Abstract
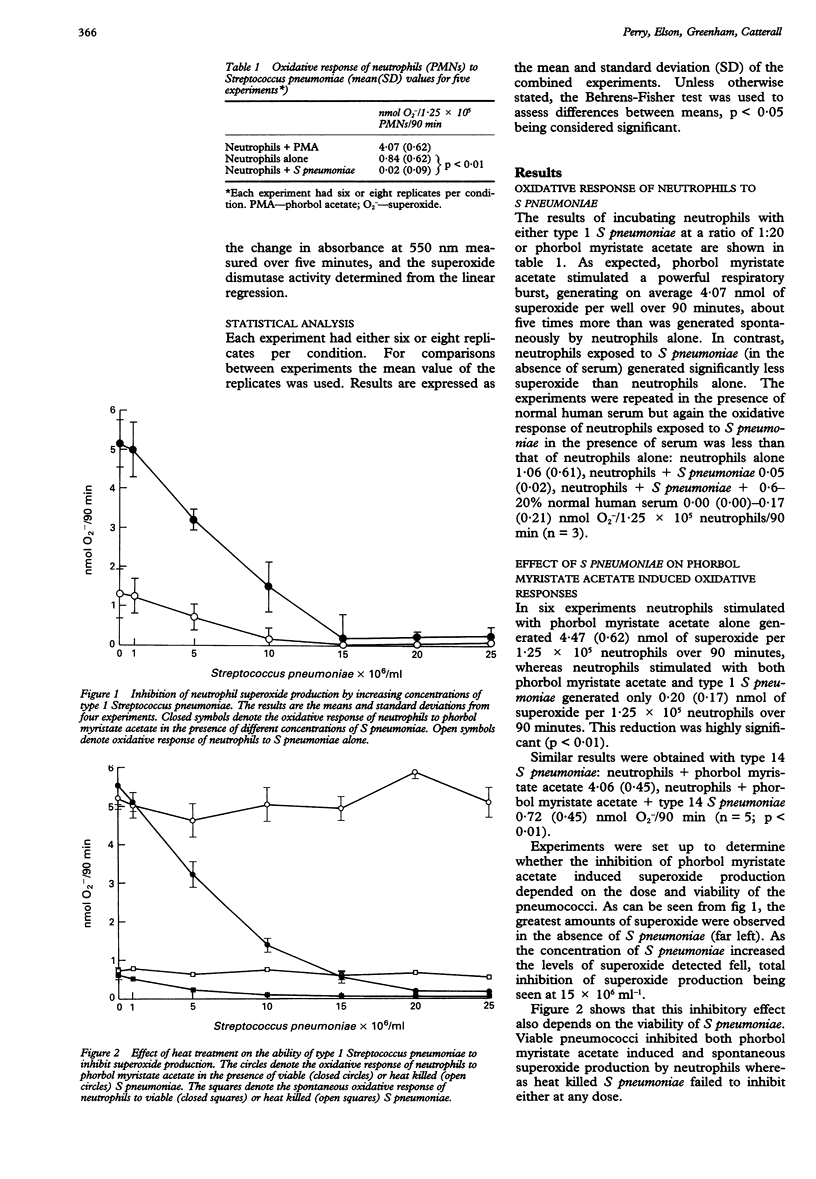
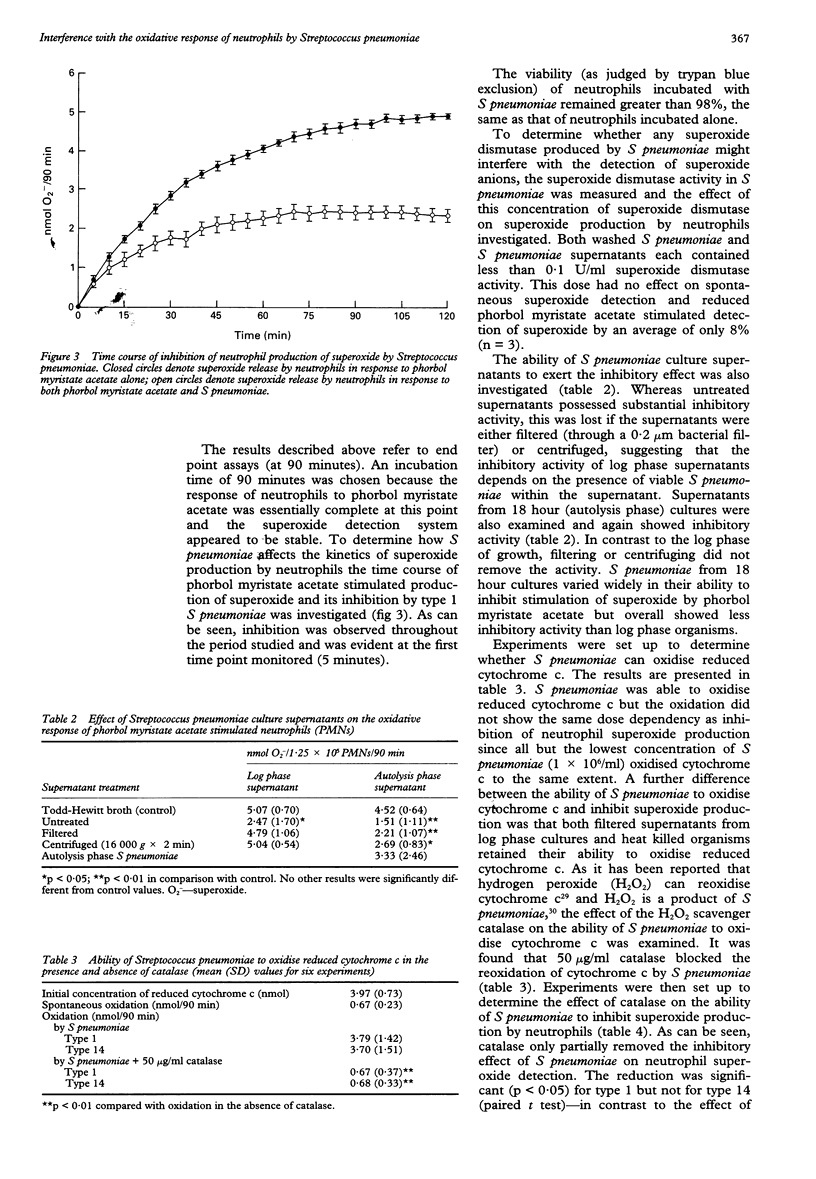
BACKGROUND--Pneumococcal infections are still a major clinical problem. Polymorphonuclear leucocytes (neutrophils) are considered to have a key role in the host's defence against Streptococcus pneumoniae but the mechanisms by which they kill the pneumococcus remain unclear. As reactive oxygen species are regarded as a major antimicrobial defence of phagocytes an attempt has been made to establish their role in the response of neutrophils to S pneumoniae. METHODS--S pneumoniae isolated from patients with bacteraemic pneumococcal pneumonia were incubated with neutrophils in suspension and superoxide production was measured by reduction of ferricytochrome c. RESULTS--S pneumoniae did not stimulate superoxide production alone or in the presence of normal human serum. Spontaneous superoxide production by neutrophils was actually abrogated by S pneumoniae, as was the powerful respiratory burst stimulated by phorbol myristate acetate. This phenomenon depended on both the dose and the viability of the bacteria. With S pneumoniae in the logarithmic phase of growth inhibitory activity was confined to the organisms themselves but with organisms undergoing autolysis it was also present in filtered supernatants, suggesting that the inhibitory activity can be attributed to a factor released during autolysis. CONCLUSIONS--S pneumoniae can interfere with the respiratory burst of neutrophils. This property may help to explain the pathogenicity of the organism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Casal J., Fenoll A., Vicioso M. D., Muñoz R. Increase in resistance to penicillin in pneumococci in Spain. Lancet. 1989 Apr 1;1(8640):735–735. doi: 10.1016/s0140-6736(89)92260-5. [DOI] [PubMed] [Google Scholar]
- Charnetzky W. T., Shuford W. W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985 Jan;47(1):234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier J. F. Oxidant-antioxidant balance. Bull Eur Physiopathol Respir. 1987 Jul-Aug;23(4):273–274. [PubMed] [Google Scholar]
- Donowitz G. R., Reardon I., Dowling J., Rubin L., Focht D. Ingestion of Legionella micdadei inhibits human neutrophil function. Infect Immun. 1990 Oct;58(10):3307–3311. doi: 10.1128/iai.58.10.3307-3311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley D. C., Simpson J. F., Meryman H. T. Isolation of large numbers of fully viable human neutrophils: a preparative technique using percoll density gradient centrifugation. Exp Hematol. 1982 Aug;10(7):591–599. [PubMed] [Google Scholar]
- Ezekowitz R. A., Dinauer M. C., Jaffe H. S., Orkin S. H., Newburger P. E. Partial correction of the phagocyte defect in patients with X-linked chronic granulomatous disease by subcutaneous interferon gamma. N Engl J Med. 1988 Jul 21;319(3):146–151. doi: 10.1056/NEJM198807213190305. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Kirk J. L., Schiffman G., Schweinle J. E., Guckian J. C. Analysis of humoral and phagocytic defenses against Streptococcus pneumoniae serotypes 1 and 3. J Lab Clin Med. 1988 Oct;112(4):487–497. [PubMed] [Google Scholar]
- Gillespie S. H. Aspects of pneumococcal infection including bacterial virulence, host response and vaccination. J Med Microbiol. 1989 Apr;28(4):237–248. doi: 10.1099/00222615-28-4-237. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. P., Koornhof H. J. Worldwide increase in pneumococcal antibiotic resistance. Lancet. 1989 Aug 19;2(8660):444–444. doi: 10.1016/s0140-6736(89)90617-x. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penicillin-resistant strains of Neisseria meningitidis in Spain. Lancet. 1988 Jun 25;1(8600):1452–1453. [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Ridgway E. J., Allen K. D. Penicillin resistance in pneumococci. J Antimicrob Chemother. 1991 Feb;27(2):251–252. doi: 10.1093/jac/27.2.251. [DOI] [PubMed] [Google Scholar]
- Robertson M. D., Seaton A., Milne L. J., Raeburn J. A. Suppression of host defences by Aspergillus fumigatus. Thorax. 1987 Jan;42(1):19–25. doi: 10.1136/thx.42.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Shapiro E. D., Berg A. T., Austrian R., Schroeder D., Parcells V., Margolis A., Adair R. K., Clemens J. D. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991 Nov 21;325(21):1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- Vandewalle P. L., Petersen N. O. Oxidation of reduced cytochrome c by hydrogen peroxide. Implications for superoxide assays. FEBS Lett. 1987 Jan 5;210(2):195–198. doi: 10.1016/0014-5793(87)81336-4. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Sword C. P., Brehm S., Dusanic D. Relationship between superoxide dismutase and pathogenic mechanisms of Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):863–872. doi: 10.1128/iai.23.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A. Complement and the host's defense against the pneumococcus. Crit Rev Microbiol. 1984;11(3):187–208. doi: 10.3109/10408418409105903. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Swift A. J. Host defense against the pneumococcus in T-lymphocyte-deficient, nude mice. Infect Immun. 1975 Nov;12(5):1222–1223. doi: 10.1128/iai.12.5.1222-1223.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. E., Abegg A. L., Travis S. J., Kobayashi G. S., Little J. R. Effects of Histoplasma capsulatum on murine macrophage functions: inhibition of macrophage priming, oxidative burst, and antifungal activities. Infect Immun. 1989 Feb;57(2):513–519. doi: 10.1128/iai.57.2.513-519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


