SYNOPSIS
Making an accurate diagnosis when evaluating a patient with a possible food allergy is particularly important both to avoid unnecessary dietary restrictions and to prevent life threatening reactions. The testing modalities used routinely in clinical practice, including skin prick testing and food specific IgE levels, have limited accuracy, and a physician-supervised oral food challenge is often required to make a definitive diagnosis. Given the labor-intensive nature of this test and the risk of inducing an allergic reaction, researchers have investigated a number of alternative diagnostic modalities to improve the accuracy of food allergy testing. Testing for IgE antibodies to particular protein components in foods has already shown promise to improve diagnostics and has entered clinical practice. Additional modalities are under study that show potential including epitope binding, T cell studies, basophil activation and others.
Keywords: Diagnosis, food allergy, skin prick testing, food specific IgE, oral food challenge, component-resolved diagnostics, epitope, basophil activation
INTRODUCTION
An accurate diagnosis of food allergies is necessary to ensure that an individual is avoiding foods that could trigger severe allergic reactions or contribute to chronic symptoms. Importantly, misdiagnosis could also result in unnecessary dietary restrictions that carry social and nutritional consequences. In 2010, a National Institute of Allergy and Infectious Diseases-sponsored expert panel published recommendations on the diagnosis of food allergy, endorsing use of the medical history and physical examination, elimination diets, skin prick testing (SPT), serum food specific IgE (sIgE) levels, and oral food challenges (OFCs).1 These key diagnostic tools, often used together, are essential for arriving at an accurate diagnosis. Unfortunately, they also carry various limitations. For example, SPTs and sIgE levels are sensitive tools for identifying the presence of food-specific IgE antibodies (sensitization) that can be associated with acute allergic reactions, but sensitization often exists without clinical consequence. Additionally, there are circumstances when these tests are negative despite the presence of a true food allergy. The medically supervised OFC is a very specific diagnostic test, but the procedure is time consuming, costly, and may result in a severe allergic reaction. In recent years, a number of testing modalities have been under investigation that may improve food allergy diagnostics, including component-resolved diagnostics (CRD), basophil activation studies, T-cell proliferation assays, and measurement of platelet activating factor (PAF).
STANDARD DIAGNOSTIC TESTS
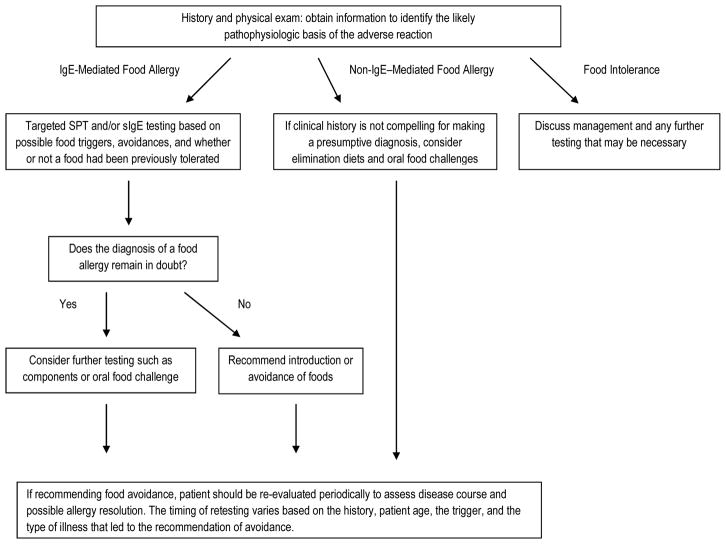
The typical diagnostic routine (Figure 1) begins with a medical history to determine whether the symptoms are potentially related to ingestion of specific foods, whether adverse reactions are allergic in nature and, if so, the likely pathophysiologic basis. Knowledge of the epidemiology of food allergy and details of the history may identify potential triggers to which simple tests, such as SPT and sIgE, can be applied and interpreted in the context of the history and a knowledge of test limitations. When the diagnosis is uncertain, an OFC can be utilized as the diagnostic “gold standard”.
Figure 1.
Food Allergy Diagnostic Algorithm
The Medical History
The evaluation for a patient with a possible food allergy begins with a thorough history and physical exam. The history should focus on possible triggers of a reaction, the quantity ingested, the time course of the reaction, whether there were ancillary/facilitating factors around the time of the reaction that might have promoted reactivity (exercise, illness, medications such as aspirin), and the specific symptoms that led to concern for an allergic reaction.2 The history is important in determining the likely pathophysiologic basis of the reaction, specifically whether the food-induced allergic reaction is IgE mediated. This is important because tests of food-specific IgE would not be diagnostic for disorders that are cell mediated/non-IgE mediated such as food protein-induced enterocolitis syndrome. Once a possible food trigger is identified, additional history can help decipher if that food is the likely culprit for the reaction. For example, a food that was not previously ingested or was ingested infrequently is more likely to have caused an acute reaction than one that had been regularly tolerated. Common (“major”) allergens such as milk, egg, wheat, soy, peanut, tree nuts, fish, and shellfish are more likely to be triggers than other foods. The history is therefore an important tool to guide allergy test selection and interpretation.
Skin Prick Testing
SPT is extremely sensitive, and has a negative predictive value of greater than 90%.3 This form of testing is often helpful to rapidly rule out an IgE-mediated food allergy. It can also help to confirm a food allergy when positive, in the setting of a recent clear history of an acute allergic reaction to the tested food (high prior probability). Unfortunately, the specificity of skin testing is not 100%; while a positive test, defined in most studies as a mean wheal diameter 3mm larger than the saline control, is able to confirm sensitization to an allergen, it does not confirm a diagnosis of food allergy. However, evaluating the SPT test result according to wheal size, rather than solely as a positive response, can add to its diagnostic utility because studies suggest that the likelihood of a reaction to the tested food increases with increasing SPT wheal size.2,4
Researchers have attempted to identify wheal diameter sizes above which virtually no one would be able to tolerate a food, but these values exist for only a few foods and vary among populations. In one Australian study of children presenting to a tertiary allergy clinic, no child was able to pass an OFC if the skin test wheal diameter was above 8 mm for milk, 7 mm for egg, or 8 mm for peanut.5 A similar study but evaluating a population-based Australian cohort of infants reported 95% predictive values for allergic reactions for egg (SPT wheal ≥ 4 mm), peanut (SPT wheal ≥ 8 mm), and sesame (SPT wheal ≥ 8mm).6 These cutoffs were not validated when researchers from Montreal, Canada looked retrospectively at children evaluated for peanut allergy. Of 140 children examined, 64 had positive SPT responses, and 18 reacted during oral peanut challenge. Of 17 patients who had an SPT wheal ≥ 10 mm, only 8 had a positive peanut challenge.7 While not specific, the SPT testing was sensitive, as all 18 patients with a positive peanut challenge had a wheal diameter ≥ 5 mm. These studies demonstrate that predictive values may vary according to different foods, ages, and populations, and that skin test results cannot be used as an isolated diagnostic tool without a thorough history and possibly other testing. Furthermore, commercial food extracts for skin testing have not been standardized, and may contain differing concentrations of relevant proteins, leading to variable results.8,9 Commercial extracts are also not available for some food allergens. Finally, in some instances, testing may be more accurate using fresh foods (especially fruits and vegetables) by doing a “prick-prick” test rather than using commercial extracts because relevant labile proteins may not be present in the commercial extracts.10,11 For ease of use in the clinical setting, fresh fruits can be frozen and used later for skin testing.12 Skin tests provide quick results and are considered highly sensitive, but they require the patient to be off antihistamines and to have an area of skin free of rash for testing.
Serum IgE Testing
Serum immunoassays that measure food-specific IgE antibodies are another common diagnostic tool used in the evaluation of IgE-mediated food allergy. Similar to SPT where larger wheal sizes correlate with increased risk of clinical allergy, higher concentrations of food sIgE correlate with increased risk of true food allergy. As with skin testing, predictive values may differ among populations for various reasons.13 Proposed cutoffs that predict 50% and 95% likelihood of a reaction to an OFC based on food specific IgE levels for egg, cow’s milk, and peanut have been calculated based on several studies of referral populations as reviewed in reference 14 (Table 1).14 A recent study examining an Australian cohort of infants revealed different predictive levels for egg (95% positive: ≥ 1.7 kUA/L) and peanut (95% positive: ≥34 kUA/L), which highlights the variability among studies due to geographical differences, patient age, referral base, test characteristics, interpretation of the OFC outcomes, and other factors.6 It is also important to note that sIgE levels that have been reported in various studies may use different test systems and that the results between these systems are not directly comparable.15 Another important limitation is that sIgE levels are not highly correlated with reaction severity.16,17
Table 1.
Predictive sIgE values for reactivity to egg, milk, and peanut
| Food | Mean age 5 years, 50% react | Mean age 5 years, ~95% react | Age <2 years, ~95% react |
|---|---|---|---|
| Egg (kUA/L) | 2 | 7 | 2 |
| Milk (kUA/L) | 2 | 15 | 5 |
| Peanut (kUA/L) | 2 (with a clinical history) 5 (without a clinical history) |
14 |
Data from Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014;133:291–307.
Limitations of SPT and sIgE
Neither SPT nor sIgE are sufficient to diagnose food allergy on their own.1 These tests are not predictive of reaction severity. Importantly, there are situations where clinical reactions occur despite negative tests either because the allergy is not IgE mediated, for example with food protein-induced enterocolitis syndrome, or because the test does not detect the relevant antigen. These limitations underscore the importance of the medical history-if the suspicion is high, a negative test may not be sufficient to exclude a food allergy.3,18 Cross reactivity among foods is often not clinically relevant and this must also be considered in test interpretation. For example, 50% of persons with peanut allergy test positive to other legumes, but 95% tolerate these legumes.19
Oral Food Challenge
Given the limitations of current testing modalities, it is often necessary to perform an OFC to make the distinction between a patient who is simply sensitized to an allergen and a patient who is clinically reactive to it. OFCs are also helpful to determine if a child has outgrown their allergy. An OFC is a physician-supervised oral provocation procedure performed in a hospital or outpatient office, where a patient ingests gradually increasing amounts of a food under medical supervision until an age-appropriate serving is reached or the feeding is terminated because of symptoms. There are a number of factors that must be considered before performing an OFC, including the patient’s medical history, age, past adverse food reactions, skin prick test [SPT] and serum food allergen-specific IgE results, and the importance of the food to the patient, both socially and nutritionally. Indications for performing the OFC can be found in Box 1.13 The OFC is usually performed when the patient’s history and test results suggest that the allergy may not exist or may have resolved. The clinician can use the medical history and test results to estimate a probability of tolerance, and the odds can then be considered in the context of the patient’s needs, weighing the risks and benefits.
Box 1. Indications for an OFC.
| Identify foods causing acute reactions for initial diagnosis of food allergy and for monitoring resolution of food allergy |
| Determine whether food allergens associated with chronic conditions such as atopic dermatitis or allergic eosinophilic esophagitis will cause immediate reactions |
| Expand the diet in persons with multiple dietary restrictions, usually because of subjective complaints such as headaches or hyperactive behaviour |
| Assess the status of tolerance to cross-reactive foods |
| Assess the effect of food processing on food tolerability, e.g. fruits and vegetables that may be tolerated in cooked form in the pollen-food allergy syndrome |
From Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, et al. Work Group report: oral food challenge testing. J Allergy Clin Immunol 2009;123:S365-83; with permission.
The OFC can be performed as an open feeding or as a single blind, or double-blind, placebo-controlled food challenge (DBPCFC). In an open challenge, the patient is aware that they are ingesting the potentially problematic food, which can lead to symptoms from anxiety, biasing the test. While an open feeding is the most common approach used clinically for OFCs, this bias must be considered if the child fails the challenge. The DBPCFC is the most specific test for the diagnosis of a food allergy.1 In this test, the food is masked in a carrier, and the food to be tested or placebo is randomly administered at different times. While the DBPCFC is currently the gold standard test for diagnosing food allergy, due to the time and labor intensive nature of this method it is typically only performed in research studies and select cases in clinical practice.20,21 A single-blind placebo-controlled OFC may be helpful if the patient is suspected of having a psychological response. The open challenge is a reasonable first choice when the need for OFC is established, as less than 1/3 of suspected foods result in a positive challenge, and many of these positive OFCs will have objective symptoms.18,20,22
How a food is processed can affect the challenge outcome. Heating can change the protein conformation of certain foods and may result in a change in allergenicity.23 This is seen with milk and egg, as well as with a number of fruits and vegetables implicated in pollen-food allergy syndrome.24–27 Based on studies of peanut, patients may be at risk of their allergy recurring if they fail to incorporate the food into their diet on a regular basis after passing an OFC.28,29
When considering whether or not to perform an OFC in the office setting, safety is a concern, and patients should be made aware of the risks and benefits of the OFC.. Lieberman et al30 reported the results of all OFCs performed between August 2008 and May 2010 at the Jaffe Food Allergy Institute, our university-based, outpatient practice. This was a retrospective chart review that included all patients referred by 9 allergists; patients were typically challenged when the likelihood of a reaction was thought to be less than 50%, though no specific cutoffs for SPT size or sIgE precluded a challenge. There were 701 OFCs performed in 521 patients who ranged in age from 8 months to 21.8 years. OFCs were performed to a wide variety of foods, most commonly milk, egg, peanut, tree nuts, soy, fish, shellfish, sesame, and wheat. Overall, 132 (18.8%) challenges elicited a reaction. The majority of those reactions were cutaneous (56.8%), and 9.1% were treated with epinephrine, with one requiring 2 doses. Since overall only 1.7% of challenges required treatment with epinephrine, the authors concluded that open OFCs are a safe and effective method of ascertaining tolerance in patients with potential food allergy. The benefits of performing OFCs, in particular eliminating unnecessary dietary restrictions, have been demonstrated in several studies. Using OFCs, Nicolaou et al31 demonstrated the high rate of false positive SPT and specific IgE to peanut. In a population-based birth cohort from the United Kingdom of 933 children, 110 (11.8%) were peanut-sensitized at 8 years of age. OFCs were performed on those children without a convincing history of reaction and who had a peanut-specific IgE ≤ 15 kUA/L, and peanut skin test wheal ≤ 8 mm. Based on OFC results, the estimated prevalence of clinical peanut allergy was only 22.4% among sensitized children. In a study from Denver of 125 children, primarily with atopic dermatitis, who were avoiding foods largely due to previous immunoassay and SPT results, 89% of 364 OFCs were passed, allowing significant dietary expansion.32 The authors concluded that in the absence of anaphylaxis, the primary reliance on serum food-specific IgE testing to make the diagnosis of food allergy, particularly in children with atopic dermatitis, is not sufficient.
EMERGING DIAGNOSTIC TESTS
Component Resolved Diagnostics (CRD)
Allergen CRD have garnered a lot of attention in recent years, with the hope of offering a more accurate assessment of allergic status. Instead of using crude allergen extracts consisting of a mixture of components, CRD measures IgE to individual allergen proteins. In recent years, a number of studies on a variety of food allergens have demonstrated that CRD can improve the specificity of allergy testing. The utility of CRD has been best demonstrated in studies on peanut allergy.33 In 2004, Koppelman et al34 suggested the importance of the peanut component, Ara h 2, in predicting reactivity or tolerance to peanut. The level of IgE to Ara h 2, one of the major peanut allergens, was most important for predicting allergic status in their cohort of 32 patients, with 26 out of 32 peanut allergic patients testing positive to this component. Similarly, Dang et al35 reported that Ara h 2 sIgE levels provide higher diagnostic accuracy than whole peanut sIgE levels. In the Australian HealthNuts study,36 11 to 15 month old infants underwent SPT to peanut, and if a positive wheal reaction was elicited, the infant was invited for a formal open OFC. Overall, 411 peanut sensitized infants underwent a peanut OFC, 137 of which were positive. Non-sensitized patients (n=140) also underwent a peanut OFC as negative controls. Another 100 peanut allergic subjects and 100 peanut tolerant subjects were randomly selected for Ara h 2 testing, and whole peanut IgE testing was also performed. Compared to currently-used whole peanut sIgE levels, Ara h 2 sIgE levels were more accurate in determining peanut allergy. An Ara h 2 s IgE level of 0.46 kUA/L provided 95% specificity and 73% sensitivity, whereas a peanut sIgE level of 6.2 kUA/L provided 95% specificity, but only 44% sensitivity. In comparison, a peanut sIgE level of 15 kUA/L provided a 95% PPV and 98% specificity, but the sensitivity dropped to 26%. An Ara h 2 level of 1.19 kUA/L provided 98% specificity, but offered sensitivity of 60%. Given the improved accuracy found in the Ara h 2 sIgE diagnostic testing, it was concluded that this test could become a preferred diagnostic tool for diagnosing peanut allergy. However, similar to the problems with food sIgE testing, predictive cutoff levels for Ara h 2 have varied from study to study. Among children in a referral population in the United States, the positive predictive value was reported to be 75% at 2 kUA/L, and the negative predictive value was 91% at 0.23 kUA/L.37 Researchers from the Netherlands examined Ara h 2 levels among children with suspected peanut allergy, and reported that a cutoff of 5 kUA/L had the best overall predictive value, with a positive predictive value of 96% and a negative predictive value of 71%.38 While the studies all report benefits to the use of Ara h 2 in the diagnosis of peanut allergy, the differing cutoffs for predictive values likely reflect differences in sensitization among different geographic locations and populations, and demonstrate that there are patients with positive test results to Ara h 2 who are still able to tolerate peanut. Ara h 2 levels also do not seem to clearly predict severity among patients with peanut allergy.39
The peanut component Ara h 6 is structurally similar to Ara h 2 and has been reported to be a relevant allergen in peanut allergy, though it is unclear if assessment of IgE levels to this component would offer additional diagnostic value to that of Ara h 2 alone as part of CRD.40 Asarnoj et al41 reported a case of a 15 year old boy who was sensitized to Ara h 8 but not to Ara h 1, 2, or 3, who developed anaphylaxis including vomiting, diarrhea, and lower respiratory obstruction following peanut ingestion. He was found to have marked sensitization to Ara h 6 (24 kUA/L) while Ara h 2 remained below 0.35 kUA/L (0.12 kUA/L). A recent study by Klemans et al,42 assessing the diagnostic value of sIgE to Ara h 6 in an adult population in the Netherlands with suspected peanut allergy, demonstrated that the discriminative ability of Ara h 6 was as good as sIgE to Ara h 2, though they did not demonstrate an added benefit to analyzing Arah6 instead of, or in addition to, the measurement of Ara h 2. Component testing to peanut is more likely to be informative if the patient has a history of no or mild reactions, a remote clinical reaction with development of birch tree pollen sensitization over time, a peanut sIgE between 0.35 and 15 kUA/L, birch pollen sensitization, or if the patient is older.33
CRD has the potential to be a very useful tool in the diagnosis of hazelnut allergy as well. It is well known that cross-reactivity between proteins found in pollens and foods can account for positive test results to a variety of plant-derived foods. Hazelnut is one such food, where Cor a 1 proteins cross-react with the birch pollen allergen, Bet v 1. In 2007, it was noted that many patients had higher than expected serum hazelnut specific IgE levels.43 At that time, the hazelnut extract in one manufacturer’s test was supplemented with additional recombinant Cor a 1 to improve the test’s sensitivity for birch-related reactions to hazelnut; however, this made it difficult to distinguish patients who had a serious allergy to hazelnut versus those who were sensitized to hazelnut only as a result of cross-reactivity from their birch pollen allergy. Phadia Immunology Reference Laboratory (PiRL) developed commercial IgE testing to the hazelnut components Cor a 1 and Cor a 8, and later added Cor a 9 and Cor a 14 to their hazelnut panel. In reports from the Mediterranean area, systemic reactions to hazelnut are generally mediated by IgE to Cor a 8, a lipid transfer protein.44,45 In reports from the US and Europe, sensitization to Cor a 9, an 11S globulin, and Cor a 14, a 2S albumin, have been associated with severe hazelnut allergy in children.46–49 In a Dutch study by Masthoff et al,48 sensitization to Cor a 9 and Cor a 14 was highly specific for identifying patients with objective symptoms to hazelnut. In this study, IgE levels of 1 kUA/L or greater to Cor a 9 or 5 kUA/L or greater to Cor a 14 in children, and 1 kUA/L or greater to Cor a 9 or 1 kUA/L or greater to Cor a 14 in adults, gave a specificity of greater than 90% for predicting true allergy to hazelnut. Similar findings were noted by our group in a birch endemic area of the US, where the specificity of hazelnut testing was greatly improved when utilizing hazelnut components that included Cor a 9 and 14.49 These components were both highly sensitive and specific, particularly when used in combination.
While a number of studies have reported the utility of component testing for allergies to egg, milk, wheat, soy, and fruits, results from these studies have varied, likely reflecting different study populations and methods, manners of sensitization, environmental exposures, and degree of sensitization to various food components.50–63 In studies on cow’s milk, hen’s egg, and shrimp component testing, CRD offered increased specificity, but decreased sensitivity, when compared to traditional SPT and serum specific IgE testing.52,53,62 From a practical standpoint, this means that CRD for these foods could be used as an adjunct to currently used allergy tests to avoid performing some OFCs, but it is not ready to be used on its own in ruling out a food allergy.
The reported utility of component testing for diagnosing wheat allergy is also quite variable. Omega-5 gliadin has been identified as a major allergen in children for provoking wheat-dependent, exercise-induced anaphylaxis.56 Studies from Finland and Japan have reported that omega-5 gliadin is a significant allergen in children with immediate reactions to wheat, and that this component can be used to predict the outcome of oral wheat challenges.57,58 A collaborative study examining wheat allergy in both American and German children found no correlation between omega-5 gliadin levels and the outcomes of oral wheat challenges, and the authors concluded that omega-5 gliadin-specific IgE levels in wheat-sensitized patients appear not to be helpful for diagnosis.59 A recent European study reported that IgE to the wheat component rTri a 36 accurately identified more patients with true wheat allergy than did IgE to omega-5 gliadin.60 While the different results reported from these studies could result from different sensitization patterns in different geographic locations, they also likely reflect different inclusion criteria and other differences in the populations tested.
FUTURE DIAGNOSTIC TESTS
A number of tests are under study and may have advantages over currently available tests for diagnosing food allergy (Table 2).
Table 2.
Emerging and Future Diagnostic Tests
| Testing Modality | Potential Advantages | Pitfalls |
|---|---|---|
| Component Resolved Diagnostics |
|
|
| Epitope Binding |
|
|
| T Cell Responses |
|
|
| Basophil Activation |
|
|
| Platelet Activating Factor and PAF Acetylhydrolase |
|
|
Epitope Binding
Just as immune responses against different proteins within a food may carry different clinical implications, the location (epitope) and strength of binding (affinity) of IgE antibodies within a protein may also have clinical ramifications. Having IgE antibodies directed to a greater number of epitopes, or to epitopes that are not easily destroyed by denaturation and digestion (e.g., sequential or linear epitopes rather than ones dependent upon folding and conformation) may be associated with clinical allergy.64 The role of sequential IgE-binding epitopes in patients with persistent milk allergy has been reported in several studies.65,66 A recent study from Finland, in which investigators compared IgE, IgG4, and IgA binding to cow’s milk epitopes in patients with early resolution versus persistent cow’s milk allergy, demonstrated that cow’s milk tolerance is associated with decreased IgE epitope binding and increased epitope binding by IgG4.67 They compared results from serum samples obtained at the time of diagnosis, 1 year later, and at follow up years later in 11 patients with IgE mediated cow’s milk allergy that was persistent to age 8–9 years, and in 12 patients who recovered by age 3 years. While the IgE epitope-binding patterns were stable over time in the patients whose allergy to cow’s milk persisted, binding decreased in those patients who recovered early.
Ayuso et al68 investigated whether the recognition of particular IgE epitopes of certain shrimp allergens are good biomarkers for clinical reactivity to shrimp. They performed DBPCFC on 37 consecutive patients with clinical histories of shrimp allergy, and analyzed their IgE binding to synthetic overlapping peptides representing the sequence of 4 allergens of the Pacific white shrimp, Lit v 1, 2, 3 and 4. The 17 patients with a positive challenge to shrimp had more intense and diverse epitope recognition to all four shrimp allergens. They concluded that IgE antibodies to these epitopes could be used as biomarkers for predicting clinical reactivity in subjects sensitized to shrimp. Studies have also demonstrated potential benefits from analyzing IgE epitope binding in studies of egg and wheat allergy.69,70 It may not be long before epitope binding assays make their way to the clinical arena.
T-Cell Responses
In recent years, researchers have examined T cell responses to food allergens, and it has been reported that analysis of allergen-specific T-cell responses may be useful for distinguishing sensitization from clinical reactivity.71,72 Flinterman et al73 examined T-cell responses to the major peanut allergens Ara h 1, 2, 3, and 6 in peanut allergic children, non-allergic peanut-sensitized children, and non-atopic adults. The peanut allergic children were the only subjects who demonstrated increased interleukin-13 production in response to Ara h 1, 3 and 6. Further studies will be necessary to confirm the utility of T-cell proliferative responses in the diagnosis of food allergy.
Basophil Activation
Several studies have reported that markers of basophil activation, specifically upregulation of cell-surface molecules such as CD63 and CD203c using flow cytometry,, may be helpful in the diagnosis of food allergy. Sato et al74 reported their findings from a Japanese cohort examining the performance of basophil activation for predicting challenge outcomes to egg and milk. In a group of 71 children with egg or milk allergy previously diagnosed by challenge outcomes or convincing history, they found that assessment of food antigen-induced CD203c expression on basophils was useful in determining whether a child will outgrew a food allergy, and in deciding whether or not to perform an OFC. In a study evaluating tolerance to baked milk among children with cow’s milk allergy, Ford et al75 compared basophil reactivity among patients with milk allergy who reacted to baked milk and those who could tolerate it. They reported that the median basophil reactivity to cow’s milk, as well as spontaneous basophil activation, were significantly higher among patients who reacted to baked milk versus those who were able to tolerate milk in a baked form. Furthermore, they reported that spontaneous basophil activation was greater among patients with more severe clinical milk reactivity; this discovery lends hope to clinicians looking for a potential test to predict the severity of a patient’s food allergy. Further studies are necessary to elucidate the utility of basophil activation for use in clinical practice.
Platelet Activating Factor (PAF) and PAF Acetylhydrolase
There is currently no means to accurately predict the severity of an allergic reaction. Researchers have examined serum PAF and PAF acetylhydrolase (PAF-AH) levels as potential markers of allergy severity. PAF is a pro-inflammatory phospholipid synthesized and secreted by mast cells, basophils, monocytes, and macrophages. It has various biological activities, including platelet activation, airway constriction, hypotension, and vascular permeation.76–78 Circulating levels of PAF are, in part, controlled by the activity of PAF-AH, an enzyme that controls activity by cleaving PAF, rendering it inactive. It has been demonstrated that anaphylactic symptoms can be mimicked by PAF injection in animals.78,79 PAF-receptor antagonists protect against anaphylaxis in mice, rabbits, and rats.80 PAF-receptor knockout mice are protected from fatal anaphylaxis in contrast to wild-type mice with intact PAF receptors.81
Vadas et al82 measured serum PAF levels and PAF-AH activity in 41 patients with anaphylaxis and 23 control patients. PAF-AH activity was also compared in 9 patients who had fatal anaphylaxis to those in non-allergic control patients, children with mild peanut allergy, and patients with nonfatal anaphylaxis. Serum PAF levels were directly correlated and serum PAF acetylhydrolase activity was inversely correlated with the severity of anaphylaxis. Further studies are necessary to establish the role of PAF and its related catabolic enzymes in the prediction and confirmation of anaphylaxis.
CONTROVERSIAL AND UNPROVEN TESTS
There are a number of tests that have been examined that are not recommended for the diagnosis of food allergy. Intradermal testing should not be used. Not only is intradermal injection of allergens overly sensitive, but it also carries a higher risk of adverse reactions than SPT.1,83 The National Institute of Allergy and Infectious Diseases expert guidelines published in 2010 also suggest that atopy patch testing (APT) should not be used in the routine evaluation of non-contact food allergy. While some studies have suggested that APT may be useful in the evaluation of food allergy in patients with eosinophilic esophagitis or atopic dermatitis, the sensitivity and specificity is variable among different studies, and there is no consensus on the appropriate reagents or methods to use, or on how to interpret these tests.1,84–87 Measuring the total serum IgE level is not recommended for routine use in making a diagnosis of food allergy.1 When researchers have examined the predictive value of the ratio of sIgE to total IgE for the diagnosis of food allergy compared with the DBPCFC, it was concluded that the ratio did not offer an advantage over sIgE alone,88 although emerging studies may suggest otherwise.89 Finally, other non-standardized tests that are not recommended for the routine evaluation of IgE-mediated food allergy include facial thermography, gastric juice analysis, applied kinesiology, allergen-specific IgG4 levels, hair analysis, and electrodermal testing.1
FUTURE CONSIDERATIONS/SUMMARY
The work up of a potential food allergy can be a complex assessment involving the clinical history, SPT, and sIgE levels, although ultimately these diagnostic tools may be inadequate to definitively diagnose a food allergy. Currently, OFCs remain the most definitive test in the diagnosis of food allergy, but they are time consuming, costly, and have the potential to elicit a severe allergic reaction. In recent years, several different testing modalities, including CRD, basophil activation studies, T-cell proliferation assays, and measurement of PAF have demonstrated potential to aid the allergist in better identifying a patient with true clinical reactivity to an allergen. While CRD is currently used clinically, the remaining tests are promising but require further evaluation before they are ready for implementation in clinical practice.
KEY POINTS.
Accurate diagnosis of food allergies is vital to identify patients who may have severe, life threatening allergic reactions, and to exclude suspected allergies that could lead to unnecessary dietary restrictions.
Traditional tests for food allergy have a number of limitations; skin prick testing and food specific IgE levels are excellent tools for detecting sensitization to foods, but often positive tests are clinically irrelevant. While oral food challenges are the gold standard for diagnosing food allergy, they are time consuming and costly, and may result in an allergic reaction.
Recent studies have identified a number of testing modalities that may improve the ability to identify true clinical reactivity/severity, including component-resolved diagnostics, basophil activation studies, T-cell proliferative responses, and measurement of platelet activating factor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacob D. Kattan, Email: jacob.kattan@mssm.edu, Assistant Professor of Allergy and Immunology, Jaffe Food Allergy Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Place Box 1198, New York, NY 10029.
Scott H. Sicherer, Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, Jaffe Food Allergy Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Place Box 1198, New York, NY 10029.
References
- 1.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAD-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;9:S1–68. [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–25. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Knight AK, Shreffler WG, Sampson HA, et al. Skin prick test to egg white provides additional diagnostic utility to serum egg white-specific IgE antibody concentration in children. J Allergy Clin Immunol. 2006;117:842–7. doi: 10.1016/j.jaci.2005.12.1304. [DOI] [PubMed] [Google Scholar]
- 5.Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg, and peanut in children. Clin Exp Allergy. 2000;30:1541–6. doi: 10.1046/j.1365-2222.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 6.Peters RL, Allen KJ, Dharmage SC, et al. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132:874–80. doi: 10.1016/j.jaci.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Pucar F, Kagan R, Lim H, Clarke AE. Peanut challenge: a retrospective study of 140 patients. Clin Exp Allergy. 2001;31:40–6. doi: 10.1046/j.1365-2222.2001.00962.x. [DOI] [PubMed] [Google Scholar]
- 8.Sampson HA. Comparative study of commercial food antigen extracts for the diagnosis of food hypersensitivity. J Allergy Clin Immunol. 1988;82:718–726. doi: 10.1016/0091-6749(88)90070-x. [DOI] [PubMed] [Google Scholar]
- 9.Hefle SL, Helm RN, Burks AW, Bush RK. Comparison of commercial peanut skin test extracts. J Allergy Clin Immunol. 1995;95:837–42. doi: 10.1016/s0091-6749(95)70127-3. [DOI] [PubMed] [Google Scholar]
- 10.Rancé F, Juchet A, Brémont F, Dutau G. Correlations between skin prick tests using commercial extracts and fresh foods, specific IgE, and food challenges. Allergy. 1997;52:1031–5. doi: 10.1111/j.1398-9995.1997.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 11.Vertege A, Mehl A, Rolinck-Werninghaus C. The predictive value of skin prick test wheal size for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:1220–6. doi: 10.1111/j.1365-2222.2005.2324.x. [DOI] [PubMed] [Google Scholar]
- 12.Begin P, Des RA, Nguyen M, Masse MS, Paradis J, Paradis L. Freezing does not alter antigenic properties of fresh fruits for skin testing in patients with birch tree pollen-induced oral allergy syndrome. J Allergy Clin Immunol. 2011;127:1624–6. doi: 10.1016/j.jaci.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–83. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121:1219–1224. doi: 10.1016/j.jaci.2007.12.1150. [DOI] [PubMed] [Google Scholar]
- 16.Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol. 2004;114:144–149. doi: 10.1016/j.jaci.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582–586. doi: 10.1067/mai.2000.104941. [DOI] [PubMed] [Google Scholar]
- 18.Sicherer SH, Wood RA. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012;129:193–7. doi: 10.1542/peds.2011-2382. [DOI] [PubMed] [Google Scholar]
- 19.Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001;108:881–90. doi: 10.1067/mai.2001.118515. [DOI] [PubMed] [Google Scholar]
- 20.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–1274. doi: 10.1016/j.jaci.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, et al. Standardization of food challenges in patients with immediate reactions to foods - position paper from the European Academy of Allergology and Clinical Immunology. Allergy. 2004;59:690–697. doi: 10.1111/j.1398-9995.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 22.Bahna SL. Blind food challenge testing with wide-open eyes. Ann Allergy. 1994;72:235–238. [PubMed] [Google Scholar]
- 23.Thomas K, Herouet-Guicheney C, Ladics G, et al. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: international workshop report. Food Chem Toxicol. 2007;45:1116–1122. doi: 10.1016/j.fct.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Eigenmann PA. Anaphylactic reactions to raw eggs after negative challenges with cooked egg. J Allergy Clin Immunol. 2000;105:587–588. doi: 10.1067/mai.2000.104255. [DOI] [PubMed] [Google Scholar]
- 25.Beyer K, Morrow E, Li XM, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107:1077–1081. doi: 10.1067/mai.2001.115480. [DOI] [PubMed] [Google Scholar]
- 26.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122:342–347. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 27.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122:977–983. doi: 10.1016/j.jaci.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Busse PJ, Nowak-Wegrzyn AH, Noone SA, Sampson HA, Sicherer SH. Recurrent peanut allergy. N Engl J Med. 2002;347:1535–1536. doi: 10.1056/NEJM200211073471921. [DOI] [PubMed] [Google Scholar]
- 29.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. Peanut allergy: recurrence and its management. J Allergy Clin Immunol. 2004;114:1195–201. doi: 10.1016/j.jaci.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman JA, Cox AL, Vitale M, Sampson HA. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128(5):1120–1122. doi: 10.1016/j.jaci.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolaou N, Poorafshar M, Murray C, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allery. J Pediatr. 2011;158:578–583. doi: 10.1016/j.jpeds.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Sicherer SH, Wood RA. Advances in diagnosing peanut allergy. J Allergy Clin Immunol Pract. 2013;1:1–13. doi: 10.1016/j.jaip.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004;34:583–90. doi: 10.1111/j.1365-2222.2004.1923.x. [DOI] [PubMed] [Google Scholar]
- 35.Dang TD, Tang M, Choo S, et al. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012;129:1056–63. doi: 10.1016/j.jaci.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 36.Osborne NJ, Koplin JJ, Martin PE, et al. The HealthNuts population-based study of paediatric food allergy: validity, safety and acceptability. Clin Exp Allergy. 2010;40:1516–22. doi: 10.1111/j.1365-2222.2010.03562.x. [DOI] [PubMed] [Google Scholar]
- 37.Keet CA, Johnson K, Savage JH, Hamilton RG, Wood RA. Evaluation of Ara h 2 IgE thresholds in the diagnosis of peanut allergy in a clinical population. J Allergy Clin Immunol Pract. 2013;1:101–3. doi: 10.1016/j.jaip.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes de Oliveira LC, Aderhold M, Brill M, et al. The value of specific IgE to peanut and its component Ara-h 2 in the diagnosis of peanut allergy. J Allergy Clin Immunol Pract. 2013;1:394–8. doi: 10.1016/j.jaip.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Klemans RJ, Otte D, Knol M, et al. The diagnostic value of specific IgE to Ara h 2 to predict peanut allergy in children is comparable to a validated and updated diagnostic prediction model. J Allergy Clin Immunol. 2013;131:157–63. doi: 10.1016/j.jaci.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Koppelman SJ, de Jong GA, Laaper-Ertmann M, et al. Purification and immunoglobulin E-binding properties of peanut allergen Ara h 6: evidence for cross-reactivity with Ara h 2. Clin Exp Allergy. 2005;35:490–7. doi: 10.1111/j.1365-2222.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- 41.Asarnoj A, Glaumann S, Elfström L, et al. Anaphylaxis to peanut in a patient predominantly sensitized to Ara h 6. Int Arch Allergy Immunol. 2012;159:209–12. doi: 10.1159/000336027. [DOI] [PubMed] [Google Scholar]
- 42.Klemans RJ, Knol EF, Bruijnzeel-Koomen CA, Knulst AC. The diagnostic accuracy of specific IgE to Ara h 6 in adults is as good as Ara h 2. Allergy. 2014 doi: 10.1111/all.12424. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Sicherer SH, Dhillon G, Laughery KA, Hamilton RG, Wood RA. Caution: the Phadia hazelnut ImmunoCAP (f17) has been supplemented with recombinant Cor a 1 and now detects Bet v 1-specific IgE, which leads to elevated values for persons with birch pollen allergy. J Allergy Clin Immunol. 2008;122:413–4. doi: 10.1016/j.jaci.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Ortolani C, Ballmer-Weber BK, Hansen KS, et al. Hazelnut allergy: a double-blind, placebo-controlled food challenge multicenter study. J Allergy Clin Immunol. 2000;105:577–81. doi: 10.1067/mai.2000.103052. [DOI] [PubMed] [Google Scholar]
- 45.Flinterman AE, Akkerdaas JH, den Hartog Jager CF, et al. Lipid transfer protein-linked hazelnut allergy in children from a non-Mediterranean birch-endemic area. J Allergy Clin Immunol. 2008;121:423–8. doi: 10.1016/j.jaci.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Beyer K, Grishina G, Bardina L, Grishin A, Sampson HA. Identification of an 11S globulin as a major hazelnut food allergen in hazelnut-induced systemic reactions. J Allergy Clin Immunol. 2002;110:517–23. doi: 10.1067/mai.2002.127434. [DOI] [PubMed] [Google Scholar]
- 47.De Knop KJ, Verweij MM, Grimmelikhuijsen M, et al. Age-related sensitization profiles for hazelnut (Corylus avellana) in a birch-endemic region. Pediatr Allergy Immunol. 2011;22:e139–49. doi: 10.1111/j.1399-3038.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 48.Masthoff LJ, Mattsson L, Zuidmeer-Jongejan L, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013;132:393–9. doi: 10.1016/j.jaci.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Kattan JD, Sicherer SH, Sampson HA. Clinical reactivity to hazelnut may be better identified by component testing than traditional testing methods. J Allergy Clin Immunol Pract. doi: 10.1016/j.jaip.2014.03.013. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartnikas LM, Sheehan WJ, Larabee KS, Petty C, Schneider LC, Phipatanakul W. Ovomucoid is not superior to egg white testing in predicting tolerance to baked egg. J Allergy Clin Immunol Pract. 2013;1:354–60. doi: 10.1016/j.jaip.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ott H, Baron JM, Heise R, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008;63:1521–8. doi: 10.1111/j.1398-9995.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- 52.D’Urbano LE, Pellegrino K, Artesani MC, et al. Performance of a component-based allergen-microarray in the diagnosis of cow’s milk and hen’s egg allergy. Clin Exp Allergy. 2010;40:1561–70. doi: 10.1111/j.1365-2222.2010.03568.x. [DOI] [PubMed] [Google Scholar]
- 53.Alessandri C, Zennaro D, Scala E, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen’s egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2012;42:441–50. doi: 10.1111/j.1365-2222.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 54.Caubet JC, Bencharitiwong R, Moshier E, Godbold JH, Sampson HA, Nowak-Wegrzyn A. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J Allergy Clin Immunol. 2012;129:739–47. doi: 10.1016/j.jaci.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 55.Caubet JC, Nowak-Wegrzyn A, Moshier E, Godbold J, Wang J, Sampson HA. Utility of casein-specific IgE levels in predicting reactivity to baked milk. J Allergy Clin Immunol. 2013;131:222–4. doi: 10.1016/j.jaci.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuo H, Dahlstrom J, Tanaka A, Kohno K, Takahashi H, Furumura M. Sensitivity and specificity of recombinant omega-5 gliadin-specific IgE measurement for the diagnosis of wheat-dependent exercise-induced anaphylaxis. Allergy. 2008;63:233–6. doi: 10.1111/j.1398-9995.2007.01504.x. [DOI] [PubMed] [Google Scholar]
- 57.Palosuo K, Varjonen E, Kekki OM, et al. Wheat omega-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J Allergy Clin Immunol. 2001;108:634–8. doi: 10.1067/mai.2001.118602. [DOI] [PubMed] [Google Scholar]
- 58.Shibata R, Nishima S, Tanaka A, Borres MP, Morita E. Usefulness of specific IgE antibodies to ω-5 gliadin in the diagnosis and follow-up of Japanese children with wheat allergy. Ann Allergy Asthma Immunol. 2011;107:337–43. doi: 10.1016/j.anai.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 59.Beyer K, Chung D, Schulz G, et al. The role of wheat omega-5 gliadin IgE antibodies as a diagnostic tool for wheat allergy in childhood. J Allergy Clin Immunol. 2008;122:419–21. doi: 10.1016/j.jaci.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Baar A, Pahr S, Constantin C, et al. Specific IgE reactivity to Tri a 36 in children with wheat food allergy. doi: 10.1016/j.jaci.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 61.Gómez-Casado C, Garrido-Arandia M, Pereira C, et al. Component-resolved diagnosis of wheat flour allergy in baker’s asthma. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.03.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.Gámez C, Sánchez-García S, Ibáñez MD, et al. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy. 2011;66:1375–83. doi: 10.1111/j.1398-9995.2011.02663.x. [DOI] [PubMed] [Google Scholar]
- 63.Ebisawa M, Brostedt P, Sjolander S, Sato S, Borres MP, Ito K. Gly m 2S albumin is a major allergen with a high diagnostic value in soybean-allergic children. J Allergy Clin Immunol. 2013;132:976–8. doi: 10.1016/j.jaci.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 64.Sampson HA. Improving in-vitro tests for the diagnosis of food hypersensitivity. Curr Opin Allergy Clin Immunol. 2002;2:257–61. doi: 10.1097/00130832-200206000-00017. [DOI] [PubMed] [Google Scholar]
- 65.Chatchatee P, Jarvinen KM, Bardina L, Beyer K, Sampson HA. Identification of IgE- and IgG-binding epitopes on alpha(s1)-casein: differences in patients with persistent and transient cow’s milk allergy. J Allergy Clin Immunol. 2001;107:379–83. doi: 10.1067/mai.2001.112372. [DOI] [PubMed] [Google Scholar]
- 66.Chatchatee P, Järvinen KM, Bardina L, Vila L, Beyer K, Sampson HA. Identification of IgE and IgG binding epitopes on beta- and kappa-casein in cow’s milk allergic patients. Clin Exp Allergy. 2001;31:1256–62. doi: 10.1046/j.1365-2222.2001.01167.x. [DOI] [PubMed] [Google Scholar]
- 67.Savilahti EM, Rantanen V, Lin JS, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol. 2010;125:1315–21. doi: 10.1016/j.jaci.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ayuso R, Sanchez-Garcia S, Pascal M, et al. Is epitope recognition of shrimp allergens useful to predict clinical reactivity? Clin Exp Allergy. 2012;42:293–304. doi: 10.1111/j.1365-2222.2011.03920.x. [DOI] [PubMed] [Google Scholar]
- 69.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159:2026–32. [PubMed] [Google Scholar]
- 70.Battais F, Mothes T, Moneret-Vautrin DA, et al. Identification of IgE-binding epitopes on gliadins for patients with food allergy to wheat. Allergy. 2005;60:815–21. doi: 10.1111/j.1398-9995.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- 71.Hoffman KM, Ho DG, Sampson HA. Evaluation of the usefulness of lymphocyte proliferation assays in the diagnosis of allergy to cow’s milk. J Allergy Clin Immunol. 1997;99:360–6. doi: 10.1016/s0091-6749(97)70054-x. [DOI] [PubMed] [Google Scholar]
- 72.Thottingal TB, Stefura BP, Simons FE, Bannon GA, Burks W, HayGlass KT. Human subjects without peanut allergy demonstrate T cell-dependent, TH2-biased, peanut-specific cytokine and chemokine responses independent of TH1 expression. J Allergy Clin Immunol. 2006;118:905–14. doi: 10.1016/j.jaci.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 73.Flinterman AE, Pasmans SG, den Hartog Jager CF, et al. T cell responses to major peanut allergens in children with and without peanut allergy. Clin Exp Allergy. 2010;40:590–7. doi: 10.1111/j.1365-2222.2009.03431.x. [DOI] [PubMed] [Google Scholar]
- 74.Sato S, Tachimoto H, Shukuya A, et al. Basophil activation marker CD203c is useful in the diagnosis of hen’s egg and cow’s milk allergies in children. Int Arch Allergy Immunol. 2010;152:54–61. doi: 10.1159/000312126. [DOI] [PubMed] [Google Scholar]
- 75.Ford LS, Bloom KA, Nowak-Wegrzyn A, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J Allergy Clin Immunol. 2013;131:180–6. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanahan DJ. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- 77.Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem. 1990;265:17381–4. [PubMed] [Google Scholar]
- 78.Imaizumi TA, Stafforini DM, Yamada Y, McIntyre TM, Prescott SM, Zimmerman GA. Platelet-activating factor: a mediator for clinicians. J Intern Med. 1995;238:5–20. doi: 10.1111/j.1365-2796.1995.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 79.Izumi T, Shimizu T. Platelet-activating factor receptor: gene expression and signal transduction. Biochem Biophys Acta. 1995;1259:317–333. doi: 10.1016/0005-2760(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 80.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 81.Ishii S, Kuwaki T, Nagase T, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–88. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 83.Bock SA, Buckley J, Holst A, May CD. Proper use of skin tests with food extracts in diagnosis of hypersensitivity to food in children. Clin Allergy. 1977;7:375–83. doi: 10.1111/j.1365-2222.1977.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 84.Mehl A, Rolinck-Werninghaus C, Staden U, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118:923–9. doi: 10.1016/j.jaci.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Keskin O, Tuncer A, Adalioglu G, Sekerel BE, Sackesen C, Kalayci O. Evaluation of the utility of atopy patch testing, skin prick testing, and total and specific IgE assays in the diagnosis of cow’s milk allergy. Ann Allergy Asthma Immunol. 2005;94:553–60. doi: 10.1016/S1081-1206(10)61133-7. [DOI] [PubMed] [Google Scholar]
- 86.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–8. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 87.Isolauri E, Turjanmaa K. Combined skin prick and patch testing enhances identification of food allergy in infants with atopic dermatitis. J Allergy Clin Immunol. 1996;97:9–15. doi: 10.1016/s0091-6749(96)70277-4. [DOI] [PubMed] [Google Scholar]
- 88.Mehl A, Verstege A, Staden U, et al. Utility of the ratio of food-specific IgE/total IgE in predicting symptomatic food allergy in children. Allergy. 2005;60:1034–9. doi: 10.1111/j.1398-9995.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 89.Gupta RS, Lau CH, Hamilton RG, Donnell A, Newhall KK. Predicting outcomes of oral food challenges by using the allergen-specific IgE-total IgE ratio. J Allergy Clin Immunol Pract. 2014;2:300–5. doi: 10.1016/j.jaip.2013.12.006. [DOI] [PubMed] [Google Scholar]