Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy in need of more effective treatment approaches. One potential therapeutic target is Wnt/β-catenin signaling, which plays important roles in PDAC tumor initiation and progression. Among Wnt inhibitors with suitable in vivo biological activity is vitamin D, which is known to antagonize Wnt/β-catenin signaling in colorectal cancer and have anti-tumor activity in PDAC. For this study the relationship between vitamin D signaling, Wnt/β-catenin activity and tumor cell growth in PDAC was investigated through the use of calcipotriol, a potent non-hypercalcemic vitamin D analog. PDAC tumor cell growth inhibition by calcipotriol was positively correlated with vitamin D receptor (VDR) expression and Wnt/β-catenin activity. Furthermore, vitamin D and Wnt signaling activity were found to be reciprocally linked through feedback regulation. Calcipotriol inhibited autocrine Wnt/β-catenin signaling in PDAC cell lines in parallel with decreased protein levels of the low density lipoprotein receptor-related protein 6 (LRP6), a requisite co-receptor for ligand-dependent canonical Wnt signaling. Decrease in LRP6 protein seen with calcipotriol was mediated through a novel mechanism involving transcriptional upregulation of low-density lipoprotein receptor adaptor protein 1 (LDLRAP1). Finally, changes in LRP6 or LDLRAP1 expression directly altered Wnt reporter activity, supporting their roles as regulators of ligand-dependent Wnt/β-catenin signaling.
Implications
This study provides a novel biochemical target through which vitamin D signaling exerts inhibitory effects on Wnt/β-catenin signaling, as well as potential biomarkers for predicting and following tumor response to vitamin D-based therapy.
Keywords: Pancreatic ductal adenocarcinoma, Wnt/β-catenin signaling, vitamin D, LRP6, LDLRAP1
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly recalcitrant malignancy with an overall five-year survival rate of 6%(1). Its poor prognosis is linked to its typically advanced clinical stage at diagnosis, rapid disease progression and resistance to cytotoxic or targeted molecular therapies(2). Improved therapeutic approaches based on a detailed understanding of the molecular and cellular basis of PDAC are needed. One therapeutic target now under consideration is Wnt/β-catenin signaling, one of twelve core pathways and processes genetically altered in most PDAC(3).
Wnt signaling plays pivotal roles in embryogenesis and adult tissue homeostasis through its regulation of diverse biological functions, including cell fate specification, proliferation and cell migration. Wnt ligands are secreted glycoproteins that signal through Frizzled (FZD) receptor and low-density lipoprotein receptor-related protein 5 or 6 (LRP5 or LRP6) co-receptor. Activation of canonical Wnt signaling (hereafter referred to as Wnt/β-catenin-dependent signaling) culminates in the cytoplasmic accumulation of β-catenin and its translocation to the nucleus where it complexes with transcription factors and co-activators to drive context-dependent target gene expression(4). Deregulation of Wnt/β-catenin signaling occurs in many types of cancer either through key driver mutations or increased Wnt ligand-mediated receptor activation. In PDAC, Wnt/β-catenin signaling is required for tumor initiation(5) and confers traits associated with “stemness” in susceptible cell lines(6). Furthermore, Wnt/β-catenin signaling supports various pro-tumorigenic phenotypes in established PDAC cell lines and has been correlated with aggressive clinical behavior in primary PDAC tumor samples(5, 7, 8).
Several Wnt inhibitors are now at various stages of preclinical and early clinical development(9). They include small molecule inhibitors, blocking antibodies, inhibitory fusion proteins, peptide antagonists and several naturally occurring compounds, including vitamin D. Calcitriol, the biologically active metabolite of vitamin D (and ligand of the vitamin D receptor), has been shown to disrupt Wnt/β-catenin signaling through multiple mechanisms. In colon cancer lines, calcitriol induces the vitamin D receptor (VDR) to directly bind β-catenin and increases E-cadherin expression to sequester β-catenin at adherens junctions, which leads to reduced β-catenin/TCF interaction and target gene expression(10). Calcitriol also indirectly increases the expression of DKK1, a secreted protein antagonist of Wnt/β-catenin signaling(11).
Vitamin D is acquired through dietary intake and ultraviolet-light mediated metabolism of its precursors in the skin. Vitamin D metabolites bind to VDR, a transcription factor which functions as an obligate heterodimer with the retinoid X receptor (RXR) to regulate the expression of target genes with vitamin D response elements. In addition to regulating calcium homeostasis, vitamin D exhibits anti-cancer action through a variety of anti-proliferative, pro-apoptotic and differentiation effects. Accordingly, vitamin D and its analogs are promising chemopreventative and chemotherapeutic agents for numerous malignancies. In relation to PDAC, some epidemiologic studies show higher intake or increased plasma levels of vitamin D correlate with decreased incidence of PDAC(12, 13). Although a published meta-analysis concludes no significant association between vitamin D levels and PDAC risk(14), a high prevalence of vitamin D insufficiency has been observed in patients with advanced pancreatic cancer(15). Published experimental studies show vitamin D and its synthetic analogs inhibit the in vitro and in vivo growth of some but not all PDAC cell lines through pro-apoptotic and anti-proliferative actions (16-18). A recent study by Sherman, et al., finds VDR also functions as a master transcriptional regulator of pancreatic stellate cell quiescence and that the vitamin D analog calcipotriol can suppress pancreatitis and mediate stromal remodeling to improve chemotherapeutic drug delivery and survival in a Kras-driven mouse model of PDAC(19).
Here we examine the potential interplay between Wnt/β-catenin and vitamin D signaling in PDAC. We show the ability of calcipotriol to inhibit PDAC cell growth strongly correlates with expression of VDR, which is correlated with and regulated by Wnt signaling. We demonstrate that calcipotriol induces expression of low-density lipoprotein receptor adaptor protein 1 (LDLRAP1), which in turn mediates rapid reduction in LRP6 protein levels and corresponding inhibition of Wnt signaling, providing an additional novel mechanism by which vitamin D can inhibit Wnt signaling activity. Our findings suggest that, in addition to potential use as stromal therapy, vitamin D analogs are suitable agents for directly targeting tumor cells in the subset of pancreatic cancers and precursor lesions defined by Wnt-dependent growth activity and higher VDR expression.
Materials and Methods
Cell lines and reagents
All cell lines were cultured as previously described(8). AsPC-1, HPAF-2, MiaPaCa-2 and PANC-1 were purchased from the American Type Culture Collection (Rockville, MD) in 2005. YAPC, Suit and HCG25 were kindly provided by Dr. Eric Collisson (University of California, San Francisco) in 2012. Cell lines have not been further authenticated since initial receipt. Calcipotriol, Bafilomycin A1 and CHIR99021 were purchased from Tocris (Minneapolis, MN).
Quantitative real-time PCR
RNA extraction, cDNA synthesis and SYBR green qPCR were performed as previously described(8) using primers listed in Supplementary Table S1. All values were normalized to ACTB housekeeping gene.
Gene knockdown
AsPC-1 cells were transfected using Lipofectamine 2000 (Life Technologies, Grand Island, NY) per manufacturer's instructions using 20nM of siRNAs purchased from GE Dharmacon (Lafayette, CO), including control (D-001810-10-05), VDR (L-003448-00-0005), LDLRAP1 (L-013025-00-0005), DKK1 (L-003843-01-0005) or combined 10nM of RXRA (L-003448-00-0005) and 10 nM RXRB (L-003444-00-0005).
Western blot and immunoprecipitation
SDS-PAGE and immunoblots were performed as previously described(20) using antibodies for β-catenin (C2206; Sigma, St. Louis, MO), tubulin (sc-5546; Santa Cruz Biotechnology, Santa Cruz, CA), VDR (sc-13133), E-cadherin (sc-21791), DKK-1 (GTX62902; GeneTex, Irvine, CA), LRP6 (CST3395; Cell Signaling Technology, Danvers, MA), RXRα (CST3085), RXRβ (CST8715), LDLRAP1 (C20125; LSBio, Seattle, WA) or LC3B (ab48394; Abcam, Cambridge, MA). For LRP6 and LC3B immunoblots, lysates were resolved on 4-20% gradient gels. Co-immunoprecipitations were performed as previously described(21). Briefly, AsPC-1 lysates were pre-cleared with A/G-PLUS agarose beads (Santa Cruz; sc-2003) and immunoprecipitated using antibody to β-catenin (BD Transduction Laboratories; 610153), VDR (Santa Cruz; sc-1008) or control isotype-matched IgG (Santa Cruz; sc-2025 or Abcam ab46540). After multiple washes, immune complexes were boiled in 6× SDS-load dye, resolved by SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting.
Cell growth assays
MTT assays (ATCC) were carried out per manufacturer's instructions in 96-well plates with initial plating of 500 (Suit2), 1,000 (Tu8988t) or 2,000 (HPAF-2, YAPC, MiaPaCa-2, AsPC-1, PANC-1) cells per well. Cells were allowed to adhere overnight and then treated with calcipotriol. Soft agar assays were performed as previously described(20).
Wnt reporter assays
Baseline Wnt reporter activity was measured in dual luciferase assays (Promega, Madison, WI) as previously described(8) by transient co-transfection with control plasmid with constitutive EF1α promoter driving Renilla expression (serving as a normalization control) and either BAR (beta-catenin activated reporter, 12 TCF response elements driving luciferase expression) or fuBAR (found unresponsive BAR, contains mutated TCF response elements). In other experiments, Wnt reporter activity was measured in AsPC-1 stably transduced with both BAR reporter and Renilla control as previously described(20).
LRP6 overexpression
LRP6 or GFP (control) expression constructs in pCS2 vector have been previously described(22) and were kindly provided by Edward De Robertis (University of California, Los Angeles). Transfections were performed with X-tremeGENE9 (Roche, Indianapolis, IN) per manufacturer's instructions.
RNA isolation, library generation, sequencing and analysis
Total RNA was isolated from human pancreatic cancer cell lines as previously described(19). Sequencing libraries were prepared from 100–500 ng of total RNA using the TruSeq RNA sample preparation kit v2 (Illumina, San Diego, CA) according to the manufacturer's protocol. Briefly, mRNA was purified, fragmented and used for first- and second-strand cDNA synthesis followed by adenylation of 3′ ends. Samples were ligated to unique adaptors and subjected to PCR amplification. Libraries were validated using the 2100 BioAnalyzer (Agilent, Santa Clara, CA), normalized and pooled for sequencing. RNA-seq libraries from three biological replicates for each experimental condition were sequenced on the Illumina HiSeq 2000 using barcoded multiplexing and a 100-bp read length. Image analysis and base calling were done with Illumina CASAVA-1.8.2. This yielded a median of 12.4 million usable reads per sample. Short read sequences were mapped to a UCSC hg19 reference sequence using the RNA-seq aligner STAR(23). Known splice junctions from hg19 were supplied to the aligner and de novo junction discovery was also permitted. Differential gene expression analysis, statistical testing and annotation were performed using Cuffdiff 2(24). Transcript expression was calculated as gene-level relative abundance in fragments per kilobase of exon model per million mapped fragments and employed correction for transcript abundance bias (25). RNA-seq data is deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA accession number SRP057571). Functional enrichment analysis was limited to gene ontology terms and examined using default parameters in the web-based Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.7; http://david.abcc.ncifcrf.gov)(26).
Microarray data analysis
Gene set enrichment analysis (GSEA) method (http://www.broadinstitute.org/gsea)(27) was used to determine whether a defined pancreatic-specific Wnt/β-catenin target gene set showed statistically significant concordance with VDR expression in a previously published gene expression microarray dataset of 25 primary human PDAC samples (deposited in NCBI GEO under accession number GSE32688). GSEA default parameter settings and the Pearson metric for ranking genes were used with VDR expression serving as a continuous phenotypic variable. The defined pancreatic-specific Wnt/β-catenin target gene set was the previously published(20) overlap of downregulated transcripts observed in AsPC-1 in response to either Wnt inhibitor ICG-001 or CTNNB1 siRNA transfection.
Statistical methods
Statistical analyses were performed in GraphPad Prism (ver 5.04, La Jolla, CA). Student t tests were used to compare continuous variables. The level of significance for all statistical tests was defined as α=0.05.
Results
PDAC growth inhibition by calcipotriol is linked to VDR expression and Wnt signaling
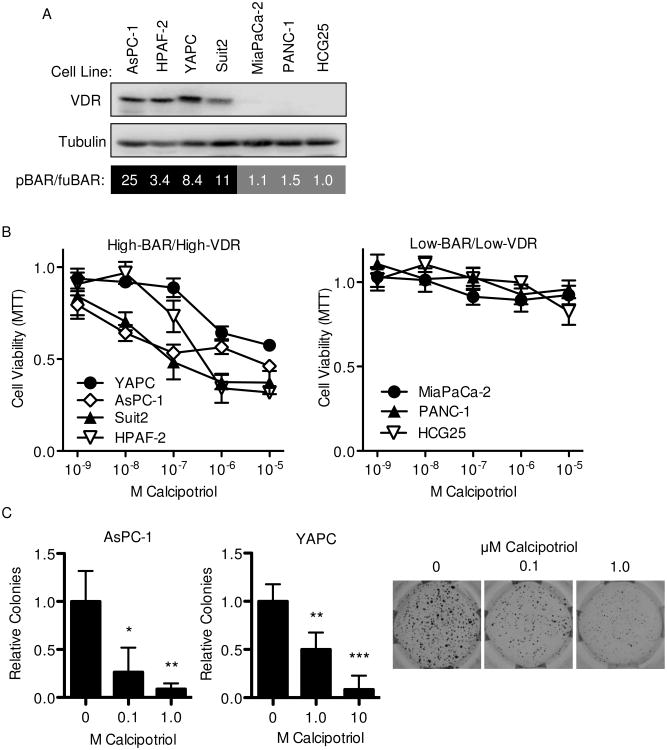
In previous work, we described a distinct subset of PDAC cell lines with higher levels of autocrine Wnt/β-catenin signaling and an anchorage-independent growth phenotype responsive to genetic or pharmacologic inhibition of Wnt(8). Given that vitamin D inhibits Wnt signaling in colon cancer(10, 11), we hypothesized that the growth-inhibitory effects of the vitamin D on PDAC tumor cells might depend on their baseline autocrine Wnt/β-catenin signaling activity. Autocrine Wnt/β-catenin activity was measured across a panel of PDAC cell lines by dual luciferase promoter-reporter assays to determine the ratio of BAR (beta-catenin activated reporter, 12 TCF response elements driving luciferase expression) to fuBAR (found unresponsive BAR, contains mutated TCF response elements)(28). AsPC1, HPAF-2, YAPC and Suit2 each had higher levels of autocrine Wnt activity (hereafter designated as High-BAR lines), while MiaPaCa-2, PANC-1 and HCG25 each had lower or absent levels of autocrine Wnt activity (hereafter designated as Low-BAR lines)(Figure 1A). Levels of autocrine Wnt activity were observed to closely parallel VDR protein expression (Figure 1A) with western blots showing higher VDR expression (High-VDR) in High-BAR lines and low or undetectable VDR expression (Low-VDR) in Low-BAR lines. Furthermore, calcipotriol inhibited anchorage-dependent growth of High-BAR/High-VDR cell lines in a dose-dependent manner at concentrations ranging from 10 nM to 1 μM, but was unable to inhibit the growth of Low-BAR/Low-VDR cell lines even at concentrations up to 10 μM (Figure 1B). Calcipotriol inhibited anchorage-independent growth of High-BAR/High-VDR cell lines in soft agar assays (Figure 1C), a phenotype we previously showed to be dependent on Wnt signaling in High-BAR PDAC lines(8). These results demonstrate PDAC growth inhibition by calcipotriol correlates with VDR expression status and might be functionally linked to underlying differences in Wnt activity, whereby Wnt/β-catenin signaling serves as a mediator and/or target of vitamin D action.
Figure 1.
Inhibition of pancreatic cancer growth by calcipotriol correlates with levels of VDR expression and autocrine Wnt/β-catenin signaling. (A) Western blot for VDR and tubulin (loading control) and mean Wnt reporter activity as indicated by BAR/fuBAR ratios and measured by dual luciferase assays 48 hours after co-transfection with control Renilla and either BAR-luciferase or fuBAR-luciferase reporter constructs in indicated PDAC cell lines (n=3 biological replicates). (B) MTT growth assays (measured at 72 hours) and (C) soft agar colony formation at indicated concentrations of calcipotriol. Representative images of AsPC-1 soft agar colony formation are also shown. Data are normalized to vehicle only and reported as mean ± standard deviation (SD) with one representative experiment of 3-5 biological repeats shown. *P<0.05, **P<0.01, ***P<0.001.
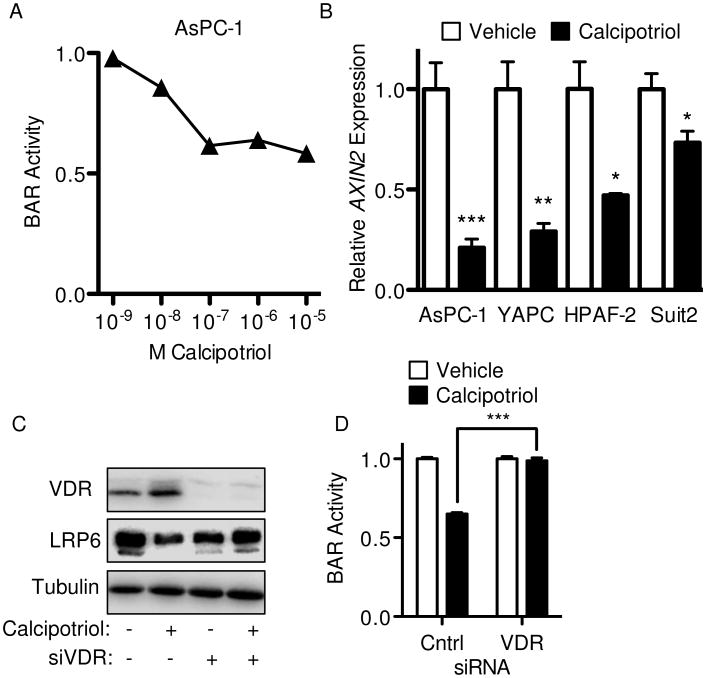
To more directly address mechanistic links between vitamin D and Wnt signaling in PDAC, AsPC-1 cells stably transduced with BAR were treated with increasing concentrations of calcipotriol. Calcipotriol significantly inhibited Wnt/β-catenin activity in a dose-dependent manner as measured by BAR reporter (Figure 2A). Calcipotriol also significantly inhibited expression of the endogenous Wnt/β-catenin transcriptional target AXIN2 across all High-BAR/High-VDR cell lines (Figure 2B). To confirm calcipotriol inhibited Wnt signaling in a VDR-dependent manner, cells were treated in the context of siRNA-mediated VDR knockdown. VDR knockdown fully rescued inhibition of BAR reporter seen with calcipotriol (Figure 2C-D), indicating Wnt inhibition by calcipotriol was VDR-dependent and did not occur through off-target or other receptor-independent effects.
Figure 2.
Calcipotriol mediates inhibition of Wnt signaling through VDR. (A) Dose response curve for calcipotriol on Wnt signaling as measured at 24 hours in AsPC-1 cells with stable BAR-luciferase reporter. (B) AXIN2 expression measured by qPCR at 24 hours following treatment with vehicle or 100 nM (AsPC-1), 5 μM (YAPC) or 1 μM (HPAF-2 and Suit2) calcipotriol. (C) VDR, LRP6 and tubulin (loading control) western blots and (D) BAR-luciferase activity for AsPC-1 transfected with either control or VDR siRNA for 48 hours and an additional 24 hour treatment with vehicle or 100 nM calcipotriol. All values were normalized to respective controls, with BAR reported as mean ± SD and qPCR reported as mean ± SEM. One representative experiment from at least three biological repeats is shown. *P<0.05, **P<0.01, ***P<0.001.
We next explored the mechanism by which calcipotriol inhibits Wnt activity in PDAC. In colon cancer cell lines, ligand-activated VDR has been shown to directly interact with β-catenin, leading to cytoplasmic sequestration of β-catenin and inhibition of β-catenin/TCF mediated transcription(10). Addressing this possibility in PDAC, cell extracts of AsPC-1 treated with either vehicle or calcipotriol were immunoprecipitated with anti- β-catenin antibody. While E-cadherin co-immunoprecipitated with β-catenin as expected, VDR failed to co-immunoprecipitate with β-catenin either in the presence of absence of calcipotriol (Supplemental Figure 1A). Reverse co-immunoprecipitation with anti-VDR antibody also failed to demonstrate an interaction between VDR and β-catenin (data not shown), suggesting Wnt inhibition by calcipotriol in does not occur through sequestration of β-catenin by ligand-activated VDR. Calcitriol has been shown to increase expression of the secreted Wnt inhibitor DKK1 through an indirect transcriptional mechanism(11). While calcipotriol increased DKK1 protein levels in AsPC-1, this increase was functionally decoupled from inhibition of Wnt signaling by calcipotriol, as inhibition of BAR activity by calcipotriol was not rescued by concurrent siRNA-mediated knockdown of DKK1 (Supplemental Figure 1B-C). Furthermore, siRNA knockdown of DKK1 paradoxically reduced BAR activity in the absence of calcipotriol, indicating DKK1 does not functionally inhibit Wnt signaling in AsPC-1 cells. Together, these results suggest calcipotriol inhibits Wnt signaling in PDAC through an alternative mechanism.
Calcipotriol reduces LRP6 protein levels
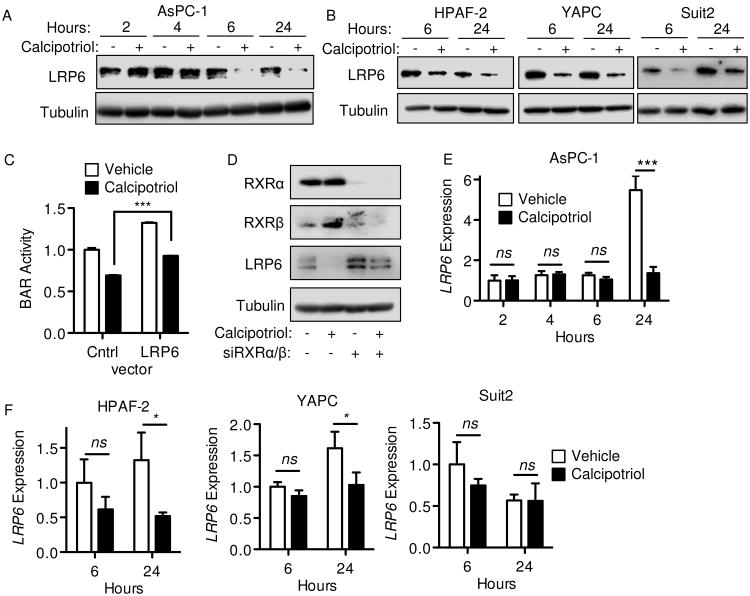
LRP6 is a requisite co-receptor for Wnt ligand-initiated canonical signaling. We noted LRP6 protein levels decreased within 6 hours of calcipotriol treatment in all High-BAR/High-VDR PDAC lines (Figure 3A-B), but not in Low-BAR/Low-VDR PDAC lines (Supplemental Figure 2A). Knockdown of VDR by siRNA blocked the decrease in LRP6 protein observed with calcipotriol (Figure 2C), indicating the decrease in LRP6 protein by calcipotriol is VDR-dependent. Transient overexpression of wild-type LRP6 increased BAR activity compared to GFP-control vector in both vehicle and calcipotriol-treated cells (Figure 3C), indicating changes in LRP6 expression directly alter Wnt reporter activity in AsPC-1. When normalized to vehicle control, calcipotriol inhibited BAR activity in control GFP or LRP6 transfected cells to a similar extent (31% and 28% relative change respectively, Figure 3C), suggesting calcipotriol may inhibit BAR activity through an LRP6-independent mechanism. Alternatively, overexpressed LRP6 might remain subject to relative downregulation by calcipotriol via the mechanism further detailed below.
Figure 3.
Calcipotriol treatment reduces LRP6 expression in PDAC. (A-B) Western blots for LRP6 or tubulin (loading control) were performed on whole cell lysates at indicated time points after cell line treatment with vehicle or 100 nM (AsPC-1), 5 μM (YAPC) or 1 μM (HPAF-2 and Suit2) calcipotriol. (C) BAR-luciferase activity for AsPC-1 transfected with full-length LRP6 expression vector or control GFP vector for 48 hours and measured after a further 24 hour treatment with vehicle or 100 nM calcipotriol. (D) RXRα, RXRβ, LRP6 and tubulin western blots on AsPC-1 whole cell lysates after control or tandem RXRA and RXRB siRNA transfection for 48 hours and further treatment with vehicle or 100 nM calcipotriol for 24 hours. (E-F) LRP6 gene expression measured by qPCR after 100 nM calcipotriol treatment for indicated times. Cells were synchronized by serum-starvation prior to calcipotriol treatment. All values were normalized to respective controls, with BAR reported as mean ± SD and qPCR reported as mean ± SEM. One representative experiment of 2-3 biological repeats is shown. *P<0.05, **P<0.01, ***P<0.001, ns = not significant.
To discriminate between genomic versus potential non-genomic mechanisms of action of VDR on LRP6 levels, the VDR transcriptional co-activators RXRα and RXRβ were knocked down in tandem by siRNA transfection. Combined RXRα/RXRβ knockdown blocked the decrease in LRP6 seen with calcipotriol, arguing again in favor of a VDR/RXR-dependent transcriptional mechanism (Figure 3D). However, while calcipotriol decreased LRP6 protein levels as early as 6 hours in each of the High-BAR/High-VDR PDAC lines, LRP6 transcript levels only differed between calcipotriol and vehicle control treatments after 24 hours in AsPC-1, HPAF-2 and YAPC (Figure 3E-F) and not at all in Suit2 (Figure 3F). This difference in transcript levels at 24 hours specifically reflected an increase in LRP6 expression in vehicle treated cells that did not occur in calcipotriol treated cells. Given LRP6 protein decreases prior to any effect on LRP6 transcript levels, we speculated that calcipotriol inhibits Wnt signaling in PDAC by regulating LRP6 protein levels indirectly through an VDR/RXR-dependent transcriptional mechanism.
LDLRAP1 mediates reduced LRP6 protein levels in response to calcipotriol
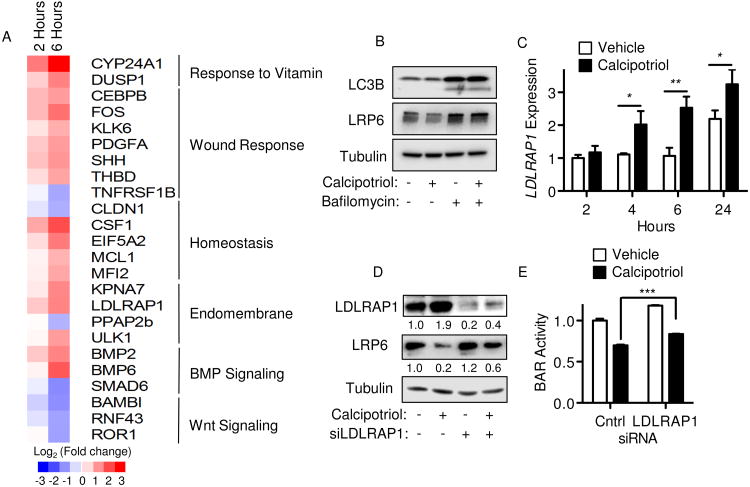
To further explore the manner in which LRP6 protein was decreased in response to calcipotriol, RNA sequencing (RNA-seq) was performed on AsPC-1 cells. Comparison of transcriptomes following calcipotriol treatment confirmed expression of known vitamin D transcriptional targets, including CYP24A1 as the most highly upregulated gene (Figure 4A and Supplemental Table 2). A total of 325 genes (220 upregulated and 105 downregulated) showed at least 1.5-fold change in expression at 6 hours (P<0.00001). Top gene ontology terms enriched in the subset of the top 220 upregulated genes included regulation of transcription from RNA polymerase II promoter, osteoblast differentiation, positive regulation of cellular biosynthetic processes and response to steroid hormone stimulus (P<0.0005). Additionally, among the top 100 calcipotriol-regulated genes at 6 hours were enrichment of categories such as the endomembrane system (i.e., LDLRAP1, ULK1), wound response (i.e., CEBPB, PDGFA, SHH, THBD), and cellular homeostasis (i.e., CLDN1, CSF1), as well as multiple genes with established roles in BMP and Wnt signaling (Figure 4A).
Figure 4.
Calcipotriol induces LDLRAP1 to regulate LRP6 protein levels. (A) Heat map of selected genes from RNA-seq after 2 or 6 hours treatment of AsPC-1 cells with vehicle or 100 nM calcipotriol. (B) Western blot for LC3B, LRP6 and tubulin (loading control) after AsPC-1 cells were treated with vehicle, 100 nM Calcipotriol, 100 nM Bafilomycin A1 or a combination of the two for 6 hours. (C) LDLRAP1 expression by qPCR after treatments described in Figure 3E. (D) Western blot for LDLRAP1, LRP6 and tubulin (loading control). AsPC-1 cells were transfected with control or LDLRAP1 siRNA for 48 hours and then treated with vehicle or 100 nM calcipotriol for 24 hours. Relative densitometry values for LDLRAP1 and LRP6 expression normalized to tubulin loading control are shown beneath corresponding blots. (E) BAR-luciferase activity in AsPC-1 cells treated as in (D). Values are shown normalized to respective controls, with BAR reported as mean ± SD and qPCR reported as mean ± SEM. One representative experiment of 3 biological repeats is shown. *P<0.05, **P<0.01, ***P<0.001.
In relation to the endomembrane system, the cytoplasmic adapter protein LDLRAP1 was significantly upregulated at 2 and 6 hours post calcipotriol treatment (Figure 4A). LDLRAP1 is required for efficient endocytosis of LDL receptors in polarized cells and is able to interact with LRP1 and LRP2 to facilitate LDL receptor clustering into clathrin-coated pits(29-31). Notably, internalization of LRP6 via clathrin antagonizes Wnt signaling(32, 33) and promotes LRP6 degradation through endosomal trafficking to lysosomes(34-36). We speculated that LDLRAP1 might also decrease LRP6 protein levels through a similar mechanism involving receptor internalization and degradation. To address this, we first assessed whether lysosomal activity was necessary to decrease LRP6 protein levels in response to calcipotriol treatment. Treatment with Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase that blocks acidification and protein degradation in lysosomes, blocked the decrease in LRP6 protein levels seen with calcipotriol treatment (Figure 4B). Bafilomycin also increased LRP6 protein levels in the absence of calcipotriol, indicating lysosomal protein degradation plays a role in the regulation of steady-state levels of LRP6 in PDAC lines. RNA-seq data was validated by qPCR in separate experiments with calcipotriol, confirming LDLRAP1 gene expression was significantly upregulated in AsPC-1 cells within 4 hours of calcipotriol treatment (Figure 4C). Western blot analysis also confirmed LDLRAP1 protein was also increased with calcipotriol (Figure 4D). Further in silico analysis of the LDLRAP1 locus identified a predicted vitamin D response element (VDRE) at the 3′ end of the LDLRAP1 gene. Evaluation of published ChIP-seq data generated from hepatic stellate cells (37) revealed VDR occupancy of the site encompassing this VDRE, indicating VDR can directly bind the LDLRAP1 gene. To further link calcipotriol-induced LDLRAP1 expression to changes in LRP6 protein and Wnt signaling, siRNA-mediated knockdown of LDLRAP1 was performed. Knockdown of LDLRAP1 significantly abrogated the reduction in LRP6 protein seen with calcipotriol treatment (Figure 4D). Concurrent evaluation of Wnt reporter showed LDLRAP1 knockdown mildly increased baseline BAR activity in the vehicle control with partial reversal of the inhibition of BAR activity seen with calcipotriol treatment (Figure 4E). Although the relative reduction in BAR activity observed with calcipotriol treatment in control versus LDLRAP 1 siRNA transfected cells was similar (30% and 31%, respectively), changes in the absolute level of BAR activity (Figure 4E) paralleled the observed changes in LRP6 expression (Figure 4D), potentially implicating a link between regulation of LRP6 expression and Wnt reporter activity by calcipotriol through LDLRAP1. In summary, LDLRAP1 is a direct transcriptional target of ligand-activated VDR that mediates reduction in LRP6 protein in coordination with the inhibition of Wnt signaling observed with calcipotriol.
VDR is regulated by Wnt signaling
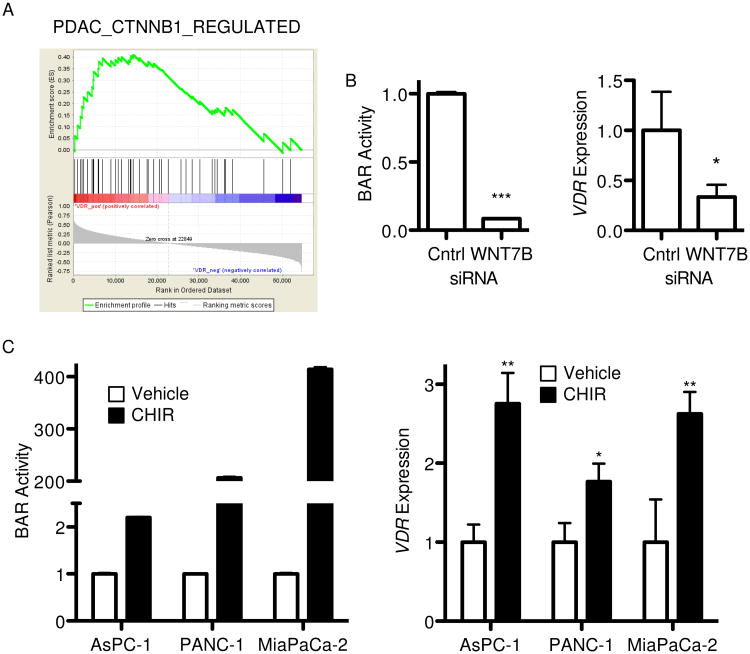
Extending upon the observed correlation between VDR expression and autocrine Wnt signaling activity across a panel of PDAC cell lines (Figure 1A), we determined whether a similar correlation exists in clinical specimens. Gene set enrichment analysis (GSEA) of a previously published cohort of 25 primary PDAC tumors(38) confirmed positive enrichment of a PDAC-specific Wnt/β-catenin target gene set in tumors with higher VDR expression (Figure 5A, normalized enrichment score = 1.39, false discovery rate q value = 0.08). To determine whether VDR is directly regulated by Wnt/β-catenin signaling in PDAC, AsPC-1 cells with stable BAR reporter were transfected with siRNA against WNT7B, a Wnt ligand previously shown to be essential for autocrine Wnt signaling activity in this cell line(8). WNT7B knockdown significantly inhibited BAR activity with a concomitant ∼70% decrease in VDR expression (Figure 5B). Addressing reciprocal activation of Wnt/β-catenin signaling, High-BAR/High-VDR and Low-BAR/Low-VDR cell lines were treated with the selective GSK3β inhibitor CHIR99021. CHIR99021 activated Wnt reporter as expected with a concomitant increase in VDR expression (Figure 5C), indicating Wnt/β-catenin signaling positively regulates VDR expression in PDAC. These data suggest a context-dependent Wnt feedback mechanism such that VDR may inhibit Wnt signaling in situations when sufficient levels of vitamin D are available to activate VDR and its downstream transcriptional targets.
Figure 5.
VDR is regulated by Wnt signaling in PDAC. (A) Gene set enrichment analysis(27) enrichment plot for PDAC-specific Wnt/β-catenin target gene set determined relative to VDR expression phenotype using a published gene expression microarray dataset of 25 primary PDAC tumor samples. The top curve shows a running enrichment score for the target gene set, while the middle portion shows a heatmap of VDR expression (red – high, blue – low) and bottom portion shows the value of the ranking metric down the list of genes. (B) BAR luciferase activity and VDR expression by qPCR48 hours after transfection of AsPC-1 cells with control or WNT7B siRNA. (C) BAR luciferase activity and VDR expression by qPCR 24 hours after treatment of indicated PDAC cell lines with vehicle or 5 μM CHIR99021 (CHIR). Values are shown normalized to respective controls, with BAR reported as mean ± SD and qPCR reported as mean ± SEM. One representative experiment of 3 biological repeats is shown. *P<0.05, **P<0.01, ***P<0.001.
Having demonstrated ligand-activated VDR inhibits Wnt/β-catenin signaling and Wnt/β-catenin positively regulates VDR transcript, we next examined whether calcipotriol might inhibit VDR gene expression through downregulation of Wnt/β-catenin signaling. Time course with AsPC-1 showed that VDR message was significantly decreased at 6 and 24 hours following calcipotriol treatment (Supplemental Figure 3A), paralleling effects on LRP6 protein expression and thus compatible with a potential mechanism for feedback inhibition at the level of VDR message(Figure 3A). Conversely, however, VDR protein levels were significantly increased within 4-6 hours and up to 24 hours following calcipotriol treatment (Supplemental Figure 3B). This paradoxical increase in VDR protein may be due to a conformational change and resulting stabilization of VDR protein that has been previously shown to occur when VDR is bound by vitamin D or vitamin D analogs (39).
Discussion
Diverse anti-tumorigenic actions of vitamin D have been shown in in vitro and in vivo preclinical studies of pancreatic cancer(16-19). In particular, non-hypercalcemic analogs of vitamin D circumventing dose-limiting side effects of hypercalcemia and hypercalciuria represent especially promising chemotherapeutic agents for PDAC and other malignancies. To date, there are only limited published data addressing the clinical efficacy of vitamin D in PDAC. In a non-randomized phase II clinical trial of advanced stage PDAC single agent vitamin D analog seocalcitol (EB1089) was well tolerated but did not improve patient survival (40). This study involved patients with advanced disease and did not address biomarkers such as VDR expression levels, Wnt signaling activity or other biochemical changes to determine their potential roles in predicting biological response to seocalcitol. Another phase II trial of advanced PDAC involving oral vitamin D (calcitriol) in combination with docetaxel demonstrated prolonged time to disease progression relative to docetaxel alone(41). Offering a potential explanation, VDR was recently shown to be a master regulator of PDAC stromal activation with calcipotriol functioning as a stromal targeting agent capable of enhancing delivery of gemcitabine chemotherapy in a genetically engineered mouse model of PDAC(19). Clinical trials focused on this exciting result are now underway utilizing the vitamin D analog paricalcitol (i.e., NCT#02030860). Importantly, newer synthetic vitamin D analogs (i.e., calcipotriol and paricalcitol) may be more efficacious as they are resistant to degradation by the product of the VDR target gene CYP24A1, which is rapidly induced by liganded VDR and is responsible for rapid feedback inhibition of naturally occurring vitamin D (i.e., calcitriol). While tumor stroma is an important target for evaluating the effects of vitamin D-based therapy in PDAC, such stromal effects also need to be cautiously interpreted in relation to any further direct actions of vitamin D on PDAC tumor cells themselves. For instance, calcitriol directly potentiates the cytotoxic activity of gemcitabine in PDAC cell lines both in vitro and in vivo(17). Furthermore, our results here demonstrate functional cross-talk between VDR and Wnt/β-catenin signaling in PDAC tumor cells that may have significant bearing on the clinical and biological response of subsets of PDAC tumors (i.e., those with higher autocrine Wnt signaling) to vitamin D analogs. Pancreatic stromal cells are also influenced by paracrine Wnt signaling(42), offering a viable signaling pathway through which vitamin D could exert its anti-stromal effects.
Our calcipotriol results are in line with previous studies showing vitamin D and its analogs inhibit the growth of certain PDAC cell lines (i.e., AsPC-1) but not others (i.e., MiaPaCa-2 and PANC-1) (18). Our study expands this observation to additional PDAC cell lines and indicates growth inhibitory activity is dependent on levels of VDR expression, which in turn is positively regulated by Wnt/β-catenin signaling. This bidirectional relationship between Wnt and vitamin D signaling could have significant bearing on identifying patients most likely to respond to vitamin D-based therapy and raises a potential confounding issue related to the use of vitamin D analogs either alone or in combination with other therapeutic Wnt inhibitors. Namely, inhibition of Wnt may reduce Wnt/β-catenin-regulated expression of VDR message, with the potential to blunt any further VDR-dependent vitamin D response. While other mechanisms (i.e., VDR protein level paradoxically increased in AsPC-1 at early time points after calcipotriol treatment) may compensate for loss of Wnt-mediated VDR expression, careful consideration should be given to the combination of and order in which various treatments (i.e., a second Wnt inhibitor drug) are used in combination with vitamin D analogs.
Calcipotriol inhibited anchorage-independent growth in cell lines with high endogenous Wnt activity, a phenotype we previously attributed to autocrine Wnt signaling in these cell lines(8). However, calcipotriol also inhibited anchorage-dependent growth, a phenotype we previously showed to be decoupled from Wnt signaling in these cell lines(8). These divergent results are not surprising given known pleiotropic anti-tumor effects attributed to vitamin D extending beyond its actions as an inhibitor of Wnt signaling. Vitamin D-mediated growth inhibitory effects in PDAC have previously been attributed to cell cycle arrest occurring via upregulation of key regulators of cell cycle such as p21 and p27(16, 18). Indeed, our RNA-seq analysis of calcipotriol treated AsPC-1 cells revealed altered expression of several key mediators of cell cycle (i.e., SKP2 and CDKN1A) as well as apoptosis (i.e., BIRC5 and BCL10).
Calcipotriol specifically inhibited Wnt/β-catenin transcriptional activity in PDAC cell lines characterized by higher levels of Wnt ligand-mediated autocrine signaling and VDR expression. Vitamin D was previously shown to inhibit Wnt/β-catenin signaling in colon cancer cell lines by mediating an interaction between VDR and β-catenin that disrupts β-catenin/TCF mediated transcription(10) and by indirectly increasing expression of the secreted Wnt antagonist DKK1(11). We were unable to detect a physical interaction between VDR and β-catenin in PDAC lines either in the presence or absence of calcipotriol. While calcipotriol did induce the expression of DKK1 in AsPC-1 cells, this could not be functionally linked to its inhibition of Wnt signaling, as DKK1 knockdown did not rescue the inhibition of Wnt signaling seen with calcipotriol. Nevertheless, our results do not exclude other proposed mechanisms of VDR and β-catenin cross-regulation such as possible re-distribution of β-catenin across the genome in response to calcipotriol treatment(43, 44).
This study provides a novel mechanism through which vitamin D could negatively regulate Wnt signaling. Protein levels of the Wnt co-receptor LRP6 were reduced as early as 6 hours post calcipotriol treatment. This reduction was dependent upon VDR transcriptional activity because knockdown of the VDR co-transcriptional activator RXR rescued the reduction of LRP6 protein seen with calcipotriol treatment. Rapid reduction of LRP6 protein was not the result of reduced LRP6 transcript levels, which were only altered 24 hours after calcipotriol treatment when LRP6 transcript levels actually increased in vehicle control and not in calcipotriol-treated cells. We speculate that the dramatic increase in LRP6 transcript seen at 24 hours in control cells may be tied to cell cycle-dependent changes in LRP6 expression(45, 46), while calcipotriol treated cells under cell cycle arrest would be expected to retain lower levels of LRP6 transcript.
LRP6 is a critical mediator of ligand-mediated Wnt/β-catenin signaling. While we show overexpression of LRP6 increased Wnt reporter activity in the absence or presence of calcipotriol (Figure 3C, this cannot be concluded to represent specific rescue of the mechanism by which calcipotriol directly inhibits the pathway. RNA-seq analysis presented here offers several additional potential regulators of Wnt signaling that could mediate calcipotriol effects, including BAMBI (BMP and activin membrane bound inhibitor) that was significantly decreased within 2 hours of calcipotriol treatment. BAMBI is a known Wnt/β-catenin agonist that promotes the interaction between Fzd and Dvl(47). We are now investigating BAMBI and other alternative targets through which vitamin D may directly inhibit Wnt signaling in pancreatic can.
This study also further focused on induction of LDLRAP1 message and protein by calcipotriol and the role of LDLRAP1 in regulating LRP6 protein levels. LDLRAP1 is an adapter protein that binds to tyrosine motifs in the cytoplasmic tail of LDL receptors, which couples them to the endocytic machinery of the clathrin coated pit(31, 48). LDLRAP1 promotes clathrin-mediated endocytosis of LRP1 and LRP2(29-31). Tyrosine motifs in the cytoplasmic tail of LDL receptor family member LRP6 are critical for its clathrin-mediated internalization(49). Our data establish an additional role for LDLRAP1 in regulating steady-state levels of LRP6 and lead us to speculate that LDLRAP1 facilitates endocytosis and lysosome-mediated degradation of LRP6.
In summary, we have elucidated a bidirectional feedback interaction between Wnt and vitamin D signaling in PDAC. Upon activation, Wnt signaling induces expression of VDR. Furthermore, ligand-activated VDR positively regulates the transcription of LDLRAP1. LDLRAP1 contributes to a rapid reduction in proteins levels of LRP6, a critical co-receptor for ligand-dependent Wnt signaling. Activation of Wnt signaling induces the expression of VDR in PDAC tumor cells, which renders those cells susceptible to vitamin D anti-tumor effects. In addressing the reciprocal relationship between vitamin D and Wnt signaling, this study offers new insights into the possible use of vitamin D as a therapeutic Wnt inhibitor, as well as possible biomarkers for predicting or following response to calcipotriol and other vitamin D analogs.
Supplementary Material
Acknowledgments
Financial Support: M. Arensman was supported by USHHS Ruth L. Kirschstein Institutional National Research Service Award #T32 CA009056 (UCLA Tumor Biology Training Grant). D. Dawson was supported by grants from the American Cancer Society (RSG-12-083-01-TBG), NIH (P01 CA163200 and P01 DK098108) and the Hirshberg Foundation for Pancreatic Cancer Research.R.M.E. is an Investigator of the Howard Hughes Medical Institute (HHMI) at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology, and is supported by National Institutes of Health (NIH) grants (DK057978, DK090962, HL088093, HL105278 and ES010337), the Leona M. and Harry B. Helmsley Charitable Trust, Ipsen/Biomeasure and the Lustgarden Foundation. R.M.E. and M.D. are supported in part by a Stand Up To Cancer Dream Team Translational Research Grant (SU2C-AACR-DT0509). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.C.L. and M.D. are funded by grants from the National Health and Medical Research Council of Australia Project Grants 512354, 632886 and 1043199.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Morris JPt, Yan W, Schofield HK, Gurney A, Simeone DM, et al. Canonical Wnt signaling Is required for pancreatic carcinogenesis. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilmer M, Recio Boiles A, Regel I, Yokoi K, Michalski CW, Wistuba II, et al. RSpo2 enhances canonical Wnt signaling to confer stemness-associated traits to susceptible pancreatic cancer cells. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-14-1327. [DOI] [PubMed] [Google Scholar]
- 7.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–32. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arensman MD, Kovochich AN, Kulikauskas RM, Lay AR, Yang PT, Li X, et al. WNT7B mediates autocrine Wnt/beta-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma. Oncogene. 2014;33:899–908. doi: 10.1038/onc.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 10.Palmer HG. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–88. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–84. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 12.Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev. 2006;15:1688–95. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- 13.Wolpin BM, Ng K, Bao Y, Kraft P, Stampfer MJ, Michaud DS, et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:82–91. doi: 10.1158/1055-9965.EPI-11-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu SL, Zhao YP, Dai MH, You L, Wen Z, Xu JW. Vitamin D status and the risk of pancreatic cancer: a meta-analysis. Chin Med J (Engl) 2013;126:3356–9. [PubMed] [Google Scholar]
- 15.Van Loon K, Owzar K, Jiang C, Kindler HL, Mulcahy MF, Niedzwiecki D, et al. 25-Hydroxyvitamin D levels and survival in advanced pancreatic cancer: findings from CALGB 80303 (Alliance) J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawa S, Nikaido T, Aoki Y, Zhai Y, Kumagai T, Furihata K, et al. Vitamin D analogues up-regulate p21 and p27 during growth inhibition of pancreatic cancer cell lines. Br J Cancer. 1997;76:884–9. doi: 10.1038/bjc.1997.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu WD, Ma Y, Flynn G, Muindi JR, Kong RX, Trump DL, et al. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle. 2010;9:3022–9. doi: 10.4161/cc.9.15.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawa S, Yoshizawa K, Nikaido T, Kiyosawa K. Inhibitory effect of 22-oxa-1,25-dihydroxyvitamin D3, maxacalcitol, on the proliferation of pancreatic cancer cell lines. J Steroid Biochem Mol Biol. 2005;97:173–7. doi: 10.1016/j.jsbmb.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arensman MD, Telesca D, Lay AR, Kershaw KM, Wu N, Donahue TR, et al. The CREB-binding protein inhibitor ICG-001 suppresses pancreatic cancer growth. Mol Cancer Ther. 2014;13:2303–14. doi: 10.1158/1535-7163.MCT-13-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano E, Cano A. Cadherin/catenin complexes in murine epidermal keratinocytes: E-cadherin complexes containing either beta-catenin or plakoglobin contribute to stable cell-cell contacts. Cell Adhes Commun. 1998;6:51–67. doi: 10.3109/15419069809069760. [DOI] [PubMed] [Google Scholar]
- 22.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–93. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011;27:2325–9. doi: 10.1093/bioinformatics/btr355. [DOI] [PubMed] [Google Scholar]
- 26.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 29.Nagai M, Meerloo T, Takeda T, Farquhar MG. The adaptor protein ARH escorts megalin to and through endosomes. Mol Biol Cell. 2003;14:4984–96. doi: 10.1091/mbc.E03-06-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mameza MG, Lockard JM, Zamora E, Hillefors M, Lavina ZS, Kaplan BB. Characterization of the adaptor protein ARH expression in the brain and ARH molecular interactions. J Neurochem. 2007;103:927–41. doi: 10.1111/j.1471-4159.2007.04854.x. [DOI] [PubMed] [Google Scholar]
- 31.Garuti R, Jones C, Li WP, Michaely P, Herz J, Gerard RD, et al. The modular adaptor protein autosomal recessive hypercholesterolemia (ARH) promotes low density lipoprotein receptor clustering into clathrin-coated pits. J Biol Chem. 2005;280:40996–1004. doi: 10.1074/jbc.M509394200. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15:37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, He X, Howe PH. Disabled-2 (Dab2) inhibits Wnt/beta-catenin signalling by binding LRP6 and promoting its internalization through clathrin. EMBO J. 2012;31:2336–49. doi: 10.1038/emboj.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 35.Maharzi N, Parietti V, Nelson E, Denti S, Robledo-Sarmiento M, Setterblad N, et al. Identification of TMEM131L as a novel regulator of thymocyte proliferation in humans. J Immunol. 2013;190:6187–97. doi: 10.4049/jimmunol.1300400. [DOI] [PubMed] [Google Scholar]
- 36.Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev Biol. 2006;293:268–83. doi: 10.1016/j.ydbio.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–13. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donahue TR, Tran LM, Hill R, Li Y, Kovochich A, Calvopina JH, et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res. 2012;18:1352–63. doi: 10.1158/1078-0432.CCR-11-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Bemd GC, Pols HA, Birkenhager JC, van Leeuwen JP. Conformational change and enhanced stabilization of the vitamin D receptor by the 1,25-dihydroxyvitamin D3 analog KH1060. Proc Natl Acad Sci U S A. 1996;93:10685–90. doi: 10.1073/pnas.93.20.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans TR, Colston KW, Lofts FJ, Cunningham D, Anthoney DA, Gogas H, et al. A phase II trial of the vitamin D analogue Seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br J Cancer. 2002;86:680–5. doi: 10.1038/sj.bjc.6600162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanke CD, Beer TM, Todd K, Mori M, Stone M, Lopez C. Phase II study of calcitriol-enhanced docetaxel in patients with previously untreated metastatic or locally advanced pancreatic cancer. Invest New Drugs. 2009;27:374–8. doi: 10.1007/s10637-008-9184-6. [DOI] [PubMed] [Google Scholar]
- 42.Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486–97. 97 e1–14. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 43.Meyer MB, Goetsch PD, Pike JW. VDR/RXR and TCF4/beta-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol Endocrinol. 2012;26:37–51. doi: 10.1210/me.2011-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 45.Olmeda D, Castel S, Vilaro S, Cano A. Beta-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol Biol Cell. 2003;14:2844–60. doi: 10.1091/mbc.E03-01-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, et al. Cell cycle control of wnt receptor activation. Dev Cell. 2009;17:788–99. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Lin Z, Gao C, Ning Y, He X, Wu W, Chen YG. The pseudoreceptor BMP and activin membrane-bound inhibitor positively modulates Wnt/beta-catenin signaling. J Biol Chem. 2008;283:33053–8. doi: 10.1074/jbc.M804039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He G, Gupta S, Yi M, Michaely P, Hobbs HH, Cohen JC. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J Biol Chem. 2002;277:44044–9. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 49.Liu CC, Kanekiyo T, Roth B, Bu G. Tyrosine-based signal mediates LRP6 receptor endocytosis and desensitization of Wnt/beta-catenin pathway signaling. J Biol Chem. 2014;289:27562–70. doi: 10.1074/jbc.M113.533927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.