Abstract
Physical activity covers not just sports but also simple everyday movements such as housework, walking and playing. Regular exercise has a great importance in maintaining good health, indeed inactivity is a risk factor for different chronic diseases. Physical exercise can play a crucial role in the treatment of rheumatic diseases, optimizing both physical and mental health, enhancing energy, decreasing fatigue and improving sleep. An exercise program for patients with rheumatic diseases aims to preserve or restore a range of motion of the affected joints, to increase muscle strength and endurance, and to improve mood and decrease health risks associated with a sedentary lifestyle. In this editorial I describe the benefits of the exercise on physical limitations and fatigue in rheumatic diseases that seem to have a short and long-term effectiveness. A literature review was conducted on PubMed, Scopus and Google Scholar using appropriate keywords based on the present editorial.
Keywords: Physical activity, Physiatric rehabilitation therapy, Rheumatic diseases, Flexibility training, Home exercise program, Knee osteoarthtritis
Core tip: In this interesting editorial, I illustrated the beneficial effects of the physical activity in our life and in rheumatic diseases, including home and gym exercise programs, flexibility training and physiatric rehabilitation therapy. Physical exercise is able to improve balance, reduce pain, activate muscle and increase functional joint stability in patients with rheumatic diseases and osteoarthritis. The benefits of the exercise on physical limitations and fatigue in rheumatic diseases that seem to have a short and long-term effectiveness.
INTRODUCTION
Physical activity covers not just sports but also simple everyday movements, no training exercise, such as housework, walking, biking and playing (Figure 1). Indeed, according to the World Health Organization, “any effort exerted by the muscle-skeletal system which results in a higher power consumption than that in the rest position” is classified as physical activity[1]. Regular exercise has a great importance in maintaining good health. Inactivity is a risk factor for chronic diseases and the benefits of a regular and moderate exercise include reduced risks of coronary artery disease, hypertension, diabetes, obesity, serum lipid abnormalities, osteoporosis and cancer[2]. Moreover, physical activity is a good way to socialize and an excellent anti-stress, decreasing the urge to smoke. Physical exercise can play a crucial role in the treatment of rheumatic diseases in optimizing both physical and mental health, enhancing energy, decreasing fatigue and improving sleep. In this way, the muscles around the affected joints become strong, the bone loss decreases and the control of joint swelling, stiffness and pain improves thanks to a better lubrication of the joint cartilage[3]. Moreover, immediately after exercising, anxiety decreases and the mood improves. An exercise program for patients with rheumatic diseases aims to preserve or restore a range of motion of the affected joints, to increase muscle strength and endurance, and to improve mood and decrease health risks associated with a sedentary lifestyle[3]. The management of these patients is multidisciplinary involving rheumatologists, radiologists, human movement scientists, rehabilitation physicians, physical therapists, sports instructors and research assistants.
Figure 1.
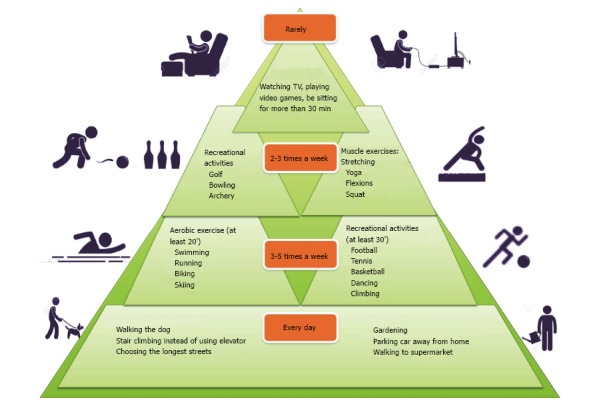
Graphic design of physical activity guidelines during our normal life in one week, suggested by the World Health Organization recommendations, to prevent the onset of some diseases related to sedentary lifestyle. This graphic represents the pyramid of the physical activity recommendation. At the base the suggestions for every day’s activities and at the top the activities to perform rarely are shown.
The aim of this editorial is to illustrate the beneficial effects of the physical activity in our life and in rheumatic diseases, including home and gym exercise programs, flexibility training and physiatric rehabilitation therapy. Physical exercise is able to improve balance, reduce pain, activate muscle and increase functional joint stability, in patients with rheumatic diseases and osteoarthritis (OA).
THE EFFECTS OF PHYSICAL ACTIVITY IN RHEUMATIC DISEASES
Rheumatic diseases are disorders affecting the musculoskeletal system and in general the connective tissues[4]. Such diseases are very different from each other, also for their severity. Some of them can affect not only joints, bones, tendons, but also other tissues and organs having thus a systemic expression[5]. Rheumatic diseases lead to pain, disability, loss of functional autonomy, reducing the quality of life, both for the side effects of drugs, and for the involvement of vital structures of the organism. Fatigue is common in patients affected by various chronic medical conditions with a low physical activity. For example, in patients with rheumatoid arthritis (RA) and fibromyalgia (FM), reduced levels of fatigue were reported in association with a higher daily physical activity[3,6]. In patients with RA, fatigue can be associated not only with the inflammation per se, but especially with pain, disability, anxiety, depressive thoughts, reduced self-efficacy, feelings of helplessness, sleep disturbances, and limitations in social functioning. Based on these findings, psychosocial factors may have an important role in the onset and persistence of fatigue[7-9]. Both cognitive-behavioral therapy and physical exercise are reported to be effective for treating fatigue in patients with RA[10]. Furthermore, exercise can enhance weight loss and promote long-term weight management in those rheumatic diseases patients who are overweight. Water is an excellent environment for the exercises and the water temperature (between 28 °C and 32 °C) can give pain relief. It allows the patient to be in a situation of reduced weight, thus allowing some patients to perform exercises that they would otherwise not be able to perform. FM is a chronic pain disorder, commonly associated with a reduced quality of life, since it is accompanied by other symptoms such as fatigue, psychological distress, cognitive disorders, no restorative sleep, poor balance, and impaired physical function[11-13]. The current treatment for FM envisages a comprehensive assessment including pharmacological and non-pharmacological therapies. The latter provide for a multimodal approach including physical activity, sleep hygiene, behavioural therapy, regular education and monitoring of treatment response[14,15]. It has been shown that regular exercise, in particular aerobic, improves pain, fatigue and sleep disturbance in patients with FM. Physical exercise is one of the most widely recognized and beneficial forms of non-pharmacological therapy[16], effective in reducing pain and depression and producing positive effects on physical function, fitness, and global health[17], particularly in patients affected by rheumatic disease[18]. No one particular form of exercise is preferred and all types may be considered. The most consistent results have been demonstrated for aerobic and strengthening exercise that, when combined with stretching, had equivalent effects on limiting pain severity among patients with FM[19]. Patients who express concerns regarding the possible worsening of pain and fatigue need to be reassured. The initiation of any exercise program, indeed, must be slow and gradual[20]. A good way to start could be an exercise regimen with hydrotherapy pool, as the warmth of the water and relative weightlessness relieves symptoms while the resistance provides a gentle workout[19]. Moreover, it has been shown that the combination of aerobic exercise, strengthening, and flexibility improves psychological health status and quality of life, preventing depression[21]. There is evidence to support the use of yoga, qi gong and tai chi (disciplines including stretching exercises) in patients with fibromyalgia[22]. Studies in which the use of these therapies resulted in improvement in fibromyalgia symptoms and physical functioning were generally small and unblended, however, given the lack of serious adverse effects and the promotion of self-efficacy, these management modalities are generally useful options. Graded exercise training and cognitive-behavioural therapy are the two interventions considered to be effective in chronic fatigue syndrome (CFS)[23]. Myalgic encephalomyelitis, commonly known as chronic fatigue syndrome, is a debilitating and complex disorder characterized by profound medically unexplained fatigue that is not improved by bed rest and that may be worsened by physical or mental activity. Symptoms affect several body systems and may include weakness, musculoskeletal pain, sleep disturbance, impaired memory and/or mental concentration, which can result in reduced participation in daily activities. Compared to healthy controls equal in age, patients with CFS are significantly less physically active[24]. After exercise therapy, patients with CFS may generally feel less fatigued, and no evidence suggests that exercise therapy may worsen outcomes. Moreover, a positive effect with respect to sleep, physical function and self-perceived general health has also been observed[25].
The most frequent degenerative rheumatic disorder in the population is OA[26]. Unfortunately, in contrast to systemic inflammatory rheumatic diseases, such as RA, the therapeutic options in OA are still limited[27]. OA is a chronic disease characterized by degenerative and productive changes of the joints (Figure 2)[28]. It is essentially linked to an imbalance between excessive cartilage damage and the ability of cartilage to “heal”, but involves and compromises the whole joint, in all its aspects, both macroscopic and microscopic changes (Figure 2)[29-31]. Risk factors include age[32,33], mechanical factors[33], obesity[34], and inflammation[35,36]. The key intervention in the management of OA is exercise therapy[37,38]. It is well known that exercise training affects the articular cartilage metabolism and modifies the cartilaginous structure by a mechanotransduction response[37-39]. Biomechanical stimulus generated by dynamic compression, during a moderate exercise, can reduce the synthesis of proteolytic enzymes, regulating the metabolic balance and preventing the progression of the disease[40,41]. Moreover, reduction in inflammation seems to be a crucial mechanism, since exercise is a potential anti-inflammatory treatment for patients with rheumatic diseases and OA[41,42]. The exercise regime could range from mild to moderate in OA patients, and also, more importantly, must be “adapted” or “tailor-made”, since the level of exercises will be dependent on the tolerability of the patients, as recommended by the American College of Rheumatology, the EULAR and OARSI guidelines[41-43]. Authors recently showed that a higher increase in muscle strength is associated to a higher increase in physical functioning[44], tolerability was assessed at every training session, for the patients who were not able to tolerate high intensity training, training intensity was adapted to a lower level[3-5].
Figure 2.
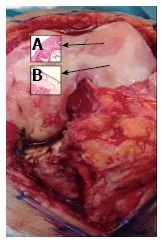
Macroscopic and microscopic signs of osteoarthritis knee hyaline cartilage. A: The microscopic observations of osteoarthritis knee hyaline cartilage showed structural alterations, reduction of cartilage thickness of the cartilage zones and subchondral bone fibrillations; B: The microscopic observations of healthy knee hyaline cartilage showed a preserved morphological structure with no sign of cartilage degradation.
Moreover, in order to preserve the articular cartilage, physicians should promote a healthy lifestyle. Physical activity (mild exercise) must be associated to a balanced diet, such as Mediterranean Diet (olive oil and red orange), in order to prevent and reduce the progression of rheumatic and OA disease[35,44-47].
Exercise programs
Exercise and physical activity are usually classified in three basic categories (Figure 3): (1) aerobic, such as walking and jogging, which are repetitive, rigorous, rhythmic, and involve the large muscles; (2) resistance, which utilizes resistance to muscular contraction to build the strength, anaerobic endurance and size of skeletal muscles; and (3) flexibility training, which keeps the body flexible, relaxes muscles and protects from physical injury[48]. Sometimes initially patients complain of the increase of pain and fatigue usually decrease with the continuation of the physical activity and can be avoided by introducing breaks in the exercise sessions.
Figure 3.
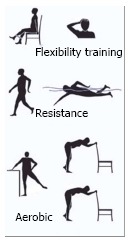
Graphic design of the three basic categories of physical activity. Aerobic (aerobic physical exercise such as walking or jogging), resistance (anaerobic endurance exercise such as marathon or cross country skiing), and flexibility training (stretching exercises to increase functional range of motion and to reduce the risk of injury).
Flexibility training
Flexibility programs can increase functional range of motion and reduce the risk of injury. Joint flexibility may decrease with age, affecting normal daily function, older adults could maintain the ability improving flexibility through stretching exercises (Figure 4)[49,50]. The 2009 American College of Sports Medicine position statement “exercise and physical activity for older adults” highlighted the lack of studies on the effects of a range of motion exercises on flexibility outcomes in older populations and also the absence of consensus about the prescription of stretching exercises[51]. Despite the lack of scientific support for the recommendation of a flexibility component in older adult exercise programs, many activity programs place a considerable emphasis on flexibility. When injuries or diseases result in a restricted range of motion of the joints, stretching exercises are used in the rehabilitation context in order to regain “normal” range of motion in the major muscle tendon groups in accordance with individualized goals[52]. For the majority of the aging population, the goals are not related to athletic performance, but rather to daily living activities. Despite the lack of research confirming the health benefits[53], it is common to find in the literature flexibility training as a presumed “component of fitness” and a beneficial adjunct to other forms of exercise. Other physical therapies can be helpful in the management of rheumatic diseases, particularly those that can be self-administered.
Figure 4.
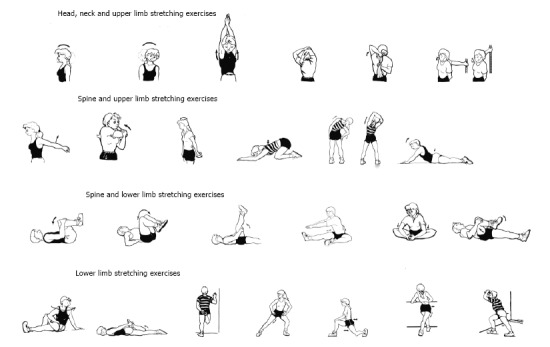
Graphic design of different stretching exercises (head, neck, spine, upper and lower limb stretching exercises). This graphic includes the stretching movement for all joints and muscles of the body such as flexion, extension, adduction, abduction, pronation, supination, intra and extra rotation, circumduction.
Physiatric rehabilitation therapy
Patients with rheumatic diseases have a high risk of progressive deterioration of articular function over the years. The main limitations are due to the pain, a reduced range of motion, the muscle wasting and the reduction in strength. Psychological motivations of anxiety and depression are often associated and could further compromise the ability to address common daily activities. Physiotherapy and kinesiology is an essential component in the overall treatment of the disease. It provides for interventions predominantly educational and preventive but also with specific measures targeted to the condition of each patient. The levels of intervention include: Training on the prevention of damage (joint protection), training in autonomy (use of aids), and specific rehabilitation treatment in relation to surgery[54]. The first step is to inform and educate the patient about the nature and consequences of the disease. The conviction of the importance of taking care of their joints is an important element in the treatment. The articular overexertion during repetitive daily activities, contributes to the deterioration of appearance and aggravation of the deformations[55]. The joint protection is based on gesture education, avoidance of incorrect movements and use of the most appropriate ones. This technique reduces the risk of joint deterioration, minimizing the efforts that exert on the joint structures in order to facilitate the movements and make them more comfortable when they are painful and tiring[56]. The program is carried out by qualified personnel in physiatric facilities. It consists of passive motion exercises, assisted active and stretching exercises to increase the extensibility. Mobilization exercises are usually associated with those of muscle strengthening. It is shown that a static or dynamic exercise program can improve strength, aerobic capacity and physical performance without increasing disease activity or aggravating joint damage[48]. In cases where surgical orthopedic treatment is indicated, physiatric preparation and follow up to the intervention is necessary - rehabilitation adapted to the type of operation, the situation of the patient[49].
Home exercise program
After the completion of the rehabilitation programme in hospital, the patient is then discharged with a home exercise program and followed up at regular intervals[57]. Home exercise program (Figure 5) should be adapted to the patient’s capacity. It is a crucial aspect of the rehabilitation program, allowing the patients to return home. The training program should be made in the patient’s home environment in order to ensure the patient’s independence. Patients improvements and general condition should be evaluated at regular intervals[58]. Moreover, the training should be gradually changed, according to the improvement of the patients to facilitate the more complex activities of daily living, and also in the late stages when the patients return to work[58]. In addition to the exercises performed during the subacute and chronic stages, shoulder, wrist, hand, hip and knee joint exercises (flexion and extension), full abduction, extension and flexion exercises for abdominal, sacrospinal, iliopsoas, gluteus maximus, gluteus minimus, hamstring, and quadriceps muscles, and resistive exercises for oblique abdominal muscles are recommended to be performed at home[59]. Exercises to increase respiratory capacity are continued. Cardiovascular moderate exercises (spinning, running) are also advised/prescribed. At the end of the rehabilitation program patients experienced less pain, improve their range of motion and were able to perform their daily work with fewer complications. Help in home exercise can come from listening to music during activity, helping in maintaining a good rhythm, body coordination, and motivation in the rehabilitation program and distracting the mind from the pain[60-62].
Figure 5.

Graphic design of some home exercise. This graphic includes different types of exercises that can be performed safely at home, using the common home furniture.
CONCLUSION
Exercise can be beneficial both physically and psychologically. It works by improving muscle trophism and capillarisation, and reducing muscle hypoxia. It also promotes the secretion of endorphins and growth hormone; increases the production of serotonin in the brain and activates adrenergic mechanisms of pain inhibition. Inactivity in patients with rheumatic diseases is very harmful both physically (reduced muscle strength, deconditioning, greater rigidity), and psychologically (fear of movement, depression, loss of self-confidence). The exercise suggested is aerobic with moderate intensity, adapted to the patient and then slowly increased according to the improvement of the conditions.
In conclusion I can assert that regular moderate physical activity (housework, slow running, walking, biking and swimming) combined with a useful stretching has a great importance in maintaining good health. Moreover, physical activity can play a crucial role in the treatment of rheumatic diseases in optimizing both physical and mental health. In fact, in patients with rheumatic diseases, physical activity is a great method to improve: pain, sleep, a range of motion, body coordination, balance, motivation, functional joint stability; also it is able to reduce the tender points, enhance energy, decrease fatigue and it is an excellent anti-stress strategy. Moreover, physical activity is a good way to socialize, an excellent anti-stress agent and the best aesthetic method for our body “mens sana in corpore sano”. We all should follow the example of some north European countries such as Germany, the Netherlands, Switzerland, Finland and others, more sensitive to disease prevention through the use of physical activity, where moderate exercise is a lifestyle. I hope with this editorial to help readers and the scientific community to better understand the importance of physical activity in our lifestyle.
It’s never too late to begin to move, there is no minimum level to have benefits: A bit of activity is better than none. The benefits begin as soon as you start to be more active.
ACKNOWLEDGEMENTS
The authors would like to thank professor Iain Halliday for commenting and making corrections to the paper. The decision to submit this paper for publication was not influenced by any the funding bodies. Furthermore, the funders had no role in the design of the study, the collection and analysis of the data, the decision to publish, or the preparation of the manuscript.
Footnotes
P- Reviewer: Maurizio T, Song J S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
Supported by A grant-in-aid from FIR 2014-2016 (COD: 314509), University of Catania.
Conflict-of-interest statement: No conflict of interest is declared by any of the authors.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 12, 2015
First decision: July 28, 2015
Article in press: August 31, 2015
References
- 1.Romano Spica V, Giampaoli S, Di Onofrio V, Liguori G. Safety of sports facilities and training of graduates in physical education. Ann Ig. 2015;27:3–10. doi: 10.7416/ai.2015.2017. [DOI] [PubMed] [Google Scholar]
- 2.Trovato GM, Catalano D, Martines GF, Pirri C, Trovato FM. Western dietary pattern and sedentary life: independent effects of diet and physical exercise intensity on NAFLD. Am J Gastroenterol. 2013;108:1932–1933. doi: 10.1038/ajg.2013.356. [DOI] [PubMed] [Google Scholar]
- 3.Rongen-van Dartel SA, Repping-Wuts H, van Hoogmoed D, Knoop H, Bleijenberg G, van Riel PL, Fransen J. Relationship between objectively assessed physical activity and fatigue in patients with rheumatoid arthritis: inverse correlation of activity and fatigue. Arthritis Care Res (Hoboken) 2014;66:852–860. doi: 10.1002/acr.22251. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Lee S, Kim WU. Applications of systems approaches in the study of rheumatic diseases. Korean J Intern Med. 2015;30:148–160. doi: 10.3904/kjim.2015.30.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landolt-Marticorena C. The need for preclinical biomarkers in systemic autoimmune rheumatic diseases. J Rheumatol. 2015;42:152–154. doi: 10.3899/jrheum.141366. [DOI] [PubMed] [Google Scholar]
- 6.Escalante A, Del Rincón I. The disablement process in rheumatoid arthritis. Arthritis Rheum. 2002;47:333–342. doi: 10.1002/art.10418. [DOI] [PubMed] [Google Scholar]
- 7.Abad VC, Sarinas PS, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008;12:211–228. doi: 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Luyster FS, Chasens ER, Wasko MC, Dunbar-Jacob J. Sleep quality and functional disability in patients with rheumatoid arthritis. J Clin Sleep Med. 2011;7:49–55. [PMC free article] [PubMed] [Google Scholar]
- 9.Trovato GM, Pace P, Salerno S, Trovato FM, Catalano D. Pain assessment in fibromyalgia and rheumatoid arthritis: influence of physical activity and illness perception. Clin Ter. 2010;161:335–339. [PubMed] [Google Scholar]
- 10.Nikolaus S, Bode C, Taal E, van de Laar MA. Fatigue and factors related to fatigue in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken) 2013;65:1128–1146. doi: 10.1002/acr.21949. [DOI] [PubMed] [Google Scholar]
- 11.Miyakoshi N. [Therapeutic exercise] Clin Calcium. 2008;18:1611–1615. [PubMed] [Google Scholar]
- 12.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 13.Mease PJ, Arnold LM, Crofford LJ, Williams DA, Russell IJ, Humphrey L, Abetz L, Martin SA. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum. 2008;59:952–960. doi: 10.1002/art.23826. [DOI] [PubMed] [Google Scholar]
- 14.Häuser W, Bernardy K, Arnold B, Offenbächer M, Schiltenwolf M. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216–224. doi: 10.1002/art.24276. [DOI] [PubMed] [Google Scholar]
- 15.Arnold LM, Clauw DJ, Dunegan LJ, Turk DC. A framework for fibromyalgia management for primary care providers. Mayo Clin Proc. 2012;87:488–496. doi: 10.1016/j.mayocp.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazzola M, Atzeni F, Salaffi F, Stisi S, Cassisi G, Sarzi-Puttini P. Which kind of exercise is best in fibromyalgia therapeutic programmes? A practical review. Clin Exp Rheumatol. 2010;28:S117–S124. [PubMed] [Google Scholar]
- 17.Busch AJ, Webber SC, Brachaniec M, Bidonde J, Bello-Haas VD, Danyliw AD, Overend TJ, Richards RS, Sawant A, Schachter CL. Exercise therapy for fibromyalgia. Curr Pain Headache Rep. 2011;15:358–367. doi: 10.1007/s11916-011-0214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masiero S, Boniolo A, Wassermann L, Machiedo H, Volante D, Punzi L. Effects of an educational-behavioral joint protection program on people with moderate to severe rheumatoid arthritis: a randomized controlled trial. Clin Rheumatol. 2007;26:2043–2050. doi: 10.1007/s10067-007-0615-0. [DOI] [PubMed] [Google Scholar]
- 19.Busch AJ, Overend TJ, Schachter CL. Fibromyalgia treatment: the role of exercise and physical activity. Int J Clin Rheumtol. 2009;4:343–380. [Google Scholar]
- 20.Hooten WM, Qu W, Townsend CO, Judd JW. Effects of strength vs aerobic exercise on pain severity in adults with fibromyalgia: a randomized equivalence trial. Pain. 2012;153:915–923. doi: 10.1016/j.pain.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Häuser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome - a systematic review. Eur J Pain. 2010;14:5–10. doi: 10.1016/j.ejpain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Mist SD, Firestone KA, Jones KD. Complementary and alternative exercise for fibromyalgia: a meta-analysis. J Pain Res. 2013;6:247–260. doi: 10.2147/JPR.S32297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreurs KM, Veehof MM, Passade L, Vollenbroek-Hutten MM. Cognitive behavioural treatment for chronic fatigue syndrome in a rehabilitation setting: effectiveness and predictors of outcome. Behav Res Ther. 2011;49:908–913. doi: 10.1016/j.brat.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Vercoulen JH, Bazelmans E, Swanink CM, Fennis JF, Galama JM, Jongen PJ, Hommes O, Van der Meer JW, Bleijenberg G. Physical activity in chronic fatigue syndrome: assessment and its role in fatigue. J Psychiatr Res. 1997;31:661–673. doi: 10.1016/s0022-3956(97)00039-3. [DOI] [PubMed] [Google Scholar]
- 25.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2015;2:CD003200. doi: 10.1002/14651858.CD003200.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Mobasheri A, Matta C, Zákány R, Musumeci G. Chondrosenescence: definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80:237–244. doi: 10.1016/j.maturitas.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Giunta S, Castorina A, Marzagalli R, Szychlinska MA, Pichler K, Mobasheri A, Musumeci G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical Evidence from in vivo and in vitro models of rat osteoarthritis. Int J Mol Sci. 2015;16:5922–5944. doi: 10.3390/ijms16035922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musumeci G, Castrogiovanni P, Mazzone V, Szychlinska MA, Castorina S, Loreto C. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur J Histochem. 2014;58:2371. doi: 10.4081/ejh.2014.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musumeci G, Trovato FM, Loreto C, Leonardi R, Szychlinska MA, Castorina S, Mobasheri A. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: a morphological, immunohistochemical and biochemical study. Acta Histochem. 2014;116:965–972. doi: 10.1016/j.acthis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Musumeci G, Castrogiovanni P, Loreto C, Castorina S, Pichler K, Weinberg AM. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: a morphological study. Int J Mol Sci. 2013;14:15767–15784. doi: 10.3390/ijms140815767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musumeci G, Szychlinska MA, Mobasheri A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: molecular markers of senescent chondrocytes. Histol Histopathol. 2015;30:1–12. doi: 10.14670/HH-30.1. [DOI] [PubMed] [Google Scholar]
- 33.Musumeci G, Castrogiovanni P, Trovato FM, Imbesi R, Giunta S, Szychlinska MA, Loreto C, Castorina S, Mobasheri A. physical activity ameliorates cartilage degeneration in a rat model of aging: a study on lubricin expression. Scand J Med Sci Sports. 2015;25:e222–e230. doi: 10.1111/sms.12290. [DOI] [PubMed] [Google Scholar]
- 34.Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16:6093–6112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musumeci G, Trovato FM, Pichler K, Weinberg AM, Loreto C, Castrogiovanni P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model. An “in vivo” and “in vitro” study on lubricin expression. J Nutr Biochem. 2013;24:2064–2075. doi: 10.1016/j.jnutbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Di Rosa M, Szychlinska MA, Tibullo D, Malaguarnera L, Musumeci G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur J Histochem. 2014;58:2423. doi: 10.4081/ejh.2014.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pichler K, Loreto C, Leonardi R, Reuber T, Weinberg AM, Musumeci G. RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training) in rat with glucocorticoid-induced osteoporosis. Histol Histopathol. 2013;28:1185–1196. doi: 10.14670/HH-28.1185. [DOI] [PubMed] [Google Scholar]
- 38.Musumeci G, Loreto C, Leonardi R, Castorina S, Giunta S, Carnazza ML, Trovato FM, Pichler K, Weinberg AM. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31:274–284. doi: 10.1007/s00774-012-0414-9. [DOI] [PubMed] [Google Scholar]
- 39.Knobloch TJ, Madhavan S, Nam J, Agarwal S, Agarwal S. Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr. 2008;18:139–150. doi: 10.1615/critreveukargeneexpr.v18.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Curr Med Chem. 2001;8:829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, Conaghan PG, Doherty M, Geenen R, Hammond A, Kjeken I, Lohmander LS, Lund H, Mallen CD, Nava T, Oliver S, Pavelka K, Pitsillidou I, da Silva JA, de la Torre J, Zanoli G, Vliet Vlieland TP; European League Against Rheumatism (EULAR) EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 44.Musumeci G, Castrogiovanni P, Coleman R, Szychlinska MA, Salvatorelli L, Parenti R, Magro G, Imbesi R. Somitogenesis: From somite to skeletal muscle. Acta Histochem. 2015;117:313–328. doi: 10.1016/j.acthis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Frasca G, Panico AM, Bonina F, Messina R, Rizza L, Musumeci G, Rapisarda P, Cardile V. Involvement of inducible nitric oxide synthase and cyclooxygenase-2 in the anti-inflammatory effects of a red orange extract in human chondrocytes. Nat Prod Res. 2010;24:1469–1480. doi: 10.1080/14786410903169987. [DOI] [PubMed] [Google Scholar]
- 46.Musumeci G, Trovato FM, Imbesi R, Castrogiovanni P. Effects of dietary extra-virgin olive oil on oxidative stress resulting from exhaustive exercise in rat skeletal muscle: a morphological study. Acta Histochem. 2014;116:61–69. doi: 10.1016/j.acthis.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Catalano D, Trovato GM, Pace P, Martines GF, Trovato FM. Mediterranean diet and physical activity: an intervention study. Does olive oil exercise the body through the mind? Int J Cardiol. 2013;168:4408–4409. doi: 10.1016/j.ijcard.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 48.Musumeci G, Loreto C, Imbesi R, Trovato FM, Di Giunta A, Lombardo C, Castorina S, Castrogiovanni P. Advantages of exercise in rehabilitation, treatment and prevention of altered morphological features in knee osteoarthritis. A narrative review. Histol Histopathol. 2014;29:707–719. doi: 10.14670/HH-29.707. [DOI] [PubMed] [Google Scholar]
- 49.Burguera M, López-Merino V, García-Civera R, Chorro J, Ruiz-Granell R, Sanchís J. [Heart rate-ventricular extrasystole relations and their dependence on circadian rhythms] Rev Esp Cardiol. 1989;42:658–665. [PubMed] [Google Scholar]
- 50.Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7:38. doi: 10.1186/1479-5868-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 52.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 53.Houston MN, Hodson VE, Adams K KE, Hoch JM. The effectiveness of whole-body-vibration training in improving hamstring flexibility in physically active adults. J Sport Rehabil. 2015;24:77–82. doi: 10.1123/JSR.2013-0059. [DOI] [PubMed] [Google Scholar]
- 54.Kim SR, Stitik TP, Foye PM, Greenwald BD, Campagnolo DI. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: a physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389. doi: 10.1097/01.phm.0000124443.31707.74. [DOI] [PubMed] [Google Scholar]
- 55.Gibbs RL, Rosen BS, Lacerte M. Published research and physiatric opinion in life care planning. Phys Med Rehabil Clin N Am. 2013;24:553–566. doi: 10.1016/j.pmr.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Moreira V, Antunes F. [Ankle sprains: from diagnosis to management. the physiatric view] Acta Med Port. 2008;21:285–292. [PubMed] [Google Scholar]
- 57.Matassi F, Duerinckx J, Vandenneucker H, Bellemans J. Range of motion after total knee arthroplasty: the effect of a preoperative home exercise program. Knee Surg Sports Traumatol Arthrosc. 2014;22:703–709. doi: 10.1007/s00167-012-2349-z. [DOI] [PubMed] [Google Scholar]
- 58.Chen H, Onishi K. Effect of home exercise program performance in patients with osteoarthritis of the knee or the spine on the visual analog scale after discharge from physical therapy. Int J Rehabil Res. 2012;35:275–277. doi: 10.1097/MRR.0b013e328355a1bd. [DOI] [PubMed] [Google Scholar]
- 59.Han AS, Nairn L, Harmer AR, Crosbie J, March L, Parker D, Crawford R, Fransen M. Early rehabilitation after total knee replacement surgery: a multicenter, noninferiority, randomized clinical trial comparing a home exercise program with usual outpatient care. Arthritis Care Res (Hoboken) 2015;67:196–202. doi: 10.1002/acr.22457. [DOI] [PubMed] [Google Scholar]
- 60.McCutchen A. Music therapy in the management of chronic osteoarthritis pain. Ky Nurse. 2007;55:10. [PubMed] [Google Scholar]
- 61.Skingley A, Vella-Burrows T. Therapeutic effects of music and singing for older people. Nurs Stand. 2010;24:35–41. doi: 10.7748/ns2010.01.24.19.35.c7446. [DOI] [PubMed] [Google Scholar]
- 62.Musumeci G. Welcome to the New Open Access Journal of Functional Morphology and Kinesiology. J Functional Morphology Kinesiology. 2016;1:1–5. [Google Scholar]


