Abstract
Therapeutic vaccines for nicotine addiction show pre-clinical efficacy. Yet, clinical evaluation of the first-generation nicotine vaccines did not meet expectations because only a subset of immunized subjects achieved effective serum antibody levels. Recent studies suggest that vaccine design affects B cell activation, and that the frequency of the hapten-specific B cell subsets contributes to vaccine efficacy against drugs of abuse. To extend this hypothesis to nicotine immunogens, we synthesized a novel hapten containing a carboxymethylureido group at the 2-position of the nicotine structure (2CMUNic) and compared its efficacy to the previously characterized 6CMUNic hapten. Haptens were conjugated to the keyhole limpet hemocyanin (KLH) carrier protein, and evaluated for efficacy against nicotine in mice using the clinically approved alum adjuvant. Using a novel fluorescent antigen-based magnetic enrichment strategy paired with multicolor flow cytometry analysis, polyclonal hapten-specific B cell subsets were measured in mice immunized with either 6CMUNic-KLH or 2CMUNic-KLH. The 6CMUNic-KLH showed significantly greater efficacy than 2CMUNic-KLH on nicotine distribution to serum and to the brain. The 6CMUNic-KLH elicited higher anti-nicotine serum antibody titers, and a greater frequency of hapten-specific B cells than 2CMUNic-KLH. Within the splenic polyclonal B cell population, a higher number of hapten-specific IgMhigh and germinal center B cells predicted greater vaccine efficacy against nicotine distribution. These early pre-clinical findings suggest that hapten structure affects activation of B cells, and that variations in the frequency of early-activated hapten-specific B cell subsets underlie individual differences in vaccine efficacy.
Keywords: antigen-specific B cells, nicotine, addiction, vaccines, biomarkers
1. INTRODUCTION
Tobacco use is one of the leading preventable causes of mortality, killing ~6 million people annually worldwide [1]. Each year, tobacco is responsible for 80–90% of deaths due to lung cancer and lower tract respiratory diseases and accounts for ~500,000 deaths in the United States [2]. Current treatments include counseling, nicotine replacement therapy, and pharmacotherapy consisting of the atypical antidepressant bupropion and the partial antagonist varenicline [3]. These treatments, although valuable, are limited by their sub-optimal clinical efficacy, patient’s acceptance and/or compliance, and concerns for side effects [3].
Immunotherapy has been studied as an alternative approach to curb tobacco use by targeting nicotine rather than the nicotine receptors in the brain [4]. Active immunization with immunogens containing nicotine-based haptens generates nicotine-specific antibodies that bind nicotine in serum, decreasing the amount of free nicotine that crosses the blood-brain barrier, thus limiting drug distribution to the brain and nicotine rewarding effects. Clinical evaluation of vaccines for substance use disorders has shown safety and proof of concept [5–9]. Yet, clinical efficacy has been shown only in the subset (~30%) of immunized subjects that achieved the highest serum antibody levels against the target drug [6,9]. Clinical data suggest that translation of nicotine vaccines will depend upon immunized subjects consistently reaching serum IgG antibody concentrations > 40μg/ml [4,10].
The immunological mechanisms underlying individual antibody responses to vaccines for substance use disorders are poorly understood. It has been proposed that addiction vaccines activate T cell-dependent B cell processes to produce drug-specific antibodies [4,11]. Immunization activates naïve antigen-specific B cells, which, in the presence of antigen-specific CD4+ T cells, will form germinal centers (GC) within the lymph nodes and spleen to generate memory B cells and antibody-secreting B cells, which produce antigen-specific antibodies [12–16]. In the GC, antigen-specific B cells go through clonal expansion, somatic hypermutation, and affinity-based selection [14,15,17,18]. These complex processes contribute to B cell heterogeneity and variability, and generation of high-affinity antibodies [14,15,17,18]. The size of the antigen-specific B and T cell populations varies greatly before and after immunization in individual mice [16,19–29], and in human subjects [30,31]. Using a cutting-edge antigen-based magnetic enrichment strategy paired to flow cytometry, we found that the frequency of the polyclonal naïve and early-activated hapten-specific B cell population, before and after immunization, correlated to the magnitude of the post-vaccination antibody response and vaccine efficacy against oxycodone in mice [11,32,33]. The size of the carrier-specific CD4+ T cell population also correlated to individual vaccine efficacy against oxycodone in mice [11]. These findings support the hypothesis that variability in the frequency of antigen-specific B and T cell subsets within the naïve or activated B and T cell repertoire underlies individual antigen-specific antibody responses and vaccine efficacy.
This study tested the extent to which the size of the early-activated hapten-specific B cell subsets predicts vaccine efficacy against nicotine by comparing two immunogens containing structurally-related nicotine-based haptens, containing the same linker attached at the 2- and 6-position of nicotine. Results showed that greater frequency of polyclonal hapten-specific B cells in the spleen soon after immunization correlated to greater vaccine efficacy against nicotine. These data show that hapten structure determines the extent of B cell activation, and that the initial size of the hapten-specific B cell response shortly after immunization is critical for subsequent individual vaccine efficacy.
2. MATERIAL AND METHODS
2.1 Ethics
Animal studies conformed to the U.S Department of Health and Human Services Guide for the Care and Use of Laboratory Animals, and the Minneapolis Medical Research Foundation Animal Care and Use Committee.
2.2 Drugs
Nicotine bitartrate was obtained through Sigma (St. Louis, MO). Drug doses and concentrations are expressed as the weight of the base, and measured as previously reported [34].
2.3 Synthesis of nicotine haptens
2.3.1 6CMUNic hapten
The 6CMUNic hapten was synthesized by attaching a carboxylmethylureido group at the 6-position of the racemic nicotine, and provided as a lithium salt as previously reported [34].
2.3.2 Two-step synthesis of the 2CMUNic hapten
A) Ethyl 2-({[3-(1-methylpyrrolidin-2-yl)pyridin-2-yl]carbamoyl}amino)acetate (base). Ethyl isocyanoacetate (0.17 g, 1.3 mmol) was added to a solution of 3-(1-methylpyrrolidin-2-yl)pyridine-2-amine (0.150 g, 0.85 mmol) in THF (20 ml) at 0 C [34]. The solution was allowed to come to room temperature while stirring was continued for an additional 2 hours. The solution was concentrated under reduced pressure and 1.0 M HCl (25 ml) was added. The suspension was extracted with CH2Cl2 (3 × 30 ml) and the aqueous layer was made basic with sat. NaHCO3. The aqueous layer was then extracted with CH2Cl2 (3 × 30 ml) and resulting organic layers were combined, dried (MgSO4), and concentrated to provide an oil. The oil was then purified using medium pressure column chromatography on silica using a mixture of CHCl3, MeOH, NH4OH in a ratio of 8:1.8:0.2 to afford ethyl 2-({[3-(1-methylpyrrolidin-2-yl)pyridin-2-yl]carbamoyl}amino)acetate (0.24 g, 92%) as a pale yellow oil. 1H NMR (300 MHz,CDCl3) δ ppm 1.26 – 1.32 (m, 3 H), 1.97 – 2.04 (m, 4 H), 2.21 – 2.28 (m, 4 H), 3.17 (dd J = XHz, 1 H), 3.17 (m, 1H) 3.34 (m, 1H), 4.17 – 4.25 (m, 4 H), 6.81 (dd, J=6, 9 Hz, 1 H), 7.36 (d, J=6 Hz, 1 H), 8.11 (d, J=6 Hz, 1 H), 10.10 (brs, 1 H), 10.69 (s, 1 H). ESI MS m/z: calculated for C15H22N4O3 306.36, Found 307.4 (M+H)+.
B) Lithium 2-({[3-(1-methylpyrrolidin-2-yl)pyridin-2-yl]carbamoyl}amino)acetate (salt). Lithium hydroxide monohydrate (0.33 g, 0.78 mmol) was added to a solution of ethyl 2-({[3-(1-methylpyrrolidin-2-yl)pyridin-2-yl]carbamoyl}amino)acetate (0.24 g, 0.78 mmol) in MeOH/THF/H2O (1:1:1, 10 ml) at 0°C. The solution was allowed to stir at 0°C until mass spectrometry analysis indicated no starting material remained (6 h). The resulting solution was concentrated using a stream of N2 and the solid was triturated with Et2O (2 × 10 ml). The solid was dried under vacuum to afford lithio 2-({[3-(1-methylpyrrolidin-2-yl)pyridin-2-yl]carbamoyl}amino)acetate (0.21 g, 94%) as a pale yellow solid. 1H NMR (300 MHz, MeOH d3) δ ppm 1.74 – 2.37 (m, 9 H), 3.31 (m, 1 H), 3.92 (s, 2 H), 6.88 (dd, J=3, 6 Hz, 1 H), 7.49 (d, J=3 Hz, 1 H), 8.17 (d, J=3 Hz, 1 H). ESI MS m/z: calculated for C13H17LiN4O3 284.24, Found 283.3 (M-H)-.
2.4 Conjugation to proteins
For ELISA and immunization studies, the 6CMUNic and 2CMUNic haptens were conjugated by carbodiimide (EDAC) chemistry to BSA, OVA (Sigma, St. Louis, MO), and KLH (Thermo Fisher, Rockford, IL) [34]. For B cell analysis, the 6CMUNic and 2CMUNic haptens were also conjugated by EDAC to the fluorescent protein phycoerythrin (PE, Prozyme, Hayward, CA) and designated as 6CMUNic-PE or 2CMUNic-PE. PE was conjugated to the fluorescent dye Alexa Fluor 647 (AF647, Life Technologies, Grand Island, NY), and conjugation verified by UV spectrometry [33]. The immunogens’ haptenization ratio (i.e., number of moles of hapten attached per mole of carrier protein) is calculated from the difference in molecular weight between the conjugated and unconjugated carrier protein measured by MALDI-TOF. Conjugation conditions resulted in a haptenization ratio >20:1 for the 6CMUNic-BSA conjugate [34], and 17 for both 2CMUNic-BSA and 2CMUNic-OVA. Due to their larger size, we did not verify haptenization ratio for haptens conjugated to either KLH (5–8 million Da), or PE (240,000 Da).
2.5 Evaluation of vaccine efficacy in mice
Naïve male BALB/c mice (Harlan Laboratories, Madison, WI) were housed with a 12hr light /12 hr dark cycle, and fed ad libitum. Mice were immunized s.c. on the scruff of the neck, on days 0, 14 and 28 with 25 μg of either 6CMUNic-KLH, 2CMUNic-KLH or unconjugated KLH absorbed to alum adjuvant (Alhydrogel85, Brenntag Biosector, Frederikssund, Denmark) in a final volume of 0.2 ml. Hapten-specific B cells were analyzed by means of spleen biopsy 14 days after the first immunization, as described [11]. Partial splenectomy does not interfere with the efficacy of addiction vaccines in mice [11]. A week after the last immunization, on day 35, mice were injected with 0.03 mg/kg nicotine i.v. in the tail vein, and at T=5′ euthanized to collect serum and brain for nicotine concentrations.
2.6 Nicotine-specific serum antibody titers
Serum IgG antibody titers were measured by ELISA using 6CMUNic-OVA and 2CMUNic-OVA as coating immunogens [34].
2.7 Hapten-specific B cell analysis
Spleen biopsy samples were processed as described [11]. Samples were incubated first with a decoy reagent consisting of PE-AF647, followed by incubation with either 6CMUNic-PE or 2CMUNic-PE, as described [11,33]. B cells bound either to the control reagent PE-AF647 or the hapten-PE conjugates were enriched by use of anti-PE mAb-conjugated magnetic beads and magnetic columns as previously described [11,33]. After enrichment, samples were stained with fluorochrome-labeled anti-mouse mAbs for B cell and non-B cell markers [11,33]. Using a flow cytometer, 6CMUNic- or 2CMUNic-specific B cells were characterized as Ighigh antibody-secreting cells (ASC), GL7high GC B cells, IgMhigh B cells, and switched immunoglobulin (swIg) B cells. The number of total lymphocytes, total B cells, 6CMUNic-specific B cells, and 2CMUNic-specific B cells was calculated for each sample using fluorescent counting beads [11,33]. Analysis of 6CMUNic- and 2CMUNic-specific B cell populations was performed on a 4-laser (405 nm, 488 nm, 561 nm, 640 nm) LSRFortessa using FACSDiva 7.0 (BD Biosciences), and processed with FlowJo 5.0 (Tree Star, Ashland, OR). This study was performed in mice because this B cell analysis strategy was recently developed in this species [25]. Notably, while it is not yet possible to perform such sensitive analysis in rats due to lack of flow cytometry reagents, we have previously analyzed hapten-specific B cells in BALB/c, C57Bl/6, and other mouse strains [11,32,35].
2.8 Statistical analysis
The mean nicotine serum and brain concentrations, and the number of hapten-specific B cells were compared by either ANOVA followed by Bonferroni post hoc across groups, or by unpaired T tests between groups. The relationship between the number of hapten-specific B cells at 14 days after vaccination and subsequent parameters of vaccine efficacy was analyzed by linear regression after performing the D’Agostino and Pearson omnibus normality test. Analyses were performed using Graph Pad 6.0 (La Jolla, CA).
3. RESULTS
3.1 Hapten synthesis
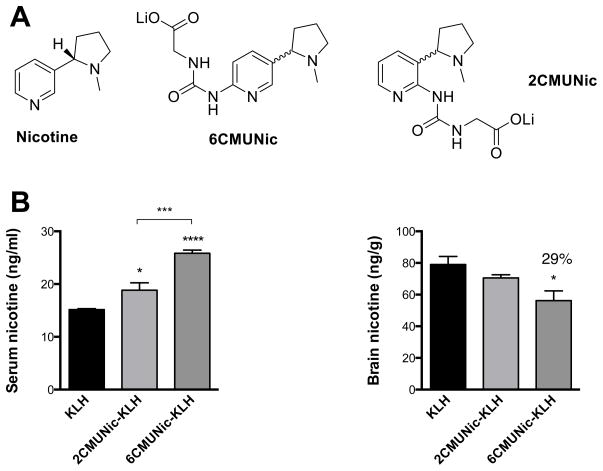
The new 2CMUNic hapten was synthesized with a two-step yield of 86% and overall purity of >95% by NMR analysis. The 2CMUNic hapten was compared to the previously characterized 6CMUNic (Figure 1A).
Figure 1. Hapten structure contributes to vaccine efficacy against nicotine.
A) The carboxymethylureido modification was attached to either the 6- or the 2-position of nicotine. B) BALB/c mice were immunized s.c. with 25 μg of either unconjugated KLH, 6CMUNic-KLH or 2CMUNic-KLH adsorbed on alum adjuvant, and administered on days 0, 14 and 21. Vaccine efficacy was evaluated 7–10 days after the last immunization. Immunized mice were challenged with 0.03 mg/kg i.v. nicotine. Data are mean±SEM, n=6 each group. Statistical symbols: *p<0.05, ***p<0.001, ****p<0.0001.
3.2 Evaluation of vaccine efficacy in vivo
Subcutaneous immunization of BALB/c mice with the 6CMUNic-KLH in alum adjuvant increased nicotine serum concentrations compared to the unconjugated KLH control group and mice immunized with the novel 2CMUNic-KLH immunogen (Fig. 1B). Consistently, immunization with 6CMUNic-KLH showed greater efficacy in reducing distribution of nicotine to the brain compared to mice immunized with either KLH or 2CMUNic-KLH. Similar results were obtained in a preliminary study performed in Holtzman rats (Supplemental material).
3.3 Analysis of hapten-specific B cells in immunized mice
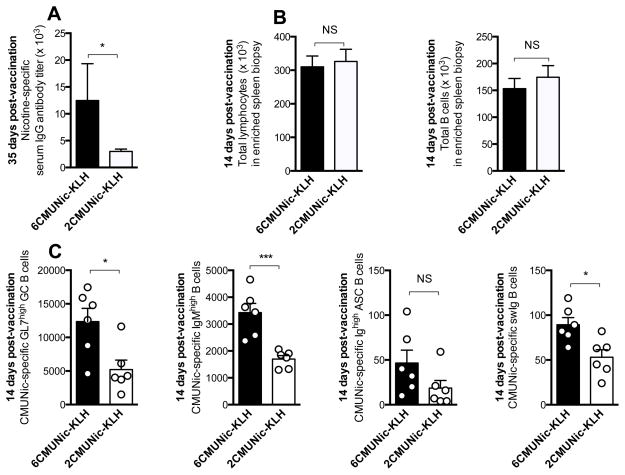
In mice, 6CMUNic-KLH induced greater CMUNic-specific serum IgG antibody titers than 2CMUNic-KLH at 7 days after the last immunization (Fig. 2A). Characterization of hapten-specific serum IgG antibody titers or concentrations by ELISA, and antibody affinity for nicotine by competitive binding ELISA, protein binding, or other similar assays, are effective strategies to evaluate successful immunization [34,36–38]. Yet, in clinical trials of nicotine vaccines, characterization of nicotine-specific serum antibodies relies on the actual presence of antibodies in plasma, which may appear weeks or months after immunization. We posit that earlier immune correlates predictive of vaccine efficacy may speed evaluation of candidate vaccines, and therefore we have analyzed the early-activated B cell subsets 14 days after immunization.
Figure 2. 6CMUNic-KLH elicits greater hapten-specific serum IgG antibody titers and splenic polyclonal hapten-specific B cells in mice.
A) 6CMUNic-KLH elicited higher serum IgG antibody titers than 2CMUNic-KLH, measured at 35 days, a week after the last immunization. The reported p value is based on analysis of log-transformed titers to meet normality requirements. In these mice, B cell analysis was performed by means of splenectomy 14 days after immunization. B) The total number of lymphocytes and B cells was not different in the spleen fragments from mice immunized with either 6CMUNic-KLH or 2CMUNic-KLH. C) Immunization with 6CMUNic-KLH was more effective in inducing GC, IgMhigh, and ASC and swIg hapten-specific B cells. Shown the number of hapten-specific B cells in individual spleen fragments after enrichment. Although B cell analysis was performed using either the 6CMUNic-PE or the 2CMUNic-PE enrichment reagents for each respective immunization group, hapten-specific B cells are reported as CMUNic-specific B cells. Data are mean±SEM, n=6 each group. Statistical symbols: *p<0.05, ***p<0.001, NS= not significant.
Soon after immunization, the 6CMUNic-KLH was more effective in stimulation of CMUNic-specific B cell subsets than the 2CMUNic-KLH. Spleen biopsy samples from mice immunized with either 6CMUNic-KLH or 2CMUNic-KLH were enriched using either the 6CMUNic-PE or 2CMUNic-PE, and the PE-AF647 decoy reagent. Bound B cells were isolated by magnetic enrichment using anti-PE magnetic microbeads and magnetic fields, and then characterized for B cell markers as previously described for oxycodone haptens [33]. The enrichment procedure was not biased toward either hapten, because the number of lymphocytes and total B cells in the spleen biopsy fragments did not differ between samples enriched with 6CMUNic-PE versus 2CMUNic-PE (Fig. 2B). However, analysis of the polyclonal early-activated hapten-specific B cell population revealed that 6CMUNic-KLH was more effective in inducing 6CMUNic-specific B cells, than 2CMUNic-specific B cells in mice immunized with 2CMUNic-KLH (Fig. 2C). Immunization with 6CMUNic-KLH induced a greater expansion of hapten-specific B cells within the GC, IgMhigh, ASC and swIg subsets compared to the less effective 2CMUNic-KLH. No differences in variance were found between groups.
3.3. The frequency of early-activated hapten-specific B cells correlate to subsequent vaccine efficacy against nicotine distribution to serum and brain
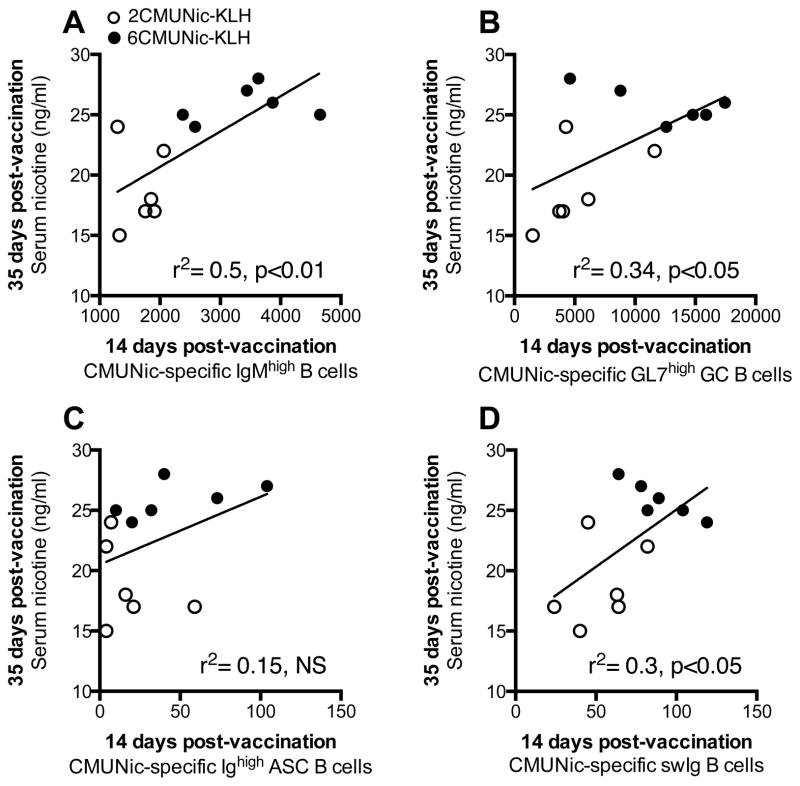
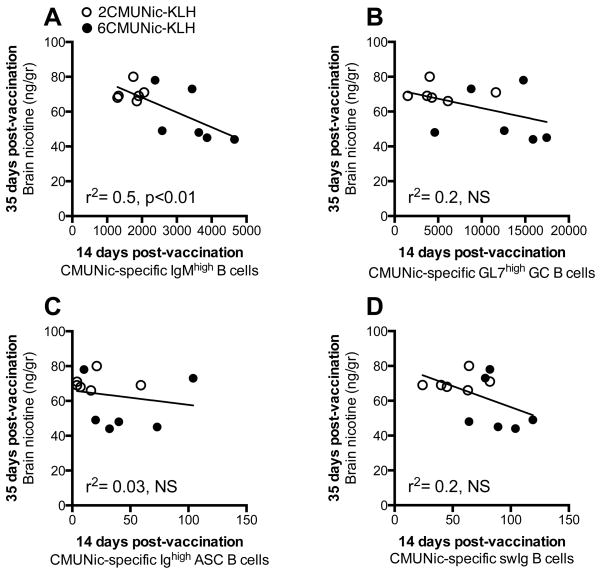
In mice, we evaluated which of the early-activated hapten-specific B cell subsets would best predict vaccine efficacy. An increasing number of CMUNic-specific IgMhigh, GC and swIg B cells 14 days after immunization in spleen correlated to greater subsequent effect on nicotine serum distribution in immunized mice (Fig. 3). However, the number of CMUNic-specific ASC B cells did not correlate to the effect of vaccination on nicotine serum distribution (Figure 3C). Among all B cell subsets, only the number of hapten-specific IgMhigh B cells correlated to subsequent vaccine efficacy in blocking nicotine distribution to the brain (Figure 4). These data indicated that within the polyclonal hapten-specific B cell population, the most reliable predictors of vaccine efficacy were the early-activated hapten-specific IgMhigh and the GC B cell subsets because these subsets correlated with two parameters of vaccine efficacy (e.g., serum IgG titers, or nicotine concentration), while the swIg and ASC B cells correlated to only one or no parameter, respectively (Table I).
Figure 3. The number of splenic early-activated CMUNic-specific B cells correlates to subsequent vaccine efficacy against nicotine serum distribution.<.
br>A greater number of hapten-specific B cells in spleen biopsies 14 days after immunization correlated to increased nicotine serum concentrations in immunized mice. Panels include mice immunized with 6CMUNic-KLH and 2CMUNic-KLH, as labeled (n=6 each group). Individual serum nicotine concentrations were significantly correlated to A) CMUNic-specific IgMhigh B cells, B) CMUNic-specific GC B cells, and D) CMUNic-specific swIgB cells, but not C) CMUNic-specific ASC B cells.
Figure 4. The number of splenic early-activated CMUNic-specific IgMhigh B cells correlates to subsequent vaccine efficacy against nicotine brain distribution.
A greater number of hapten-specific B cells in spleen biopsies 14 days after immunization correlated to decreased nicotine brain concentrations in immunized mice. Panels include mice immunized with 6CMUNic-KLH and 2CMUNic-KLH, as labeled (n=6 each group). Individual brain nicotine concentrations were significantly correlated to A) CMUNic-specific IgMhigh B cells, but not B) CMUNic-specific GC B cells, C) CMUNic-specific ASC B cells, and D) CMUNic-specific swIgB cells.
Table I.
Hapten-specific IgMhigh and GC B cell subsets predict vaccine efficacy against nicotine
| B cell subset | Analysis Time point | Nicotine-specific IgG titers at 35 days | Vaccine efficacy on serum nicotine | Vaccine efficacy on brain nicotine |
|---|---|---|---|---|
| IgMhigh | 14 days | r2= 0.23, NS | r2=0.5, p< 0.01 | r2= 0.44, p<0.05 |
| swIg | 14 days | r2= 0.1, NS | r2=0.3, p< 0.05 | r2=0.2, p< NS |
| GC | 14 days | r2= 0.34, p<0.05 | r2=0.34, p< 0.05 | r2=0.2, p< NS |
| ASC | 14 days | r2= 0.2, NS | r2= 0.15, NS | r2= 0.03, p< NS |
Shown the regression coefficient (r2) between numbers of hapten-specific B cells 14 days after the first immunization and vaccine efficacy in mice with 6CMUNic-KLH and 2CMUNic-KLH (n=12 total sample size). Mice were immunized s.c. in alum on days 0, 14 and 28. Serum antibodies and vaccine effects on nicotine distribution were analyzed a week after the last immunization. NS= not significant.
4. DISCUSSION
One limitation to translation of vaccines against nicotine dependence is that efficacy is shown in the fraction of immunized subjects that achieved the highest nicotine-specific serum antibody titers [10]. A better understanding of the immunological mechanisms underlying individual variability will likely guide design of more effective vaccines and suggest new strategies to predict vaccine efficacy. Here, we have tested the hypothesis that the frequency of early-activated hapten-specific B cells predicts the most effective hapten and individual responses to immunization against nicotine.
To test this hypothesis, we have synthesized a novel nicotine-based hapten containing a linker at the 2-position on the pyridine ring of the racemic nicotine structure and we compared its efficacy to the previously characterized 6CMUNic hapten containing the same linker at the 6-position. The 2CMUNic hapten conjugated to the KLH carrier protein was less effective than 6CMUNic-KLH in both mice and rats, using either the clinically relevant alum adjuvant, or the model Freund’s adjuvant, and delivered by either the s.c. or the i.p route of administration.
The current study is the first report of 6CMUNic-KLH administered s.c. in alum adjuvant in mice. Previously, the 6CMUNic-KLH showed proof of efficacy in BALB/c mice immunized i.m. and i.d., using either alum, monophosphoryl lipid A (MPLA), or laser-based adjuvants [39]. In that study, immunization with 6CMUNic-KLH blocked ~70% brain distribution of i.v. 0.01 mg/kg nicotine compared to KLH control [39]. Here, s.c. immunization of BALB/c mice with 6CMUNic-KLH in alum blocked ~30% brain distribution of i.v. 0.03 mg/kg nicotine compared to KLH. In rats, the quantity and quality of the anti-nicotine antibody response, and the magnitude of immunization effect in blocking nicotine distribution to the brain depended upon route of immunization and adjuvant [34,40,41]. In rats, i.p. immunization with either 3′AminoNic-rEPA, also known as the clinical-stage NicVax, or 6CMUNic-KLH in Freund’s adjuvant blocked ≥70% brain distribution of i.v. 0.03 mg/kg nicotine [34,41], while s.c. immunization with 3′AminoNic-rEPA in alum blocked ~30% brain distribution of i.v. 0.03 mg/kg nicotine [41].
Immunogens showing different efficacy are ideal models to study antigen-specific B cells. Structurally similar nicotine-based haptens containing linkers at the 1′-, 3′- and 6-positions were equally effective when conjugated to maleimide-activated KLH (mKLH), rEPA, or KLH carrier proteins [34,41]. The 1′SNic-mKLH, 3′AminoNic-rEPA and 6CMUNic-KLH generated distinct, and non-overlapping, nicotine-specific serum antibodies suggesting that these immunogens stimulated expansion of independent B cell populations [34,41]. In contrast, the 2CMUNic-KLH was not as effective as 6CMUNic-KLH, suggesting that placing a linker at the 2-position may negatively affect B cell activation. In a previous report, nicotine haptens containing a 2-position linker function were not as effective as nicotine haptens containing the same functionality at the 6-position [37]. Notably, a direct comparison with results obtained with the 2CMUNic hapten may be complicated because these previously tested immunogens (i.e. [37]) contained different hapten and linker chemistry, the diphtheria toxoid as carrier, and were tested in BALB/c female mice immunized i.m. using alum combined with B class CpG as adjuvant [37].
Several studies showed that nicotine hapten design is crucial for eliciting nicotine-specific antibodies and to achieve vaccine efficacy [37,38,42]. We hypothesized that the in vivo efficacy of immunogens containing structurally distinct nicotine haptens may lie in the ability of B cells to discriminate between haptens, and in the size or phenotype of the initial polyclonal hapten-specific B cell population. To address this hypothesis, our group has recently developed a sensitive fluorescent antigen-based enrichment strategy paired with flow cytometry analysis to detect scarce numbers of hapten-specific B cells before and soon after vaccination [35]. This approach showed that naïve B cells can discriminate between structurally-related opioid haptens, and that the number of naïve and early-activated hapten-specific B cells correlated with conjugate immunogen efficacy against oxycodone in mice [11,32,33]. In the present study, we have applied the same strategy to analyze the number and phenotype of B cells specific for the 2CMUNic and the 6CMUNic haptens appearing soon after vaccination, and test their relevance to vaccine efficacy against nicotine.
The 2CMUNic-KLH immunogen was not as effective as the previously characterized 6CMUNic-KLH. Soon after immunization it was apparent that 6CMUNic-KLH was more effective in inducing expansion of the polyclonal hapten-specific B cell population compared to 2CMUNic-KLH. The 6CMUNic-KLH immunogen elicited greater number of splenic hapten-specific IgMhigh, GC, and swIg B cells compared to 2CMUNic-KLH 14 days after a single immunization. These data are consistent with hapten-specific B cell responses to structurally-related oxycodone vaccines [33]. As previously shown in BALB/c mice, an oxycodone conjugate immunogen adsorbed on alum adjuvant elicited selective expansion of the polyclonal hapten-specific B cell subsets in peripheral lymph nodes and spleen 14 days after immunization compared to naïve mice and mice immunized with a less effective immunogen [33].
The novelty of the current study is that B cell analysis was performed by spleen biopsy to allow for both between- and within-subject analysis. Indeed, the number of hapten-specific B cells appearing 14 days after immunization correlated with greater efficacy of vaccines on distribution of nicotine to serum and to the brain at 35 days after the first vaccination (i.e. 5 weeks later). These data suggest that variations in the frequency of early-activated hapten-specific B cell subsets are immune correlates, or biomarkers, predictive of vaccine efficacy against nicotine. The observed individual variability in the number of hapten-specific B cells is consistent with other reports showing that before and after immunization the frequency of protein-specific B cells, or peptide-specific T cells, varies greatly across individual subjects from inbred mouse strains [16,19–29]. Similar, or greater, individual variability in the polyclonal hapten-specific B cell population size has also been observed in blood, lymph nodes and spleens in various mouse strains before and shortly after immunization with oxycodone vaccines [11,32,35]. The origin of such variability is still poorly understood. Variability in the frequency of hapten-specific B cells in naïve (i.e., non-immunized) mice argues against a technical issue of vaccine delivery, or local vaccine distribution after immunization. In fact, multiple mechanisms may underlie the post-immunization individual variability in the hapten-specific B cell subsets, and contribute to individual vaccine efficacy. For instance, apoptosis and antigen affinity affect the heterogeneity of the primary immune response by limiting differentiation of a single naïve B cell shortly after immunization [28]. As well, clonal selection, affinity for antigen, and peptide chemistry regulate the size and diversity of the peptide-specific T cell repertoire shortly after immunization [21–24,29]. Individual variability of epitope specific T cells has also been found in neonatal inbred mice [43], suggesting that this is not an age-related effect on the adaptive immune response. In immunized mice, the frequency of antigen-specific GC B and CD4+ T cells is highly correlated [20]. Consistently, the frequency of both hapten-specific B cells and carrier-specific T cells, and the magnitude of early GC formation, correlated with individual vaccine efficacy against oxycodone in mice [11].
Within the polyclonal B cell population, distinct hapten-specific B cell subsets were predictive of vaccine efficacy. The hapten-specific IgMhigh and GL7high GC B cells were the best predictors of post-immunization serum IgG antibody titers and vaccine efficacy against nicotine distribution to serum and to the brain. The hapten-specific IgMhigh and GL7high GC B cell subsets were also the most abundant and easiest to detect, which may explain why these B cell phenotypes were the best predictors of vaccine success. In a similar study, hapten-specific IgMhigh B cells in spleen and blood, before and soon after vaccination, were also the most robust predictive markers of vaccine efficacy against oxycodone in mice [11]. As shown in this study, analysis of antigen-specific B cells supports pre-clinical vaccine development, and screening of lead vaccines. In clinical settings, analysis of antigen-specific B cell subsets in blood is expected to reveal biomarkers that support patient stratification in adaptive clinical trials, and identify subjects most likely to achieve vaccine success in personalized medicine approaches.
These data are consistent with similar reports of oxycodone vaccines [11,32,33], and provide further insights in the immunological mechanisms underlying efficacy of vaccines for substance use disorders. Yet, more studies are needed to fully explore the relationship between vaccine design and activation of the naïve and early-activated B cell subsets, and between the frequency of specific antigen-specific B cell populations and individual responses to vaccines. Analysis of antigen-specific B and T cells provides complementary tools to accelerate development of immunogens containing novel haptens, carriers, and delivery platforms. For instance, use of capsid proteins derived from disrupted adenoviruses [44], trimeric coiled-coil peptides containing B and T cell epitopes [45], or nanoparticle-based platforms [46,47] are appealing approaches to enhance B and T cell responses, and augment generation of anti-nicotine serum antibodies. A better understanding of the T cell-dependent B cell processes underlying effective post-vaccination antibody responses, will be critical for implementation of rational vaccine design, and mechanism-based immunization strategies to increase the efficacy of vaccines for substance use disorders, and for other challenging targets such as HIV, malaria, or cancer.
Supplementary Material
Highlights.
Two immunogens containing structurally-related nicotine haptens were compared in mice
Hapten-specific B cells were analyzed by antigen-based magnetic enrichment and flow cytometry
The most effective immunogen elicited more hapten-specific B cells shortly after immunization
Early frequency of hapten-specific B cells correlated with vaccine efficacy against nicotine
Acknowledgments
This study was supported by NIH DA034487 (Pravetoni), and a MMRF Translational Research Program Award (Pravetoni). The authors thank Theresa Harmon and Danielle Burroughs for outstanding technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Tobacco. 2014. Fact sheet No 339. [Google Scholar]
- 2.CDC. Wide-ranging OnLine Data for Epidemiologic Research (WONDER) 2014. [Google Scholar]
- 3.Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2013 reviews. Addiction. 2014 Sep;109(9):1414–1425. doi: 10.1111/add.12633. [DOI] [PubMed] [Google Scholar]
- 4.Pentel PR, LeSage MG. New directions in nicotine vaccine design and use. Adv Pharmacol. 2014;69:553–580. doi: 10.1016/B978-0-12-420118-7.00014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, Maurer P, Bachmann MF, Cerny T. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3(6):e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, Rennard SI. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011 Mar;89(3):392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, Horwith G, Pentel PR. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005 Nov;78(5):456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Kosten TR, Rosen M, Bond J, Settles M, Roberts JS, Shields J, Jack L, Fox B. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002 Jan 15;20(7–8):1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 9.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009 Oct;66(10):1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahim RE, Kessler PD, Kalnik MW. Therapeutic vaccines against tobacco addiction. Expert Rev Vaccines. 2013 Mar;12(3):333–342. doi: 10.1586/erv.13.13. [DOI] [PubMed] [Google Scholar]
- 11.Megan Laudenbach FB, Vervacke Jeffrey S, Distefano Mark D, Titcombe Philip J, Mueller Daniel L, Tubo Noah J, Griffith Thomas S, Pravetoni Marco. The frequency of naïve and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. J Immunol. 2015 Jun 15;194(12) doi: 10.4049/jimmunol.1500385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015 Mar;15(3):149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 13.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 14.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol. 2012 Jan;12(1):24–34. doi: 10.1038/nri3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunol Cell Biol. 2008 Feb;86(2):133–138. doi: 10.1038/sj.icb.7100148. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends Immunol. 2012 Dec;33(12):590–597. doi: 10.1016/j.it.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Victora GD, Mesin L. Clonal and cellular dynamics in germinal centers. Curr Opin Immunol. 2014 Jun;28:90–96. doi: 10.1016/j.coi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 19.Lofano G, Mancini F, Salvatore G, Cantisani R, Monaci E, Carrisi C, Tavarini S, Sammicheli C, Rossi Paccani S, Soldaini E, Laera D, Finco O, Nuti S, Rappuoli R, De Gregorio E, Bagnoli F, Bertholet S. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J Immunol. 2015 Aug 15;195(4):1617–1627. doi: 10.4049/jimmunol.1402604. [DOI] [PubMed] [Google Scholar]
- 20.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013 Mar 21;38(3):596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J Immunol. 2010 Oct 15;185(8):4705–4713. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu HH, Moon JJ, Takada K, Pepper M, Molitor JA, Schacker TW, Hogquist KA, Jameson SC, Jenkins MK. Positive selection optimizes the number and function of MHCII-restricted CD4+ T cell clones in the naive polyclonal repertoire. Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):11241–11245. doi: 10.1073/pnas.0902015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012 May 1;188(9):4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007 Aug;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011 Mar 4;331(6021):1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor JJ, Martinez RJ, Titcombe PJ, Barsness LO, Thomas SR, Zhang N, Katzman SD, Jenkins MK, Mueller DL. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J Exp Med. 2012 Oct 22;209(11):2065–2077. doi: 10.1084/jem.20112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012 Mar 12;209(3):597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor JJ, Pape KA, Steach HR, Jenkins MK. Humoral immunity. Apoptosis and antigen affinity limit effector cell differentiation of a single naive B cell. Science. 2015 Feb 13;347(6223):784–787. doi: 10.1126/science.aaa1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single Naive CD4(+) T Cells from a Diverse Repertoire Produce Different Effector Cell Types during Infection. Cell. 2013 May 9;153(4):785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becattini S, Latorre D, Mele F, Foglierini M, De Gregorio C, Cassotta A, Fernandez B, Kelderman S, Schumacher TN, Corti D, Lanzavecchia A, Sallusto F. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science. 2015 Jan 23;347(6220):400–406. doi: 10.1126/science.1260668. [DOI] [PubMed] [Google Scholar]
- 31.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009 Jul 6;206(7):1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One. 2014;9(5):e96547. doi: 10.1371/journal.pone.0096547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor JJ, Laudenbach M, Tucker AM, Jenkins MK, Pravetoni M. Hapten-specific naive B cells are biomarkers of vaccine efficacy against drugs of abuse. J Immunol Methods. 2014 Jan 23; doi: 10.1016/j.jim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, Earley CA, Pentel PR. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol. 2012 Feb 15;83(4):543–550. doi: 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor JJ, Laudenbach M, Tucker AM, Jenkins MK, Pravetoni M. Hapten-specific naive B cells are biomarkers of vaccine efficacy against drugs of abuse. J Immunol Methods. 2014 Mar;405:74–86. doi: 10.1016/j.jim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCluskie MJ, Thorn J, Mehelic PR, Kolhe P, Bhattacharya K, Finneman JI, Stead DR, Piatchek MB, Zhang N, Chikh G, Cartier J, Evans DM, Merson JR, Davis HL. Molecular attributes of conjugate antigen influence function of antibodies induced by anti-nicotine vaccine in mice and non-human primates. Int Immunopharmacol. 2015 Apr;25(2):518–527. doi: 10.1016/j.intimp.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Pryde DC, Jones LH, Gervais DP, Stead DR, Blakemore DC, Selby MD, Brown AD, Coe JW, Badland M, Beal DM, Glen R, Wharton Y, Miller GJ, White P, Zhang N, Benoit M, Robertson K, Merson JR, Davis HL, McCluskie MJ. Selection of a novel anti-nicotine vaccine: influence of antigen design on antibody function in mice. PLoS One. 2013;8(10):e76557. doi: 10.1371/journal.pone.0076557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lockner JW, Lively JM, Collins KC, Vendruscolo JC, Azar MR, Janda KD. A conjugate vaccine using enantiopure hapten imparts superior nicotine-binding capacity. J Med Chem. 2015 Jan 22;58(2):1005–1011. doi: 10.1021/jm501625j. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012 Dec 17;31(1):159–164. doi: 10.1016/j.vaccine.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornish KE, de Villiers SH, Pravetoni M, Pentel PR. Immunogenicity of individual vaccine components in a bivalent nicotine vaccine differ according to vaccine formulation and administration conditions. PLoS One. 2013;8(12):e82557. doi: 10.1371/journal.pone.0082557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Villiers SH, Cornish KE, Troska AJ, Pravetoni M, Pentel PR. Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine. 2013 Oct 29; doi: 10.1016/j.vaccine.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Baraznenok I, Malmerfelt A, Marcus MM, Johansson AM, Svensson TH. Nicotine hapten structure, antibody selectivity and effect relationships: results from a nicotine vaccine screening procedure. Vaccine. 2010 Mar 2;28(10):2161–2168. doi: 10.1016/j.vaccine.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 43.Nelson RW, Rajpal MN, Jenkins MK. The Neonatal CD4+ T Cell Response to a Single Epitope Varies in Genetically Identical Mice. J Immunol. 2015 Jul 15; doi: 10.4049/jimmunol.1500405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De BP, Pagovich OE, Hicks MJ, Rosenberg JB, Moreno AY, Janda KD, Koob GF, Worgall S, Kaminsky SM, Sondhi D, Crystal RG. Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity. Hum Gene Ther. 2013 Jan;24(1):58–66. doi: 10.1089/hum.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller KD, Roque R, Clegg CH. Novel Anti-Nicotine Vaccine Using a Trimeric Coiled-Coil Hapten Carrier. PLoS One. 2014;9(12):e114366. doi: 10.1371/journal.pone.0114366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desai RI, Bergman J. Effects of the Nanoparticle-based Vaccine, SEL-068, on Nicotine Discrimination in Squirrel Monkeys. Neuropsychopharmacology. 2015 Mar 6; doi: 10.1038/npp.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser CC, Altreuter DH, Ilyinskii P, Pittet L, LaMothe RA, Keegan M, Johnston L, Kishimoto TK. Generation of a universal CD4 memory T cell recall peptide effective in humans, mice and non-human primates. Vaccine. 2014 May 19;32(24):2896–2903. doi: 10.1016/j.vaccine.2014.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.