Abstract
Obesity is a global concern, affecting both developed and developing countries. Although there are large variations in obesity and breast cancer rates worldwide and across racial/ethnic groups, most studies evaluating the impact of obesity on breast cancer risk and survival have been conducted in non-Hispanic white women in the United States or Europe. Given the known racial/ethnic differences in tumor hormone receptor subtype distribution, obesity prevalence, and risk factor profiles, we reviewed published data for women of African, Hispanic, and Asian ancestry in the United States and their countries of origin. Although the data are limited, current evidence suggests a stronger adverse effect of obesity on breast cancer risk and survival in women of Asian ancestry. For African Americans and Hispanics, the strength of the associations appears to be more comparable to that of non-Hispanic whites, particularly when accounting for subtype and menopausal status. Central obesity seems to have a stronger impact in African-American women than general adiposity as measured by body mass index. International data from countries undergoing economic transition offer a unique opportunity to evaluate the impact of rapid weight gain on breast cancer. Such studies should take into account genetic ancestry, which may help elucidate differences in associations between ethnically admixed populations. Overall, additional large studies that use a variety of adiposity measures are needed, because the current evidence is based on few studies, most with limited statistical power. Future investigations of obesity biomarkers will be useful to understand possible racial/ethnic biological differences underlying the complex association between obesity and breast cancer development and progression.
Keywords: obesity, central adiposity, weight gain, breast cancer, subtypes, ethnicity, Hispanics, African American, Asian, Africa
Introduction
Breast cancer is the most common cancer among women worldwide, with an estimated 1.7 million new cases diagnosed in 2012, and represents 25% of all cancer diagnoses (1). Incidence rates vary widely across geographic regions, with the highest rates in North America, Australia, and northern and western Europe and the lowest rates in large parts of Africa and Asia. Mortality rates do not differ as much due to better survival in developed countries, where incidence rates are also the highest (1). Although the variation in incidence rates may reflect differences in screening and reporting practices and access to care, genetic, lifestyle, and environmental factors likely contribute to these differences.
Obesity, defined as a having a high BMI (in kg/m2; ≥30), is a modifiable lifestyle factor known to affect breast cancer risk and survival (2, 3), with a high prevalence worldwide, which more than doubled between 1980 and 2014 (4). The prevalence of obesity is highest in South Africa, followed by North Africa and the Middle East and North America (5). Over the next 2 decades, low- and middle-income countries are expected to experience the largest proportional increase in overweight and obesity rates (5).
In the United States, there are large differences in both breast cancer rates and the prevalence of obesity across racial/ethnic groups. Breast cancer incidence is higher in non-Hispanic white (NHW)10 women than in other groups, with the lowest rates observed in Asian Americans/Pacific Islanders (6). Five-year breast cancer–specific survival rates are lowest in African Americans (AAs; 78.9%), followed by Hispanics (87%) and NHWs (88.6%), and are highest in Asian Americans (91.4%) (6), although they vary among Asian-American subgroups (7). Notably, the groups with poorer survival also have a higher prevalence of general (8, 9) and central (10) obesity (Figure 1), as discussed below in more detail for each racial/ethnic group.
FIGURE 1.
Age-adjusted prevalence of obesity by race/Hispanic origin in women aged >20 y: United States, 2011–2012. NH, non-Hispanic. Adapted from reference 9.
Current Evidence for Obesity and Breast Cancer Risk
The evidence for the impact of obesity on breast cancer risk comes largely from studies conducted in NHW women in the United States and Europe. These studies have shown that obesity and weight gain during adulthood are associated with increased postmenopausal breast cancer risk, particularly among women not using menopausal hormone therapy (HT), whereas a higher BMI is associated with reduced risk in premenopausal women (2). Obesity during adolescence and early adulthood has been associated with reduced risk of both pre- and postmenopausal breast cancer (11, 12). Central obesity, in most studies defined as a high waist-to-hip ratio (WHR), has been associated with increased breast cancer risk in postmenopausal women (2) but less consistently in premenopausal women (13, 14). However, a meta-analysis showed that the association with WHR became weaker and nonsignificant among postmenopausal women after adjusting for BMI, whereas the association persisted in premenopausal women (15).
Current Evidence for Obesity and Breast Cancer Survival
Few studies have evaluated the impact of obesity on breast cancer prognosis in racial/ethnic minorities. In NHW women, there is growing evidence that both pre- and post-diagnostic obesity, as well as post-diagnostic weight gain, are associated with higher breast cancer–specific and overall mortality in both pre- and postmenopausal women (3, 16–19). The role of central adiposity in survival after breast cancer diagnosis has received little attention, but a recent meta-analysis based on 4 studies found elevated all-cause mortality for those with high WHR around the time of diagnosis (3).
Breast Cancer Subtypes
Breast cancer is a heterogeneous disease with several major subtypes defined by tumor hormone receptor (HR) status or molecular intrinsic markers, with growing evidence of different etiologic and prognostic profiles (20). Because most studies are limited to clinical information from cancer registries, which do not have information on molecular subtypes, the classification of subtypes is commonly based on HR status as defined by estrogen receptor (ER) and progesterone receptor (PR) status. Some studies also include human epidermal growth factor receptor 2 (HER2) status, which has been a required data element in SEER (Surveillance, Epidemiology, and End Results) cancer registries since 2010 (21), and allows to differentiate a subset of ER-negative (ER−) tumors known as triple-negative breast cancer, which are negative for ER, PR, and HER2 and are more difficult to treat and have a worse prognosis (22, 23). The distribution of subtypes varies widely across racial/ethnic groups, with AAs and Hispanics being more likely to have ER− and triple-negative breast cancer than NHW women (24).
Given racial/ethnic differences in subtype distribution, obesity prevalence, and risk factor profiles, it is important to evaluate tumor subtypes to fully understand the impact of obesity. Few studies, however, examined subtype-specific associations by race/ethnicity, and most did not have sufficient statistical power to do so. The overall evidence, based primarily on NHW women, indicates that associations with obesity are stronger (increasing risk for postmenopausal women and decreasing risk for premenopausal women) for risk of ER-positive (ER+)/PR+ than of ER−/PR− tumors (25–27). Few studies have evaluated the association of obesity with the risk of triple-negative breast cancer (23), but a recent meta-analysis reported increased risk for premenopausal women and inconsistent and mostly null associations for postmenopausal women (28).
Only a handful of studies, conducted primarily in NHW women, have evaluated associations with breast cancer prognosis by HR subtype and most had limited statistical power, particularly for ER− tumors. For prediagnosis BMI, the increased risk of all-cause mortality and breast cancer–specific mortality appears to be limited to HR+ tumors (3). However, there is some evidence that BMI around the time of diagnosis increases breast cancer mortality for both HR+ and HR− tumors (3, 17). A few studies evaluated the impact of BMI at diagnosis on overall survival and disease-free survival among women with triple-negative breast cancer and produced inconsistent results (29–34). In the California Breast Cancer Survivorship Consortium, a higher WHR was associated with increased breast cancer–specific mortality only for HR+ tumors (35).
Possible Biological Mechanisms
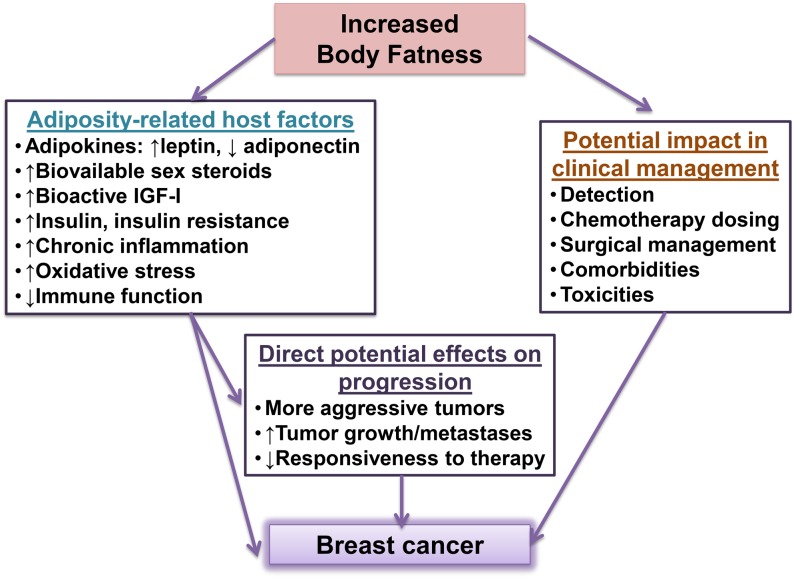
Obesity may be associated with breast cancer risk and progression through several overlapping pathways, including increased aromatase-mediated estrogen production, high circulating insulin/insulin-like growth factor I (IGF-I), altered adipokine concentrations (high leptin and low adiponectin), and chronic inflammation (18), resulting in significant increases in local and circulating proinflammatory cytokines, which, in turn, activates the innate and humoral immune system (36, 37). Moreover, obesity may affect disease management by having an impact on treatment efficacy and treatment decisions (e.g., lowering chemotherapy dosing or affecting surgical outcomes) by increasing adverse treatment effects and/or by affecting related comorbidities (38) (Figure 2). Important differences in obesity biomarkers (39), disease presentation (6), and management (38) across racial/ethnic groups have been described.
FIGURE 2.
Potential adverse effects of body fatness on breast cancer risk and survival. IGF-I, insulin-like growth factor I.
Here we summarize the current evidence on the impact of general and central obesity on breast cancer risk and survival in women of different racial/ethnic backgrounds. For each racial/ethnic group, we included publications identified through PubMed, meta-analyses, and review articles without conducting a systematic search. The focus of our review is on women of African, Hispanic, and Asian ancestry in the United States and in their countries of origin. In addition, we discuss possible racial/ethnic differences in underlying biological mechanisms in these populations.
Obesity and Breast Cancer in AA Women
AA women are diagnosed with breast cancer at an earlier age and with more aggressive features, such as high-grade, advanced-stage, and ER− tumors, including triple-negative breast cancer, than other racial/ethnic groups (22). AAs also have a higher prevalence of overall and central obesity [defined as a waist circumference (WC) ≥88 cm] than do other racial/ethnic groups, with rates of 71% and 91%, respectively, projected by 2020 (40).
Obesity and breast cancer risk
Studies reporting on the association of several anthropometric measures with breast cancer risk (41–54) are shown in Table 1.
TABLE 1.
Studies reporting on the association between anthropometric factors and breast cancer risk in African-American women1
| Premenopausal |
Postmenopausal |
|||||||||
| First author, year (ref) | Age, y | Study design | Exposure | Contrast | Cases/noncases, n | Risk estimate | 95% CI | Cases/noncases, n | Risk estimate | 95% CI |
| Young-adult BMI | ||||||||||
| Mayberry, 1994 (41)2 | 20–54 | PCC | BMI (kg/m2) age 18 y | ≥30.70 vs. <23.80 | 177/137 | 0.52 | 0.2, 1.4 | 313/348 | 1.52 | 0.9, 2.7 |
| Zhu, 2005 (42) | 20–64 | PCC | BMI age 18 y | ≥30 vs. <25 | 109/109 | 1.84 | 0.27, 12.45 | 158/148 | 1.35 | 0.20, 9.15 |
| Palmer, 2007 (43) | 21–69 | PC | BMI age 18 y | ≥25 vs. <20 | 491/42,538 | 0.68 | 0.46, 0.98 | 442/9542 | 0.55 | 0.37, 0.82 |
| Berstad, 2010 (44) | 35–64 | PCC | BMI age 18 y | ≥25 vs. <20 | 733/688 | 0.67 | 0.47, 0.96 | 639/712 | 0.80 | 0.54, 1.19 |
| ER+/PR+ | 257/688 | 0.50 | 0.30, 0.87 | 225/712 | 0.80 | 0.46, 1.41 | ||||
| ER−/PR− | 230/688 | 0.96 | 0.59, 1.57 | 158/712 | 0.55 | 0.27, 1.14 | ||||
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | BMI in 20s | High vs. low quantiles3 | 154/160 | 0.87 | 0.41, 1.86 | 243/315 | 0.934 | 0.59, 1.45 |
| ER+/PR+ (tertiles)3 | 57/160 | 0.34 | 0.13, 0.92 | 108/315 | 0.734 | 0.39, 1.36 | ||||
| White, 2012 (47) | 45–75 | PC | BMI age 21 y | ≥30 vs. <20 | — | — | — | 523/14,669 | 0.73 | 0.36, 1.48 |
| Bandera, 2013 (48) | 20–75 | PCC | BMI age 20 y | ≥30 vs. <25 | 469/482 | 0.77 | 0.42, 1.40 | 509/476 | 0.88 | 0.43, 1.81 |
| Robinson, 2014 (49)5 | 20–74 | PCC | BMI age 18 y | ≥25 vs. <22 | 336/327 | 1.26 | 0.75, 2.11 | 425/367 | 1.26 | 0.80, 1.98 |
| ER+/PR+ | 142/327 | 1.114 | 0.62, 2.00 | 173/367 | 1.474 | 0.82, 2.63 | ||||
| ER−/PR− | 153/327 | 1.284 | 0.73, 2.24 | 99/367 | 1.144 | 0.56, 2.35 | ||||
| Bandera, 2015 (50) | 20–75 | Pooled6 | BMI age 18–21 y | ≥30 vs. <20 | 1125/4053 | 0.78 | 0.55, 1.10 | 1923/7465 | 0.67 | 0.45, 1.01 |
| ER+ | 674/4053 | 0.65 | 0.42, 1.01 | 1338/7465 | 0.62 | 0.38, 1.01 | ||||
| ER− | 451/4053 | 1.00 | 0.63, 1.58 | 585/7465 | 0.78 | 0.44, 1.41 | ||||
| TN | 224/4053 | 1.08 | 0.59, 1.98 | 255/7465 | 0.68 | 0.29, 1.56 | ||||
| Recent BMI | ||||||||||
| Schatzkin, 1987 (51) | 25–70 | PCC | BMI | ≥30 vs. ≤24 | 221/334 | 1.2 | 0.7, 2.1 | 310/233 | 2.5 | 1.5, 4.4 |
| Mayberry, 1994 (41)2 | 20–54 | PCC | Adult BMI | ≥32.3 vs. <24.9 | 177/137 | 3.92 | 1.3, 11.6 | 313/348 | 0.82 | 0.4, 1.4 |
| Brinton, 1997 (52)2 | 20–54 | PCC | Current BMI | <22.0 vs. >28.8 | Not specified/102 | 1.32 | 0.5, 3.1 | NS/194 | 0.82 | 0.3, 1.9 |
| Zhu, 2005 (42) | 20–64 | PCC | Recent BMI | ≥30 vs. <25 | 110/110 | 2.49 | 0.87, 7.59 | 161/150 | 2.32 | 1.04, 5.19 |
| Palmer, 2007 (43) | 21–69 | PC | Current BMI | ≥35 vs. <25 | 495/42,538 | 0.87 | 0.62, 1.21 | 454/9542 | 0.99 | 0.72, 1.36 |
| Berstad, 2010 (44) | 35–64 | PCC | Recent BMI | ≥35 vs. <25 | 733/688 | 0.81 | 0.56, 1.19 | 639/712 | 1.26 | 0.85, 1.85 |
| ER+/PR+ | 257/688 | 0.63 | 0.36, 1.11 | 225/712 | 1.83 | 1.08, 3.09 | ||||
| ER−/PR− | 230/688 | 1.12 | 0.66, 1.91 | 158/712 | 0.58 | 0.27, 1.24 | ||||
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | Current BMI | ≥30 vs. <25 | 154/160 | 0.65 | 0.35, 1.23 | 243/315 | 1.074 | 0.66, 1.73 |
| ER+/PR+ | 57/160 | 0.32 | 0.13, 0.81 | 108/315 | 1.464 | 0.74, 2.89 | ||||
| White, 2012 (47) | 45–75 | PC | Baseline BMI | ≥30 vs. <20 | — | — | — | 523/8595 | 1.244 | 0.90, 1.70 |
| Bandera, 2013 (53) | 20–75 | PCC | Current BMI | ≥30 vs. <25 | 469/482 | 0.927 | 0.54, 1.56 | 509/476 | 1.007 | 0.58, 1.72 |
| ER+/PR+ | 195/482 | 1.347 | 0.68, 2.68 | 206/476 | 1.047 | 0.50, 2.18 | ||||
| ER−/PR− | 102/482 | 1.267 | 0.53, 3.00 | 101/476 | 0.377 | 0.15, 0.96 | ||||
| Robinson, 2014 (49) | 20–74 | PCC | Recent BMI | ≥35 vs. <25 | 340/327 | 1.09 | 0.67, 1.78 | 432/375 | 1.06 | 0.66, 1.70 |
| ER+/PR+ | 143/327 | 0.844 | 0.44, 1.62 | 177/375 | 1.284 | 0.64, 2.55 | ||||
| ER−/PR− | 155/327 | 1.134 | 0.59, 2.16 | 100/375 | 0.634 | 0.27, 1.48 | ||||
| Bandera, 2015 (50) | 20–75 | Pooled6 | Recent BMI | ≥35 vs. <25 | 1149/4087 | 0.837 | 0.66, 1.05 | 2025/7973 | 1.087 | 0.88, 1.34 |
| ER+ | 691/4087 | 0.817 | 0.61, 1.07 | 1413/7973 | 1.317 | 1.02, 1.67 | ||||
| ER− | 458/4087 | 0.897 | 0.64, 1.24 | 612/7973 | 0.757 | 0.54, 1.04 | ||||
| TN | 227/4087 | 1.137 | 0.71, 1.80 | 264/7973 | 0.607 | 0.39, 0.93 | ||||
| Weight gain | ||||||||||
| Palmer, 2007 (43) | 21–69 | PC | Weight gain since age 18 y | ≥25 vs. <10 kg | 490/42,538 | 1.178 | 0.90, 1.52 | 443/9542 | 1.098 | 0.81, 1.488 |
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | Weight gain (kg) since 20s | High vs. none/stable9 | 153/158 | 0.52 | 0.22, 1.22 | 231/297 | 1.134 | 0.54, 2.39 |
| ER+/PR+ | 61/158 | 0.31 | 0.09, 1.04 | 104/297 | 1.274 | 0.47, 3.42 | ||||
| White, 2012 (47) | 45–75 | PC | Weight gain since age 21 y | >22.7 vs. ≤3.64 kg | — | — | — | 523/8595 | 1.46 | 1.09, 1.97 |
| Bandera, 2013 (48) | 20–75 | PCC | Weight gain since age 20 y | ≥34.6 vs. <13.82 kg | 407/434 | 1.4910 | 0.81, 2.73 | 439/427 | 1.4210 | 0.80, 2.53 |
| Robinson, 2014 (49) | 20–74 | PCC | Weight gain since age 18 y | ≥55 vs. ≤25 pounds | 336/326 | 0.6810 | 0.39, 1.19 | 429/375 | 0.8410 | 0.50, 1.40 |
| ER+/PR+ | 141/326 | 0.764 | 0.44, 1.30 | 175/375 | 0.864 | 0.48, 1.57 | ||||
| ER−/PR− | 154/326 | 0.774 | 0.44, 1.35 | 100/375 | 0.634 | 0.31, 1.29 | ||||
| WHR or WC | ||||||||||
| Palmer, 2007 (43) | 21–69 | PC | WHR | ≥0.87 vs. <0.71 | 429/42,538 | 1.19 | 0.87, 1.64 | 382/9542 | 0.99 | 0.72, 1.37 |
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | WC (cm) | High vs. low quartile11 | 154/160 | 1.09 | 0.47, 2.52 | 243/315 | 2.174,10 | 1.05, 4.49 |
| Tertile 3 vs. tertile 112 ER+/ER+ | 57/160 | 0.87 | 0.32, 2.35 | 108/315 | 3.364,10 | 1.10, 10.28 | ||||
| Bandera, 2013 (53) | 20–75 | PCC | WHR | >0.92 vs. ≤0.82 | 469/482 | 1.3810 | 0.89, 2.12 | 509/476 | 1.4810 | 0.97, 2.26 |
| ER+/PR+ | 192/482 | 1.3310 | 0.76, 2.32 | 205/470 | 1.6110 | 0.90, 2.90 | ||||
| ER−/PR− | 94/482 | 1.5110 | 0.74, 3.05 | 100/470 | 1.2610 | 0.63, 2.50 | ||||
| Robinson, 2014 (49) | 20–74 | PCC | WHR | ≥0.84 vs. <0.77 | 341/325 | 2.1510 | 1.34, 3.47 | 434/380 | 1.4910 | 0.90, 2.47 |
| ER+/PR+ | 145/325 | 1.424 | 0.80, 2.51 | 176/380 | 1.484 | 0.70, 3.12 | ||||
| ER−/PR− | 155/325 | 2.324 | 1.26, 4.29 | 105/380 | 0.924 | 0.40, 2.10 | ||||
| Bandera, 2015 (50) | 20–75 | Pooled6 | WHR | >0.84 vs. ≤0.74 | 1081/3648 | 1.2610 | 0.99, 1.60 | 1380/4621 | 1.2610 | 1.02, 1.56 |
| ER+ | 643/3648 | 1.3510 | 1.01, 1.80 | 930/4621 | 1.2410 | 0.97, 1.60 | ||||
| ER− | 438/3648 | 1.1410 | 0.81, 1.59 | 450/4621 | 1.3110 | 0.93, 1.83 | ||||
| TN | 222/3648 | 1.4010 | 0.85, 2.31 | 214/4621 | 1.6010 | 0.94, 2.73 | ||||
ER, estrogen receptor; HER2, human epidermal growth receptor 2; PC, prospective cohort study; PCC, population-based case-control study; PR, progesterone receptor; ref, reference; TN, triple negative (ER−, PR−, HER2− WC, waist circumference; WHR, waist-to-hip ratio; +, positive; −, negative.
Mayberry and Branch (54) and Brinton et al. (52) stratified analyses by age (20–39 and 40–54 y), not by menopausal status. Total number of cases in Brinton et al. (52) was 281, but the numbers of cases in the 2 age categories were not provided.
Premenopausal women: quartile 4 vs. quartile 1, BMI >24.9 vs. ≤20.9; for ER+/PR+ analyses: tertile 3 vs. tertile 1, BMI >24.0 vs. ≤21.5. Postmenopausal women: tertile 3 vs. tertile 1, BMI >23.7 vs. ≤21.2. Same cutoffs for ER+/PR+ analyses.
Never/noncurrent users of menopausal hormone therapy.
Hall et al. (54) and Robinson et al 2014 (49) are from the same study, the Carolina Breast Cancer Study; therefore, only the most recent publication is included in the table.
Bandera et al. (50) includes data from the Black Women’s Health Study (43), the Carolina Breast Cancer Study (49), the Women’s Circle of Health Study (53), and the Multiethnic Cohort Study (47), with additional recruitment since the individual published reports.
Further adjusted for WHR or WC.
Further adjusted for young-adult BMI.
Premenopausal women: weight gain >20 kg vs. none/stable; postmenopausal women: ≥30 kg vs. none/stable.
Further adjusted for recent BMI.
Premenopausal women: quartile 4 vs. quartile 1, WC >98.0 vs. ≤78.7 cm; postmenopausal women: tertile 3 vs. tertile 1, WC >96.4 vs. ≤85.0 cm.
Premenopausal women: tertile 3 vs. tertile 1, WC >93.5 vs. ≤81.2 cm; postmenopausal women: tertile 3 vs. tertile 1, WC >96.4 vs. ≤85.0 cm.
Young-adult BMI.
As reported in NHW women, having a high BMI as a young adult was associated with reduced risk of pre- and postmenopausal breast cancer in the Black Women’s Health Study (43). The Multiethnic Cohort evaluated the association in postmenopausal women and suggested an inverse association, but CIs included the null value (47). Case-control data tend to suggest a reduced risk for premenopausal women (41, 44, 45, 48), although individual results were significant in only one study (44). For postmenopausal women, there was greater inconsistency in the findings. Two studies reported nonsignificant elevated risk estimates for pre- and postmenopausal women (42, 49). Few studies evaluated associations by menopausal status and HR status and statistical power was limited. Inverse associations with young-adult BMI appear to be stronger for ER+PR+ (44, 45) or ER+ (50) breast cancer in premenopausal women but not in all studies (49). The AMBER Consortium (50) pooled data from the Black Women’s Health Study (43), the Multiethnic Cohort (47), the Carolina Breast Cancer Study (49), and the Women’s Circle of Health Study (48) and also found a borderline inverse association with ER+ breast cancer in postmenopausal women (50).
Recent BMI.
The few studies that evaluated the impact of recent BMI on breast cancer risk in AA women have generated inconsistent results (41–47, 49–53), with most showing nonsignificant or null results. For HR+ tumors, all but 1 (53) of the 5 studies that stratified by HR and menopausal status (44–46, 49, 50, 53) suggested an inverse association with BMI in premenopausal women and an elevated risk among postmenopausal women, consistent with the evidence for NHWs. Interestingly, the 4 studies reporting on the association with BMI for HR− breast cancer suggested an inverse association for postmenopausal women (44, 49, 50, 53). The association for triple-negative tumors was only evaluated in the AMBER Consortium, which found an inverse association for postmenopausal women (50). In contrast to findings in NHWs, in the 3 studies that evaluated effect modification by HT use, results remained essentially the same after excluding HT users (47, 49, 53). Similar findings were reported in the AMBER Consortium, which included these 3 studies plus the Black Women’s Health Study (43), with additional recruitment since the individual studies were published. The lower frequency of HT use among AA women (47, 49, 55) and/or use of different formulations by AAs compared with NHW women (47, 56) may explain the lack of effect modification by HT in AA women. Furthermore, AA women are more likely to have undergone a hysterectomy and to receive estrogen-only HT, which is not as strongly associated with breast cancer risk as the combined regime of estrogen plus progesterone (56).
Weight gain.
Only 2 cohort studies (43, 47) and 3 case-control studies (45, 46, 48, 49) reported on the association of weight gain with breast cancer, with inconsistent and mostly nonsignificant results, with the exception of the Multiethnic Cohort, which reported a strong positive association with higher weight gain among postmenopausal women (47). In the AMBER Consortium (50), the highest risk estimate was observed for obese postmenopausal women with ER+ breast cancer who were lean as young adults (OR: 1.91; 95% CI: 1.32, 2.75).
Central obesity.
Most studies reported an elevated breast cancer risk of high WHR among premenopausal (49, 50, 53) and postmenopausal (46, 49, 50, 53) women. The few studies that stratified by HR subtype tended to support elevated risks of both HR+ and HR− tumors (49, 50, 53).
Obesity and breast cancer survival
AA women have the worst 5-y breast cancer survival rate of all racial/ethnic groups (57). Reasons for this well-documented disparity in mortality are likely to be multifactorial, including possible differences in biological factors, health care access and quality, cultural and behavioral factors, as well as socioeconomic status (38). Although socioeconomic status may be an important determinant, it does not seem to fully explain the increased breast cancer mortality in AA women (35, 58). Furthermore, clinical trials have shown higher mortality rates in AAs among patients with breast cancer receiving identical treatment protocols, even after adjustment for prognostic factors (59), which suggests that biological and behavioral factors may play important roles.
To our knowledge, only 3 studies evaluated the association of obesity with breast cancer survival in AA women and reported no association between prediagnosis obesity and breast cancer–specific mortality or all-cause mortality (35, 60, 61). Only one of these studies (60) evaluated the impact by subtype in AA women and found a suggestion of decreased breast cancer mortality for ER− tumors, although these analyses were based on small numbers. In the California Breast Cancer Survivorship Consortium (35), although BMI was not associated with breast cancer–specific or all-cause mortality in AA women, those with higher WHR had worse overall survival.
Obesity and Breast Cancer in Non-US Black Women
Although breast cancer incidence rates are lower in Africa than in the rest of the world, mortality rates in some countries (e.g., Nigeria and Ethiopia) are among the highest worldwide (62). Cancer is a growing public health problem in Africa due to aging, population growth, and the increasing prevalence of risk factors such as obesity as a consequence of the economic transition (63). The prevalence of obesity has been increasing rapidly in some African countries, particularly in southern sub-Saharan Africa, where the rates are highest (42%) among South African women (64). Obesity prevalence is also very high in some areas of the Caribbean, such as the Bahamas (48%) (64). Similar to US women of African ancestry, breast cancer in African women tends to be diagnosed at an earlier age and has more aggressive features, including a higher proportion of HR− tumors (63). Although there are methodologic challenges in determining the distribution of HR subtypes of breast cancer in Africa, recent studies suggest that the proportion of ER− tumors is similar to that reported in AA women (63).
Obesity and breast cancer risk
Recent BMI.
To our knowledge, only 2 studies in Africa (65, 66) and one in Barbados (67) evaluated the association of BMI with premenopausal breast cancer and results were comparable to those reported in AA women in the United States and in NHWs (Table 2). Two earlier reports (68, 69) from one of the studies in Nigeria (66, 70) were not included in the table. In contrast, for postmenopausal women, the 3 studies suggested an inverse association, although none of the risk estimates was significant and none of the studies evaluated HR subtypes.
TABLE 2.
Studies reporting on the association between anthropometric factors and breast cancer risk in women of African ancestry: non-US studies1
| Premenopausal |
Postmenopausal |
|||||||||
| First author, year (ref) | Age, y | Study design | Country | Contrast | Cases/noncases, n | Risk estimate | 95% CI | Cases/noncases, n | Risk estimate | 95% CI |
| Recent BMI | ||||||||||
| Okobia, 2006 (65) | Not specified | HCC | Nigeria | Above vs. below median2 | 142/not specified | 0.82 | 0.49, 1.36 | 108/NS | 0.76 | 0.44, 1.32 |
| Nemesure, 2009 (67) | ≥21 | PCC | Barbados | ≥30 vs. < 25 | 77/1653 | 0.44 | 0.19, 1.01 | 142/2864 | 0.70 | 0.38, 1.28 |
| Ogundiran, 2010 (66)5 | ≥18 | PCC | Nigeria | ≥28 vs. < 21 | 682/819 | 0.70 | 0.50, 0.98 | 505/278 | 0.76 | 0.48, 1.21 |
| WHR | ||||||||||
| Okobia, 2006 (65) | Not specified | HCC | Nigeria | Above vs. below median2 | 142/not specified | 2.566 | 1.48, 4.41 | 108/not specified | 2.06 | 1.10, 3.64 |
| Nemesure, 2009 (67) | ≥21 | PCC | Barbados | ≥0.85 vs. < 0.80 | 76/1653 | 0.86 | 0.37, 1.98 | 143/2864 | 2.17 | 1.02, 4.60 |
| Ogundiran, 2012 (70)5 | ≥18 | PCC | Nigeria | ≥0.87 vs. < 0.77 | 702/819 | 2.127 | 1.49, 2.99 | 518/278 | 2.267 | 1.39, 3.68 |
HCC, hospital-based case-control study; PCC, population-based case-control study; ref, reference; WHR, waist-to-hip ratio.
Dichotomized variable based on means for controls: premenopausal women: BMI:24.83 kg/m2, WHR: 0.88; postmenopausal women: BMI: 25.03 kg/m2, WHR: 0.90.
Age < 50 y (unknown menopausal status).
Age ≥50 y (unknown menopausal status).
Adebamowo et al. (68, 69) were earlier reports for Ogundiran et al. (66, 70) and were therefore not included in the table because they correspond to the same study.
Adjusted for age only.
Further adjusted for BMI.
Central obesity.
In premenopausal women, high WHR was associated with increased risk in the 2 Nigerian studies (65, 70), but not in the small study from Barbados (67). For postmenopausal women, the results were more consistent, with 2-fold higher risks reported for Nigerian women (65, 70) and black women from Barbados (67) (Table 2).
Obesity and breast cancer survival
To our knowledge, no studies in African women have evaluated the impact of obesity on breast cancer survival.
Obesity and Breast Cancer in US Hispanic Women
Although breast cancer incidence is lower in US Hispanics than in NHW women, the risk is higher in US-born than in foreign-born Hispanic women, with the risk increasing over successive generations living in the United States (71). The prevalence of overweight and general and central obesity is high both in Hispanic female youth and adults (8, 10), but mean BMI varies little by country of birth or by acculturation in Hispanic women living in Northern California (71). Few studies have assessed the association between body size and breast cancer risk in US Hispanic women (45–47, 72–77), and sample sizes were relatively small, except for the Breast Cancer Health Disparities Study (BCHDS) (76, 77), which harmonized and pooled data from 2 case-control studies conducted in the Four Corners region (Arizona, Colorado, New Mexico, Utah) (74) and the San Francisco Bay Area (45, 46).
Obesity and breast cancer risk
Studies evaluating the relation of BMI, weight gain, and measures of body fat distribution with breast cancer risk are shown in Table 3.
TABLE 3.
Studies reporting on the association between anthropometric factors and breast cancer risk in US Hispanic women1
| Premenopausal |
Postmenopausal |
|||||||||
| First author, year (ref) | Age, y | Study design | Exposure | Contrast | Cases/controls, n | Risk estimate | 95% CI | Cases/controls, n | Risk estimate | 95% CI |
| Young-adult BMI | ||||||||||
| Wenten, 2002 (73) | 30–74 | PCC | BMI (kg/m2) age 18 y | ≥25 vs. <18.5 | 114/145 | 1.34 | 0.38, 4.70 | 138/172 | 0.47 | 0.11, 1.95 |
| Slattery 2007 (74) | 25–79 | PCC | BMI age 30 y | ≥30 vs. <25 | 311/312 | 0.46 | 0.25, 0.84 | 171/2292,3 | 1.19 | 0.56, 2.54 |
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | BMI in 20s | High vs. low quantile4 | 370/458 | 0.65 | 0.41, 1.04 | 346/642 | 0.63 | 0.45, 0.90 |
| >23.7 vs. ≤21.2 ER+PR+ | — | — | — | 174/642 | 0.80 | 0.51, 1.25 | ||||
| John, 2015 (76, 77)5 | 25–79 | Pooled PCC6 | BMI in 20s | Quartile 4 vs. quartile 17 ER+PR+ | 280/726 | 0.59 | 0.38, 0.93 | 270/906 | 0.718 | 0.46, 1.099 |
| Tertile 3 vs. tertile 110 ER−PR− | 135/726 | 0.50 | 0.31, 0.81 | 146/1198 | 0.79 | 0.49, 1.289 | ||||
| White, 2012 (47) | 45–7511 | Cohort | BMI age 21 y | ≥30 vs. 20–29.9 | 465/18,378 | 0.70 | 0.33, 1.49 | |||
| Recent BMI | ||||||||||
| Mayberry, 1994 (72) | 20–54 | PCC | Adult BMI | ≥32.3 vs. <24.9 | 148/167 | 1.312 | 0.5, 3.2 | |||
| Wenten, 2002 (73) | 30–74 | PCC | Usual BMI | ≥30 vs. <22 | 114/145 | 1.64 | 0.52, 5.11 | 138/172 | 1.32 | 0.47, 3.72 |
| Slattery, 2007 (74) | 25–79 | PCC | Current BMI | ≥30 vs. <25 | 327/329 | 0.96 | 0.63, 1.46 | 177/2382,3 | 0.80 | 0.44, 1.45 |
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | Current BMI | ≥30 vs. <25 | 375/483 | 0.52 | 0.35, 0.77 | 375/7048 | 0.77 | 0.53, 1.12 |
| ER+PR+ | — | — | — | 190/7048 | 1.23 | 0.75, 2.01 | ||||
| John, 2015 (76, 77)5 | 25–79 | Pooled PCC6 | Current BMI | ≥30 vs. <25 ER+PR+ | 285/763 | 0.63 | 0.44, 0.92 | 293/95713 | 0.91 | 0.55, 1.51 |
| ER−PR− | 142/763 | 0.71 | 0.45, 1.12 | 152/1260 | 0.60 | 0.32, 1.11 | ||||
| White, 2012 (47) | 45–7511 | Cohort | Baseline BMI | ≥30 vs. 20–29.9 | 465/18,378 | 1.27 | 0.99, 1.61 | |||
| Weight gain | ||||||||||
| Wenten, 2002 (73) | 30–74 | PCC | Weight gain since 18 y | >14 vs. <4 kg | 114/145 | 1.87 | 0.82, 4.24 | 138/172 | 2.46 | 0.98, 6.17 |
| Slattery, 2007 (74) | 25–79 | PCC | Weight gain since 15 y | >25 vs. ≤5 kg | 315/322 | 1.38 | 0.76, 2.52 | 163/2162,3 | 0.76 | 0.35, 1.65 |
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | Weight gain (kg) since 20s | High vs. low14 | 308/458 | 0.35 | 0.21, 0.59 | 328/6238 | 0.91 | 0.50, 1.66 |
| ER+PR+15 | 175/458 | 0.30 | 0.17, 0.52 | 168/6238 | 1.43 | 0.70, 2.94 | ||||
| John, 2015 (76, 77)5 | 25–79 | Pooled PCC6 | Weight gain (kg) since 20s | Quartile 4 vs. quartile 116 ER+PR+ | 242/662 | 0.82 | 0.54, 1.24 | 251/84213 | 1.75 | 1.02, 3.02 |
| Tertile 3 vs. tertile 117 ER−PR− | 126/662 | 0.86 | 0.54, 1.35 | 135/1110 | 1.08 | 0.57, 2.02 | ||||
| WC, WHR, WHtR | ||||||||||
| Slattery, 2007 (74) | 25–79 | PCC | WC | >40 vs. <35 inches | 323/329 | 1.04 | 0.66, 1.62 | 264/37522,3 | 0.81 | 0.47, 1.39 |
| WHR | >0.9 vs. <0.8 | 1.25 | 0.72, 2.18 | 0.77 | 0.39, 1.50 | |||||
| John, 2011 and 2013 (45, 46) | 35–79 | PCC | WC (cm) | High vs. low quantile18 | 363/471 | 0.74 | 0.47, 1.17 | 355/6788 | 1.79 | 1.14, 2.819 |
| ER+PR+ | 172/471 | 0.64 | 0.39, 1.05 | 181/6788 | 2.03 | 1.11, 3.709 | ||||
| WHtR | High vs. low quantile19 | 363/471 | 0.74 | 0.47, 1.17 | 355/6788 | 1.55 | 0.96, 2.509 | |||
| ER+PR+ | 172/471 | 0.61 | 0.37, 1.02 | 181/6788 | 1.83 | 0.97, 3.479 | ||||
| John, 2015 (76, 77)5 | 25–79 | Pooled PCC6 | WC (cm) | Tertile 3 vs. tertile 120 | 71/560 | 2.2721 | 0.92, 5.619 | 122/883 | 1.2321 | 0.63, 2.419 |
| WHR | Tertile 3 vs. tertile 122 | 71/560 | 1.9621 | 0.95, 4.069 | 122/882 | 0.9321 | 0.57, 1.53 | |||
ER, estrogen receptor; PC, prospective cohort study; PCC, population-based case-control study; PR, progesterone receptor; ref, reference; WC, waist circumference; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio; +, positive; −, negative.
Includes in situ breast cancers (82–83% were invasive).
No recent postmenopausal hormone therapy use.
Premenopausal women: quartile 4 vs. quartile 1, BMI >24.9 vs. ≤20.9; postmenopausal women: tertile 3 vs. tertile 1, BMI > 23.7 vs. ≤21.2.
Excludes in situ breast cancers.
Pooled data from the San Francisco Bay Area Breast Cancer Study (45, 46) and the 4-Corners Breast Cancer Study (74).
Premenopausal women: quartile 4 vs. quartile 1, BMI >25.3 vs. <20.9; postmenopausal women: quartile 4 vs. quartile 1: BMI >25.1 vs. <21.0.
Never/noncurrent users of postmenopausal hormone therapy.
Further adjusted for current BMI.
Premenopausal women: tertile 3 vs. tertile 1, BMI >24.3 vs. <21.5; postmenopausal women: tertile 3 vs. tertile 1, BMI >23.8 vs. <21.2.
At baseline.
Risk estimate for women ages 20–54 y.
Never/noncurrent/nonrecent users of postmenopausal hormone therapy.
Premenopausal women: weight gain >20 vs. <3.1 kg; postmenopausal women: weight gain ≥30 vs. <3 kg.
Premenopausal women: weight gain > 10 vs. <3.1 kg; postmenopausal women: weight gain ≥30 vs. <3 kg.
Premenopausal women: quartile 4 vs. quartile 1, weight gain >20.4 vs. < 7.5 kg; postmenopausal women: quartile 4 vs. quartile 1, weight gain >23.6 vs. <9.1 kg.
Premenopausal women: tertile 3 vs. tertile 1, weight gain >17.3 vs. < 9.1 kg; postmenopausal women: tertile 3 vs. tertile 1, weight gain >20.4 vs. <10.7 kg.
Premenopausal women: quartile 4 vs. quartile 1, WC >98 vs. ≤78.7 cm; postmenopausal women: tertile 3 vs. tertile 1, WC >96.4 vs. ≤85 cm.
Premenopausal women: quartile 4 vs. quartile 1, WHtR >0.61 vs. 0.50; postmenopausal women: tertile 3 vs. tertile 1, WHtR >0.61 vs. 0.54.
Premenopausal women: tertile 3 vs. tertile 1, WC >0.94 vs. <82.9 cm; postmenopausal women: tertile 3 vs. tertile 1, WC >96.6 vs. <86.4 cm.
Restricted to subjects measured <12 mo after diagnosis/selection into the study.
Premenopausal women: tertile 3 vs. tertile 1, WHR >0.85 vs. <0.80.
Young-adult BMI.
For premenopausal Hispanic women, the BCHDS found strong inverse associations, both for ER+PR+ and ER−PR− breast cancer (76), with risk reductions of 41% and 50% for high and low young-adult BMI categories, respectively. The pooled analysis also found an inverse association with ER+PR+ breast cancer among postmenopausal women not using HT (77).
Recent BMI.
In premenopausal Hispanic women, reports for current obesity include nonsignificant positive (72, 73), significant inverse (45), and null (74) associations. In the BCHDS, risk reductions associated with overweight and obesity were limited to ER+PR+ tumors, but they were seen only in women with high young-adult BMI (76). For postmenopausal women not currently using HT, there was no evidence of a positive association with obesity for breast cancer overall (46, 74, 77) or for ER+PR+ tumors (46, 77). However, a 2-fold increased risk of ER+PR+ breast cancer with obesity was found for Hispanics with low young-adult BMI (46). In the Multiethnic Cohort, a borderline positive association with BMI at baseline did not remain after adjustment for weight gain (47).
Weight gain.
For premenopausal breast cancer, findings for the comparison of high with low or no weight gain include nonsignificant positive associations for breast cancer overall (73, 74, 76) and similarly strong inverse associations for breast cancer overall and ER+PR+ tumors (45). The BCHDS found no association with weight gain. Similarly, when considering young-adult and current BMI jointly as an indicator of weight gain, current overweight or obesity was not associated with ER+PR+ breast cancer risk among women with low young-adult BMI (76). In postmenopausal women not using HT, weight gain was associated with a suggestive increased risk of ER+PR+ tumors only (46). The BCHDS pooled analysis found a 75% increased risk of ER+PR+ disease associated with the highest (compared with lowest) quartile of weight gain (77), but no association for ER−PR− breast cancer. Furthermore, weight gain (per 5 kg) was associated with a 42% increase in risk of ER+PR+ breast cancer only among women with low young-adult BMI. This finding suggests that among postmenopausal Hispanics with elevated young-adult BMI, additional weight gain attenuates the protective effect associated with young-adult obesity.
Central obesity.
Only 2 studies measured WC and hip circumference (HC) in US Hispanic women (45, 46, 74). Most previous analyses in premenopausal women did not adjust for current BMI and found no association between measures of central obesity (WC, WHR, and waist-to-height ratio) and risk of breast cancer (45, 74). In contrast, the BCHDS detected borderline 2-fold higher risks associated with WC and WHR after adjustment for current BMI and limiting the analysis to women measured <12 mo after diagnosis or selection into the study (76). In postmenopausal women, only one study found positive associations with WC and waist-to-height ratio after adjustment for current BMI (46), whereas the larger BCHDS found no evidence for an association between central obesity and postmenopausal breast cancer risk (77).
Obesity and breast cancer survival
The California Breast Cancer Survivorship Consortium is the only study that reported on the association between obesity and breast cancer survival in Hispanic women. Extreme obesity (BMI ≥40) was the only measure associated with survival, with 2-fold higher risks found for both breast cancer–specific and all-cause mortality (35).
Obesity and Breast Cancer in Latin America
Breast cancer is the most common cancer among women in Latin American countries (78), with incidence rates (per 100,000 women) ranging from 15 (in Guatemala) to 90.7 (in Uruguay) (3). Changes in reproductive and lifestyle factors and the nutritional transition likely play an important role in the sharp increase in breast cancer incidence rates. Latin American women are more likely to develop breast cancer at a younger age and be diagnosed at an advanced stage, possibly due to limited access to health care (79).
Obesity has been increasing sharply in Latin America. Currently, the prevalence in women is 20–30% in most countries, with the highest prevalence in Mexico (64). Among Mexican women of reproductive age, the prevalence of obesity increased from 25% in 1980 to 35% in 2012 (80).
Obesity and breast cancer risk
Only 2 case-control studies conducted in Latin America evaluated the association of obesity with breast cancer risk, including the Cáncer de Mama study (CAMA) from Mexico (81) and a small hospital-based case-control study in postmenopausal women from Uruguay (82) (Table 4).
TABLE 4.
Studies reporting on the association between anthropometric factors and breast cancer risk in Latin America1
| Premenopausal |
Postmenopausal |
|||||||||
| First author, year (ref) | Age, y | Study design | Country of origin | Contrast | Cases/noncases, n | Risk estimate* | 95% CI | Cases/noncases, n | Risk estimate | 95% CI |
| Recent BMI | ||||||||||
| Amadou, 2014 (81) | 35–69 | PCC | Mexico | ≥30 vs. <25 kg/m2 | 415/476 | 0.48 | 0.32, 0.72 | 583/598 | 0.75 | 0.51, 1.12 |
| Ronco, 2012 (82) | 23–69 | HCC | Uruguay | Obese vs. nonobese | — | — | — | 165/261 | 0.77 | 0.48, 1.25 |
| WHR, WC | ||||||||||
| Amadou 2014 (81) | 35–69 | PCC | Mexico | WHR: ≥0.93 vs. <0.85 | 415/476 | 0.712 | 0.49, 1.03 | 583/598 | 0.562 | 0.38, 0.82 |
| WC: ≥103 vs. <93 cm | 0.45 | 0.27, 0.74 | 0.62 | 0.40, 0.95 | ||||||
| Change in body shape | ||||||||||
| Amadou, 2014 (81) | 35–69 | PCC | Mexico | Trajectory3 | 415/476 | 583/598 | ||||
| Gr2 vs. Gr1 | 1.31 | 0.83, 2.08 | 1.55 | 1.05, 2.20 | ||||||
| Gr3 vs. Gr1 | 1.28 | 0.76, 2.14 | 1.71 | 1.10, 2.67 | ||||||
| Gr4 vs. Gr1 | 1.65 | 0.98, 2.75 | 1.46 | 0.93, 2.29 | ||||||
| Gr5 vs. Gr1 | 0.83 | 0.30, 2.28 | 2.20 | 0.98, 4.94 | ||||||
Gr, group; HCC, hospital-based case-control study; PCC, population-based case-control study; ref, reference; WC, waist circumference; WHR, waist-to-hip ratio.
Further adjusted for recent BMI.
Trajectory of body shape throughout life: Gr1, constantly low; Gr2, constantly midrange; Gr3, moderate increase; Gr4, sharp increase; Gr5, constantly high.
Recent BMI.
In the CAMA study, BMI was inversely associated with the risk of premenopausal breast cancer and was not associated with the risk of postmenopausal breast cancer (81). Similarly, in the Uruguayan study, obesity was not associated with postmenopausal breast cancer risk (82).
Central obesity.
Measures of central obesity (WC, HC, WHR) have been shown to be inversely associated with breast cancer risk in premenopausal Mexican women (81). In postmenopausal women, larger WC, HC, and WHR were associated with reduced breast cancer risk, but after stratification by time since menopause, risk reductions were limited to women <10 y after menopause; no inverse associations remained in women ≥10 y after menopause.
Change in body shape.
In the CAMA study (81), body shape at specific ages was not associated with breast cancer risk; however, compared with women who remained slim over the life span (from age 8 y to current age), an increase in body size over the life span was associated with increased risk, particularly among postmenopausal women. This study emphasizes the importance of considering the evolution of body shape throughout life instead of examining body shape at specific ages when studying the association between obesity and breast cancer risk.
Obesity and breast cancer survival
To our knowledge, the only study evaluating the impact of obesity on breast cancer outcomes in Latin America was a retrospective study conducted at the National Institute of Oncology of Mexico, which included 819 patients with breast cancer (stages IIB–IIIB) treated with neoadjuvant chemotherapy and followed for 28 mo (83). Obesity at diagnosis was related to poor survival (OR: 1.79; 95% CI: 1.09, 2.96).
Obesity and Breast Cancer in Women of Asian Ancestry
Although breast cancer incidence remains lower in many Asian countries compared with Western countries (62), the incidence in Japanese Americans and other Asian-American groups is now similar to that in NHW women (84, 85). Research among Japanese migrants has shown that breast cancer incidence increased over several generations due to changes in lifestyle factors and increases in body weight (86). In comparison to other racial/ethnic groups, BMI continues to be lower in Asian-American women despite increases in body weight over the past 30 y (87). In the Multiethnic Cohort, only 7% of Japanese-American women and men were obese at cohort entry, whereas the respective proportions for NHW women and men were 20% and 15% (88).
Obesity and breast cancer risk
Studies of anthropometric measures and breast cancer risk in women of Asian ancestry (47, 89–107) are shown in Table 5.
TABLE 5.
Studies reporting on the association between anthropometric factors and breast cancer risk in Asian and Asian-American women1
| Premenopausal |
Postmenopausal |
|||||||||
| First author, year (ref) | Age at study entry, y | Study design | Ethnicity | Contrast | Cases/noncases, n | Risk estimate | 95% CI | Cases/noncases, n | Risk estimate | 95% CI |
| Recent BMI (kg/m2) | ||||||||||
| Kyogoku, 1990 (89) | 45–79 | PCC | Japanese | Quartile 4 vs. quartile 1 | — | — | — | 121/242 | 1.10 | 0.50, 2.50 |
| Ziegler, 1996 (90) | 20–55 | PCC | Asian-American | >31.3 vs. <22.9 | 421/656 | 1.60 | 0.87, 2.94 | 112/237 | 1.78 | 0.57, 5.58 |
| Hu, 1997 (91) | 20 to ≥65 | PCC | Japanese | >23 vs. <21 | 87/202 | 0.45 | 0.22, 0.92 | 67/159 | 1.98 | 0.86, 4.55 |
| Ng, 1997 (92) | 45–69 | PCC | Chinese | >27.5 vs. ≤21 | 74/297 | 0.60 | 0.30, 1.20 | 130/585 | 1.20 | 0.70, 2.30 |
| Tung, 1999 (93) | 29 to ≥80 | PCC | Japanese | >25 vs. <20 | 190/119 | 0.98 | 0.46, 2.06 | 186/282 | 1.90 | 1.10, 3.24 |
| Shu, 2001 (94) | 25–64 | PCC | Chinese | ≥28.0 vs. <20.7 | 952/990 | 1.10 | 0.70, 1.70 | 501/562 | 2.00 | 1.20, 3.20 |
| Hirose, 2001 (95) | 30 to ≥70 | PC | Japanese | ≥25 vs. <20 | 861/9622 | 1.18 | 0.91, 1.50 | 604/4991 | 2.17 | 1.60, 2.90 |
| Kuriyama, 2005 (96) | ≥40 | PC | Japanese | ≥30 vs. <252 | 33/5355 | 0.84 | 0.24, 2.88 | 65/9601 | 2.67 | 1.03, 6.92 |
| Chow, 2005 (97) | 24–85 | PCC | Chinese | 27–31 vs. <19 | 68/212 | 1.32 | 0.39, 4.43 | 130/129 | 2.06 | 1.08, 3.93 |
| Wu, 2006 (98) | 47 ± 10 | PC | Chinese | >26.2 vs. <21.6 | — | — | — | 104/11,7263 | 1.903 | 1.00, 3.403 |
| Tian, 2007 (99) | 22–87 | PCC | Chinese | >24.5 vs. ≤24.5 | 141/141 | 0.60 | 0.32, 1.09 | 103/103 | 2.94 | 1.53, 5.68 |
| Iwasaki, 2007 (100) | 40–69 | PC | Japanese | ≥30 vs. <19 | 201/20,670 | 1.35 | 0.53, 3.47 | 229/28,939 | 2.28 | 0.94, 5.53 |
| ER+/PR+ (cont) | 62/20,809 | 1.04 | 0.98, 1.11 | 65/29,103 | 1.08 | 1.01, 1.15 | ||||
| ER−/PR− (cont) | 41/20,830 | 1.02 | 0.93, 1.13 | 41/29,127 | 0.95 | 0.84, 1.06 | ||||
| Wu, 2007 (101) | 25–74 | PCC | Asian-American | >24.60 vs. ≤20.43 | 572/613 | 0.67 | 0.46, 0.98 | 705/547 | 1.35 | 0.95, 1.93 |
| Mathew, 2008 (102) | NA | PCC | Indian | ≥30 vs. <25 | 898/1182 | 1.58 | 0.46, 5.42 | 968/691 | 1.07 | 0.33, 3.45 |
| Shin, 2009 (103) | 20–70 | PCC | Chinese | ≥25.0 vs. ≤20.9 | 2080/1959 | 1.30 | 1.10, 1.50 | 1368/1511 | 1.80 | 1.40, 2.20 |
| Kawai, 2010 (104) | 40–64 | PC | Japanese | ≥25.9 vs. <20.5 | — | — | — | 108/9998 | 2.54 | 1.16, 5.55 |
| Suzuki, 2011 (105) | 40–69 | PC | Japanese | ≥24.0 vs. 20.0 to <24.0 | 220/17666 | 1.01 | 0.63, 1.61 | 232/23,476 | 0.77 | 0.52, 1.14 |
| White, 2012 (47) | 40–75 | PC | Japanese-American | ≥30 vs. <20 | — | — | — | 921/21,396 | 1.59 | 1.24, 2.05 |
| Suzuki, 2013 (106) | 40–79 | PC | Japanese | ≥29.0 vs. 20.0–23.9 | 62/8131 | 0.62 | 0.08, 4.58 | 172/28,033 | 2.13 | 1.09, 4.16 |
| Kawai, 2013 (107) | ≥30 | PCC | Japanese | ≥30.0 vs. <18.5 | — | — | — | — | — | — |
| ER+/PR+ | 250/1039 | 0.55 | 0.22, 1.34 | 277/1756 | 6.24 | 2.68, 14.5 | ||||
| ER−/PR− | 95/1039 | 0.65 | 0.19, 2.28 | 142/1756 | 2.43 | 0.74, 7.95 | ||||
| Weight gain | ||||||||||
| Kyogoku, 1990 (89) | 45–79 | PCC | Japanese | > 4 vs. ≤0 kg/m2 | — | — | — | 121/242 | 1.00 | 0.50, 2.10 |
| Shu, 2001 (94) | 25–64 | PCC | Chinese | > 5.65 vs. < 1.15 kg | 952/990 | 1.10 | 0.90, 1.40 | 501/562 | 1.50 | 1.10, 2.10 |
| Hirose, 2001 (95) | 30 to ≥70 | PC | Japanese | >3 vs. 0 kg/m2 | 861/9622 | 0.89 | 0.73, 1.10 | 604/4991 | 1.34 | 1.00, 1.70 |
| Wu, 2007 (101) | 25–74 | PCC | Asian-American | >20 vs. ≤10 kg | 572/613 | 1.48 | 0.52, 4.25 | 705/547 | 2.23 | 1.00, 4.94 |
| Shin, 2009 (103) | 20–70 | PCC | Chinese | ≥15 vs. ≤0 | 2080/1959 | 1.40 | 1.10, 1.70 | 1368/1511 | 1.80 | 1.40, 2.40 |
| Kawai, 2010 (104) | 40–64 | PC | Japanese | ≥12 vs. ± 2 kg | — | — | — | 108/9998 | 1.55 | 0.70, 3.45 |
| White, 2012 (47) | 40–75 | PC | Japanese-American | >22.5 vs. 3.7–9.1 kg | — | — | — | 921/21,396 | 2.05 | 1.40, 3.00 |
| Suzuki, 2013 (106) | 40–79 | PC | Japanese | ≥10 kg and BMI ≥24 vs. <10 kg and BMI <24 | 62/8131 | 1.88 | 0.85, 4.16 | 172/28,033 | 2.55 | 1.47, 4.42 |
| WHR | ||||||||||
| Ng, 1997 (92) | 45–69 | PCC | Chinese | >0.86 vs. <0.75 | 74/297 | 6.40 | 2.40, 16.9 | 130/585 | 6.10 | 2.70, 14.2 |
| Shu, 2001 (94) | 25–64 | PCC | Chinese | ≥0.865 vs. <0.764 | 952/990 | 1.80 | 1.30, 2.60 | 501/562 | 1.60 | 1.00, 2.50 |
| Wu, 2006 (98) | 47 ± 10 | PC | Chinese | >0.85 vs. <0.77 | — | — | — | 104/11,7223 | 0.603 | 0.30, 1.203 |
| Tian, 2007 (99) | 22–87 | PCC | Chinese | >0.81 vs. <0.81 | 140/141 | 1.40 | 0.81, 2.41 | 102/103 | 3.64 | 1.88, 7.05 |
| Wu, 2007 (101) | 25–74 | PCC | Asian-American | >0.84 vs. ≤0.76 | 564/606 | 1.20 | 0.82, 1.77 | 687/548 | 1.48 | 1.02, 2.15 |
| Mathew, 2008 (102) | NA | PCC | Indian | >0.85 vs. ≤0.85 | 898/1182 | 0.92 | 0.74, 1.13 | 968/691 | 0.74 | 0.57, 0.97 |
| Shin, 2009 (103) | 20–70 | PCC | Chinese | ≥0.84 vs. ≤0.76 | 2080/1962 | 2.30 | 1.90, 2.80 | 1372/1512 | 2.20 | 1.70, 2.80 |
cont, continuous; ER, estrogen receptor; NA, not available; PC, prospective cohort study; PCC, population-based case-control study; PR, progesterone receptor; ref, reference; WHR, waist-to-hip ratio; +, positive; −, negative.
Highest category for premenopausal women is smaller (27.5–29.9).
Pre- and postmenopausal women combined.
Recent BMI.
For premenopausal women, only 2 studies reported significant inverse associations with recent BMI (91, 101) and one showed a significantly elevated risk (102). A systematic review reported a risk estimate of 1.05 (95% CI: 1.01, 1.09) for Asians compared with 0.93 (95% CI: 0.91, 0.95) for NHWs (108). In the Multiethnic Cohort, postmenopausal breast cancer risk associated with obesity (BMI ≥30 compared with <25) was higher for Japanese than for NHWs with HRs of 1.59 (95% CI: 1.24, 2.05) and 1.38 (95% CI: 1.24, 1.53), respectively (47). The respective values for overweight women were 1.23 (95% CI: 1.05, 1.45) and 1.34 (95% CI: 1.15, 1.56). These results largely agree with reports for postmenopausal women from Japan (91, 93, 95, 96, 100, 104–106), China (94, 97–99, 103), South India (102), and for Asian Americans in other US studies (90–101). Whereas the risk of developing breast cancer was approximately twice as high in the highest than in the lowest BMI category in investigations of women of Asian ancestry, a summary risk estimate of 1.27 (95% CI: 1.03, 1.55) was reported for mostly NHW Western populations when comparing the highest with the lowest BMI category (110). Moreover, a meta-analysis that separated studies by geographic area (110) found a summary RR estimate of 1.31 (95% CI: 1.15, 1.48) for the Asia-Pacific region, whereas the RRs for North America and Europe were only 1.15 (95% CI: 1.05, 1.19) and 1.09 (95% CI: 1.04, 1.14), respectively. In analyses stratified by HR status, only ER+/PR+ breast cancer appears to be associated with BMI in postmenopausal Japanese women (25, 100, 107), in agreement with findings for NHWs (27). In the Multiethnic Cohort, the association with BMI among postmenopausal Japanese Americans was limited to HT nonusers (47), whereas in Asian Americans from California, the BMI association was not modified by HT use (101).
Weight gain.
In the Multiethnic Cohort, gaining >22.7 kg since age 21 y was associated with a 2-fold higher risk of breast cancer in postmenopausal Japanese-American women compared with a 50% increased risk in NHW women (47). Studies in Asian-American, Japanese, and Chinese women with varying categories of weight gain found significantly elevated risks between 1.16 and 2.70, although some null findings were also reported (Table 5).
Central obesity.
Higher WHR was associated with increased breast cancer risk both in premenopausal (92, 94, 99, 103), and postmenopausal (92, 94, 99, 103) Chinese women. A positive association was also reported for postmenopausal Asian-American women from California (101). In Indian postmenopausal women, WHR was associated with a significantly reduced risk (102).
Obesity and breast cancer survival
Although the evidence is limited, an increasing number of studies indicate that survival in Asian patients with breast cancer may be affected to a greater degree by excess adiposity than in NHW women. A study from China reported a risk of 1.30 (95% CI: 1.00, 1.80) for overall mortality/recurrence among women with a BMI >25.53 compared with <21.23 (111). In a Japanese study, breast cancer–specific mortality was higher in women with a BMI of ≥25.8 than in women with a BMI ≥21.2 to <23.3 (HR: 1.46; 95% CI: 0.81, 2.64) (112). In a comparative study in US and Chinese women, a >10% weight gain after breast cancer diagnosis did not affect breast cancer–specific survival in NHW women (HR: 1.03; 95% CI: 0.84, 1.26) but did increase mortality among breast cancer survivors in China (HR: 1.25; 95% CI: 0.88, 1.77) (113). Furthermore, the California Breast Cancer Survivorship Consortium showed a significant association of high WHR with breast cancer–specific mortality in Asian Americans (HR: 2.21; 95% CI: 1.21, 4.03) but not in other racial/ethnic groups (35). In contrast, the Multiethnic Cohort detected no significant interaction of ethnicity with BMI status related to survival (62).
Conclusions
The impact of BMI and central obesity on breast cancer risk and survival in AA women is not well understood. The overall evidence suggests that in AAs central adiposity may have a stronger impact than BMI. BMI may not be a good marker of adiposity in AA women. For a given BMI, they tend to have more lean mass and lower fat mass than do NHW women (114), and for a given amount of body fat AAs have less visceral adipose tissue and more subcutaneous adipose tissue than do NHWs (115). Interestingly, despite lower visceral tissue, AA women are more likely to be insulin resistant than are NHW women at the same BMI category (116). Furthermore, important differences in obesity-related biomarkers have been reported for AA women compared with NHW women, even after adjusting for BMI, including higher leptin and lower adiponectin concentrations (39). Because the proportion of ER− and triple-negative tumors in AA women is highest among all racial/ethnic groups, evaluating associations by subtype is critical to understanding the impact of general and central obesity in this population.
For Hispanic women, evidence suggests that associations with body size are similar to those reported for NHWs. The available data emphasize the importance of considering breast cancer subtypes, as well as modifying factors such as menopausal status and HT use. Weight gain appears to be a better predictor of postmenopausal ER+PR+ breast cancer risk than recent BMI but only among Hispanics with a low young-adult BMI and those not using HT. Young-adult BMI has a major impact on breast cancer risk, particularly in premenopausal Hispanics, with inverse associations found for both ER+PR+ and ER−PR− subtypes. Data on the role of central obesity in Hispanics are sparse. Central obesity, adjusted for BMI, is a strong risk factor for premenopausal breast cancer and possibly for postmenopausal women.
Only a few studies have been conducted in women of African ancestry and Latinas outside of the United States, and they were small with no information on tumor subtypes. Interestingly, both in AA and Hispanic women 2 pooled analyses (50, 77) reported that among postmenopausal women, those who were lean as young adults and became obese later in life had the highest risk of HR+ breast cancer. This finding emphasizes the importance of maintaining a healthy weight in these populations. A current initiative including 4 Latin American countries (Chile, Colombia, Costa Rica, and Mexico) is underway at the International Agency for Research on Cancer to explore the association between obesity and breast cancer phenotypes on the basis of immunohistochemistry and tumor sequencing among premenopausal women. Similarly, several population-based, case-control studies are ongoing in African countries (e.g., South Africa and Ghana) to better understand the role of obesity and lifestyle factors in the etiology of breast cancer. Studies in countries undergoing economic transition offer an important opportunity to evaluate the impact of obesity and possible biological mechanisms.
Breast cancer risk and obesity rates have increased in women of Asian ancestry, although to a greater degree among migrants to the United States than among women living in Asia. Most of the available data come from studies in women of Japanese and Chinese ancestry, both living in Japan or China and in the United States. The association of BMI, weight gain, and WHR with breast cancer risk appears to be stronger in postmenopausal Asian and Asian-American women than in NHWs, suggesting that their risk of developing breast cancer increases at relatively low BMIs. Many of the risk estimates (Table 5) for the highest compared with the lowest BMI categories are increased by ∼2-fold (93, 94, 99, 104, 106), whereas meta-analyses in NHW women reported 10–13% higher obesity-associated risks (25, 109, 110). The evidence for premenopausal women is inconsistent; only a few reports in Asian women (91, 98) showed an inverse association with obesity that is well established for NHW premenopausal women (108).
The stronger adverse effects of obesity on breast cancer risk and mortality in women of Asian ancestry (14) may be the result of a higher proportion of visceral than subcutaneous adipose tissue among Asians compared with NHWs (117). This hypothesis is supported by the finding that WHR as a marker for central obesity is a stronger predictor of risk and survival in Asians than in other racial/ethnic groups. Visceral adipose tissue plays an important role in lipid and glucose metabolism due to the production of various hormones and cytokines (118) and is more strongly associated with an adverse metabolic risk profile than subcutaneous fat, even after controlling for standard anthropometric indexes (119). A comparative investigation reported a higher trunk-to-periphery fat ratio (1.41 compared with 1.11; P = 0.0002) and twice the prevalence of fatty liver (51% compared with 24%; P = 0.05) for Japanese Americans than for NHWs (117). A greater amount of visceral fat has also been reported for Filipina (120) and Chinese (121) women than for NHWs. The higher proportion of visceral fat in Asians may lead to a different pattern of secreted adipokines and inflammatory markers, which may mediate diverse adverse health effects. In Hawaii, Japanese-American women had significantly lower serum concentrations of leptin, adiponectin, and C-reactive protein than did NHWs after adjustment for BMI (39, 122). Also, in the Study of Women’s Health across the Nation, Chinese and Japanese Americans had lower adiponectin concentrations than did NHW women (123).
Data on the impact of obesity on mortality in racial/ethnic minority populations are very limited. In AA women, central obesity seems to have a greater impact on breast cancer prognosis than does BMI. However, the impact of obesity by subtype has not yet been adequately evaluated. In Hispanics, morbid obesity, but not lower obesity levels, has been shown to double all-cause mortality. The small body of literature on breast cancer survival in women of Asian ancestry indicates that the prognosis may also be worse than in NHWs of similar BMI. Clearly, this is an important area that requires further research in order to understand the impact of obesity on breast cancer survival across racial/ethnic groups and to tailor efforts aimed at reducing breast cancer mortality disparities.
Overall, the evaluation of body size associations with breast cancer risk across racial/ethnic groups is limited by several factors: the data are sparse for AA and Hispanic women and individual studies tended to have small sample sizes. Furthermore, few studies included 2 racial/ethnic groups for direct comparisons of associations (44–48), included multiple measures of both overall and central adiposity, assessed associations with breast cancer subtypes defined by HR status, or considered HT use in postmenopausal women. The impact of genetic ancestry in AA and Hispanic women has not been examined.
The association of obesity with breast cancer risk and prognosis is complex, with opposing effects depending on time windows of obesity and tumor subtypes. Large data sets are needed to thoroughly evaluate the complex relation between excess body fat and breast cancer in combination with modifying factors. Ideally, comparisons of associations with body size measures across racial/ethnic groups should be obtained from the same study that used a single method to assess body size or used standardized methods across populations. Current evidence, albeit limited, suggests that the impact of adiposity may vary by race/ethnicity. Obesity-related biomarkers are starting to provide insight into biological mechanisms. Given the limitation of BMI as a measure of adiposity, additional anthropometric measures, in particular from imaging, are needed to understand the relation of race/ethnicity and adiposity with circulating obesity-related biomarkers and their association with breast cancer risk and survival. Improving availability and standardizing subtype information are critical for future studies, which is particularly problematic in studies conducted in developing countries. International studies in economically developing countries offer unique opportunities to evaluate the impact of rapid weight gain on breast cancer risk and survival. Such studies should also take into account genetic ancestry, which may help elucidate differences in associations among ethnically admixed populations.
Given the increasing prevalence of obesity, which disproportionately affects US racial/ethnic minorities and developing countries, understanding its impact on breast cancer throughout a woman’s life course is crucial in tailoring preventive efforts to reduce disparities in breast cancer development and survival.
Acknowledgments
All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: AA, African American; BCHDS, Breast Cancer Health Disparities Study; CAMA, Cáncer de Mama Study; ER, estrogen receptor; HC, hip circumference; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; HT, hormone therapy; IGF-I, insulin-like growth factor I; NHW, non-Hispanic white; PR, progesterone receptor; WC, waist circumference; WHR, waist-to-hip ratio; +, positive; −, negative.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project report summary. Food, nutrition, physical activity, and the prevention of breast cancer. London; World Cancer Research Fund International; 2010. [Google Scholar]
- 3. World Cancer Research Fund. Continuous update project report. Diet, nutrition, physical activity and breast cancer survivors. London; World Cancer Research Fund International; 2014. [Google Scholar]
- 4. World Health Organization. Overweight and obesity. Fact sheet no. 311. [Internet][updated 2015 Jan; cited 2015 30 Aug]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 5.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9:13–27. [DOI] [PubMed] [Google Scholar]
- 6.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52–62. [DOI] [PubMed] [Google Scholar]
- 7.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health 2010;100:861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA 2014;312:189–90. [DOI] [PubMed] [Google Scholar]
- 9.Odgen CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. Hyattsville (MD): National Center for Health Statistics; 2013. NCHS Data Brief No. 131. [cited 2015 Aug 30]. Available from: http://www.cdc.gov/nchs/data/databriefs/db131.pdf. [Google Scholar]
- 10.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA 2014;312:1151–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruder EH, Dorgan JF, Kranz S, Kris-Etherton PM, Hartman TJ. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer 2008;8:334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuemmeler BF, Pendzich MK, Tercyak KP. Weight, dietary behavior, and physical activity in childhood and adolescence: implications for adult cancer risk. Obes Facts 2009;2:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev 2007;28:763–77. [DOI] [PubMed] [Google Scholar]
- 14.Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol 2013;Feb 3 (Epub; DOI: 10.1155/2013/906495). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev 2003;4:157–73. [DOI] [PubMed] [Google Scholar]
- 16.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123:627–35. [DOI] [PubMed] [Google Scholar]
- 17.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 2012;134:769–81. [DOI] [PubMed] [Google Scholar]
- 18.Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr 2012;32:311–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst 2014;106:djt351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichman ME, Altekruse S, Li CI, Chen VW, Deapen D, Potts M, Wu XC, Morrell D, Hafterson J, Phipps AI, et al. . Feasibility study for collection of HER2 data by National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program central cancer registries. Cancer Epidemiol Biomarkers Prev 2010;19:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin 2013;63:151–66. [DOI] [PubMed] [Google Scholar]
- 23.Gierach GL, Burke A, Anderson WF. Epidemiology of triple negative breast cancers. Breast Dis 2010;32:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, et al. . Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 2015;107:djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis. Int J Cancer 2009;124:698–712. [DOI] [PubMed] [Google Scholar]
- 26.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, et al. . Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst 2011;103:250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 2014;36:114–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 2013;137:307–14. [DOI] [PubMed] [Google Scholar]
- 29.Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 2011;117:4132–40. [DOI] [PubMed] [Google Scholar]
- 30.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer 2012;12:364–72. [DOI] [PubMed] [Google Scholar]
- 31.Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res 2013;184:253–9. [DOI] [PubMed] [Google Scholar]
- 32.Tait S, Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Body mass index, diabetes, and triple-negative breast cancer prognosis. Breast Cancer Res Treat 2014;146:189–97. [DOI] [PubMed] [Google Scholar]
- 33.Turkoz FP, Solak M, Petekkaya I, Keskin O, Kertmen N, Sarici F, Arik Z, Babacan T, Ozisik Y, Altundag K. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON 2013;18:335–41. [PubMed] [Google Scholar]
- 34.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, et al. . Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer 2012;118:5937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwan ML, John EM, Caan BJ, Lee VS, Bernstein L, Cheng I, Gomez SL, Henderson BE, Keegan TH, Kurian AW, et al. . Obesity and mortality after breast cancer by race/ethnicity: the California Breast Cancer Survivorship Consortium. Am J Epidemiol 2014;179:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol 2012;8:709–16. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med 2013;64:45–57. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, Drake BF. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst 2013;105:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto Y, Conroy SM, Ollberding NJ, Kim Y, Lim U, Cooney RV, Franke AA, Wilkens LR, Hernandez BY, Goodman MT, et al. . Ethnic differences in serum adipokine and C-reactive protein levels: the multiethnic cohort. Int J Obes 2014;38:1416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beydoun MA, Wang Y. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring) 2009;17:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayberry RM. Age-specific patterns of association between breast cancer and risk factors in black women, ages 20 to 39 and 40 to 54. Ann Epidemiol 1994;4:205–13. [DOI] [PubMed] [Google Scholar]
- 42.Zhu K, Caulfield J, Hunter S, Roland CL, Payne-Wilks K, Texter L. Body mass index and breast cancer risk in African American women. Ann Epidemiol 2005;15:123–8. [DOI] [PubMed] [Google Scholar]
- 43.Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev 2007;16:1795–802. [DOI] [PubMed]
- 44.Berstad P, Coates RJ, Bernstein L, Folger SG, Malone KE, Marchbanks PA, Weiss LK, Liff JM, McDonald JA, Strom BL, et al. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev 2010;19:1532–44. [DOI] [PMC free article] [PubMed]
- 45.John EM, Sangaramoorthy M, Phipps AI, Koo J, Horn-Ross PL. Adult body size, hormone receptor status, and premenopausal breast cancer risk in a multiethnic population: the San Francisco Bay Area Breast Cancer Study. Am J Epidemiol 2011;173:201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.John EM, Phipps AI, Sangaramoorthy M. Body size, modifying factors, and postmenopausal breast cancer risk in a multiethnic population: the San Francisco Bay Area Breast Cancer Study. Springerplus 2013;2:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White KK, Park SY, Kolonel LN, Henderson BE, Wilkens LR. Body size and breast cancer risk: the Multiethnic Cohort. Int J Cancer 2012;131:E705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bandera EV, Chandran U, Zirpoli G, Ciupak G, Bovbjerg DH, Jandorf L, Pawlish K, Freudenheim JL, Ambrosone CB. Body size in early life and breast cancer risk in African American and European American women. Cancer Causes Control 2013;24:2231–43.49. [DOI] [PMC free article] [PubMed]
- 49.Robinson WR, Tse CK, Olshan AF, Troester MA. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993–2001. Cancer Causes Control 2014;25:1101–17. [DOI] [PMC free article] [PubMed]
- 50.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, Haiman CA, Park SY, Olshan AF, Ambrosone CB, et al. . Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat 2015;150:655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schatzkin A, Palmer JR, Rosenberg L, Helmrich SP, Miller DR, Kaufman DW, Lesko SM, Shapiro S. Risk factors for breast cancer in black women. J Natl Cancer Inst 1987;78:213–7. [PubMed] [Google Scholar]
- 52.Brinton LA, Benichou J, Gammon MD, Brogan DR, Coates R, Schoenberg JB. Ethnicity and variation in breast cancer incidence. Int J Cancer 1997;73(3):349–55. [DOI] [PubMed]
- 53.Bandera EV, Chandran U, Zirpoli G, Gong Z, McCann SE, Hong CC, Ciupak G, Pawlish K, Ambrosone CB. Body fatness and breast cancer risk in women of African ancestry. BMC Cancer 2013;13:475. [DOI] [PMC free article] [PubMed]
- 54.Hall IJ, Newman B, Millikan RC, Moorman PG. Body size and breast cancer risk in black women and white women: the Carolina Breast Cancer Study. Am J Epidemiol 2000;151:754–64. [DOI] [PubMed] [Google Scholar]
- 55.Chandran U, Zirpoli G, Ciupak G, McCann SE, Gong Z, Pawlish K, Lin Y, Demissie K, Ambrosone CB, Bandera EV. Racial disparities in red meat and poultry intake and breast cancer risk. Cancer Causes Control 2013;24:2217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou N, Hong S, Wang W, Olopade OI, Dignam JJ, Huo D. Hormone replacement therapy and breast cancer: heterogeneous risks by race, weight, and breast density. J Natl Cancer Inst 2013;105:1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. American Cancer Society. Cancer facts and figures. Atlanta (GA): American Cancer Society; 2014. [Google Scholar]
- 58.Maskarinec G, Sen C, Koga K, Conroy SM. Ethnic differences in breast cancer survival: status and determinants. Womens Health (Lond Engl) 2011;7:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 2009;101:984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Y, Ma H, Malone KE, Norman SA, Sullivan-Halley J, Strom BL, Marchbanks PA, Spirtas R, Burkman RT, Deapen D, et al. . Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol 2011;29:3358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat 2011;129:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.International Agency for Research on Cancer/WHOGLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 [cited 2015 Apr 8]. Available from: http//globocan.iarc.fr.
- 63.Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev 2014;23:953–66. [DOI] [PubMed] [Google Scholar]
- 64.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. . Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okobia MN, Bunker CH, Zmuda JM, Osime U, Ezeome ER, Anyanwu SN, Uche EE, Ojukwu J, Kuller LH. Anthropometry and breast cancer risk in Nigerian women. Breast J 2006;12:462–6. [DOI] [PubMed] [Google Scholar]
- 66.Ogundiran TO, Huo D, Adenipekun A, Campbell O, Oyesegun R, Akang E, Adebamowo C, Olopade OI. Case-control study of body size and breast cancer risk in Nigerian women. Am J Epidemiol 2010;172:682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nemesure B, Wu SY, Hennis A, Leske MC; Barbados National Cancer Study Group Body size and breast cancer in a black population—the Barbados National Cancer Study. Cancer Causes Control 2009;20:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adebamowo CA, Ogundiran TO, Adenipekun AA, Oyesegun RA, Campbell OB, Akang EE, Rotimi CN, Olopade OI. Waist-hip ratio and breast cancer risk in urbanized Nigerian women. Breast Cancer Res 2003;5:R18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adebamowo CA, Ogundiran TO, Adenipekun AA, Oyesegun RA, Campbell OB, Akang EU, Rotimi CN, Olopade OI. Obesity and height in urban Nigerian women with breast cancer. Ann Epidemiol 2003;13:455–61. [DOI] [PubMed] [Google Scholar]
- 70.Ogundiran TO, Huo D, Adenipekun A, Campbell O, Oyesegun R, Akang E, Adebamowo C, Olopade OI. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control 2012;23:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev 2005;14(12):2905–13. [DOI] [PubMed]
- 72.Mayberry RM, Branch PT. Breast cancer risk factors among Hispanic women. Ethn Dis 1994;4:41–6. [PubMed] [Google Scholar]
- 73.Wenten M, Gilliland FD, Baumgartner K, Samet JM. Associations of weight, weight change, and body mass with breast cancer risk in Hispanic and non-Hispanic white women. Ann Epidemiol 2002;12:435–44. [DOI] [PubMed]
- 74.Slattery ML, Sweeney C, Edwards S, Herrick J, Baumgartner K, Wolff R, Murtaugh M, Baumgartner R, Giuliano A, Byers T. Body size, weight change, fat distribution and breast cancer risk in Hispanic and non-Hispanic white women. Breast Cancer Res Treat 2007;102:85–101. [DOI] [PubMed] [Google Scholar]
- 75.Sangaramoorthy M, Phipps AI, Horn-Ross PL, Koo J, John EM. Early-life factors and breast cancer risk in Hispanic women: the role of adolescent body size. Cancer Epidemiol Biomarkers Prev 2011;20:2572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.John EM, Sangaramoorthy M, Hines LM, Stern MC, Baumgartner KB, Giuliano AR, Wolff RK, Slattery ML. Overall and abdominal adiposity and premenopausal breast cancer risk among Hispanic women: the Breast Cancer Health Disparities Study. Cancer Epidemiol Biomarkers Prev 2015;24:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.John EM, Sangaramoorthy M, Hines LM, Stern MC, Baumgartner KB, Giuliano AR, Wolff RK, Slattery ML. Body size throughout adult life influences postmenopausal breast cancer risk among Hispanic women: the Breast Cancer Health Disparities Study. Cancer Epidemiol Biomarkers Prev 2015;24:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 2011;378:1461–84. [DOI] [PubMed] [Google Scholar]
- 79.Justo N, Wilking N, Jonsson B, Luciani S, Cazap E. A review of breast cancer care and outcomes in Latin America. Oncologist 2013;18:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kroker-Lobos MF, Pedroza-Tobias A, Pedraza LS, Rivera JA. The double burden of undernutrition and excess body weight in Mexico. Am J Clin Nutr 2014;100: Suppl:1652S–8S. [DOI] [PubMed] [Google Scholar]
- 81.Amadou A, Torres Mejia G, Fagherazzi G, Ortega C, Angeles-Llerenas A, Chajes V, Biessy C, Sighoko D, Hainaut P, Romieu I. Anthropometry, silhouette trajectory, and risk of breast cancer in Mexican women. Am J Prev Med 2014; 46(3, Suppl 1)S52–64. [DOI] [PubMed] [Google Scholar]
- 82.Ronco AL, De Stefani E, Deneo-Pellegrini H, Quarneti A. Diabetes, overweight and risk of postmenopausal breast cancer: a case-control study in Uruguay. Asian Pac J Cancer Prev 2012;13:139–46. [DOI] [PubMed] [Google Scholar]
- 83.Arce-Salinas C, Aguilar-Ponce JL, Villarreal-Garza C, Lara-Medina FU, Olvera-Caraza D, Alvarado Miranda A, Flores-Diaz D, Mohar A. Overweight and obesity as poor prognostic factors in locally advanced breast cancer patients. Breast Cancer Res Treat 2014;146:183–8. [DOI] [PubMed] [Google Scholar]
- 84.Pike MC, Kolonel LN, Henderson BE, Wilkens LR, Hankin JH, Feigelson HS, Wan PC, Stram DO, Nomura AM. Breast cancer in a multiethnic cohort in Hawaii and Los Angeles: risk factor-adjusted incidence in Japanese equals and in Hawaiians exceeds that in whites. Cancer Epidemiol Biomarkers Prev 2002;11:795–800. [PubMed] [Google Scholar]
- 85.American Cancer Society; Cancer Research Center of HawaiiHawaii Department of Health. Hawaii cancer facts & figures 2010. [cited 2015 Aug 30]. Available from: http://www uhcancercenter org/research/research-highlightsreports.
- 86.Kolonel LN, Wilkens LR. Migrant studies. In: Schottenfeld D, Fraumeni JF, editors. In: Cancer epidemiology and prevention. New York: Oxford University Press; 2006. p. 189–201.
- 87.Maskarinec G, Takata Y, Pagano I, Carlin L, Goodman MT, Le ML, Nomura AM, Wilkens LR, Kolonel LN. Trends and dietary determinants of overweight and obesity in a multiethnic population. Obesity (Silver Spring) 2006;14:717–26. [DOI] [PubMed] [Google Scholar]
- 88.Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kyogoku S, Hirohata T, Takeshita S, Hirota Y, Shigematsu T. Anthropometric indicators of breast cancer risk in Japanese women in Fukuoka. Jpn J Cancer Res 1990;81:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ziegler RG, Hoover RN, Nomura AMY, West DW, Wu AH, Pike MC, Lake AJ, Horn-Ross PL, Kolonel LN, Siiteri PK, et al. . Relative weight, weight change, height, and breast cancer risk in Asian-American women. J Natl Cancer Inst 1996;88:650–60. [DOI] [PubMed] [Google Scholar]
- 91.Hu YH, Nagata C, Shimizu H, Kaneda N, Kashiki Y. Association of body mass index, physical activity, and reproductive histories with breast cancer: a case-control study in Gifu, Japan. Breast Cancer Res Treat 1997;43:65–72. [DOI] [PubMed] [Google Scholar]
- 92.Ng EH, Gao F, Ji CY, Ho GH, Soo KC. Risk factors for breast carcinoma in Singaporean Chinese women: the role of central obesity. Cancer 1997;80:725–31. [DOI] [PubMed] [Google Scholar]
- 93.Tung HT, Tsukuma H, Tanaka H, Kinoshita N, Koyama Y, Ajiki W, Oshima A, Koyama H. Risk factors for breast cancer in Japan, with special attention to anthropometric measurements and reproductive history. Jpn J Clin Oncol 1999;29:137–46. [DOI] [PubMed] [Google Scholar]
- 94.Shu XO, Jin F, Dai Q, Shi JR, Potter JD, Brinton LA, Hebert JR, Ruan Z, Gao YT, Zheng W. Association of body size and fat distribution with risk of breast cancer among Chinese women. Int J Cancer 2001;94:449–55. [DOI] [PubMed] [Google Scholar]
- 95.Hirose K, Tajima K, Hamajima N, Takezaki T, Inoue M, Kuroishi T, Miura S, Tokudome S. Association of family history and other risk factors with breast cancer risk among Japanese premenopausal and postmenopausal women. Cancer Causes Control 2001;12:349–58. [DOI] [PubMed] [Google Scholar]
- 96.Kuriyama S, Tsubono Y, Hozawa A, Shimazu T, Suzuki Y, Koizumi Y, Suzuki Y, Ohmori K, Nishino Y, Tsuji I. Obesity and risk of cancer in Japan. Int J Cancer 2005;113:148–57. [DOI] [PubMed] [Google Scholar]
- 97.Chow LW, Lui KL, Chan JC, Chan TC, Ho PK, Lee WY, Leung LH, Sy WM, Yeung CC, Yung AK. Association between body mass index and risk of formation of breast cancer in Chinese women. Asian J Surg 2005;28:179–84. [DOI] [PubMed] [Google Scholar]
- 98.Wu MH, Chou YC, Yu JC, Yu CP, Wu CC, Chu CM, Yang T, Lai CH, Hsieh CY, You SL, et al. . Hormonal and body-size factors in relation to breast cancer risk: a prospective study of 11,889 women in a low-incidence area. Ann Epidemiol 2006;16:223–9. [DOI] [PubMed] [Google Scholar]
- 99.Tian YF, Chu CH, Wu MH, Chang CL, Yang T, Chou YC, Hsu GC, Yu CP, Yu JC, Sun CA. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr Relat Cancer 2007;14:669–77. [DOI] [PubMed] [Google Scholar]
- 100.Iwasaki M, Otani T, Inoue M, Sasazuki S, Tsugane S. Body size and risk for breast cancer in relation to estrogen and progesterone receptor status in Japan. Ann Epidemiol 2007;17:304–12. [DOI] [PubMed] [Google Scholar]
- 101.Wu AH, Yu MC, Tseng CC, Pike MC. Body size, hormone therapy and risk of breast cancer in Asian-American women. Int J Cancer 2007;120:844–52. [DOI] [PubMed] [Google Scholar]
- 102.Mathew A, Gajalakshmi V, Rajan B, Kanimozhi V, Brennan P, Mathew BS, Boffetta P. Anthropometric factors and breast cancer risk among urban and rural women in South India: a multicentric case-control study. Br J Cancer 2008;99:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin A, Matthews CE, Shu XO, Gao YT, Lu W, Gu K, Zheng W. Joint effects of body size, energy intake, and physical activity on breast cancer risk. Breast Cancer Res Treat 2009;113:153–61. [DOI] [PubMed] [Google Scholar]
- 104.Kawai M, Minami Y, Kuriyama S, Kakizaki M, Kakugawa Y, Nishino Y, Ishida T, Fukao A, Tsuji I, Ohuchi N. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Br J Cancer 2010;103:1443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki R, Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, Tsugane S. Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status—the Japan Public Health Center-based Prospective Study. Int J Cancer 2011;129:1214–24. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki S, Kojima M, Tokudome S, Mori M, Sakauchi F, Wakai K, Fujino Y, Lin Y, Kikuchi S, Tamakoshi K, et al. . Obesity/weight gain and breast cancer risk: findings from the Japan collaborative cohort study for the evaluation of cancer risk. J Epidemiol 2013;23:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawai M, Kakugawa Y, Nishino Y, Hamanaka Y, Ohuchi N, Minami Y. Anthropometric factors, physical activity, and breast cancer risk in relation to hormone receptor and menopausal status in Japanese women: a case-control study. Cancer Causes Control 2013;24:1033–44. [DOI] [PubMed] [Google Scholar]
- 108.Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev 2013;14:665–78. [DOI] [PubMed] [Google Scholar]
- 109.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, et al. . Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000;152:514–27. [DOI] [PubMed] [Google Scholar]
- 110.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- 111.Tao MH, Shu XO, Ruan ZX, Gao YT, Zheng W. Association of overweight with breast cancer survival. Am J Epidemiol 2006;163:101–7. [DOI] [PubMed] [Google Scholar]
- 112.Kwan ML, Chen WY, Kroenke CH, Weltzien EK, Beasley JM, Nechuta SJ, Poole EM, Lu W, Holmes MD, Quesenberry CP Jr, et al. . Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat 2012;132:729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, Poole EM, Kroenke CH, Weltzien EK, Flatt SW, et al. . Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev 2012;21:1260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 2009;89:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL Jr, Ravussin E, Ryan DH, Smith SR, Bouchard C. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr 2010;91:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goedecke JH, Levitt NS, Evans J, Ellman N, Hume DJ, Kotze L, Tootla M, Victor H, Keswell D. The role of adipose tissue in insulin resistance in women of African ancestry. J Obes 2013;2013:952916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim U, Ernst T, Buchthal SD, Latch M, Albright CL, Wilkens LR, Kolonel LN, Murphy SP, Chang L, Novotny R, et al. . Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes 2011;1:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 2008;57:1269–75. [DOI] [PubMed] [Google Scholar]
- 119.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. . Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 120.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res 2005;13:1458–65. [DOI] [PubMed] [Google Scholar]
- 121.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 2007;86:353–9. [DOI] [PubMed] [Google Scholar]
- 122.Conroy SM, Chai W, Lim U, Franke AA, Cooney RV, Maskarinec G. Leptin, adiponectin, and obesity among Caucasian and Asian women. Mediators Inflamm 2011;2011:253580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khan UI, Wang D, Sowers MR, Mancuso P, Everson-Rose SA, Scherer PE, Wildman RP. Race-ethnic differences in adipokine levels: the Study of Women’s Health Across the Nation (SWAN). Metabolism 2012;61:1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]