Abstract
Objectives:
The aims of this study were to determine the etiology, clinical features, and predictors of outcome of new-onset refractory status epilepticus.
Methods:
Retrospective review of patients with refractory status epilepticus without etiology identified within 48 hours of admission between January 1, 2008, and December 31, 2013, in 13 academic medical centers. The primary outcome measure was poor functional outcome at discharge (defined as a score >3 on the modified Rankin Scale).
Results:
Of 130 cases, 67 (52%) remained cryptogenic. The most common identified etiologies were autoimmune (19%) and paraneoplastic (18%) encephalitis. Full data were available in 125 cases (62 cryptogenic). Poor outcome occurred in 77 of 125 cases (62%), and 28 (22%) died. Predictors of poor outcome included duration of status epilepticus, use of anesthetics, and medical complications. Among the 63 patients with available follow-up data (median 9 months), functional status improved in 36 (57%); 79% had good or fair outcome at last follow-up, but epilepsy developed in 37% with most survivors (92%) remaining on antiseizure medications. Immune therapies were used less frequently in cryptogenic cases, despite a comparable prevalence of inflammatory CSF changes.
Conclusions:
Autoimmune encephalitis is the most commonly identified cause of new-onset refractory status epilepticus, but half remain cryptogenic. Outcome at discharge is poor but improves during follow-up. Epilepsy develops in most cases. The role of anesthetics and immune therapies warrants further investigation.
Status epilepticus (SE) is the second most common neurologic emergency.1 Up to 40% of SE cases are refractory (RSE) to first- and second-line treatments.2,3 New-onset RSE (NORSE) is a rare but challenging condition, characterized by the occurrence of a prolonged period of refractory seizures with no readily identifiable cause in otherwise healthy individuals.4 Approximately 40 adult cases have been reported, describing a febrile illness–related NORSE syndrome.4–9 It is likely that some of the cases of SE attributed to a “possible” encephalitis would qualify as NORSE.2,3,10,11 The absence of a proven etiology was mandatory in the early series but some have suggested that autoimmune encephalitis4,12–14 may emerge as a common cause of NORSE.4,14,15 Viral encephalitis is another plausible etiology, but viruses are rarely identified in cases of encephalitis with RSE.16
RSE is associated with substantial morbidity and mortality,2,3,11,13,17 including in patients with encephalitis.18 Anesthetic medications have been associated with worse outcome in prior studies, although a causal relationship was not established.19,20 Anecdotal evidence suggests that immune therapies might be effective in autoimmune cases of SE12 and in NORSE.7,8
This study aimed to describe a large cohort of patients with NORSE and determine its etiology, clinical features, response to treatment, and prognostic features to help guide management and plan prospective treatment trials.
METHODS
Study design.
A multicenter retrospective study across 13 academic medical centers belonging to the Critical Care EEG Monitoring Research Consortium (CCEMRC) was performed. Prospective EEG databases were searched between January 1, 2008, and December 31, 2013, for patients with RSE.21 These dates were selected because of the availability of anti-NMDA receptor (NMDAR) antibodies testing.
The inclusion criteria were as follows: (1) age 18 years or older; (2) SE refractory to appropriate doses of 2 lines of antiseizure treatment21; (3) no definite etiology identified by history and ancillary tests within the first 48 hours after admission (thus excluding acute brain injury, bacterial meningitis or abscess, herpes encephalitis, known seizure disorder, acute medical condition); (4) ≥24 hours of continuous EEG (CEEG) monitoring; and (5) paraneoplastic/autoimmune panel ordered. Nonspecific abnormalities on MRI or on CSF analysis (pleocytosis and elevated protein levels) were not considered exclusionary. Data from medical charts, EEG reports, imaging reports, and results of laboratory tests and pathology were collected using a data dictionary. Collected clinical variables included age, sex, presence and type of prodromal symptoms, presence and type of seizures and SE before admission, consciousness level on admission, duration of SE, duration of hospital and intensive care unit (ICU) stay, and ultimate control of SE. We also collected the number and type of antiseizure medications, anesthetics, and immune therapies received. Complications included need for vasopressors, severe acidosis (defined as the lowest pH during SE <7.2), renal dysfunction (defined as the highest creatinine level >1.2 mg/dL), hepatic dysfunction (defined as bilirubin level >2 mg/dL), cardiac injury (defined as the highest troponin I level >0.4 UI/L), need for mechanical ventilation, respiratory dysfunction (defined as the lowest Pa/Fio2 ratio <300 mm Hg), pulmonary embolism, pneumonia, urinary tract infection, bowel ischemia, gastrointestinal bleeding, anemia (defined as the lowest hemoglobin level <8 g/dL or transfusion), thrombocytopenia (defined as platelet count <150 × 103/mL), hypernatremia (defined as the highest sodium level >150 mmol/L), and hyponatremia (defined as the lowest sodium level <130 mmol/L). CEEG variables included time, presence, and type of seizures, SE, periodic discharges, and sporadic epileptiform discharges. Imaging variables included presence and location of abnormalities on brain MRI including fluid-attenuated inversion recovery, diffusion-weighted imaging, and gadolinium-enhanced images (on either initial MRI or repeat MRI, when available).
Identified etiologies were classified as autoimmune (presence of an autoantibody in the absence of a neoplasm), paraneoplastic (newly diagnosed neoplasm, with or without the presence of an antibody), infectious (presence of a pathogen demonstrated by culture, PCR, or serologic tests), or other.
The primary outcome measure was poor outcome (score >3 on the modified Rankin Scale [mRS]) at discharge. Secondary outcome measures included mortality at discharge, and functional outcome and presence of seizures or use of antiseizure medications at time of last follow-up.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the institutional review board at each institution, which granted us waiver of consent given the retrospective nature of the study.
Statistical analysis.
Statistical analyses were performed with R (The R Foundation, Vienna, Austria). Chi-square, Fisher, and Mann–Whitney tests were used as appropriate. The association between outcome and number of anesthetics was tested using the Cochran-Armitage trend test with the assumption that outcome worsened with an increasing number of anesthetics. Results are presented as median (interquartile range) or number (percent). Adjustment for multiple comparisons was performed with the false detection rate.22 A p value <0.0036 was considered significant and a p value between 0.0036 and 0.05 was considered a trend. We performed a multivariate logistic regression to establish the independent association of the use of anesthetics with both functional outcome measures (mRS score >3 and death), adjusting for known confounders (severity of SE, using the Status Epilepticus Severity Score [STESS], duration of SE, and number of complications).19
RESULTS
Demographics and etiology.
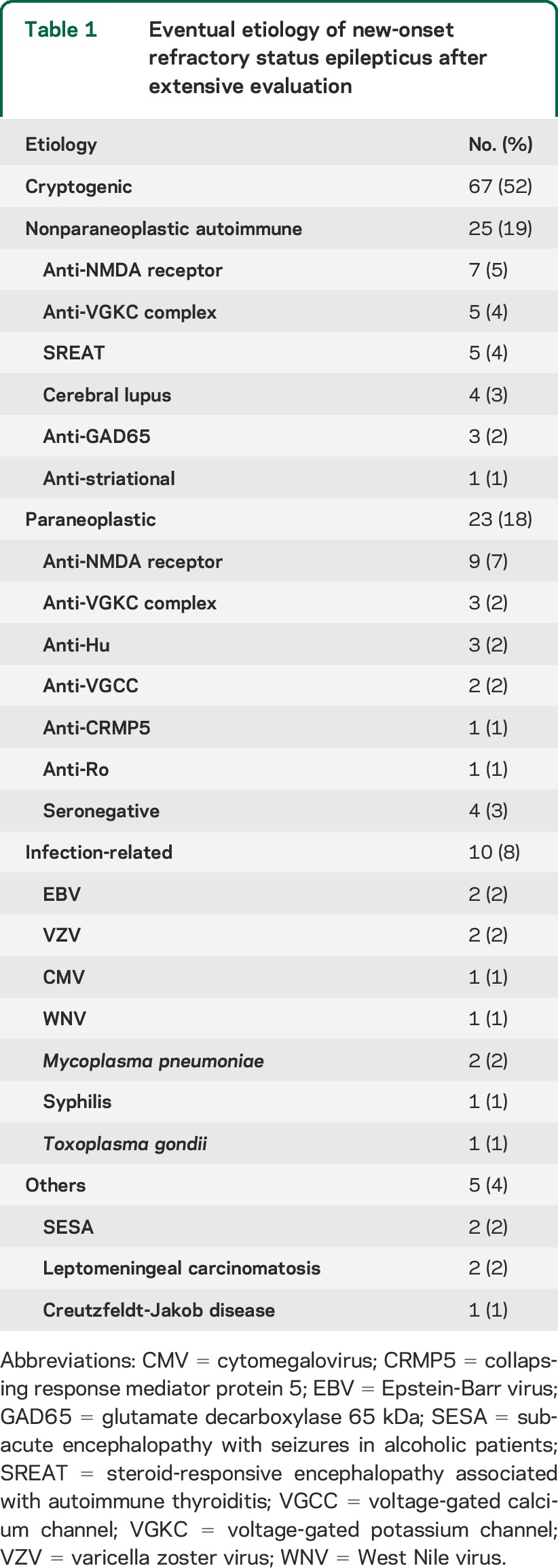
We identified 130 cases fulfilling our criteria among 675 cases with RSE. An etiology was eventually found in 63 of 130 cases (47%) (table 1). Nonparaneoplastic autoimmune (25/63 [40%]) and paraneoplastic (19/63 [30%]) cases represented more than two-thirds of this group, while infectious cases were less common (10/63 [16%]). The most frequent etiologies were encephalitis with anti-NMDAR antibodies, of which half were associated with an ovarian teratoma, and with anti–voltage-gated potassium channel complex (VGKCC) antibodies, of which a third were paraneoplastic (see table e-1 on the Neurology® Web site at Neurology.org). Herpes viruses (excluding herpes simplex virus 1) were the most frequent infectious agents (5/10 [50%]). Other causes are detailed in table 1. Sixty-seven of 130 cases (52%) remained cryptogenic despite an extensive workup. Among the cryptogenic cases, 66 of 67 benefited from a paraneoplastic panel (comprising anti-Hu in 66, anti-CRMP5/CV-2 in 59, anti-amphiphysin in 57, and anti-Ma2/Ta in 41). Anti-VGKCC, anti-NMDAR, anti-GAD65, anti-AMPAR, and anti-GABAB-receptor antibodies were tested in 48, 42, 40, 5, and 5 cryptogenic cases, respectively. An evaluation for an occult neoplasm was performed in 63 of 66 cases (43 with thoracoabdominal CT scan, and 20 with both whole-body fluorodeoxyglucose-PET and CT scan). Anti-NMDAR antibody testing was obtained in 87 of 130 (67%), including 48 of 67 (67%) cryptogenic cases.
Table 1.
Eventual etiology of new-onset refractory status epilepticus after extensive evaluation
Age ranged from 18 to 81 years and followed a bimodal distribution, with peaks at 28.5 and 65.5 years; 62 of 130 patients (48%) were older than 50 years. There was a female predominance (83:47).
Complete data about treatment, complications, and outcome at discharge were available in 125 of 130 cases (96%) (63/125 [50%] cryptogenic).
Clinical presentation.
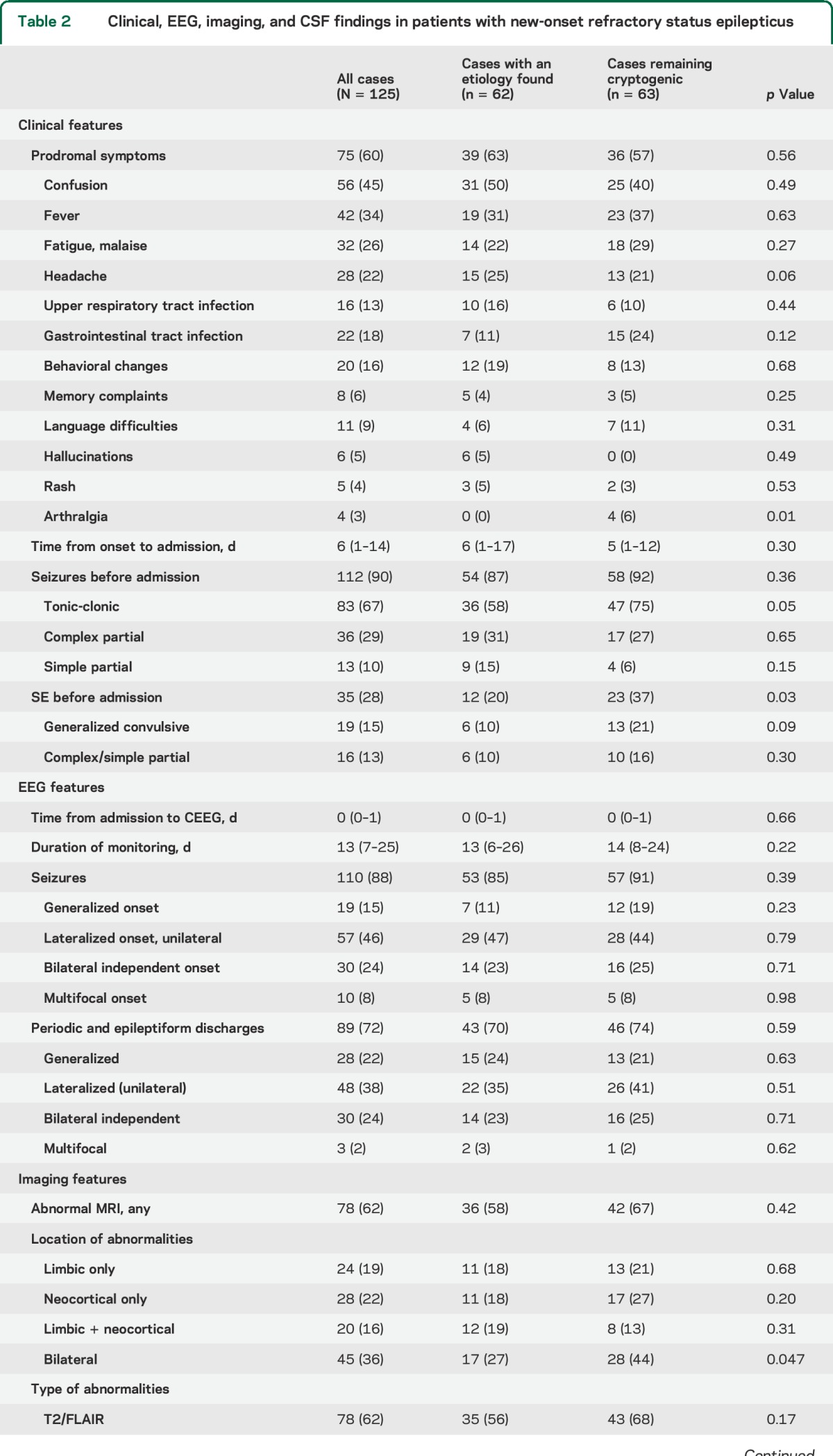
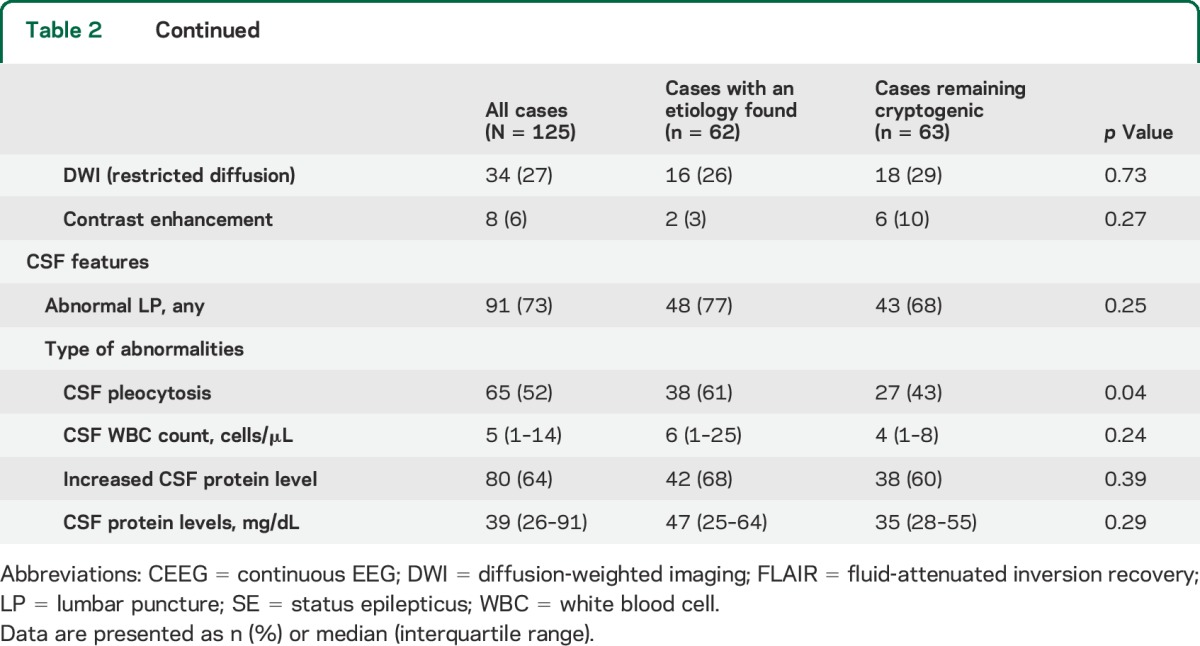
Prodromal symptoms preceded the onset of NORSE in 75 of 125 cases (60%) and started 6 days (interquartile range [IQR] 1–14 days) before admission (table 2). They included confusion (45%), fever (34%), fatigue (26%), headache (22%), symptoms of gastrointestinal (18%) or upper respiratory (13%) tract infection, and behavioral changes (16%). Hallucinations did not occur in the cryptogenic group but were present in 4 of 16 cases (25%) of anti-NMDAR encephalitis and 1 of 8 cases (13%) of anti-VGKCC encephalitis. None of the patients who were not tested for anti-NMDAR presented typical EEG or clinical features of the syndrome. Seizures occurred before admission in 90% of cases, of which 28% were SE (table 2).
Table 2.
Clinical, EEG, imaging, and CSF findings in patients with new-onset refractory status epilepticus
EEG.
Seizures occurred during CEEG in 88% of cases (table 2). Patients who did not have seizures during CEEG presented with generalized convulsive RSE and were treated with anesthetics before monitoring. Unilateral (46%) seizure onset was more common than bilateral independent (24%), generalized (15%), and multifocal (8%) onsets. Periodic or epileptiform discharges were observed in 72% of cases and were more frequently lateralized (39%) than bilateral independent (24%), generalized (22%), or multifocal (2%). There was no difference in EEG findings between cryptogenic cases and cases with a proven etiology.
Imaging and CSF.
All patients underwent brain MRI and this was repeated in 24% of cases (table 2). Abnormalities were found in 62% of cases and predominated in limbic (19%) and neocortical structures (22%) or both (16%). Abnormalities were more commonly identified on fluid-attenuated inversion recovery images (62%). No significant difference was found between cryptogenic cases and cases with an identified etiology.
CSF analyses were available in all cases and were repeated in 42% of cases (table 2). An abnormal CSF, with pleocytosis and/or elevated protein levels, was found in 73% of cases. There were no significant differences between cryptogenic cases and cases with an identified etiology.
Treatment.
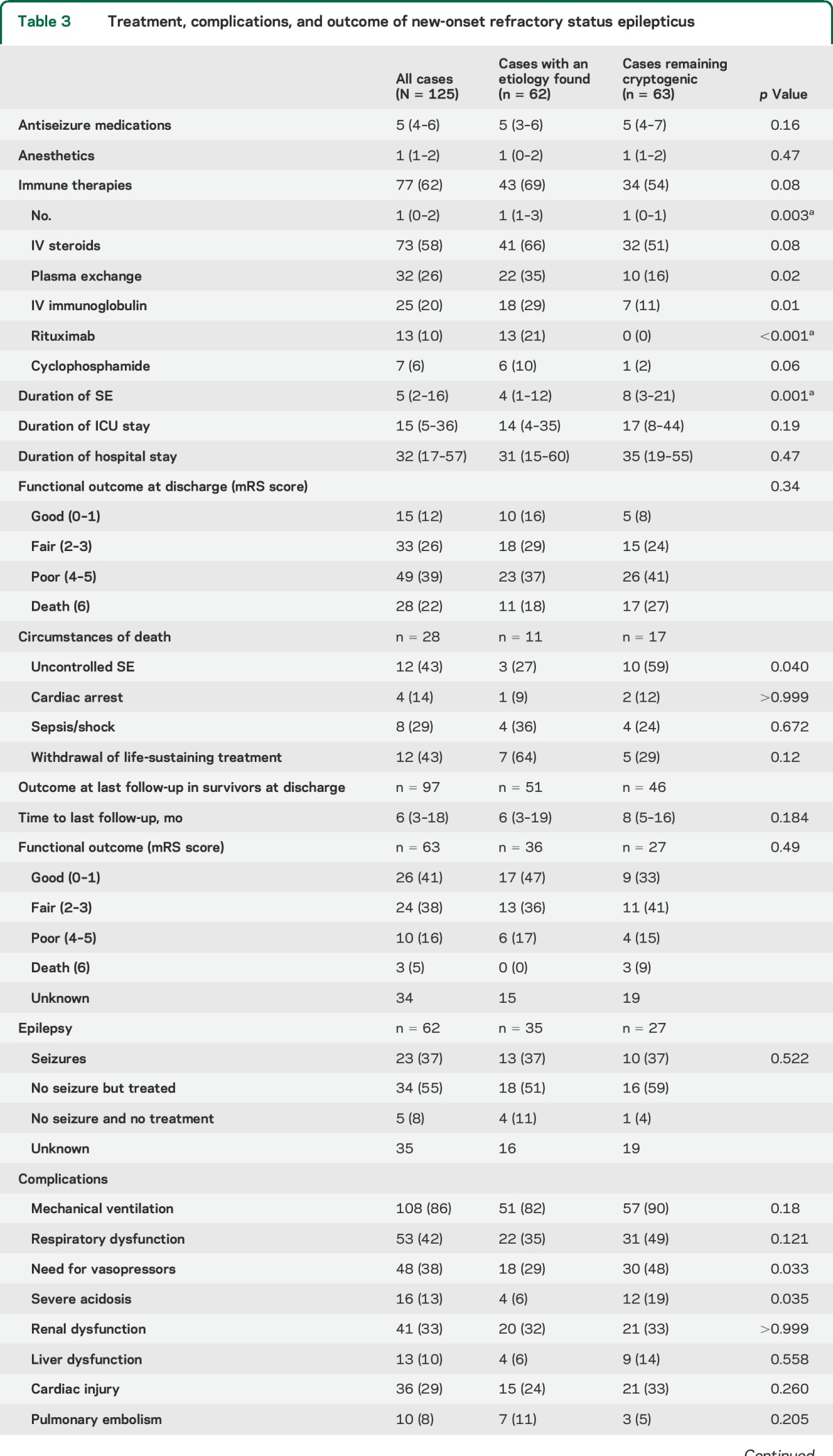
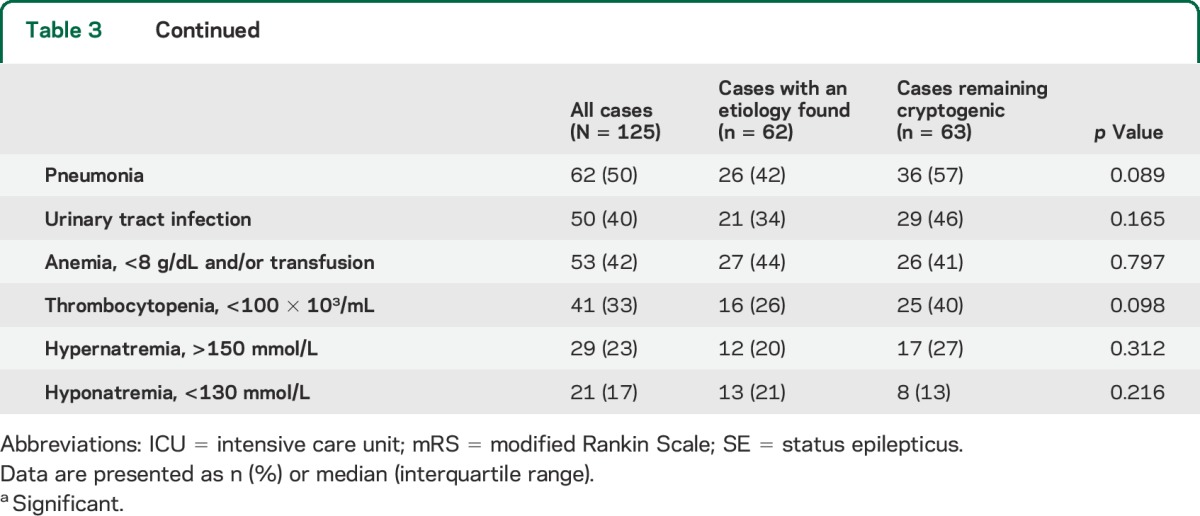
Patients received a median of 5 (IQR 4–6; range 4–11) antiseizure medications (table 3). Continuous anesthetics were used in 96 of 125 cases (77%) (median 1; IQR 0–2; range 0–4). The number and type of antiseizure medications and anesthetic drugs was similar in cryptogenic cases and cases with a proven etiology. Immune therapies were used in 77 of 125 cases (62%). The number of immune therapies used per patient was significantly lower in cryptogenic cases (1 [0–1] vs 1 [1–3]; p = 0.003).
Table 3.
Treatment, complications, and outcome of new-onset refractory status epilepticus
Outcome and complications.
The duration of SE was longer in cryptogenic cases than in cases with a proven etiology (8 [3–21] vs 4 [1–8]; p < 0.001) although the durations of ICU stay and hospital stay were not different (table 3). Overall, 77 of 125 cases (62%) achieved a poor outcome (mRS score 0–3) at discharge among which 28 (22%) died in the hospital; there were no differences in outcome between groups.
A higher STESS and duration of SE were associated with poor outcome (table 4). However, 11 of 48 patients (23%) with SE longer than 1 week and 2 of 14 patients (14%) with SE longer than 1 month achieved a good or fair outcome (mRS score 0–3) at discharge. Similarly, 8 of 28 patients (29%) with a STESS >2 achieved a good or fair outcome at discharge. Complications occurred in 84 of 125 cases (67%) and were associated with poor outcome and mortality (table 4).
Table 4.
Predictors of outcome in new-onset refractory status epilepticus
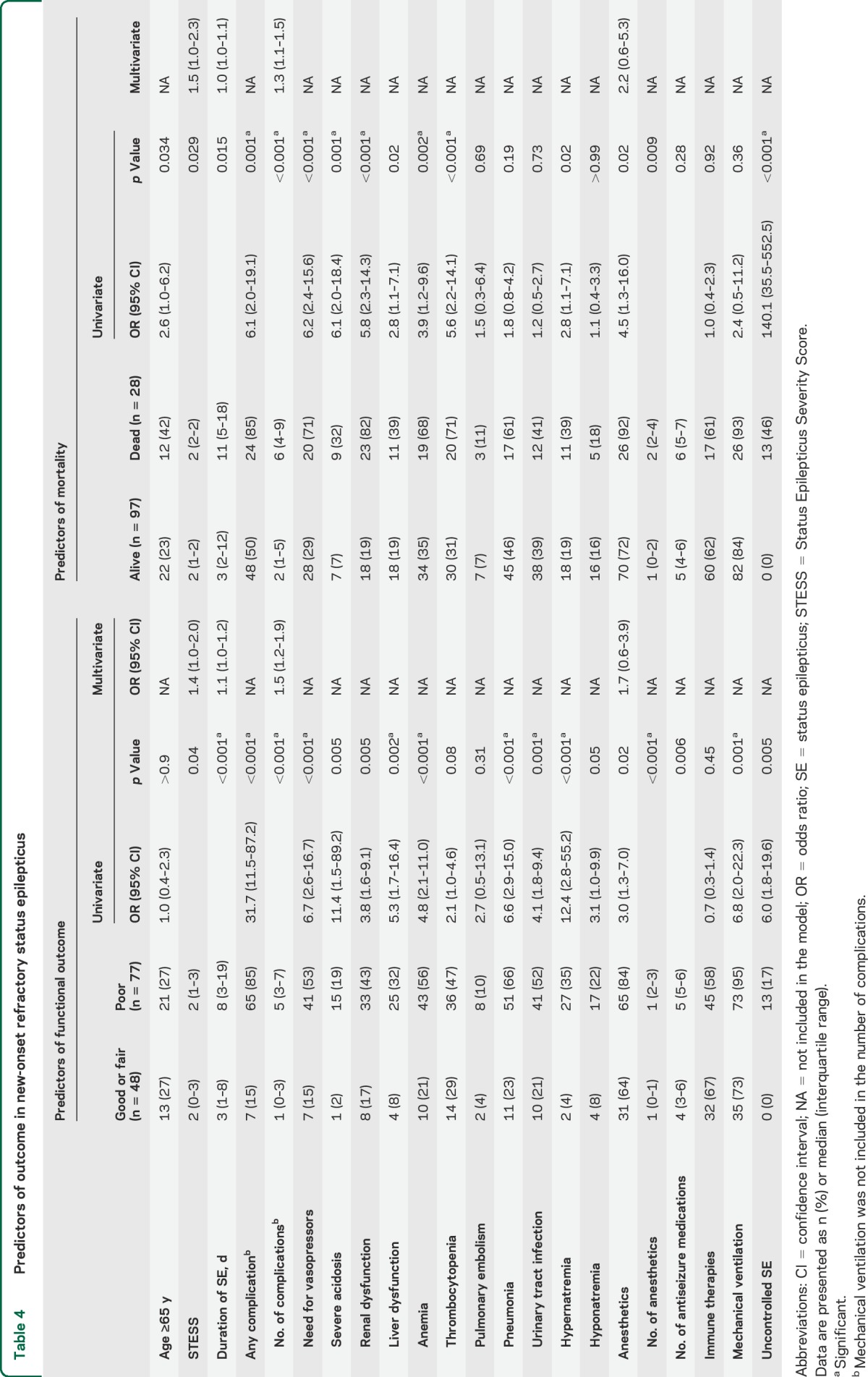
The use of anesthetics tended to be associated with poor outcome (65/77 [84%] vs 31/48 [64%]; p = 0.02) and the risk significantly increased with the number of anesthetics used, either sequentially or concomitantly (13/29 [45%], 28/48 [58%], 17/27 [63%], 8/9 [89%], and 12/12 [100%] for 0, 1, 2, 3, and 4 anesthetics, respectively; p < 0.001). Similar relationships were found with mortality (2/29 [6%], 5/48 [10%], 8/27 [29%], 5/9 [55%], and 8/12 [67%] for 0, 1, 2, 3, and 4 anesthetics, respectively; p < 0.001 [table 4]). Complications were also more frequent with anesthetics (table e-2). The duration of SE tended to be longer in patients who received anesthetics (5 [3–16] days vs 2 [0–10] days; p = 0.008) and was associated with a higher complication rate (table e-3). Anesthetics were used in all patients who had failure to control SE (13/96 [12%] vs 0/29 [0%]; p = 0.04). Withdrawal of life-sustaining treatment occurred almost exclusively in this group (11/96 [11%] vs 1/29 [3%]; p = 0.29). The number of nonanesthetic antiseizure medications was similar in patients who received anesthetic drugs and those who did not (table e-2).
When controlling for STESS, burden of complications, and duration of SE, the use of anesthetics was not significantly associated with either poor outcome or mortality (table 4).
Follow-up data were available in 63 of 97 survivors (65%) with a median follow-up time of 9 (4–19) months. Functional outcome at time of last follow-up was good or fair (mRS score 0–3) in 50 of 63 patients (79%) and improved compared with functional outcome at discharge in 36 of 63 patients (57%) (table 3). An average of 92% of patients across all groups with follow-up data had seizures (37%) or were receiving antiseizure medications (55%).
DISCUSSION
In this multicenter retrospective study of patients with NORSE, we found that (1) an etiology could ultimately be identified in 48% of cases; (2) the most frequent etiologies were nonparaneoplastic autoimmune encephalitis (19%) and paraneoplastic encephalitis (18%); (3) CSF abnormalities, most often mild, occurred as frequently in cryptogenic cases as in cases with an identified etiology; (4) despite receiving fewer immune therapies and experiencing longer episodes of SE, cryptogenic cases had a similar outcome to cases with an etiology, with 61% of cases overall achieving a poor outcome (mRS score 4–6) and a mortality of 22%; and (5) anesthetic use was not associated with poor outcome when controlling for confounders.
Our definition of NORSE was broader than previously suggested,4–6 as we did not limit the inclusion to cryptogenic cases. The definition of cryptogenic is likely to evolve with time as new etiologies of SE are discovered. Most published cases of NORSE predate the discovery of anti-NMDAR antibodies,23 which was the single most common etiology in our series. We did not restrict our inclusion to cases treated with anesthetics as refractory nonconvulsive SE may be treated with sequential trials of nonanesthetic medications.21,24
We found 130 cases of NORSE among 675 cases of RSE. This proportion compares well with other series, in which the reported proportion of RSE due to suspected encephalitis or to an unknown etiology varied between 16% and 22%.3,13
Autoimmune encephalitis, either nonparaneoplastic or paraneoplastic, was the most common cause of NORSE (37%), whereas identifiable infections were uncommon (8%). This agrees with previous studies that have demonstrated that autoimmune disorders are the most frequently identified cause of encephalitis whereas a viral agent is rarely found.3,10,16,18,25,26 The finding that many cases of NORSE are of autoimmune origin suggests that these etiologies should be aggressively sought.
More than half of the cases remained cryptogenic despite an extensive, albeit variable, workup, similar to prior studies of encephalitis with RSE.16 Although many cryptogenic cases benefited from a comprehensive antibody panel and an evaluation for occult neoplasm, these analyses were not systematically performed and the proportion of autoimmune and paraneoplastic cases may be underestimated. Cryptogenic cases presented with clinical features similar to autoimmune cases, suggesting a similar etiology. It is possible that some cryptogenic cases correspond to autoimmune encephalitis associated with antibodies yet to be identified. Recent studies have shown an intrathecal production of proconvulsant cytokines in the CSF of children with febrile illness–related epilepsy syndromes, a pediatric disorder akin to NORSE.27
Cryptogenic cases received fewer immune therapies than cases with a proven autoimmune etiology. Although we did not study the effect of immune therapies in our series, because of a high variability in doses and delay to treatment (data not shown), small case series have suggested that high-dose steroids, IV immune globulins, and plasma exchanges may be beneficial in cryptogenic NORSE.7–9 Our findings indicate that half of these patients do not receive such treatments. While the reasons for this difference were not specifically examined in this study, we suspect that physicians hesitate to treat patients with unknown etiology of NORSE with potentially hazardous immune-suppressing treatments in the absence of a well-established inflammatory cause. Postponing immune therapies might be detrimental since early treatment is associated with better outcome in autoimmune encephalitis.28,29
The role of anesthetics for RSE has been questioned.19,20 The majority of patients in our series received anesthetics. The overall mortality was lower compared with most series of refractory and super-refractory SE,2,3,11,13,17,30 probably because of the absence of acute structural brain injury that independently affects outcome. Decisions to withdraw life-sustaining treatment and treatment failure occurred mostly in the group of patients who received anesthetics and accounted for most of the deaths in our series. However, after adjusting for confounders (STESS, complications, and duration of SE), the use of anesthetics was not significantly associated with poor outcome, suggesting that the severity and duration of SE are the dominant reasons for both the use of anesthetic medications and a higher rate of complications and mortality.31 However, duration of SE and complications remained independently associated with outcome. A recent study found a better outcome of patients with RSE treated with high doses of midazolam, compared with low doses, further indicating that the severity of RSE itself is the main determinant of poor outcome, rather than the use of anesthetics.32
CONCLUSION
In this multicenter retrospective study, half of the cases of NORSE had an identifiable cause, most of which were autoimmune. Cryptogenic cases had a similar, albeit slightly more severe, course than cases with a proven etiology. Inflammatory CSF findings were equally common in both groups, but immune therapies were less frequently prescribed in cryptogenic cases. Anesthetic drugs were not associated with poor outcome, when adjusting for confounders. Further studies are required to clarify the etiology of currently cryptogenic cases of NORSE, and to determine the role of early immune therapy and anesthetics in its treatment.
Supplementary Material
GLOSSARY
- CCEMRC
Critical Care EEG Monitoring Research Consortium
- CEEG
continuous EEG
- ICU
intensive care unit
- IQR
interquartile range
- mRS
modified Rankin Scale
- NMDAR
NMDA receptor
- NORSE
new-onset refractory status epilepticus
- RSE
refractory status epilepticus
- SE
status epilepticus
- STESS
Status Epilepticus Severity Score
- VGKCC
voltage-gated potassium channel complex
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Critical Care EEG Monitoring Research Consortium (CCEMRC), Evan Fertig, Susan T. Herman, Linda Huh, Jong Woo Lee, Tobias Loddenkemper, Kevin Chapman, Nicholas S. Abend, Jessica Carpenter, Stephen Hantus, Jan Claassen, Aatif M. Husain, Nicolas Gaspard, Suzette M. LaRoche, Eva K. Ritzl, Tennile Gofton, Courtney Wusthoff, Joshua Goldstein, Brandon M. Westover, Sara Hocker, Jonathan Halford, Jennifer Jones, Elizabeth E. Gerard, Sarah E. Schmitt, Korwyn Williams, Cecil D. Hahn, Jerzy P. Szaflarski, Andreas Kramer, Leslie Rudzinski, Jennifer Hopp, Ram Mani, Giridhar P Kalamangalam, Puneet Gupta, Mark S. Quigg, Kevin F Haas, Adam Ostendorf, Deepti Zutshi, and Lawrence J. Hirsch
AUTHOR CONTRIBUTIONS
Nicolas Gaspard: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis, study supervision. Brandon P. Foreman: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data. Vincent Alvarez: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Christian Cabrera Kang: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. John C. Probasco: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Amy C. Jongeling: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Emma Meyers: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Alyssa Espinera: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Kevin F. Haas: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. Sarah E. Schmitt: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data. Elizabeth E. Gerard: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. Teneille Gofton: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. Peter W. Kaplan: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. Jong W. Lee: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. Benjamin Legros: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Jerzy P. Szaflarski: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Brandon M. Westover: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Suzette M. LaRoche: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Lawrence J. Hirsch: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, study supervision.
STUDY FUNDING
The CCEMRC received funding from the American Epilepsy Society's Research Infrastructure Award.
DISCLOSURE
N. Gaspard is a clinician master specialist of the National Fund for Scientific Research and received royalties for authoring chapters for UpToDate–Neurology. B. Foreman, V. Alvarez, and C. Cabrera Kang report no disclosures relevant to the manuscript. J. Probasco serves on the editorial board for The Neurohospitalist and received a grant as a section editor for Oxford University Press. A. Jongeling, E. Meyers, A. Espinera, K. Haas, and S. Schmitt report no disclosures relevant to the manuscript. E. Gerard received funding from SAGE Pharmaceuticals and UCB Pharma. T. Gofton reports no disclosures relevant to the manuscript. P. Kaplan received funding from the Qatar National Research Foundation, and royalties from Wiley Blackwell and Demos Publications for authoring textbooks on epilepsy, seizures, and EEG. J.W. Lee received research funding from the National Institute of Neurological Disorders and Stroke, American Epilepsy Society, UCB, Inc., the Duke Clinical Research Institute, and Sunovion, Inc. He is a consultant for SleepMed and Advance Medical. B. Legros received travel grants from UCB Pharma and Medtronic, speaker honoraria from UCB Pharma, and consultation fees for advising from UCB Pharma and Pfizer. J. Szaflarski received funding from NIH, Shor Foundation for Epilepsy Research, Epilepsy Foundation of America, Department of Defense, UCB Biosciences, Epilepsy Study Consortium, University of Alabama at Birmingham, Compumedics Neuroscan Inc., Food and Drug Administration, American Epilepsy Society, SAGE Therapeutics Inc.; had consulting activity for SAGE Therapeutics Inc., Biomedical Systems Inc., Elite Medical Experts LLC; and served as an editorial board member for Epilepsy and Behavior, Journal of Epileptology, Restorative Neurology and Neuroscience, Journal of Medical Science, and Folia Medica Copernicana. B. Westover received research support from the NIH (NIH–National Institute of Neurological Disorders and Stroke, 1K23NS090900-01), the Phyllis & Jerome Lyle Rappaport Foundation, and the Andrew David Heitman Neuroendovascular Research Fund. S. LaRoche received royalties from Demos Publishing for editing the book Handbook of ICU EEG Monitoring. L. Hirsch received research support for investigator-initiated studies from UCB Pharma, Eisai, Upsher-Smith, Sunovion, and Lundbeck; consultation fees for advising from Lundbeck, Upsher-Smith, NeuroPace, Natus, and Allergan; honoraria for speaking from Natus and NeuroPace; and royalties for authoring chapters for UpToDate–Neurology and from Wiley for coauthoring the book Atlas of EEG in Critical Care, by Hirsch and Brenner, 2010. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology 1998;50:735–741. [DOI] [PubMed] [Google Scholar]
- 2.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205–210. [DOI] [PubMed] [Google Scholar]
- 3.Holtkamp M, Othman J, Buchheim K, Meierkord H. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry 2005;76:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costello DJ, Kilbride RD, Cole AJ. Cryptogenic new onset refractory status epilepticus (NORSE) in adults: infectious or not? J Neurol Sci 2009;277:26–31. [DOI] [PubMed] [Google Scholar]
- 5.Van Lierde I, Van Paesschen W, Dupont P, Maes A, Sciot R. De novo cryptogenic refractory multifocal febrile status epilepticus in the young adult: a review of six cases. Acta Neurol Belg 2003;103:88–94. [PubMed] [Google Scholar]
- 6.Wilder-Smith EP, Lim EC, Teoh HL, et al. The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore 2005;34:417–420. [PubMed] [Google Scholar]
- 7.Gall CR, Jumma O, Mohanraj R. Five cases of new onset refractory status epilepticus (NORSE) syndrome: outcomes with early immunotherapy. Seizure 2013;22:217–220. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Saldivar C, Maganti RK. Plasma exchange in cryptogenic new onset refractory status epilepticus. Seizure 2013;22:70–73. [DOI] [PubMed] [Google Scholar]
- 9.Khawaja AM, Dewolfe JM, Miller DM, Szaflarski JP. New onset refractory status epilepticus (NORSE): the potential role for immunotherapy. Epilepsy Behav 2015;47:17–23. [DOI] [PubMed] [Google Scholar]
- 10.Holtkamp M, Othman J, Buchheim K, Masuhr F, Schielke E, Meierkord H. A “malignant” variant of status epilepticus. Arch Neurol 2005;62:1428–1431. [DOI] [PubMed] [Google Scholar]
- 11.Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia 2010;51:251–256. [DOI] [PubMed] [Google Scholar]
- 12.Davis R, Dalmau J. Autoimmunity, seizures, and status epilepticus. Epilepsia 2013;54(suppl 6):46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutter R, Marsch S, Fuhr P, Rüegg S. Mortality and recovery from refractory status epilepticus in the intensive care unit: a 7-year observational study. Epilepsia 2013;54:502–511. [DOI] [PubMed] [Google Scholar]
- 14.Bleck TP. Less common etiologies of status epilepticus. Epilepsy Curr 2010;10:31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hainsworth JB, Shishido A, Theeler BJ, Carroll CG, Fasano RE. Treatment responsive GABA(B)-receptor limbic encephalitis presenting as new-onset super-refractory status epilepticus (NORSE) in a deployed U.S. soldier. Epileptic Disord 2014;16:486–493. [DOI] [PubMed] [Google Scholar]
- 16.Glaser CA, Gilliam S, Honarmand S, et al. Refractory status epilepticus in suspect encephalitis. Neurocrit Care 2008;9:74–82. [DOI] [PubMed] [Google Scholar]
- 17.Hocker SE, Britton JW, Mandrekar JN, Wijdicks EF, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol 2013;70:72–77. [DOI] [PubMed] [Google Scholar]
- 18.Thakur KT, Motta M, Asemota AO, et al. Predictors of outcome in acute encephalitis. Neurology 2013;81:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutter R, Marsch S, Fuhr P, Kaplan PW, Rüegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology 2014;82:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchi NA, Novy J, Faouzi M, Stähli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med 2015;43:1003–1009. [DOI] [PubMed] [Google Scholar]
- 21.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300. [Google Scholar]
- 23.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol 2010;17:348–355. [DOI] [PubMed] [Google Scholar]
- 25.Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis 2006;43:1565–1577. [DOI] [PubMed] [Google Scholar]
- 26.Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology 2015;84:359–366. [DOI] [PubMed] [Google Scholar]
- 27.Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J Neurol Neurosurg Psychiatry 2015;86:820–822. [DOI] [PubMed] [Google Scholar]
- 28.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133:1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilbride RD, Reynolds AS, Szaflarski JP, Hirsch LJ. Clinical outcomes following prolonged refractory status epilepticus (PRSE). Neurocrit Care 2013;18:374–385. [DOI] [PubMed] [Google Scholar]
- 31.Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia 1994;35:27–34. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez A, Lantigua H, Lesch C, et al. High-dose midazolam infusion for refractory status epilepticus. Neurology 2014;82:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


