]. Static historphometric analysis was performed on von Kossa and TRAP stained-sections, for osteoblasts and osteoclast, respectively. Dynamic histomorphometric measurements were performed in unstained bone sections of femoral midshaft [27]. Histomorphometric analysis was performed using OsteoMeasure high resolution digital video system (OsteoMetrics Inc., Decatur, GA, USA). The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (ASBMR) [29].
Apoptosis
Osteocyte and osteoblast apoptosis was detected in vertebral bone sections by TdT-mediated dUTP nick-end labeling (TUNEL) using a modified version of a commercial kit (EMD Millipore, Billerica, MA) in sections counterstained with 2% methyl green, as previously described [18].
RNA extraction and quantitative PCR (qPCR)
RNA was purified from lumbar vertebrae using TRIzol (Invitrogen, Grand Island, NY). qPCR was performed as described [30], using the house-keeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the ΔCt method [30]. Primers and probes were commercially available (Applied Biosystems, Foster City, CA) or were designed using the Assay Design Center (Roche Applied Science, Indianapolis, IN).
Biomechanical testing
Three-point bending testing of the femoral midshaft was performed following previously published protocols [27]. Briefly, bones were thawed to room temperature, hydrated in 0.9% saline, and loaded to failure at 2 mm/min with force versus displacement data collected at 10 Hz using a servo-hydraulic test system (TestResources Inc., Shakopee, MN, USA). Femora were loaded to failure in an anterior-posterior direction with the upper contact area at the mid-diaphysis (50% total bone length) and the bottom contact points centered around this point and separated by 8 mm. Structural mechanical properties, ultimate load, stiffness, and energy to failure were determined from the load-deformation curves using standard definitions while material-level estimations of ultimate stress, modulus, and toughness were calculated using standard equations. Cross-sectional moment of inertia and anterior-posterior diameter were determined by μCT and were used to calculate material-level properties, as previously described [31].
Statistical analysis
Data were analyzed by using SigmaPlot (Systat Software Inc., San Jose, CA). Differences were considered significant for p<0.05. All values are reported as the mean ± standard deviation. Differences were evaluated by two-way ANOVA, with post-hoc analysis using Student-Newman-Keuls Method.
Results
Global absence of Cx43 CT domain decreases cancellous bone, independently of whether or not osteocytes express full length Cx43
As previously reported [18], deletion of Cx43 from osteocytes in Cx43fl/fl; DMP1-8kb-Cre did not alter body weight (Fig. 1B) or BMD at any site (Fig. 1C) between 1 and 4 months of age. Similarly, ubiquitous expression of the truncated Cx43 lacking the CT domain did not alter weight or BMD up to 4 months of age (Figs. 1B and C).
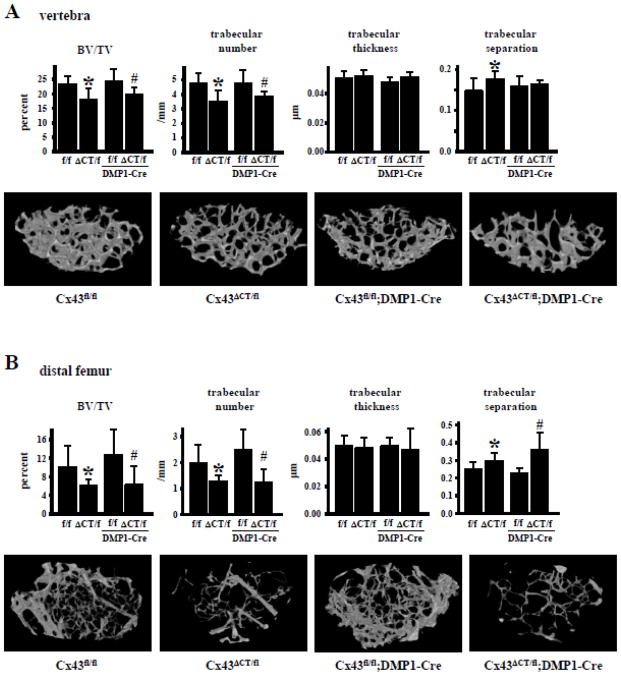
Analysis of bone microarchitecture of 4.5-month-old mice by μCT revealed that Cx43ΔCT/fl mice exhibit 30% lower cancellous (BV/TV) and trabecular number (Tb.N), and higher trabecular separation (Tb.Sp) in L4 vertebrae compared to Cx43fl/fl mice, without changes in trabecular thickness (Tb.Th) (Fig. 2A). A similar phenotype was found in the cancellous bone of the distal femora; with a more profound decrease in BV/TV and trabecular number in Cx43ΔCT/fl mice, compared to Cx43fl/fl littermates (Fig. 2B). Furthermore, BV/TV and trabecular number were lower in mice expressing only the truncated mutant and lacking the full length Cx43 in osteocytes (Cx43ΔCT/fl;DMP1-8kb-Cre mice) compared to mice lacking Cx43 from osteocytes (Cx43fl/fl;DMP1-8kb-Cre mice) in both vertebra and distal femur, whereas trabecular separation was only increased in the distal femur (Fig. 2). This evidence suggests that expression of Cx43 and its CT domain of Cx43 in osteocytes is not required for achieving proper cancellous bone architecture, as mice lacking Cx43 from osteocytes, but not mice expressing the Cx43ΔCT exhibit normal cancellous bone volume. On the other hand, expression of Cx43ΔCT or deletion of full length Cx43 from osteocytes did not alter tissue material density in the cancellous bone of the vertebrae, or in cancellous and cortical bone of the femur (Table 1).
Fig. 2. Absence of CT domain of Cx43 results in low cancellous bone volume.
Cancellous bone microarchitecture was assessed in Cx43fl/fl, Cx43ΔCT/fl, Cx43fl/fl;DMP1-8kb-Cre, and Cx43ΔCT/fl;DMP1-8kb-Cre mice at 4.5 months of age by μCT in (A) L4 vertebrae and (B) distal femora. Representative reconstructed 3D μCT images are shown. Bars represent mean ± s.d., n=7–12. *p<0.05 versus Cx43fl/fl mice, and #p<0.05 versus Cx43fl/fl;DMP1-8kb-Cre mice by two-way ANOVA.
Table 1.
Global deletion of Cx43 CT domain or removal of the full length Cx43 from osteocytes does not affect bone material density in cancellous and cortical bone.
| Material density (pixels) | Cx43fl/fl | Cx43ΔCT/fl | Cx43fl/fl; DMP1-8kb-Cre | Cx43ΔCT/fl; DMP1- 8kb-Cre |
|---|---|---|---|---|
| cancellous bone lumbar vertebra | 129.04 ± 9.50 | 135.50 ± 3.05 | 130.92 ± 1.65 | 134.08 ± 2.17 |
| cancellous bone distal femur | 128.93 ± 3.78 | 127.81 ± 4.34 | 127.81 ± 5.10 | 128.37 ± 6.00 |
| cortical bone femoral mid- diaphysis | 185.11 ± 2.59 | 184.85 ± 2.93 | 181.56 ± 4.04 | 180.25 ± 7.44 |
n=7–12. No significant differences were found among groups.
Absence of Cx43 CT domain leads to reduced osteoblast numbers
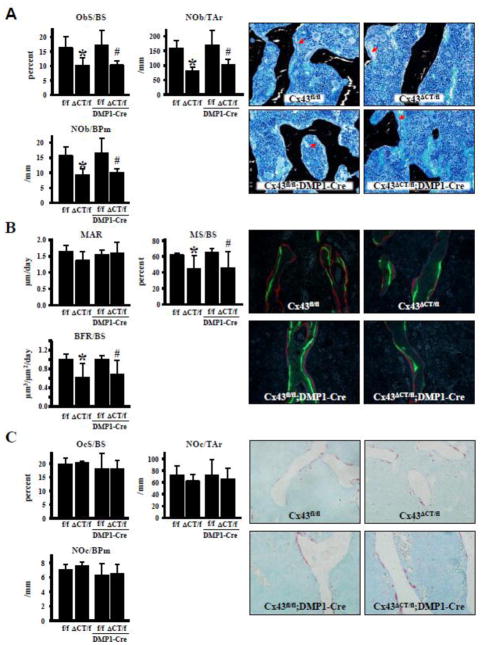
The decrease in bone volume in cancellous bone is accompanied by a decrease in osteoblast number and surface in Cx43ΔCT/fl (Fig. 3A). Deletion of the full allele of Cx43 from osteocytes in Cx43ΔCT/fl;DMP1-8kb-Cre mice did not further affect osteoblast number and surface. Consistent with the changes in osteoblast number, mineralizing surface was significantly decreased in Cx43ΔCT/fl and Cx43ΔCT/fl;DMP1-Cre mice, resulting in reduced bone formation rate, even though mineral apposition rate was not changed (Fig. 3B). Osteoclast surface and number were not affected by the truncated Cx43 (Fig. 3C).
Fig. 3. Deletion of Cx43 CT domain leads to decreased osteoblasts and bone formation, without affecting osteoclasts.
Cell surface and number were measured in bone sections from lumbar vertebra obtained from 4.5 month-old-mice. (A) Osteoblasts were identified in bone sections stained by von Kossa/McNeal. Surface covered by osteoblasts (ObS/BS), number of osteoblasts per tissue area (NOb/TAr), and per bone surface (NOb/BPm) are reported. Representative images of von Kossa/McNeal stained bone sections are shown. Red arrows point at rows of osteoblasts. (B) Mineral apposition rate (MAR), mineralizing surface (MS/BS) and bone formation rate (BFR) were measured in unstained vertebral bone sections. Representative fluorescent images are shown. (C) Osteoclasts were identified in vertebral bone sections stained for TRAPase/Toluidine blue. Surface covered by osteoclasts (OcS/BS), osteoclast number per tissue area (NOc/TAr), and per bone surface (NOc/BPm) are reported. Representative images of bone sections stained for TRAP (red) are shown. Bars represent mean ± s.d., n=4. *p<0.05 versus Cx43fl/fl mice, and #p<0.05 versus Cx43fl/fl;DMP1-8kb-Cre mice by two-way ANOVA.
Taken together, these pieces of evidence suggest that the decrease in bone volume is due to defective osteoblastic bone formation, rather than to an increase in osteoclastic bone resorption.
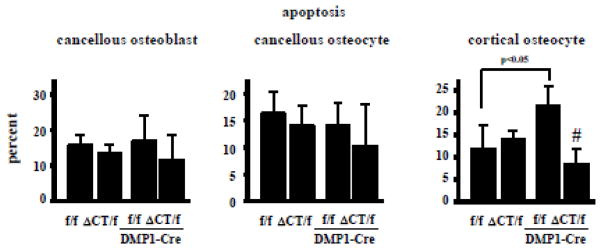
Expression of the truncated Cx43 did not alter the prevalence of apoptosis of cancellous osteoblasts and osteocytes, or of cortical osteocytes (Fig. 4). As found before, Cx43fl/fl;DMP1-8kb-Cre exhibit increased cortical osteocyte apoptosis without changes in the prevalence of apoptosis of cancellous osteocytes and osteoblasts [16, 18]. Cx43 lacking the C-terminus tail was able to compensate for the absence of osteocytic full-length Cx43 maintaining cortical osteocyte viability, suggesting that Cx43 C-terminus domain is not required for the survival of cortical osteocytes.
Fig. 4. Deletion of Cx43 CT reverses the increased cortical osteocyte apoptosis in mice lacking osteocytic Cx43.
Apoptotic (TUNEL positive) cancellous osteoblasts and cancellous and cortical osteocytes were scored in vertebral bone. Bars represent mean ± s.d., n=4–9. #p<0.05 versus Cx43fl/fl;DMP1-8kb-Cre mice by two-way ANOVA.
Cortical bone geometry and strength are preserved in mice expressing Cx43 lacking the CT domain in the absence osteocytic Cx43
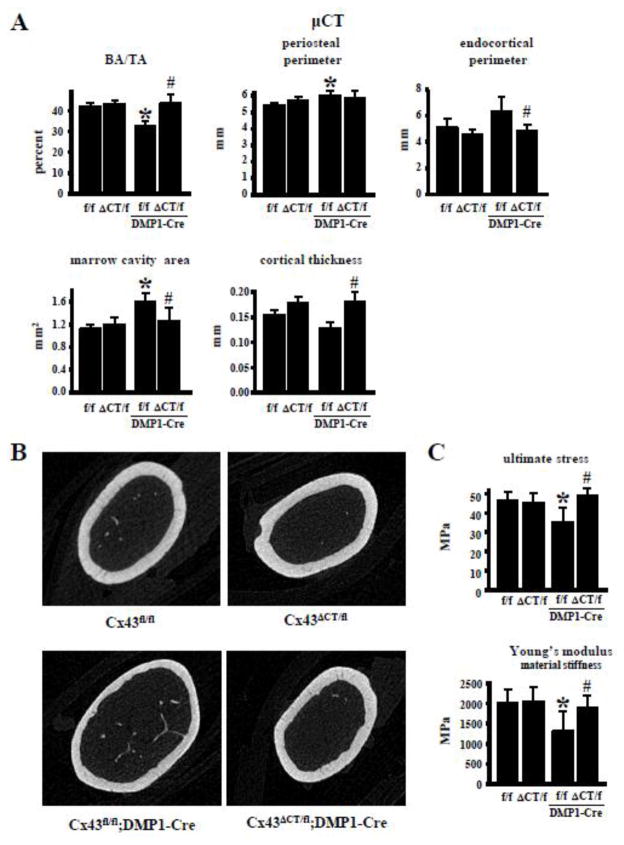
Structural and biomechanical studies of the cortical bone of the femur shows that global expression of the truncated Cx43 has no effect on its own, except for an increase in mechanical stiffness, and ultimate and yield load compared to Cx43fl/fl mice (Figs. 5 and 6A). As found before [18], deletion of Cx43 from osteocytes results in reduced cortical bone area, and increased periosteal perimeter and marrow cavity area in the femoral midshaft (Fig. 5A). In addition, Cx43fl/fl;DMP1-8kb-Cre mice exhibit decreased material properties (Fig. 5C), and no change in mechanical properties (Fig. 6) at this site. Some of the changes in bone geometry and mechanical properties found in mice lacking Cx43 in osteocytes were absent in mice expressing the Cx43ΔCT mutant (Figs. 5 and 6). In particular, the decrease in cortical bone area/tissue area, ultimate stress and material stiffness (elastic modulus), and the increase in periosteal perimeter and marrow cavity area at the mid-diaphysis found in Cx43fl/fl;DMP1-Cre mice were not present in mice expressing only the truncated mutant of Cx43 in osteocytes (Cx43ΔCT/fl;DMP1-Cre mice). This suggests that the function of Cx43 CT is not required for proper cortical bone structure and mechanical function.
Fig. 5. Expression of truncated Cx43 is sufficient to maintain Cx43 function in cortical bone in mice lacking full length osteocytic Cx43.
(A) Bone microarchitecture was evaluated in the femoral midshaft by μCT. n=9–10. * p<0.05 versus Cx43fl/fl mice, and #p<0.05 versus Cx43fl/fl:DMP1-8kb-Cre mice by two-way ANOVA. (B) Representative 3D μCT images of the femoral mid-diaphysis are shown. (C) Biomechanical properties were measured in femoral bone by 3-point bending testing. Bars represent mean ± s.d. n=7–10. *p<0.05 versus Cx43fl/fl mice, and #p<0.05 versus Cx43fl/fl;DMP1-8kb-Cre mice by two-way ANOVA.
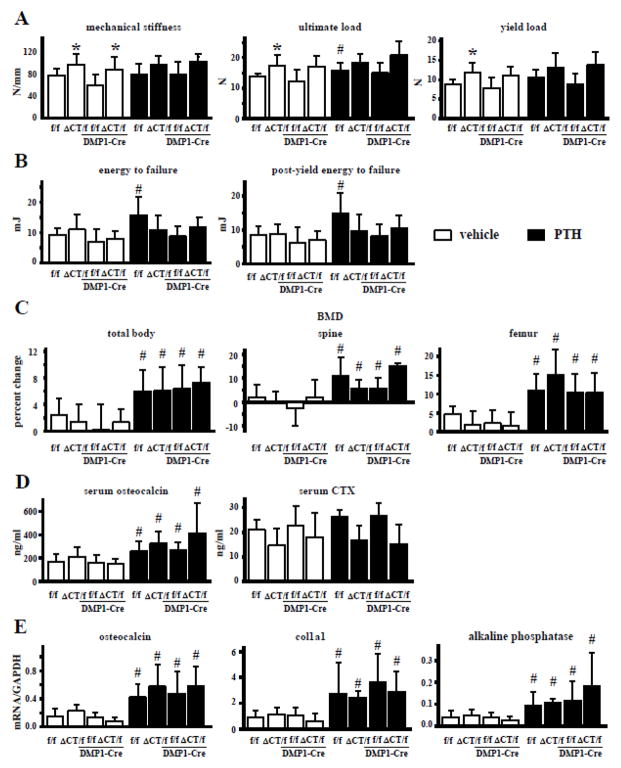
Fig. 6. Absence of Cx43 CT domain prevents the increase in resistance to fractures induced by intermittent PTH administration.
(A and B) 3-point bending analysis of cortical bone of the femoral mid-diaphysis. (A) Bone material properties were assessed at the femoral midshaft. n=5–13. (B) Energy to failure and post-energy to failure were measured in the femoral midshaft. n=5–11. (C) Total body, spinal, and femoral BMD were measured after intermittent PTH administration by DXA in Cx43fl/fl, Cx43ΔCT/fl, Cx43fl/fl;DMP1-8kb-Cre, and Cx43ΔCT/fl;DMP1-8kb-Cre mice. n=7–14 (D) Circulating levels for osteocalcin and CTX were measured by ELISA. n=7–14 (E) Expression of the indicated genes was measured by qPCR in whole lumbar vertebrae. Bars represent mean ± s.d. n=4–14. *p<0.05 versus Cx43fl/fl mice and Cx43fl/fl;DMP1-8kb-Cre for Cx43ΔCT/fl and Cx43ΔCT/fl;DMP1-8kb-Cre mice, respectively; and #p<0.05 versus corresponding vehicle-treated mice; by two-way ANOVA.
Expression of truncated Cx43 increased femoral mechanical properties in the presence and absence of full length Cx43 in the osteocytes (Fig. 6A). Stiffness was higher in Cx43ΔCT/fl and Cx43ΔCT/fl;DMP1-Cre mice compared to Cx43fl/fl and Cx43fl/fl;DMP1-Cre littermates, respectively. A similar tendency was observed for ultimate load and yield load, although in this case only the difference between Cx43ΔCT/fl and Cx43fl/fl mice, but not between Cx43ΔCT/fl;DMP1-Cre and Cx43fl/fl;DMP1-Cre mice reached significance (Fig. 6A). Energy to failure and post-yield energy to failure, measurements that indicate the resistance of bone to fracture, were not affected by expressing the truncated Cx43 molecule (Fig. 6B).
Intermittent PTH administration led to a small but significant increase in ultimate load in Cx43fl/fl mice (Fig. 6A). On the other hand, PTH increased energy to failure and post-yield energy to failure in Cx43fl/fl mice, but not in littermates expressing Cx43ΔCT and/or lacking full length Cx43 in osteocytes (Fig. 6B). This suggests that the presence of Cx43 CT domain in osteocytes is required for the improvements in mechanical properties induced by intermittent PTH administration.
Expression of full length Cx43 in osteocytes and the scaffolding CT domain of the protein are dispensable for the anabolic action of intermittent PTH administration
Intermittent PTH administration increased total body, spinal, and femoral BMD (Fig. 6C) and cancellous bone volume (Table 2) in the vertebra and femur.
Table 2.
Expression of Cx43 CT domain in osteocytes does not alter the effects of intermittent PTH administration in cancellous bone
| FEMUR | |||||
|---|---|---|---|---|---|
| Cx43fl/fl | Cx43ΔCT/fl | Cx43fl/fl; DMP1- 8kb-Cre | Cx43ΔCT/fl; DMP1-8kb-Cre | ||
| BV/TV (percent) | vehicle | 10.50 ± 3.42 | 5.84 ± 1.29* | 11.71 ± 3.80 | 5.22 ± 2.39§ |
| PTH | 17.23 ± 6.61# | 9.53 ± 3.14# | 26.67 ± 12.82# | 9.08 ± 4.91# | |
| VERTEBRA | |||||
| Cx43fl/fl | Cx43ΔCT/fl | Cx43fl/fl; DMP1- 8kb-Cre | Cx43ΔCT/fl; DMP1-8kb-Cre | ||
| BV/TV (percent) | vehicle | 23.23 ± 3.02 | 18.06 ± 3.86* | 24.35 ± 4.73 | 19.90 ± 2.25§ |
| PTH | 29.98 ± 3.45# | 24.20 ± 4.13# | 31.99 ± 5.39# | 25.02 ± 1.97# | |
| ObS/BS (percent) | vehicle | 16.27 ± 3.80 | 10.13 ± 2.63* | 17.04 ± 5.40 | 10.30 ± 1.48§ |
| PTH | 26.31 ± 0.92# | 22.43 ± 5.45# | 25.08 ± 3.26# | 20.65 ± 2.17# | |
| NOb/Tar (/mm) | vehicle | 159.09 ± 26.90 | 80.37 ± 13.31* | 169.54 ± 51.85 | 102.16 ± 19.34§ |
| PTH | 260.07 ± 69.64# | 215.13 ± 43.93# | 312.41 ± 65.91# | 218.81 ± 12.98# | |
| NOb/BPm (/mm) | vehicle | 15.64 ± 3.00 | 9.34 ± 1.9*4 | 16.53 ± 4.90 | 10.07 ± 1.30§ |
| PTH | 24.73 ± 3.25# | 22.30 ± 4.11# | 24.67 ± 2.62# | 21.23 ± 1.13# | |
| MAR (μm/day) | vehicle | 1.64 ± 0.19 | 1.37 ± 0.27 | 1.54 ± 0.15 | 1.60 ± 0.32 |
| PTH | 1.61 ± 0.15 | 2.02 ± 0.70 | 1.71 ± 0.20 | 1.80 ± 0.18 | |
| MS/BS (percent) | vehicle | 61.66 ± 2.57 | 44.15 ± 16.78* | 65.23 ± 4.87 | 45.64 ± 20.18§ |
| PTH | 67.10 ± 4.19# | 64.92 ± 11.23# | 72.30 ± 0.65# | 65.42 ± 5.36# | |
| BFR/BS (μm3/μm2/day) | vehicle | 0.97 ± 0.10 | 0.62 ± 0.30* | 1.00 ± 0.09 | 0.69 ± 0.30§ |
| PTH | 1.08 ± 0.07# | 1.26 ± 0.26# | 1.17 ± 0.13# | 1.17 ± 0.11# | |
n=3–11.
p<0.05 versus Cx43fl/fl,
p<0.05 versus Cx43fl/fl;DMP1-8kb-Cre and
p<0.05 versus vehicle-treated mice of the same genotype, by two-way ANOVA.
Moreover, PTH increased osteoblast number and bone formation rate in lumbar vertebra independently of the mouse genotype when compared to the corresponding vehicle-treated littermates (Table 2). In addition, serum osteocalcin and the expression of the osteoblastic genes osteocalcin, collagen 1a1 and alkaline phosphatase were also increased in all mice that received PTH injection, independently of the genotype (Figs. 6D and E). On the other hand, neither the genotype nor the hormonal treatment altered the levels of the resorption marker CTX, even though there was a tendency towards lower CTX level in mice expressing the truncated Cx43, which did not reach significance (Fig. 6D).
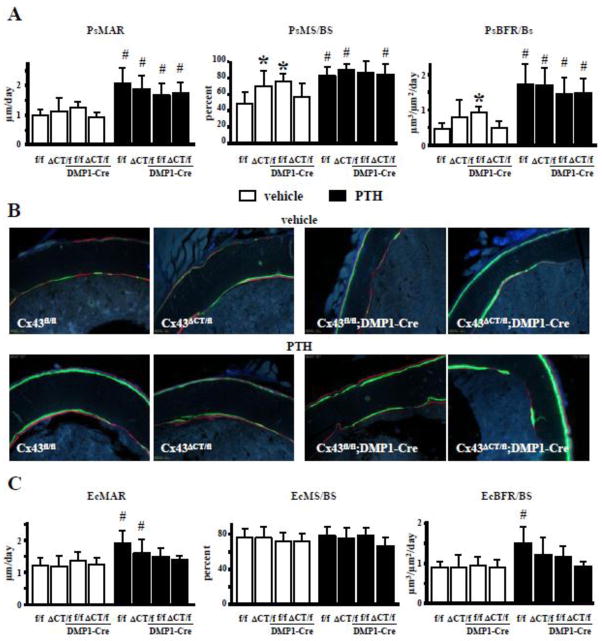
Increased bone formation induced by intermittent PTH administration on endocortical surfaces is prevented by deleting Cx43 from osteocytes and by expressing the truncated Cx43 lacking the CT domain
Periosteal mineralizing surface, but not mineral apposition rate, was increased in vehicle-treated mice expressing the truncated form of Cx43 lacking the CT domain in all tissues (Figs. 7A and B), similar to what is observed in Cx43fl/fl;DMP1-Cre mice lacking Cx43 in osteocytes (Figs. 7A and B and [18]). However, the difference in bone formation rate between Cx43ΔCT/fl mice and Cx43fl/fl littermate controls did not reach significance. Further, the presence of the truncated form of the molecule in mice with osteocytic deletion of the full length allele of Cx43 (Cx43ΔCT/fl;DMP1-Cre mice) was sufficient to maintain MS/BS and BFR, which were similar to control mice. On the other hand, deletion of Cx43 CT did not alter bone formation on the endocortical bone surface (Figs. 7B and C).
Fig. 7. Cx43 CT is involved in intermittent PTH-induced bone formation on endocortical but not periosteal surface.
(A) Periosteal mineral apposition rate (PsMAR), mineralizating surface (PsMS/BS), and bone formation rate (PsBFR/BS) were measured in unstained sections from femoral midshaft at 4.5 months of age. (B) Representative images show green and red fluorochrome labels. (C) Endosteal mineral apposition rate (EcMAR), mineralizating surface (EcMS/BS), and bone formation rate (EcBFR/BS) were measured in the same bone section as (A). Bars represent mean ± s.d., n=7/group. *p<0.05 versus Cx43fl/fl mice, and #p<0.05 versus corresponding vehicle-treated mice, by two-way ANOVA.
Intermittent PTH administration increased bone formation in both the periosteal and endocortical surfaces in Cx43fl/fl control mice, with about 300% higher BFR on the periosteum, due to increases in both MAR and MS/BS (Figs. 7A and B). The increased in BFR induced by PTH was only about 50% on the endosteum, and was only due to elevated MAR (Figs. 7C and B). Further, expression of the truncated form of Cx43 in Cx43ΔCT/fl and Cx43ΔCT/fl;DMP1-Cre mice abolished the effect of intermittent PTH administration on bone formation on the endocortical surface without affecting the response to the hormone on the periosteal surface (Fig. 7). Deletion of Cx43 from osteocytes prevented the increase in MS/BS on the periosteal surface induced by intermittent PTH, but BFR remained higher due to the increase in MAR (Fig. 7A). On the other hand, the increase in BFR induced by PTH on the endocortical surface was abolished in Cx43fl/fl;DMP1-Cre mice due to lower MAR (Fig. 7B). Taken together, these pieces of evidence suggest that expression of Cx43 in osteocytes, and in particular its CT domain, is required for the effect of PTH on bone formation on the endocortical bone surface.
Discussion
The findings of the present study demonstrate that Cx43 exerts a complex role on bone homeostasis through different domains of the protein. Absence of the cytoplasmic CT domain of Cx43 known to regulate channel closure and intracellular signaling induces a profound reduction in cancellous bone volume in the spine as well as in the long bones, as a consequence of low osteoblast number and bone formation. However, it does not affect cortical bone. These findings demonstrate that the CT domain of Cx43 exert distinct functions in osteoblastic cells in the two bone envelopes. Furthermore, the expression of the truncated form of Cx43 lacking the CT domain compensates for deletion of Cx43 from osteocytes and normalizes the increase in osteocyte apoptosis and the alterations on cortical bone geometry and mechanical properties, suggesting that in cortical bone osteocyte apoptosis, bone geometry, and material strength are closely correlated. Our findings suggest that Cx43 trasnmembrane, cytoplasmic, and/or amino domains, but not the CT domain, are required in osteocytes to control osteocyte survival and bone formation and resorption in cortical bone, resulting in proper bone geometry. However, we cannot rule out the possibility that Cx43ΔCT expressed in other cells compensates for the absence of the full length molecule in osteocytes. Moreover, mice expressing the truncated form of Cx43 respond to PTH with increased bone formation on cancellous and periosteal bone surfaces, but not on endocortical bone surface, demonstrating that the CT domain is involved in part in the anabolic response to the hormone. This evidence indicates that the CT domain of Cx43 participates in cancellous bone acquisition and has a dominant negative effect over the full length molecule in this compartment, but it is dispensable for reaching proper cortical bone geometry. Furthermore, the CT domain of Cx43 participates in the response to intermittent PTH administration in cortical but not cancellous bone.
At this point it is not possible to determine whether the observed phenotype is due to the expression of one copy of the truncated Cx43 or the combination of the truncated form of Cx43 with the wild type allele. We can, however, discard the possibility that the phenotype is due to the presence of only one allele of full length Cx43 mice, since Cx43flox/− mice do not exhibit decreased cancellous bone volume, bone formation, or osteoblast number [12].
The precise molecular mechanism underlying the differential phenotype in cancellous and cortical bone of mice lacking the Cx43 CT domain is not known. The CT domain of Cx43 has scaffolding functions that regulate intracellular signaling [3] and osteoblasts in cancellous and cortical bone are likely influenced by different stimuli that trigger particular signaling pathways. Therefore, the different phenotype of these mice in cancellous versus cortical bone might result from regulation of different signaling pathways in the two envelopes.
Cx43 expression is required for the development and function of several cell types, including bone cells [10]. In addition to its well-recognized function in cell-to-cell communication through gap junction channels, Cx43 hemichannels are also active in unopposed plasma membranes, mediating the exchange of molecules between the cells and the extracellular milieu [1, 32]. Cx43 also acts as a scaffolding protein with the ability to foster interactions among molecules that participate in different intracellular signaling pathways, thereby regulating cell function [33]. This “channel independent” function is exerted by the CT domain of the connexin. Studies aiming to investigate the role of Cx43 CT domain are challenged by the early lethality of mice expressing the truncated form of Cx43 [22]. Similarly to mice with complete absence of Cx43 [34], mice homozygous for Cx43ΔCT die soon after birth [22]. However, whereas Cx43−/− mice die due to cardiac malformation [34], Cx43ΔCT/ΔCT die due to defective epidermal barrier and dehydration [22], suggesting that the CT domain of the molecule is required for some, but not all functions of the gap junction protein. Consistent with the disparate role of the CT domain of Cx43 in different cell populations, we now demonstrate that the Cx43 CT domain is involved in achieving proper osteoblast number and bone formation in cancellous bone but not in osteoblast function in cortical bone, in osteoclast function, in the regulation of the expression of RANKL and OPG, or in the effects of osteocytic Cx43 on cortical bone geometry.
The disparate role of Cx43 domains in different compartment is further supported by a recent study with two mouse models expressing mutated forms of Cx43 in osteocytes; one with impaired cell-to-cell gap junction channel activity, but normal hemichannel function, and the other unable to form functional channels and hemichannels [35]. Mice with impaired gap junction activity in osteocytes but that still retained hemichannel function do not exhibit major differences in the skeleton, compared to wild type littermates. On the other hand, mice with impaired channel and hemichannel activity exhibit a cortical bone phenotype that resembles that of mice with deletion of Cx43 from osteocytes [18, 31] with increased marrow cavity area and periosteal apposition; but without changes in cancellous bone architecture. Taken together with the current study, this evidence support a differential effect of Cx43 domains in cancellous versus cortical bone.
Ex vivo studies pioneered by Civitelli and colleagues demonstrated that cells from bones of Cx43−/− mice showed delayed ossification, reduced expression of osteoblast markers, and deficient mineralization compared to wild type cells [11]. Tissue-specific deletion of Cx43 provided a detailed account of the role of Cx43 in each bone cell type, demonstrating that Cx43 expression is required for normal osteoblastic gene expression and function [11, 12, 18, 36–39], as well as for osteoclast differentiation and function [8, 40–42]. We found that the cancellous bone mass phenotype of our Cx43ΔCT/fl mice is closer to that of mice lacking Cx43 in osteoblast progenitors [12], suggesting that Cx43 CT domain is required early in osteoblast differentiation. However, since the Cx43ΔCT/fl do not exhibit low BMD or reduced femoral length unlike the mice lacking Cx43 in cells expressing dermo1-Cre [38], it is likely that the Cx43 CT domain is not required for proper function of osteochondroprogenitors.
Unlike Cx43 removal from early osteoblast progenitors, deletion of Cx43 from osteocytes does not affect cancellous bone but has a significant effect on cortical bone, with decreased bone volume, and increased total bone and marrow cavity area ([18] and this manuscript). Our previous study demonstrated that these effects of Cx43 deletion result from increased periosteal bone formation and endocortical bone resorption. Furthermore, deletion of osteocytic Cx43 results in altered bone material-level mechanical properties, with decreased stiffness, and increased osteocyte apoptosis. All these changes are absent when the truncated Cx43 lacking the CT is expressed, suggesting that the transmembrane pore and/or the cytoplasmic amino-terminal domains are sufficient to maintain osteocyte viability and to restrain bone formation and bone resorption, leading to the normalization of the bone geometry, and to prevent the decrease in bone material properties of the cortical bone. On the other hand, Cx43 pore is not sufficient to mediate the role of the connexin on cancellous bone acquisition, suggesting that the scaffolding function of the CT domain or the regulation of channel activity are needed for proper osteoblast function in this bone envelope.
Even though previous studies using reporter mice show that DMP-8kb promoter not only targets osteocytes but also some osteoblasts [43], we showed that Cre recombinase under the control of the 8kb fragment of the DMP1 promoter is expressed in osteocytes and not in osteoblasts in our DMP1-8kb-Cre mice. This was evidenced by demonstrating that Cre is expressed in cells also expressing the osteocyte marker sclerostin and not in cells expressing the osteoblast marker keratocan [18]. Further, when the DMP1-8kb-Cre mice were used to delete Cx43 (Cx43fl/fl;DMP1-8kb-Cre mice), immunostaining with an anti-Cx43 antibody showed that Cx43 is still present in osteoblasts on the bone surface but it is absent from osteocytes within bone [18]. Therefore, the DMP1-8kb-Cre appears to selectively delete Cx43 from osteocytes and not from osteoblasts.
In the current study, we found that expression of the truncated Cx43 leads to decrease osteoblast number in cancellous bone without affecting osteoclast number or the levels of the resorption marker CTX in the circulation. This suggests that the Cx43 CT domain is intrinsically required for osteoblast but not for osteoclast differentiation and function. Consistent with the role of Cx43 in osteoblast differentiation, osteoblasts derived from mice with global deletion of Cx43 exhibit reduced expression of genes present in mature osteoblasts [11]. Further, overexpression of full-length Cx43 but not a truncated form of Cx43 lacking the C-terminus domain stimulates the expression of osteoblastic genes in MC3T3 osteoblastic cells in vitro [44]. Our findings also suggest that the Cx43ΔCT allele might act as a dominant negative for the full length molecule, as the phenotype is revealed even in the presence of one copy of the full-length Cx43 allele. Similarly, astrocytes from mice expressing one copy of the truncated Cx43 and one of the full length (wild type) allele (Cx43ΔCT/+) mice exhibit defective channel activity confirming that one allele of the mutant acts as dominant negative over the full length Cx43 [23]. However, because mice used in the current study express the mutated molecule ubiquitously [22], future studies using mice with cell-specific expression of the truncated Cx43 will be required to identify the cell in which Cx43 CT domain exert its function regulating cancellous and cortical bone acquisition.
In spite of the effect of expression of the truncated Cx43 on the basal bone phenotype, Cx43ΔCT/fl mice respond to intermittent PTH administration with a comparable increase in bone mass, bone formation, gene expression, and circulating osteocalcin as control littermates that express only full length Cx43. Similar normal response to pharmacological treatments is observed in several genetically modified mice with low bone mass. Examples of this are mice lacking LRP5 treated with interemittent PTH [45] or with anti-sclerostin antibodies [46], and mice overexpressing DKK1 treated with intermittent PTH [47]. Taken together these pieces of evidence indicate that different stimuli are involved in bone mass acquisition and the response to pharmacological treatments.
We found that, unlike deletion of Cx43 from osteoblastic cells [12], deletion of Cx43 from osteocytes did not impair the increase in bone mass and cancellous bone formation in response to intermittent PTH administration. This contrasts with the lack of anabolic effect of mice lacking the PTH receptor in osteocytes, which do not respond to intermittent administration of the hormone [48, 49]. Taken together, these pieces of evidence suggest that while expression of Cx43 in osteocytes is not required for the increase in bone mass induced by intermittent administration of PTH, other osteocytic molecules activated downstream of the PTH receptor are responsible for the increase in bone mass induced by the hormonal treatment.
An additional finding that supports a complex role of Cx43 on the anabolic effect of intermittent PTH is the fact that bone formation on the endocortical surface of the femur is not increased in mice lacking Cx43 in osteocytes. Furthermore, in contrast to the ability of Cx43ΔCT to retain the function of full-length osteocytic Cx43 in cortical bone, expression of the truncated molecule in osteocytes cannot compensate for the lack of full length Cx43 in Cx43ΔCT/fl;DMP1-Cre mice on PTH-induced endocortical bone formation. Similarly, the increase in the amount of energy that the bone absorbs until it fails in the presence of PTH is absent in mice lacking Cx43 in osteocytes, or expressing only the truncated connexin in these cells. The reason for an abnormal response to PTH but normal cortical bone in mice lacking Cx43 CT domain might be due to the fact that different mechanisms control cortical bone formation in response to the hormone versus under normal conditions. Nevertheless, our evidence suggests that part of the PTH effect on cortical bone requires the presence of Cx43 CT domain in osteocytes.
The reason why bone cells from cortical versus cancellous bone of Cx43ΔCT mice respond differently to PTH is unknown. However, it could be due to the influence of different stimuli, to the fact that endogenous Cx43 is expressed at different levels in the two envelopes (lower in cancellous than cortical bone [18, 38]), or to both reasons. In any event, bone cells from these compartments are also differentially affected by expression of Cx43ΔCT. Moreover, several studies by the groups of Roberto Civitelli, Henry Donahue, Jean Jiang, and us consistently demonstrated that manipulation of Cx43 in osteoblastic cells affects cortical and cancellous bone in a different manner [18, 35, 38, 39, 50, 51].
In summary, mice lacking Cx43 CT exhibit normal cortical bone but a marked decrease in cancellous bone accrual due to decreased osteoblast number and bone formation. In addition, Cx43 lacking the CT domain can compensate for the absence of full length Cx43 in osteocytes. Further, mice lacking full length Cx43 in osteocytes or expressing the mutated Cx43 lacking the CT domain do not exhibit increased endocortical bone formation and bone strength induced by intermittent PTH administration, whereas they show a normal anabolic response to the hormone in cancellous bone or on the periosteal surface of cortical bone.
Conclusion
Our study establishes the requirement of distinct domains of Cx43 in different bone envelopes (cortical versus cancellous) and surfaces (endocortical versus periosteal) and in the regulation of hormonal responses by Cx43.
Highlights.
Cx43 channels allow communication among bone cells, however, the role of its C-terminal domain (CT) on the skeleton is unknown.
Removal of Cx43 CT results in decreased cancellous bone mass due to reduced osteoblast number and activity.
Removal of Cx43 CT has no effect on cortical bone geometry or mechanical properties.
The effects of PTH were less in mice lacking the Cx43 C-terminal domain compared to wild-type animals.
Cx43 CT is involved in bone acquisition; and osteocytic Cx43 is dispensable for some but not all PTH anabolic actions
Acknowledgments
The authors thank Meloney Cregor for technical assistance. This research was supported by the National Institutes of Health R01-AR053643 to LIP. μCT studies were performed using equipment obtained with the NIH grant S10-RR023710 (PI: James Williams, Department of Anatomy and Cell Biology, Indiana University School of Medicine). RPC received a scholarship from Coordination of Improvement of Higher Level Personnel (CAPES), Brazil (PDEE# 1065/11-4).
Abbreviations
- Cx43
connexin 43
- CT
Cx43 carboxy-terminus
- Cx43ΔCT
Cx43 mutant lacking the CT domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rafael Pacheco-Costa, Email: rafa.pacheco@ig.com.br.
Hannah M. Davis, Email: hannahd@indiana.edu.
Chad Sorenson, Email: csorenso@iupui.edu.
Mary C. Hon, Email: mchon@iupui.edu.
Iraj Hassan, Email: irhassan@umail.iu.edu.
References
- 1.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 2.Bargiello TA, Tang Q, Oh S, Kwon T. Voltage-dependent conformational changes in connexin channels. Biochim Biophys Acta. 2012;1818:1807–1822. doi: 10.1016/j.bbamem.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herve JC, Derangeon M, Sarrouilhe D, Giepmans BN, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta. 2012;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. http://dx.doi.org/10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans WH, Martin PE. Gap junctions: structure and function. Mol Membr Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 7.Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91:1888–1896. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilvesaro J, Väänänen K, Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J Bone Min Res. 2000;15:919–926. doi: 10.1359/jbmr.2000.15.5.919. [DOI] [PubMed] [Google Scholar]
- 9.Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 10.Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–166. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung D, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–4198. doi: 10.1242/?jcs.03162. [DOI] [PubMed] [Google Scholar]
- 13.Thi MM, Urban-Maldonado M, Spray DC, Suadicani SO. Characterization of human telomerase reverse transcriptase (hTERT) immortalized osteoblast cell lines generated from wildtype and connexin43-null mouse calvaria. Am J Physiol Cell Physiol. 2010;299:C994–C1006. doi: 10.1152/ajpcell.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima F, Niger C, Hebert C, Stains JP. Connexin43 Potentiates Osteoblast Responsiveness to Fibroblast Growth Factor 2 via a Protein Kinase C-Delta/Runx2-dependent Mechanism. Mol Biol Cell. 2009;20:2697–2708. doi: 10.1091/mbc.E08-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. ERK Acts in Parallel to PKC delta to Mediate the Connexin43-dependent Potentiation of Runx2 Activity by FGF2 in MC3T3 Osteoblasts. Am J Physiol Cell Physiol. 2012;302:C1035–C1044. doi: 10.1152/ajpcell.00262.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23:1712–1721. doi: 10.1359/jbmr.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57:76–83. doi: 10.1016/j.bone.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun L, Rhee Y, Bellido T, Plotkin LI. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Min Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with βarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112:2920–2930. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 21.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. http://dx.doi.org/10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, Grummer R, Kretz M, Lewalter T, Tiemann K, Winterhager E, Herzog V, Willecke K. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozoriz MG, Bechberger JF, Bechberger GR, Suen MW, Moreno AP, Maass K, Willecke K, Naus CC. The connexin43 C-terminal region mediates neuroprotection during stroke. J Neuropathol Exp Neurol. 2010;69:196–206. doi: 10.1097/NEN.0b013e3181cd44df. [DOI] [PubMed] [Google Scholar]
- 24.Maass K, Shibayama J, Chase SE, Willecke K, Delmar M. C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res. 2007;101:1283–1291. doi: 10.1161/CIRCRESAHA.107.162818. [DOI] [PubMed] [Google Scholar]
- 25.Cina C, Maass K, Theis M, Willecke K, Bechberger JF, Naus CC. Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. J Neurosci. 2009;29:2009–2021. doi: 10.1523/JNEUROSCI.5025-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theis M, de Wit C, Schlaeger TM, Eckardt D, Kruger O, Doring B, Risau W, Deutsch U, Pohl U, Willecke K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29:1–13. doi: 10.1002/1526-968X(200101)29:1<1::AID-GENE1000>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Pacheco-Costa R, Hassan I, Reginato RD, Davis HM, Bruzzaniti A, Allen MR, Plotkin LI. High Bone Mass in Mice Lacking Cx37 Due to Defective Osteoclast Differentiation. J Biol Chem. 2014;289:8508–8520. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 29.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien CA, Plotkin LI, Galli C, Goellner J, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, Plotkin LI. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int. 2012;91:215–224. doi: 10.1007/s00223-012-9628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta. 2005;1711:208–214. doi: 10.1016/j.bbamem.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Gu S, Riquelme MA, Burra S, Callaway D, Cheng H, Guda T, Schmitz J, Fajardo RJ, Werner SL, Zhao H, Shang P, Johnson ML, Bonewald LF, Jiang JX. Connexin43 Channels are Essential for Normal Bone Structure and Osteocyte Viability. J Bone Miner Res. 2015;30:550–562. doi: 10.1002/jbmr.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Nieto D, Li L, Kohler A, Ghiaur G, Ishikawa E, Sengupta A, Madhu M, Arnett J, Santho R, Dunn SK, Fishman GI, Gutstein DE, Civitelli R, Barrio LC, Gunzer M, Cancelas JA. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood. 2012;119:5144–5154. doi: 10.1182/blood-2011-07-368506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, Civitelli R. Osteoblast Connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell. 2011;22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Paul EM, Sathyendra V, Davidson A, Bronson S, Srinivasan S, Gross TS, Donahue HJ. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilvesaro J, Tavi P, Tuukkanen J. Connexin-mimetic peptide Gap 27 decreases osteoclastic activity. BMC Musculoskelet Disord. 2001;2:10. doi: 10.1186/1471-2474-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matemba SF, Lie A, Ransjo M. Regulation of osteoclastogenesis by gap junction communication. J Cell Biochem. 2006;99:528–537. doi: 10.1002/jcb.20866. [DOI] [PubMed] [Google Scholar]
- 42.Sternlieb M, Paul E, Donahue HJ, Zhang Y. Ablation of connexin 43 in osteoclasts leads to decreased in vivo osteoclastogenesis. J Bone Miner Res. 2012;27:S53. [Google Scholar]
- 43.Kalajzic I, Matthews BG, Torreggiani E, Harris MA, Divieti PP, Harris SE. In vitro and in vivo approaches to study osteocyte biology. Bone. 2013;54:296–306. doi: 10.1016/j.bone.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebert C, Stains JP. An intact connexin43 is required to enhance signaling and gene expression in osteoblast-like cells. J Cell Biochem. 2013;114:2542–2550. doi: 10.1002/jcb.24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The WNT co-receptor LRP5 is essential for skeletal mechanotransduction, but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 46.Kedlaya R, Veera S, Horan DJ, Moss RE, Ayturk UM, Jacobsen CM, Bowen ME, Paszty C, Warman ML, Robling AG. Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci Transl Med. 2013;5:211ra158. doi: 10.1126/scitranslmed.3006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao GQ, Wu JJ, Troiano N, Insogna K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab. 2010;29:141–148. doi: 10.1007/s00774-010-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu X, McAndrews K, Delgado-Calle J, Olivos N, Ben-Awadh A, Kim W, Pacheco-Costa R, Richardson D, Peacock M, Plotkin LI, Bellido T. Osteocytic PTH receptor is required for bone anabolism induced by intermittent PTH administration, but is dispensable for bone resorption and the loss of bone induced by chronic PTH elevation. J Bone Miner Res. 2013;28:S233. [Google Scholar]
- 49.Saini V, Marengi DA, Barry KJ, Fulzele KS, Heiden E, Liu X, Dedic C, Maeda A, Lotinun S, Baron R, Pajevic PD. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288:20122–20134. doi: 10.1074/jbc.M112.441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS ONE. 2012;7:e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27:2359–2372. doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]