Abstract
Background
Primary prostate cancers are infiltrated with PD-1 expressing CD8+ T cells. However, in early clinical trials, men with mCRPC did not respond to PD-1 blockade as a monotherapy. One explanation for this unresponsiveness could be that prostate tumors generally do not express PD-L1, the primary ligand for PD-1. However, lack of PD-L1 expression in prostate cancer would be surprising, given that PTEN loss is relatively common in prostate cancer and several studies have shown that PTEN loss correlates with PD-L1 up-regulation - constituting a mechanism of innate immune resistance. This study tested whether prostate cancer cells were capable of expressing PD-L1, and whether the rare PD-L1 expression that occurs in human specimens correlates with PTEN loss.
Methods
Human prostate cancer cell lines were evaluated for PD-L1 expression and loss of PTEN by flow cytometry and western blotting, respectively. Immunohistochemical (IHC) staining for PTEN was correlated with PD-L1 IHC using a series of resected human prostate cancer samples.
Results
In vitro, many prostate cancer cell lines up-regulated PD-L1 expression in response to inflammatory cytokines, consistent with adaptive immune resistance. In these cell lines, no association between PTEN loss and PD-L1 expression was apparent. In primary prostate tumors, PD-L1 expression was rare, and was not associated with PTEN loss.
Conclusions
These studies show that some prostate cancer cell lines are capable of expressing PD-L1. However, in human prostate cancer, PTEN loss is not associated with PD-L1 expression, arguing against innate immune resistance as a mechanism that mitigates anti-tumor immune responses in this disease.
INTRODUCTION
Antibody-mediated blockade of the PD-1 / PD-L1 interaction is a promising clinical strategy in several tumor types(1–3), and PD-1 blocking reagents have recently been approved for both melanoma and squamous lung cancer. Immunologically, these agents function by blocking the interaction between PD-1 on a partially activated or “exhausted” CD8+ T cell and its primary ligand (PD-L1), which is expressed on tumor cells as well as myeloid cells in the tumor microenvironment. That interaction inhibits CD8+ T cell function, so blocking PD-1 or PD-L1 can lead to T cell activation, proliferation, and tumor cell lysis(4,5). Unfortunately, Phase I data from men with metastatic castrate resistant prostate cancer (mCRPC) suggest that PD-1 blockade is less effective in prostate cancer than other tumor types, with 0 out of 17 patients showing objective responses in the initial Phase I study(6,7).
The relative lack of objective responses to PD-1 blockade in prostate cancer could be due to the observation that PD-L1 expression appears to be rare in prostate cancer, at least in the data accumulated thus far(2). This is not the case for immune-sensitive diseases like melanoma, where PD-L1 expression is common(8), and expression of PD-L1 correlates with response to PD-L1 blockade(9,10). Several studies show that PD-L1 expression can be mediated by pro-inflammatory factors secreted by immune cells, a phenomena which has been termed ‘adaptive immune resistance’(1,11,12). This hypothesis is supported by the co-localization of immune cells and PD-L1 expressing tumor cells suggesting that signals from infiltrating immune cells trigger tumor cells to up-regulate PD-L1(8). Other data suggest that PD-L1 up-regulation may be driven by oncogene expression, a process termed ‘innate immune resistance’. This hypothesis suggests that PD-L1 is constitutively expressed in response to aberrant signaling in other pathways. For example, Parsa et al showed that loss of the tumor suppressor PTEN, and associated PI3K activation, was associated with PD-L1 expression in both glioblastoma(13) and prostate cancer(14). To date, the relationship of ‘innate immune resistance’ whereby PD-L1 is constitutively up-regulated when PTEN is lost has not been further explored in depth in prostate cancer, especially in primary tumor specimens.
In prostate cancer PTEN loss is a relatively common occurrence and has been established as a predictor of poor clinical outcomes and progression to mCRPC(15–18). Based on the lack of clinical responses to anti-PD-1 therapy in patients with mCRPC, we hypothesized that PTEN loss might not be associated with PD-L1 expression in prostate cancer. In the current study we demonstrate that prostate cancer occasionally expresses PD-L1, and that this expression has no clear relationship to PTEN loss or activation of the PI3K pathway in this disease.
MATERIALS/METHODS
Cell Culture
Human prostate cancer cell lines were grown in a monolayer under standard culture conditions with 5% CO2 in a 37°C incubator. Tissue culture media was supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. CWR22Rv1(19), E006AA(20), DU145(21) LNCAP(21), and PC3(21) were grown in RPMI 1640 medium (Invitrogen, Grand Island, New York, USA); LAPC-4(21) was grown in Iscove’s Modified Dulbecco’s Medium (Invitrogen, Grand Island, New York, USA); and VCAP(22) was grown in Dulbecco’s Modified Eagle Medium (Invitrogen, Grand Island, New York, USA). Cell lines were a generous gift from Dr. John Isaacs, PhD, Johns Hopkins University. Cell lines have been previously described. STR and mycoplasma testing was performed on all cell lines. For stimulation experiments, cells were plated at a density of ~5,000/cm2 into 100cm2 tissue culture dishes and allowed to adhere for 24hours. After 24 hours cell cultures were treated with recombinant human interferon gamma (IFN-γ) (300–02, Pepro Tech, Rocky Hill, Connecticut, USA) at a concentration of 100units/mL for 48 hours prior to harvest(23) or with 10μM bicalutamide (B9061, Sigma Aldrich, St. Louis, Missouri, USA) for 48 hours(24). All stimulation experiments were repeated at least 3 times.
Flow Cytometry
Cells were stained with phycoerythrin (PE) labeled mouse anti-human PD-L1 (CD274, clone MIH 1, 12-5983-41, Ebiosciences, San Diego, CA, USA), PE-labeled mouse anti-human PD-L2 (CD273, clone MIH18 clone, 12-5888-41, Ebiosciences, San Diego, CA, USA), or fluorescein (FITC) labeled mouse anti-human HLA DR, DP, DQ (clone Tu39, 555558, Becton Dickinson, Franklin Lakes, New Jersey, USA). Antibodies were diluted 1:200 and staining was performed in FACS buffer for 15 minutes at room temperature. The same procedure was performed using isotype control antibodies, PE-labeled mouse IgG1 kappa or FITC-labeled mouse IgG2a kappa. Cells were analyzed using a BD FACS Calibur (Becton Dickinson, Franklin Lakes, New Jersey, USA) and FlowJo software (Tree Star, Ashland, Oregon, USA). Flow cytometric analyses were repeated at least 3 times.
Western Blotting
Cells were grown with or without IFN-γ as above and lysed directly in tissue culture flasks using ice cold RIPA buffer (R0278, Sigma Aldrich, St. Louis, Missouri, USA) supplemented with Sigmafast protease inhibitor tablets (S8820-2TAB, Sigma Aldrich, St. Louis, Missouri, USA). Protein quantitation was performed using a Coomassie Blue (Fisher Scientific, Waltham, MA USA) chromogenic assay. 80ug protein/well was resolved by SDS-Page and transferred to a PVDF membrane. Primary antibodies included either p-AKT Ser473 (clone D9E, 9188, Cell Signaling, Danvers, Massachusetts, USA) or PTEN (clone D4.3 XP 9271, Cell Signaling, Danvers, Massachusetts, USA) at a concentration of 1:1000. Protein was detected using horseradish-peroxidase linked anti-rabbit IgG as a secondary, and developed using the Amersham ECL Detection Kit (RPN2232, GE Healthcare UK Limited, Little Chalfont, Buckinghamshire, UK). These blots were subsequently stripped and re-incubated with anti-actin antibody at a concentration of 1:5000 (A2066, Sigma Aldrich, St. Louis, Missouri, USA). Images were obtained using Biospectrum Imaging Center/VisionWorks LS software (UVP, Upland, CA, USA). Western blot analysis was performed twice.
Human Prostate Samples
20 whole mount paraffin embedded primary prostate cancer specimens were obtained from a prostatectomy database maintained by the Department of Pathology at the University of Colorado under IRB approved protocol, 00-812. Informed consent was obtained for tissue acquisition of each sample. 11 of these samples were treated with leuprolide prior to prostatectomy and 9 received no pre-treatment. This was done at the discretion of the treating physician and not part of a clinical trial.
Immunohistochemistry
Immunohistochemistry for PD-L1 was performed by the Johns Hopkins Pathology Core using the 5H1 clone of the mouse anti-human CD274 monoclonal antibody with a mouse IgG1 isotype as a negative control(8). Immunohistochemistry for PTEN was also performed by the Core Laboratory utilizing rabbit anti-human PTEN antibody clone D4.3 XP (9188, Cell Signaling, Danvers, Massachusetts, USA) at a 1:50 dilution as previously described(16).
Scoring
Scoring was performed by two independent pathologists (AMD, RAA). PTEN status was scored using a validated dichotomous scoring system(16). PD-L1 scoring has also been previously described(8). Briefly, samples in which > 5% of malignant epithelial cells show membrane staining were designated as PD-L1 positive.
RESULTS
IFN-gamma Up-Regulates PD-L1 (Adaptive Immune Resistance)
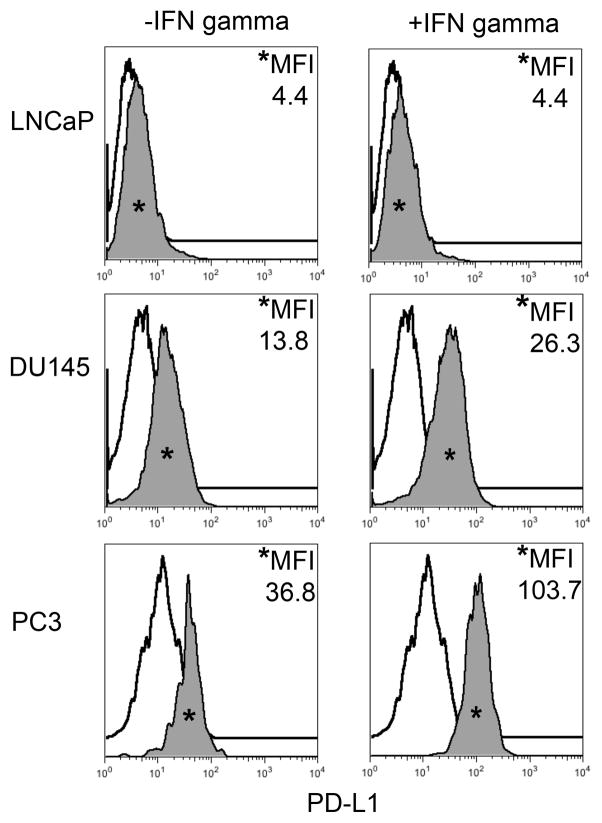
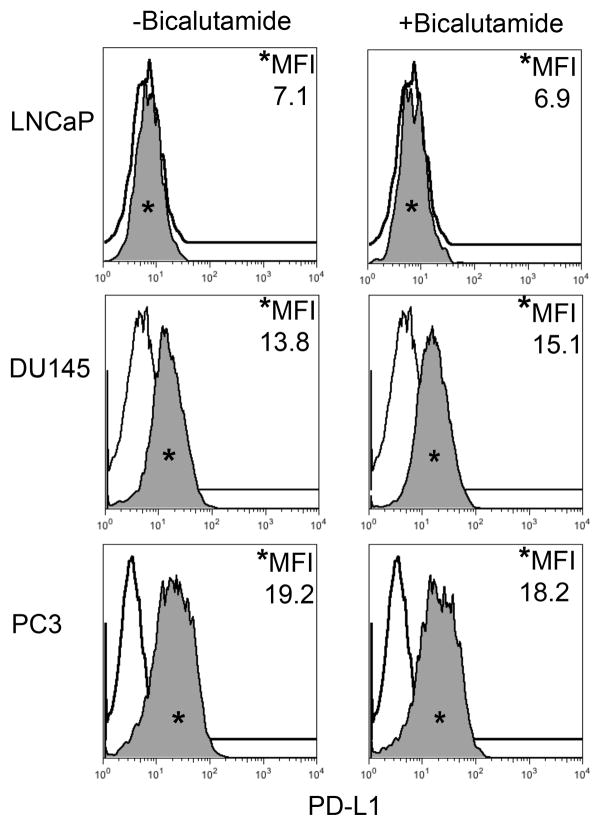
Cytokine driven up-regulation of PD-L1 has been implicated in the ability of tumors to evade detection and destruction by tumor-specific cytotoxic CD8+ T cells. To assess the potential of human prostate cancer to demonstrate ‘adaptive immune resistance’(1,11,12), PD-L1 expression was assayed in seven human prostate cancer cell lines: CWR22Rv1, DU145, E006AA, LAPC-4, LNCaP, PC3, and VCaP by flow cytometry. We also quantified expression of the second major ligand for PD-1 (PD-L2), as well as Class II MHC, which may contribute to immune evasion by binding to the immune checkpoint molecule LAG-3 on exhausted CD8+ T cells(25). To broadly simulate a TH1-driven immune response, cells were cultured in the presence of IFN-γ for 48 hours; IFN-γ is a known mediator of PD-L1 expression in human cancer cell lines and has also been shown to induce MHC(1,23,26). The majority of prostate cancer cell lines tested up-regulated PD-L1 in response to IFN-γ, suggesting that prostate cancer cells are indeed capable of ‘adaptive immune resistance’. Three representative lines are shown in Figure 1, additional cell lines are shown in supplementary Figure 1. Interestingly, two of the cell lines, Du145 and PC3, demonstrated PD-L1 expression at baseline, suggesting the possibility of ‘innate immune resistance’. All of the cell lines examined expressed PD-L1 to some degree except for the two lines derived from lymph node metastases, LAPC-4 and LNCaP, (Table 1, Supplementary Fig 1). Prostate cancer is a hormone sensitive tumor and alterations in the PD-1 pathway have been linked to steroid hormones(24,27). To determine whether activity of the androgen receptor contributes to expression of PD-L1 or other immune-related molecules, cells were treated with the androgen receptor (AR) antagonist, bicalutamide. As expected, the two cell lines that do not express AR showed no effects of bicalutamide treatment (Figure 2). The other cell lines tested also evinced no changes in immune-related cell surface markers either. (Table 2; Supplementary Figure 2).
FIGURE 1. Human Prostate Cancer Cell Lines Display Varying Expression of PD-L1 in Response to IFN-γ.
Histograms representing PD-L1 surface expression as detected by flow cytometry in human prostate cancer cell lines with and without exposure to IFN-γ. Open histograms represent cells stained with an isotype IgG1 antibody tagged with a matching PE fluorochrome. Shaded histograms represent PD-L1. Quantitative measurements reflect mean fluorescence index. LNCaP does not express PD-L1 at rest or in response to IFN-γ. DU145 expresses PD-L1 at rest and has little response to IFN-γ indicating an innate immune resistance phenotype. PC3 expresses PD-L1 at rest but robustly up-regulates this expression in response to IFN-γ displaying a more classic adaptive immune resistance phenotype.
TABLE 1.
Response to IFN-γ
| Source | Cell Line | PD-L1 Expression Pattern | PD-L2 Expression Pattern | MHCII Expression Pattern |
|---|---|---|---|---|
| Primary | CWR22Rv1 | Inducible | None | Inducible |
| E006AA | Inducible | Inducible | Inducible | |
| LN | LAPC-4 | None | None | None |
| LNCaP | None | None | None | |
| Bone | PC3 | Constitutive & Inducible | Inducible | Inducible |
| VCaP | Inducible | None | None | |
| Brain | DU145 | Constitutive & Inducible | None | None |
FIGURE 2. Human Prostate Cancer Cell Lines Display No Change in PD-L1 Expression in Response to Bicalutamide.
Histograms representing PD-L1 surface expression as detected by flow cytometry in human prostate cancer cell lines with and without exposure to bicalutamide. Open histograms represent cells stained with an isotype IgG1 antibody tagged with a matching PE fluorochrome. Shaded histograms represent PD-L1. Quantitative measurements reflect mean fluorescence index. LNCaP, DU145, and PC3 displayed no changes in PD-L1 expression upon exposure to bicalutamide.
TABLE 2.
Response to Bicalutamide
| Source | Androgen Receptor | Cell Line | PD-L1 Expression Pattern | PD-L2 Expression Pattern | MHCII Expression Pattern |
|---|---|---|---|---|---|
| Primary | Positive | CWR22Rv1 | None | None | None |
| Positive | E006AA | None | None | None | |
| LN | Positive | LAPC-4 | None | None | None |
| Positive | LNCaP | None | None | None | |
| Bone | Negative | PC3 | Constitutive | None | None |
| Positive | VCaP | None | None | None | |
| Brain | Negative | DU145 | Constitutive | None | None |
Expression of Immunologic Markers Is Independent of PTEN Status
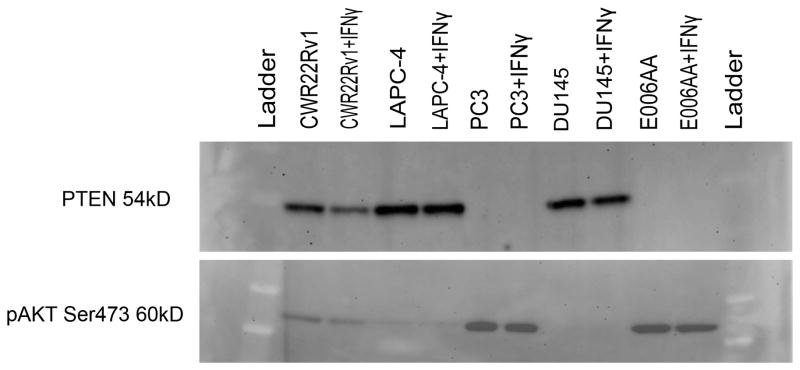
Loss of the tumor suppressor PTEN has been linked to increased expression of PD-L1 in glioblastoma(13) and prostate cancer(14). In patients, PTEN loss occurs in up to 60–70% of primary prostate cancer cases and portends a less favorable outcome(15–18). Mechanistically, loss of PTEN de-represses the PI3K pathway leading to transcriptional activation of several downstream targets and is associated with mTORC2 signaling activation and the phosphorylation of AKT to its active form, phospho-AKT (p-AKT S473)(28). To determine whether PTEN loss and PD-L1 up-regulation are linked in prostate cancer cell lines, PTEN and p-AKT levels were evaluated by western blotting, and correlated with PD-L1 protein expression. Consistent with previously published reports, PTEN expression was intact in CWR22Rv1, DU145, and LAPC-4 and absent in E006AA and PC3(28) (Fig 3). All lines exhibiting PTEN loss had coordinate up-regulation of p-AKT. A paired evaluation with and without IFN-γ was done on each sample. As expected PTEN status was not affected by IFN-γ since this is a genetic loss of function mutation. Importantly, p-AKT expression was not altered by exposure of cell lines to IFN-γ indicating that IFN-γ was not activating PI3K by another mechanism. Perhaps most significantly, there was no correlation between PTEN status and PD-L1 expression in these cell lines (Table 3), arguing against PTEN loss driving innate immune resistance in vitro.
FIGURE 3. Activation of the PI3K/MAPK Pathway Does Not Correlate with PD-L1 Expression in Human Prostate Cancer Cell Lines.
Western Blot quantitating PTEN and p-AKT protein in CWR22Rv1, LAPC-4, PC3, DU145, and E006AA with and without exposure to IFN-γ.
TABLE 3.
PD-L1 Expression is Independent of PTEN Status in Human Prostate Cancer Cell Lines
| PTEN Status | Cell Line | PD-L1 Expression Pattern |
|---|---|---|
| Intact | CWR22Rv1 | Inducible |
| DU145 | Constitutive & Inducible | |
| LAPC-4 | None | |
| VCaP | Inducible | |
| Loss | E006AA | Inducible |
| LNCaP | None | |
| PC3 | Constitutive & Inducible |
PD-L1 Expression in Human Prostate Cancer Samples is Independent of PTEN Status
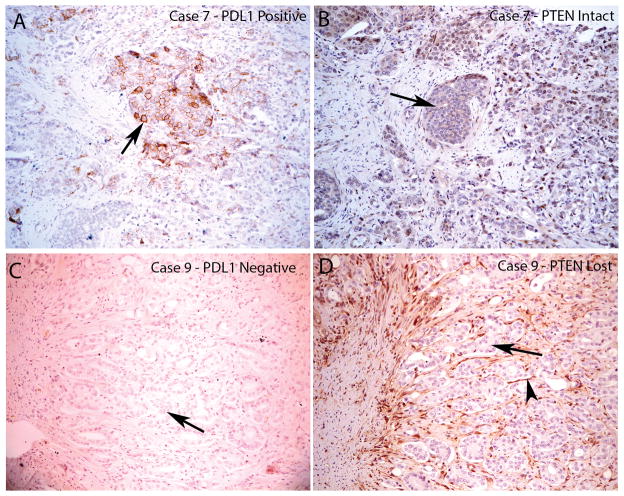
To determine whether there was an association between PTEN status and PD-L1 expression in patients, 20 paraffin embedded whole mount primary prostate cancer samples were stained for both PTEN and PD-L1, using previously validated IHC protocols(8,16). Broadly consistent with previously reported results, 25% (5/20) of samples demonstrated loss of PTEN (Table 4). For these studies, PD-L1 “positivity” was defined as 5% membrane staining, based on previous work showing a correlation between marker positivity and clinical response to blocking antibody(8,10). Using this cutoff, 3/20 samples (15%,) had focal areas of PD-L1 positivity, although in only 2 of the 3 positive samples was plasma membrane staining clearly observed on malignant epithelial cells (Supplementary Table 1). Strikingly, none of these PD-L1 positive samples demonstrated loss of PTEN, i.e. consistent with the cell line data, none of the 5 samples with PTEN loss were PD-L1 positive (Fig 4; Table 4). Of note, 11/20 patients in this series had received anti-androgen hormonal therapy prior to surgery. Again similar to our cell line results, none of the hormonally treated samples were positive for PD-L1. Interestingly, several of the samples in this series had PD-L1 expression in the 1% or lower range, the clinical significance of those low levels of staining is less clear(10) (Supplementary Table 1). Some of this low-level staining appeared to occur in areas of proliferative inflammatory atrophy (PIA) a proposed pre-cancerous inflammatory state of the prostate(29). Interestingly, some of this low-level staining occurred on infiltrating immune cells (Figure 5), and this finding has been correlated with response to PD-1 blockade in some clinical studies (30). Taken together, these results support previous data showing that PD-L1 expression in prostate cancers is rare, and that when expression does occur, it is not associated with regions of PTEN loss.
TABLE 4.
PTEN vs PD-L1 Expression in Human Prostate Cancer Samples
| Sample | PTEN Status | PD-L1 Expression |
|---|---|---|
| 1 | Intact | Present |
| 2 | Lost | None |
| 3 | Intact | None |
| 4 | Not Evaluable | Not Evaluable |
| 5 | Not Evaluable | Not Evaluable |
| 6 | Lost | None |
| 7 | Intact | Present |
| 8 | Intact | Present |
| 9 | Lost | None |
| 10 | Intact | None |
| 11 | Intact | None |
| 12 | Intact | None |
| 13 | Lost | None |
| 14 | Intact | None |
| 15 | Intact | None |
| 16 | Intact | None |
| 17 | Intact | None |
| 18 | Intact | None |
| 19 | Intact | None |
| 20 | Lost | None |
FIGURE 4. Loss of PTEN Does Not Correlate with PD-L1 Expression in Resected Primary Prostate Carcinomas.
PD-L1 and PTEN staining in tumor lesions from two different patients. A. Case number 8 showing focally positive PD-L1 area. Arrow indicates positive tumor cell staining which is predominantly on the plasma membrane. B, similar region from adjacent section of case number 8 showing intact PTEN staining in all tumor cells (arrow shows a group of tumor cells with intact PTEN staining). C, case number 9 showing negative staining for PDL1 in all tumor cells (arrow shows tumor cells without PD-L1 staining). D, adjacent region showing PTEN loss in nearly all of the tumor cells (arrow shows tumor cells with negative PTEN staining; arrowhead shows stromal element with strongly positive PTEN staining). All images taken at 100 X original magnification.
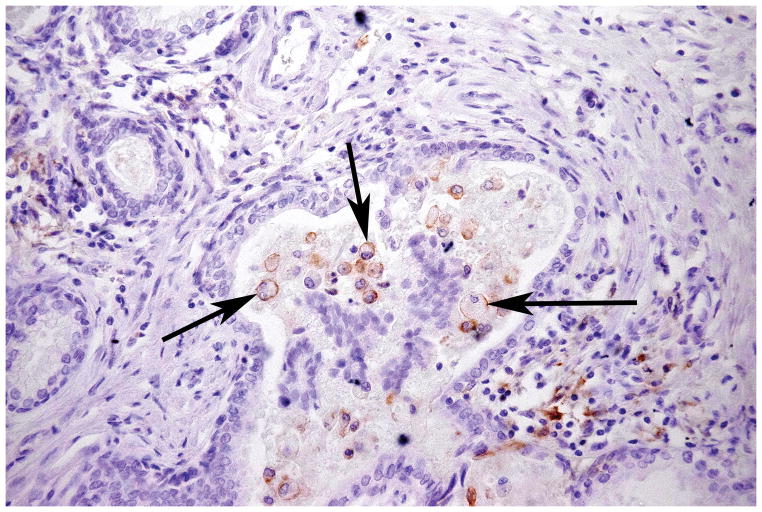
FIGURE 5. PD-L1 Is Expressed on Infiltrating Inflammatory Cells.
Surface expression of PD-L1 demonstrated by macrophages (arrows) infiltrating the lumen of a benign gland from prostatectomy Case number 1. Image taken at 200 X original magnification.
DISCUSSION
Immune checkpoint blockade with anti-PD-1 or anti-PD-L1 is emerging as a promising treatment modality in several tumor types(1–3), but to date blocking this interaction has been relatively disappointing in prostate cancer. The primary reason for this is likely that prostate cancer patients have little or no PD-L1 expression in their tumors, as demonstrated by several previous IHC studies and as confirmed here.
PD-L1 expression can be driven by two major mechanisms. In the most common, ‘adaptive immune resistance’, PD-L1 expression on tumor cells is driven by immune cell production of pro-inflammatory cytokines such as IFN-γ. PD-L1 up-regulation in turn, protects the tumor cells from CD8+ T cell mediated attack by binding to PD-1 on tumor-specific cytotoxic T cells. Our data confirm that prostate cancer cells can express PD-L1 in vitro, in response to pro-inflammatory signals. In human samples, though, PD-L1 expression was relatively rare, confirming previous data(2) and suggesting that the paucity of PD-L1 expression in patients may be due to a locally immunosuppressive environment that very effectively dampens CD8+ T cell production of IFN-γ, as has been clearly demonstrated in several animal models(31,32).
The second major mechanism underlying PD-L1 expression is known as ‘innate immune resistance’, in which tumor cells autonomously up-regulate PD-L1, potentially under the influence of oncogenic pathways(12). In the case of prostate cancer, previous data suggested that loss of PTEN, a common event in prostate cancer, is potentially associated with PD-L1 expression(14). If that were the case, then PD-L1 expression in prostate cancer might be assumed to be fairly widespread, since PTEN loss is a common event in prostate cancer. We tested this hypothesis in both cell lines and a small case series, and could not confirm the notion that PD-L1 expression is associated with PTEN loss, despite using well-validated staining protocols for both markers. Although there were only 5 samples of PTEN negative prostate cancer in this series, if ‘innate immune resistance’ were a major mechanism driving PD-L1 expression in prostate cancer it stands to reason that a few PD-L1 positive cells would have been seen. The reasons for this discrepancy are not immediately obvious, and a larger case series or TMA study could be considered to explore this association further. Nevertheless, these data do support the conclusion that ‘innate immune resistance’ triggered by PTEN loss and subsequent PI3K pathway activation is not likely to be a major underlying mechanism driving PD-L1 expression in prostate cancer. It should be noted that a role for “innate immune resistance” has recently been challenged in melanoma as well, where prior suggestions that common mutations in melanoma (i.e. BRAF V600E) were associated with PD-L1 expression were not supported in studies using patient samples(33).
The ability of androgen-ablation to modulate a pro-inflammatory tumor microenvironment in prostate cancer has been demonstrated by several previous studies (34,35). For this reason it has been postulated that androgen ablation might increase PD-L1 expression on tumor cells by attracting infiltrating immune cells and triggering and adaptive immune response(34,36,37). Therefore it is surprising that in this study PD-L1 expression on prostate cancer cells remained rare and was not increased in those which were pre-treated with androgen-ablation. There was also no evidence of increased immune infiltration in androgen ablated samples(Supplementary Figure 1). This suggests that if inflammation occurred in response to androgen ablation, it may have been transient and was not captured by this study. The elegant work by Mercader et al indicates that immune infiltration peaks within 2 weeks of anti-androgen administration(34), and patients included in this study had been ablated for several months prior to surgery. These findings suggest that androgen ablation alone may not be sufficient to overcome the suppressive immune microenvironment of prostate cancer outside of a narrow therapeutic window. It is also important to note that from a translational standpoint anti-PD-1 therapy has never been administered to newly diagnosed patients with prostate cancer. All of the Phase I and II studies have been done in patients with mCRPC(7,9). Therefore it is possible that the lack of efficacy observed in clinical studies reflects the intrinsic biological differences between primary and metastatic prostate tumors.
In summary, these data suggest that treatments directed at the PD-1 / PD-L1 interaction are unlikely to be successful as monotherapies in prostate cancer. Nevertheless, it is possible that the acute inflammation driven by androgen-ablation(34,35) could transiently increase PD-L1 expression, suggesting a strategy in which blockade is combined with androgen-ablation. Similar effects could be mediated by prostate cancer specific vaccination, although, the PD-L1 staining data above suggest that if PD-L1 expression does occur, it may be transient in nature. It is also possible that one of the many other immune checkpoint / ligand pairs are of greater importance than the PD-1 / PD-L1 axis in prostate cancer, ongoing studies in our lab and many others are actively investigating that hypothesis.
Supplementary Material
Supplemental File # 1 (Figure S-1). PD-L2 expression was observed rarely and only in response to IFN-γ in the primary cell line, E006AA, and the metastatic bone cell line, PC3. The expression of MHCII closely mirrored that of PD-L1. IFN-γ induced expression of MHCII in all lines except, LAPC-4, LNCaP, and DU145 where it was absent.
Supplemental File # 2 (Figure S-2). Expression of PD-L1, PD-L2, and MHCII was not altered by bicalutamide in any cell line.
Supplemental File # 3. Full Western Blot with size markers and actin quantitation.
Supplemental File # 4. Low levels of PD-L1 expression not meeting established criteria for positivity were detected on >50% of samples often in association with PIA.
Acknowledgments
We would like to thank John T Isaacs, PhD for donating the human prostate cancer cell lines for this study and Susan Dalrymple, BS for instructing us on maintaining them. We would also like to thank Olesya Chornoguz, PhD for instruction regarding Western blotting for phosphor-AKT.
Funding:
CGD: National Institutes of Health R01 CA127153, the Patrick C. Walsh Fund, the One-in-Six Foundation, the Koch Foundation, and the Prostate Cancer Foundation.
AMD is the Virginia and Warren Schwerin Scholar and is supported by 1P50CA58236-15, the Patrick C. Walsh Fund, and the Prostate Cancer Foundation.
AvB and MSL: NIH P30CA046934.
This work was also supported by the NIH P30 CA006973 to the Johns Hopkins Sidney Kimmel Cancer Center.
Footnotes
Supplementary information is available at http://www.nature.com/pcan/index.html.
CONFLICT OF INTEREST
The authors declare competing financial interests. Charles G. Drake has consulted for Amplimmune, Bristol Myers Squibb (BMS), Merck, and Roche-Genentech, all of whom have either anti-PD-1 or anti-PD-L1 reagents in various stages of clinical development. In addition, Drs. Anders and Drake have received sponsored research funding from BMS. The first author Dr. Allison M. Martin has no conflicts of interest to declare.
References
- 1.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 2.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature Reviews Immunology. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 3.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nature reviews Clinical oncology. 2014;11(1):24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: Survival and long-term safety in a phase I trial. JOURNAL OF CLINICAL ONCOLOGY: AMER SOC CLINICAL ONCOLOGY 2318 MILL ROAD, STE 800, ALEXANDRIA, VA 22314 USA; 2013.
- 7.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010 Jul 1;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012 Mar 28;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). JOURNAL OF CLINICAL ONCOLOGY: AMER SOC CLINICAL ONCOLOGY 2318 MILL ROAD, STE 800, ALEXANDRIA, VA 22314 USA; 2013.
- 11.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 12.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012 Feb 13;209(2):201–209. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 14.Crane C, Panner A, Murray J, Wilson S, Xu H, Chen L, et al. PI (3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28(2):306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sircar K, Yoshimoto M, Monzon FA, Koumakpayi IH, Katz RL, Khanna A, et al. PTEN genomic deletion is associated with p_Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. J Pathol. 2009;218(4):505–513. doi: 10.1002/path.2559. [DOI] [PubMed] [Google Scholar]
- 16.Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011 Oct 15;17(20):6563–6573. doi: 10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto M, Ding K, Sweet JM, Ludkovski O, Trottier G, Song KS, et al. PTEN losses exhibit heterogeneity in multifocal prostatic adenocarcinoma and are associated with higher Gleason grade. Modern Pathology. 2013;26(3):435–447. doi: 10.1038/modpathol.2012.162. [DOI] [PubMed] [Google Scholar]
- 18.Antonarakis ES, Keizman D, Zhang Z, Gurel B, Lotan TL, Hicks JL, et al. An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer. 2012;118(24):6063–6071. doi: 10.1002/cncr.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pretlow TG, Wolman SR, Micale MA, Pelley RJ, Kursh ED, Resnick MI, et al. Xenografts of primary human prostatic carcinoma. J Natl Cancer Inst. 1993 Mar 3;85(5):394–398. doi: 10.1093/jnci/85.5.394. [DOI] [PubMed] [Google Scholar]
- 20.Koochekpour S, Maresh GA, Katner A, Parker_Johnson K, Lee T, Hebert FE, et al. Establishment and characterization of a primary androgen_responsive African_American prostate cancer cell line, E006AA. Prostate. 2004;60(2):141–152. doi: 10.1002/pros.20053. [DOI] [PubMed] [Google Scholar]
- 21.Russell PJ, Kingsley EA. Prostate Cancer Methods and Protocols. Springer; 2003. Human prostate cancer cell lines; pp. 21–39. [DOI] [PubMed] [Google Scholar]
- 22.Korenchuk S, Lehr JE, MClean L, Lee YG, Whitney S, Vessella R, et al. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001 Mar-Apr;15(2):163–168. [PubMed] [Google Scholar]
- 23.Haile ST, Bosch JJ, Agu NI, Zeender AM, Somasundaram P, Srivastava MK, et al. Tumor cell programmed death ligand 1-mediated T cell suppression is overcome by coexpression of CD80. J Immunol. 2011 Jun 15;186(12):6822–6829. doi: 10.4049/jimmunol.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Romigh T, He X, Tan M, Orloff M, Silverman R, et al. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene. 2011;30(42):4327–4338. doi: 10.1038/onc.2011.144. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg MV, Drake CG. Cancer Immunology and Immunotherapy. Springer; 2011. LAG-3 in cancer immunotherapy; pp. 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994 Jul 1;265(5168):106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 27.Lin PY, Sun L, Thibodeaux SR, Ludwig SM, Vadlamudi RK, Hurez VJ, et al. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J Immunol. 2010 Sep 1;185(5):2747–2753. doi: 10.4049/jimmunol.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X, Chen S, Asara JM, Balk SP. Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits. J Biol Chem. 2010 May 14;285(20):14980–14989. doi: 10.1074/jbc.M109.085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. The American journal of pathology. 1999;155(6):1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Soria J, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degl’Innocenti E, Grioni M, Boni A, Camporeale A, Bertilaccio MT, Freschi M, et al. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur J Immunol. 2005;35(1):66–75. doi: 10.1002/eji.200425531. [DOI] [PubMed] [Google Scholar]
- 32.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008 Sep 2;105(35):13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014 Jul 1;20(13):3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001 Dec 4;98(25):14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson A, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348(1):9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12(4957):71. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 37.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nature Reviews Immunology. 2010;10(8):580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File # 1 (Figure S-1). PD-L2 expression was observed rarely and only in response to IFN-γ in the primary cell line, E006AA, and the metastatic bone cell line, PC3. The expression of MHCII closely mirrored that of PD-L1. IFN-γ induced expression of MHCII in all lines except, LAPC-4, LNCaP, and DU145 where it was absent.
Supplemental File # 2 (Figure S-2). Expression of PD-L1, PD-L2, and MHCII was not altered by bicalutamide in any cell line.
Supplemental File # 3. Full Western Blot with size markers and actin quantitation.
Supplemental File # 4. Low levels of PD-L1 expression not meeting established criteria for positivity were detected on >50% of samples often in association with PIA.