Abstract
The complexity of gene expression control by non-coding RNA has been highlighted by the recent progress in the field of riboswitches. Discovered a decade ago, riboswitches represent a diverse group of non-coding mRNA regions that possess a unique ability to directly sense cellular metabolites and modulate gene expression through formation of alternative metabolite-free and metabolite-bound conformations. Such protein-free metabolite sensing domains utilize sophisticated three-dimensional folding of RNA molecules to discriminate between a cognate ligand from related compounds so that only the right ligand would trigger a genetic response. Given the variety of riboswitch ligands ranging from small cations to large coenzymes, riboswitches adopt a great diversity of structures. Although many riboswitches share structural principles to build metabolite-competent folds, form precise ligand-binding pockets, and communicate a ligand-binding event to downstream regulatory regions, virtually all riboswitch classes possess unique features for ligand recognition, even those tuned to recognize the same metabolites. Here we present an overview of the biochemical and structural research on riboswitches with a major focus on common principles and individual characteristics adopted by these regulatory RNA elements during evolution to specifically target small molecules and exert genetic responses.
Keywords: RNA structure, X-ray crystallography, metabolite, gene expression, transcription, non-coding RNA
INTRODUCTION
The ability of RNA to fold into complex three-dimensional (3-D) structures was proven many years ago by determination of the X-ray structure of a tRNA molecule [1]. Although in the case of tRNA, the robust 3-D structure is formed with the help of several modified nucleotides, most other cellular RNA molecules adopt complex spatial folds using only four building blocks composed of similar nucleobases and identical sugars and phosphates. This sharply contrasts with proteins, which build their 3-D structures using twenty amino acids, each with a unique side chain. The large range of chemical properties of amino acids also makes proteins versatile binders of small molecules. Although tRNA was shown to interact with naturally occurring polyamines [2], the ability of RNA to specifically bind other small organic molecules in the biologically relevant context was not demonstrated until the discovery of catalytic RNAs.
The discovery of ribozymes changed the perception of RNA molecules from inert aids, which carry genetic information and provide scaffolding and transporting functions, to more active participants, which independently or synergistically with proteins perform catalytic reactions in cells. One of the first discovered ribozymes [3], self-splicing group I introns, also recruit a guanosine cofactor and Mg2+ cations to play a pivotal role in the splicing reaction, suggesting that RNAs can specifically interact with small molecules. Later, Systematic Evolution of Ligands by Exponential Enrichment (SELEX) experiments revealed that recognition by RNA molecules is not restricted to nucleosides [4–6]. In vitro selected RNAs termed aptamers can bind strongly and specifically to chemical compounds with different properties, despite the presence of multiple negatively charged groups in RNA. These findings paved the road for the discovery of riboswitches [7–9], non-coding mRNA regions capable of specific binding to cellular metabolites. However, in contrast to the SELEX RNAs, riboswitches do not merely bind small molecules, interactions with these ligands also trigger modulation of expression of associated genes.
In addition to SELEX RNAs, several other breakthroughs and observations contributed to the discovery of riboswitches. An interplay between RNA conformations in the attenuator region as a means to control gene expression involving small molecules was first discovered in the Escherichia coli trp operon [10]. Later, RNA elements in the 5′-UTR of mRNAs encoding for aminoacyl tRNA genes were found to specifically bind non-aminoacylated tRNA molecules and activate transcription of the downstream genes [11]. These regulatory elements, coined T-boxes, share many features with metabolite-sensing riboswitches and represent, in essence, riboswitches specific to large ligands, uncharged tRNAs. Lastly, a number of laboratories reported feedback regulation of various operons in bacteria but failed to identify protein factors involved in the gene expression control (see references in [12]). These systems were later shown to be controlled by riboswitches.
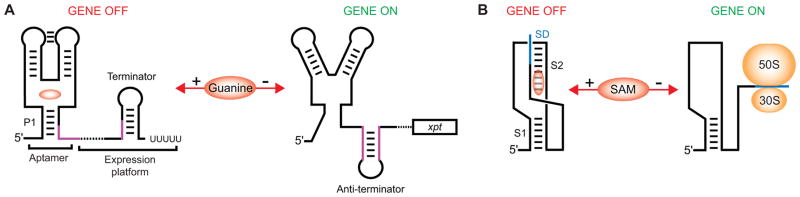
A typical riboswitch, such as a guanosine-responding RNA (Fig. 1A) [13], consists of two regions, an evolutionarily conserved metabolite-binding or aptamer domain followed by a variable expression platform that carries gene expression signals such as a transcription terminator or a ribosome-binding site (RBS). A salient feature of riboswitches is their ability to fold into two alternative conformations in response to the absence or presence of a cognate ligand at the over-threshold concentration [14, 15]. Metabolite binding stabilizes a ligand-bound conformation of the metabolite sensor, thus impacting the folding of the downstream expression platform. In the absence of a cognate ligand, the metabolite-sensing domain does not form a stable structure and becomes engaged in alternative pairing with a region in the expression platform, resulting in a different conformation of the entire riboswitch. The two conformations of the riboswitch oppositely direct expression of the associated genes.
Fig. 1.
Typical mechanisms of the riboswitch-mediated gene expression control. (A), Transcription termination mechanism of the guanine-specific xpt riboswitch [73]. Guanine binding stabilizes the structure of the junctional metabolite-sensing domain, including regulatory helix P1, and facilitates the formation of a Rho-independent transcriptional terminator that aborts transcription elongation. In the absence of guanine, the metabolite sensor does not adopt the ligand-bound conformation, and the 3′ region of P1 (magenta color) makes alternative base pairing with the region in the expression platform inducing formation of an anti-terminator hairpin. This hairpin prevents formation of the transcription terminator and allows RNA polymerase to transcribe the entire xpt gene. (B), Regulation of translation initiation by the SAM-II riboswitch [52, 53]. SAM binding stabilizes the formation of the pseudoknot-based fold including stem S2 that engages in base pairing with the Shine-Dalgarno sequence (blue color) of the downstream gene. In the absence of SAM, S2 is disrupted and the ribosome can access the Shine-Dalgarno sequence and initiate translation of the gene.
Despite a simple interplay between alternative conformations to exert gene expression control, riboswitches employ a large variety of mechanisms for gene regulation [16]. Here, we will highlight the diverse mechanistic repertoire of riboswitches adopted for control of transcription, translation, splicing and mRNA degradation. We will also overview architectural principles used for building the riboswitch structures and will provide insights on molecular features employed by natural riboswitches for specific ligand recognition.
Diversity of riboswitch ligands and regulatory mechanisms
To date, riboswitches encompass more than a dozen classes and several subclasses that target a large range of cellular compounds [12, 17]. The most abundant and wide-spread riboswitches recognize coenzymes and related compounds: adenosylcobalamin (AdoCbl) [8], thiamine pyrophosphate (TPP) [7, 9], flavin mononucleotide (FMN) [7, 18], S-adenosylmethionine (SAM) [19–26], S-adenosylhomocysteine (SAH) [27], tetrahydrofolate (THF) [28] and molybdenum/tungsten cofactors (Moco/Tuco) [29]. The second largest group combines purines and their derivatives [30, 31], such as adenine [32], guanine [13], pre-queuosine-1 (preQ1) [33, 34], deoxyguanosine (dG) [35], cyclic-di-GMP (c-di-GMP) [36, 37], and cyclic-di-AMP (c-di-AMP) [38]. A number of riboswitches respond to the amino acids lysine [39–41], glycine [42] and glutamine [43]. Riboswitches are also capable of sensing metal cations such as Mg2+ [44, 45] and the halide anion F− [46]. The phosphorylated sugar glucosamine-6-phosphate (GlcN6P) serves as the primary ligand for a special glmS riboswitch-ribozyme [47]. Given that there are also additional putative riboswitches that await independent confirmation and a large number of ‘orphan’ riboswitches [25, 48, 49], which lack identified ligands, the list of cellular metabolites triggering a riboswitch response will continue to grow.
Riboswitches are found in all three domains of life with the vast majority of examples identified across many bacterial species [50]. Most riboswitches are present in Gram-positive bacteria, while several riboswitches appear to be species- or small-group-specific regulators [51]. Many riboswitches turn off expression of genes through a two-state feedback mechanism [14, 15] highlighted by a schematic in Fig. 1A. When in excess in the cell, a metabolite binds to and stabilizes a metabolite-sensing domain of a riboswitch, thereby inducing formation of a downstream hairpin that serves as a Rho-independent transcription terminator. The riboswitch therefore induces premature transcription termination of the downstream genes that are involved in metabolism, utilization or transport of the riboswitch ligand or related compounds. If a cognate metabolite is not present at the threshold concentration, the folding of a stable metabolite-sensing domain is disrupted and a segment of the riboswitch, often a region of the domain-closing helix P1, participates in alternative base pairing. Thus, instead of a transcription terminator, the riboswitch forms an anti-terminator hairpin that supports transcription of the downstream genes and biosynthesis of proteins. Many riboswitches use a similar mechanism to control translation initiation through sequestration of an RBS and an initiation codon within a hairpin formed by the expression platform. In several riboswitches, such as the SAM-II riboswitch [19, 52, 53], the sequestering hairpin is eliminated and the region carrying the RBS becomes an integral part of the ligand-bound metabolite-sensing domain. In this case, the ligand binding directly contributes to occluding the RBS and preventing translation initiation (Fig. 1B).
A two-state switch mechanism is not the only option used by riboswitches to control gene expression. The adenine-sensing riboswitch from the thermophile Vibrio vulnificus can adopt three stable states, a ligand-bound conformation with an accessible RBS and two distinct ligand-free conformations, in which an RBS is sequestered by the formation of a hairpin [54]. One of the ligand-free conformations cannot interact with the ligand and requires conversion to a ligand-receptive form by a change in ambient temperature. The second riboswitch form, predisposed to ligand binding, interacts with adenine when the metabolite concentration exceeds a threshold, freeing the RBS for translation.
An unconventional mechanism has been proven for a glmS riboswitch-ribozyme, which cleaves the 5′-UTR off glmS mRNA using the bound metabolite as a reaction cofactor [47, 55, 56]. The remaining portion of the mRNA has a 5′ hydroxylated RNA end susceptible to the 5′-to-3′-directional hydrolytic activity of RNase J [57]. Similarly, the lysine-sensing riboswitch from the E. coli lysC gene opens mRNA for degradation [58]. Upon lysine binding, the riboswitch adopts a conformation that not only inhibits translation initiation but also exposes RNase E cleavage sites located in the riboswitch expression platform. However, in the absence of lysine, the riboswitch folds into the alternative conformation that simultaneously allows translation initiation and sequesters RNase E cleavage sites [58].
Several bacterial riboswitches exploit other regulatory options. An FMN riboswitch does not form a Rho-independent transcription termination but instead employs Rho-dependent transcription termination [59]. Bacterial riboswitches generally operate through cis-acting control mechanisms, while two SAM-I riboswitches from Listeria monocytogenes, SreA and SreB, function in trans by binding to the 5′-UTR of prfA mRNA and inhibiting expression of a virulence regulator [60]. The vitamin B12 riboswitch from the same bacterium controls transcription of AspocR, an antisense RNA which in its full-length form inhibits expression of a transcription factor via direct interactions with its mRNA [61]. Some c-di-GMP riboswitches explore an even more complex regulatory approach [36]. These RNA elements are located adjacent to group I self-splicing introns such that c-di-GMP binding to its aptamer induces conformational changes that allows GTP to attack the intron’s 5′ splice site. The resulting self-excision of the intron brings two distantly located parts of the RBS together to reconstitute a full-length RBS for efficient translation of the open reading frame (ORF).
In contrast to bacterial riboswitches which commonly repress gene expression by terminating transcription or by blocking ribosome binding, eukaryotic riboswitches regulate gene expression by alternative splicing. Thus far, all known eukaryotic riboswitches respond to TPP [50] and typically reside within introns of pre-mRNAs. In fungi [62, 63] and algae [64], in the absence of TPP, a sequence within the aptamer domain, either a segment of helix P1 or an internal sequence, blocks alternative splicing sites and facilitates removal of the entire intron. The resulting mRNA is normally translated. In the presence of TPP, the aptamer domain folds into a stable structure and opens alternative splice sites, resulting in mRNAs that contain either an internal stop codon [64] or short upstream ORFs [62], which preclude expression of the main ORF. In higher plants [65, 66], a TPP riboswitch controls splicing at a single 5′ splice site to either remove or retain the intron. Intron excision, when TPP is abundant, also removes a polyadenylation signal thereby producing unstable mRNA. In the absence of TPP, alternative base pairing sequesters the splice site and retains the intron with the processing signal, yielding a stable transcript.
Riboswitch architectures from top to bottom
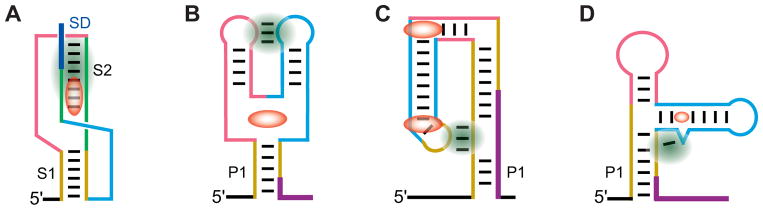
Continuous efforts from several laboratories have culminated in determination of the 3-D structures for the metabolite-sensing domains of virtually all major riboswitch types and several subclasses. Given the variety of the riboswitch ligands, these studies revealed amazing variability of the riboswitch architectures with similarities only in three closely related adenine, guanine and dG riboswitches. Despite great structural diversity, all riboswitches can be divided in two large groups, pseudoknotted and junctional riboswitches, based on the folding principle that defines the overall riboswitch architecture (Fig. 2) [12, 67].
Fig. 2.
Schematics of architectures observed in riboswitch structures. Ligands are shown in red color. Long-distance tertiary interactions reinforced by ligand binding are highlighted in green color. (A), The pseudoknot type of the metabolite sensing domain, exemplified by the SAM-II riboswitch [52]. (B), The ‘regular’ architecture of junctional riboswitches observed in purine riboswitches. The fold is stabilized by ligand binding in the center of the three-way junction. (C), The ‘inverted’ architecture of junctional riboswitches stabilized in the THF riboswitch by binding to two ligand molecules [89]. (D), Glycine riboswitch is an example of the ‘regular’ architecture stabilized by tertiary interactions that span shorter distance [87].
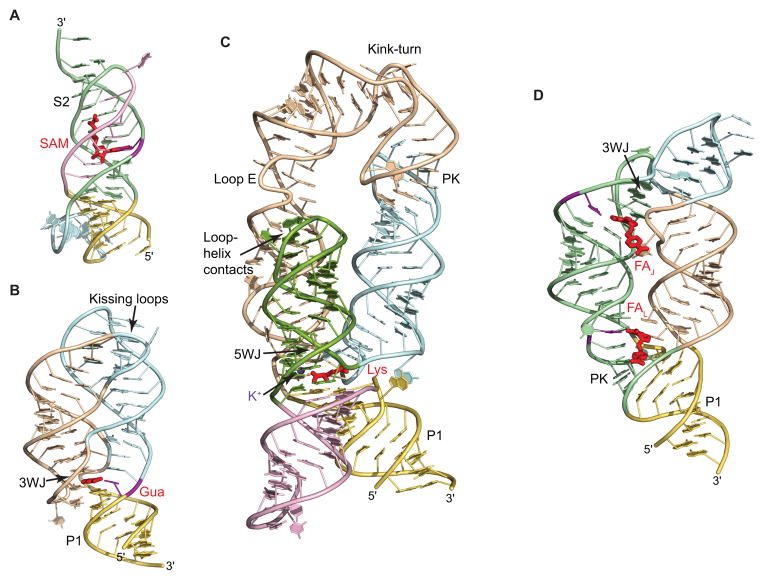
Pseudoknotted riboswitches, such as preQ1 classes I and II (preQ1-I and preQ1-II) [68–72], SAM-II [52] (Fig. 3A) and others, are built predominantly on the basis of a single pseudoknot, a knot-shaped conformation formed through base pairing between a loop of an RNA stem-loop structure and an outside region (Fig. 2A). Pseudoknots in riboswitches usually constitute a stack of two helices connected by short loops, which sometimes contain extra elements, for instance, a hairpin in the fluoride riboswitch. Ligands interact with pseudoknots in the junctional region that connect pseudoknot helices, as in preQ1-I [70–72] and fluoride [46] riboswitches, or are positioned along the groove of a helix and trapped by nucleotides of the loop, as in a SAM-II riboswitch (Fig. 3A) [52].
Fig. 3.
Examples of the riboswitch structures. RNA is shown in ribbon representation and is color-coded according to Fig. 2. Ligands are shown in red color. Nucleotides important for discrimination of the ligand are in violet. 3WJ and 5WJ, three-way and five-way junctions, respectively. PK, pseudoknot. (A) Structure of the SAM-II riboswitch bound to SAM highlights a riboswitch fold based on a pseudoknot conformation [52]. A key feature of the structure is the stabilization of stem S2 by binding to SAM. (B) Structure of the guanine riboswitch bound to guanine (Gua) shows ‘regular’ architecture of junctional riboswitches [73]. The ligand binds in the center of the three-way junction and holds together the junctional fold. Tertiary interactions between apical loops provide correct alignment of stems and reinforce the structure. (C) Structure of the lysine riboswitch bound to lysine [79]. The structure consists of two-helical and three-helical bundles positioned below and above the ligand-bound five-way junction. One of the helices reverses its orientation through a kink-turn and is anchored in place by pseudoknot interactions. Ligand-bound potassium cation is shown by a violet sphere. (D), Structure of the THF riboswitch bound to two molecules of folinic acid (FA) highlights the ‘inverted’ architecture of junctional riboswitches, centered on the three-way junction and stabilized by pseudoknot interactions [89]. Ligand molecules stabilize both the junction and the pseudoknot.
Junctional riboswitches (Fig. 2B–D) contain a multihelical junction which connects a various number of helices, e.g. three in purine (Fig. 3B) [73, 74] and TPP [75–77] riboswitches, five in lysine (Fig. 3C) [78, 79] and six in FMN [80] riboswitches. According to the basic principles of RNA folding, riboswitch helices often stack on each other in the junctional region and pack side-to-side above and below the junction, thus forming helical bundles [81–83]. Ligands usually bind riboswitches of this type within junctions, as in purine (Fig. 3B) [73, 74], lysine (Fig. 3C) [78, 79] and c-di-GMP-I riboswitches [84, 85], or in the regions adjacent to the junction, as in glycine riboswitches [86, 87]. TPP and some other ligands bind at a distance from the junction, but are nevertheless able to hold the helical stems emerging from the junction, thereby reinforcing the ligand-bound conformation [75, 76].
Junctional riboswitches can be further divided into two subgroups in respect to the mutual location of the key junction and the domain-closing helix, typically the regulatory helix P1 (Fig. 2B) [12, 67]. In several riboswitches, helix P1 originates in the centrally positioned junction and the regulatory mechanism involves ligand-mediated organization and stabilization of the junction that contribute to zipping up the regulatory helix (Fig. 2B,D). In these Y-shaped or ‘normal’ junctional riboswitches, ligands can be directly involved in the formation of the regulatory helix by interacting with the residues of P1, as in the purine [73, 74, 88] and lysine [78, 79] riboswitches (Fig. 3B,C). Alternatively, ligands can contribute indirectly by displacing a residue from the ligand-bound pocket and inserting it into the junctional region, as in glycine riboswitches [87], or can stabilize the junction and P1 remotely by anchoring the junctional stems, as in TPP riboswitches [75, 76].
The second group contains junctional riboswitches characterized by the h-shaped or ‘inverted’ architecture, where the multihelical junction is located on the periphery, whereas P1 or the helix which closes the sensing domain is positioned far from the junction and is connected to the junction through an irregular helix (Fig. 2C). In these riboswitches, the domain-closing helix is stabilized through long distance tertiary interactions with a hairpin protruding from the peripheral junction. Examples of ‘inverted’ junctional architectures are THF (Fig. 3D) [89, 90], glmS [55, 56] and Mg2+-II (M-box) [45] riboswitches. In these riboswitches, ligands can be bound either in the junctional region and/or in the vicinity of the regulatory helix.
Several riboswitches, for instance, SAM-I [91], c-di-GMP-II [92], lysine [78, 79], glmS [55, 56], and THF [89, 90] contain both multihelical junctions and pseudoknots and therefore might be considered as a mixed type. We prefer to assign these RNAs to the junctional category since their long helices connected by junctions basically define the global architecture while their pseudoknots play a somewhat secondary role in riboswitch scaffolding by either anchoring components of the junctional fold through tertiary contacts or participating in building ligand-binding pockets.
Practically all structures of junctional riboswitches available to date share a common feature: the riboswitch conformations are reinforced by long distance tertiary interactions which join and lock up otherwise freely moving helices (Fig. 2B–D). These interactions may include loop-loop contacts between apical loops of hairpins (Fig. 3B) and are technically classified as pseudoknot interactions if formed predominantly by Watson-Crick pairing, as in the lysine riboswitch (Fig. 3C) [78, 79]. Pseudoknot interactions are also observed as a result of base pairing between an apical loop and an internal loop in the c-di-GMP-II [92], SAM-I [91] and THF [89, 90] riboswitches (Fig. 3D). In the purine [73, 74] and dG [88] riboswitches, loop-loop interactions involve both Watson-Crick and non-canonical base pairs as well as additional base stacking interactions and hydrogen bonds between bases and the RNA backbone. TPP [75, 76], c-di-GMP-I [84, 85], lysine [78, 79] (Fig. 3C), Mg2+-II [45] and glmS [55, 56] riboswitches contain another variation of long-distance contacts, tertiary interactions between the minor groove of helices and apical loops of hairpins. Such interactions do not require a contact between hairpins of the same length and therefore do not restrict the length of the longer stem. Long-distance contacts are most unusual in the FMN riboswitch [80]. The scaffold of this RNA is reinforced by a pair of symmetrical double contacts, each includes loop-loop and loop-helix interactions. Interestingly, with the exception of the ‘normal’ c-di-GMP-II [92] and ‘inverted’ THF [89] and Mg2+-II sensors [45], ligands are not directly involved in the formation of long-distance tertiary contacts in the junctional riboswitches (Fig. 3D).
Two RNAs, SAM-III [93] and the glycine [86, 87] responsive elements, diverge from other junctional riboswitches. In these RNAs, tertiary contacts are formed between adjacent regions and therefore span much shorter distances. In the glycine riboswitch (Fig. 2D), a nucleotide loops out of the ligand-binding pocket positioned slightly above the junction and intercalates between the junction and P1, thus assisting base pairing in P1. In the SAM-III riboswitch, SAM is embedded in the heart of the junction and is kept in place by a short loop region which folds back from the stem that protrudes from the junction.
Remarkably, certain metabolites can be recognized by more than one riboswitch fold. For instance, at least five riboswitch classes bind SAM [94] and two classes interact with c-di-GMP [36, 37] and preQ1 [33, 34]. Structures determined for three classes of SAM [52, 91, 93], and both classes of each c-di-GMP [84, 85, 92] and preQ1 [34, 68, 70, 71] riboswitches demonstrate great diversity in the RNA scaffolds, in recognition of the ligands, and even in the conformation of bound ligands in complexes with RNAs that target the same metabolite. One of the most astonishing examples is three SAM-specific riboswitches, SAM-I [91], SAM-II [52] and SAM-III [93], which structurally have little in common. These RNAs adopt a four-way junctional architecture (SAM-I), a pseudoknot fold (SAM-II), and a three-way junctional conformation (SAM-III), suggesting that the SAM binding folds emerged independently during evolution.
Despite differences in overall conformations and a number of unique structural features, riboswitches share structural elements and motifs also observed in other RNAs. For instance, many tertiary contacts found in riboswitches [81] include A-minor [95] and related base triples, ribose zippers and tetraloops. Helical stems in several riboswitches bend at kink-turn motifs [78, 79, 96–98] (Fig. 3C). Some riboswitches, including the aforementioned FMN riboswitch [80], contain larger recurrent motifs, such as T-loops, first identified in the tRNA structure [99, 100]. These motifs participate in the formation of large structural domains, such as T-loop PK domain, found in ribosomes [101].
Selectivity for small ligands from the standpoint of RNA
Some can take for granted that the specificity of small molecule recognition has been demonstrated a long time ago for ribosomes that use rRNA for interaction with antibiotics, for RNA aptamers selected in vitro for binding to the small molecule of choice, and for some self-splicing ribozymes that require guanosine for their reactions [102]. While these examples have conclusively shown that RNA molecules are capable of specific targeting of small molecules, it has not been clear whether RNA can use binding of small molecules as a driving force or a trigger to modulate gene expression. It was also not clear what level of selectivity RNA molecules would require functioning as intracellular sensors of metabolites and effectors of genetic control.
Riboswitches have several key differences from other RNAs that target small molecules. Ribosomes are large particles that contain long RNAs folded with assistance of many proteins. Because of the size and specific functional properties, ribosomes contain plenty of pockets built with distinctive conformations of RNA chains and suitable for specific recognition of small molecules. These sites are targeted by antibiotics which do not need to be distinguished from similar molecules of the cellular origin and in a number of examples display modest specificity and affinity for rRNA [103, 104]. Many rRNA-binding antibiotics, especially aminoglycosides, are decorated with positively charged groups that increase affinity for negatively-charged RNA molecules and make these compounds capable of binding to various RNAs with micromolar and sub-micromolar dissociation constants [105]. Likewise, group I self-splicing introns also do not show exquisite specificity for their small molecule cofactor guanosine [3]. In addition to the nucleoside, phosphate-carrying guanosine nucleotides can be efficiently docked into the guanosine-binding site and participate in the first step of splicing.
Because of their small size and exquisite ligand specificity, riboswitches have more in common with small-molecule-binding RNA aptamers produced in SELEX experiments than with large ribosomes and self-splicing ribozymes. Like riboswitches, some SELEX aptamers can control expression of genes in vivo by binding to small molecules of non-cellular origin if placed in the right genetic context [106, 107]. Moreover, SELEX aptamers can replace riboswitch aptamers within natural riboswitches and successfully direct their expression platforms thus generating chimeric riboswitches capable of robustly controlling gene expression [108, 109]. However, it remains to be investigated how effectively in vitro selected aptamers that bind a riboswitch ligand can replace natural riboswitch aptamers responsive to the same ligand. Indeed, synthetic aptamers were typically selected for high binding affinity and specificity that did not imply the ability to stabilize the regulatory helix or sequester an RNA segment bearing the RBS. Therefore, in the construction of artificial riboswitches, a significant hurdle has been to couple a metabolite sensor and an expression platform enabling their efficient communication. The problem can be alleviated by careful engineering of communicating sequences [108, 109] and a combination of in vitro and in vivo selection strategies [110]. Unlike SELEX aptamers, modern riboswitches naturally evolved for both ligand binding and gene regulation, although aptamer domains might have been co-opted from ancient RNAs with different activities.
Comparison of the available structures of SELEX aptamers and riboswitches specific to the same or similar compounds did not reveal obvious similarities, although one would expect at least the major ligand recognition means to be similar [80, 97, 98]. Given the small selection window, direction of the selective pressure applied in SELEX experiments, and other factors specific to the in vitro selection, this observation is not particularly surprising and one more time emphasizes versatility of RNA sequences, especially in light of several distinct structures recognizing the same ligand in the SAM and other riboswitch families. Therefore, the finding that a bacterium and several vertebrates harbor adenosine aptamers of the same structural family as those discovered using in vitro selections is of particular interest to the RNA research community [111]. Although the biological function of the identified aptamers has not been understood yet, these results demonstrate that the convergent molecular evolution of adenosine binding encompasses genomic sequences.
In addition to achieving high specificity, riboswitches face other challenges. First, riboswitches have to respond to a specific threshold concentration which would ensure an adequate genetic response. Since concentrations of cellular metabolites may vary in cells, riboswitches of the same family but controlling different operons in the genome or riboswitches in different species can be tuned to different concentrations of the ligand [112]. Alternatively, riboswitches can be placed in tandem arrangements to achieve better control in response to cognate metabolites [113]. Second, riboswitches have to bind metabolites which can be present in cells at low concentrations, as second messengers, or higher millimolar concentrations, as amino acids. Given such large difference in affinity, structural principles evolved for one riboswitch type cannot be transplanted into another riboswitch type, thus demanding multiple structural solutions to be emerged during evolution. Third, organisms may have an advantage from the sensor that integrates positive and negative chemical signals by recognizing different ligands and relaying the overall metabolic state of the cell to the gene expression machinery [114]. Therefore, in a certain biological context, exquisite ligand specificity may be less beneficial than broader selectivity.
Solutions to the challenge of cellular metabolite binding
In cells, riboswitches face a serious challenge to discriminate a cognate ligand from its precursors, derivatives, and other similar compounds. To accomplish this difficult task, the riboswitch structures form highly specific ligand-binding pockets. Since riboswitch ligands are chemically diverse, various riboswitches use different strategies for selective binding. These strategies however include a combination of several intermolecular forces and binding principles that are often shared by different riboswitch types.
Shape complementarity
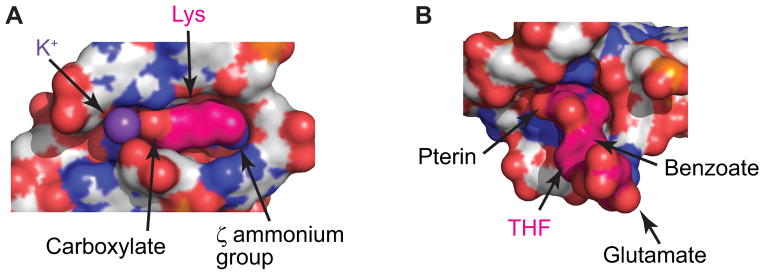
One of the simplest strategies to discriminate against larger compounds is to form a tight ligand binding pocket which could exclusively fit a cognate ligand and hold it by van der Waals and other interactions. The best examples to illustrate this approach are glycine and lysine riboswitches. The glycine riboswitch has a small pocket compatible with only very small molecules while the slightly larger alanine or derivatives of glycine cannot be accommodated [79]. The lysine riboswitch contains a tight pocket to fit an extended amino acid with the shape of lysine (Fig. 4A) [79, 115]. Smaller amino acids, such as glycine or alanine, can in principle be accommodated in the pocket but bulkier amino acids with larger or branched side chains do not have sufficient room.
Fig. 4.
Examples of ligand-binding pockets. RNA (grey) and ligands (hot pink) are shown in surface representation with heteroatoms depicted in the following colors: oxygen, red; nitrogen, blue; phosphorus, orange. All views are from the top in respect to the Fig. 3C,D views. (A), Tight lysine-bound pocket of the lysine riboswitch shows good shape complementarity with bound lysine [79]. A potassium cation (violet sphere) mediates interactions between the carboxylate of lysine and RNA. Nucleotides from the front are removed to visualize the pocket. (B) Semi-open pocket of the THF riboswitch bound to THF [90]. The pocket is located in the junctional region.
Probably the best solution for ligand binding is to create a binding pocket with perfect shape complementarity to the entire ligand. Some ligand-binding pockets, for instance, in lysine [79, 115] and purine riboswitches [73, 74], indeed encapsulate bound ligands such that up to 98% of the ligand surface is in contact with RNA and only a small percentage of the surface remains exposed to solution. Nevertheless RNA does not need to envelop the entire ligand for specific recognition. The TPP riboswitch structures [75, 76] have shown that the riboswitch forms two ligand binding pockets for each extremity of TPP whereas the middle region of the ligand is not recognized by RNA and is largely exposed to solution. Riboswitches also do not require deep and tight ligand binding pockets, especially if a riboswitch can respond to several similar compounds of the same class. For instance, the THF riboswitch adopts a 3-D structure with a shallow semi-open ligand binding pocket for binding to the pterin moiety of THF (Fig. 4B) [89, 90]. The middle region, an amino benzoate moiety, is positioned on the RNA surface in stacking with a guanosine while another terminus of the coenzyme, a glutamyl moiety, does not make specific interactions with RNA [90]. Such ligand recognition implies that the riboswitch can potentially respond to a number of coenzymes related to THF if they contain the pterin moiety and carry a chemical group in the middle capable of stacking with a guanosine base.
Direct hydrogen bonding
In proteins, binding pockets for small molecules often contain hydrophobic amino acids and employ hydrophobic forces for interactions with ligands. RNA has difficulties in creating a hydrophobic environment since all parts of a nucleotide contain heteroatoms capable of making hydrogen bonds or electrostatic interactions. Fortunately, known riboswitch ligands carry plenty of heteroatoms and many riboswitches exploit them for intermolecular interactions, as illustrated by adenine and guanine riboswitches [73, 74] (Fig. 5A,B), which recognize all oxygen and nitrogen atoms of the bound ligands.
Fig. 5.
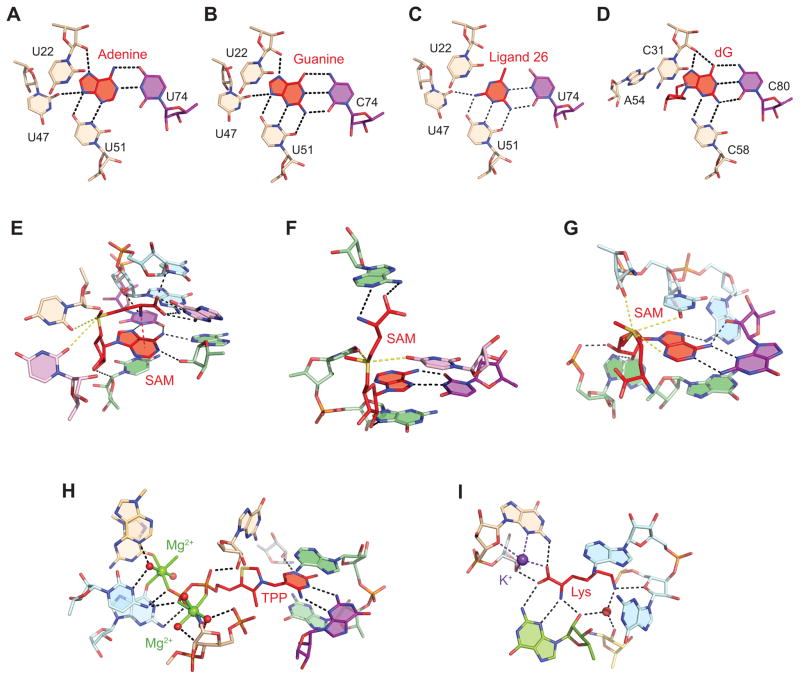
Recognition of different metabolites by riboswitches. The views show only nucleotides involved in ligand binding. Ligands are in red. Hydrogen bonds are depicted by dashed black lines. Carbon atoms of RNA are color-coded as in Figs. 2 and 3 while heteroatoms are shown in atomic colors. (A), Adenine riboswitch bound to adenine [73]. The discriminatory nucleotide is in violet. (B), Guanine riboswitch bound to guanine [73]. (C), Adenine riboswitch bound to a synthetic compound identified by virtual ligand screening [116]. (D), dG riboswitch bound to dG [88]. (E), SAM-I riboswitch bound to SAM [91]. Electrostatic interactions involving the sulfonium group are depicted by yellow dashed lines. Cation-π interaction is shown by red dashed line. (F), SAM-II riboswitch bound to SAM [52]. (G), SAM-III riboswitch bound to SAM [93]. (H), TPP riboswitch bound to TPP and two magnesium cations (green) [75]. The view does not show all intermolecular interactions. Water molecules are shown as pink spheres. Coordination bonds of cations are shown with green sticks. (I), Lysine riboswitch bound to lysine and a potassium cation [79].
Direct hydrogen bonding not only contributes to the affinity of interactions, it is the most important means for specificity of recognition. Riboswitch ligands have both hydrogen bond donors and acceptors and require a certain pattern of the groups that donate or accept hydrogen bonds for specific binding. In addition, a set of hydrogen bonds provides certain geometric constrains and ensures correct orientation of the ligand in the pocket. A change in the hydrogen bonding pattern, for instance, by mutating a single nucleotide that contributes to the specific recognition of the ligand can lead to dramatic consequences. For instance, replacement of a uridine by a cytosine at position 74 of the adenine riboswitch switches specificity of the RNA from the adenine to the guanine sensor and vice versa since pyrimidine 74 is the sole discriminatory residue that makes Watson-Crick base pairing with the bound purine (Fig. 5A,B) [13, 32]. Other edges of the ligands are bound by the same nucleotides in both adenine and guanine riboswitches [73, 74]. The purine binding pocket can accommodate smaller compounds not present in cells (Fig. 5C) [116], although binding is weaker possibly because of the loss of direct hydrogen bonds. The pocket is re-arranged in the deoxyguanosine (dG) riboswitch to provide room for the sugar moiety of dG (Fig. 5D) [88, 117]. The dG riboswitch retains the discriminatory cytosine but has two substitutions which prevent interactions of the RNA with N9 of guanine (U47A) and preserve direct hydrogen bonding with the sugar edge of dG (U51C).
Many other riboswitch ligands also contain nucleobases or similar aromatic moieties and employ their Watson-Crick or Watson-Crick-like edges for specific recognition of ligands. Surprisingly, in addition to purine and dG riboswitches, only c-di-GMP-I [84, 85], preQ1-I [70–72] and FMN [80] riboswitches make Watson-Crick type base pairing with nucleotides of RNA. Other nucleobase-containing ligands either use a Watson-Crick edge for non-canonical pairing with Watson-Crick (THF [90]) and sugar (SAM-I [91], TPP [75, 76], c-di-GMP-II [92], SAM-III [93]) (Fig. 5E,G,H) edges of RNA bases and interactions with sugars (SAM-I) (Fig. 5E) and water molecules (c-di-GMP-I [84, 85]).
Non-Watson-Crick edges of nucleobases in riboswitch ligands also make important contributions to binding selectivity in all nucleobase-containing ligands. For instance, as mentioned earlier, the N2-N3-N9 (‘sugar edge’) of the ligand in the guanine riboswitch is bound by a uridine that blocks binding of nucleosides, which can interact with the RNA when uridne 51 is replaced by a cytosine (Fig. 5B,D) [88]. Remarkably, different classes of SAM (Fig. 5E–G), preQ1 [68, 70–72] and c-di-GMP [84, 85, 92] riboswitches interacting with the same ligands utilize very different hydrogen bond patterns for specific recognition of nucleobases. This observation supports the suggestion that different classes of riboswitches targeting the same ligand have risen independently from each other during evolution.
Direct hydrogen bonding is not restricted to nucleobases of the ligands and is especially important for recognition of amino acids and sugars that do not contain aromatic rings. Both main chain carboxylate and ammonium groups of glycine [87] and lysine [78, 79] (Fig. 5I) form hydrogen bonds with nucleobases of the conserved residues in the corresponding riboswitches. The ammonium group of the lysine side chain makes less specific hydrogen bonding and electrostatic interactions with the sugar-phosphate backbone of RNA but nevertheless serves as a recognition determinant to discriminate lysine against amino acids with shorter side chains. Alanine and glycine can fit their side chains into the lysine binding pocket but cannot anchor the side chains in the interior of the pocket through hydrogen bonding.
Stacking interactions
Another important type of interactions between aromatic rings of ligands and RNA bases is the π-π stacking interaction. These interactions are critical for maintaining DNA and RNA helices and often participate in tertiary contacts that reinforce more complex RNA structures. Although practically all aromatic rings of riboswitch ligands are involved in stacking with RNA bases, several riboswitches appear to use these interactions for selective and high affinity ligand binding that facilitates formation of the riboswitch structure. For instance, intercalation of aromatic rings of the ligands between bases that belong to different structural elements in the TPP (Fig. 5H) [75, 76], FMN [80] and preQ1-I [68, 70–72] riboswitches ensures stability of the ligand-bound RNA conformation. In c-di-GMP riboswitches [84, 85, 92], an adenine base is intercalated between two purine bases of the ligand to reduce conformational dynamics in the ligand, maximize binding affinity and maintain the overall RNA structure.
Recognition of negatively charged groups
One of the common traits in riboswitch ligands is the presence of negatively charged phosphate and carboxylate groups. To bind these moieties, negatively charged RNA molecules employ the same strategy used for RNA folding - neutralization of phosphate charges by metal cations such as Mg2+. Indeed, in the TPP (Fig. 5H) [75, 76], FMN [80] and glmS [55, 56] riboswitches, interactions between phosphates and RNA are mediated by one or two Mg2+ cations which bind RNA through direct coordination and water-mediated bonds. A Mg2+ cation also bridges the carboxylate of glycine with the backbone of the glycine riboswitch [87]. In contrast to these riboswitches, lysine sensors utilize a different ion, a monovalent K+ cation that mediates interactions with the carboxylate group of lysine (Fig. 5I) [79]. Because of its larger size, a K+ cation can form more coordination bonds than Mg2+. In the lysine riboswitch, the K+ cation holds three nucleotides in the same RNA strand.
Mg2+ cations have been found to be critical in binding of the fluoride anion to the riboswitch. A structural study has shown that the negatively charged F− anion is directly coordinated by three Mg2+ cations, with the anion positioned slightly out of the plane formed by the cations [118]. The fluoride ion does not contact RNA whereas Mg2+ cations interact with several phosphates from two distinct segments of the RNA thus joining the helices of the pseudoknot and holding the conformation of the riboswitch together.
Mg2+ cations do not only facilitate ligand recognition; they serve as specific ligands in two Mg2+-sensing riboswitches that control genes encoding for ion transporters [44, 45]. The structure of the M-box riboswitch reveals several Mg2+ cations participating in stabilization of the ‘inverted’ riboswitch architecture and the regulatory helix through long-distance tertiary contacts [45]. These cations facilitate parallel alignment and bridge distant RNA elements so that the tertiary interactions contribute to the formation of the regulatory helix.
Recognition of positively charged groups
Electrostatic forces take part in recognition of positive groups in several riboswitch ligands. For instance, the positively charged ammonium group of the lysine side chain is positioned in the negatively charged environment and, in addition to hydrogen bonding, is locked in place by electrostatic interactions [78, 79]. As mentioned earlier, these interactions help the riboswitch discriminate against amino acids with short side chains.
Electrostatic interactions account for discrimination between the SAM and SAH ligands by SAM riboswitches. SAH is formed by demethylation of SAM in SAM-dependent catalytic reactions and is deprived of not only the methyl group, but also the positive charge on the sulfur atom [27, 94]. Since the extra methyl group of SAM cannot add much affinity to SAM binding and the SAM-binding pocket cannot pose sterical constraints on SAH binding, SAM riboswitches evolved a strategy to specifically recognize the positively charged sulfonium group of SAM. In all three SAM structures (Fig. 5E–G), the sulfonium group makes electrostatic interactions with oxygen atoms of uridine bases, the type of interactions incompatible with the chemical structure of SAH [52, 91, 93]. Interestingly, recognition of the sulfonium group is a common principle for ligand binding by very different SAM riboswitches. SAM-I [91], SAM-II [52] and SAM-III [93] riboswitches all recognize the sulfonium group but use distinct strategies to recognize adenosyl and carboxylate moieties of SAM and trap bound ligands in different conformations.
Riboswitches also exhibit less common electrostatic interactions. For instance, a cation/π interaction, which can be viewed as a case of amino-aromatic interactions [119], stabilizes a ‘folded over’ conformation of SAM in the SAM-I riboswitch (Fig. 5E) [91, 120]. In this case, the amino group of the methionine moiety at one end points toward the adenine ring at the other end of the ligand. In the glmS riboswitch, two methyl groups of GlcN6P point at the rings of the guanosine base [55, 56], thus forming C-H/π interactions [121], typically observed in carbohydrate-protein complexes [122]. RNA is capable of other types of interactions [123] and it will not be surprising to see further means of specific ligand recognition by riboswitches.
Metabolite sensing and genetic control
A plethora of structural principles used for ligand binding and RNA folding practically serves a single purpose – to selectively bind the cognate ligand and reject non-cognate ligands at the time scale sufficient to choose the pathway of the riboswitch folding and gene expression control. Obviously, folding of a transcriptional riboswitch into a structure capable of ligand binding is restricted in time by the rate of transcription. Although the RNA polymerase can pause, allowing the RNA folding to catch up [124], many riboswitches can bind their cognate ligand prior to achieving thermodynamic equilibrium with the ligand. These riboswitches operate by kinetic control and require ligand concentrations higher than their Kd to commit to the ligand-binding pathway of folding and regulation. The same riboswitch placed in a different genetic context, for instance, to control translation initiation, may reach equilibrium with the ligand prior to making the genetic decision [125, 126] (reviewed in [127]). Such riboswitches may even function as true switches, turning translation on and off by associating and dissociating with the ligand [128]. In contrast, transcription does not re-initiate after termination and transcriptional riboswitches operate as molecular fuses.
The picture of riboswitch-metabolite binding is further complicated by the fact that the ligand should be recognized by a metastable riboswitch conformation, capable of committing to either the ligand-bound or -unbound folding pathway. The nature of this conformation is of particular interest since available X-ray structures in the metabolite-bound and -unbound states poorly describe this conformation (reviewed in [129]). In the ligand-bound structures, RNA can completely envelop bound ligands, demonstrating that RNA adjusts its conformation after initial ligand recognition [73, 74, 78, 79], according to the induced-fit concept of ligand binding. On the other hand, because of crystallization restrains, the riboswitch structures in the apo state were reported similar to the ligand-bound structures and, therefore, are incompatible with ligand binding [78, 79]. For some riboswitches that bind small ligands, adjustments in the RNA conformation can be local, as validated by obtaining ligand-bound structures after soaking ligands into the crystals of the apo riboswitches [87]. Large ligands may require larger rearrangements in RNA [130]. Nevertheless, the apo riboswitch conformation should be to some extent similar to the ligand-bound structures to take full advantage of the ligand binding and discriminating potentials. However, biochemical and biophysical studies have revealed a multitude of RNA conformations in the apo state [53, 131–137], supporting a ‘conformational selection’ concept of ligand recognition [138]. This concept postulates that macromolecules adopt a dynamic ensemble of pre-existing conformations in the apo state. Among many available conformations, ligand molecules capture only conformations that are competent of ligand binding and thus shift the equilibrium toward the bound state. Available structural and biochemical data suggest that riboswitches most likely utilize both conformational selection and induced fit to first select the appropriate RNA conformation from the ensemble of apo conformations and then trap the bound ligand by adjusting RNA conformation locally to maximize binding affinity.
The glmS riboswitch-ribozyme exerts genetic control via ligand-dependent cleavage and not through alternative conformations of the metabolite-sensing domain. Since GlcN6P catalyses the RNA cleavage, a pre-formed apo RNA structure cannot change the genetic output allowing ligand recognition of the largely pre-formed ligand-unbound conformation [55, 56]. Therefore, the glmS riboswitch-ribozyme does not appear to follow the common principle of metabolite recognition and folding.
Perspectives in structural and structure-function studies of riboswitches
Many critical questions in the riboswitch field have been addressed by structural and structure-guided biochemical, genetic, and biophysical studies during approximately a decade of research [12]. These works revealed novel RNA architectures, broadened the collection of structural motifs and added novel principles of small molecule recognition to the repertoire of ligand-RNA interactions. The structures agreed with the results of biochemical studies and in addition uncovered many unexpected features [139–141]. One of the most surprising outcomes of the structural studies is the divergence and unpredictability of riboswitch folds, which are difficult to envision based on the secondary structure schematics. Therefore, despite many available riboswitch structures, accurate prediction of a 3-D fold for a riboswitch remains a challenging task, even if the structure was determined for a related RNA [142]. Thus, 3-D structures cannot be predicted for many orphan riboswitches. Some of these riboswitches are involved in the control of important genetic circuits and their structures will be essential to understand regulatory pathways in bacteria [17, 25, 48, 49].
X-ray structures offer ‘frozen’ views on riboswitches and despite many hints at the mechanistic principles employed to modulate gene expression, structural information has to be complemented by other methods to provide detailed insights on the mechanism of riboswitch function. One avenue of research includes elucidation of the riboswitch dynamics. The size of the majority of riboswitches exceeds the limits for routine NMR studies [143] and the dynamics of riboswitches is currently mostly tackled by various single-molecule approaches [53, 132, 144] with the support of small angle X-ray scattering [145, 146] and other techniques [53, 133, 136, 147]. These studies have the potential to trace the entire riboswitch mechanism, starting from co-transcriptional RNA folding and ending by translation or degradation of the message. With the discovery of novel riboswitches and their subsequent 3-D structure determination, these approaches will ensure our progress towards the understanding of the riboswitch-mediated genetic circuits and consideration of riboswitches for biotechnological and medicinal applications.
Acknowledgments
This work was supported by the New York University Medical Center funds.
References
- 1.Kim SH, Suddath FL, Quigley GJ, McPherson A, Sussman JL, Wang AH, Seeman NC, Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 2.Quigley GJ, Teeter MM, Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978;75:64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cech TR, Zaug AJ, Grabowski PJ. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981;27:487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- 4.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 5.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 6.Robertson DL, Joyce GF. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature. 1990;344:467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 7.Mironov AS, Gusarov I, Rafikov R, Lopez LE, Shatalin K, Kreneva RA, Perumov DA, Nudler E. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. Cell. 2002;111:747–756. doi: 10.1016/s0092-8674(02)01134-0. [DOI] [PubMed] [Google Scholar]
- 8.Nahvi A, Sudarsan N, Ebert MS, Zou X, Brown KL, Breaker RR. Genetic control by a metabolite binding mRNA. Chem Biol. 2002;9:1043. doi: 10.1016/s1074-5521(02)00224-7. [DOI] [PubMed] [Google Scholar]
- 9.Winkler W, Nahvi A, Breaker RR. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- 10.Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981;289:751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- 11.Grundy FJ, Henkin TM. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell. 1993;74:475–482. doi: 10.1016/0092-8674(93)80049-k. [DOI] [PubMed] [Google Scholar]
- 12.Serganov A, Nudler E. A decade of riboswitches. Cell. 2013;152:17–24. doi: 10.1016/j.cell.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal M, Boese B, Barrick JE, Winkler WC, Breaker RR. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell. 2003;113:577–586. doi: 10.1016/s0092-8674(03)00391-x. [DOI] [PubMed] [Google Scholar]
- 14.Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 15.Nudler E, Mironov AS. The riboswitch control of bacterial metabolism. Trends Biochem Sci. 2004;29:11–17. doi: 10.1016/j.tibs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Henkin T. Riboswitch RNAs: using RNA to sense cellular metabolism. Genes Dev. 2008;22:3383–3390. doi: 10.1101/gad.1747308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breaker RR. Prospects for riboswitch discovery and analysis. Mol Cell. 2011;43:867–879. doi: 10.1016/j.molcel.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler WC, Cohen-Chalamish S, Breaker RR. An mRNA structure that controls gene expression by binding FMN. Proc Natl Acad Sci U S A. 2002;99:15908–15913. doi: 10.1073/pnas.212628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbino KA, Barrick JE, Lim J, Welz R, Tucker BJ, Puskarz I, Mandal M, Rudnick ND, Breaker RR. Evidence for a second class of S-adenosylmethionine riboswitches and other regulatory RNA motifs in alpha-proteobacteria. Genome Biol. 2005;6:R70. doi: 10.1186/gb-2005-6-8-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epshtein V, Mironov AS, Nudler E. The riboswitch-mediated control of sulfur metabolism in bacteria. Proc Natl Acad Sci U S A. 2003;100:5052–5056. doi: 10.1073/pnas.0531307100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs RT, Grundy FJ, Henkin TM. The SMK box is a new SAM-binding RNA for translational regulation of SAM synthetase. Nat Struct Mol Biol. 2006;13:226–233. doi: 10.1038/nsmb1059. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel BA, Grundy FJ, Artsimovitch I, Henkin TM. Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA. Proc Natl Acad Sci U S A. 2003;100:3083–3088. doi: 10.1073/pnas.0630422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poiata E, Meyer MM, Ames TD, Breaker RR. A variant riboswitch aptamer class for S-adenosylmethionine common in marine bacteria. RNA. 2009;15:2046–2056. doi: 10.1261/rna.1824209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg Z, Regulski EE, Hammond MC, Barrick JE, Yao Z, Ruzzo WL, Breaker RR. The aptamer core of SAM-IV riboswitches mimics the ligand-binding site of SAM-I riboswitches. RNA. 2008;14:822–828. doi: 10.1261/rna.988608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg Z, Wang JX, Bogue J, Yang J, Corbino K, Moy RH, Breaker RR. Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes. Genome Biol. 2010;11:R31. doi: 10.1186/gb-2010-11-3-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JX, Breaker RR. Riboswitches that sense S-adenosylmethionine and S-adenosylhomocysteine. Biochem Cell Biol. 2008;86:157–168. doi: 10.1139/O08-008. [DOI] [PubMed] [Google Scholar]
- 27.Wang JX, Lee ER, Morales DR, Lim J, Breaker RR. Riboswitches that sense S-adenosylhomocysteine and activate genes involved in coenzyme recycling. Mol Cell. 2008;29:691–702. doi: 10.1016/j.molcel.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ames TD, Rodionov DA, Weinberg Z, Breaker RR. A eubacterial riboswitch class that senses the coenzyme tetrahydrofolate. Chem Biol. 2010;17:681–685. doi: 10.1016/j.chembiol.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regulski EE, Moy RH, Weinberg Z, Barrick JE, Yao Z, Ruzzo WL, Breaker RR. A widespread riboswitch candidate that controls bacterial genes involved in molybdenum cofactor and tungsten cofactor metabolism. Mol Microbiol. 2008;68:918–932. doi: 10.1111/j.1365-2958.2008.06208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batey RT. Structure and mechanism of purine-binding riboswitches. Q Rev Biophys. 2012;45:345–381. doi: 10.1017/S0033583512000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JN, Breaker RR. Purine sensing by riboswitches. Biol Cell. 2008;100:1–11. doi: 10.1042/BC20070088. [DOI] [PubMed] [Google Scholar]
- 32.Mandal M, Breaker RR. Adenine riboswitches and gene activation by disruption of a transcription terminator. Nat Struct Mol Biol. 2004;11:29–35. doi: 10.1038/nsmb710. [DOI] [PubMed] [Google Scholar]
- 33.Meyer MM, Roth A, Chervin SM, Garcia GA, Breaker RR. Confirmation of a second natural preQ1 aptamer class in Streptococcaceae bacteria. RNA. 2008;14:685–695. doi: 10.1261/rna.937308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth A, Winkler WC, Regulski EE, Lee BW, Lim J, Jona I, Barrick JE, Ritwik A, Kim JN, Welz R, Iwata-Reuyl D, Breaker RR. A riboswitch selective for the queuosine precursor preQ1 contains an unusually small aptamer domain. Nat Struct Mol Biol. 2007;14:308–317. doi: 10.1038/nsmb1224. [DOI] [PubMed] [Google Scholar]
- 35.Kim JN, Roth A, Breaker RR. Guanine riboswitch variants from Mesoplasma florum selectively recognize 2′-deoxyguanosine. Proc Natl Acad Sci U S A. 2007;104:16092–16097. doi: 10.1073/pnas.0705884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science. 2010;329:845–848. doi: 10.1126/science.1190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson JW, Sudarsan N, Furukawa K, Weinberg Z, Wang JX, Breaker RR. Riboswitches in eubacteria sense the second messenger c-di-AMP. Nat Chem Biol. 2013;9:834–839. doi: 10.1038/nchembio.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grundy FJ, Lehman SC, Henkin TM. The L box regulon: lysine sensing by leader RNAs of bacterial lysine biosynthesis genes. Proc Natl Acad Sci U S A. 2003;100:12057–12062. doi: 10.1073/pnas.2133705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Regulation of lysine biosynthesis and transport genes in bacteria: yet another RNA riboswitch? Nucleic Acids Res. 2003;31:6748–6757. doi: 10.1093/nar/gkg900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudarsan N, Wickiser JK, Nakamura S, Ebert MS, Breaker RR. An mRNA structure in bacteria that controls gene expression by binding lysine. Genes Dev. 2003;17:2688–2697. doi: 10.1101/gad.1140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal M, Lee M, Barrick JE, Weinberg Z, Emilsson GM, Ruzzo WL, Breaker RR. A glycine-dependent riboswitch that uses cooperative binding to control gene expression. Science. 2004;306:275–279. doi: 10.1126/science.1100829. [DOI] [PubMed] [Google Scholar]
- 43.Ames TD, Breaker RR. Bacterial aptamers that selectively bind glutamine. RNA Biol. 2011;8:82–89. doi: 10.4161/rna.8.1.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg2+ Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 45.Dann CE, 3rd, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. Structure and mechanism of a metal-sensing regulatory RNA. Cell. 2007;130:878–892. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Breaker RR. Fluoride enhances the activity of fungicides that destabilize cell membranes. Bioorg Med Chem Lett. 2012;22:3317–3322. doi: 10.1016/j.bmcl.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg Z, Barrick JE, Yao Z, Roth A, Kim JN, Gore J, Wang JX, Lee ER, Block KF, Sudarsan N, Neph S, Tompa M, Ruzzo WL, Breaker RR. Identification of 22 candidate structured RNAs in bacteria using the CMfinder comparative genomics pipeline. Nucleic Acids Res. 2007;35:4809–4819. doi: 10.1093/nar/gkm487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, Wickiser JK, Breaker RR. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci U S A. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudarsan N, Barrick JE, Breaker RR. Metabolite-binding RNA domains are present in the genes of eukaryotes. RNA. 2003;9:644–647. doi: 10.1261/rna.5090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrick JE, Breaker RR. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol. 2007;8:R239. doi: 10.1186/gb-2007-8-11-r239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbert SD, Rambo RP, Van Tyne D, Batey RT. Structure of the SAM-II riboswitch bound to S-adenosylmethionine. Nat Struct Mol Biol. 2008;15:177–182. doi: 10.1038/nsmb.1371. [DOI] [PubMed] [Google Scholar]
- 53.Haller A, Rieder U, Aigner M, Blanchard SC, Micura R. Conformational capture of the SAM-II riboswitch. Nat Chem Biol. 2011;7:393–400. doi: 10.1038/nchembio.562. [DOI] [PubMed] [Google Scholar]
- 54.Reining A, Nozinovic S, Schlepckow K, Buhr F, Furtig B, Schwalbe H. Three-state mechanism couples ligand and temperature sensing in riboswitches. Nature. 2013;499:355–359. doi: 10.1038/nature12378. [DOI] [PubMed] [Google Scholar]
- 55.Klein DJ, Ferre-D’Amare AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 56.Cochrane JC, Lipchock SV, Strobel SA. Structural investigation of the GlmS ribozyme bound to its catalytic cofactor. Chem Biol. 2007;14:97–105. doi: 10.1016/j.chembiol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins JA, Irnov I, Baker S, Winkler WC. Mechanism of mRNA destabilization by the glmS ribozyme. Genes Dev. 2007;21:3356–3368. doi: 10.1101/gad.1605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caron MP, Bastet L, Lussier A, Simoneau-Roy M, Masse E, Lafontaine DA. Dual-acting riboswitch control of translation initiation and mRNA decay. Proc Natl Acad Sci U S A. 2012;109:E3444–3453. doi: 10.1073/pnas.1214024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hollands K, Proshkin S, Sklyarova S, Epshtein V, Mironov A, Nudler E, Groisman EA. Riboswitch control of Rho-dependent transcription termination. Proc Natl Acad Sci U S A. 2012;109:5376–5381. doi: 10.1073/pnas.1112211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, Mandin P, Repoila F, Buchrieser C, Cossart P, Johansson J. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell. 2009;139:770–779. doi: 10.1016/j.cell.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 61.Mellin JR, Tiensuu T, Becavin C, Gouin E, Johansson J, Cossart P. A riboswitch-regulated antisense RNA in Listeria monocytogenes. Proc Natl Acad Sci U S A. 2013;110:13132–13137. doi: 10.1073/pnas.1304795110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheah MT, Wachter A, Sudarsan N, Breaker RR. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:391–393. doi: 10.1038/nature05769. [DOI] [PubMed] [Google Scholar]
- 63.Li S, Breaker RR. Eukaryotic TPP riboswitch regulation of alternative splicing involving long-distance base pairing. Nucleic Acids Res. 2013;41:3022–3031. doi: 10.1093/nar/gkt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Croft MT, Moulin M, Webb ME, Smith AG. Thiamine biosynthesis in algae is regulated by riboswitches. Proc Natl Acad Sci U S A. 2007;104:20770–20775. doi: 10.1073/pnas.0705786105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bocobza S, Adato A, Mandel T, Shapira M, Nudler E, Aharoni A. Riboswitch-dependent gene regulation and its evolution in the plant kingdom. Genes Dev. 2007;21:2874–2879. doi: 10.1101/gad.443907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wachter A, Tunc-Ozdemir M, Grove BC, Green PJ, Shintani DK, Breaker RR. Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant Cell. 2007;19:3437–3450. doi: 10.1105/tpc.107.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serganov A, Patel DJ. Metabolite recognition principles and molecular mechanisms underlying riboswitch function. Annu Rev Biophys. 2012;41:343–370. doi: 10.1146/annurev-biophys-101211-113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liberman JA, Salim M, Krucinska J, Wedekind JE. Structure of a class II preQ1 riboswitch reveals ligand recognition by a new fold. Nat Chem Biol. 2013;9:353–355. doi: 10.1038/nchembio.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenkins JL, Krucinska J, McCarty RM, Bandarian V, Wedekind JE. Comparison of a preQ1 riboswitch aptamer in metabolite-bound and free states with implications for gene regulation. J Biol Chem. 2011;286:24626–24637. doi: 10.1074/jbc.M111.230375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang M, Peterson R, Feigon J. Structural insights into riboswitch control of the biosynthesis of queuosine, a modified nucleotide found in the anticodon of tRNA. Mol Cell. 2009;33:784–790. doi: 10.1016/j.molcel.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 71.Klein DJ, Edwards TE, Ferre-D’Amare AR. Cocrystal structure of a class I preQ1 riboswitch reveals a pseudoknot recognizing an essential hypermodified nucleobase. Nat Struct Mol Biol. 2009;16:343–344. doi: 10.1038/nsmb.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spitale RC, Torelli AT, Krucinska J, Bandarian V, Wedekind JE. The structural basis for recognition of the PreQ0 metabolite by an unusually small riboswitch aptamer domain. J Biol Chem. 2009;284:11012–11016. doi: 10.1074/jbc.C900024200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serganov A, Yuan YR, Pikovskaya O, Polonskaia A, Malinina L, Phan AT, Hobartner C, Micura R, Breaker RR, Patel DJ. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem Biol. 2004;11:1729–1741. doi: 10.1016/j.chembiol.2004.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Batey RT, Gilbert SD, Montange RK. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature. 2004;432:411–415. doi: 10.1038/nature03037. [DOI] [PubMed] [Google Scholar]
- 75.Serganov A, Polonskaia A, Phan AT, Breaker RR, Patel DJ. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature. 2006;441:1167–1171. doi: 10.1038/nature04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thore S, Leibundgut M, Ban N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science. 2006;312:1208–1211. doi: 10.1126/science.1128451. [DOI] [PubMed] [Google Scholar]
- 77.Edwards TE, Ferre-D’Amare AR. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure. 2006;14:1459–1468. doi: 10.1016/j.str.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 78.Garst AD, Heroux A, Rambo RP, Batey RT. Crystal structure of the lysine riboswitch regulatory mRNA element. J Biol Chem. 2008;283:22347–22351. doi: 10.1074/jbc.C800120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serganov A, Huang L, Patel DJ. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature. 2008;455:1263–1267. doi: 10.1038/nature07326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serganov A, Huang L, Patel DJ. Coenzyme recognition and gene regulation by a flavin mononucleotide riboswitch. Nature. 2009;458:233–237. doi: 10.1038/nature07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serganov A. Determination of riboswitch structures: light at the end of the tunnel? RNA Biol. 2010;7:98–103. doi: 10.4161/rna.7.1.10756. [DOI] [PubMed] [Google Scholar]
- 82.Lescoute A, Westhof E. Topology of three-way junctions in folded RNAs. Rna. 2006;12:83–93. doi: 10.1261/rna.2208106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lescoute A, Westhof E. The interaction networks of structured RNAs. Nucleic Acids Res. 2006;34:6587–6604. doi: 10.1093/nar/gkl963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kulshina N, Baird NJ, Ferre-D’Amare AR. Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nat Struct Mol Biol. 2009;16:1212–1217. doi: 10.1038/nsmb.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith KD, Lipchock SV, Ames TD, Wang J, Breaker RR, Strobel SA. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat Struct Mol Biol. 2009;16:1218–1223. doi: 10.1038/nsmb.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Butler EB, Xiong Y, Wang J, Strobel SA. Structural basis of cooperative ligand binding by the glycine riboswitch. Chem Biol. 2011;18:293–298. doi: 10.1016/j.chembiol.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang L, Serganov A, Patel DJ. Structural insights into ligand recognition by a sensing domain of the cooperative glycine riboswitch. Mol Cell. 2010;40:774–786. doi: 10.1016/j.molcel.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pikovskaya O, Polonskaia A, Patel DJ, Serganov A. Structural principles of nucleoside selectivity in a 2′-deoxyguanosine riboswitch. Nat Chem Biol. 2011;7:748–755. doi: 10.1038/nchembio.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trausch JJ, Ceres P, Reyes FE, Batey RT. The structure of a tetrahydrofolate-sensing riboswitch reveals two ligand binding sites in a single aptamer. Structure. 2011;19:1413–1423. doi: 10.1016/j.str.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang L, Ishibe-Murakami S, Patel DJ, Serganov A. Long-range pseudoknot interactions dictate the regulatory response in the tetrahydrofolate riboswitch. Proc Natl Acad Sci U S A. 2011;108:14801–14806. doi: 10.1073/pnas.1111701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Montange RK, Batey RT. Structure of the S-adenosylmethionine riboswitch regulatory mRNA element. Nature. 2006;441:1172–1175. doi: 10.1038/nature04819. [DOI] [PubMed] [Google Scholar]
- 92.Smith KD, Shanahan CA, Moore EL, Simon AC, Strobel SA. Structural basis of differential ligand recognition by two classes of bis-(3′-5′)-cyclic dimeric guanosine monophosphate-binding riboswitches. Proc Natl Acad Sci U S A. 2011;108:7757–7762. doi: 10.1073/pnas.1018857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu C, Smith AM, Fuchs RT, Ding F, Rajashankar K, Henkin TM, Ke A. Crystal structures of the SAM-III/SMK riboswitch reveal the SAM-dependent translation inhibition mechanism. Nat Struct Mol Biol. 2008;15:1076–1083. doi: 10.1038/nsmb.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edwards AL, Reyes FE, Heroux A, Batey RT. Structural basis for recognition of S-adenosylhomocysteine by riboswitches. RNA. 2010;16:2144–2155. doi: 10.1261/rna.2341610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nissen P, Ippolito JA, Ban N, Moore PB, Steitz TA. RNA tertiary interactions in the large ribosomal subunit: the A-minor motif. Proc Natl Acad Sci U S A. 2001;98:4899–4903. doi: 10.1073/pnas.081082398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klein DJ, Schmeing TM, Moore PB, Steitz TA. The kink-turn: a new RNA secondary structure motif. EMBO J. 2001;20:4214–4221. doi: 10.1093/emboj/20.15.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peselis A, Serganov A. Structural insights into ligand binding and gene expression control by an adenosylcobalamin riboswitch. Nat Struct Mol Biol. 2012;19:1182–1184. doi: 10.1038/nsmb.2405. [DOI] [PubMed] [Google Scholar]
- 98.Johnson JE, Jr, Reyes FE, Polaski JT, Batey RT. B12 cofactors directly stabilize an mRNA regulatory switch. Nature. 2012;492:133–137. doi: 10.1038/nature11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagaswamy U, Fox GE. Frequent occurrence of the T-loop RNA folding motif in ribosomal RNAs. RNA. 2002;8:1112–1119. doi: 10.1017/s135583820202006x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krasilnikov AS, Mondragon A. On the occurrence of the T-loop RNA folding motif in large RNA molecules. RNA. 2008;9:640–643. doi: 10.1261/rna.2202703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jaeger L, Verzemnieks EJ, Geary C. The UA_handle: a versatile submotif in stable RNA architectures. Nucleic Acids Res. 2009;37:215–230. doi: 10.1093/nar/gkn911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vicens Q. RNA’s coming of age as a drug target. J Incl Phenom Macrocycl Chem. 2009;65:171–188. [Google Scholar]
- 103.Hermann T. Drugs targeting the ribosome. Curr Opin Struct Biol. 2005;15:355–366. doi: 10.1016/j.sbi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Y, Gregor VE, Sun Z, Ayida BK, Winters GC, Murphy D, Simonsen KB, Vourloumis D, Fish S, Froelich JM, Wall D, Hermann T. Structure-guided discovery of novel aminoglycoside mimetics as antibacterial translation inhibitors. Antimicrob Agents Chemother. 2005;49:4942–4949. doi: 10.1128/AAC.49.12.4942-4949.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hermann T, Tor Y. RNA as a target for small-molecule therapeutics. Expert Opin Ther patents. 2205;15:49–62. [Google Scholar]
- 106.Suess B, Fink B, Berens C, Stentz R, Hillen W. A theophylline responsive riboswitch based on helix slipping controls gene expression in vivo. Nucleic Acids Res. 2004;32:1610–1614. doi: 10.1093/nar/gkh321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suess B. Engineered riboswitches control gene expression by small molecules. Biochem Soc Trans. 2005;33:474–476. doi: 10.1042/BST0330474. [DOI] [PubMed] [Google Scholar]
- 108.Ceres P, Garst AD, Marcano-Velazquez JG, Batey RT. Modularity of select riboswitch expression platforms enables facile engineering of novel genetic regulatory devices. ACS Synth Biol. 2013;2:463–472. doi: 10.1021/sb4000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ceres P, Trausch JJ, Batey RT. Engineering modular ‘ON’ RNA switches using biological components. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sinha J, Reyes SJ, Gallivan JP. Reprogramming bacteria to seek and destroy an herbicide. Nat Chem Biol. 2010;6:464–470. doi: 10.1038/nchembio.369. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Vu MM, Jameson NE, Masuda SJ, Lin D, Larralde-Ridaura R, Luptak A. Convergent evolution of adenosine aptamers spanning bacterial, human, and random sequences revealed by structure-based bioinformatics and genomic SELEX. Chem Biol. 2012;19:1247–1254. doi: 10.1016/j.chembiol.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tomsic J, McDaniel BA, Grundy FJ, Henkin TM. Natural variability in S-adenosylmethionine (SAM)-dependent riboswitches: S-box elements in bacillus subtilis exhibit differential sensitivity to SAM In vivo and in vitro. Journal of bacteriology. 2008;190:823–833. doi: 10.1128/JB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sudarsan N, Hammond MC, Block KF, Welz R, Barrick JE, Roth A, Breaker RR. Tandem riboswitch architectures exhibit complex gene control functions. Science. 2006;314:300–304. doi: 10.1126/science.1130716. [DOI] [PubMed] [Google Scholar]
- 114.Watson PY, Fedor MJ. The glmS riboswitch integrates signals from activating and inhibitory metabolites in vivo. Nat Struct Mol Biol. 2011;18:359–363. doi: 10.1038/nsmb.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garst AD, Porter EB, Batey RT. Insights into the regulatory landscape of the lysine riboswitch. J Mol Biol. 2012;423:17–33. doi: 10.1016/j.jmb.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daldrop P, Reyes FE, Robinson DA, Hammond CM, Lilley DM, Batey RT, Brenk R. Novel ligands for a purine riboswitch discovered by RNA-ligand docking. Chem Biol. 2011;18:324–335. doi: 10.1016/j.chembiol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edwards AL, Batey RT. A structural basis for the recognition of 2′-deoxyguanosine by the purine riboswitch. J Mol Biol. 2009;385:938–948. doi: 10.1016/j.jmb.2008.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ren A, Rajashankar KR, Patel DJ. Fluoride ion encapsulation by Mg2+ ions and phosphates in a fluoride riboswitch. Nature. 2012;486:85–89. doi: 10.1038/nature11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burley SK, Petsko GA. Weakly polar interactions in proteins. Adv Protein Chem. 1988;39:125–189. doi: 10.1016/s0065-3233(08)60376-9. [DOI] [PubMed] [Google Scholar]
- 120.Lu C, Ding F, Chowdhury A, Pradhan V, Tomsic J, Holmes WM, Henkin TM, Ke A. SAM recognition and conformational switching mechanism in the Bacillus subtilis yitJ S box/SAM-I riboswitch. J Mol Biol. 2010;404:803–818. doi: 10.1016/j.jmb.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brandl M, Weiss MS, Jabs A, Suhnel J, Hilgenfeld R. C-H...pi-interactions in proteins. J Mol Biol. 2001;307:357–377. doi: 10.1006/jmbi.2000.4473. [DOI] [PubMed] [Google Scholar]
- 122.Muraki M. The importance of CH/pi interactions to the function of carbohydrate binding proteins. Protein Pept Lett. 2002;9:195–209. doi: 10.2174/0929866023408751. [DOI] [PubMed] [Google Scholar]
- 123.Auffinger P, Bielecki L, Westhof E. Anion binding to nucleic acids. Structure. 2004;12:379–388. doi: 10.1016/j.str.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 124.Wickiser JK, Winkler WC, Breaker RR, Crothers DM. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol Cell. 2005;18:49–60. doi: 10.1016/j.molcel.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 125.Lemay JF, Desnoyers G, Blouin S, Heppell B, Bastet L, St-Pierre P, Masse E, Lafontaine DA. Comparative study between transcriptionally- and translationally-acting adenine riboswitches reveals key differences in riboswitch regulatory mechanisms. PLoS Genet. 2011;7:e1001278. doi: 10.1371/journal.pgen.1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suddala KC, Rinaldi AJ, Feng J, Mustoe AM, Eichhorn CD, Liberman JA, Wedekind JE, Al-Hashimi HM, Brooks CL, 3rd, Walter NG. Single transcriptional and translational preQ1 riboswitches adopt similar pre-folded ensembles that follow distinct folding pathways into the same ligand-bound structure. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haller A, Souliere MF, Micura R. The dynamic nature of RNA as key to understanding riboswitch mechanisms. Acc Chem Res. 2011 doi: 10.1021/ar200035g. [DOI] [PubMed] [Google Scholar]
- 128.Smith AM, Fuchs RT, Grundy FJ, Henkin TM. The SAM-responsive SMK box is a reversible riboswitch. Mol Microbiol. 2010;78:1393–1402. doi: 10.1111/j.1365-2958.2010.07410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liberman JA, Wedekind JE. Riboswitch structure in the ligand-free state. Wiley Interdiscip Rev RNA. 2012;3:369–384. doi: 10.1002/wrna.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vicens Q, Mondragon E, Batey RT. Molecular sensing by the aptamer domain of the FMN riboswitch: a general model for ligand binding by conformational selection. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heppell B, Blouin S, Dussault AM, Mulhbacher J, Ennifar E, Penedo JC, Lafontaine DA. Molecular insights into the ligand-controlled organization of the SAM-I riboswitch. Nat Chem Biol. 2011;7:384–392. doi: 10.1038/nchembio.563. [DOI] [PubMed] [Google Scholar]
- 132.Lemay JF, Penedo JC, Tremblay R, Lilley DM, Lafontaine DA. Folding of the adenine riboswitch. Chem Biol. 2006;13:857–868. doi: 10.1016/j.chembiol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 133.Lang K, Rieder R, Micura R. Ligand-induced folding of the thiM TPP riboswitch investigated by a structure-based fluorescence spectroscopic approach. Nucleic Acids Res. 2007;35:5370–5378. doi: 10.1093/nar/gkm580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rieder U, Kreutz C, Micura R. Folding of a transcriptionally acting preQ1 riboswitch. Proc Natl Acad Sci U S A. 2010;107:10804–10809. doi: 10.1073/pnas.0914925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stoddard CD, Montange RK, Hennelly SP, Rambo RP, Sanbonmatsu KY, Batey RT. Free state conformational sampling of the SAM-I riboswitch aptamer domain. Structure. 2010;18:787–797. doi: 10.1016/j.str.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haller A, Altman RB, Souliere MF, Blanchard SC, Micura R. Folding and ligand recognition of the TPP riboswitch aptamer at single-molecule resolution. Proc Natl Acad Sci U S A. 2013;110:4188–4193. doi: 10.1073/pnas.1218062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Souliere MF, Altman RB, Schwarz V, Haller A, Blanchard SC, Micura R. Tuning a riboswitch response through structural extension of a pseudoknot. Proc Natl Acad Sci U S A. 2013;110:E3256–3264. doi: 10.1073/pnas.1304585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boehr DD, Nussinov R, Wright PE. The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol. 2009;5:789–796. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Serganov A, Patel DJ. Towards deciphering the principles underlying an mRNA recognition code. Curr Opin Struct Biol. 2008;18:120–129. doi: 10.1016/j.sbi.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Serganov A. The long and the short of riboswitches. Curr Opin Struct Biol. 2009;19:251–259. doi: 10.1016/j.sbi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Serganov A, Patel DJ. Molecular recognition and function of riboswitches. Curr Opin Struct Biol. 2012;22:279–286. doi: 10.1016/j.sbi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cruz JA, Blanchet MF, Boniecki M, Bujnicki JM, Chen SJ, Cao S, Das R, Ding F, Dokholyan NV, Flores SC, Huang L, Lavender CA, Lisi V, Major F, Mikolajczak K, Patel DJ, Philips A, Puton T, Santalucia J, Sijenyi F, Hermann T, Rother K, Rother M, Serganov A, Skorupski M, Soltysinski T, Sripakdeevong P, Tuszynska I, Weeks KM, Waldsich C, Wildauer M, Leontis NB, Westhof E. RNA-Puzzles: a CASP-like evaluation of RNA three-dimensional structure prediction. RNA. 2012;18:610–625. doi: 10.1261/rna.031054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Al-Hashimi HM, Walter NG. RNA dynamics: it is about time. Curr Opin Struct Biol. 2008;18:321–329. doi: 10.1016/j.sbi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Greenleaf WJ, Frieda KL, Foster DA, Woodside MT, Block SM. Direct observation of hierarchical folding in single riboswitch aptamers. Science. 2008;319:630–633. doi: 10.1126/science.1151298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lipfert J, Das R, Chu VB, Kudaravalli M, Boyd N, Herschlag D, Doniach S. Structural transitions and thermodynamics of a glycine-dependent riboswitch from Vibrio cholerae. J Mol Biol. 2007;365:1393–1406. doi: 10.1016/j.jmb.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baird NJ, Ferre-D’Amare AR. Idiosyncratically tuned switching behavior of riboswitch aptamer domains revealed by comparative small-angle X-ray scattering analysis. RNA. 2010;16:598–609. doi: 10.1261/rna.1852310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feng J, Walter NG, Brooks CL., 3rd Cooperative and directional folding of the preQ1 riboswitch aptamer domain. J Am Chem Soc. 2011;133:4196–4199. doi: 10.1021/ja110411m. [DOI] [PMC free article] [PubMed] [Google Scholar]