Abstract
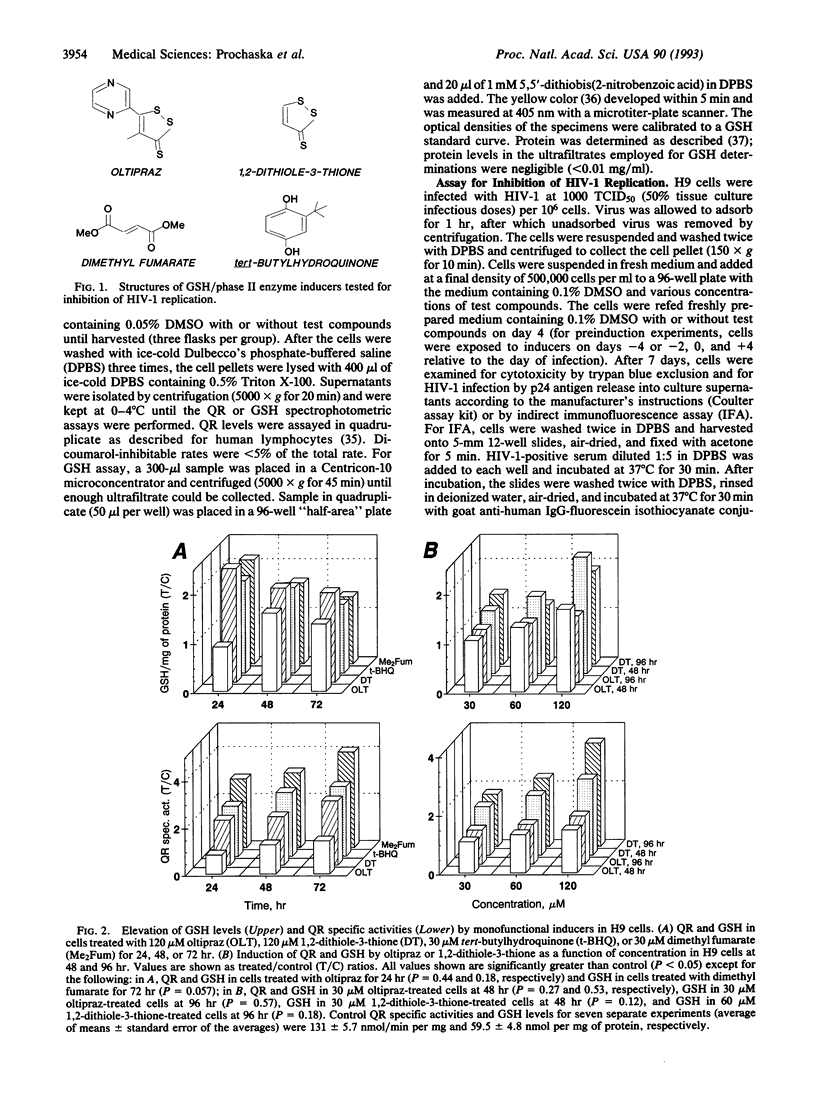
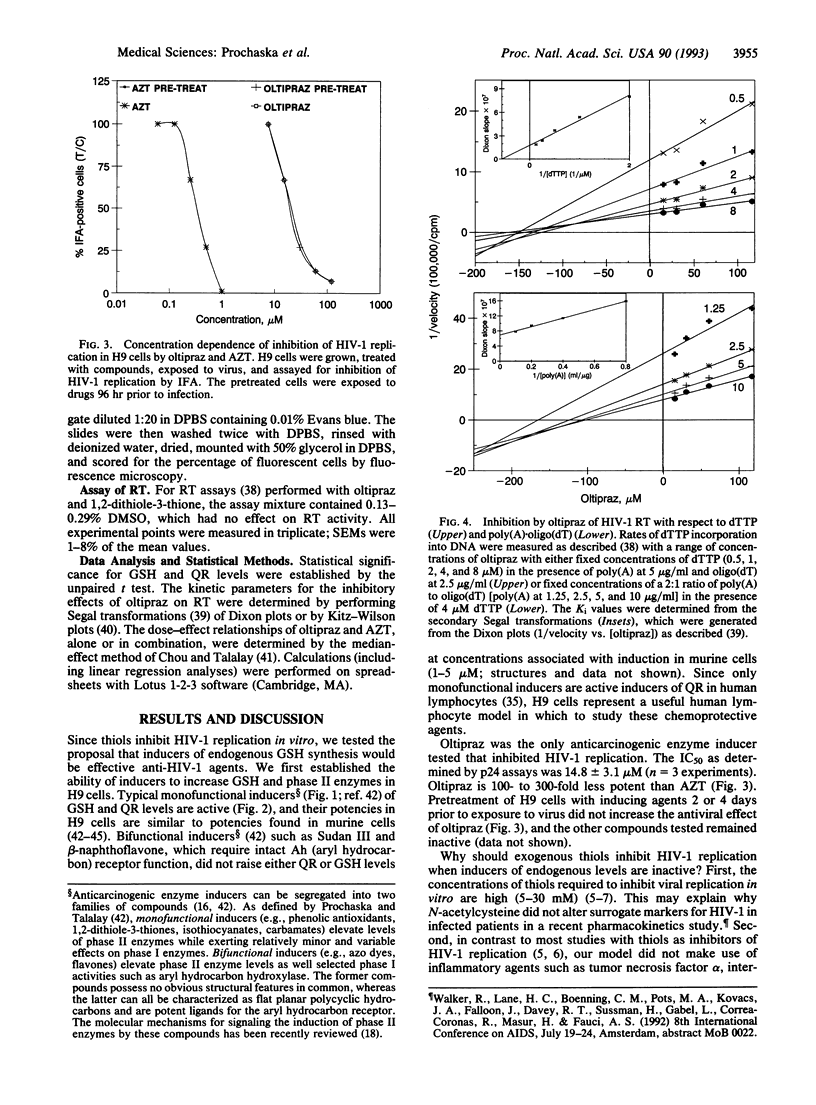
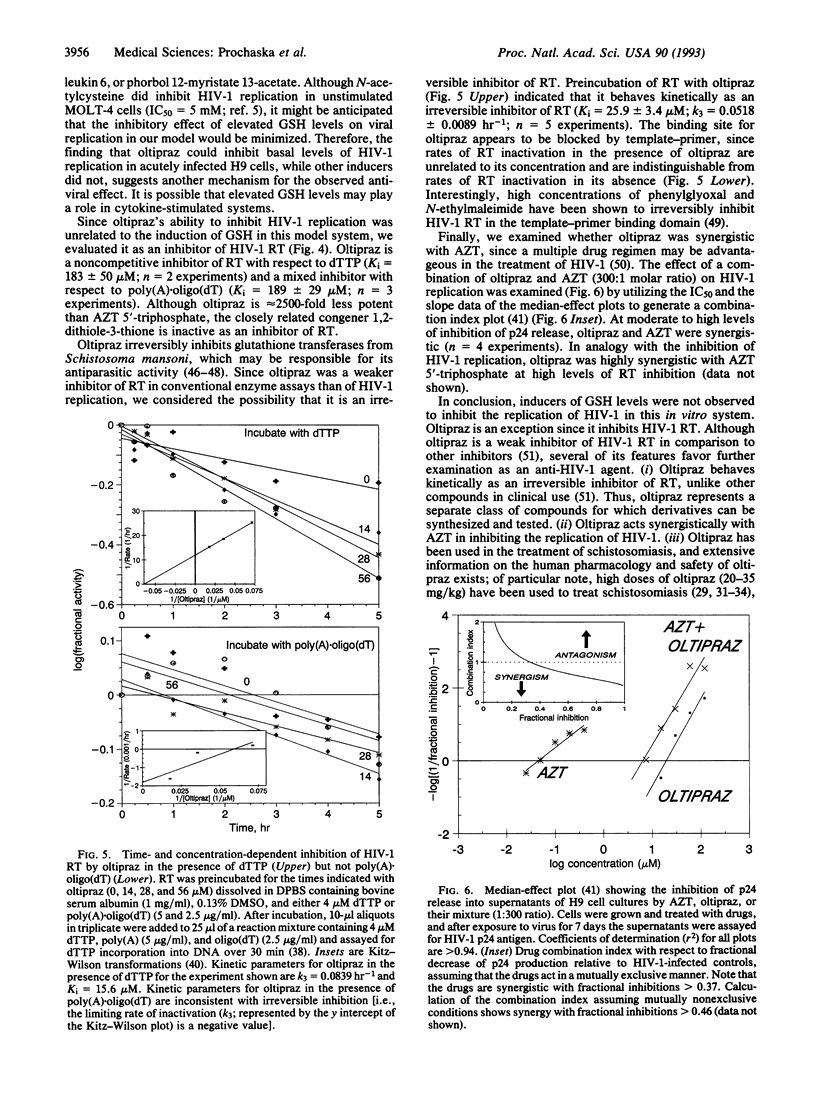
Glutathione depletion may play a pivotal role in the pathogenesis of human immunodeficiency virus type-1 (HIV-1) infection. Since certain compounds prevent experimental carcinogenesis by elevating the levels of glutathione and phase II detoxication enzymes, we compared the potencies of several inducers with their ability to inhibit basal levels of HIV-1 replication in H9 cutaneous T-cell lymphoma cells. All monofunctional inducers tested elevated the levels of glutathione and quinone reductase, a marker for phase II enzyme induction. However, only oltipraz [4-methyl-5-(2-pyrazinyl)-1,2-dithiole-3-thione] was effective at inhibiting HIV-1 replication (IC50 = 14.8 +/- 3.1 microM). The antiviral effect of oltipraz was potentiated by 3'-azido-3'-deoxythymidine. Thus, 1,2-dithiole-3-thiones represent a hitherto unrecognized class of anti-HIV-1 agents. Oltipraz behaves kinetically as an irreversible inhibitor of HIV-1 reverse transcriptase in the template-primer binding domain. Oltipraz has been used to treat schistosomiasis in humans and is undergoing clinical evaluation as an anticarcinogen. Thus, oltipraz (and other 1,2-dithiole-3-thiones) may have therapeutic utility in HIV-1-infected individuals, not only because of their antiretroviral activity, but also by preventing the development of HIV-1-associated neoplasms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H. M., Homeida M. M., Sulaiman S. M., Bennett J. L. Diet-controlled blood levels of oltipraz in healthy male subjects. J Antimicrob Chemother. 1984 May;13(5):465–470. doi: 10.1093/jac/13.5.465. [DOI] [PubMed] [Google Scholar]
- Ansher S. S., Dolan P., Bueding E. Biochemical effects of dithiolthiones. Food Chem Toxicol. 1986 May;24(5):405–415. doi: 10.1016/0278-6915(86)90205-x. [DOI] [PubMed] [Google Scholar]
- Archer S. The chemotherapy of schistosomiasis. Annu Rev Pharmacol Toxicol. 1985;25:485–508. doi: 10.1146/annurev.pa.25.040185.002413. [DOI] [PubMed] [Google Scholar]
- Baker D. H., Wood R. J. Cellular antioxidant status and human immunodeficiency virus replication. Nutr Rev. 1992 Jan;50(1):15–18. doi: 10.1111/j.1753-4887.1992.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Bella H., Rahim A. G., Mustafa M. D., Ahmed M. A., Wasfi S., Bennett J. L. Oltipraz--antischistosomal efficacy in Sudanese infected with Schistosoma mansoni. Am J Trop Med Hyg. 1982 Jul;31(4):775–778. doi: 10.4269/ajtmh.1982.31.775. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Batzinger R. P., Ou S. Y., Bueding E., Cha Y. N., Talalay P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978 Dec;38(12):4486–4495. [PubMed] [Google Scholar]
- Bieder A., Decouvelaere B., Gaillard C., Depaire H., Heusse D., Ledoux C., Lemar M., Le Roy J. P., Raynaud L., Snozzi C. Comparison of the metabolism of oltipraz in the mouse, rat and monkey and in man. Distribution of the metabolites in each species. Arzneimittelforschung. 1983;33(9):1289–1297. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buhl R., Jaffe H. A., Holroyd K. J., Wells F. B., Mastrangeli A., Saltini C., Cantin A. M., Crystal R. G. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989 Dec 2;2(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- De Clercq E. HIV inhibitors targeted at the reverse transcriptase. AIDS Res Hum Retroviruses. 1992 Feb;8(2):119–134. doi: 10.1089/aid.1992.8.119. [DOI] [PubMed] [Google Scholar]
- De Long M. J., Dolan P., Santamaria A. B., Bueding E. 1,2-Dithiol-3-thione analogs: effects on NAD(P)H:quinone reductase and glutathione levels in murine hepatoma cells. Carcinogenesis. 1986 Jun;7(6):977–980. doi: 10.1093/carcin/7.6.977. [DOI] [PubMed] [Google Scholar]
- Dröge W., Eck H. P., Mihm S. HIV-induced cysteine deficiency and T-cell dysfunction--a rationale for treatment with N-acetylcysteine. Immunol Today. 1992 Jun;13(6):211–214. doi: 10.1016/0167-5699(92)90156-2. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eck H. P., Gmünder H., Hartmann M., Petzoldt D., Daniel V., Dröge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol Chem Hoppe Seyler. 1989 Feb;370(2):101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- Flexner C., Broyles S. S., Earl P., Chakrabarti S., Moss B. Characterization of human immunodeficiency virus gag/pol gene products expressed by recombinant vaccinia viruses. Virology. 1988 Oct;166(2):339–349. doi: 10.1016/0042-6822(88)90504-1. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Prochaska H. J., Yang L. Y. Induction of NAD(P)H:quinone reductase in human peripheral blood lymphocytes. Carcinogenesis. 1991 Dec;12(12):2393–2396. doi: 10.1093/carcin/12.12.2393. [DOI] [PubMed] [Google Scholar]
- Ho W. Z., Douglas S. D. Glutathione and N-acetylcysteine suppression of human immunodeficiency virus replication in human monocyte/macrophages in vitro. AIDS Res Hum Retroviruses. 1992 Jul;8(7):1249–1253. doi: 10.1089/aid.1992.8.1249. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kalebic T., Kinter A., Poli G., Anderson M. E., Meister A., Fauci A. S. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):986–990. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardaman M. W., Fenwick A., el Igail A. B., el Tayeb M., Bennett J. L., Daffalla A. A. Field trials with Oltipraz against Schistosoma mansoni in the Gezira Irrigated Area, Sudan. J Trop Med Hyg. 1985 Apr;88(2):95–100. [PubMed] [Google Scholar]
- Kelloff G. J., Boone C. W., Malone W. F., Steele V. E. Chemoprevention clinical trials. Mutat Res. 1992 Jun;267(2):291–295. doi: 10.1016/0027-5107(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Egner P. A., Dolan P. M., Groopman J. D., Roebuck B. D. Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res. 1987 Aug 15;47(16):4271–4277. [PubMed] [Google Scholar]
- Kong X. B., Zhu Q. Y., Ruprecht R. M., Watanabe K. A., Zeidler J. M., Gold J. W., Polsky B., Armstrong D., Chou T. C. Synergistic inhibition of human immunodeficiency virus type 1 replication in vitro by two-drug and three-drug combinations of 3'-azido-3'-deoxythymidine, phosphonoformate, and 2',3'-dideoxythymidine. Antimicrob Agents Chemother. 1991 Oct;35(10):2003–2011. doi: 10.1128/aac.35.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. G., Moon R. C. Characterization of effective chemopreventive agents in mammary gland in vitro using an initiation-promotion protocol. Anticancer Res. 1991 Mar-Apr;11(2):593–596. [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991;51(2):155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Mitchell L. L., Cooperman B. S. Active site studies of human immunodeficiency virus reverse transcriptase. Biochemistry. 1992 Aug 25;31(33):7707–7713. doi: 10.1021/bi00148a035. [DOI] [PubMed] [Google Scholar]
- Nare B., Smith J. M., Prichard R. K. Differential effects of oltipraz and its oxy-analogue on the viability of Schistosoma mansoni and the activity of glutathione S-transferase. Biochem Pharmacol. 1991 Aug 22;42(6):1287–1292. doi: 10.1016/0006-2952(91)90267-9. [DOI] [PubMed] [Google Scholar]
- Nare B., Smith J. M., Prichard R. K. Mechanisms of inactivation of Schistosoma mansoni and mammalian glutathione S-transferase activity by the antischistosomal drug oltipraz. Biochem Pharmacol. 1992 Mar 17;43(6):1345–1351. doi: 10.1016/0006-2952(92)90512-h. [DOI] [PubMed] [Google Scholar]
- Nare B., Smith J. M., Prichard R. K. Oltipraz-induced decrease in the activity of cytosolic glutathione S-transferase in Schistosoma mansoni. Int J Parasitol. 1991 Dec;21(8):919–925. doi: 10.1016/0020-7519(91)90167-6. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B., Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Rao C. V., Tokomo K., Kelloff G., Reddy B. S. Inhibition by dietary oltipraz of experimental intestinal carcinogenesis induced by azoxymethane in male F344 rats. Carcinogenesis. 1991 Jun;12(6):1051–1055. doi: 10.1093/carcin/12.6.1051. [DOI] [PubMed] [Google Scholar]
- Roebuck B. D., Liu Y. L., Rogers A. E., Groopman J. D., Kensler T. W. Protection against aflatoxin B1-induced hepatocarcinogenesis in F344 rats by 5-(2-pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz): predictive role for short-term molecular dosimetry. Cancer Res. 1991 Oct 15;51(20):5501–5506. [PubMed] [Google Scholar]
- Roederer M., Ela S. W., Staal F. J., Herzenberg L. A., Herzenberg L. A. N-acetylcysteine: a new approach to anti-HIV therapy. AIDS Res Hum Retroviruses. 1992 Feb;8(2):209–217. doi: 10.1089/aid.1992.8.209. [DOI] [PubMed] [Google Scholar]
- Roederer M., Staal F. J., Raju P. A., Ela S. W., Herzenberg L. A., Herzenberg L. A. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K. C. Schistosomiasis drug therapy and treatment considerations. Drugs. 1991 Sep;42(3):379–405. doi: 10.2165/00003495-199142030-00004. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Venegas P. L., Wattenberg L. W. Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents. J Natl Cancer Inst. 1982 Mar;68(3):493–496. [PubMed] [Google Scholar]
- Sparnins V. L., Wattenberg L. W. Enhancement of glutathione S-transferase activity of the mouse forestomach by inhibitors of Benzo[a]pyrene-induced neoplasia of the forestomach. J Natl Cancer Inst. 1981 Apr;66(4):769–771. [PubMed] [Google Scholar]
- Spencer S. R., Wilczak C. A., Talalay P. Induction of glutathione transferases and NAD(P)H:quinone reductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res. 1990 Dec 15;50(24):7871–7875. [PubMed] [Google Scholar]
- Staal F. J., Ela S. W., Roederer M., Anderson M. T., Herzenberg L. A., Herzenberg L. A. Glutathione deficiency and human immunodeficiency virus infection. Lancet. 1992 Apr 11;339(8798):909–912. doi: 10.1016/0140-6736(92)90939-z. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twerdok L. E., Rembish S. J., Trush M. A. Induction of quinone reductase and glutathione in bone marrow cells by 1,2-dithiole-3-thione: effect on hydroquinone-induced cytotoxicity. Toxicol Appl Pharmacol. 1992 Feb;112(2):273–281. doi: 10.1016/0041-008x(92)90197-z. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W., Bueding E. Inhibitory effects of 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (Oltipraz) on carcinogenesis induced by benzo[a]pyrene, diethylnitrosamine and uracil mustard. Carcinogenesis. 1986 Aug;7(8):1379–1381. doi: 10.1093/carcin/7.8.1379. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of neoplasia by minor dietary constituents. Cancer Res. 1983 May;43(5 Suppl):2448s–2453s. [PubMed] [Google Scholar]
- Zhang Y., Talalay P., Cho C. G., Posner G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quay B., Malinverni R., Lauterburg B. H. Glutathione depletion in HIV-infected patients: role of cysteine deficiency and effect of oral N-acetylcysteine. AIDS. 1992 Aug;6(8):815–819. [PubMed] [Google Scholar]
- el Igail A. B., el Tayeb M., Kardaman M. W., Daffalla A. A., Dixon H. G., Fenwick A. Dose-finding trial using Oltipraz to treat schoolchildren infected with Schistosoma mansoni in Gezira, Sudan. J Trop Med Hyg. 1985 Apr;88(2):101–104. [PubMed] [Google Scholar]



