Abstract
Cardiac cells express more than one isoform of the Na, K-ATPase (NKA), the heteromeric enzyme that creates the Na+ and K+ gradients across the plasmalemma. Cardiac isozymes contain one catalytic α-subunit isoform (α1, α2, or α3) associated with an auxiliary β-subunit isoform (β1 or β2). Past studies using biochemical approaches have revealed minor kinetic differences between isozymes formed by different α-β isoform combinations; these results make it difficult to understand the physiological requirement for multiple isoforms. In intact cells, however, NKA enzymes operate in a more complex environment, which includes a substantial transmembrane potential. We evaluated the voltage dependence of human cardiac NKA isozymes expressed in Xenopus oocytes, and of native NKA isozymes in rat ventricular myocytes, using normal mammalian physiological concentrations of Na+o and K+o. We demonstrate that although α1 and α3 pumps are functional at all physiologically relevant voltages, α2β1 pumps and α2β2 pumps are inhibited by ∼75% and ∼95%, respectively, at resting membrane potentials, and only activate appreciably upon depolarization. Furthermore, phospholemman (FXYD1) inhibits pump function without significantly altering the pump’s voltage dependence. Our observations provide a simple explanation for the physiological relevance of the α2 subunit (∼20% of total α subunits in rat ventricle): they act as a reserve and are recruited into action for extra pumping during the long-lasting cardiac action potential, where most of the Na+ entry occurs. This strong voltage dependence of α2 pumps also helps explain how cardiotonic steroids, which block NKA pumps, can be a beneficial treatment for heart failure: by only inhibiting the α2 pumps, they selectively reduce NKA activity during the cardiac action potential, leading to an increase in systolic Ca2+, due to reduced extrusion through the Na/Ca exchanger, without affecting resting Na+ and Ca2+ concentrations.
Introduction
Cellular excitability and secondary active transport are among the many processes driven by the Na+ and K+ gradients built by the Na, K-ATPase (NKA or Na/K pump), a membrane protein that transports three Na+ ions out of the cell in exchange for importing two K+ ions at the cost of the hydrolysis of one ATP molecule (1). NKA is a P-type IIC ATPase formed by the obligatory association of a catalytic α subunit, which contains all the machinery for ion transport and ATP hydrolysis, with an accessory β-subunit, which is essential for membrane targeting and protein stability (2).
Vertebrates express multiple subunit isoforms with distinct tissue distributions. There are four α and three β subunits (reviewed in (3)): α1 is ubiquitous; α2 is present in all muscle types (4, 5, 6), glia (4, 7), and neurons (8); α3 is expressed in neurons (9, 10) and in the heart, especially in the conductive system (11); α4 is present in testis (12). This article focuses on those isoforms expressed in the heart. Cardiac cells express β1 and β2 (13) and coimmunoprecipitation experiments showed that β1 associates with α1 and α2, whereas β2 interacts with α2 and α3 (14). This association pattern can be even more intricate as some tissues express NKA trimers when a member of the single transmembrane domain FXYD protein family (isoforms 1 through 7) associates with the αxβx dimer. FXYD1 (aka phospholemman) participates in oxidative- (15) and kinase- (16) mediated regulation of the cardiac NKA formed by the α1 and α2 isoforms (17). Thus, mature NKA multimers, even within a single tissue, exhibit substantial molecular-level complexity.
A central question that remains unanswered is how the molecular variety translates into functional diversity? Several studies that have investigated NKA kinetics show that the isozymes exhibit relatively small differences in their affinity for ligands (see (3) for review). In addition, ion transport by all isoforms is electrogenic and hence affected by transmembrane voltage (18). Nearly all the voltage dependence arises from the transit of ions into and out of their binding sites through access channels (19). In the presence of both Na+o and K+o most of the voltage dependence arises from Na+o rebinding to externally facing sites. This induces voltage-dependent inhibition (VDI) at negative voltages and a positive slope in the current-voltage relationship (IP-V) (20, 21, 22, 23): The larger the [Na+]o the stronger the VDI. Under external physiological conditions for mammals, where the [K+]o does not saturate the K+ binding sites, competition between Na+ and K+ is significant and VDI is even greater than at near-saturating [K+]o. Therefore, the chosen concentrations of Na+ and K+ can impact transport. The extent of VDI in cardiac cells, due to the individual sensitivities of the expressed isoforms, is presently unknown.
The comparison of physiological data from prior reports is confounded by the fact that, depending on the preparation, ionic conditions varied, as did the complexity of the underlying NKA isoforms. Using batrachian ionic conditions (≤100 mM Na+o), α2-subunits showed greater VDI than α1 and α3 pumps (24, 25), but these experiments failed to present concrete evidence for the role of voltage in determining the functional state of the α2 pumps. On the other hand, studies in cardiac myocytes have either measured the voltage dependence of all the pumps in the myocyte (20, 26, 27, 28), or have measured the activity of α1 and non-α1 pumps only at 0 mV (29, 30, 31, 32).
Here, we present a study of the voltage dependence of cardiac isozymes under mammalian physiological concentrations of Na+o (∼150 mM) and K+o (∼4.5 mM). Our results demonstrate that the voltage dependence of pumps including the α2 isoform is unusually steep over the physiological range. This characteristic suggests that they serve as a reservoir of pumping activity during the cardiac action potential but are inactive at ventricular resting potentials. In addition, we show that association with the β2 subunit exacerbates this voltage dependence. Furthermore, although FXYD1 does not significantly alter the voltage dependence of the isozymes, it affects the apparent affinity for K+o, which in turn depends on the external [Na+]o. Finally, using rat cardiac myocytes, where the contribution of different native isozymes can be distinguished, we demonstrate the relevance of isoform-specific voltage dependence to cardiac physiology.
Materials and Methods
Cell preparation
Oocytes were isolated enzymatically, injected with a mixture of human αxßx cRNA, and maintained for 3–6 days postinjection as described previously (22).
Rat ventricular myocytes were isolated from 1 to 3 months old rats with slight modification of methods previously described for Guinea pigs (33). Briefly, the heart was removed under pentobarbital anesthesia and rapidly mounted on a Langendörf system and perfused with oxygenated Tyrode solution consisting of (in mM): 1.8 CaCl2, 145 NaCl, 5 KCl, 1 MgCl2, and 10 HEPES (pH 7.4) at 35°C. Once the coronaries were blood-free the heart was perfused with Ca2+-free Tyrode for 5 min, followed by application of Ca2+-free Tyrode with enzyme solution (1 mg/mL collagenase type 1A and 0.2 mg/mL protease type XIV (Sigma, St. Louis, MO)) until the digestion was obvious to sight and tact (∼7 min). A solution containing (in mM): 70 K-glutamate, 25 KCl, 20 taurine, 10 KH2PO4, 1 MgCl2, 0.5 EGTA, 20 glucose, and 10 HEPES (pH 7.3) was used to wash the enzyme, cut the heart, disperse the myocytes, and store them for up to 36 h at 4°C.
Molecular biology
Human NKA isoforms were amplified from the neuroblastoma cell line SHSY5Y cDNA using isoform-specific polymerase chain reaction primers. Sequences were validated by DNA sequencing and the open reading frames were subcloned into the Bgl II restriction enzyme site in the pBSTA vector for oocyte expression (34). Plasmids were linearized with Not I before in vitro transcription with T7 RNA polymerase. FXYD1, purchased from the mammalian gene collection (http://mgc.nci.nih.gov) was subcloned into the pSD5 vector and linearized with Bgl II before transcription with SP6.
Electrophysiology and solutions
Two-electrode voltage-clamp recordings were performed using either an OC-725C amplifier (Warner Instruments, Hamden, CT) or a CA-1B High Performance Oocyte Clamp amplifier (Dagan, Minneapolis, MN) in combination with a Digidata 1440 A/D board, a Minidigi 1A, and pClamp 10 software (Molecular Devices, Sunnyvale, CA). Signals were filtered at 2 kHz and digitized at 10 kHz. Resistance of both microelectrodes (filled with 3 M KCl) was 0.2–0.8 MΩ. Oocytes were Na-loaded by 20–30 min incubation in either a solution containing (in mM): 150 HEPES, 20 tetraethylammonium-Cl, and 0.2 EGTA (pH 7.2 with NaOH) or 90 Na-sulfamate, 20 Na-HEPES, 20 TEA-Cl, and 0.2 EGTA (pH 7.2). Both solutions produced indistinguishable results. The signals from α3 pumps were reduced with longer than 30-min incubations by a mechanism that was not studied further. After the 30-min incubation, oocytes were kept in 90 mM Na+ solution until recording. All experiments were performed at room temperature (RT, 22–24°C) except those in Fig. 5 where a TC-10 temperature controller (Dagan) was used to set the temperature between 24 and 34°C.
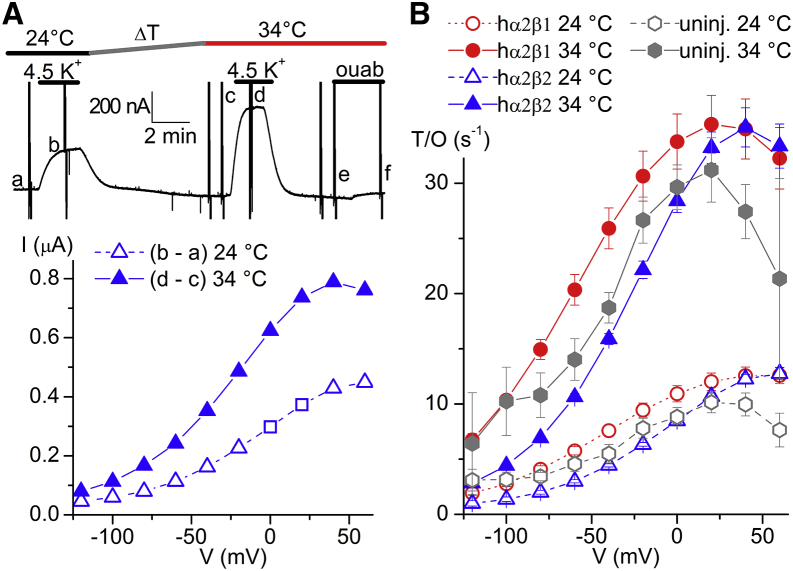
Figure 5.
(A) Continuous trace at −50 mV (top) and IP-V curves (bottom) from an oocyte expressing α2β2 pumps bathed by 125 mM Na+o in which the T was increased from 24°C to 34°C. The small caps indicate when the current-voltage relationships shown subtracted in the IP-V curves obtained. (B) Turnover rate as a function of V for α2β1, α2β2, and uninjected oocytes (average of four oocytes each with measurements at both T). To account for rundown, the IP at each T was divided by the total charge at the same T. For example, in (A) the Q-V at 24°C was obtained by subtracting the traces in a minus the traces in f, and that at 34°C by subtracting traces in e minus traces in f. Rundown, was present in nearly all experiments. For injected oocytes, only those with currents ≥200 nA at 0 mV at both T, and without significant changes in linear capacitance (obvious in the transient currents and in Q-V curves) were included in (B). To see this figure in color, go online.
The 90 mM Na+ solution for two-electrode voltage-clamp (TEVC) was composed of (in mM): 130 methane sulfonic acid (MS), 90 NaOH, 35 N-methyl D-glucamine (NMG), 10 HEPES, 5 Ba(OH)2, 0.5 Ca(OH)2, 1 Mg(OH)2 (pH 7.6, with NMG, 250–260 mOsm/kg). Temperature-controlled experiments were performed at 125 mM Na+ (where 35 mM Na+ was substituted for the NMG of the 90 mM Na+ solution). The 150 mM Na+ solution was used in TEVC and patch-clamp experiments and contained (in mM): 130 MS, 150 NaOH, 10 HEPES, 5 BaCl2, 1 CdCl2, 1 MgCl2 (pH 7.4, 300–310 mOsm/kg).
A common strategy to completely eliminate contribution of endogenous pumps to the signals is to introduce ouabain resistance conferring mutations naturally occurring in the rat α1 subunit (35). However, such strategy produces significant changes in the affinity for both Na+o and K+o (36). Because the relevance of our results requires studying wild-type pumps under normal external mammalian solutions, we decided not to introduce any mutation of this sort. Therefore, to be sure that at least 90% of pump signals come from the exogenously expressed pumps we only used experiments that presented >200 nA of K+-induced pump current. Small endogenous pump currents are shown in Fig. S1 in the Supporting Material along with averages from injected oocytes.
Turnover rates were estimated by dividing the pump current by the total ouabain-sensitive charge measured in the presence of Na+ without K+, essentially as described by Tavraz et al. (37). To obtain the total charge, the ouabain-sensitive transient currents when the pulses were turned off were integrated, the QOFF-V plots were fitted with a Boltzmann distribution QOFF = Qmin + Qtot/(1 + e(V-V1/2)/k), where Qtot is the total charge, Qmin the charge moved by negative pulses, V1/2 is the center of the distribution, and k is the slope factor.
Dose response curves from all experiments at each voltage were fitted globally with the Hill equation I = Imax × ([K+]n/([K0.5]n + [K+]n). The half maximal concentration (K0.5) was shared and the Hill number was fixed (n = 1.6). In the few cases where unblocking of K+-channel currents (38) at 4.5 or 10 mM K+ was observed, evident as a second rising phase at V ≥ 0 mV in the K+-induced current versus voltage curve, only ouabain-sensitive currents were considered.
Whole-cell patch-clamp recording of rat ventricular myocytes was performed with an Axopatch 200A, a Digidata 1322 A/D board, and pClamp 10 software (all from Molecular Devices), with acquisition at 10 kHz, filtered at 2 kHz. Pipettes had resistances between 1 and 3 MΩ when filled with intracellular solution containing (in mM): 130 L-glutamic acid, 10 HEPES, 10 EGTA, 10 tetraethylammonium-Cl, 5 MgATP, 1 MgCl2, and 85 NaOH, pH 7.4 with CsOH (∼65 mM). The 150 mM external solution was heated to 35°C with an in-line heater connected to a temperature controller TC344B (Warner Instruments).
K+ was added from a 450 mM K-MS stock. Bath electrodes were AgCl pellets connected by 3 M KCl agar bridges. NKA-mediated current was identified by inhibition with ouabain at concentrations of 200 μM in oocytes, and 5 μM for non-α1 pumps or 5 mM ouabain for α1 pumps in rat ventricular myocytes.
Western blotting
Membranes from ventricular tissue were prepared as described by Fuller, et al. (39). Protein electrophoresis was performed using Mini-Protean Tgx gels 7.5% (BioRad, Hercules CA) in Mini Protean Tetra System (BioRad). Protein was transferred onto 0.22 μm polyvinylidene fluoride membrane using Hoefer TE77. Transferred membranes were blocked with Tris-Buffered Saline (TBS) supplemented with 5% milk and Tween 20 (TBST) for 1 h, incubated overnight with TBS supplemented with 1% milk, 1% bovine serum albumin (BSA) and containing the TED primary antibody (40) diluted 1:100 (generous gift of Thomas A. Pressley, TTUHSC) followed by 1 h in TBST supplemented with 1% milk, 1% BSA and containing the horseradish peroxidase antirabbit (1:1000 dilution). Imaging was performed at the TTUHSC Molecular Biology Core Facility with Luminol (Santa Cruz Biotechnology, Dallas, TX) on an ImageQuant LAS 4000 (GE Healthcare, Pitsburgh, PA).
Immunofluorescence
After isolation cells were allowed to attach to poly-L-lysine-coated coverglass, fixed with paraformaldehyde for 15 min at RT, washed, permeabilized with triton X-100 for 10 min at RT, washed with phosphate buffered saline (PBS), and blocked in PBS supplemented with 3% BSA for 1 h. Samples were then incubated with primary antibody (PBS supplemented with 1% BSA) overnight, washed, and incubated with secondary antibody (PBS supplemented with 1% BSA) for 1 h. Permeabilized cells, blocked and treated only with secondary antibody were used as controls in each myocyte preparation (not shown). Images were acquired at the TTUHSC Image Analysis Core Facility on a Nikon Ti-E microscope.
Results
Function of human NKA isozymes in Xenopus oocytes with mammalian osmolality
As mentioned above, all previous characterizations of NKA function in oocytes were made at 80–100 mM Na+o, with variable K+o concentrations (24, 25). We first evaluated whether these batrachian ionic conditions might have led to an underestimation of the level of VDI observed at negative voltages with some isoforms, particularly α2 pumps. TEVC was used to address the effect of switching from 90 mM Na+o to 150 mM Na+o on the voltage dependence of the current induced by 4.5 mM K+o, the normal mammalian serum K+o concentration (Figs. 1 and 2).
Figure 1.
TEVC recording of a Xenopus oocyte 5 days after injection with human α2β1 cRNAs. (A) Continuous recording at −50 mV. Application of a 4.5 mM K+-induced outward pump current (IP) in 90 as well as in 150 mM Na+ (note significantly smaller current at 150 mM Na+). 100 μM ouabain was applied after the 10-min break inhibiting K+-induced IP. Sharp vertical deflections correspond to 100 ms pulses used to obtain the current versus voltage (I-V) relationships. (B) I-V curves obtained at the times indicated by small cap letters in (A). (C) Voltage dependence of 4.5 mM K+-induced currents at 90 mM Na+, 150 mM Na+, and 150 mM Na+ with ouabain. Note the stronger VDI at 150 mM Na+. To see this figure in color, go online.
Figure 2.
Voltage dependencies of all four cardiac isoforms at 4.5 mM K+. (A) Voltage dependence of 4.5 mM K+-induced IP normalized to the value at +40 mV in 90 mM Na+ to illustrate the effect of 150 mm Na+, for α1β1 (squares, n = 7), α2β1 (circles, n = 7), α2β2 (triangles, n = 16), and α3β2 (stars, n = 9). (B) Turnover rate (T/O), for α1β1 n = 11; α2β1 n = 8; α2β2 n = 8; and α3β2 n = 6. To see this figure in color, go online.
Fig. 1 A shows a representative current recorded on a slow time base, from an oocyte expressing α2β1 pumps held at −50 mV. The application of 4.5 mM K+o induced outward currents in both 90 mM and 150 mM external Na+. Sharp vertical deflections in the continuous trace correspond to the application of brief (100 ms) voltage pulses. The current at the end of those pulses is plotted as a function of voltage in Fig. 1 B. Measurements in the absence of K+ at the times marked a, c, and e in Fig. 1 A were then subtracted from those marked b, d, and f, to obtain the K+-induced currents plotted in Fig. 1 C. Thus, Fig. 1 C displays the K+-induced currents with 90 mM or 150 mM Na+o in the absence of ouabain and in 150 mM Na+o after inhibition with 100 μM ouabain. Lack of current after ouabain demonstrates that K+o-induced current (IP) is due to the Na/K pump. The activity of α2β1 pumps expressed in this oocyte is highly dependent on voltage within the physiological voltage range; from −90 mV to +40 mV.
We performed similar experiments on oocytes expressing human α1β1, α2β1, α2β2, and α3β2 pumps because evidence suggests that these isoforms are present, and interact, in rat and guinea pig ventricular myocytes (14). Fig. 2 A plots the K+-induced currents in 150 mM Na+o normalized to the same currents induced in 90 mM Na+o at +40 mV. To minimize the contribution of endogenous IP to the signals (∼25 nA, Fig. S1) we limit our analysis to oocytes showing currents ≥200 nA at our normalization point. It is evident that switching from 90 to 150 mM Na+o induces a strong current reduction in Na/K pumps created by all cardiac isoform combinations (α1β1, α2β1, α2β2, and α3β2) and this effect is present over the entire range of physiological membrane potentials. Furthermore, 150 mM Na+ induces a dramatic IP reduction for all α2-containing pumps at negative voltages. Endogenous pumps are responsible for most of the IP remaining at −80 mV in α2β2 expressing oocytes (cf. Fig. S1, gray hexagons).
The inhibitory effect of 150 mM Na+o can be better appreciated when the data is presented as turnover rate (Fig. 2 B). To obtain the turnover rate (T/O) under physiological conditions, one must estimate the number of pumps in each oocyte, and this was accomplished by measuring the total charge (Qtot) moved by NKAs in the absence of K+ (37). To be thorough, we also studied the function of α1β2 and α3β1 pumps. The association of any α-subunit with β2 shifts their voltage dependence toward positive voltages (Fig. S2).
To better understand how hypo- and hyperkalemia could affect cardiac pumps under physiological Na+, we investigated the dose dependence for K+o activation in 150 mM Na+o (Fig. 3). A continuous current record from an oocyte expressing α1β1 being held at −50 mV is shown in Fig. 3 A as an illustration of our experimental design. K+ induces current in a concentration-dependent manner and saturates at ∼10 mM. Fig. 3 B displays the K+-induced currents as a function of voltage. Fig. 3 C shows the current as a function of the K+ concentration at two voltages, where the continuous lines are fits of the Hill equation to the data.
Figure 3.
Dose response for K+ in an α1β1 expressing oocyte. (A) Continuous recording four days after injection. Step changes in [K+] produced a concentration-dependent increase in outward current. (B) Voltage dependence of K+-induced ouabain-sensitive currents at different [K+]. (C) The IP at different voltages were plotted against [K+] and fitted with a Hill equation (line plots). Only two voltages are shown for clarity. The K0.5 and the maximal turnover (Imax/Qtot) are plotted as a function of voltage in Fig. 4. To see this figure in color, go online.
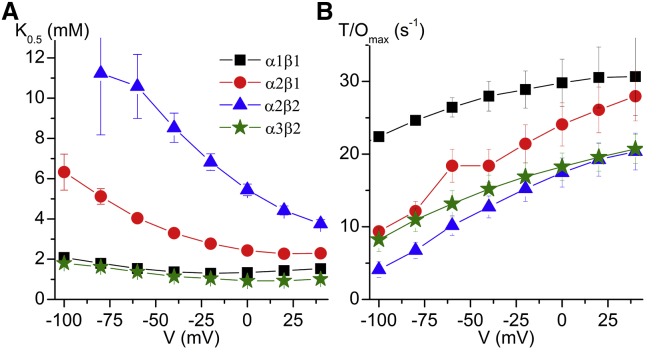
The voltage dependence of the Hill equation parameters fitted to the data from all cardiac isoforms combinations are presented in Fig. 4. The K0.5 for K+o and the maximal turnover rates (T/Omax) as a function of transmembrane voltage are shown in Fig. 4, A and B, respectively. It must be noted that α2β2 pumps have an extremely low apparent affinity for K+o, especially at negative voltages (K0.5 ≥ 10 mM at −60 mV); because of this strong voltage dependence neither the K0.5 nor the Imax can be accurately determined below −60 mV. To extend our analysis beyond the cardiac isoforms combinations, we studied α1β2 and α3β1 (Fig. S3). The α1β2 isozyme has a lower affinity for K+ than α1β1, but only at negative potentials.
Figure 4.
Voltage dependence of apparent affinity for K+ and maximum turnover rate. (A) K0.5 as a function of voltage at 150 mM Na+. α1β1 (squares, n = 4), α2β1 (circles, n = 3), α2β2 (triangle, n = 6), α3β2 (stars, n = 3). (B) Maximum turnover rate (Imax/Qtot) as a function of voltage. The Imax measured in the same experiments at +40 mV averaged 332 ±71 nA (α1β1), 251 ±24 nA (α2β1), 599 ±69 nA (α2β2), and 338 ±81 nA (α3β2). Note uncertainty of K0.5 for α2β2 at V ≤ −60 mV due to unreliable measurements for α2β2 at very negative potentials because of small endogenous pump signals (c.f. Fig. S1). To see this figure in color, go online.
The question arises of whether the lower than physiological temperature (T) used for the previous experiments (22–24°C) could have affected the NKA’s voltage dependence. We measured the voltage dependence of pump function at 24°C and 34°C (Fig. 5), but with 125 mM Na+ (oocytes could not withstand 150 mM Na+ at high T). The top trace in Fig. 5 A shows a continuous recording at −50 mV (interrupted by 100 ms-long voltage pulses responsible for the sharp vertical deflections at this slow time base) from an oocyte injected with α2β2. The IP-V curve in the same oocyte is shown at the bottom. Fig. 5 B illustrates the mean turnover rate at each T measured in uninjected oocytes (uninj., gray hexagons) and in oocytes injected with α2β1 (circles), or α2β2 (triangles). Both, the lack of a strong effect on voltage dependence and the 2.5-fold change in NKA turnover rate, are consistent with previous electrophysiological studies addressing the effect of T on pump function (41, 42, 43).
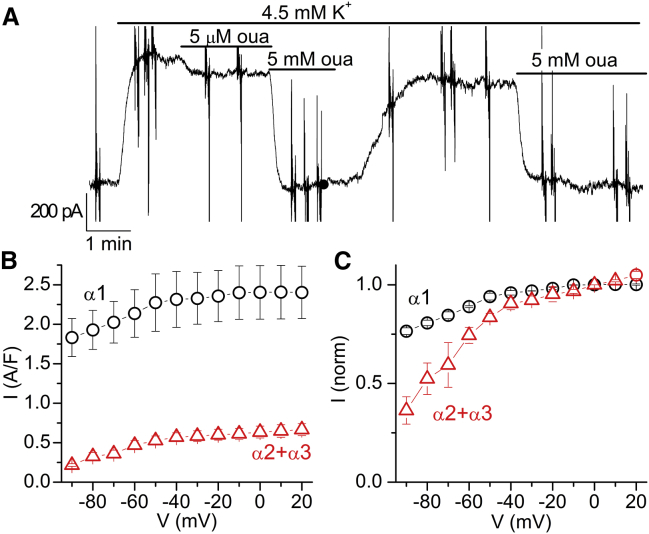
The striking difference in voltage dependence among the human isoforms carries profound physiological implications. We tested whether the differences in VDI that we measured in oocytes can also be observed in native rat ventricular myocytes (Fig. 6). In rodents the α1 subunit has a relatively low affinity for ouabain (IC50 ∼100 μM), whereas α2 and α3 pumps are far more sensitive (IC50 < 1 μM) (44). Thus, the function of the α1 isoform can be readily separated from α2 and α3 using different ouabain concentrations (5 mM and 5 μM).
Figure 6.
Voltage dependence of NKA isoforms in rat cardiac myocytes. (A) Current from a myocyte bathed by 145 mM Na+ at −40 mV. K+ application (4.5 mM) activates outward IP, which was inhibited by a subsequent addition of ouabain; 5 μM inhibited non-α1 (α2 + α3) and 5 mM inhibited all isoforms. (B) IP-V curve for the α1 and non-α1 pumps. IP/cell capacitance accounts for myocyte size variation. (C) IP-V curve for both components normalized to 0 mV. 85 mM Na+i, 5 mM MgATP, T = 35°C (n = 4, from three different animals aged 7, 8, and 16 weeks). To see this figure in color, go online.
Fig. 6 A shows a continuous recording from a ventricular myocyte bathed by 145 mM Na+o held at −40 mV. Perfusion of 4.5 mM K+o activated outward IP, which was inhibited by different ouabain concentrations. Application of 5 μM ouabain inhibited the current due to non-α1 pumps and 5 mM ouabain inhibited all isoforms. Although a small rundown of the current cannot be ruled out, it is clear that only α1 pumps recover significantly after washout, due to the faster ouabain unbinding from this isoform; the unbinding time constant at 37°C is ∼4 min for α2 pumps and ∼100 min for α3 pumps (45). The current from α1 pumps is obtained by subtracting the current in 5 mM ouabain from that in 5 μM ouabain. The component from non-α1 pumps is obtained by subtracting the current in 5 μM ouabain from that without ouabain. The IP-V curves measured at the end of 100-ms step pulses (• = α1; Δ = α2 + α3) are shown in Fig. 6, B and C. The non-α1 component (α2 + α3) is ∼20% of the total, consistent with previous reports at RT with similar ionic conditions (30). Fig. 6 C displays α1 and non-α1 components normalized to the current at 0 mV. Note that the stronger voltage dependence of the non-α1 component is consistent with a greater abundance of α2 pumps than α3 pumps in adult rat myocytes (46).
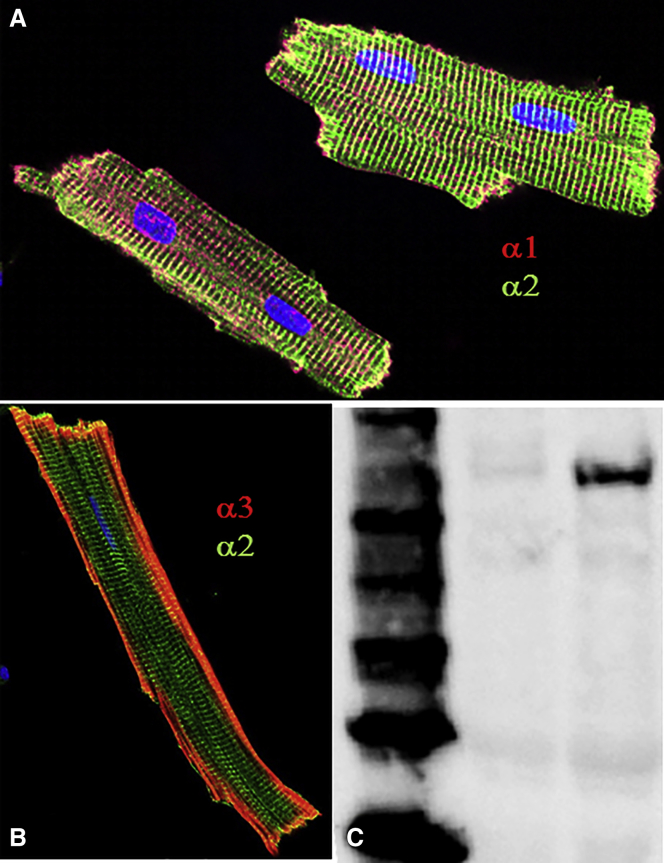
We tested whether the incomplete inhibition by voltage of the non-α1 component could be explained by a small percentage of α3 pumps, whose presence in adult rat hearts is still under debate. Coimmunostaining of ventricular myocytes with a monoclonal α1 antibody (α1-C464.6, Santa Cruz Biotech) and a rabbit-raised polyclonal α2 antibody (HERED (40)) always produced the same staining pattern (Fig. 7 A): α2 pumps appear mainly confined to T-tubules, whereas α1 pumps are present everywhere, consistent with previous functional (30) and immunostaining reports (14, 47). We observed even α3 staining of the nontubular membrane with a monoclonal commercial α3 antibody (XVIF9-G10, Santa Cruz Biotech, Fig. 7 B). Immunostaining with this antibody produced identical results in all myocytes isolated from 11 adult animals (aged 3 weeks to 1 year). To confirm the presence of α3, we also performed Western blots with a rabbit polyclonal antibody (TED (40)). Some (e.g., Fig. 7 C), but not all, heart preparations showed a clear α3 band, consistent with previously reported animal-to-animal variation (11).
Figure 7.
NKA catalytic subunit isoforms in ventricular myocytes. (A) Isolated myocytes stained against α1 (red) and α2 (green). Nuclei were stained with DAPI (blue). (B) Another representative myocyte stained against α3 (red) and α2 (green). (C) Western blot showing the ladder (left lane), boiled left ventricle membrane preparation (middle lane), and 65°C treated membrane preparation (right lane) probed with rabbit polyclonal antibody against α3 (TED).
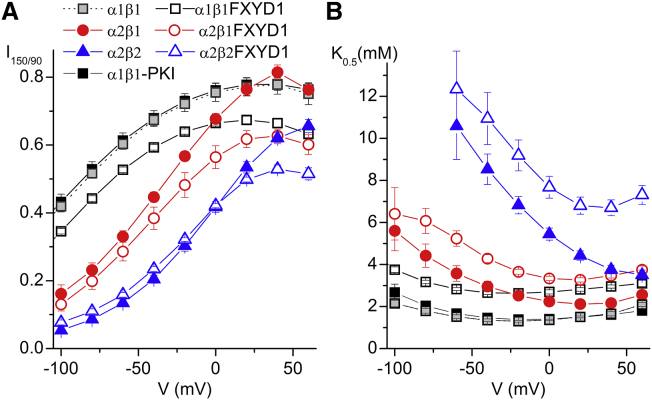
In the heart, transient modulation of NKA function by kinase-dependent phosphorylation is mediated by FXYD1 (48, 49). It has been shown that dephosphorylated FXYD1 interacts with α1 and α2 pumps (16) and reduces their activity by lowering the apparent affinity for intracellular Na+ and for extracellular K+ (16, 29, 50, 51). To ensure that FXYD1 was not phosphorylated by the variable and relatively high basal protein kinase A (PKA) activity in oocytes (52), we injected a PKA inhibitor peptide (PKI) 2 h before recording. PKI injection had no effect on the functional parameters of the three isozymes in the absence of FXYD1 (for clarity, only the results of the control with PKI-injected α1β1 pumps are shown in Fig. 8).
Figure 8.
Effect of FXYD1 on α1 and α2 isozymes. (A) Voltage dependence of 4.5 mM K+-induced IP at 150 mM Na+ normalized to the signal measured at +40 mV in 90 mM Na+. (B) K0.5 for K+ activation as a function of voltage obtained in dose response experiments. Note that the K0.5 for all pumps is increased by FXYD1. All FXYD1 experiments were performed after injection of PKI; no significant effect after PKI injection was observed for α1β1 (gray squares), α2β1 or α2β2 (PKI injected α2 pumps were excluded for clarity). To see this figure in color, go online.
We tested whether switching from 90 mM to 150 mM Na+ had a distinct effect on α1β1, α2β1, and α2β2 pumps interacting with FXYD1 (Fig. 8). Fig. 8 A shows the current induced by 4.5 mM K+ in 150 mM Na+, normalized to the current in 90 mM Na+ at +40 mV (as in Fig. 2 A). For all three isoform combinations, FXYD1 causes a more pronounced reduction in pump current by switching to 150 mM Na+, but this effect appears to be voltage independent. Fig. 8 B shows that pumps associated with FXYD1 have a reduced apparent affinity for K+o. Thus, Na+o is a more effective competitor for K+o and more potently reduces pump current. The fold change in K+o affinity induced by FXYD1 that we report here, however, is significantly larger than that in previous reports in oocytes (16, 17).
Discussion
Our data provide conclusive evidence that the difference in the voltage dependence between the two main cardiac muscle NKA catalytic subunit isoforms is dramatic and relevant for ion homeostasis in myocytes. Furthermore, we demonstrate that the β1 and β2 subunits are important regulators of kinetics and this allows us to hypothesize a mechanism underlying kinetic differences observed between different preparations in previous reports, including significant differences on the magnitude of the inhibitory effects of FXYD1. These findings and their implications for the beneficial action of cardiotonic steroids in heart failure will be discussed next.
Which pump isozymes and isoforms are present in the heart?
The presence of α1, α2, β1, and β2 is well accepted in ventricular myocytes of several mammalian species (11). The α3 isoform has also been reported in human ventricle, and reports have also shown that it is abundant in neonatal animals, but the amount diminishes as the animals approach adulthood (46). In this report, we present data that shows that there is a very small fraction of α3 pumps in adult rat myocytes: immunostaining with α3 antibodies (Fig. 7 B), Western blots showing detection in membrane preparations from some animals, but not all (Fig. 7 C), and pump current versus voltage plots where a small amount of non-α1 component resists VDI at −100 mV (Fig. 6, B and C). These observations are fully consistent with a previous report using Western blots that showed variable α3 presence in adult rats (11).
There are many possible combinations for functional pumps because all the αxβx form functional pumps in vitro (24). Nevertheless, studies in brain (53) and heart (14) indicate that α1 does not associate with β2. Furthermore, Madin-Darby canine kidney cells expressing the α1, β1, and β2 show a clear preference to form the α1β1 isozyme (53) indicating that α1β2 is not physiologically relevant. How much of the ventricular α2 component may be formed by β2 isoforms? Because the K0.5 for K+o for α2β2 pumps in oocytes at mammalian physiological concentrations (Fig. 4 B, ∼10 mM at −20 mV) is much higher than the K0.5 values previously reported in myocytes for non-α1 pumps (29) we think that most α2 pumps in ventricular myocytes must be associated with β1 with a low expression level of β2, as previously suggested (13).
Functional characteristics of different isozymes in mammalian solutions
Several studies have investigated the kinetic differences between NKA isozymes, both biochemically and electrophysiologically. When coexpressed with β1, the affinity for Na+i is highest in α2, intermediate in α1, and lowest in α3 subunits (54, 55). All α subunits presented similar apparent affinities for K+o when measurements were taken without Na+o. However, the affinities for K+o differed in the presence of 100 mM Na+o, where the lower affinity for K+o corresponds to the pump with the highest Na+ affinity (α2) and the highest apparent affinity for K+o, to the catalytic subunit with lowest Na+ affinity (α3) (25). In other words, because external Na+ competes with K+ for the ion-binding sites, the pump’s apparent affinity for K+o is highly dependent on the concentration of Na+.
Our experiments highlight the importance of the actual Na+o concentration when trying to understand the physiological role of specific pumps. Indeed, we measured significantly (∼2- to 3-fold) lower apparent affinities for K+ at mammalian Na+ concentrations than those previously reported for mammalian pump isoforms expressed in Xenopus oocytes (24, 25, 37, 56, 57). For instance, Larsen et al. (57) reported that the K0.5 for K+ of α2β2, at −100 mV with 70 mM Na+, was ∼7 mM, although we estimate that it is on the order of 15 mM with 150 mM Na+ (based on extrapolation). We think that due to the lower Na+ concentration used in previous reports the physiologically significant lower affinity for α2β1 pumps at negative voltages could not be observed. It is also possible that the use of ouabain-resistant pumps with altered function masked this observation.
The turnover rates we calculated are comparable to those previously measured in oocytes at RT. For example, the turnover rate of human α2β1 pumps at −30 mV, 90 mM Na+, and 10 mM K+ reported by Tavraz et al. (37) was 13 s−1 nearly identical to the values we observe: 14 s−1 at −20 mV and 11 s−1 at −40 mV. Crambert et al. (24) reported the maximum turnover rate for all nine wild-type human isoforms at −50 mV and 90 mM Na+ to be ∼30 s−1 for α1β1 and α1β2; ∼22 s−1 for α2β1 and α2β2; and ∼10 s−1 for α3β1 and α3β2. At −40 mV and 150 mM Na+ we report 19.5 ± 2.5 s−1 for α1β1, 7.2 ± 0.4 s−1 for α1β2, 11.2 ± 1.2 s−1 for α2β1, 4.1 ± 0.4 s−1 for α2β2, 18.5 ± 1.3 s−1 for α3β1, and 12.8 ± 1.1 s−1 for α3β2. The slightly lower turnover rates we report here for α1 and α2 pumps could be accounted for by the difference in ionic composition as Crambert et al. used 80 mM Na+ with nearly saturating 10 mM K+.
Voltage dependence of cardiac NKA isozymes at physiological Na+ concentrations: the role of α2 pumps and the β2 subunit
All pumps are subject to inhibition at negative potentials. This VDI depends on the concentration of external Na+. The pump-current versus voltage curves shift to the right as the Na+ concentration (20), or the Na+ affinity of the sites in the pump is increased, but it shifts to the left as the concentration of K+ (as a competitor for Na+) is increased. Thus, the physiologic relevance of this process must be assessed at normal Na+o and K+o concentrations. Our results (Fig. 2) show that at physiological Na+o and K+o, α2 pumps, when associated with β1, are ∼75% inhibited at membrane potentials corresponding to the resting potentials in ventricular myocytes (∼−75 mV (58)). When they associate with the β2 subunit, they are ∼95% inhibited (percentages obtained after subtraction of endogenous currents, c.f. Fig. S1). We confirmed the stronger voltage dependence of α2-pumps (∼70% of non-α1 pumps) in rat myocytes (Fig. 5). Our results strongly suggest heart α2 pumps are not meant to pump continuously. This is in contrast to the α1 pumps that perform a housekeeping role. Based on our data, we propose that α2 pumps are inhibited at the resting membrane potential and do not significantly contribute to Na+ pumping (nor to Ca2+ handling). During the cardiac action potential they are highly active when there is a need for increased Na+ extrusion due to the large Na+ influx through Na+ channels and to the inversion of the Na/Ca exchanger (NCX) function due to the depolarized membrane potential.
It is therefore expected that this safety net function of the α2 NKA also occurs in other tissues where it is highly expressed. For instance, α2 pumps constitute >70% of the pumps in skeletal muscle, being principally targeted to the T-tubules (59) where it must associate with β1 and β2 subunits (60). In this muscle type the resting potentials can be as low as −90 mV, meaning α2 pumps will be completely inactive, consistent with the minimal effect of NKA α2 inhibition in the skeletal muscle resting potential (61). Although the skeletal muscle action potentials are very brief, a train of activity produces accumulation of K+ in the T-tubule leading to a transient depolarization. This combination of elevated K+o and depolarization would activate α2 pumps to clear K+o from the tubule. Consistent with this idea, a recent report by Di Franco et al. (62) showed a reduced apparent affinity for K+ for α2, compared to α1 pumps, in mouse skeletal muscle fibers.
Previous reports have indicated that β subunits have relatively minor consequences on NKA kinetics (24, 63, 64). Our results contradict this idea. We show that compared to β1 isozymes, β2 isozymes induce drastic changes in pump function, significantly shifting the IP-voltage curve toward depolarized potentials due to an increased affinity for Na+o. Within the physiologic voltage range, the effect is most pronounced when associated with α2, as α2β2 pumps are >80% inhibited below −60 mV due to the very low affinity for K+ at this voltage (Fig. 4 A). This suggests that β2 association with α2 is important to ensure that these pumps will activate only when there is a need to clear elevated external K+. It is easy to envision how a mix of α2β1 and α2β2 can contribute when there is a need to clear different amounts of K+o accumulation. Larsen et al. (57) suggested a similar mechanism in glia, where the depolarization induced by the increase in K+o produces activation of the α2β2 pumps when they are needed to clear K+ from the extracellular space after K+o concentration increases following a train of action potentials or injury. Our results reinforce their proposal and show that differential interaction of α2 with β1 and β2 may provide two safety net pumps with different K+o affinities.
NKA α2 pumps, NCX, and the action of digitalis
Cardiotonic steroids (aka digitalis), such as ouabain and digoxin, are specific inhibitors of the NKA that have been used to treat heart failure since the 18th century (65). Cardiotonic steroids' therapeutic action occurs through NKA inhibition as the decrease in Na+ electrochemical gradient reduces Ca2+ extrusion via NCX (66). This in turn leads to a higher Ca2+ load within the sarcoplasmic reticulum, thus allowing a larger Ca2+ release during the systole (67). Recent reports indicate that human α2 pumps have a slightly higher affinity for the clinically preferred cardiotonic steroids than α1 pumps (68). It is widely believed that the actions of cardiotonic steroids on cardiac contraction are mediated through the α2 subunit, which occurs at a higher density in the T-tubules ((31) and Fig. 6 A). Although both α1 and α2 interact with NCX (69, 70), α2 and NCX interact within membrane micro-domains (71, 72), and it was also shown that α2 preferentially modulates Ca2+ transients (73, 74).
The results described previously have led to the idea that by targeting the differentially expressed α2 subunit, cardiotonic steroids could increase Ca2+i and contraction force by binding preferentially to only ∼25% of the total pumps, which account for up to 50% of the pumps in the T-tubules (31). Another reason why binding to α2 mediates the beneficial effects of cardiotonic steroids comes from their strong VDI; as α2 pumps are inactive at resting potentials, their inhibition would not affect the Ca2+ extrusion capacity at rest, but it would affect [Ca2+] handling during systole. The change in driving force due to inhibition of 50% of the tubular pumps may favor backward Ca2+ transport by NCX, also increasing systolic Ca2+.
Hypokalemia, a pathophysiologic condition that increases the risk of ventricular arrhythmias, is another condition where the lower K+-affinity and stronger VDI of α2 pumps appears to play an important role. It has been recently shown that hypokalemia hyperpolarizes rat ventricular myocytes and reduces the non-α1 pumping component, leading to larger Ca2+ transients with negligible changes in basal Ca2+ (cf. Figs. 1 and 2 in (74)) as we propose therapeutic concentrations of digitalis do.
The inhibitory effect of FXYD1
It has been shown that FXYD1 interacts with the α1 and α2 isoforms, but not with the α3 isoform (17), leading to partial inhibition of NKA. Förster resonance energy transfer experiments suggest that the relief of this inhibition through phosphorylation of FXYD1 by PKA and protein kinase C involves a separation of FXYD1 and α subunit at the intracellular ends of their transmembrane segments (51). The inhibition by FXYD1 at saturating Na+i reflects, at least in part, a reduction of the NKA’s affinity for K+o. According to previous reports, FXYD1 induced a smaller increase in the K0.5 for K+o in oocytes at 90 mM Na+o (∼40%, 17) than in transgenic mice at 120 mM Na+o (∼100%, 29). This difference brought into question the physiologic relevance of oocyte experiments.
Our results demonstrate that human FXYD1 induces physiologically relevant changes in apparent affinity for K+o of human αβ isozymes, and again, quantitatively reconcile data obtained in different systems. In better agreement with the mice myocytes experiments, at −20 mV and 150 mM Na+o, the K0.5 without and with FXYD1 varied, respectively, from 1.3 mM to 2.9 mM for α1β1 and from 2.5 to 3.7 for α2β1. Both the injection of PKI to inhibit all basal PKA activity and the use of mammalian [Na+]o may have contributed to the observed differences. Finally, the finding that dephosphorylated FXYD1 does not affect the voltage dependence (neither the position of the IP-V curve nor that of the K0.5-V curve shift with respect to voltage) indicates that FXYD1 inhibition affects a completely different pathway. The larger inhibitory effect of 150 mM Na+o at 4.5 mM K+o may simply reflect the increased binding of Na+o secondary to the reduction in K+o affinity. A small increase in Na+ binding affinity, however, cannot be ruled out by our experiments.
Conclusion
This work demonstrates a previously unnoted relevance for the NKA isozyme-specific voltage dependence. In particular, their voltage dependence allows α2 pumps to act as a safety net which, in the heart, will only increase Na+ extrusion upon depolarization during an action potential. In this context, inhibition of α2 pumps with cardiotonic steroids should only increase intracellular Na+ levels (and thus intracellular [Ca2+]) during depolarization (controlled by α2 and α1 pumps), but not at rest (dependent on α1 pumps). On the other hand, although voltage dependence is apparently irrelevant for FXYD1-mediated regulation, future studies will test the possibility of voltage dependence being an important regulatory step for NKA function, as a small shift in the current voltage curve, particularly for α2 pumps, could have highly relevant consequences for pumping capacity.
Author Contributions
C.M.S., D.G.G., D.J.M., and P.A. performed and analyzed experiments. A.B. performed experiments, J.J.R. cloned human subunits and helped write the article, and P.A. and D.G.G. designed research and wrote the article.
Acknowledgments
We thank Dr. Thomas A. Pressley for providing the isoform specific antibodies HERED (α2) and TED (α3), Dr. Charitha Galva for subcloning FXYD1 into PSD5 vector, Drs. Luis Reuss and Craig Gatto for comments on the manuscript, and the TTUHSC Image Analysis Core Facility for technical help.
This work was supported by grants from National Institute of Neurological Disorders and Stroke (NINDS) National Institutes of Health (NIH): R15 NS081570-01A to P.A. and R01 NS064259 to J.J.R.
Editor: Ian Forster.
Footnotes
Christopher M. Stanley and Dominique G. Gagnon contributed equally to this work.
Three figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)00952-2.
Supporting Material
References
- 1.Sen A.K., Post R.L. Stoichiometry and localization of adenosine triphosphate-dependent sodium and potassium transport in the erythrocyte. J. Biol. Chem. 1964;239:345–352. [PubMed] [Google Scholar]
- 2.Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 2001;33:425–438. doi: 10.1023/a:1010623724749. [DOI] [PubMed] [Google Scholar]
- 3.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Schwinger R.H., McDonough A.A. Regional expression of sodium pump subunits isoforms and Na+-Ca++ exchanger in the human heart. J. Clin. Invest. 1996;98:1650–1658. doi: 10.1172/JCI118960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlowski J., Lingrel J.B. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic alpha isoform and beta subunit mRNAs. J. Biol. Chem. 1988;263:10436–10442. [PubMed] [Google Scholar]
- 6.Zahler R., Sun W., Kashgarian M. Na-K-ATPase alpha-isoform expression in heart and vascular endothelia: cellular and developmental regulation. Am. J. Physiol. 1996;270:C361–C371. doi: 10.1152/ajpcell.1996.270.1.C361. [DOI] [PubMed] [Google Scholar]
- 7.Juhaszova M., Blaustein M.P. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc. Natl. Acad. Sci. USA. 1997;94:1800–1805. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moseley A.E., Lieske S.P., Lingrel J.B. The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J. Biol. Chem. 2003;278:5317–5324. doi: 10.1074/jbc.M211315200. [DOI] [PubMed] [Google Scholar]
- 9.Bøttger P., Tracz Z., Lykke-Hartmann K. Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J. Comp. Neurol. 2011;519:376–404. doi: 10.1002/cne.22524. [DOI] [PubMed] [Google Scholar]
- 10.Parekh A., Campbell A.J., Lawson S.N. Immunostaining for the α3 isoform of the Na+/K+-ATPase is selective for functionally identified muscle spindle afferents in vivo. J. Physiol. 2010;588:4131–4143. doi: 10.1113/jphysiol.2010.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweadner K.J., Herrera V.L., Repke K.R. Immunologic identification of Na+,K(+)-ATPase isoforms in myocardium. Isoform change in deoxycorticosterone acetate-salt hypertension. Circ. Res. 1994;74:669–678. doi: 10.1161/01.res.74.4.669. [DOI] [PubMed] [Google Scholar]
- 12.Blanco G., Mercer R.W. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 13.Hensley C.B., Bersohn M.M., McDonough A.A. Amiodarone decreases Na,K-ATPase alpha 2 and beta 2 expression specifically in cardiac ventricle. J. Mol. Cell. Cardiol. 1994;26:417–424. doi: 10.1006/jmcc.1994.1052. [DOI] [PubMed] [Google Scholar]
- 14.Harada K., Lin H., Inoue M. Subunit composition and role of Na+,K+-ATPases in ventricular myocytes. J. Physiol. Sci. 2006;56:113–121. doi: 10.2170/physiolsci.rp001905. [DOI] [PubMed] [Google Scholar]
- 15.Bibert S., Liu C.C., Rasmussen H.H. FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit. J. Biol. Chem. 2011;286:18562–18572. doi: 10.1074/jbc.M110.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bibert S., Roy S., Geering K. Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K-ATPase isozymes. J. Biol. Chem. 2008;283:476–486. doi: 10.1074/jbc.M705830200. [DOI] [PubMed] [Google Scholar]
- 17.Crambert G., Fuzesi M., Geering K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc. Natl. Acad. Sci. USA. 2002;99:11476–11481. doi: 10.1073/pnas.182267299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Weer P., Gadsby D.C., Rakowski R.F. Voltage dependence of the Na-K pump. Annu. Rev. Physiol. 1988;50:225–241. doi: 10.1146/annurev.ph.50.030188.001301. [DOI] [PubMed] [Google Scholar]
- 19.Bezanilla F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 20.Gadsby D.C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J. Gen. Physiol. 1989;94:511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C., Capendeguy O., Horisberger J.D. A third Na+-binding site in the sodium pump. Proc. Natl. Acad. Sci. USA. 2005;102:12706–12711. doi: 10.1073/pnas.0505980102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaragatupalli S., Olivera J.F., Artigas P. Altered Na+ transport after an intracellular alpha-subunit deletion reveals strict external sequential release of Na+ from the Na/K pump. Proc. Natl. Acad. Sci. USA. 2009;106:15507–15512. doi: 10.1073/pnas.0903752106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratheal I.M., Virgin G.K., Artigas P. Selectivity of externally facing ion-binding sites in the Na/K pump to alkali metals and organic cations. Proc. Natl. Acad. Sci. USA. 2010;107:18718–18723. doi: 10.1073/pnas.1004214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crambert G., Hasler U., Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- 25.Horisberger J.D., Kharoubi-Hess S. Functional differences between alpha subunit isoforms of the rat Na,K-ATPase expressed in Xenopus oocytes. J. Physiol. 2002;539:669–680. doi: 10.1113/jphysiol.2001.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakao M., Gadsby D.C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J. Gen. Physiol. 1989;94:539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peluffo R.D., Berlin J.R. Electrogenic K+ transport by the Na(+)-K+ pump in rat cardiac ventricular myocytes. J. Physiol. 1997;501:33–40. doi: 10.1111/j.1469-7793.1997.033bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peluffo R.D., Hara Y., Berlin J.R. Quaternary organic amines inhibit Na,K pump current in a voltage-dependent manner: direct evidence of an extracellular access channel in the Na,K-ATPase. J. Gen. Physiol. 2004;123:249–263. doi: 10.1085/jgp.200308872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han F., Tucker A.L., Bers D.M. Extracellular potassium dependence of the Na+-K+-ATPase in cardiac myocytes: isoform specificity and effect of phospholemman. Am. J. Physiol. Cell Physiol. 2009;297:C699–C705. doi: 10.1152/ajpcell.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Despa S., Bers D.M. Functional analysis of Na+/K+-ATPase isoform distribution in rat ventricular myocytes. Am. J. Physiol. Cell Physiol. 2007;293:C321–C327. doi: 10.1152/ajpcell.00597.2006. [DOI] [PubMed] [Google Scholar]
- 31.Berry R.G., Despa S., Shattock M.J. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc. Res. 2007;73:92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Gao J., Mathias R.T., Baldo G.J. Two functionally different Na/K pumps in cardiac ventricular myocytes. J. Gen. Physiol. 1995;106:995–1030. doi: 10.1085/jgp.106.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell T.J., Zugarramurdi C., Artigas P. Sodium and proton effects on inward proton transport through Na/K pumps. Biophys. J. 2014;106:2555–2565. doi: 10.1016/j.bpj.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liman E.R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 35.Price E.M., Lingrel J.B. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988;27:8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- 36.Vedovato N., Gadsby D.C. The two C-terminal tyrosines stabilize occluded Na/K pump conformations containing Na or K ions. J. Gen. Physiol. 2010;136:63–82. doi: 10.1085/jgp.201010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavraz N.N., Friedrich T., Dichgans M. Diverse functional consequences of mutations in the Na+/K+-ATPase alpha2-subunit causing familial hemiplegic migraine type 2. J. Biol. Chem. 2008;283:31097–31106. doi: 10.1074/jbc.M802771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong C.M., Swenson R.P., Jr., Taylor S.R. Block of squid axon K channels by internally and externally applied barium ions. J. Gen. Physiol. 1982;80:663–682. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuller W., Eaton P., Shattock M.J. Differential centrifugation separates cardiac sarcolemmal and endosomal membranes from Langendorff-perfused rat hearts. Anal. Biochem. 2001;293:216–223. doi: 10.1006/abio.2001.5127. [DOI] [PubMed] [Google Scholar]
- 40.Pressley T.A. Phylogenetic conservation of isoform-specific regions within alpha-subunit of Na(+)-K(+)-ATPase. Am. J. Physiol. 1992;262:C743–C751. doi: 10.1152/ajpcell.1992.262.3.C743. [DOI] [PubMed] [Google Scholar]
- 41.Galarza-Muñoz G., Soto-Morales S.I., Rosenthal J.J. Physiological adaptation of an Antarctic Na+/K+-ATPase to the cold. J. Exp. Biol. 2011;214:2164–2174. doi: 10.1242/jeb.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillo J.P., De Giorgis D., Bezanilla F. Energy landscape of the reactions governing the Na+ deeply occluded state of the Na+/K+-ATPase in the giant axon of the Humboldt squid. Proc. Natl. Acad. Sci. USA. 2011;108:20556–20561. doi: 10.1073/pnas.1116439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedrich T., Bamberg E., Nagel G. Na+,K(+)-ATPase pump currents in giant excised patches activated by an ATP concentration jump. Biophys. J. 1996;71:2486–2500. doi: 10.1016/S0006-3495(96)79442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shull G.E., Greeb J., Lingrel J.B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986;25:8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien W.J., Lingrel J.B., Wallick E.T. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch. Biochem. Biophys. 1994;310:32–39. doi: 10.1006/abbi.1994.1136. [DOI] [PubMed] [Google Scholar]
- 46.Lucchesi P.A., Sweadner K.J. Postnatal changes in Na,K-ATPase isoform expression in rat cardiac ventricle. Conservation of biphasic ouabain affinity. J. Biol. Chem. 1991;266:9327–9331. [PubMed] [Google Scholar]
- 47.McDonough A.A., Zhang Y., Frank J.S. Subcellular distribution of sodium pump isoform subunits in mammalian cardiac myocytes. Am. J. Physiol. 1996;270:C1221–C1227. doi: 10.1152/ajpcell.1996.270.4.C1221. [DOI] [PubMed] [Google Scholar]
- 48.Despa S., Bossuyt J., Bers D.M. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ. Res. 2005;97:252–259. doi: 10.1161/01.RES.0000176532.97731.e5. [DOI] [PubMed] [Google Scholar]
- 49.Fuller W., Howie J., Shattock M.J. FXYD1 phosphorylation in vitro and in adult rat cardiac myocytes: threonine 69 is a novel substrate for protein kinase C. Am. J. Physiol. Cell Physiol. 2009;296:C1346–C1355. doi: 10.1152/ajpcell.00523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han F., Bossuyt J., Bers D.M. Role of phospholemman phosphorylation sites in mediating kinase-dependent regulation of the Na+-K+-ATPase. Am. J. Physiol. Cell Physiol. 2010;299:C1363–C1369. doi: 10.1152/ajpcell.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bossuyt J., Despa S., Bers D.M. Isoform specificity of the Na/K-ATPase association and regulation by phospholemman. J. Biol. Chem. 2009;284:26749–26757. doi: 10.1074/jbc.M109.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Artigas P., Al’aref S.J., Andersen O.S. 2,3-butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: the role of bilayer material properties. Mol. Pharmacol. 2006;70:2015–2026. doi: 10.1124/mol.106.026070. [DOI] [PubMed] [Google Scholar]
- 53.Tokhtaeva E., Clifford R.J., Vagin O. Subunit isoform selectivity in assembly of Na,K-ATPase α-β heterodimers. J. Biol. Chem. 2012;287:26115–26125. doi: 10.1074/jbc.M112.370734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanco G. The NA/K-ATPase and its isozymes: what we have learned using the baculovirus expression system. Front. Biosci. 2005;10:2397–2411. doi: 10.2741/1705. [DOI] [PubMed] [Google Scholar]
- 55.Jewell E.A., Lingrel J.B. Comparison of the substrate dependence properties of the rat Na,K-ATPase alpha 1, alpha 2, and alpha 3 isoforms expressed in HeLa cells. J. Biol. Chem. 1991;266:16925–16930. [PubMed] [Google Scholar]
- 56.Koenderink J.B., Geibel S., Friedrich T. Electrophysiological analysis of the mutated Na,K-ATPase cation binding pocket. J. Biol. Chem. 2003;278:51213–51222. doi: 10.1074/jbc.M306384200. [DOI] [PubMed] [Google Scholar]
- 57.Larsen B.R., Assentoft M., MacAulay N. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia. 2014;62:608–622. doi: 10.1002/glia.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koumi S., Backer C.L., Sato R. beta-Adrenergic modulation of the inwardly rectifying potassium channel in isolated human ventricular myocytes. Alteration in channel response to beta-adrenergic stimulation in failing human hearts. J. Clin. Invest. 1995;96:2870–2881. doi: 10.1172/JCI118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radzyukevich T.L., Neumann J.C., Heiny J.A. Tissue-specific role of the Na,K-ATPase α2 isozyme in skeletal muscle. J. Biol. Chem. 2013;288:1226–1237. doi: 10.1074/jbc.M112.424663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson C.B., McDonough A.A. Skeletal muscle Na,K-ATPase alpha and beta subunit protein levels respond to hypokalemic challenge with isoform and muscle type specificity. J. Biol. Chem. 1996;271:32653–32658. doi: 10.1074/jbc.271.51.32653. [DOI] [PubMed] [Google Scholar]
- 61.Gruener R., Stern L.Z., Gerdes C. Electrophysiologic properties of intercostal muscle fibers in human neuromuscular diseases. Muscle Nerve. 1979;2:165–172. doi: 10.1002/mus.880020303. [DOI] [PubMed] [Google Scholar]
- 62.Di Franco M., Hakimjavadi H., Lingrel J.B., Heiny J.A. Na,K-ATPase alpha2 activity in mammalian skeletal muscle T-tubules is acutely stimulated by extracellular K. J. Gen. Physiol. 2015;146:281–294. doi: 10.1085/jgp.201511407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaisser F., Jaunin P., Horisberger J.D. Modulation of the Na,K-pump function by beta subunit isoforms. J. Gen. Physiol. 1994;103:605–623. doi: 10.1085/jgp.103.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanco G., Koster J.C., Mercer R.W. Kinetic properties of the alpha 2 beta 1 and alpha 2 beta 2 isozymes of the Na,K-ATPase. Biochemistry. 1995;34:319–325. doi: 10.1021/bi00001a039. [DOI] [PubMed] [Google Scholar]
- 65.Withering W. J and J Robinson; London: 1785. An Account of the Foxglove and Some of its Medical Uses: With Practical Remarks on Dropsy and Other Diseases. [Google Scholar]
- 66.Baker P.F., Blaustein M.P., Steinhardt R.A. The influence of calcium on sodium efflux in squid axons. J. Physiol. 1969;200:431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wier W.G., Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J. Gen. Physiol. 1984;83:395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katz A., Lifshitz Y., Karlish S.J. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J. Biol. Chem. 2010;285:19582–19592. doi: 10.1074/jbc.M110.119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohler P.J., Davis J.Q., Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dostanic I., Schultz Jel. J., Lingrel J.B. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J. Biol. Chem. 2004;279:54053–54061. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 71.Song H., Lee M.Y., Blaustein M.P. An N-terminal sequence targets and tethers Na+ pump alpha2 subunits to specialized plasma membrane microdomains. J. Biol. Chem. 2006;281:12929–12940. doi: 10.1074/jbc.M507450200. [DOI] [PubMed] [Google Scholar]
- 72.Golovina V., Song H., Blaustein M. Regulation of Ca2+ signaling by Na+ pump alpha-2 subunit expression. Ann. N. Y. Acad. Sci. 2003;986:509–513. doi: 10.1111/j.1749-6632.2003.tb07236.x. [DOI] [PubMed] [Google Scholar]
- 73.Despa S., Lingrel J.B., Bers D.M. Na(+)/K)+)-ATPase α2-isoform preferentially modulates Ca2(+) transients and sarcoplasmic reticulum Ca2(+) release in cardiac myocytes. Cardiovasc. Res. 2012;95:480–486. doi: 10.1093/cvr/cvs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aronsen J.M., Skogestad J., Sjaastad I. Hypokalaemia induces Ca(2+) overload and Ca(2+) waves in ventricular myocytes by reducing Na(+),K(+)-ATPase α2 activity. J. Physiol. 2015;593:1509–1521. doi: 10.1113/jphysiol.2014.279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.