Abstract
Rationale: The influence of particulate air pollution on respiratory health starts in utero. Fetal lung growth and structural development occurs in stages; thus, effects on postnatal respiratory disorders may differ based on timing of exposure.
Objectives: We implemented an innovative method to identify sensitive windows for effects of prenatal exposure to particulate matter with a diameter less than or equal to 2.5 μm (PM2.5) on children's asthma development in an urban pregnancy cohort.
Methods: Analyses included 736 full-term (≥37 wk) children. Each mother’s daily PM2.5 exposure was estimated over gestation using a validated satellite-based spatiotemporal resolved model. Using distributed lag models, we examined associations between weekly averaged PM2.5 levels over pregnancy and physician-diagnosed asthma in children by age 6 years. Effect modification by sex was also examined.
Measurements and Main Results: Most mothers were ethnic minorities (54% Hispanic, 30% black), had 12 or fewer years of education (66%), and did not smoke in pregnancy (80%). In the sample as a whole, distributed lag models adjusting for child age, sex, and maternal factors (education, race and ethnicity, smoking, stress, atopy, prepregnancy obesity) showed that increased PM2.5 exposure levels at 16–25 weeks gestation were significantly associated with early childhood asthma development. An interaction between PM2.5 and sex was significant (P = 0.01) with sex-stratified analyses showing that the association exists only for boys.
Conclusions: Higher prenatal PM2.5 exposure at midgestation was associated with asthma development by age 6 years in boys. Methods to better characterize vulnerable windows may provide insight into underlying mechanisms.
Keywords: fine particulate matter, asthma, prenatal exposure, sensitive windows, sex difference
At a Glance Commentary
Scientific Knowledge on the Subject
The influence of ambient fine particulate matter (PM2.5) on respiratory health starts in utero and may be sex specific. Fetal lung growth and structural development occurs in stages. Thus, effects on early childhood respiratory disorders may differ based on timing of exposure, albeit the effect of exposure timing remains poorly understood in human studies.
What This Study Adds to the Field
This is the first study to leverage weekly PM2.5 exposure estimates over gestation combined with data-driven statistics to characterize susceptibility windows, removing the subjectivity that currently guides the decision of when to assess exposure effects. These data demonstrate that increased prenatal PM2.5 exposure at mid-gestation (16–25 wk gestation) was associated with asthma development by age 6 years in boys. A more definitive understanding of the temporal effects of in utero toxins on outcomes in early childhood may provide clues as to the underlying mechanisms being perturbed based on current understanding of the cellular differentiation, proliferation, or physiologic function changes occurring progressively over pregnancy that impact respiratory outcomes.
Toxic exposures in critical developmental windows may result in permanently altered changes in respiratory and interrelated systems (e.g., immune, autonomic, neuroendocrine) at the cellular, structural, and/or functional level that manifest in childhood disorders (e.g., asthma) (1, 2). The fetus is particularly vulnerable because of immature immune, neuroendocrine, and xenobiotic detoxification systems and antioxidant defenses (3–6). Prenatal development of the respiratory system is a multievent process progressing sequentially from early gestation (7), thus toxins may have variable impact depending on timing of exposure (1).
Air pollution exposure impacts the developing respiratory system, with evidence particularly implicating prooxidants, such as particulate matter with an aerodynamic diameter less than or equal to 2.5 μm (PM2.5). Epidemiologic studies link prenatal particulate air pollution with childhood wheeze, asthma, and altered lung function (4, 8–10). Animal studies link prenatal PM with cytokine disruption, increased IgE, impaired lung growth, and airway hyperresponsiveness in offspring (11–14). Gestational exposure to PM may enhance maternal systemic oxidative stress and proinflammatory cytokine production (15) resulting in placental and endothelial dysfunction, and increased fetal oxidative stress with consequent effects on fetal immune and lung development (7, 14, 16–18).
Human studies have not extensively elucidated sensitive windows, largely because of variable methods for exposure assignment and lack of temporal resolution. Epidemiologic studies have historically used more crude and less time resolved indices (e.g., “high polluted” vs. “low polluted” areas, levels at closest monitor, proximity to roads, traffic density), because it is cost prohibitive to collect repeated exposure data over pregnancy using personal monitoring (19–21). Thus studies have considered relatively arbitrary assignment of exposure windows (e.g., averaged over pregnancy, within clinically defined trimesters, personal monitoring at discrete time points) rather than being grounded in an understanding of developmental processes relevant to the respiratory system. Because the processes involved in programming respiratory outcomes do not necessarily occur within clinically defined trimesters and sensitive periods remain largely unknown (7), research that allows flexibility in identifying sensitive windows may be particularly informative. More recently developed approaches (e.g., spatiotemporal land-use regression [LUR], multiscale air quality deterministic chemistry models, and so forth) for deriving spatiotemporally resolved exposure profiles (22–24) allow researchers to estimate exposure patterns at a higher temporal resolution.
Overlapping animal and human research suggest that prenatal air pollution exposure may have sex-specific effects. Animal studies demonstrate sex differences in lung growth and airway development (25, 26). In humans, females display earlier fetal breathing and surfactant production, which may in part be the basis for the reduction in forced expiratory flow rates that predispose males to airway diseases in early childhood (27, 28). Prenatal air pollution induces fetal oxidative stress (29), and in turn influences gene expression and physiologic events crucial for lung maturation (30). Boys may be more vulnerable to prenatal oxidant injury (31) and thus may have an exaggerated response to in utero air pollution exposure.
To address these research gaps, we leveraged daily prenatal PM2.5 measures available over pregnancy and applied advanced statistical methods (e.g., distributed lag models [DLMs]) to more precisely identify sensitive windows in relation to childhood asthma onset by age 6 years in an ethnically mixed lower socioeconomic status urban pregnancy cohort. Effect modification by sex was also examined. Findings from these analyses have been previously reported in the form of an abstract (32).
Methods
Participants were from the Asthma Coalition on Community, Environment and Social Stress (ACCESS) project, a pregnancy cohort designed to examine the effects of perinatal exposure to physical toxins and psychosocial stress on urban childhood respiratory health (33). In brief, English- or Spanish-speaking pregnant women (≥18 yr old) receiving care at Brigham and Women's Hospital, Boston Medical Center, and affiliated community health centers were enrolled at 28.4 ± 7.9 weeks gestation between August 2002 and July 2009. Among pregnant women approached who were eligible, 989 (78.1%) agreed to enroll. Based on screening data, mothers who declined versus enrolled were slightly less likely to be ethnic minorities (78.9% and 81.5% Hispanic or African American, respectively) or to have a high school education or less (57.7% vs. 60.6%, respectively) and slightly more likely to report an income level less than $20,000 annually (37.7% vs. 35.2%, respectively); there were no significant differences between groups on these covariates. Of those enrolled, 955 gave birth to a singleton live born infant and continued follow-up. Procedures were approved by human studies committees at the Brigham and Women’s Hospital and Boston Medical Center; written consent was obtained in the subject’s primary language.
Daily Prenatal PM2.5 Levels
Mothers’ prenatal exposure to PM2.5, an index of ambient pollution from traffic and other sources, was estimated based on residence over the pregnancy (i.e., at enrollment and updated if they moved) using a novel spatiotemporal model incorporating moderate resolution imaging spectroradiometer satellite-derived aerosol optical depth (AOD) measurements at a 10 × 10 km spatial resolution and layering this remote sensing data with traditional LUR predictors to yield residence-specific estimates of daily PM2.5 as detailed previously (22). The model was run using day-specific calibrations of AOD data using ground PM2.5 measurements from 78 monitoring stations covering New England and LUR and meteorologic variables (temperature, wind speed, visibility, elevation, distance to major roads, percent open space, point emissions, and area emissions). This approach incorporates highly resolved spatial information from the LUR data and important spatiotemporal data from the remote sensing satellite data.
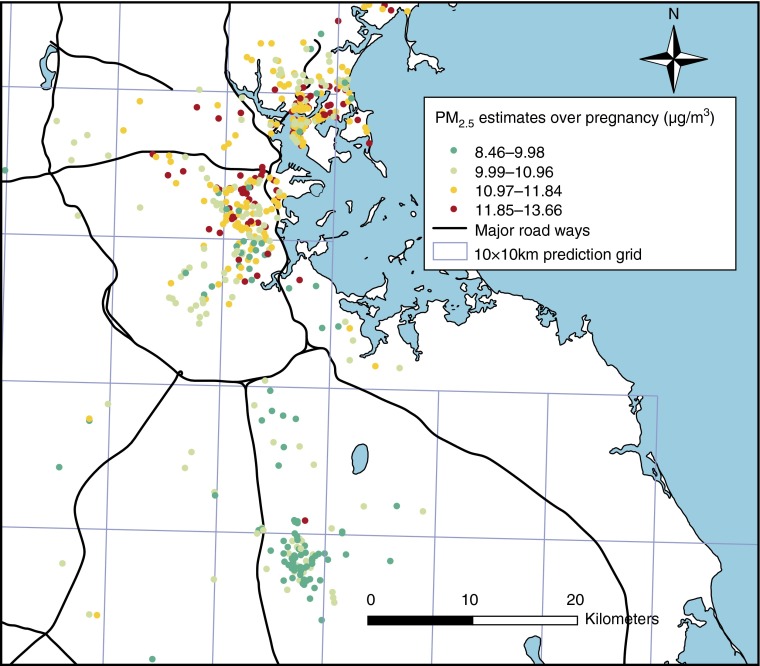
The AOD-PM2.5 relationship was calibrated for each day using data from grid cells with both monitor and AOD values using mixed models with random slopes for day, nested within region. For days without AOD data (because of cloud coverage, snow, and so forth), the model was fit with a smooth function of latitude and longitude and a random intercept for each cell (similar to universal Kriging). The “out of sample” 10-fold cross validation R2 for daily values was 0.83 and 0.81 for days with and without available AOD data, respectively. For use in the health effect models, to reduce potential noise caused by day-to-day PM2.5 variation, daily levels were averaged into weekly exposure profiles. Predicted overall prenatal PM2.5 levels at participant’s residence in relation to the 10 × 10 km grids for which AOD data were available are shown in Figure 1. Although levels were higher around major roadways as anticipated, there was reasonable heterogeneity.
Figure 1.
Predicted daily particulate matter with a diameter less than or equal to 2.5 μm (PM2.5) levels for Asthma Coalition on Community, Environment and Social Stress participants averaged over pregnancy. This figure demonstrates predicted daily PM2.5 levels for study participants based on residence and averaged throughout the gestation period. The 10 × 10 km aerosol optical depth grid used to predict daily PM2.5 levels is also depicted.
Asthma
Maternal-reported clinician-diagnosed asthma was ascertained from birth up to age 6 years through telephone and face-to-face interviews at approximately 3-month intervals for the first 24 months then annually thereafter. Mothers were asked, “Has a doctor or nurse ever said that your child had asthma?” Most of these children were given a diagnosis of asthma after the age of 3 years (78.6%) (see Figure E1 in the online supplement).
Covariates
Maternal age, race, education, and prepregnancy height and weight, and child’s sex were ascertained by questionnaire; date of birth, gestational age, and birth weight were obtained by medical record review. A validation analysis on a subset of 121 ACCESS women showed no difference in the level of agreement/disagreement for height and weight when comparing values measured early in pregnancy (<10 wk) with self-report (34). Women were asked about smoking at enrollment and in the third trimester and classified as prenatal smokers if smoking at either visit. Mothers reported postnatal smoking and whether others smoked in the home at each postpartum interview. Household crowding was calculated by dividing the number of persons living in the home by the number of rooms based on maternal report in pregnancy. Maternal atopy was defined by self-reported doctor-diagnosed asthma, eczema, and/or hay fever. Body mass index was calculated by dividing weight by height squared (kg/m2); obesity was defined as body mass index greater than or equal to 30 kg/m2 (35).
Because prenatal stress may covary with pollution and has been associated with asthma (36), this was also considered as a confounder. We measured stress using the Crisis in Family Systems-Revised survey administered prenatally within 2 weeks of enrollment (37, 38). This survey assesses life events experienced across 11 domains (e.g., financial, relationships, violence, housing, discrimination/prejudice). Mothers endorsed events experienced in the past 6 months and rated each as positive, negative, or neutral. The number of domains with one or more negative event was summed to create a continuous negative life events (NLEs) domain score, with higher scores indicating greater stress. Because birth weight and gestational age may be on the pathway between prenatal PM and asthma risk, birth weight for gestational age z score (39) was considered in sensitivity analyses.
Statistical Analysis
Analyses included 736 singleton full-term (gestational age ≥37 wk) children with two or more postnatal interviews followed up to age 6 years and air pollution exposure data. Table E1 shows that there were no differences across covariates when comparing those included in the analysis with the whole sample. To explore sensitive windows, we constructed an exposure lag space (40) incorporating weekly averages of daily PM2.5 predictions at each subject’s residence throughout the gestational period. We fit DLMs to estimate the time-varying association between the probability of child’s asthma onset and the estimated PM2.5 level during a given week in pregnancy. Specifically, we fit the logistic regression DLM:
where is the probability of a report of clinician diagnosed asthma, is the estimated PM2.5 level for week j of pregnancy, and , …, are confounders for subject i. Models included maternal age, race and ethnicity, education, prepregnancy obesity, prenatal and postnatal tobacco smoke exposure, prenatal NLEs, and child's sex. Without additional structure on the coefficients, the estimates of the week-specific effects are typically unstable because of collinearity among the weekly pollution averages. Therefore, we fit constrained DLMs that assume these effects are a smooth function of j (wk), such = h(j). We modeled this smooth function using b-splines. A sensitive window was identified when the pointwise 95% confidence bands did not contain zero.
Next, to assess whether the sensitive window of prenatal PM2.5 exposure on childhood asthma onset was different between boys and girls, sex-stratified DLMs were performed, adjusting for maternal age, race and ethnicity, education, prepregnancy obesity, prenatal and postnatal smoking status, and prenatal NLEs. We then constructed a difference curve by subtracting the effect estimates of the DLM curve for the girls from the effect estimates of the DLM curve for the boys ([log odds of boys] − [log odds of girls]), and calculated the associated pooled standard error to derive the 95% pointwise confidence interval of the difference curve. For additional sensitivity analyses, we also examined sex-specific associations between prenatal PM2.5 levels averaged across the DLM-identified sensitive windows using multivariable-adjusted logistic regression models, and fitting the interaction model using the following equation: logit(asthma) = intercept + β1(PM2.5) + β2(male) + β3(male × PM2.5) + β4x1i + β5x2i, where x1i, x2i, . . . are covariates. Finally, because prenatal and postnatal PM2.5 levels were correlated (Spearman r = 0.82), we performed sensitivity analyses by including postnatal PM2.5 levels in the models. We also fit models including birth weight adjusted for gestational age. DLMs were implemented using the dlnm package in R version 3.0.1 (Vienna, Austria) (40), and other analyses were performed in SAS version 9.1.3 (SAS Institute, Inc., Cary, NC).
Results
Most mothers were ethnic minority (54% Hispanic, 30% African American), had less than or equal to 12 years of education (66%), and did not smoke in pregnancy (80%); the distribution of these covariates did not differ by sex (Table 1). There were 110 asthma cases among the 736 children included in the final analysis. We present the distribution of asthma cases relative to all ACCESS participants in the online supplement (see Figure E2). There were also no significant sex difference in terms of gestational age at birth, maternal age, atopy, obesity, prenatal stress, and PM2.5 exposure. Boys were more likely to be diagnosed with asthma compared with girls (18% vs. 12%; chi-square test, P = 0.02). Birth weight for gestational age z score was significantly lower in girls than boys (Wilcoxon rank sum test, P = 0.003).
Table 1.
ACCESS Participant Characteristics
| All Children (n = 736) | Boys (n = 374) | Girls (n = 362) | |
|---|---|---|---|
| Ever had asthma up to 6 yr old, n (%) | |||
| No | 626 (85.0) | 307 (82.1) | 319 (88.1) |
| Yes | 110 (15.0) | 67 (17.9) | 431 (1.9) |
| Race/ethnicity, n (%) | |||
| Black | 218 (29.6) | 119 (31.8) | 99 (27.4) |
| Hispanic | 395 (53.7) | 191 (51.1) | 204 (6.4) |
| White/other | 123 (16.7) | 64 (17.1) | 59 (16.3) |
| Maternal education, n (%) | |||
| >12 yr | 251 (34.1) | 128 (34.2) | 123 (34) |
| ≤12 yr | 485 (65.9) | 246 (65.8) | 239 (66.0) |
| Maternal smoking status, n (%) | |||
| Never smoked | 590 (80.2) | 303 (81) | 287 (79.0) |
| Smoked prenatally, but not postnatally | 36 (4.9) | 19 (5.1) | 17 (4.7) |
| Did not smoke prenatally, but smoked postnatally | 42 (5.7) | 19 (5.1) | 23 (6.4) |
| Smoked both prenatally and postnatally | 68 (9.2) | 33 (8.8) | 13 (9.7) |
| Maternal atopy, n (%)* | 262 (35.6) | 131 (35.0) | 131 (36.2) |
| Maternal obese, n (%)† | 205 (27.9) | 97 (26.0) | 108 (30.0) |
| Maternal age at enrollment, yr, median (IQR) | 25.5 (22.3–30.7) | 25.6 (22.4–31.3) | 25.4 (22.2–30.3) |
| Birth weight for gestational age z score, mean ± SD | −0.16 ± 1.06 | −0.07 ± 1.09 | −0.26 ± 1.03 |
| Averaged prenatal PM2.5 level, μg/m3, median (IQR) | 11.2 (10.2–11.8) | 11.2 (10.2–11.9) | 11.0 (10.2–11.7) |
| Prenatal negative life events score, mean ± SD‡ | 2.40 ± 2.00 | 2.37 ± 1.99 | 2.43 ± 2.03 |
| Household crowding, median (IQR)§ | 1 (0.60–1.25) | 1 (0.60–1.25) | 1 (0.67–1.25) |
Definition of abbreviations: ACCESS = Asthma Coalition on Community, Environment and Social Stress; IQR = interquartile range; PM2.5 = particulate matter with a diameter less than or equal to 2.5 μm.
Ever self-reported doctor-diagnosed asthma, eczema, and/or hay fever.
Prepregnancy obesity: body mass index ≥ 30 kg/m2.
Assessed using Crisis in Family Systems-Revised survey (37, 38), a multiitem survey summarized into a continuous score.
Household crowding indexed as number of people in the home divided by number of rooms in the home (person per room).
Distributed Lag Models
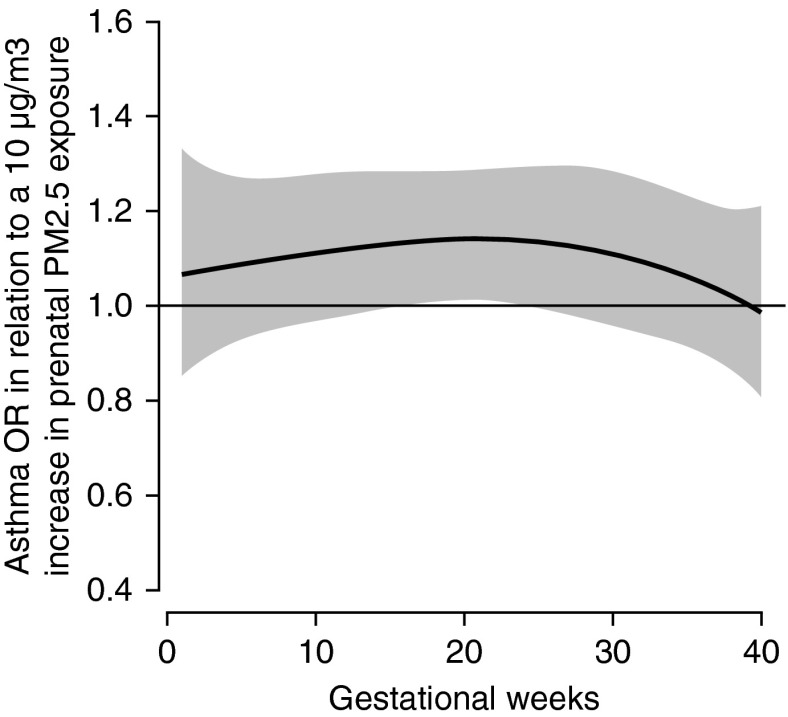
Figure 2 shows the association between prenatal PM2.5 and children's asthma onset using DLMs, adjusting for maternal age, race and ethnicity, education, prepregnancy obesity, prenatal and postnatal tobacco smoke exposure, prenatal NLEs, and child's sex. We observed a significant sensitive window of PM2.5 exposure around mid-pregnancy on asthma onset by age 6 years, specifically during 16–25 weeks gestation (Figure 2). Sensitivity analyses additionally including averaged postnatal PM2.5 levels and birth weight adjusted for gestational age did not materially change these results (data not shown).
Figure 2.
Association between weekly particulate matter with a diameter less than or equal to 2.5 μm (PM2.5) levels over gestation and asthma onset. This figure demonstrates the association between PM2.5 over gestation and asthma onset by age 6 years using a distributed lag model assuming week-specific effects, adjusting for child's sex, maternal age at enrollment, race and ethnicity, education, prepregnancy obesity, prenatal and postnatal smoking, and prenatal stress. The y-axis shows the odds ratio (OR) of asthma in relation to a 10 μg/m3 increase in prenatal PM2.5 exposure; the x-axis depicts gestational age in weeks. The solid line shows the predicted OR, and the gray area indicates the 95% confidence interval. A sensitive window is identified when the estimated pointwise 95% confidence interval does not include zero.
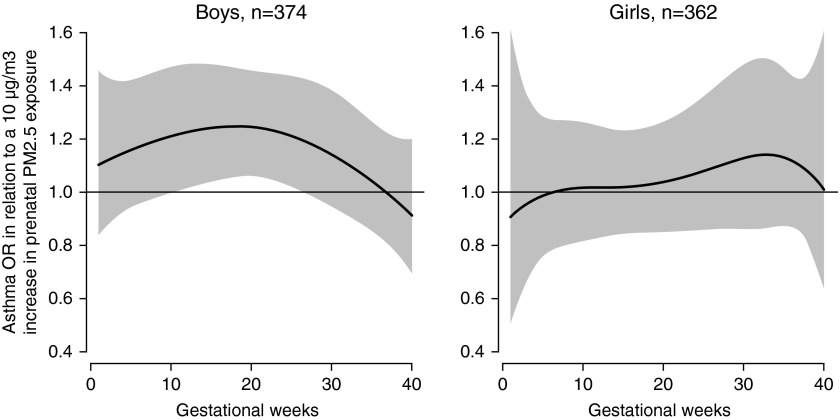
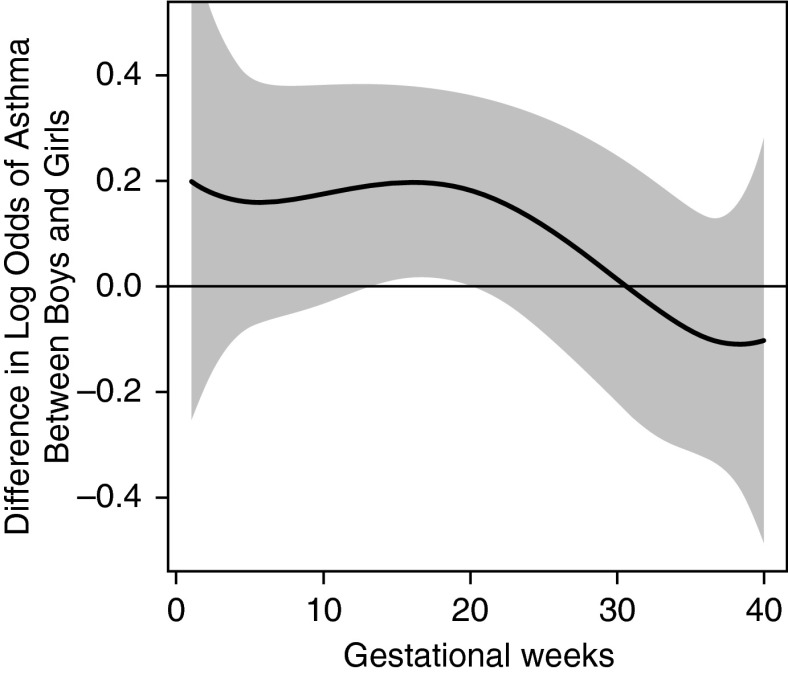
Figure 3 demonstrates the sex-specific association between prenatal PM2.5 and children's asthma onset using DLMs, adjusting for maternal age, race and ethnicity, education, prepregnancy obesity, prenatal and postnatal tobacco smoke exposure, and prenatal NLEs. When stratified by sex, we observed a significant sensitive exposure window between 12 and 26 weeks gestation among boys but not girls (Figure 3). To examine the interaction between prenatal PM2.5 and sex, we also constructed a DLM demonstrating the difference between boys and girls; the difference curve showed that associations were significantly stronger in boys from 14–20 weeks gestation compared with girls (Figure 4). Finally, we fit a multivariable logistic regression model including a PM2.5 × sex interaction term using PM2.5 level averaged over this identified sensitive window, and found a significant interaction between PM2.5 and sex (P = 0.01). Details on the full regression model can be found in the online supplement (see Tables E2A and E2B).
Figure 3.
Association between weekly particulate matter with a diameter less than or equal to 2.5 μm (PM2.5) levels over gestation and asthma onset. This figure demonstrates the association between PM2.5 over gestation and asthma onset by age 6 years using distributed lag models assuming week-specific effects, stratified by sex. The models adjusted for maternal age at enrollment, race and ethnicity, education, prepregnancy obesity, prenatal and postnatal smoking status, and prenatal stress. The y-axis shows the odds ratio (OR) of asthma in relation to a 10 μg/m3 increase in prenatal PM2.5 exposure; the x-axis depicts gestational age in weeks. The solid line shows the predicted OR, and the gray area indicates the 95% confidence interval. A sensitive window is identified when the estimated pointwise 95% confidence interval does not include zero.
Figure 4.
Association between weekly particulate matter with a diameter less than or equal to 2.5 μm (PM2.5) levels over gestation and the difference in asthma onset between boys and girls. This figure demonstrates the association between PM2.5 over gestation and the difference in asthma onset between sex using a distributed lag model assuming week-specific effects, adjusting for maternal age at enrollment, race and ethnicity, education, prepregnancy obesity, prenatal and postnatal smoking status, and prenatal stress. The y-axis shows the differences in log odds of asthma between boys and girls ([log odds of boys] − [log odds of girls]) in relation to a 10 μg/m3 increase in prenatal PM2.5 exposure; the x-axis is gestational age in weeks. The solid line shows the predicted odds ratio, and the gray area indicates the 95% confidence interval.
Discussion
These data add to a growing literature linking prenatal particulate air pollution exposure to children's respiratory health. This is the first study to leverage weekly PM2.5 exposure estimates over gestation combined with data-driven statistics to characterize susceptibility windows, removing the subjectivity that currently guides the decision of when to assess exposure effects. These data demonstrate that increased prenatal PM2.5 exposure in mid-gestation (16–25 wk gestation) was associated with development of childhood asthma in these urban children, but only in boys.
Although previous studies link higher prenatal exposure to PM with adverse pulmonary outcomes, such as asthma (41–43), the sensitive window with the greatest impact has not been well elucidated. A more definitive understanding of the temporal effects of toxins on outcomes in the offspring may provide clues as to the underlying mechanisms being perturbed based on current understanding of the cellular differentiation, proliferation, or physiologic function changes occurring progressively over pregnancy. This study demonstrates the applicability of advanced statistical modeling to illustrate the pattern of associations throughout the pregnancy based on the data per se rather than assigning a priori exposure time points relevant to the exposure of interest. The identified sensitive window coincides with the late pseudoglandular and canalicular phases of fetal lung development (7). Several essential tissues and their functions are shaped during these lung development phases. During the pseudoglandular stage (5–17 wk), the conducting airways develop smooth muscle, mucous glands form, and acinar outlines appear (44).
The airways continue to develop during the canalicular stage (17–26 wk) and capillaries, thin-walled terminal saccules, and alveolar epithelium begin to appear. Type II cells may undergo differentiation to type I cells in this period, with subsequent surfactant production (7, 42, 45). For example, the airway epithelium, formed during the canalicular phase, can secrete an array of innate immune molecules implicated in reactive airway disorders, such as asthma (46). Specifically, the airway epithelium may be a major source of IL-25, which regulates immune-mediated inflammatory airway diseases and the response to infections, and IL-33 and thymic stromal lymphopoietin, which also influence asthma development (47). Moreover, recent studies have found that epithelial barrier, epithelial mesenchymal transition, and mesenchymal phenotype are associated with lung function and allergic pulmonary diseases over the life course (47–50). Factors involved in airway epithelial function and migration have been increasingly implicated in the links between PM, impaired lung growth, and asthma risk (51, 52).
Previous human studies have suggested that sex differences in lung development may be related to differential maturation in males relative to females in terms of surfactant synthesis, airway size, and airway resistance, which also begin during the late pseudoglandular and canalicular phases (28, 53). Fetal breathing, a critical determinant of lung development, and surfactant production occur earlier in females as compared with males (54, 55). Sex differences result in lower specific airway resistance and higher size-corrected flow rates and specific airway conductance in female infants (56–59) and predispose male infants to childhood respiratory diseases including asthma (60, 61). Infant males, therefore, may have a pulmonary phenotype more susceptible to the deleterious effects of prenatal air pollution exposure.
Moreover, a leading mechanism underlying the link between prenatal PM2.5 exposure and childhood asthma is thought to involve oxidative stress pathways and proinflammatory cytokine production (15, 62). The developing fetus is particularly vulnerable to oxidative stress because fetal antioxidant capabilities do not increase until the third trimester. Murine models of oxidative stress at embryonic Day 16, equivalent to the canalicular phase in human lung development, demonstrate reductions in peripheral airway number, branching complexity, and alveolarization (63). This coupled with evidence to support an increased susceptibility of the male fetus to maternal oxidative stress (31) may contribute to the observed greater risk in males. It is also possible that the antioxidant properties of female sex hormones (64, 65) may mitigate damaging effects of prenatal ambient PM exposure. Future studies are needed to corroborate our findings and further examine these mechanisms to better understand observed sex differences.
We note several strengths of this study. We assessed prenatal maternal daily particulate air pollution using a validated state-of-the-art hybrid spatiotemporal LUR model incorporating satellite-derived AOD measures based on mothers’ residence during pregnancy. We then leveraged these exposure estimates to implement a data-driven, advanced statistical method to objectively identify susceptibility windows for PM. Also, these analyses included a lower socioeconomic status ethnically mixed inner-city cohort that may be more highly exposed to ambient pollution and be at greater risk for asthma. Finally, this is the first study to examine sex-specific effects of prenatal particulate air pollution on childhood asthma development.
We also acknowledge some limitations. Although we adjusted for several factors known to be important in asthma development, we did not have data on dietary and other environmental factors that may covary with air pollution, such as temperature. Further studies may therefore consider sex-specific joint or interactive associations among additional environmental factors. Children's doctor-diagnosed asthma was reported by mothers. Most of these children were given a diagnosis of asthma after the age of 3 years (78.6%) (see Figure E1), which reduces the likelihood that cases represented wheezing respiratory illnesses other than asthma (e.g., early transient wheeze), although this remains a possibility. As we follow this cohort it will be informative to see if similar associations hold for more objective measures including respiratory function (e.g., spirometry, airway reactivity). Finally, although we focused on a higher-risk sample, our results may not be generalizable to the overall U.S. population.
In summary, we demonstrate that advanced statistical methods when combined with highly temporally resolved exposure data can identify susceptibility windows to environmental exposures that may enhance the ability to find effects and identify vulnerable groups. Increased PM exposure around mid-gestation may be particularly relevant to childhood asthma development, especially among boys. A more definitive characterization of vulnerable windows may provide insight into underlying mechanisms when coupled with the understanding of lung growth, airway structural and functional development, and asthma pathophysiology.
Footnotes
Supported by grants R01 ES010932, U01 HL072494, and R01 HL080674 (R.J.W., principal investigator [PI]) for the Asthma Coalition on Community, Environment, and Social Stress project. The CLEAN Air Center dedicated to air pollution estimates was funded by EPA RD 83479801, and phenotyping and biostatistical support was funded by P30 ES023515 (R.O.W., PI) and P30 ES000002 (B.A.C., PI of Biostatistics Core).
Author Contributions: H-.H.L.H. planned and conducted statistical analysis, participated in the interpretation of results, and took the lead on writing the manuscript. Y.-H.M.C. participated in the interpretation of results and revision of the manuscript. B.A.C. provided statistical expertise and contributed to revision of the manuscript. I.K., J.S., A.L., and R.O.W. participated in data interpretation and revision of the manuscript. R.J.W. contributed to project conception, design, supervising analyses, data interpretation, and leading the writing of the manuscript along with H.-H.L.H.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201504-0658OC on July 15, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dietert RR, Etzel RA, Chen D, Halonen M, Holladay SD, Jarabek AM, Landreth K, Peden DB, Pinkerton K, Smialowicz RJ, et al. Workshop to identify critical windows of exposure for children’s health: immune and respiratory systems work group summary. Environ Health Perspect. 2000;108:483–490. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright RJ. Perinatal stress and early life programming of lung structure and function. Biol Psychol. 2010;84:46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElroy MC, Postle AD, Kelly FJ. Catalase, superoxide dismutase and glutathione peroxidase activities of lung and liver during human development. Biochim Biophys Acta. 1992;1117:153–158. doi: 10.1016/0304-4165(92)90073-4. [DOI] [PubMed] [Google Scholar]
- 4.Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–1071. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 5.Dietert RR. Maternal and childhood asthma: risk factors, interactions, and ramifications. Reprod Toxicol. 2011;32:198–204. doi: 10.1016/j.reprotox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Sly PD, Holt PG. Role of innate immunity in the development of allergy and asthma. Curr Opin Allergy Clin Immunol. 2011;11:127–131. doi: 10.1097/ACI.0b013e32834487c6. [DOI] [PubMed] [Google Scholar]
- 7.Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacol Ther. 2007;114:129–145. doi: 10.1016/j.pharmthera.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Jedrychowski WA, Perera FP, Maugeri U, Mroz E, Klimaszewska-Rembiasz M, Flak E, Edwards S, Spengler JD. Effect of prenatal exposure to fine particulate matter on ventilatory lung function of preschool children of non-smoking mothers. Paediatr Perinat Epidemiol. 2010;24:492–501. doi: 10.1111/j.1365-3016.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jedrychowski WA, Perera FP, Spengler JD, Mroz E, Stigter L, Flak E, Majewska R, Klimaszewska-Rembiasz M, Jacek R. Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int J Hyg Environ Health. 2013;216:395–401. doi: 10.1016/j.ijheh.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, Wright RJ. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133:713–722.e14. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 2008;38:57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada K, Suzaki Y, Leme A, Ito T, Miyamoto K, Kobzik L, Kimura H. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A. 2007;70:688–695. doi: 10.1080/15287390600974692. [DOI] [PubMed] [Google Scholar]
- 13.Mauad T, Rivero DH, de Oliveira RC, Lichtenfels AJ, Guimarães ET, de Andre PA, Kasahara DI, Bueno HM, Saldiva PH. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178:721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida S, Takano H, Nishikawa M, Miao H, Ichinose T. Effects of fetal exposure to urban particulate matter on the immune system of male mouse offspring. Biol Pharm Bull. 2012;35:1238–1243. doi: 10.1248/bpb.b110708. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Deng F, Guo X, Lv P, Zhong M, Liu C, Wang A, Tzan K, Jiang SY, Lippmann M, et al. Association of systemic inflammation with marked changes in particulate air pollution in Beijing in 2008. Toxicol Lett. 2012;212:147–156. doi: 10.1016/j.toxlet.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backes CH, Nelin T, Gorr MW, Wold LE. Early life exposure to air pollution: how bad is it? Toxicol Lett. 2013;216:47–53. doi: 10.1016/j.toxlet.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prescott SL. The development of respiratory inflammation in children. Paediatr Respir Rev. 2006;7:89–96. doi: 10.1016/j.prrv.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Verhein KC, Fryer AD, Jacoby DB. Neural control of airway inflammation. Curr Allergy Asthma Rep. 2009;9:484–490. doi: 10.1007/s11882-009-0071-9. [DOI] [PubMed] [Google Scholar]
- 19.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Gehring U, Koletzko S, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med. 2007;64:8–16. doi: 10.1136/oem.2006.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, et al. GINI Study Group; LISA Study Group. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 21.Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M, Rundle A, Kinney PL, Perera FP, Miller RL. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ Res. 2011;111:1222–1229. doi: 10.1016/j.envres.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–6275. [Google Scholar]
- 23.Ma Z, Hu X, Huang L, Bi J, Liu Y. Estimating ground-level PM2.5 in China using satellite remote sensing. Environ Sci Technol. 2014;48:7436–7444. doi: 10.1021/es5009399. [DOI] [PubMed] [Google Scholar]
- 24.Warren J, Fuentes M, Herring A, Langlois P. Spatial-temporal modeling of the association between air pollution exposure and preterm birth: identifying critical windows of exposure. Biometrics. 2012;68:1157–1167. doi: 10.1111/j.1541-0420.2012.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L272–L278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 26.Chang HY, Mitzner W. Sex differences in mouse models of asthma. Can J Physiol Pharmacol. 2007;85:1226–1235. doi: 10.1139/Y07-116. [DOI] [PubMed] [Google Scholar]
- 27.Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, Zeldin DC. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18:308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jedrychowski W, Spengler JD, Maugeri U, Miller RL, Budzyn-Mrozek D, Perzanowski M, Flak E, Mroz E, Majewska R, Kaim I, et al. Effect of prenatal exposure to fine particulate matter and intake of Paracetamol (acetaminophen) in pregnancy on eczema occurrence in early childhood. Sci Total Environ. 2011;409:5205–5209. doi: 10.1016/j.scitotenv.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Land SC, Wilson SM. Redox regulation of lung development and perinatal lung epithelial function. Antioxid Redox Signal. 2005;7:92–107. doi: 10.1089/ars.2005.7.92. [DOI] [PubMed] [Google Scholar]
- 31.Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med. 2013;26:259–262. doi: 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- 32.Chiu YH, Coull BA, Kloog I, Schwartz J, Hsu HH, Wright RO, Wright RJ. Identifying prenatal windows of susceptibility to particulate air pollution on childhood asthma onset in a prospective urban birth cohort [abstract] Am J Respir Crit Care Med. 2014;189:A2441. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian S, Wright R. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cien Saude Colet. 2008;13:1729–1742. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright RJ, Fisher K, Chiu YH, Wright RO, Fein R, Cohen S, Coull BA. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–1193. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention Overweight and obesity 2012[accessed 2015 Mar 15]. Available from: http://www.cdc.gov/obesity/adult/defining.html
- 36.Mathilda Chiu YH, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. 2012;186:147–154. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry C, Shalowitz M, Quinn K, Wolf R. Validation of the Crisis in Family Systems-Revised, a contemporary measure of life stressors. Psychol Rep. 2001;88:713–724. doi: 10.2466/pr0.2001.88.3.713. [DOI] [PubMed] [Google Scholar]
- 38.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33:1381–1402. [PMC free article] [PubMed] [Google Scholar]
- 39.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29:2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright RJ, Brunst KJ. Programming of respiratory health in childhood: influence of outdoor air pollution. Curr Opin Pediatr. 2013;25:232–239. doi: 10.1097/MOP.0b013e32835e78cc. [DOI] [PubMed] [Google Scholar]
- 42.Miller MD, Marty MA. Impact of environmental chemicals on lung development. Environ Health Perspect. 2010;118:1155–1164. doi: 10.1289/ehp.0901856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stelmach I, Bobrowska-Korzeniowska M, Smejda K, Majak P, Jerzynska J, Stelmach W, Polańska K, Sobala W, Krysicka J, Hanke W. Risk factors for the development of atopic dermatitis and early wheeze. Allergy Asthma Proc. 2014;35:382–389. doi: 10.2500/aap.2014.35.3786. [DOI] [PubMed] [Google Scholar]
- 44.Boyden EA. Development and growth of the airways. Lung biology in health and disease development of the lung. New York: M Dekker; 1977. pp. 3–35.
- 45.Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol. 1984;46:617–628. doi: 10.1146/annurev.ph.46.030184.003153. [DOI] [PubMed] [Google Scholar]
- 46.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO GABRIEL Consortium. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roos AB, Mori M, Grönneberg R, Österlund C, Claesson HE, Wahlström J, Grunewald J, Eklund A, Erjefält JS, Lundberg JO, et al. Elevated exhaled nitric oxide in allergen-provoked asthma is associated with airway epithelial iNOS. PLoS One. 2014;9:e90018. doi: 10.1371/journal.pone.0090018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohal SS, Ward C, Walters EH. Importance of epithelial mesenchymal transition (EMT) in COPD and asthma. Thorax. 2014;69:768. doi: 10.1136/thoraxjnl-2014-205582. [DOI] [PubMed] [Google Scholar]
- 50.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 52.Iwanaga K, Elliott MS, Vedal S, Debley JS. Urban particulate matter induces pro-remodeling factors by airway epithelial cells from healthy and asthmatic children. Inhal Toxicol. 2013;25:653–660. doi: 10.3109/08958378.2013.827283. [DOI] [PubMed] [Google Scholar]
- 53.Ishak N, Sozo F, Harding R, De Matteo R. Does lung development differ in male and female fetuses? Exp Lung Res. 2014;40:30–39. doi: 10.3109/01902148.2013.858197. [DOI] [PubMed] [Google Scholar]
- 54.Torday JS, Nielsen HC. The sex difference in fetal lung surfactant production. Exp Lung Res. 1987;12:1–19. doi: 10.3109/01902148709068811. [DOI] [PubMed] [Google Scholar]
- 55.Boddy K, Dawes GS. Fetal breathing. Br Med Bull. 1975;31:3–7. doi: 10.1093/oxfordjournals.bmb.a071237. [DOI] [PubMed] [Google Scholar]
- 56.Quanjer PH, Borsboom GJ, Brunekreef B, Zach M, Forche G, Cotes JE, Sanchis J, Paoletti P. Spirometric reference values for white European children and adolescents: Polgar revisited. Pediatr Pulmonol. 1995;19:135–142. doi: 10.1002/ppul.1950190209. [DOI] [PubMed] [Google Scholar]
- 57.Clausen JL, Coates AL, Quanjer PH. Measurement of lung volumes in humans: review and recommendations from an ATS/ERS workshop. Eur Respir J. 1997;10:1205–1206. doi: 10.1183/09031936.97.10061205. [DOI] [PubMed] [Google Scholar]
- 58.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official statement of the European Respiratory Society. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 59.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 60.Demissie K, Marcella SW, Breckenridge MB, Rhoads GG. Maternal asthma and transient tachypnea of the newborn. Pediatrics. 1998;102:84–90. doi: 10.1542/peds.102.1.84. [DOI] [PubMed] [Google Scholar]
- 61.Air Pollution and Chronic Respiratory Disease. Air pollution and chronic respiratory disease. I. Methods and material (author’s transl) Bull Eur Physiopathol Respir. 1982;18:87–99. [PubMed] [Google Scholar]
- 62.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dieperink HI, Blackwell TS, Prince LS. Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res. 2006;59:185–190. doi: 10.1203/01.pdr.0000196371.85945.3a. [DOI] [PubMed] [Google Scholar]
- 64.Bellanti F, Matteo M, Rollo T, De Rosario F, Greco P, Vendemiale G, Serviddio G. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol. 2013;1:340–346. doi: 10.1016/j.redox.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Massafra C, Gioia D, De Felice C, Muscettola M, Longini M, Buonocore G. Gender-related differences in erythrocyte glutathione peroxidase activity in healthy subjects. Clin Endocrinol (Oxf) 2002;57:663–667. doi: 10.1046/j.1365-2265.2002.01657.x. [DOI] [PubMed] [Google Scholar]