Background: Hyaluronan has been linked to asthma severity and inflammation.
Results: We characterized the hyaluronan levels and its heavy chain modification in an experimental model of human asthma exacerbation.
Conclusion: These data implicate hyaluronan and its heavy chain modification in human asthma severity.
Significance: Repetitive asthma exacerbations exacerbate hyaluronan pathobiology, which contribute to the chronic inflammation associated with this disease.
Keywords: asthma, extracellular matrix, human, hyaluronan, lung, TSG-6, heavy chain
Abstract
Hyaluronan (HA) is a large (>1500 kDa) polysaccharide of the extracellular matrix that has been linked to severity and inflammation in asthma. During inflammation, HA becomes covalently modified with heavy chains (HC-HA) from inter-α-inhibitor (IαI), which functions to increase its avidity for leukocytes. Our murine model of allergic pulmonary inflammation suggested that HC-HA may contribute to inflammation, adversely effecting lower airway remodeling and asthma severity. Our objective was to characterize the levels of HA and HC-HA in asthmatic subjects and to correlate these levels with asthma severity. We determined the levels and distribution of HA and HC-HA (i) from asthmatic and control lung tissue, (ii) in bronchoalveolar lavage fluid obtained from non-severe and severe asthmatics and controls, and (iii) in serum and urine from atopic asthmatics after an experimental asthma exacerbation. HC-HA distribution was observed (i) in the thickened basement membrane of asthmatic lower airways, (ii) around smooth muscle cells of the asthmatic submucosa, and (iii) around reserve cells of the asthmatic epithelium. Patients with severe asthma had increased HA levels in bronchoalveolar lavage fluid that correlated with pulmonary function and nitric oxide levels, whereas HC-HA was only observed in a patient with non-severe asthma. After an experimental asthma exacerbation, serum HA was increased within 4 h after challenge and remained elevated through 5 days after challenge. Urine HA and HC-HA were not significantly different. These data implicate HA and HC-HA in the pathogenesis of asthma severity that may occur in part due to repetitive asthma exacerbations over the course of the disease.
Introduction
Asthma is a chronic inflammatory disease that has increased in prevalence over the past decades. A subset of asthmatics have severe disease characterized by a fixed obstructive component on spirometry and are often on high dose inhaled corticosteroids or chronic oral steroids. The lower airways in these patients are characterized by basement membrane thickening, severe airway smooth muscle hypertrophy, and increased deposition of extracellular matrix (1).
Hyaluronan (HA)2 is a glycosaminoglycan in which the disaccharide (glucuronic acid-β1,3-N-acetylglucosamine-β1,4-) is repeated several thousand times. HA is a major component and important constituent of the extracellular matrix (2, 3). In asthma, studies have linked HA levels in the bronchoalveolar lavage fluid (BAL) with inflammation and have been shown to increase after histamine challenge (4, 5). Our published data in a mouse model of asthma revealed increased HA in BAL of mice during the course of inflammatory cell recruitment (6, 7). Increased HA deposition was detected in the peribronchioles and perivascular regions in the lung. Furthermore, the HA was later replaced with a collagen extracellular matrix during a chronic exposure model.
During inflammatory and developmental processes, HA can be covalently modified with the heavy chains (HCs) of inter-α-inhibitor (IαI) to form an HC-HA complex (8, 9). The transfer of HCs to HA is accomplished by the enzyme tumor necrosis factor-stimulated gene 6 (TSG-6) (10, 11). We and others have shown that HC substitution of HA significantly increases leukocyte adhesion to HA (12, 13), thereby potentially defining a mechanism whereby HC-HA could direct inflammatory events. Indeed, TSG-6 null mice had reduced HA in the lungs after acute exposure, less inflammation, and a reduction in lower airway hyperresponsiveness (14). Additionally, Liang et al. (15) published data indicating deranged HA homeostasis in asthma with an increased HA production by human asthmatic airway fibroblasts.
Based on these data, we hypothesized that HA and the covalent modification of HA with HCs promote leukocyte adhesion to HA matrices in asthmatic lower airways, promoting chronic inflammation that can result in aberrant remodeling of the lower airways and contributing to asthma severity as a result of repeated asthma exacerbations. To test our hypothesis, we measured HA and HC-HA: (i) distribution in asthmatic lower airways, (ii) levels in BAL fluid of controls, non-severe, and severe asthmatics, and (iii) levels in the serum and urine of asthmatics that underwent an aerosolized whole lung allergen provocation.
Experimental Procedures
Subject Enrollment and Characterization
All recruited subjects gave informed written consent by signing a Cleveland Clinic Institutional Review Board-approved consent document. All subjects were part of the Cleveland Clinic Severe Asthma Research Program. To qualify, all subjects were screened by history, physical examination, spirometry (before and after 2 puffs of inhaled albuterol), methacholine provocation, and allergy prick skin testing to a standard panel of aeroallergens. All subjects were nonsmokers (<5-packs/year) between ages 18 and 55 yr. Subjects were classified as healthy controls if they were free of respiratory symptoms and had normal baseline spirometry, a negative methacholine challenge test, and negative skin tests. Subjects were classified as allergic asthmatics if they had two or more positive skin tests and met the criteria for asthma. None of the asthmatics had atopic dermatitis/eczema. One asthmatic patient had nasal polyps and chronic sinusitis. Severe asthma was defined by the proceedings of the American Thoracic Society Workshop on Refractory Asthma (16), with major and minor characteristics previously described (17). To distinguish nonsevere and severe asthma, we evaluated all individuals with asthma on the basis of severity of airflow limitation, as classified by forced expiratory volume (%FEV1) and FEV1/FVC ratio. BAL fluid was collected from the instillation of three 50-ml aliquots of normal saline solution warmed to 37 °C into the lingula and withdrawn by gentle hand aspiration. Lung tissue for histological and biochemical analysis was procured from endobronchial biopsies during a bronchoscopy with Institutional Review Board approval and from rare large surgical biopsies. Cadaver lung tissue was procured from a patient with acute severe asthma. The tissue for the microscopic and electrophoretic assays was from similar regions in some, but not all, subjects.
Lung Function and Fraction of Exhaled NO
Spirometry and fractional exhaled NO analyses were done consistent with American Thoracic Society standards and as previously described (18).
Allergy Skin Testing
Allergy skin testing was done with the skin prick method as previously described (17).
Experimental Asthma Exacerbation
This method has been previously described (19–22). Asthmatics with >2-positive skin tests (Allergic asthmatics, group 4) and whose positive skin tests included reactions to either ragweed or grass pollen were selected for this procedure (whole lung allergen challenge (WLAC)). They were exposed to aerosolized whole lung allergen provocation when out of season. Escalating concentrations of allergen were aerosolized to determine the provocative dose, which causes a 20% decline in FEV1 (PD20). For grass pollen challenge, a stock solution of allergen (10,000 bioequivalent allergy units (BAU/ml)) was diluted with saline to produce eight concentrations (1, 3.6, 10, 31.6, 100, 316, 1000, and 3162 BAU/ml) for inhalation. For ragweed pollen challenge, a stock solution of allergen (22 antigen E units/ml) was diluted with saline to produce eight concentrations (0.002, 0.008, 0.02, 0.8, 0.22, 0.7, 2.2, and 7 antigen E units/ml) for inhalation. The patient inhaled five breaths at each concentration range. The concentration of allergen producing a 20% fall in FEV1 was determined. At the end of the challenge, two puffs of inhaled albuterol were administered via a metered dose inhaler.
ELISA-like Assay for Quantification of HA
HA was quantified using an HA test kit (Corgenix, Broomfield, CO) according to the manufacturer's instructions. The HA test kit uses a capture molecule known as “hyaluronan-binding protein” (HABP). Diluted BAL, serum, and urine samples were incubated in HABP-coated microwells, which allows the HA present in samples to react with the immobilized HABP. After removal of unbound BAL molecules by washing with PBS, HABP conjugated with horseradish peroxidase solution was added to the microwells to form complexes with the immobilized HA. After washing with PBS, a chromogenic substrate of tetramethylbenzidine and hydrogen peroxide was added to develop color reaction. The intensity of the color was measured in absorbance units with a spectrophotometer at 450 nm. HA levels in urine and control samples were determined against a reference curve prepared from the reagent blank and the HA reference solution provided with the kit. For more information on the sensitivity, specificity, and molecular weight accuracy of this technique please consult our review by Haserodt et al. (23).
Immunohistochemistry of HA
HA distribution on paraffin sections of healthy control and asthmatic human lung tissue was assessed by probing the sections with a biotinylated HABP (385911; EMD Millipore, Billerica, MA) used at a 1:100 dilution in PBS containing 1% BSA. A streptavidin horseradish peroxidase construct was then applied following the manufacturer's recommendations for the VECTASTAIN Elite ABC Kit, using the 3,3′-diaminobenzidine substrate.
Immunofluorescent Microscopy Hyaluronan's Heavy Chain Modification
De-indentified human lung tissues of asthmatics and control non-asthmatic patients from paraffin-embedded sections were deparaffinized and blocked for 30 min in PBS with 1% BSA. HA, IαI, and the common leukocyte antigen (CD45) were simultaneously labeled with the following primary antibodies and binding protein in the blocking solution. (i) HA was labeled with a biotinylated hyaluronan binding protein (5 μg/ml; 385911; EMD Millipore), (ii) IαI was labeled with a rabbit polyclonal antibody against IαI (1:100; A0301; Dako, Glostrup, Denmark), and (iii) CD45 was labeled with a mouse monoclonal antibody (1:100; M070; Dako). After a 45-min incubation at room temperature, the slides were washed in PBS 3 times, 10 min each wash. The following secondary antibodies and binding proteins were simultaneously applied in the blocking solution: (i) streptavidin conjugated to Alexa Fluor® 633 (1:500; S21375; Life Technologies); (ii) Cy3 donkey anti-rabbit (1:400; 711-165-152; Jackson ImmunoResearch, West Grove, PA), (iii) donkey anti-mouse Alexa 488 (1:250; A21202; Life Technologies). After a-45 min incubation at room temperature, the slides were washed in PBS 3 times, 10 min each wash. Vectashield fluorescent mounting medium with DAPI (H-1200; Vector Laboratories, Burlingame, CA) was applied before placing the coverslip. Imaging was done by confocal (Fig. 2) and standard (Fig. 3) fluorescent microscopy. Grayscale images were pseudocolored using ImageJ software (Rasband, W.S., ImageJ, United States National Institutes of Health, Bethesda, MD).
FIGURE 2.
Accumulation of the heavy chain modification of hyaluronan in asthmatic airways. Paraffin lung sections of a healthy control donor (A–F) and a patient with severe asthma (G–L) were probed with a biotinylated HABP (green; panels A, D, G, and J) and an antibody against IαI (red; panels B, E, H, and K). DAPI stained nuclei are shown in blue. An overlay of the images is shown in panels C, F, I, and L. Fluorescent secondary antibody controls are shown in panels D–F and J–L. The airway submucosas are indicated with white arrows. Magnification was 20×. The magnification bar is shown in the lower right corner of panel L (200 μm). These images were representative of six healthy control subjects and three asthmatics.
FIGURE 3.
Expression of the heavy chain donor inter-α-inhibitor by reserve cells of the airway epithelium. Shown is a paraffin lung section of a biopsy from an asthmatic individual probed with a biotinylated HABP (green; panel A) and an antibody against IαI (red; panel B). DAPI-stained nuclei are shown in blue. An overlay of the images is shown in panel C. Magnification was 40×. The magnification bar is shown in the upper right corner of panel C (40 μm). These images were representative of three asthmatic subjects from bronchial biopsies.
TSG-6 Activity Assay
This assay is based on the assay developed by Wisniewski et al. (24) with the exception that a Li-Cor (Lincoln, NE) infrared secondary antibody and the Li-Cor Odyssey scanner were used instead of the ELISA-based detection. In this assay, HA was covalently attached to 8-well strips of a 96-well plate as follows. An aliquot (97.52 μl) of 46 mm Sulfo-NHS (106627-54-7; Thermo Scientific) was added to 5.3 ml of 1700-kDa HA (LifeCore Biomedical, Chaska, MN) at 200 μg/ml in water followed by the addition of 5.3 ml of 1.87 mm of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) (25952-53-8; Thermo Scientific). An aliquot (100 μl/well) was transferred to each well of the 96 wells on a Nunc NH-Covalink plate (Thermo Scientific) and incubated at room temperature for 2 h followed by an overnight incubation at 4 °C. The wells were washed twice with 2 m NaCl (200 μl per well) followed by washing with PBS 3 times. Gentamicin in PBS (50 μg/ml) was added to each well (200 μl/well), and the sealed plate was stored at 4 °C. On the day of the assay, the wells of the plate were blocked for 1 h with 100 μl of 5% nonfat milk in PBS and then rinsed 3 times in PBS. To 91 μl of each BAL sample was added 4 μl of a commercial source of human serum (Equitech Bio, Kerrville, TX) and 5 μl of 10 mm MgCl2. Positive controls included the addition of 0.15 μg/ml recombinant human TSG-6 (2104-TS-050; R&D Systems, Minneapolis, MN). Negative controls included 4 μl of serum and 5 μl of 10 mm MgCl2 added to 91 μl of PBS. The BAL was incubated on the HA-coated NH-Covalink plates for 2 h at 37 °C. The reaction was stopped by the addition of 50 μl of 20 mm EDTA to each well. The wells were washed with PBST (0.05% Tween 20 in PBS) 3 times, and then an aliquot (100 μl) of a rabbit polyclonal antibody, raised against IαI, (diluted 1:1000 in Li-Cor blocking buffer containing 0.05% Tween 20), was added to each well and incubated for 1 h at room temperature. The wells were washed 3 times with PBST followed by the addition of 100 μl of an infrared anti-rabbit secondary antibody from Li-Cor diluted 1:2000 in Li-Cor blocker containing 0.05% Tween 20 and incubated at room temperature for 45 min. The wells were washed three times in PBST followed by imaging on a Li-Cor Odyssey CLx infrared scanner.
Hyaluronidase Extraction of Heavy Chains from Asthmatic Lung Tissue and BAL Fluid
This method has been previously described and is presented here with minor changes (25). Human lung tissue from a patient who died from acute severe asthma was cut with a scalpel and transferred to a preweighed 1.5-ml tube that was prechilled on dry ice. The weight of the lung tissue was recorded, and pre-chilled PBS was added to the tubes such that 100 μl of cold PBS was added for every 30 mg of tissue. The tissue was minced with scissors, on ice, in PBS for ∼1 min. A 50-μl aliquot of the minced tissue suspension was transferred to four new prechilled 1.5-ml new tubes. To each of these four tubes was added (i) PBS (10 μl), (ii) Streptomyces hyaluronidase (10 μl of a 0.5 TRU/ml stock; 389561-100U, EMD Millipore), (iii) an HA oligosaccharide 14 monosaccharides in length (2 μl of a 1 mg/ml stock; Seikagaku) with 8 μl PBS, and (iv) HA14 (same as tube 3) and recombinant TSG-6 (2 μl of a 0.250 μg/ml stock; R&D Systems). Tubes 1 and 2 were incubated on ice for 30 min and then centrifuged at 13,200 rpm at 4 °C for 5 min. The supernatants were transferred to new prechilled 1.5-ml tubes and incubated for another 30 min at 37 °C. Tubes 3 and 4 were incubated for 2 h at 37 °C and centrifuged at 13,200 rpm at 4 °C for 5 min, and the supernatants were transferred to new tubes. Then 25 μl of each supernatant was added per lane on 4–15% Mini-PROTEAN TGX gels (Bio-Rad) and blotted using the Bio-Rad nitrocellulose and Trans-Blot Turbo System. The blot was blocked for 1 h with Li-Cor blocking buffer (927-40000; Li-Cor) and then probed with a rabbit polyclonal antibody against IαI (A0301, Dako North America, Inc. Carpinteria, CA, 1:8000 dilution). The secondary antibody was IRDYE anti-rabbit 800CW (926-32211, Li-Cor) used at 1:15,000. The blot was washed and imaged on an Odyssey Infrared Imaging System (Li-Cor). The method used to measure HC-HA in BAL fluid was similar. An aliquot (2.5 μl) of Streptomyces hyaluronidase (0.5 TRU/ml) or PBS as a negative control was added to 25 μl of BAL fluid and incubated on ice for 30 min followed by a second incubation at 37 °C for 30 min. The samples were then analyzed by Western blot, as described above for the lung tissue HC-HA.
Statistical Analysis
Quantitative data were summarized as the means ± S.E. unless otherwise noted; categorical data were summarized by frequencies. Two-tailed t test statistics and analysis of variance were used when appropriate.
Results
Distribution of Hyaluronan in Asthmatic and Control Lower Airways
Although the presence of HA in the pulmonary secretions (26, 27), (5, 28), and cultured cells (15, 29) of asthmatics has been demonstrated, to our knowledge the distribution of HA in asthmatic lung tissue has only been partially characterized (15). To address this we probed paraffin blocks of healthy control and asthmatic lung tissue with a biotinylated HABP using standard IHC methods (Fig. 1; n = 6 for healthy controls and n = 3 for asthmatics). HA was observed in the thickened basement membrane and surrounding the smooth muscle cells of the airway submucosa in asthmatic tissue, although it was comparably absent in the mucus and goblet cells of the airway epithelium. HA was also found in the basement membrane and the airway submucosa of control subjects, albeit to a much lesser extent considering the lack of the smooth muscle cell hypertrophy and basement membrane thickening in the control tissue.
FIGURE 1.
Distribution of hyaluronan in asthmatic airways. Paraffin lung sections of a healthy control donor (A–C) and a patient with severe asthma (D–F) were probed with a biotinylated HABP and processed for immunohistochemistry (brown 3,3′-diaminobenzidine stain). Nuclei were counterstained with hematoxylin. Panels B and E portray a zoom of the 10× images of panels A and D, respectively. Panels C and F show negative controls that were probed with the horseradish peroxidase streptavidin secondary and developed with 3,3′-diaminobenzidine but lacked the HABP primary. The magnification bar is shown in the lower right corner of panel F (200 μm). These images are representative of six healthy control subjects and three asthmatics.
Pathological Redistribution of Inter-α-inhibitor Heavy Chains from Normal Lower Airway Epithelium to Airway Smooth Muscle Hyaluronan Matrices in Asthmatic Airways
In Fig. 2 we examined the distribution of IαI (indicative of the covalent HC modification of HA) with HA in asthmatic and healthy control lung tissue (n = 6 for healthy controls and n = 3 for asthmatics). Colocalization by immunofluorescent microscopy of HA (HABP) with an antibody against IαI was observed in the thickened basement membrane around asthmatic airways but not within the basement membranes of non-asthmatic airways. This colocalization was also observed around smooth muscle cells in the airway submucosa of asthmatic, but not control, tissue rather than in the airway epithelium where HA and IαI levels were minimal. The colocalization was observed in the matrix surrounding the smooth muscle cell myofibrils rather than in the myofibrils themselves. Although the airway epithelium staining was essentially negative for HA, HC-HA, and IαI, regions of “reserve” epithelial cells stained positive for IαI (Fig. 3; n = 3), suggesting that these cells might secrete their own HC donor for use in the remodeling of the damaged epithelium by this cell population (Fig. 3). Although the co-localization of IαI with HA is not sufficient proof that the HA in these regions is covalently modified with HCs (that will be demonstrated later in the manuscript), the striking colocalization is at least suggestive that the HC modification of HA may be in these regions.
Co-localization of Leukocytes with HC-HA Matrices in an Acute Severe Asthmatic Lower Airway
We used immunofluorescent microscopy to examine the distribution of leukocytes within HC-HA matrices in lung tissue from a patient with acute severe asthma (Fig. 4; n = 3). As apparent in the hematoxylin and eosin (H&E) and trichrome stains (Fig. 4, E and F), the airways of this patient displayed significant smooth muscle proliferation, epithelial metaplasia, and mucus gland hypertrophy, including airway obstruction by a significant mucus plug. HA was distributed throughout the submucosa region and distributed around, but not within, serous mucus glands (Fig. 4A). Furthermore, HA was largely absent within the mucus of the airway lumen. IαI (i.e. HC) distribution was almost exclusively present in the submucosa region, displaying a striking colocalization with HA indicative of pathological HC-HA matrices (Fig. 4B). Using the common leukocyte antigen CD45 as a generic marker of inflammatory cells, we found large numbers of leukocytes in the submucosa region of this lung colocalizing with and, embedded within, pathological HC-HA matrices.
FIGURE 4.
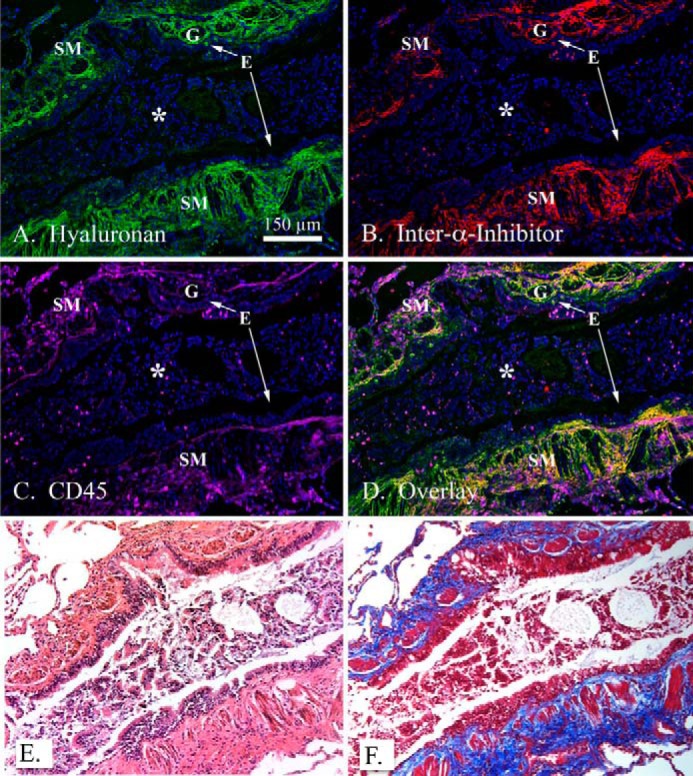
Co-localization of leukocytes within hyaluronan matrices modified with heavy chains in asthmatic airways. A paraffin lung section of a patient with acute severe asthma was probed with a hyaluronan binding protein (green; A), an antibody against IαI (red; panel B), and the common leukocyte antigen CD45 (panel C; magenta). DAPI-stained nuclei are shown in blue. The overlay is shown in panel D. H&E and trichrome staining from the same region shown in panels A–D are shown in panels E and F, respectively. Magnification is 10×. A magnification bar is portrayed as a white line in panel A (150 μm). The airway epithelium is identified by an E, airway smooth muscle by SM, submucosal glands by G, and a mucus plug by an asterisk in panels A–D. These images are representative of three asthmatic replicates.
Hyaluronidase Extraction of Inter-α-inhibitor Heavy Chains from the Lungs of a Patient with Acute Severe Asthma
To obtain biochemical evidence that HA is covalently modified with HCs from IαΙ in asthmatic lower airways (HC-HA), we extracted acute severe asthmatic lung tissue with Streptomyces hyaluronidase (specific for HA) (Fig. 5A; n = 1). Treatment of the minced lung tissue with hyaluronidase induced a HC gel shift from HC-HA (that is too large to enter the gel) to a band at ∼83 kDa representative of free HCs, indicating the presence of HC-HA in the lung tissue. In a similar experiment with healthy control lung tissue, the 83-kDa HC band was largely absent in the hyaluronidase extracts, indicating the relative absence of HC-HA in the control lung tissue (Fig. 5B; n = 4). TSG-6 is the enzyme that transfers HCs to HA (10, 11). To test for endogenous TSG-6 activity, we added an HA oligosaccharide (oligo), 14 monosaccharides in length, to the minced lung tissue as an artificial HC acceptor (Fig. 5A, lane 3). If endogenous TSG-6 activity were present, endogenous TSG-6 would transfer HCs from endogenous IαI to the HA oligo, as observed by the appearance of a band at ∼83 kDa. Although it is possible that the 83-kDa HC band after treatment of the minced lung tissue with HA14 (lane 3) is somewhat stronger than the negative control in lane 1 (Fig. 5A), the difference is minor, demonstrating a lack of endogenous TSG-6 activity. In contrast, the addition of recombinant TSG-6 (Fig. 5A, lane 4) as a positive control demonstrated that conditions were permissible to detect endogenous TSG-6 activity, if it were present. These data suggest that the immunolocalization of HCs in the immunostained sections of Fig. 2 is likely to be HC-HA matrix rather than IαI alone.
FIGURE 5.
The pathological heavy chain modification of HA is present in lung tissue from a patient with acute severe asthma. Lung tissue from an individual with acute severe asthma was minced in a normalized volume of PBS (per wet weight of tissue) and incubated with or without Streptomyces hyaluronidase, an HA14 oligomer as a heavy chain acceptor, and TSG-6 to transfer heavy chains (panel A). The proteins were electrophoretically separated, transferred, and stained with an antibody against IαI (green). Molecular weight standards are shown in red. Lane 1 shows untreated lung tissue. The HC-HA complex was too large to enter the gel, but when digested with hyaluronidase (HAase) (lane 2) the heavy chains were released and migrated as a single band at ∼83 kDa. In lane 3, an HA oligo, 14 monosaccharides in length, was added to the minced tissue to detect endogenous TSG-6 activity. As a positive control, exogenous recombinant TSG-6 was also added with the HA14 (lane 4). Panel B shows four replicates of healthy control (Ctrl) lung tissue treated with and without hyaluronidase (similar to the asthmatic tissue in lanes 1 and 2 of panel A). The green lobules in the schematic models represent HCs attached to bikunin (blue rectangle) via a single chondroitin sulfate chain (black line). The schematic with two HCs represents IαI. The schematic with one HC represents pre-IαI. The asthmatic tissue in panel A was from a rare, large surgical biopsy of a single patient. Surgical biopsies of asthmatics are not a recommended diagnostic procedure as asthma is determined by other clinical criteria. For this reason we are unable to present more replicates from the asthmatic cohort.
Altered Bronchoalveolar Lavage Hyaluronan and Exhaled Nitric Oxide Levels Correspond to Pulmonary Function in Human Asthma
To determine if HA levels are related to asthma severity, we measured HA levels in BAL fluid and urine of control, non-severe, and severe asthmatics (Table 1 and Fig. 6; n = 18 healthy controls, n = 14 non-severe asthmatics, and n = 10 severe asthmatics). We compared these levels to exhaled nitric oxide (Fig. 6B) and pulmonary function (Fig. 6, C and D). HA levels in the BAL were ∼20% higher for non-severe asthmatics (p = 0.25) and ∼33% higher for severe asthmatics (p = 0.05) compared with controls (Fig. 6A) (as determined by an ELISA-like assay). Total BAL protein levels and cell counts are listed in Table 1. HA urine levels were not statistically different, and HC-HA was below the limit of detection in urine (data not shown). Exhaled nitric oxide was ∼56% higher for non-severe asthmatics (p = 0.07) and ∼52% higher for severe asthmatics (p = 0.03) compared with controls. %FEV1 for this cohort of patients was ∼29% lower for patients with severe asthma compared with controls (p < 0.0001) and ∼21% lower when the %FEV1 for severe asthmatics was compared with non-severe asthmatics (p = 0.009) (Fig. 4C). A measurement of airway obstruction (FEV1/FVC) was ∼15% lower for patients with severe asthma compared with controls (p = 0.003) and ∼11% lower when severe asthmatics were compared with non-severe asthmatics (p = 0.05) (Fig. 6D).
TABLE 1.
Demographic data for control and asthmatic individuals
AA, African American; C, white (Caucasian), n = number of individuals with available data; AQLQ, asthma quality of life questionnaire; PC, provocative concentration for methacholine. Data are presented as the means and S.E. Total cells and differentials are from BAL.
| Healthy (n = 18) | Non severe asthma (n = 14) | Severe asthma (n = 10) | |
|---|---|---|---|
| Gender (M/F) | 5/13 | 5/9 | 3/7 |
| Age | 33.3 (2.7) | 33.1 (2.5) | 41.8 (2.5) |
| Race. AA/C/others | 8/9/1 | 2/9/3 | 7/3/0 |
| Height, cm | 168.7 (2.8) | 169.2 (2.2) | 167.5 (3.8) |
| Weight, kg | 82.9 (6.8) | 82.5 (5.5) | 93.6 (5.8) |
| Duration of asthma, years | N/A | 17.5 (2.9) | 22.3 (3.9) |
| Total AQLQ score | 7.0 (0.01) | 5.4 (0.2) | 3.3 (0.3)a |
| PC20, mg/ml | N/A | 4.73 (2.10) | 1.56 (0.49)a |
| Atopy (no/yes) | 13/5 | 3/11 | 0/10a |
| IgE levels, IU/ml | 52.1 (19.3) | 287.7 (87.6) | 282.6 (109.4)a |
| No. positive skin tests | 2.23 (0.68) | 4.0 (0.50) | 4.71 (0.78)a |
| Inhaled steroid, % (n) | 0 (18) | 42.9 (6) | 100 (10) |
| Bronchoalveolar lavage | |||
| Total protein (μg/ml BAL) | 100.6 (9.8) | 83.2 (21.1) | 98.9 (29.6) |
| Total cell count, millions | 4.4 (0.9) | 3.9 (0.9) | 5.5 (2.3) |
| % Alveolar macrophages | 91.0 (1.5) | 92.7(1.4) | 90.1 (3.4) |
| % Lymphocytes | 5.6 (1.3) | 3.6 (0.9) | 5.7 (2.2) |
| % Neutrophils | 3.3 (0.7) | 3.0 (1.0) | 3.9 (2.0) |
| % Eosinophils | 0.1 (0.03) | 0.7 (0.4) | 0.3 (0.3) |
a p < 0.05.
FIGURE 6.

Altered bronchoalveolar lavage hyaluronan and exhaled nitric oxide levels correspond to pulmonary function in human asthma. BAL HA levels (A), exhaled nitric oxide (B), and pulmonary function (%FEV1 and FEV1/FVC, panels C and D, respectively) are presented from a cohort of control subjects (n = 18) and in non-severe (n = 14) and severe (n = 10) asthmatic subjects. Statistically significant p values are annotated on the individual panels.
HC-HA Levels and TSG-6 Activity in Asthmatic Bronchoalveolar Lavage Fluid
Using a method similar to the hyaluronidase extraction procedure described in Fig. 5, we measured HC-HA levels in the BAL fluid from healthy controls, non-severe asthma, and severe asthmatic subjects (Fig. 7, A (n = 8 healthy controls, n = 5 non-severe asthmatics, n = 5 severe asthmatics) and B (n = 10 healthy controls, n = 5 non-severe asthmatics, n = 5 severe asthmatics)). Full-length IαI (250 kDa) was detected in most subjects, although it was generally higher in asthmatic individuals. IαI is primarily found in serum, so it is most likely that the IαI detected in BAL is from serum exudates, although we have not ruled out the possibility that it is derived from the airway epithelium. HC-HA was only detected in a single non-severe asthmatic, as evident by the appearance of an 83-kDa band in the hyaluronidase-treated group (Fig. 7B, lane 6). It is also interesting that this subject had the highest levels of IαI, including IαI isoforms containing three, or one, HCs in contrast to the standard two HCs of IαI. We have seen this phenomenon when TSG-6 is incubated in the presence of IαI when HA levels are relatively low (30). This results in the TSG-6-mediated shuffling of HCs among IαI molecules, also resulting in the release of free HCs. In Fig. 7C we examined the activity of TSG-6 in this patient cohort. Indeed, the same non-severe asthmatic that had HC-HA in the BAL as well as evidence of TSG-6 activity also gave a strongly positive signal in our TSG-6 activity assay (asterisk), whereas the activity was not detected in any of the other patients. The amount of recombinant TSG-6 used in the positive control was 0.15 μg/ml, so the amount of active TSG-6 in the BAL of this patient was close to that level.
FIGURE 7.
Identification of the heavy chain modification of hyaluronan and TSG-6 activity in asthmatic bronchoalveolar lavage fluid. Equal portions of BAL fluid from healthy control (lanes 1–3; n = 3), non-severe asthma (lanes 4–6; n = 3), and severe asthma (lanes 7–9; n = 3) subjects were treated with (panel B) and without (panel A) Streptomyces hyaluronidase and analyzed by Western blot, probing the blot with an antibody against IαI (green). Molecular weight standards are shown in red. Additional replicates (n = 5 for healthy controls, n = 2 for non-severe asthmatics, and n = 2 for severe asthmatics) were run on separate gels (data not shown). The green lobules in the schematic models represent HCs attached to bikunin (blue rectangle) via a single chondroitin sulfate chain (black line). The schematic with two HCs represents IαI. The schematic with one HC represents pre-IαI. The scanned image of three eight-well strips representing the activity of TSG-6 in BAL fluid is shown in panel C. Healthy controls (orange circles; n = 10), non-severe asthmatic (blue circles; n = 5), and severe asthmatic (purple circles; n = 5) are indicated. An aliquot of recombinant TSG-6 was added as a positive control (green circles; n = 2). Negative control includes an aliquot of human serum in PBS but without the addition of recombinant TSG-6 (red circles; n = 2). The asterisk indicates the same patient as presented in panels A and B, lane 6.
Altered Serum Hyaluronan Levels in Experimental Asthma Exacerbation
To determine if asthma exacerbation leads to increased HA, we measured serum and urine HA levels (by an ELISA-like assay) in atopic asthmatics after experimental asthma exacerbation utilizing WLAC (Table 2). Serum HA levels increased ∼33% at 4 h after WLAC (range 11–59%), peaking at ∼47% 5 days after WLAC (range 4.7–65%) (Fig. 8; n = 8). HA levels in all 8 patients increased from baseline 4 h after WLAC although this trend did not reach statistical significance. HA levels remained elevated after 5 days in 7 of the 8 patients, which was statistically significant (p = 0.02). Urine HA levels after WLAC were not statistically significant for the 4-h or 5-day time points (data not shown).
TABLE 2.
Demographic data for allergen sub-study
“Before” means before allergen challenge. “After” means 4 h after allergen inhalation. AA, African American; C, Caucasian. Data are presented as the means (S.E.). Total cells and differentials are from BAL. * p < 0.05.
FIGURE 8.
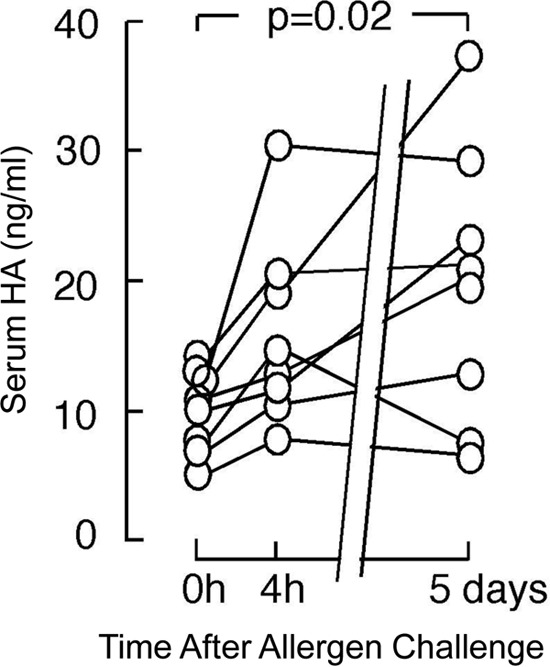
Altered serum hyaluronan levels in whole lung allergen challenge. Serum HA levels were measured by an ELISA-like assay previous to (0 h), 4 h, and 5 days after WLAC to ragweed among allergic individuals (n = 8). A statistically significant p value between 0 h and 5 days is annotated on the graph.
Discussion
The primary conclusions from this study are: (i) a pathological form of HA, covalently modified with HCs from IαI, was present in the thickened basement membrane and the submucosa of human asthmatic lower airways; (ii) leukocytes were found embedded within the HC-HA matrices of the asthmatic airway submucosa; (iii) reserve cells of the airway epithelium stained positively for IαI, suggesting that they may make their own HC donor during remodeling of the damaged airway epithelium; (iv) elevated BAL HA levels were associated with asthma severity, including NO production and pulmonary function; (v) HC-HA and TSG-6 activity were detected in the BAL of an asthmatic patient with non-severe asthma; (vi) HA serum levels after allergen challenge in a model of experimental asthma were elevated 4 h after challenge and remained elevated for at least 5 days thereafter.
Hyaluronan's Heavy Chain Modification in Asthmatic Lower Airways
Acute severe asthma is a life-threatening condition of airway obstruction in asthma that is unresponsive to bronchodilators or steroids. The present data indicate that the formation of HC-HA in asthmatic airways may contribute to exacerbations associated with asthma, and our previous mouse data further support this conclusion (14). We have previously demonstrated that mice lacking the ability to form HC-HA (TSG-6−/−) develop less inflammation and lower airway hyperresponsiveness and accumulate less HA in their airways after allergen challenge in a model of allergic asthma (14). The mechanism whereby HC-HA directs inflammatory events in asthma is not fully known, but it is likely to involve the increased avidity of leukocytes to HC-HA matrices rather than to HA alone (12, 13). We demonstrated in Fig. 4 that leukocytes were embedded within HC-HA matrices of the asthmatic airway submucosa. Although it is not likely that HC-HA matrices play a direct role in leukocyte recruitment to the airways, they are likely to affect leukocyte behavior and activation after receptor-mediated docking within these matrices. Among these receptors is the HA receptor CD44. Blocking antibodies against this receptor have been shown to prevent leukocyte adhesion to HC-HA matrices (13, 31). The interaction between HC-HA and CD44 has been observed to promote CD44 “capping” and the digestion of HA by monocytes (32), which could release low molecular weight fragments that have been described as proinflammatory (33). That being said, there is evidence that monocyte incubation with HC-HA induces an anti-inflammatory M2 phenotype (34). This is supported by literature describing an anti-inflammatory role for TSG-6 in different disease models (10, 35, 36). Thus, the contribution that HC-HA makes toward inflammation in asthma remains to be clarified, but we believe the data more strongly support a model for a proinflammatory effect that could adversely affect airway remodeling.
Increased HA Levels in Severe Asthma
Our data confirm previous reports (26, 27) linking higher BAL HA levels to asthma severity. Others have demonstrated the presence of HA in asthmatic BAL fluid (5) and sputum (28). The origin of HA in pulmonary secretions is not certain. Options include direct secretion from the airway epithelium, airway smooth muscle cells, goblet cells, mucus glands, or a combination of these. Among these options, the direct secretion from the airway epithelium and airways smooth muscle cells are the most likely, and several studies have demonstrated the ability of the epithelium and airways smooth muscle cells to secrete HA in vitro (3, 37, 38). The relatively negative staining of HA in goblet cell and mucous gland cell populations indicates that these cells are less likely to be involved in the secretion of HA into the airway lumen. Elevated levels of HA in the BAL of severe asthmatics could be caused by an autocrine mechanism whereby the inflamed epithelium releases proinflammatory cytokines or other mediators such as nitric oxide that act upon the epithelium itself and/or airways smooth muscle cells to induce HA secretion. Another proinflammatory mediator could be the degradation of high molecular weight HA into low molecular weight HA by epithelial derived hyaluronidase activity (39). Alternatively, leukocytes in the lung or airway lumen might release cytokines in asthmatic individuals that stimulate apical HA secretion by the airway epithelium or locally by airways smooth muscle cells. The correlation of elevated levels of exhaled nitric oxide in asthmatics, with elevated levels of HA in BAL suggests the possibility that the HA response might be partly caused by the formation of reactive nitrogen species, which can modify proteins and adversely affect their functional activities (40). The asthmatic HC-HA staining shown in Fig. 2 was taken from a subject who had been prescribed prednisone (5 mg/day) and a high dose of inhaled corticosteroid/long-acting β-agonist combination drug. Similar results, although with somewhat lower levels of HC-HA, were obtained from an asthmatic patient who had been prescribed an inhaled corticosteroid inhaler. Thus, steroid use does not appear to be able to prevent the formation of HC-HA in asthmatic airways. We interpret the presence of IαI in the BAL of the non-severe and severe asthmatic subjects (Fig. 7) as largely indicative of serum exudates leaking into the airway surface liquid as a result of inflammation. The presence of HC-HA and TSG-6 activity in one of the non-severe asthmatics, but not severe asthmatics, could suggest that the production of HC-HA by TSG-6 is an early event that is less abundant during chronic inflammation associated with severe asthma. Although this may be true, it should be acknowledged that other factors could influence these results, such as the technical difficulty of effectively recovering a lavage from the lower airways and that HC-HA may be primarily deposited in the lower airway submucosa (supported by Figs. 2 and 4) rather than at the epithelial surface.
Elevated HA Serum Levels after Allergen Challenge
The elevated serum HA levels in a model of experimental asthma exacerbation (Fig. 8) are most likely the result of airway HA entering the circulatory system in inflamed regions where vascular dilation is present. It is not yet clear whether the HA that enters the circulation is simply flushed from the submucosal connective tissue or whether it is being actively degraded by leukocytes that release fragments of HA into the circulation. HA injected into the circulatory system is removed by the liver at a rate of 0.3–1.0 μg min−1/kg body weight (41). Circulating HA is normally derived from the lymph (41). Our observation that serum HA levels remained elevated for 5 days suggests a steady, continuous release of HA into the circulatory system after WLAC rather than a 5-day retention time of HA in serum after an initial burst of HA induced by WLAC. This provides important evidence that the effect of allergen challenge on matrix remodeling is prolonged several days beyond initial stimulation. It has yet to be determined if the total tissue HA levels are being depleted over the 5-day period or whether they remain at a steady state due to continuous synthesis and turnover of HA. HC-HA levels in asthmatic and control serum were below the limit of detection (not shown), indicating that the HA that accumulates in the serum is not modified with HCs and that the HC modification of HA might retard its turnover in asthmatic tissues, thereby enriching HA matrices with HC-HA in asthmatic airways.
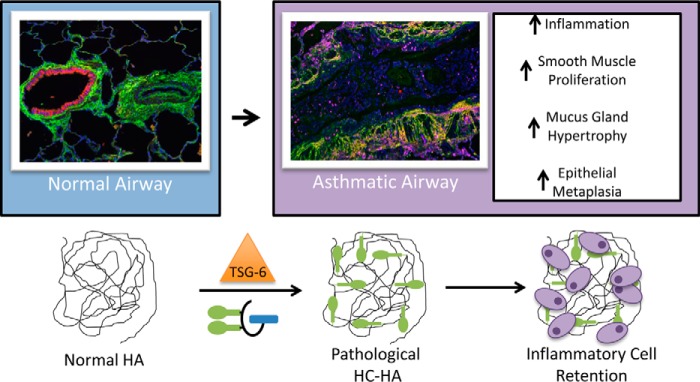
Model of HA in Asthmatic Lower Airways
Altogether, our data support a model for a pathological form of HA (i.e. HC-HA) in asthmatic airways, which may contribute to the inflammation, smooth muscle proliferation, mucus gland hypertrophy, and epithelial metaplasia associated with asthmatic exacerbations (Fig. 9). Our previous observations that mice lacking the ability to form HC-HA (TSG-6−/−) developed a milder form of exacerbations associated with asthma identified the formation of HC-HA matrices as a therapeutic target for the treatment of asthma (14). The present manuscript supports these observations in human asthmatic tissue, BAL, and serum, linking the accumulation of HA and HC-HA to asthma severity and providing further evidence for their role in the pathogenesis of this disease.
FIGURE 9.
Model for the role of the pathological heavy chain modification of hyaluronan in asthma. Under normal conditions, HA is present in the submucosa of airways and pulmonary vasculature as a very large, linear, glycosaminoglycan of the extracellular matrix, lacking any protein modifications. During asthma, the enzyme TSG-6 covalently transfers HCs from IαI to HA in the airway submucosa, forming the pathological HC-HA matrix. This structure promotes inflammatory cell adhesion and retention in the submucosa region of asthmatic airways. It may also influence smooth muscle cell proliferation, mucus gland hypertrophy, and epithelial metaplasia, leading to airway obstruction by the excessive production of mucus.
Author Contributions
M. E. L., M. A. A., S. C. E., and V. C. H. were all involved in the conception design, analysis, and interpretation of the experiments and data. M. E. L. also wrote the manuscript and compiled the figures for this manuscript and created Fig. 9. M. E. L. and B. M. were directly involved in the experiments, data acquisition, and figure preparation for Figs. 1, 2, 3, 4, 5, and 7. A. K. M., L. M. R., A. S., and M. A. A. were involved in the data acquisition, analysis, and figure preparation for Figs. 6 and 8 as well as the data acquisition and analysis of the urine samples. R. D. designed and performed the allergen challenge experiments at Vanderbilt University and provided us with the urine samples that were analyzed. S. C. was responsible for logging, de-identifying, aliquoting, and storing human samples obtained from the Cleveland Clinic whole lung allergen challenge model as well as those shipped from Dr. Dworski at Vanderbilt University. She was also responsible for creating Tables 1 and 2 and assisted with Fig. 6. C. F. provided de-identified lung tissue and provided the pathologic analysis of human tissue for Figs. 1–4. D. G., D. L., R. A. D., and S. C. E. were involved in the design and execution of the human whole lung allergen challenge model at the Cleveland Clinic. All authors provided critical feedback and reviews of this manuscript during preparation.
Acknowledgments
We acknowledge the Imaging Core of the Lerner Research Institute, Cleveland Clinic, and S. Swaidani for assistance with the processing and staining of tissues. We acknowledge the Cleveland Clinic Program of Excellence in Glycoscience (PEG) Resource Core for assistance with the hyaluronan imaging, HC-HA extractions, and TSG-6 activity assay, especially Valbona Cali.
This work was supported, in whole or in part, by National Institutes of Health Grants HL081064 and HL103453 (NHLBI). This work was also supported in part by Case Western Reserve University/Cleveland Clinic CTSA Grant UL1TR000439 and the National Center for Advancing Translational Sciences (NCATS). The authors declare that they have no conflict of interest with the contents of this article.
- HA
- hyaluronan
- BAL
- bronchoalveolar lavage fluid
- HC
- heavy chain
- IαI
- inter-α-inhibitor
- HC-HA
- heavy-chain hyaluronan
- inter-α-inhibitor
- (IαI)
- TSG
- tumor necrosis factor-stimulated gene
- WLAC
- whole lung allergen challenge
- HABP
- hyaluronan-binding protein
- IαI
- inter-α-inhibitor
- %FEV1
- forced expiratory volume
- FVC
- forced vital capacity.
References
- 1. Elias J. A., Lee C. G., Zheng T., Ma B., Homer R. J., Zhu Z. (2003) New insights into the pathogenesis of asthma. J. Clin. Invest. 111, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraser J. R., Laurent T. C., Laurent U. B. (1997) Hyaluronan: its nature, distribution, functions, and turnover. J Intern. Med. 242, 27–33 [DOI] [PubMed] [Google Scholar]
- 3. Forteza R., Lieb T., Aoki T., Savani R. C., Conner G. E., Salathe M. (2001) Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 15, 2179–2186 [DOI] [PubMed] [Google Scholar]
- 4. Söderberg M., Bjermer L., Hällgren R., Lundgren R. (1989) Increased hyaluronan (hyaluronic acid) levels in bronchoalveolar lavage fluid after histamine inhalation. Int. Arch. Allergy Appl. Immunol. 88, 373–376 [DOI] [PubMed] [Google Scholar]
- 5. Sahu S., Lynn W. S. (1978) Hyaluronic acid in the pulmonary secretions of patients with asthma. Biochem. J. 173, 565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng G., Swaidani S., Sharma M., Lauer M. E., Hascall V. C., Aronica M. A. (2013) Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology 23, 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng G., Swaidani S., Sharma M., Lauer M. E., Hascall V. C., Aronica M. A. (2011) Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol 30, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fries E., Kaczmarczyk A. (2003) Inter-α-inhibitor, hyaluronan, and inflammation. Acta Biochim. Pol. 50, 735–742 [PubMed] [Google Scholar]
- 9. Milner C. M., Tongsoongnoen W., Rugg M. S., Day A. J. (2007) The molecular basis of inter-α-inhibitor heavy chain transfer on to hyaluronan. Biochem. Soc. Trans. 35, 672–676 [DOI] [PubMed] [Google Scholar]
- 10. Milner C. M., Higman V. A., Day A. J. (2006) TSG-6: a pluripotent inflammatory mediator? Biochem. Soc. Trans. 34, 446–450 [DOI] [PubMed] [Google Scholar]
- 11. Wisniewski H.-G., Vilcek J. (2004) Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 15, 129–146 [DOI] [PubMed] [Google Scholar]
- 12. Lauer M. E., Cheng G., Swaidani S., Aronica M. A., Weigel P. H., Hascall V. C. (2013) Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J. Biol. Chem. 288, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhuo L., Kanamori A., Kannagi R., Itano N., Wu J., Hamaguchi M., Ishiguro N., Kimata K. (2006) SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 281, 20303–20314 [DOI] [PubMed] [Google Scholar]
- 14. Swaidani S., Cheng G., Lauer M. E., Sharma M., Mikecz K., Hascall V. C., Aronica M. A. (2013) TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J. Biol. Chem. 288, 412–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang J., Jiang D., Jung Y., Xie T., Ingram J., Church T., Degan S., Leonard M., Kraft M., Noble P. W. (2011) Role of hyaluronan and hyaluronan-binding proteins in human asthma. J. Allergy Clin. Immunol. 128, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Thoracic Society (2000) Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am. J. Respir. Crit. Care. Med. 162, 2341–2351 [DOI] [PubMed] [Google Scholar]
- 17. Comhair S. A., Ricci K. S., Arroliga M., Lara A. R., Dweik R. A., Song W., Hazen S. L., Bleecker E. R., Busse W. W., Chung K. F., Gaston B., Hastie A., Hew M., Jarjour N., Moore W., Peters S., Teague W. G., Wenzel S. E., Erzurum S. C. (2005) Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am. J. Respir. Crit. Care. Med. 172, 306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lara A., Khatri S. B., Wang Z., Comhair S. A., Xu W., Dweik R. A., Bodine M., Levison B. S., Hammel J., Bleecker E., Busse W., Calhoun W. J., Castro M., Chung K. F., Curran-Everett D., Gaston B., Israel E., Jarjour N., Moore W., Peters S. P., Teague W. G., Wenzel S., Hazen S. L., Erzurum S. C., and National Heart, Lung, and Blood Institute's Severe Asthma Research Program (2008) Alterations of the arginine metabolome in asthma. Am. J. Respir. Crit. Care. Med. 178, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khatri S. B., Ozkan M., McCarthy K., Laskowski D., Hammel J., Dweik R. A., Erzurum S. C. (2001) Alterations in exhaled gas profile during allergen-induced asthmatic response. Am. J. Respir. Crit. Care. Med. 164, 1844–1848 [DOI] [PubMed] [Google Scholar]
- 20. Khatri S. B., Hammel J., Kavuru M. S., Erzurum S. C., Dweik R. A. (2003) J. Appl. Physiol. (1985) 95, 436–440; discussion 435 [DOI] [PubMed] [Google Scholar]
- 21. Comhair S. A., Bhathena P. R., Dweik R. A., Kavuru M., Erzurum S. C. (2000) Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 355, 624. [DOI] [PubMed] [Google Scholar]
- 22. Dweik R. A., Comhair S. A., Gaston B., Thunnissen F. B., Farver C., Thomassen M. J., Kavuru M., Hammel J., Abu-Soud H. M., Erzurum S. C. (2001) NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl. Acad. Sci. U.S.A. 98, 2622–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haserodt S., Aytekin M., Dweik R. A. (2011) A comparison of the sensitivity, specificity, and molecular weight accuracy of three different commercially available hyaluronan ELISA-like assays. Glycobiology 21, 175–183 [DOI] [PubMed] [Google Scholar]
- 24. Wisniewski H.-G., Colón E., Liublinska V., Karia R. J., Stabler T. V., Attur M., Abramson S. B., Band P. A., Kraus V. B. (2014) TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthritis Cartilage 22, 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lauer M. E., Loftis J., de la Motte C., Hascall V. C. (2015) Analysis of the heavy-chain modification and TSG-6 activity in pathological hyaluronan matrices. Methods Mol. Biol. 1229, 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vignola A. M., Chanez P., Campbell A. M., Souques F., Lebel B., Enander I., Bousquet J. (1998) Airway inflammation in mild intermittent and in persistent asthma. Am. J. Respir. Crit. Care. Med. 157, 403–409 [DOI] [PubMed] [Google Scholar]
- 27. Bousquet J., Chanez P., Lacoste J. Y., Enander I., Venge P., Peterson C., Ahlstedt S., Michel F. B., Godard P. (1991) Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J. Allergy Clin. Immunol. 88, 649–660 [DOI] [PubMed] [Google Scholar]
- 28. Ayars A. G., Altman L. C., Potter-Perigo S., Radford K., Wight T. N., Nair P. (2013) Sputum hyaluronan and versican in severe eosinophilic asthma. Int. Arch. Allergy Immunol. 161, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Westergren-Thorsson G., Chakir J., Lafrenière-Allard M.-J., Boulet L.-P., Tremblay G. M. (2002) Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int. J. Biochem. Cell Biol. 34, 1256–1267 [DOI] [PubMed] [Google Scholar]
- 30. Lauer M. E., Glant T. T., Mikecz K., DeAngelis P. L., Haller F. M., Husni M. E., Hascall V. C., Calabro A. (2013) Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J. Biol. Chem. 288, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de la Motte C. A., Hascall V. C., Drazba J., Bandyopadhyay S. K., Strong S. A. (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-α-trypsin inhibitor is crucial to structure and function. Am J Pathol 163, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang A., de la Motte C., Lauer M., Hascall V. (2011) Hyaluronan matrices in pathobiological processes. FEBS J. 278, 1412–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stern R., Asari A. A., Sugahara K. N. (2006) Hyaluronan fragments: an information-rich system. Eur. J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 34. He H., Zhang S., Tighe S., Son J., Tseng S. C. (2013) Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J. Biol. Chem. 288, 25792–25803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bárdos T., Kamath R. V., Mikecz K., Glant T. T. (2001) Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-α-stimulated gene-6) in murine models of experimental arthritis. Am. J. Pathol. 159, 1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wisniewski H. G., Vilcek J. (1997) TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 8, 143–156 [DOI] [PubMed] [Google Scholar]
- 37. Forteza R. M., Casalino-Matsuda S. M., Falcon N. S., Valencia Gattas M., Monzon M. E. (2012) Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. J. Biol. Chem. 287, 42288–42298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauer M. E., Erzurum S. C., Mukhopadhyay D., Vasanji A., Drazba J., Wang A., Fulop C., Hascall V. C. (2008) Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. J. Biol. Chem. 283, 26283–26296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monzón M. E., Manzanares D., Schmid N., Casalino-Matsuda S. M., Forteza R. M. (2008) Hyaluronidase expression and activity is regulated by proinflammatory cytokines in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 39, 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghosh S., Erzurum S. C. (2012) Modulation of asthma pathogenesis by nitric oxide pathways and therapeutic opportunities. Drug Discov. Today Dis. Mech. 9, e89–e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fraser J. R. E., Laurent T. C. (1989) Turnover and metabolism of hyaluronan. Ciba Found. Symp. 143, 41–59 [PubMed] [Google Scholar]