Abstract
Objective:
To determine the association of lipoprotein-associated phospholipase A2 (Lp-PLA2) measured in the acute period and the short-term risk of recurrent vascular events in patients with TIA or minor stroke.
Methods:
We measured Lp-PLA2 activity (Lp-PLA2-A) in a subset of 3,201 participants enrolled in the CHANCE (Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events) trial. Participants with TIA or minor stroke were enrolled within 24 hours of symptom onset and randomized to single or dual antiplatelet therapy. In the current analysis, the primary outcome was defined as the composite of ischemic stroke, myocardial infarction, or death within 90 days.
Results:
The composite endpoint occurred in 299 of 3,021 participants (9.9%). The population average Lp-PLA2-A level was 209 ± 59 nmol/min/mL (95% confidence interval [CI] 207–211). Older age, male sex, and current smoking were associated with higher Lp-PLA2-A levels. Lp-PLA2-A was significantly associated with the primary endpoint (adjusted hazard ratio 1.07, 95% CI 1.01–1.13 for every 30 nmol/min/mL increase). Similar results were seen for ischemic stroke alone. Adjustment for low-density lipoprotein cholesterol attenuated the association between Lp-PLA2-A and the primary endpoint (adjusted hazard ratio 1.04, 95% CI 0.97–1.11 for every 30 nmol/min/mL increase).
Conclusions:
Higher levels of Lp-PLA2-A in the acute period are associated with increased short-term risk of recurrent vascular events.
Lipoprotein-associated phospholipase A2 (Lp-PLA2), an enzyme associated with metabolism of low-density lipoprotein to proinflammatory mediators, has been linked to atherosclerotic plaque inflammation and instability.1,2 In pathologic studies of plaque specimens obtained following carotid endarterectomy, expression of Lp-PLA2 is increased in patients with recent stroke or TIA compared with asymptomatic patients.3 Both Lp-PLA2 mass and activity can be easily measured in blood samples, and elevated levels have been shown to predict long-term risk of stroke and myocardial infarction.4 Less is known about the relationship between Lp-PLA2 levels and the short-term risk of recurrent vascular events, although several small studies have suggested that Lp-PLA2-A, but not Lp-PLA2 mass, may predict short-term risk of recurrent stroke after TIA.5,6 The aim of the present study was to examine the association of Lp-PLA2-A with short-term risk of recurrent vascular events in a large cohort of patients with acute TIA or minor stroke.
METHODS
The study population consisted of participants enrolled in the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial for whom stored blood samples were available for testing. Details about study rationale, design, and results have been published previously.7,8 Briefly, CHANCE was a randomized, double-blind, multicenter trial conducted in China that enrolled patients with acute TIA or minor stroke within 24 hours of symptom onset. TIA participants were required to be at moderate to high risk of stroke as categorized by an ABCD2 score of ≥4. The ABCD2 score is a clinical risk score that stratifies risk of stroke after TIA.9 Stroke participants were required to have minor stroke as defined by an NIH Stroke Scale score of ≤3. Participants were randomized to one of 2 groups. The first received clopidogrel (loading dose of 300 mg followed by 75 mg daily for 3 months) plus aspirin (loading dose of 75–300 mg followed by 75 mg daily for 21 days), while the second group received aspirin (loading dose of 75–300 mg followed by 75 mg daily for 3 months). The selection of the aspirin loading dose between 75 and 300 mg was at the discretion of the treating investigator in both groups. The primary outcome measure was the risk of any stroke, including both ischemic and hemorrhagic stroke, within a 3-month period. Secondary outcomes included incidence of any vascular event or death. For the current analysis, the primary outcome measure was specified as a composite of ischemic stroke, myocardial infarction, and death, with ischemic stroke alone as a secondary outcome measure.
Standard protocol approvals, registrations, and patient consents.
The CHANCE trial is registered with clinicaltrials.gov (NCT00979589). All of the participants or their legal proxies provided written informed consent. The CHANCE protocol was approved by the ethics committee at each study center.
Blood sampling.
A subset of 73 CHANCE centers participated in blood sample collection. Venous blood samples were obtained under sterile conditions within 48 hours of symptom onset. Samples were collected in serum-separation tubes and centrifuged within 2 hours at 1,500g for 15 minutes. Serum was extracted and samples were stored at −80°C until testing was performed. Lp-PLA2-A was measured with an automated enzyme assay system run on a Hitachi 7,600 analyzer (PLAC test for Lp-PLA2-A; diaDexus Inc., San Francisco, CA). The intra-assay coefficient of variation for the Lp-PLA2-A assay is ≤2.9% and the interassay coefficient of variation is ≤3.0%. Low-density lipoprotein cholesterol (LDL-C) was measured using a Roche Modular P800 system (Roche, Basel, Switzerland). All assays were performed at a central laboratory at Tiantan Hospital with laboratory personnel blinded to clinical data.
Statistical analysis.
Variables were summarized using means and 95% conference intervals (CIs) for continuous variables, with frequencies and percentages for categorical variables. Student t test was used to compare group differences for continuous variables, and χ2 tests for dichotomous variables. Cox proportional hazard models were applied to examine the association between patient characteristics and the primary endpoint. Lp-PLA2-A levels were dichotomized to ≤225 or >225 nmol/min/mL. This threshold was prespecified based on prior studies of acute stroke and TIA that suggested a substantially increased risk of recurrence associated with levels >150 nmol/min/mL using an older microplate Lp-PLA2-A assay run at room temperature.5,10,11 The newer-generation Lp-PLA2-A assay used in the present study runs at 37°C on automated analyzers and produces values approximately 1.5 times higher than the previous microplate assay. The choice of the 225 nmol/min/mL threshold was thus based on a calculation of the level from the newer-generation assay equivalent to the 150 nmol/min/mL threshold using the older-generation assay. To examine whether there is a nonlinear relationship between Lp-PLA2-A levels and outcome, we also tested the association between the quadratic and cubic term and the primary endpoint. A treatment by Lp-PLA2-A level interaction was also tested. Multivariable models were constructed with adjustment for imbalances between groups in demographic factors, risk factors, enrolling event (TIA vs stroke), and study intervention. Variables were included in the model if there was imbalance across groups with p < 0.10. All variables that were associated with the composite outcome in univariate analysis with p < 0.10 were also included in the final model. A post hoc analysis adjusting for LDL-C in a subset of patients with available LDL-C measurements was also performed. All tests were 2-sided. An association was considered statistically significant if p < 0.05. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
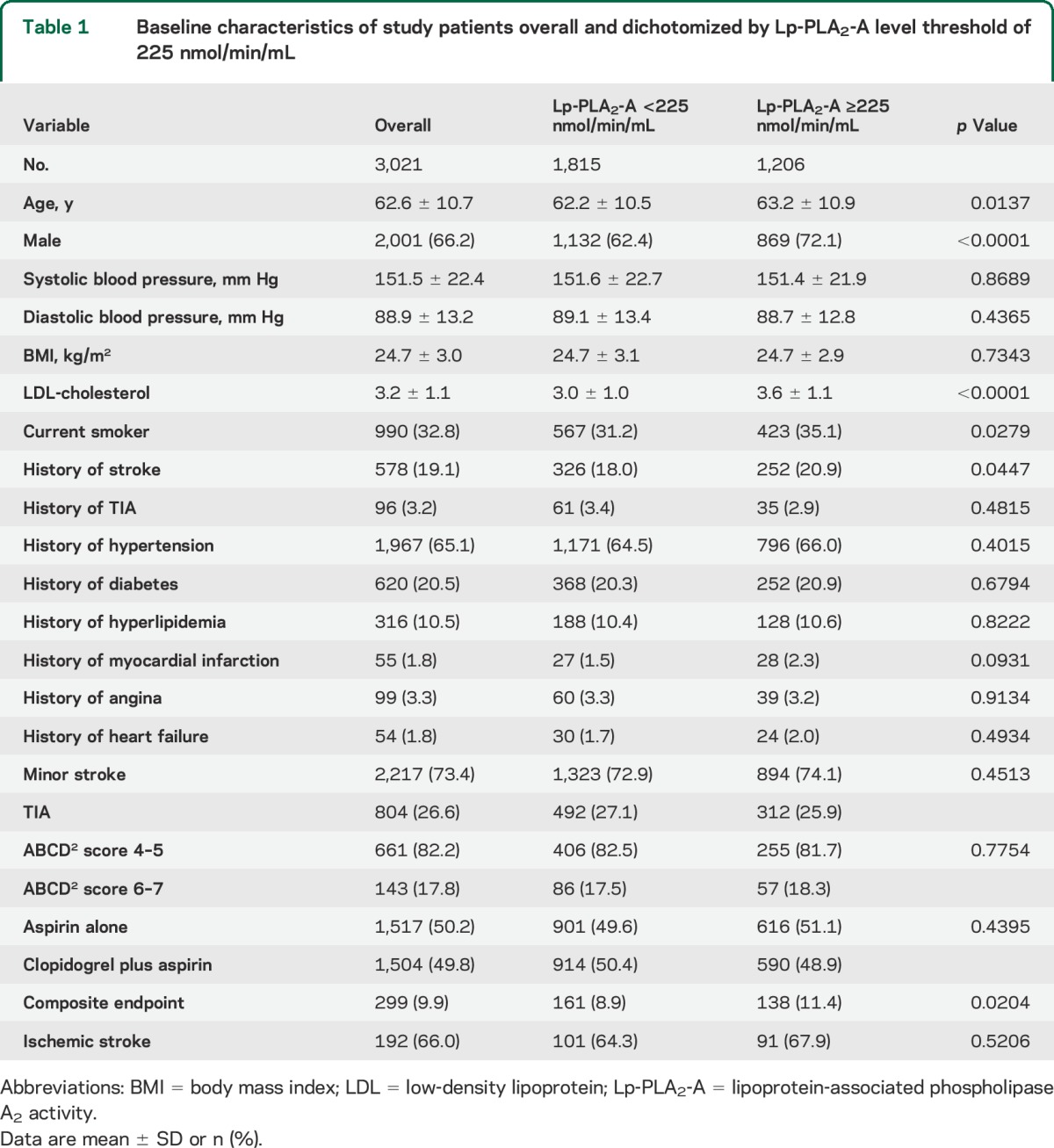
From 2009 to 2012, 5,170 patients were enrolled in the CHANCE trial. Of these, 3,021 patients from 73 sites participated in the blood biomarker substudy. Among the 3,021 participants, the mean Lp-PLA2-A level was 209.3 nmol/min/mL (95% CI 207.2–211.4) and 1,206 (39.9%) had Lp-PLA2-A levels ≥225 nmol/min/mL. Baseline characteristics of the 3,021 included patients are shown in table 1. Comparing those with Lp-PLA2-A levels above vs below the 225 nmol/min/mL threshold, those with higher levels were more likely to be older, be male, smoke, have a history of prior stroke, and have higher LDL-C (table 1).
Table 1.
Baseline characteristics of study patients overall and dichotomized by Lp-PLA2-A level threshold of 225 nmol/min/mL
Outcomes.
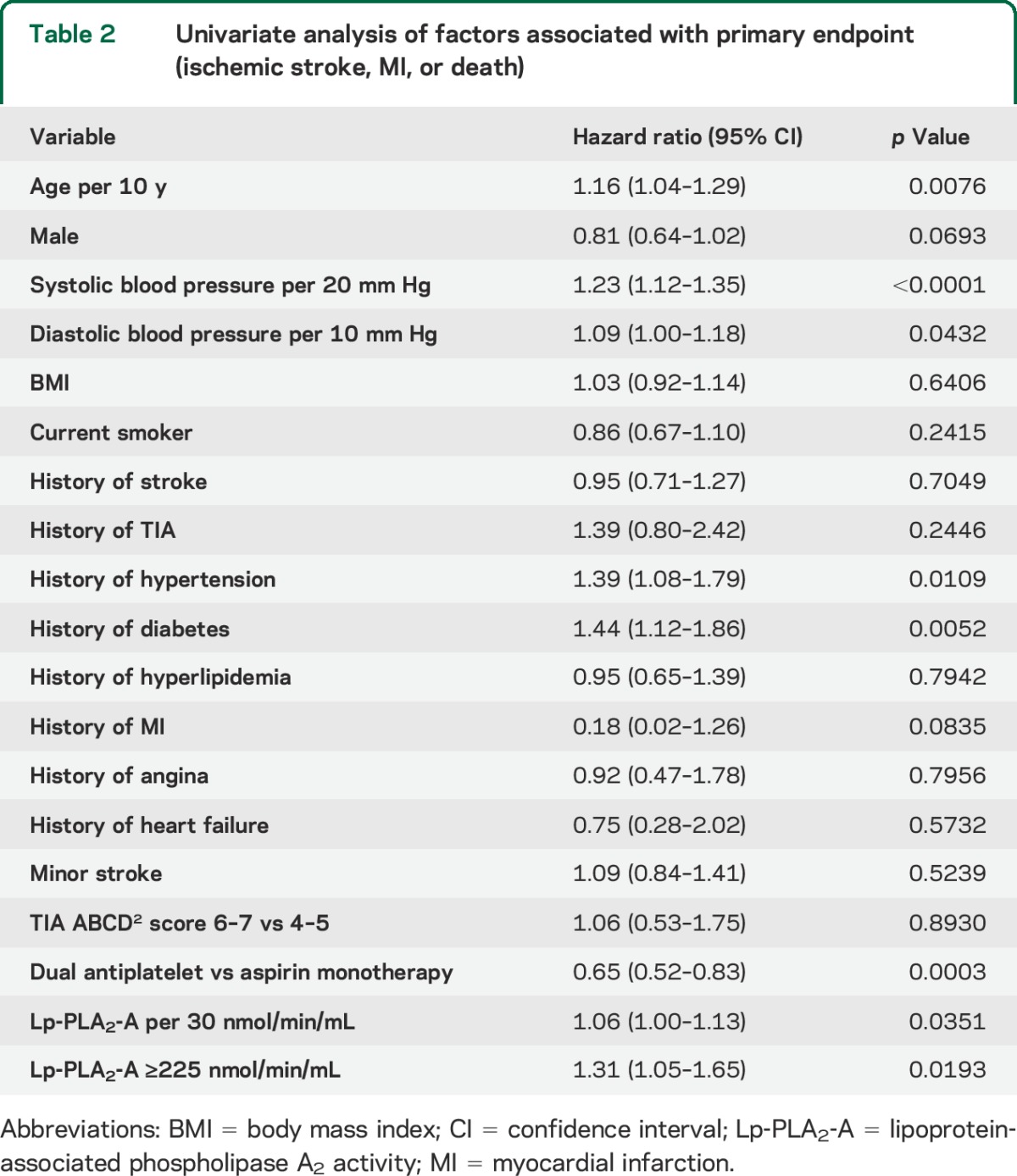
The composite endpoint of ischemic stroke, myocardial infarction, or death occurred in 299 of 3,021 participants (9.9%). Ischemic stroke occurred in 291 participants (9.6%), myocardial infarction in 2 participants (0.07%), and death in 6 participants (0.2%). Older age, higher systolic and diastolic blood pressures, history of hypertension, history of diabetes, higher Lp-PLA2-A level, and assignment to aspirin alone were associated with a greater risk of the composite outcome (table 2). Nearly identical results were seen for ischemic stroke alone (table e-1 on the Neurology® Web site at Neurology.org).
Table 2.
Univariate analysis of factors associated with primary endpoint (ischemic stroke, MI, or death)
Lp-PLA2-A levels and outcome.
When tested as a linear variable, Lp-PLA2-A levels were higher in older patients, men, current smokers, and those with a history of myocardial infarction (table 3). After adjustment for age, sex, systolic blood pressure, hypertension, diabetes, prior myocardial infarction, and antiplatelet treatment, Lp-PLA2-A level was significantly linearly associated with the primary endpoint (hazard ratio [HR] 1.07, 95% CI 1.01–1.13, per 30 nmol/min/mL increase) (table 4). This linear association also indicated that the treatment assignment did not modify the effect of Lp-PLA2-A on the given outcome (p = 0.32 for the treatment by Lp-PLA2-A interaction). Using a threshold Lp-PLA2-A level of 225 nmol/min/mL, we found that the patient group with Lp-PLA2-A at or above this level had a 30% increased risk of the primary endpoint (adjusted HR 1.30, 95% CI 1.03–1.63, p = 0.026). Similar results were found for ischemic stroke alone (table e-2).
Table 3.
Lp-PLA2-A levels by patient characteristics
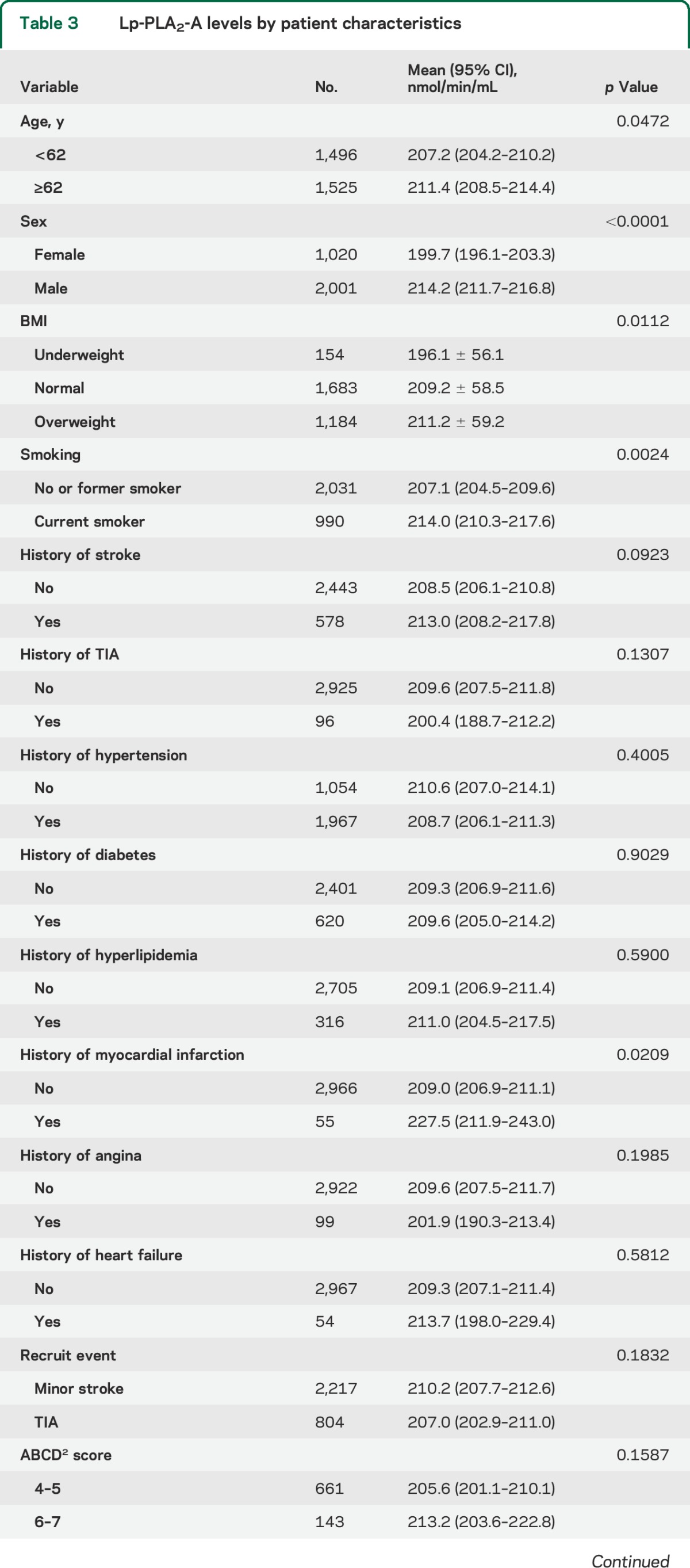
Table 4.
Multivariable analysis of factors associated with primary endpoint (ischemic stroke, MI, or death)
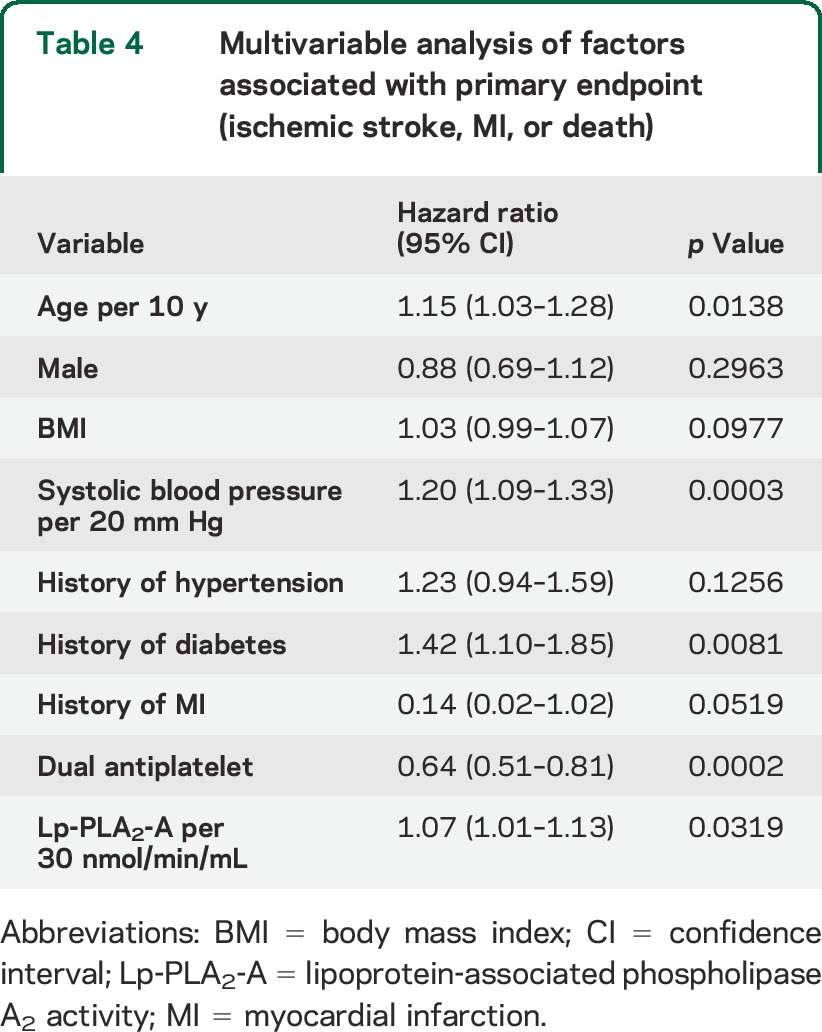
For the test of the goodness of the multivariable model fit, we found that neither the quadratic (p = 0.86) nor the cubic (p = 0.62) trend of Lp-PLA2-A level was found to be associated with the primary endpoint, which meant that the model was constructed successfully.
Interaction between LDL-C and Lp-PLA2-A.
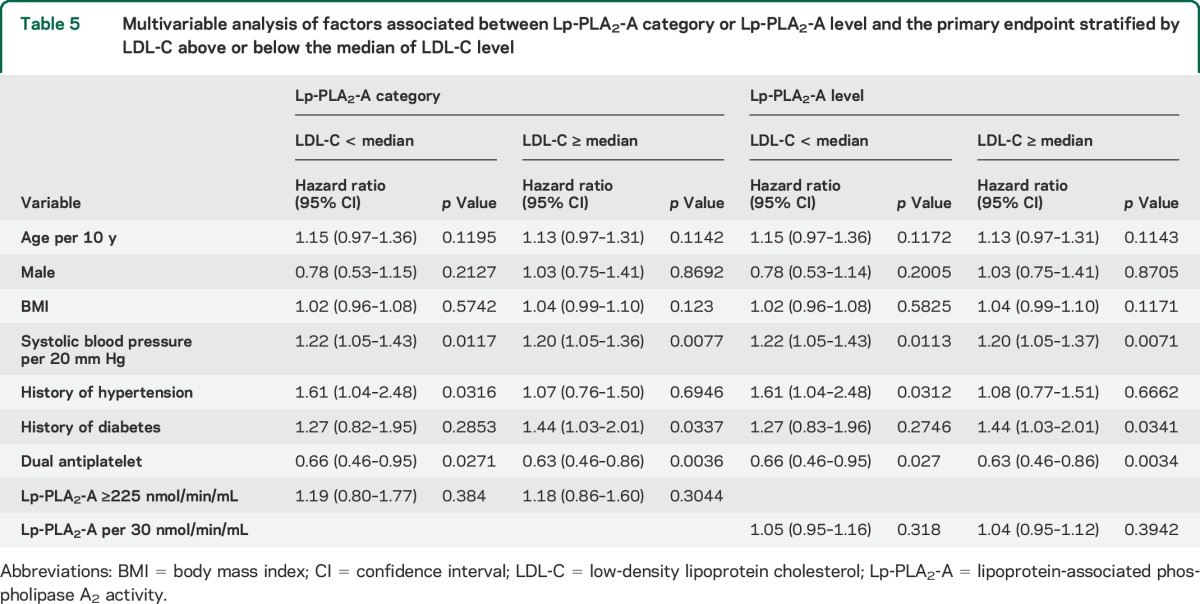
LDL-C levels were available in a subset of 2,936 patients. Mean LDL-C was 3.22 (SD: 1.07) and median LDL-C was 3.12 (IQR: 2.49–3.82). LDL-C and Lp-PLA2-A were highly correlated (Pearson r = 0.36, p < 0.0001). In univariate analysis, LDL-C was associated with the primary endpoint (HR 1.14, 95% CI 1.02–1.27, p = 0.025). When LDL-C was entered into the multivariable model, the association between Lp-PLA2-A and the primary endpoint was attenuated and was no longer significant (adjusted HR 1.04, 95% CI 0.97–1.11). Analysis comparing the association between Lp-PLA2-A, used as a categorical or continuous variable, and the primary endpoint stratified by LDL-C above or below the median LDL-C level showed no significant difference between those with high compared with low LDL-C (table 5).
Table 5.
Multivariable analysis of factors associated between Lp-PLA2-A category or Lp-PLA2-A level and the primary endpoint stratified by LDL-C above or below the median of LDL-C level
DISCUSSION
Considerable data support an association between Lp-PLA2 measured in clinically stable patients and the long-term risk of stroke and other vascular events. In a pooled, collaborative analysis of 79,036 participants in 32 separate prospective studies, Lp-PLA2-A and mass were both associated with a continuous increased risk of stroke and myocardial infarction.4 Much less is known about the short-term risk of recurrence associated with Lp-PLA2 levels drawn in the setting of an acute vascular event. Several small studies have examined Lp-PLA2 levels measured in the acute period after TIA or stroke. For example, a US study of patients with acute TIA (n = 167) found that elevated Lp-PLA2 activity and mass were both associated with a large vessel TIA etiology, and that Lp-PLA2-A was associated with early recurrent stroke or death.5 Similar findings were reported in a Spanish cohort with acute TIA, with Lp-PLA2-A being associated both with large vessel disease and with early recurrent cerebrovascular events within 7 and 30 days.6 A somewhat larger study including patients with both acute stroke and TIA found Lp-PLA2-A to be associated with recurrent events at 6 months.11 The results of the present study are consistent with these prior data. Lp-PLA2-A level was significantly associated with the primary endpoint such that the risk of recurrence increased 7% for every 30 nmol/min/mL increase of Lp-PLA2-A.
In contrast, 2 studies examining Lp-PLA2-A measured in the setting of acute coronary syndromes (ACS) did not find an association with outcome. In the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial, there was no relationship between baseline Lp-PLA2-A and vascular events within 16 weeks.12 In the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) trial, there was no relationship between baseline Lp-PLA2-A and recurrent events over an average of 2 years of follow-up.13 There are important differences between the TIA/minor stroke and ACS populations, as well as differences in individual studies, which might account for these discrepant findings. Stroke and TIA have heterogeneous causes, ranging from cardioembolism to large vessel atherosclerotic stenosis to small vessel disease, in contrast to ACS, which has a more homogeneous pathophysiology. Risk of recurrent events is dramatically higher in stroke and TIA due to large vessel stenosis as opposed to other causes.14 As a marker of unstable atherosclerotic plaque, Lp-PLA2-A may therefore be identifying those TIA and stroke patients with a large vessel mechanism and thus higher risk. In contrast, as most patients with ACS have large vessel atherosclerotic disease with unstable plaque, the ability to discriminate risk among patients on this basis may be reduced. In addition, differences in the timing of blood sampling might be important, as Lp-PLA2-A levels change dynamically early after a vascular event. In a study of longitudinal samples collected in the NOMAS (Northern Manhattan Study), Lp-PLA2-A levels dropped about 10% in the days following acute ischemic stroke and about 30% following acute myocardial infarction.15 Samples in the MIRACL study were obtained 24 to 96 hours after hospital admission, and in PROVE-IT within 10 days of the index event; in contrast, in CHANCE and other TIA/minor stroke studies, samples were obtained <48 hours from symptom onset.
The observed association of Lp-PLA2-A with short-term vascular risk in our cohort is consistent with the hypothesis that Lp-PLA2-A, as measured in peripheral blood, may reflect plaque instability and therefore early risk of recurrence. This suggests that therapies designed to suppress Lp-PLA2 might have a role in the treatment of acute cerebrovascular disease.16 Statin therapy significantly reduces Lp-PLA2 levels, and this could represent one potential biological mechanism by which early administration of statin therapy might reduce the risk of recurrence in patients with acute TIA or minor stroke. However, the specific Lp-PLA2 inhibitor darapladib has been tested in patients with coronary artery disease in 2 recently published large randomized studies (SOLID-TIMI and STABILITY), neither of which demonstrated benefit.17,18 The results of these trials suggest the possibility that Lp-PLA2 may be merely a marker of unstable plaque rather than having an important mechanistic role in clinical atherothrombotic events.
We found a strong correlation between LDL-C and Lp-PLA2, and the association between Lp-PLA2 and vascular events was attenuated after adjustment for LDL-C. Some prior studies have suggested that the association between Lp-PLA2 and vascular events is strongest in patients with lower LDL-C levels,19 whereas others have found no significant difference in the predictive value of Lp-PLA2 based on baseline LDL-C level.20 In our cohort, there was no evidence of variability in the association between Lp-PLA2 and vascular risk based on baseline LDL-C.
Our study has several limitations. First, data on specific TIA/stroke mechanisms were not available, so we were not able to examine whether Lp-PLA2-A levels correlated with a large vessel atherosclerotic mechanism as has been reported in earlier studies. Second, we did not have detailed information on baseline and subsequent statin use. Statin therapy both lowers Lp-PLA2 levels and has been associated with an attenuation of the risk associated with elevated Lp-PLA2-A.16 For instance, in the JUPITER trial, rosuvastatin lowered Lp-PLA2-A levels by 33%; while there was a significant relationship between Lp-PLA2-A and cardiovascular events in patients randomized to placebo, there was no relationship in those receiving rosuvastatin.21 Third, comparison with prior studies of Lp-PLA2 may be limited by the different populations studied. The CHANCE trial was conducted exclusively in China, whereas most of the earlier data are derived from Western populations. Different patterns of atherosclerotic disease, for instance the greater prevalence of intracranial stenosis as opposed to extracranial stenosis in Asian populations, may limit extrapolation to other groups. Fourth, patients with a known cardioembolic source at presentation were excluded from enrollment in CHANCE, so the population of TIA/stroke patients in the present study may differ from that included in purely observational studies that had broader inclusion criteria.
Our results demonstrate an association between Lp-PLA2-A measured in the acute period after TIA or minor stroke and the short-term risk of recurrent vascular events. This association remained significant after adjustment for traditional prognostic factors. Future studies to better understand the mechanistic link between Lp-PLA2-A levels and early recurrence might lead to greater insight into the biological basis of early recurrent cerebrovascular events.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all participating investigators for their support.
GLOSSARY
- ABCD2
age, blood pressure, clinical features, duration of TIA, and presence of diabetes
- ACS
acute coronary syndrome
- CHANCE
Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events
- CI
confidence interval
- HR
hazard ratio
- LDL-C
low-density lipoprotein cholesterol
- Lp-PLA2
lipoprotein-associated phospholipase A2
- Lp-PLA2-A
lipoprotein-associated phospholipase A2 activity
- MIRACL
Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering
- PROVE-IT
Pravastatin or Atorvastatin Evaluation and Infection Therapy
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: CHANCE investigators, Yongjun Wang, S.Claiborne Johnston, Yilong Wang, Xingquan Zhao, Zhimin Wang, Haiqin Xia, Guiru Zhang, Xudong Ren, Chunling Ji, Guohua Zhang, Jianhua Li, Bohua Lu, Liping Wang, Shutao Feng, Dali Wang, Weiguo Tang, Juntao Li, Hongtian Zhang, Guanglai Li, Baojun Wang, Yuhua Chen, Ying Lian, Bin Liu, Junfang Teng, Rubo Sui, Lejun Li, Zhiling Yuan, Dawei Zang, Zuneng Lu, Li Sun, Dong Wang, Liying Hou, Dongcai Yuan, Yongliang Cao, Hui Li, Xiuge Tan, Huicong Wang, Haisong Du, Mingyi Liu, Suping Wang, Qiuwu Liu, Zhong Zhang, Qifu Cui, Runqing Wang, Jialin Zhao, Jiewen Zhang, Jianping Zhao, Qi Bi, Xiyou Qi, Junyan Liu, Changxin Li, Ling Li, Xiaoping Pan, Junling Zhang, Derang Jiao, Zhao Han, Dawei Qian, Jin Xiao, Yan Xing, Huishan Du, Guang Huang, Yongqiang Cui, Yan Li, Lianyuan Feng, Lianbo Gao, Bo Xiao, Yibin Cao, Yiping Wu, Jinfeng Liu, Zhiming Zhang, Zhengxie Dong, Limin Wang, Li He, Xinchen Wang, Xueying Guo, Ming Wang, Xiaosha Wang, Jiandong Jiang, Renliang Zhao, Shengnian Zhou, Hao Hu, Maolin He, Fengchun Yu, Quping Ouyang, Jingbo Zhang, Anding Xu, Xiaokun Qi, Lei Wang, Fuming Shi, Fuqiang Guo, Jianfeng Wang, Fengli Zhao, Ronghua Dou, Dongning Wei, Qingwei Meng, Yilu Xia, Shimin Wang, Zhangcang Xue, Yuming Xu, Liping Ma, Chun Wang, Jiang Wu, Yifeng Du, Yinzhou Wang, Lijun Xiao, Fucong Song, Wenli Hu, Zhigang Chen, Qingrui Liu, Jiemin Zhang, Mei Chen, Xiaodong Yuan, Zhihui Liu, Guozhong Li, Xiaohong Li, and Tingchen Tian
AUTHOR CONTRIBUTIONS
Jinxi Lin performed the experiments, interpreted the data, and wrote drafts of the manuscript. Hongwei Zheng interpreted the data and commented on drafts. Brett L. Cucchiara supervised the analysis, interpreted the data, and commented on drafts. Jiejie Li performed the experiments and interpreted the data. Xianhong Liang performed the experiments and interpreted the data. Xingquan Zhao and Chunxue Wang supervised the analysis. Hao Li and Michael T. Mullen conducted the statistical analyses and interpreted the data. S. Claiborne Johnston designed and supervised the analysis. Yilong Wang and Yongjun Wang formulated the research question and designed and supervised the analysis.
STUDY FUNDING
Supported by the Ministry of Science and Technology of the People's Republic of China (2011BAI08B02 to Yilong Wang; 2006BAI01A10 to Xingquan Zhao; 2012ZX09303-005-001 to Yongjun Wang); Beijing Biobank of Cerebral Vascular Disease (D131100005313003 to Yongjun Wang); the National Natural Science Foundation of China (81322019 to Yilong Wang; 81471211 to Yongjun Wang); Bejing Institute for Brain Disorders (BIBD_PXM2014_014226_000016 to Yongjun Wang); and Seed Grant of International Alliance of Translational Neuroscience (PXM2014_014226_000006 to Yongjun Wang). The PLAC assay kits for Lp-PLA2-A are provided by diaDexus. This work was supported in part by funding from the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03 to Jizong Zhao) and the Ministry of Science and Technology of the People's Republic of China (2011BAI08B01 to Gaifen Liu).
DISCLOSURE
J. Lin and H. Zheng report no disclosures relevant to the manuscript. B. Cucchiara is a consultant for Bayer 2013–present, Bristol-Myers Squibb 2014–present, Boehringer Ingelheim 2013–2014, diaDeduxus 2010, receives royalties from the publication Intraventricular Hemorrhage UpToDate and Antiplatelet Therapy for Secondary Stroke Prevention 2009–2010, is funded by NIH U01S062835 as Bristol-Myers Squibb, principal investigator (PI), 2012–present, also serves as local PI, 2010–present, Biogen, local PI, 2013–present. J. Li received research support from the National Natural Science Foundation of China (81200844) and Beijing Postdoctoral Working Funding (2014ZZ-10). X. Zhao received research support from the Ministry of Science and Technology of the People's Republic of China (2006BAI01A10). X. Liang, C. Wang, and H. Li report no disclosures relevant to the manuscript. M. Mullen is funded by AHRQ R01HS010914 and NINDS R01 NS060653-06. C. Johnston is the Dean of the Dell Medical School at The University of Texas at Austin, Professor and Vice Chancellor of Research at The University of California at San Francisco, performs research study “SOCRATES” supported by AstraZeneca, received “Drug and Placebo” from Sanofi, and serves as expert witness in “Sarie Francis” trial. Yilong Wang received research support from the Ministry of Science and Technology of the People's Republic of China (2011BAI08B02) and the National Natural Science Foundation of China (81322019). Yongjun Wang received research support from the Ministry of Science and Technology of the People's Republic of China (2012ZX09303-005-001), Beijing Biobank of Cerebral Vascular Disease (D131100005313003), the National Natural Science Foundation of China (81471211), Beijing Institute for Brain Disorders (BIBD_PXM2014_014226_000016), and Seed Grant of International Alliance of Translational Neuroscience (PXM2014_014226_000006). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Garcia-Garcia HM, Serruys PW. Phospholipase A2 inhibitors. Curr Opin Lipidol 2009;20:327–332. [DOI] [PubMed] [Google Scholar]
- 2.Wilensky RL, Shi Y, Mohler ER, III, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med 2008;14:1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannheim D, Herrmann J, Versari D, et al. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke 2008;39:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 2010;375:1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucchiara BL, Messe SR, Sansing L, et al. Lipoprotein-associated phospholipase A2 and C-reactive protein for risk-stratification of patients with TIA. Stroke 2009;40:2332–2336. [DOI] [PubMed] [Google Scholar]
- 6.Delgado P, Chacon P, Penalba A, et al. Lipoprotein-associated phospholipase A(2) activity is associated with large-artery atherosclerotic etiology and recurrent stroke in TIA patients. Cerebrovasc Dis 2012;33:150–158. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Johnston SC. Rationale and design of a randomized, double-blind trial comparing the effects of a 3-month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J 2010;160:380–386.e1. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 9.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283–292. [DOI] [PubMed] [Google Scholar]
- 10.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. Lipoprotein-associated phospholipase A2 activity and risk of recurrent stroke. Cerebrovasc Dis 2009;27:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furie K, Parides M, Greer D, et al. Lipoprotein-associated phospholipase A2 activity predicts early stroke recurrence. Stroke 2007;38:458. [Google Scholar]
- 12.Ryu SK, Mallat Z, Benessiano J, et al. Phospholipase A2 enzymes, high-dose atorvastatin, and prediction of ischemic events after acute coronary syndromes. Circulation 2012;125:757–766. [DOI] [PubMed] [Google Scholar]
- 13.O'Donoghue M, Morrow DA, Sabatine MS, et al. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction) trial. Circulation 2006;113:1745–1752. [DOI] [PubMed] [Google Scholar]
- 14.Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke 2012;43:1013–1017. [DOI] [PubMed] [Google Scholar]
- 15.Elkind MS, Leon V, Moon YP, Paik MC, Sacco RL. High-sensitivity C-reactive protein and lipoprotein-associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke 2009;40:3233–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida K, Cucchiara B. Therapeutic options to reduce Lp-PLA2 levels and the potential impact on vascular risk reduction. Curr Treat Options Cardiovasc Med 2013;15:313–321. [DOI] [PubMed] [Google Scholar]
- 17.O'Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014;312:1006–1015. [DOI] [PubMed] [Google Scholar]
- 18.White HD, Held C, Stewart R, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 19.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Int Med 2006;166:2073–2080. [DOI] [PubMed] [Google Scholar]
- 20.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 2005;111:570–575. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, MacFadyen JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A(2) mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin Chem 2012;58:877–886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


