Background: NF45-NF90 and NF45-NF110 possess double-stranded RNA binding domains.
Results: NF45-NF90 and NF45-NF110 occupy the c-fos gene dynamically and enhance its transcriptional induction by extracellular stimuli.
Conclusion: NF45-NF90 and NF45-NF110 function as coactivators and are involved in various steps of gene expression.
Significance: Analysis of RNA binding coactivators is important for understanding the coordination among different steps of gene expression.
Keywords: c-Fos, gene regulation, RNA binding protein, transcription, transcription coactivator
Abstract
The c-fos gene is rapidly induced to high levels by various extracellular stimuli. We used a defined in vitro transcription system that utilizes the c-fos promoter to purify a coactivator activity in an unbiased manner. We report here that NF45-NF90 and NF45-NF110, which possess archetypical double-stranded RNA binding motifs, have a direct function as transcriptional coactivators. The transcriptional activities of the nuclear factor (NF) complexes (NF45-NF90 and NF45-NF110) are mediated by both the upstream enhancer and core promoter regions of the c-fos gene and do not require their double-stranded RNA binding activities. The NF complexes cooperate with general coactivators, PC4 and Mediator, to elicit a high level of transcription and display multiple interactions with activators and the components of the general transcriptional machinery. Knockdown of the endogenous NF90/NF110 in mouse cells shows an important role for the NF complexes in inducing c-fos transcription. Chromatin immunoprecipitation assays demonstrate that the NF complexes occupy the c-fos enhancer/promoter region before and after serum induction and that their occupancies within the coding region of the c-fos gene increase in parallel to that of RNAPII upon serum induction. In light of their dynamic occupancy on the c-fos gene as well as direct functions in both transcription and posttranscriptional processes, the NF complexes appear to serve as multifunctional coactivators that coordinate different steps of gene expression to facilitate rapid response of inducible genes.
Introduction
The c-fos gene is an immediate early gene that is induced rapidly by diverse extracellular signals including growth factors, cytokines, and cellular stress (1). These signals are transmitted via cascades of kinases to transcriptional activators such as SRF,3 Elk-1, CREB, and ATF1, bound on the serum response element (SRE) and the cAMP response elements (CREs) of the c-fos enhancer/promoter (2, 3). The activators promote formation of the preinitiation complex, which consists of general transcription factors (TFIIA, TFIIB, TFIID, TFIIE, TFIIF, and TFIIH) and RNAPII, and also facilitate the subsequent steps of transcription (4). This activation process is believed to require physical and functional interactions among activators, the basal transcriptional machinery, and a third class of factors termed coactivators or coregulators (5).
Several factors have been proposed to serve as coactivators for the activators bound on the c-fos gene. For instance, p300/CBP functions as a bridging factor, a scaffold, and a histone acetyltransferase for SRF, Elk-1, and CREB, integrating multiple signals to regulate their target genes (6). The Med23 subunit of Mediator, originally identified as an E1A-interacting protein, interacts with Elk-1 and is required for activating transcription of SRE-containing genes such as egr-1 and c-fos (7, 8). A family of potent coactivators, transducers of regulated CREB activity (TORCs), bind the DNA binding domain of CREB and facilitate the interaction between CREB and TAF4 (TBP-associated factor 4), enhancing transcription of CRE-containing genes regardless of the phosphorylation status of CREB (9). In addition to these coactivators, we previously reported a coactivator-like activity termed transcriptional regulator of c-fos (TREF), which stimulates transcription from the c-fos promoter in vitro. One component of the TREF activity was identified as heterogeneous nuclear ribonucleoprotein R (10), an RNA binding protein that can potentially interact with various RNAs, raising the possibility that heterogeneous nuclear ribonucleoprotein R serves as a coactivator for the c-fos gene through a novel mechanism that differs from those of p300/CBP, TORCs (transducers of regulated CREB activity), and Mediator.
To further clarify the molecular identity of the TREF activities, we purified another component of TREF and identified it as the complex of NF45 and NF90. NF90 and its splicing variant NF110 contain double-stranded RNA binding motifs (dsRBMs), which have been demonstrated experimentally to bind dsRNAs (11–13). Consistently, NF90 binds the adenylate uridylate-rich elements present in a number of mRNAs to regulate the stability, nuclear export, and cellular distribution (14–22) of these mRNAs; moreover, NF90 is also known to modulate the rate of translation (17, 20, 23). NF90 and NF110 bind to genomic RNAs of various viruses and are not only involved in cellular defense against viral infection but also are utilized as host factors for viral replication (24, 25). In addition to the functions involving their dsRNA binding activities, NF90 and NF110 have been implicated in regulating transcription. Indeed, several studies suggested that the NF45-NF90 complex binds specific DNA sequences (26–29) and activates transcription in cell-based assays (30–35). Given the role for the NF complexes in mRNA stabilization, however, it has been unresolved if the NF complexes have a direct transcriptional function to increase mRNA levels.
Using a highly purified in vitro transcription system, we show that the NF complexes have a direct transcriptional function as a coactivator. This coactivator activity does not require dsRNA binding activities, which are essential for the mRNA stabilizing activity of the NF complexes. Knockdown of the endogenous NF90/NF110 in mouse cells shows that the NF complexes play an important role in rapid induction of c-fos transcription. The NF complexes are present on the c-fos enhancer/promoter region before serum induction, and their occupancies within the coding region increase in parallel to that of RNAPII upon serum induction. Consistent with their occupancy on the c-fos enhancer/promoter and their role as a coactivator, the NF complexes interact with the activators and the general transcriptional machinery. Given their dynamic occupancy on the c-fos gene and well known functions in posttranscriptional processes, the NF complexes may coordinate multiple steps in gene expression by dynamically associating with diverse machineries that regulate gene expression.
Experimental Procedures
Plasmids
Based on the published DNA sequences (33), the coding regions of human NF45, NF90, and NF110 were amplified from HeLa cell mRNA using a PCR-based method (10). The cDNA clones were tagged with the triple (3×) FLAG at their C termini by PCR and then subcloned into pVL1392 (BD Biosciences) for baculovirus expression or pcDNA3 (Invitrogen) for transient expression in mammalian cells. The cDNAs encoding NF45, NF90, and NF110 tagged with GST at their N termini were created by a PCR-based method and subcloned into pVL1392. The baculovirus vector for expressing firefly luciferase was created by using the cDNA amplified from pGL3-basic vector (Promega). PCR-based mutagenesis was used to create the cDNAs encoding NF90(FA) and NF110(FA) by mutating Phe-432 and Phe-559 into alanine, and those encoding NF90(AP) and NF110(AP) by mutating Ala-458 and Ala-588 into proline. The cDNAs of general transcription factors were tagged N-terminally with the FLAG epitope using corresponding cDNAs (36), and the derived cDNAs were subcloned into pVL1392. RNGTT (RNA guanylyl transferase and 5′-phosphatase), Oct-1/POU2F1, and USF (upstream transcription factor) were similarly tagged with the FLAG epitope using cDNAs amplified by PCR using HeLa cell mRNA and subcloned into pVL1392. All the cDNA clones were sequenced entirely to exclude PCR-derived errors. The vectors for expressing FLAG-tagged SRF, Elk-1, CREB, and ATF1 were described previously (10).
Protein Purification
Baculovirus transfer vectors were cotransfected into Sf9 cells with linearized baculoviral DNA (BD Biosciences) to produce the recombinant viruses, which were used for infecting High Five cells. The cell extracts were prepared essentially as described (10). For expressing the complexes of NF45-NF90 and NF45-NF110, High Five cells were coinfected with two different baculoviruses expressing each subunit. All the purification procedures used BC buffer, which consists of 20 mm Hepes-KOH, pH 7.9, 1 mm EDTA, and 10% glycerol, with the number in the parentheses indicating the concentration (mm) of KCl. The extract was loaded onto a HiTrap Q column equilibrated with BC(100) containing 0.5 mm PMSF and 1 mm DTT, and the bound proteins were eluted in a linear gradient from 0.1 m KCl to 1.0 m KCl. The NF45, NF90, NF110, and their complexes were eluted at 300–500 mm KCl. The fractions containing the NF proteins were then subjected to a Sephacryl S300 gel filtration column (GE Healthcare) in BC(500) containing 0.5 mm PMSF. Because all the NF proteins contained a 3×FLAG tag, appropriate fractions were bound to anti-FLAG M2-agarose (Sigma) in BC(300) containing 1 mm PMSF and 0.01% Triton X-100, and the bound proteins were eluted in the same buffer containing 0.1 mg/ml 3×FLAG peptide. Extracts for general transcription factors were prepared similarly. For GST pulldown assays, the extract was purified partially on a HiTrap Q column to remove nucleic acids. Extracts for GST fusion proteins were prepared similarly and used directly for binding the fusion proteins to glutathione-Sepharose 4B (GE Healthcare). Purification of the transcription factors used for in vitro transcription was performed as described (10).
Antibodies
The cDNAs encoding the C-terminal regions of NF45 (residues 162–390), NF90 (residues 353–706), and NF110 (residues 725–898) were amplified and subcloned into pET15b, and the resultant plasmids were transformed into BL21(DE3)pLysS. The recombinant proteins, induced by 1 mm isopropyl 1-thio-β-d-galactopyranoside, were separated by preparative SDS-PAGE, and after excision of the appropriate portion of the gel the proteins were eluted using the Model 422 electro-eluter (Bio-Rad). The purified proteins were then used to immunize rabbits to obtain anti-sera. The crude antisera were purified through columns in which the C-terminal regions of NF45, NF90, and NF110 were cross-linked to Sepharose 4B by dimethyl pimelimidate (Pierce). The bound antibodies were eluted in 0.2 m glycine-HCl, pH 2.5, and dialyzed against phosphate-buffered saline. The anti-RPB2 antibody was prepared in essentially the same manner using the residues 1–306 of RPB2 as purified antigen.
In Vitro Transcription
In vitro transcription assays were performed essentially as described previously using the templates containing the c-fos promoter, pfMC2AT, and five GAL4-binding sites, pG5HMC2AT (10, 37). To allow the inclusion of additional factors in the reactions, however, the volume of transcription assays was increased to 50 μl instead of 25 μl.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assayswere performed as previously described (38) with some modifications. To avoid inactivating anti-NF antibodies, 1% SDS in the lysis buffer and the ChIP dilution buffer and 0.1% SDS in low salt buffer and high salt buffer were substituted by 1% and 0.1% Nonidet P-40, respectively; moreover, 1% deoxycholate in LiCl Buffer was omitted. C3H10T1/2 cells, grown in a 100-mm culture dish, were starved in DMEM containing 0.5% fetal bovine serum (FBS) for 24 h and then stimulated by the addition of 20% FBS for 30 or 60 min. Then, the cells were fixed, collected after wash, and suspended in 150 μl of the lysis buffer containing Protease Inhibitor Mixture (Sigma). The soluble extract, prepared by sonication, was diluted and used for immunoprecipitation, in which antigen-purified anti-NF45, NF90/NF110, NF110, and RPB2 antibodies as well as non-immune rabbit IgG (Sigma) were mixed with the diluted extract. After the addition of recombinant Protein A-Sepharose CL-4B (GE Healthcare), the mixture was rotated for 1 h. The mixture was then washed successively in low salt buffer, high salt buffer, LiCl buffer, and Tris-EDTA buffer, and DNA was isolated for quantitative PCR analyses. The primers used for quantitative PCR were as follows: 5′-GACTGAAACGCTATTTCACTGTAC-3′ and 5′-TCCCACCACTACAAAACCTGAGAG-3′ for the −3,400 region, 5′-CCAACCTCAGTCCTAAAGTTTCTC-3′ and 5′-GGTCATTGTCCAGCAATCTGGAAC-3′ for the −600 region,5′-GCGAGCTGTTCCCGTCAATCCCTC-3′ and 5′-AGACCTTCCGCGTGTAGGATTTCG-3′ for the −300 region, 5′-TTGAAAGCCTGGGGCGTAGAGTTG-3′ and 5′-GGCTCTATCCAGTCTTCTCAGTTG-3′ for the transcription start site, 5′-GGTTAGAAAAACTGCTTCACCGAG-3′ and 5′-TTTCTCTTCCTCTTCAGGAGATAG-3′ for the +1,600 region, 5′-ATTAACCTGGTGCTGGATTGTATC-3′ and 5′-ATTGACGCTGAAGGACTACAGTAC-3′ for the +3,000 region, 5′-TCCTAAGCCAGTGAGTGGCACAGC-3′ and 5′-ACACAACTATGTCAGAAGCAAATG-3′ for the transcription start site of the β-globin gene, and 5′-ACCCTTGGACCCAGCGGTACTTTG-3′ and 5′-AAGGTGCCCTTGAGGCTGTCCAAG-3′ for the +400 region of the β-globin gene. For PCR reactions, Power SYBR Green PCR master mix and ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) were utilized.
GST Pulldown Assays
GST fusion proteins were expressed by infecting insect cells with the appropriate baculoviruses. When expressing GST-fused NF45-NF90 or NF45-NF110, the cells were co-infected with the baculovirus expressing GST-NF45 and that expressing either NF90 or NF110. GST-VP16 was expressed in Escherichia coli as described (37). GST pulldown assays for purified proteins, the TREF fraction, or HeLa nuclear extract were performed essentially as described (38) except that the bound proteins were eluted from the Sepharose beads prebound with GST activators using an equal volume of BC(1000) containing 0.5 mm PMSF and 1 mm DTT.
RNA Interference (RNAi) Assays
∼5 × 105 C3H10T1/2 cells in a 35-mm culture dish were transfected with Stealth RNAiTM siRNA for NF90/NF110 (Invitrogen, MSS205464) or a negative control duplex (Invitrogen, medium GC duplex) using LipofectamineTM 2000 (Invitrogen), and then the cells were cultured in fresh DMEM containing 10% FBS for 48 h to knock down NF90/NF110 expression before cell lysates were prepared. To induce c-fos transcription, the siRNA-transfected cells were starved in DMEM containing 0.5% FBS for 24 h before the addition of the medium containing 20% FBS, 25 ng/ml anisomycin, or 5 ng/ml EGF. The cells were then collected at the indicated time points to prepare total cellular RNAs and cell lysates. The efficiency of the NF90/NF110 knockdown was estimated by immunoblots of the cell lysates with antibodies against NF90/NF110. The isolated total cellular RNAs were used for measuring the amount of the c-fos mRNA by quantitative RT-PCR. The primers for amplifying the c-fos mRNA were 5′-GAGGACCTTACCTGTTCGTGAAA-3′ and 5′-CCAGATGTGGATGCTTGCAA-3′. The primers for the α-tubulin RNA were 5′-GGTTCCCAAAGATGTCAATGCT-3′ and 5′-CAAACTGGATGGTACGCTTGGT-3′. The amounts of the α-tubulin mRNA, which were essentially invariable under the tested conditions, were used for normalization.
Cell Transfection and Reporter Assays
3.3 × 104 HEK293 cells in a 48-well plate were transfected with 356 ng of plasmid DNA using LipofectamineTM 2000 (Invitrogen), and the cells were washed and then lysed. The lysates were used for assaying the luciferase and β-galactosidase activities according to the manufacturer's instructions (Promega). Each experiment was performed at least three times, and the values of the luciferase activities were normalized with those of β-galactosidase activities.
Results
The NF45-NF90 Complex Is a Component of the TREF Activity
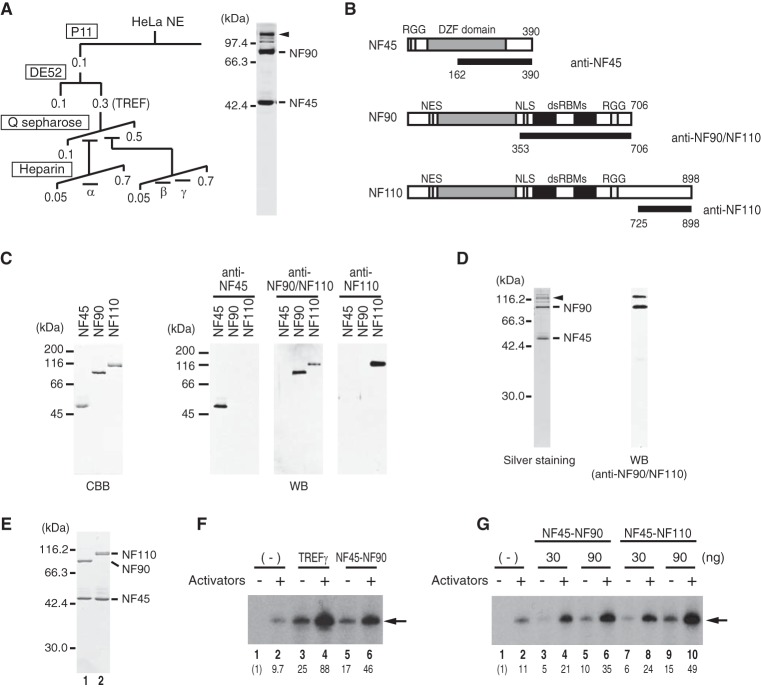
To identify the molecular component(s) of the TREF activity, we purified the TREF fraction derived from HeLa nuclear extracts (Fig. 1A) by monitoring its coactivator activity using the c-fos template, pfMC2AT (10). The purification through four different columns revealed that TREF could be separated into three distinct activities termed TREFα, TREFβ, and TREFγ (Fig. 1A). TREFα was identified as heterogeneous nuclear ribonucleoprotein R, an RNA-binding protein that possesses three RNA recognition motifs and an arginine-glycine-glycine-rich box (10). In this study we purified TREFγ to near homogeneity, and its peak fractions revealed two major polypeptides that were identified as NF45 and NF90 by mass spectrometric analyses (Fig. 1, A and B). To obtain direct evidence of the transcriptional activity of NF45 and NF90, FLAG-tagged NF45 and NF90 were co-expressed in insect cells and purified to near homogeneity (Fig. 1E). Because NF45 and NF90 interact with each other to form a stable complex, we will hereafter refer to their complex as NF45-NF90. Recombinant NF45-NF90 stimulated transcription from the c-fos promoter in a dose-dependent manner and to a similar but slightly lesser extent as compared with native TREFγ (Fig. 1F), eliciting a ∼5-fold stimulation over the activator-dependent c-fos transcription (lanes 2 and 6).
FIGURE 1.
The NF complexes possess a direct transcriptional activity as coactivators. A, purification scheme of the TREF activities from HeLa cell nuclear extracts (NE). The numbers indicate the molar concentration of KCl in the elution buffer. In a silver-stained SDS-polyacrylamide gel of the purified TREFγ, the positions of NF45 and NF90 are indicated on the right, and the arrowhead indicates the position of NF110. B, structures of NF45, NF90, and NF110. The number of amino acid residues is shown at the C terminus of each NF protein. RGG, DZF, NES, NLS, and dsRBM indicate arginine-glycine-glycine rich, dimerization zinc finger, nuclear export signal, nuclear localization signal, and double-stranded RNA-binding motif, respectively. The regions of the NF proteins used for immunizing rabbits are indicated by a black bar under the schematic structures of NF45, NF90, and NF110. Because 353–706 of NF90 is identical to 353–706 of NF110, the antibody raised against this region is designated as anti-NF90/NF110. C, NF45, NF90, and NF110 were separated by SDS-PAGE and stained with Coomassie Brilliant Blue (CBB). The antibodies were tested for their reactivity with NF45, NF90, and NF110. Anti-NF45 and anti-NF110 detected NF45 and NF110, respectively (WB). As expected, anti-NF90/NF110 detected both NF90 and NF110. D, the immunoblot with anti-NF110 (WB) revealed that the TREFγ fraction contained a small amount of NF110 as indicated by an arrowhead (left panel, silver staining). E, SDS-PAGE analysis of purified recombinant NF45-NF90 (lane 1) and NF45-NF110 (lane 2). F, comparison of the transcriptional activities of TREFγ and NF45-NF90 using in vitro transcription of pfMC2AT. Activators indicates 20 ng of SRF, 10 ng of Elk-1, 20 ng of CREB, 20 ng of ATF1, and 200 ng of PC4 (lanes 2, 4, and 6). Semi-quantitative immunoblots with anti-NF45 antibody were used to estimate the amounts of the NF complexes in the TREFγ and recombinant NF45-NF90, and the reactions contained 6.7 μl of TREFγ (lanes 3 and 4) and 90 ng of NF45-NF90 (lanes 5 and 6), respectively. The arrow indicates the position of the transcript, and the relative levels of the transcripts are indicated below each lane. G, in vitro transcription reactions were performed as in F in the presence of the indicated amounts of NF45-NF90 and NF45-NF110. The relative transcription levels are indicated below each lane.
The gene for NF90 also encodes an alternatively spliced mRNA, which is translated into NF110 that diverges from NF90 at the C-terminal region (Fig. 1B) (33, 39). Because silver-stained SDS-PAGE gel of the TREFγ fraction revealed a substoichiometric amount of the ∼110-kDa protein (Fig. 1A, arrowhead) that reacted with an anti-NF90/110 antibody (Fig. 1, C and D), we tested if NF45-NF110 possesses the TREFγ activity as well. Recombinant NF45-NF110, prepared similarly by the baculovirus system (Fig. 1E), stimulated transcription from the c-fos promoter as in the case of NF45-NF90 (Fig. 1G). Dose-response experiments (Fig. 1G) showed that NF45-NF110 enhanced transcription slightly better than NF45-NF90 in the presence (lanes 2, 4, 6, 8, and 10) or absence (lanes 1, 3, 5, 7, and 9) of the activators. These results show that both NF45-NF90 and NF45-NF110 function as a coactivator for transcription from the c-fos promoter in vitro.
The Transcriptional Activities of the NF Complexes Vary on Different Promoters
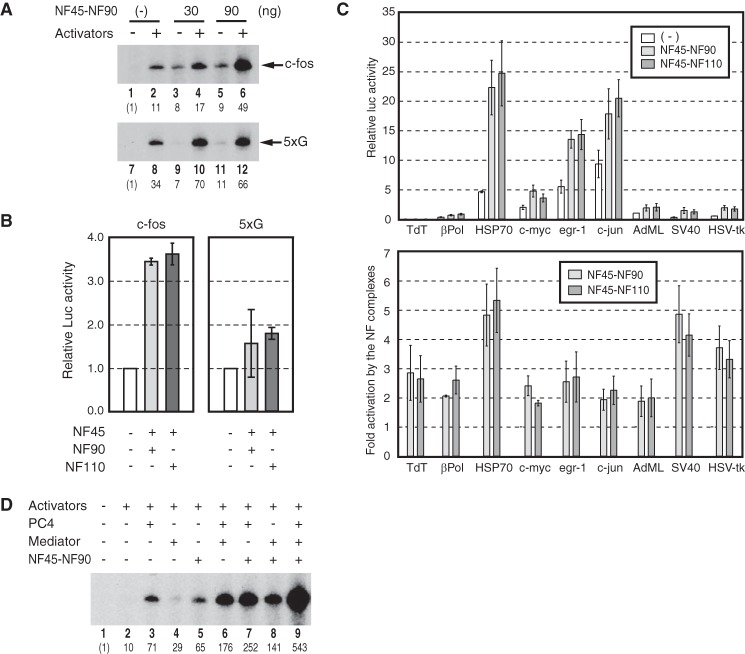
Because TREF stimulates transcription from the c-fos promoter but has little stimulatory activity toward GAL4-VP16-dependent transcription (10), we tested if the NF complexes display a selective activity toward the c-fos promoter as well. As shown in Fig. 2A, in vitro transcription assays showed that 30 and 90 ng of NF45-NF90 stimulated activator-dependent transcription from the c-fos promoter by 1.5- and 4.5-fold over that in the absence of NF45-NF90 (compare lane 2 versus lanes 4 and 6). In contrast, NF45-NF90 stimulated GAL4-VP16-dependent transcription by 2.1- and 1.9-fold under the same condition (Fig. 2A, compare lane 8 versus lanes 10 and 12). Thus, although TREF does not stimulate transcription from the 5xG promoter at all (10), NF45-NF90 does do so, albeit to a lesser extent than the c-fos promoter, indicating that some components in TREF other than NF45-NF90 may repress transcription from the 5xG promoter. In cell-based assays using the c-fos promoter fused to the luciferase gene, NF45-NF90 and NF45-NF110 stimulated c-fos transcription by ∼3.5-fold (Fig. 2B). Using the same assays, we found that other promoters were also stimulated by both NF45-NF90 and NF45-NF110 to different extents (2–5-fold) (Fig. 2C). In contrast, the 5xG promoter fused to the luciferase gene was stimulated by <2-fold (Fig. 2B). These in vitro and in vivo studies indicate that the coactivator activities of NF45-NF90 and NF45-NF110 are less effective toward GAL4-VP16-dependent transcription than c-fos transcription and that their transcriptional effects vary among different promoters.
FIGURE 2.
The activity of the NF complexes on different promoters and the functional cooperation among the NF complexes, PC4, and Mediator. A, transcription reactions contained SRF, Elk-1, CREB, ATF1, and PC4 (lanes 2, 4, and 6) for the c-fos template pfMC2AT (c-fos) as in Fig. 1D and 25 ng of GAL4-VP16 and 200 ng of PC4 (lanes 8, 10, and 12) for pG5HMC2AT, which contains five GAL4-binding sites (5xG). The reactions included either 30 ng (lanes 3 and 4) or 90 ng (lanes 5 and 6) of NF45-NF90. The positions of the transcripts are indicated on the right, and the relative levels of the transcripts are indicated below each lane. B, HEK293 cells were transfected with the indicated combinations of the plasmids expressing either NF45, NF90, and NF110 together with the luciferase gene fused to the c-fos promoter (left panel). The same sets of experiments were performed using the luciferase gene fused to the promoter containing five GAL4-binding sites and the plasmid expressing GAL4-VP16 (right panel). The relative levels of the luciferase activities are shown in each panel. The bars indicate S.E. of three independent experiments. C, HEK293 cells were transfected with the luciferase reporter gene fused to the indicated promoters. The reporter genes were cotransfected with the empty expression vector (white bars), expression vectors for NF45 and NF90 (gray bars), or those for NF45 and NF110 (dark gray bars). The upper panel indicates the relative levels of the luciferase activities, and the lower panel indicates the -fold activation by NF45-NF90 (gray bars) and NF45-NF110 (dark gray bars). The bars indicate S.E. of three independent experiments. D, in vitro transcription assays contained the indicated combinations of 200 ng of PC4 (lanes 3, 6, 7, and 9), ∼30 ng of human Mediator (lanes 4, 6, 8, and 9), and 100 ng of NF45-NF90 (lanes 5, 7, 8, and 9). The relative levels of transcript are shown below each lane.
The NF Complexes Cooperate with PC4 and Mediator
We also tested if NF45-NF90 cooperates with PC4 and Mediator to stimulate c-fos transcription in vitro, as observed for TREF (10). When NF45-NF90, PC4, and Mediator were tested individually, each coactivator stimulated activator-dependent transcription from the c-fos promoter by 3–7-fold, with the strongest stimulation displayed by PC4 (Fig. 2D, compare lane 2 versus lanes 3–5). NF45-NF90 showed a somewhat weaker activity than PC4, yet it had a stronger activity than Mediator (Fig. 2D, lanes 3–5). Although their individual transcriptional stimulation was moderate, the combined effects by two of the coactivators were more pronounced (Fig. 2D, lanes 6–8), indicating that they cooperate with each other to stimulate c-fos transcription. Indeed, when all three coactivators were included simultaneously in the reaction (Fig. 2D, lane 9), the level of c-fos transcription increased by 54-fold over that in the absence of any coactivator (compare lane 2 versus lane 9) and by 543-fold over its basal level in the absence of activators and coactivators (compare lane 1 versus lane 9). These results demonstrate that both NF45-NF90 and NF45-NF110 cooperate with PC4 and Mediator to stimulate c-fos transcription in vitro.
Double-stranded RNA Binding Activity Is Dispensable for the Coactivator Activity of the NF Complexes in Vitro
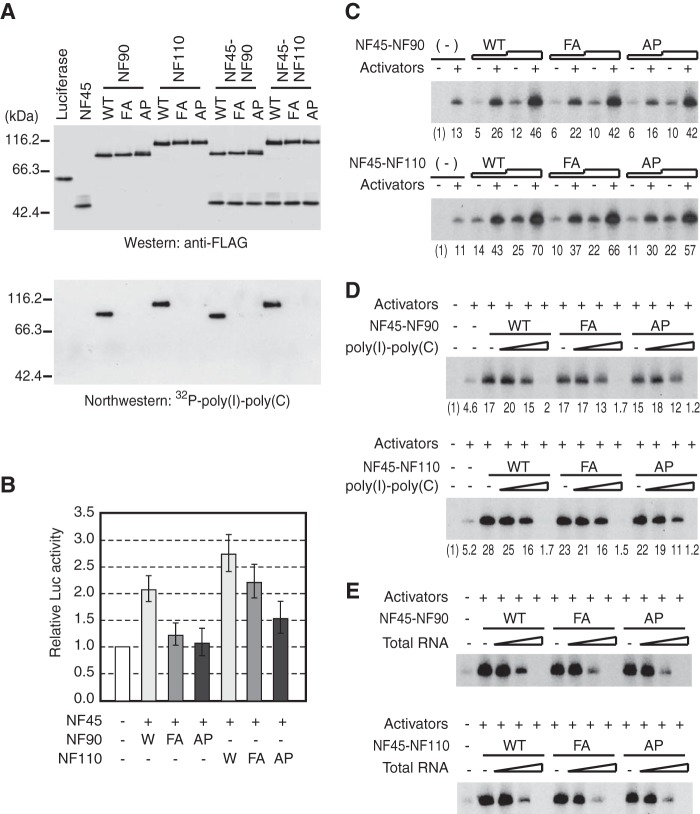
Previous in vivo studies showed that the dsRNA binding activity of NF110 is important for increasing the expression of the reporter gene in cell-based assays (31). In these assays, however, the increased expression could be caused by the mRNA stabilization by NF90 and NF110 (14, 16, 17, 19). Moreover, NF110, which has a weaker dsRNA binding activity than NF90, showed a stronger effect on gene expression than NF90 in similar cell-based assays (31, 33). To clarify the relationship between dsRNA binding and transcriptional activities of the NF complexes, we created NF90(FA), in which two phenylalanine residues, Phe-432 and Phe-559, were mutated into alanine and NF90(AP), in which two alanine residues, Ala-458 and Ala-588, were mutated into proline as well as the corresponding NF110 mutants described previously (31). As expected, NF90(FA), NF90(AP), NF110(FA), and NF110(AP) showed virtually no binding to 32P-labeled poly(I)·poly(C) in Northwestern assays (Fig. 3A). When introduced into C3H10T1/2 cells together with NF45, the dsRBM mutants, especially NF90(AP) and NF110(AP), showed lower levels of the reporter gene expression than their wild-type counterparts (Fig. 3B), in essential agreement with the previous study (31).
FIGURE 3.
The NF complexes do not require their dsRNA binding activities to function as a coactivator. A, partially purified NF proteins as well as control luciferase tagged with a FLAG epitope were separated by SDS-PAGE and detected by immunoblots using anti-FLAG M2 antibody (upper panel). The same sets of proteins were tested for binding to 32P-labeled poly(I)·poly(C) (lower panel). B, HEK293 cells were transfected with the reporter gene plasmid containing the c-fos promoter fused to the luciferase gene together with the indicated combinations of the plasmids expressing NF45, NF90, N90(FA), NF90(AP), NF110, NF110(FA), or NF110(AP). Relative levels of the luciferase activities are shown, and the bars indicate S.E. of three independent experiments. C, in vitro transcription reactions using the c-fos promoter were performed in the presence of NF45-NF90, NF45-NF90(FA), and NF45-NF90(AP) (upper panel) or in the presence of NF45-NF110, NF45-NF110(FA), and NF45-NF110(AP) (lower panel) as indicated above each lane. The NF complexes added to reactions were either 33 ng or 100 ng. SRF, Elk-1, CREB, ATF1, and PC4 were included in the reactions indicated by +. The relative levels of transcripts are indicated below each panel. D, in vitro transcription reactions were performed as in C, and the reactions contained 5, 50, or 500 ng of poly(I)·poly(C). E, in vitro transcription reactions were performed as in C, and the reactions contained 5, 50, or 500 ng of total cellular RNA.
However, when these mutants were tested directly in in vitro transcription assays, in which there is no post-transcriptional effect, NF45-NF90(FA), NF45-NF90(AP), NF45-NF110(FA), and NF45-NF110(AP) stimulated transcription from the c-fos promoter to the levels comparable with those of their wild-type counterparts (Fig. 3C), indicating that the intact dsRBMs are dispensable for the direct coactivator activity of the NF complexes observed in vitro. We also tested the effect of RNA on the coactivator activities of the NF complexes by including poly(I)·poly(C) or total cellular RNA. Increasing the amounts of poly(I)·poly(C) (Fig. 3D) or total cellular RNA (Fig. 3E) did not augment the coactivator activities of the NF complexes but instead gradually repressed the levels of transcription (Fig. 3D). This repressive effect of poly(I)·poly(C) (Fig. 3D) and total cellular RNA (Fig. 3E) is unrelated to the dsRNA binding activity of the NF complexes because the repression was also observed regardless of their dsRNA binding activities. Thus, although there remains a possibility that some specific RNAs such as small NF90-associated RNAs (40) regulate the activities of the NF complexes via their dsRBMs, these results indicate that the NF complexes possess a coactivator activity that is independent of their dsRNA binding activity.
NF90 and NF110 Are Important for Rapid Induction of c-fos Transcription
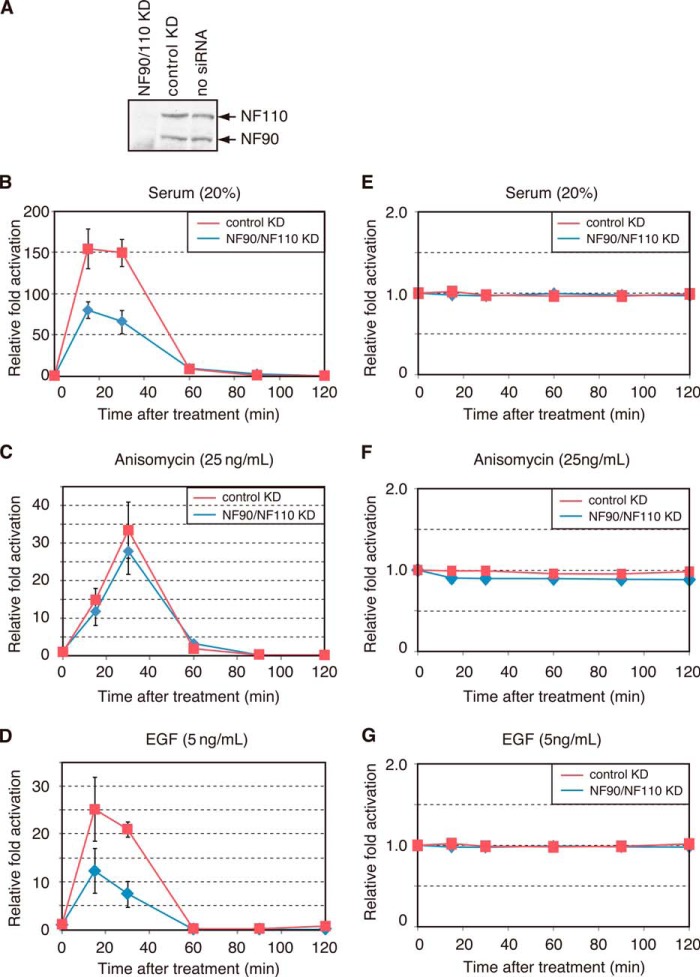
Next, to obtain the in vivo evidence that NF45-NF90 and NF45-NF110 are required for c-fos transcription, we knocked down the endogenous NF90/NF110 and analyzed the level of c-fos transcription. When C3H10T1/2 cells were transfected with siRNA specific for the mouse NF90/NF110 gene, the amounts of NF90 and NF110 decreased to undetectable levels, whereas control siRNA did not have any effect (Fig. 4A). We then analyzed induction of the c-fos mRNA in cells stimulated with 20% fetal bovine serum after 24 h of serum starvation. Fig. 4B shows that the knockdown reduced the level of the c-fos mRNA, especially during 15–30 min after serum stimulation and that the level of the c-fos mRNA returned virtually to that in the control cells after 90 min. Because serum may contain various stimuli that can induce c-fos transcription, we tested two well defined stimuli, anisomycin and EGF, whose effects are mediated by the JNK/p38 and Erk1/2 subfamilies of MAP kinases, respectively. As shown in Fig. 4C, RNAi knockdown of NF90/NF110 had no significant effect on anisomycin-induced c-fos transcription. By contrast, EGF induction of the c-fos gene was markedly reduced upon NF90/NF110 knockdown (Fig. 4D). In all the RNAi knockdown experiments, the levels of the α-tubulin mRNA remained comparable in cells treated with control or NF90/NF110-specific siRNA (Fig. 4, E–G). Thus, NF90 and NF110 play an important role in rapidly inducing c-fos transcription, especially in response to serum or EGF stimulation.
FIGURE 4.
siRNA-mediated knockdown of NF90 and NF110 diminishes rapid induction of the c-fos gene. A, C3H10T1/2 cells were treated with siRNA specific for NF90/NF110 (NF90/NF110 KD) or control siRNA (control KD) for 48 h. The cell lysates were separated by SDS-PAGE and analyzed by immunoblots using anti-NF90/NF110 to determine the amounts of NF90 and NF110 in the cells. B–D, C3H10T1/2 cells were treated with siRNA for 48 h, and then the cells were starved for 24 h before stimulation by 20% serum, 25 ng/ml anisomycin, or 5 ng/ml EGF. The levels of the c-fos mRNA were determined by quantitative RT-PCR before (0 min) and after 20% serum stimulation (B), 25 ng/ml anisomycin (C), or 5 ng/ml EGF (D) (15 min, 30 min, 45 min, 60 min, 90 min, and 120 min). The p values for the differences in the c-fos mRNA levels between NF90/NF110 and control knockdown experiments were: p = 0.0331 (15 min, 20% serum), p = 0.0054 (30 min, 20% serum), p = 0.0026 (30 min, 5 ng/ml EGF). The p values were >0.05 at all the other time points for 20% serum, 25 ng/ml anisomycin, and 5 ng/ml EGF. E–G, NF90/NF110 knockdown and c-fos induction was performed as in B–D. The expression of α-tubulin after treatment by 20% serum (E), 25 ng/ml anisomycin (F), or 5 ng/ml EGF (G) was analyzed by quantitative RT-PCR. The graphs show relative -fold activations compared with 0 min, which were calculated and averaged from five experiments. The error bars indicate the S.E.
The NF Complexes Dynamically Occupy the c-fos Gene
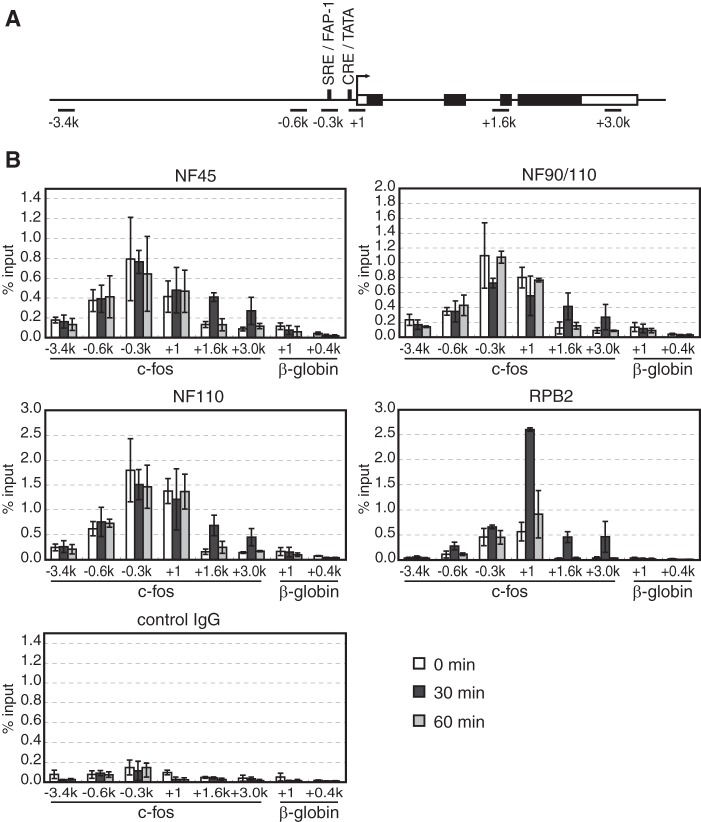
In view of the multiple roles for the NF complexes in transcription (Fig. 4) and posttranscriptional processes (39, 41), we wished to know the temporal occupancy of NF45, NF90, and NF110 on the c-fos gene during its transcriptional induction. C3H10T1/2 cells were starved and then treated with 20% FBS for 0, 30, and 60 min before the cells were fixed with formaldehyde, and the chromatin was immunoprecipitated by anti-NF45, anti-NF90/NF110, or anti-NF110 antibodies (Fig. 1C). The precipitated DNA was then used for quantitative PCR with sets of primers designed to amplify ∼100-bp regions around −3,400, −600, −300, +1, +1,600, and +3,000 of the c-fos gene (Fig. 5A). In addition, sets of primers for the +1 and +400 regions of the β-globin gene, which is not expressed in C3H10T1/2 cells, were used as controls.
FIGURE 5.
Dynamic occupancies of NF45, NF90, and NF110 on the c-fos gene. A, structure of the c-fos gene. The boxes indicate the exons of the c-fos gene; the white boxes indicate its untranslated regions. The positions of SRE/FAP-1, CRE, the TATA box, and the transcription start site are indicated. The black bars indicate the positions of the amplified regions by PCR in the ChIP assays. B, ChIP assays were performed with C3H10T1/2 cells collected before (0 min) and after serum stimulation (30 min and 60 min). Antibodies against NF45, NF90/NF110, NF110, and RPB2 as well as control IgG were used for the ChIP assays as indicated above each panel. The error bars indicate S.E. of three independent experiments.
Under the starved condition (0 min), the NF proteins were present from the upstream regions (−600 and −300) to the transcription start site (+1), whereas much lesser amounts of the NF proteins were observed in the far upstream region (−3,400) and the downstream regions (+1,600 and +3,000) within the c-fos gene (Fig. 5B). RNAPII also showed a similar occupancy to those of the NF proteins. As expected, 30 min after serum addition when the c-fos gene is induced maximally, the RNAPII occupancy increased dramatically at the start site (+1) and in the downstream regions (+1,600 and +3,000) of the c-fos gene. Concomitantly, the occupancies of the NF proteins increased in a similar manner to that of RNAPII in the coding regions (+1,600 and +3,000), raising the possibility of some physical association between RNAPII and the NF proteins. Whereas the occupancies of the NF proteins remained largely unchanged in the upstream regions (−600 and −300) and at the start site (+1), slight reductions in the occupancies of NF90 and NF110 were observed in the −300 and +1 regions, which could possibly reflect transfer of the NF proteins from the promoter to the elongating RNAPII. After 60 min the occupancies of the NF proteins and RNAPII returned rapidly to the pre-induction levels. As the control, no significant occupancies of the NF proteins and RNAPII were observed on the β-globin gene in C3H10T1/2 cells.
These experiments demonstrate that the NF proteins are associated with the regulatory and core promoter regions of the c-fos gene before induction and that the occupancies of the NF proteins undergo dynamic changes during induction of c-fos transcription. These results suggest that the NF proteins, probably as the complexes of NF45-NF90 and NF45-NF110, may travel along the gene with elongating RNAPIIs, further substantiating their dual roles in transcription and posttranscriptional processes.
NF45-NF90 and NF45-NF110 Stimulate Transcription through Both the Upstream Regulatory Elements and the Core Promoter
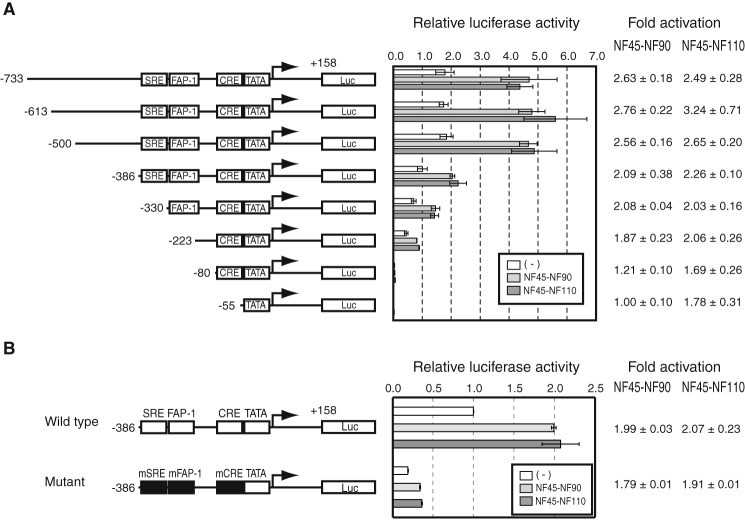
Our in vitro analyses indicated that the NF proteins stimulate both activator-dependent and basal transcription (Figs. 1 and 2). In addition, we observed a marked occupancy of the NF proteins on the c-fos gene from the −600 to +1 region (Fig. 5), which encompasses from the upstream regulatory elements to the core promoter. Thus, to delineate the c-fos promoter region that mediates the transcriptional activity of the NF proteins, we tested a series of deletion mutants of the c-fos regulatory elements (Fig. 6A). Deletion of the upstream region from −733 to −500 did not affect the luciferase activity at all. However, further deletion from −500 to −55, including the known regulatory elements (SRE, FAP-1, and CRE), reduced the level of the luciferase activity dramatically regardless of the presence of the NF complexes, in accord with the critical roles of these elements in c-fos transcription. Importantly, the 2.5–3.0-fold stimulation by NF45-NF90 or NF45-NF110 observed for the c-fos promoter (from −733 to +158) decreased gradually as the region downstream from −500 was removed progressively. These results indicate that the NF complexes act broadly through the regulatory elements between −500 and −55, the region that conforms approximately with the occupancy of the NF proteins on the endogenous c-fos gene (Fig. 5). Moreover, introduction of point mutations into the individual regulatory elements (SRE, FAP-1, and CRE) also decreased the luciferase activity while still allowing an ∼1.8-fold stimulation in the presence of NF45-NF90 and NF45-NF110 (Fig. 6B). Finally, the reporter gene with only the core promoter (−55 to +158) was stimulated ∼1.8-fold by NF45-NF110 but not by NF45-NF90, indicating that NF45-NF110 has a direct stimulatory effect on the core promoter as well. These results indicate that the NF complexes stimulate c-fos transcription through both the upstream regulatory elements and the core promoter, in agreement with the occupancies of the c-fos enhancer/promoter region by the NF proteins in cells.
FIGURE 6.
The NF complexes act through the upstream regulatory elements and the core promoter of the c-fos gene. A, the reporter gene plasmids contained deletion mutants of the c-fos enhancer/promoter region as indicated on the left. Each reporter plasmid was transfected into C3H10T1/2 cells together with the plasmid expressing NF45 and that expressing NF90 or NF110. The middle panel shows the relative levels of the luciferase activities in the presence of NF45-NF90 or NF45-NF110. The columns on the right indicate the values of -fold activation by NF45-NF90 and NF45-NF110. B, the structure of the reporter gene that harbors the wild-type or mutant c-fos gene promoter is indicated on the left. The filled boxes indicate the mutated SRE, FAP-1, and CRE. The wild-type or mutant reporter gene was introduced into HEK293 cells with the expression vectors for NF45 and NF90 or those with NF45 and NF110. The middle panel shows the relative levels of the luciferase activities in the presence of NF45-NF90 or NF45-NF110. The bars indicate S.E. of three independent experiments. The columns on the right indicate the values of -fold activation by NF45-NF90 and NF45-NF110.
Multiple Interactions of the NF Proteins with Activators and the Basal Transcriptional Machinery
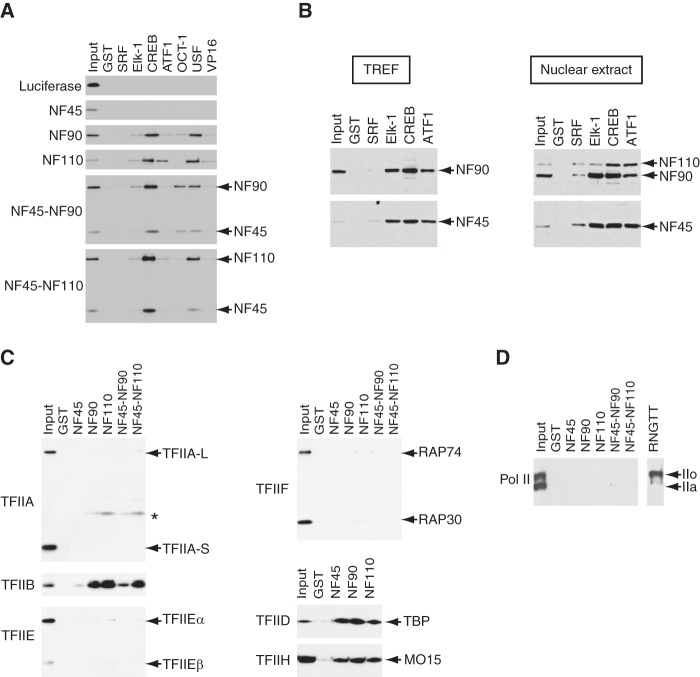
The coactivator function of the NF complexes and their occupancy on the c-fos gene on its enhancer/promoter region suggested that they might interact directly with activators and the basal transcriptional machinery. We, therefore, expressed and purified recombinant FLAG-tagged NF proteins and their complexes (NF45-NF90 and NF45-NF110) as well as the firefly luciferase protein for a control. The tagged proteins were then allowed to interact with various GST-fused activators pre-bound to glutathione-Sepharose beads, and the bound proteins were eluted and analyzed by immunoblotting with anti-FLAG M2 antibody. As shown in Fig. 7A, NF45-NF90 and NF45-NF110 interacted with CREB, Elk-1, ATF1, and USF to varying degrees, and NF45-NF90 interacted with Oct-1/POU2F1. The observed interactions are specific because the luciferase protein did not interact with any activators, and none of the NF proteins or their complexes interacted with GST alone (Fig. 7A). Consistent with its role as a functional regulator of NF90 (32), NF45 appeared to modulate the interactions of NF90 and NF110 with the activators, although NF45 did not interact with any activators by itself. For instance, NF45 enhanced the interaction between NF90 and Oct-1/POU2F1, whereas it diminished the interaction between NF110 and ATF1 (Fig. 7A). Finally, the NF proteins showed very weak interactions with the VP16 activation domain, which agrees with their minimal stimulatory activity toward GAL4-VP16-dependent transcription (Fig. 2, A and B).
FIGURE 7.
The NF proteins interact with activators and the components of the basal transcriptional machinery. A, FLAG-tagged NF proteins or their complexes were tested for interactions with GST-fused activators retained on glutathione-Sepharose 4B. The bound proteins were separated by SDS-PAGE and visualized by immunoblots using anti-FLAG antibody. B, the TREF fraction (2.24 mg) or HeLa nuclear extract (4.67 mg) was used in place of purified NF complexes for interaction assays as performed in A. Anti-NF90/NF110 or anti-NF45 antibody was used to detect the NF proteins in the bound proteins. The amounts of bound proteins were 2.1%, 1.9%, 1.9%, 2.3%, and 2.0% of the TREF fraction for GST, GST-SRF, GST-Elk-1, GST-CREB, and GST-ATF1, respectively. Similarly, the amounts of bound proteins were 1.5%, 1.2%, 1.3%, 1.1%, and 1.0% of HeLa nuclear extract for GST, GST-SRF, GST-Elk-1, GST-CREB, and GST-ATF1, respectively. C, FLAG-tagged general transcription factors were tested for interactions with GST-fused NF proteins. In TFIIH and TFIID, MO15 and TBP were tagged with FLAG. In the complexes of NF45-NF90 and NF45-NF110, NF45 was expressed as a GST fusion protein and heterodimerized in insect cells with NF90 and NF110, respectively. The asterisk indicates a nonspecific band. D, partially purified RNAPII from HeLa cells were tested for interactions with the NF proteins. IIo and IIa indicate the positions of hyperphosphorylated and unphosphorylated forms of RNAPII, respectively. RNGTT indicates RNA guanylyltransferase and 5′-phosphatase.
As these assays were performed with purified proteins, we further examined the specificity by testing if the activators interact with the NF complexes in the extracts. Instead of purified NF complexes, we first incubated the TREF fraction (Fig. 1A) with GST-fused activators bound to glutathione-Sepharose beads. After washing, the bound proteins, which corresponded to 1.9–2.3% of the proteins contained in the TREF fraction (data not shown), were tested for the presence of the NF complexes by immunoblots using anti-NF90/NF110 or anti-NF45 antibodies. As shown in the left panel of Fig. 7B, the bound fractions contained the NF complexes. We then used HeLa nuclear extract to perform the same experiments, and 1.0–1.5% of the proteins were bound to GST-fused activators (data not shown). Similar to the results with the TREF fraction, the NF complexes were detected in the bound fractions (Fig. 7B, right panel). These results show that the NF complexes interact specifically with the activators, albeit to different degrees.
Next, we tested the interactions between the NF proteins and the components of the basal transcriptional machinery. Because we wished to test if the NF proteins interact with multiprotein complexes including TFIID and TFIIH, we prepared NF45, NF90, and NF110 as well as their complexes (NF45-NF90 and NF45-NF110) as GST fusion proteins. In NF45-NF90 and NF45-NF110, only NF45 was expressed as a GST fusion protein, which formed heterodimers with non-tagged NF90 or NF110. As shown in Fig. 7C, the NF proteins showed interactions with TFIIB, TFIID, and TFIIH but not with TFIIA, TFIIE, and TFIIF. Although NF45 did not interact with any activators (Fig. 7A), it interacted with TFIID and TFIIH as efficiently as did its heterodimeric partners, NF90 and NF110 (Fig. 7C). NF45 reduced the interaction of NF90 with TFIIB, again indicating a possible regulatory role for NF45 in the context of the NF complexes. Collectively, the NF proteins have multiple interactions with both activators and the basal transcriptional machinery, in agreement with their direct transcriptional function and their occupancies on both the upstream regulatory elements and the core promoter (Figs. 1, 2, and 5).
Finally, despite the similar dynamic occupancies of RNAPII and the NF proteins on the c-fos gene (Fig. 5), neither individual NF proteins (NF45, NF90, and NF110) nor their complexes (NF45-NF90 and NF45-NF110) showed any interaction with RNAPII under the condition where the capping enzyme, RNGTT, bound specifically to the phosphorylated IIO (Fig. 7D). This result suggests that interactions between the NF complexes and RNAPII, if any, are likely to be indirect.
Discussion
The NF Complexes Are Multifunctional Coactivators
In this study we purified TREFγ and ascribed its activity to the NF complexes, which consist of either NF45 and NF90 or NF45 and NF110 (39). The NF complexes were identified initially as a DNA binding factor that binds the ARRE site (26, 27), the CCAAT-binding site (28), and an MHC-I site (29). However, independent studies identified the NF proteins by means of their RNA binding activity and named them as ILF3 (42), DRBP76 (13), or TCP80 (23). Indeed, NF90 and NF110 possess two dsRBMs, which, as the name implies, is a modular nucleic acid binding motif that binds duplexed RNA but not ssRNA, dsDNA, or single-stranded DNA (11–13). Consistent with the presence of dsRBMs, the NF complexes do not bind dsDNA under the conditions where their binding to dsRNA can be demonstrated unambiguously (33, 43, 44). Given the absence of any consensus DNA sequence among the presumptive NF binding DNA elements described above (26–29), it remained to be defined if the NF complexes regulate transcription by binding directly to specific DNA sequences (45). Further complicating their role in transcription is that the NF complexes stabilize mRNA through binding to specific RNA sequences (14, 19). Thus, the transcriptional stimulation by the NF complexes observed in cell-based assays may be attributed to posttranscriptional effects (39, 41). Thus, despite several reports that the NF complexes increase the level of mRNA in vivo (30–35), it has been unclear whether they have a direct function in transcription.
Combined with in vitro transcription assays, our unbiased identification and purification of the TREFγ activity from cell extract demonstrate beyond the presumed role in transcription by earlier studies that the NF complexes possess a direct function in transcription from the c-fos promoter. Earlier (31) as well as our own studies (Fig. 3B) using cell-based assays show that the mutations of dsRBMs drastically diminish the ability of the NF complexes to stimulate transcription as measured by mRNA levels. However, the NF complexes stimulated transcription in vitro even when its dsRBMs were mutated (Fig. 3C), demonstrating that the NF complexes possess a direct transcriptional function that is separate from the posttranscriptional functions that require intact dsRBMs. Consistent with the direct transcriptional function, the NF proteins interact physically with the components of the basal transcriptional machinery, notably with TFIID and TFIIB that are pivotal in transcriptional regulation. Because the NF complexes exert the transcriptional effect through multiple cis-regulatory elements (Fig. 6), probably by interacting with various activators (Fig. 7A), they are likely to function as a transcriptional coactivator. Given that knockdown of NF90/NF110 attenuates the induction of c-fos transcription upon serum or EGF stimulation (Fig. 4D) together with their previously demonstrated roles in posttranscriptional processes (39, 41), the NF complexes may coordinate transcription and post-transcriptional processes to elicit rapid response of inducible genes.
Coordination between transcription and posttranscriptional processes has been reported for numerous proteins (46, 47). For example, PGC-1 and CoAA, initially identified as transcriptional coactivators, were later shown to function in posttranscriptional processes as well (48, 49). PGC-1 possesses an RNA recognition motif and an RS domain, both of which are RNA binding domains that interact with splicing factors and are essential for coupling transcription and mRNA processing (48, 50). Similarly, two RS domains in CoAA play a role in linking transcription and splicing in a promoter-preferential manner (50). Furthermore, another coactivator, RNA helicase A, possesses dsRBMs and an arginine-glycine-glycine-rich domain (41, 51), which are RNA binding motifs also present in NF90 and NF110. Interestingly, RNA helicase A copurifies with NF45-NF90 through several column chromatographies and interacts directly with NF90 and NF110 (11, 33), suggesting a functional partnership between the NF complexes and RNA helicase A. On the whole, the prevalence of RNA binding motifs in these coactivators suggests that the functional coordination between transcription and posttranscriptional processes may entail direct interactions between these RNA binding coactivators and the RNA transcript emerging from RNAPII.
How the NF complexes participate in multiple aspects of RNA metabolism, however, remains to be elucidated. One possibility is that NF90 and NF110 have separate roles in distinct steps of gene expression; for example, NF110 may function at earlier steps than does NF90. For instance, although NF110 and NF90 are distributed in both nucleus and cytoplasm (14, 27, 28, 33, 52), NF110 tends to be more abundant in nucleus, whereas NF90 is more readily observed in cytoplasm. In addition, NF110 appears to stimulate gene expression slightly better than NF90 in vivo (31, 33) and in vitro (Fig. 1G); by contrast, NF90, but not NF110, is implicated in mRNA export and stabilization (14, 19). An alternative, but not mutually exclusive, possibility is that the NF complexes serve as a scaffold that assembles different proteins to acquire functions besides their intrinsic activities. Consistent with this, the NF proteins have been shown to interact with multiple proteins such as Yin Yang 1 (30, 53), protein kinase R (13, 30, 54, 55), protein-arginine methyltransferase 1 (11, 33, 53, 56), RNA helicase A (11, 33), survival of motor neuron (30), and FUS/TLS RNA-binding protein (30) and have been isolated as components of RNAPII-interacting proteins as well as integral components of H complex (57, 58).
Although we identified the NF complexes from their transcriptional activity on the c-fos promoter, our own data and those from others (26, 32, 33, 35) indicate that the NF complexes stimulate transcription of other genes as well. In NF90−/− knock-out mice, T cells show severely impaired expression of IL-2, which is regulated at multiple steps of gene expression by the NF complexes (59). Moreover, up-regulation of NF90 in cancer cells increases mRNAs of interferon-regulated genes, which are also prototypical inducible genes (34). These observations make it highly likely that a subset of inducible genes require the NF complexes for their prompt expression, which requires coordination among the multiple steps from transcription to translation.
Dynamic Occupancy of the NF Complexes within the c-fos Gene
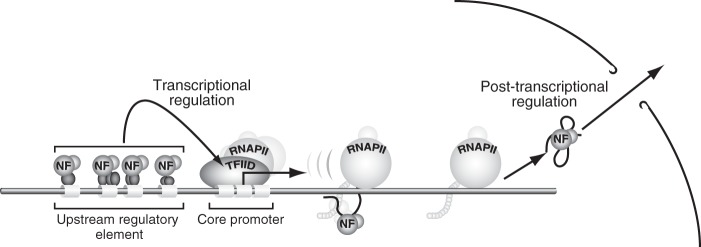
The NF complexes have been shown to possess both nuclear export and nuclear localization signals, which permit shuttling between nucleus and cytoplasm (14, 52, 60, 61). In a significant extension of the earlier studies that showed the occupancy of NF90 on the IL-2 gene (59) and their association with chromatin (33, 62), our ChIP analyses revealed that the NF complexes are present on the intragenic and enhancer/promoter regions of the c-fos gene. Moreover, the occupancy of the NF proteins in the intragenic region parallels both temporally and spatially with that of RNAPII during c-fos induction (Fig. 5). This dynamic occupancy implies that the NF complexes move along the c-fos gene in association with transcribing RNAPII, which is entirely consistent with their dual roles in transcription and post-transcriptional processes (Fig. 8).
FIGURE 8.
Dynamic localization of the NF complexes during transcription and posttranscriptional processes. The NF complexes are present on the upstream regulatory elements as well as on the promoter. Upon the activation of the c-fos gene, they are transferred to RNAPII and become associated indirectly with RNAPII. After reaching the downstream of the gene, they bind to the mRNA and exported into cytoplasm.
The dynamic occupancy along the c-fos gene raises the intriguing possibility that the NF complexes may act on the very same transcript whose transcription initiation they stimulated in the first place. This would explain why cell-based assays showed that the mutations of the dsRBMs drastically reduce the stimulatory effect of the NF complexes on transcription in vivo (Fig. 3B) (31). Because the mutated dsRBMs can compromise the posttranscriptional effects of the NF complexes, their stimulatory effect on transcription initiation might have been largely cancelled out in the cell-based assays, giving a misleading impression that the transcriptional activity requires dsRNA binding.
The NF complexes may be recruited to the promoter region via direct interactions with the activators and/or the preinitiation complex (Fig. 8). NF90 and NF110 showed numerous interactions with the activators and the basal transcriptional machinery (Fig. 7), supporting the ChIP data that showed the occupancy of the NF complexes on the enhancer/promoter region (Fig. 5). Although the ChIP data also suggest that the NF complexes may be transferred from the promoter to the elongating RNAPII, if and how this occurs remains unclear. However, the transfer of the NF complexes may be critical for their functions. Indeed, when NF90 is tethered to the promoter region as Gal4-NF90, Gal4-NF90 appears to act like a dominant negative form and inhibits transcription (32), presumably because Gal4-NF90 cannot be transferred from the promoter region to the elongating RNAPII.
Given the parallel occupancies of the NF proteins and RNAPII, it was somewhat unexpected to find that the NF proteins do not interact directly with purified RNAPII irrespective of the phosphorylation status of its CTD (Fig. 7D). Three lines of evidence, however, strongly indicate an indirect physical association between RNAPII and the NF complexes, probably mediated by their associated proteins and/or RNA transcript. First, a proteomic analysis revealed that the NF complexes are present in H complex together with heterogeneous nuclear ribonucleoproteins and other types of RNA-binding proteins (63). H complex is believed to bind nascent RNA that has just emerged from RNAPII before the assembly of splicing factors (57, 58). Second, the NF complexes are co-precipitated with the phosphorylated (and thus elongating) RNAPII and various RNA-binding proteins by an anti-CTD antibody (64). Third, the NF complexes are immunoprecipitated by an anti-Chd1 antibody, which also precipitates RNAPII and numerous proteins involved in splicing and other RNA processing steps (65). Interestingly, Chd1 binds the H3-K4me3 (66, 67), whose presence on the chromatin (68, 69) overlaps with the occupancy of the NF complexes (Fig. 5).
In conclusion, this study reports that the NF complexes have a direct function in stimulating transcription as a coactivator. Given their roles in multiple steps of gene expression and the dynamic occupancy on the c-fos gene, it will be essential to elucidate the structure and function of the proteins that associate with the NF complexes during distinct steps of gene expression.
Author Contributions
T. N. and A. F. designed and performed all the experiments. M. S. and K. N. provided technical assistance and experimental materials. T. N., A. F. and K. H. analyzed the data, wrote the paper, and prepared the figures. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We are grateful to other members of the laboratory for helpful discussion and Michiyo Takeuchi for technical support.
This work was supported in part by grants-in-aid for Scientific Research (to T. N., A. F., M. S., K. N., and K. H.) and Special Coordination Funds for Promoting Science and Technology (to A. F. and K. N.) of the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors declare that they have no conflicts of interest with the contents of this article.
- SRF
- serum response factor
- TREF
- transcriptional regulator of c-fos
- RNAPII
- RNA polymerase II
- NF
- nuclear factor
- dsRBM
- double-stranded RNA binding motif
- CRE
- cAMP-responsive element
- CREB
- CRE-binding protein
- SRE
- serum response element
- Elk-1
- Ets-related transcription factor
- ATF1
- activating transcription factor 1
- FAP-1
- c-fos AP-1 site
- PC4
- positive cofactor 4
- dsRNA
- double-stranded RNA.
References
- 1.Treisman R. (1995) Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 14, 4905–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price M. A., Hill C., and Treisman R. (1996) Integration of growth factor signals at the c-fos serum response element. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 551–559 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., and Prywes R. (2000) Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene 19, 1379–1385 [DOI] [PubMed] [Google Scholar]
- 4.Roeder R. G. (1996) The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 21, 327–335 [PubMed] [Google Scholar]
- 5.Malik S., and Roeder R. G. (2000) Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem. Sci. 25, 277–283 [DOI] [PubMed] [Google Scholar]
- 6.Chan H. M., and La Thangue N. B. (2001) p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114, 2363–2373 [DOI] [PubMed] [Google Scholar]
- 7.Boyer T. G., Martin M. E., Lees E., Ricciardi R. P., and Berk A. J. (1999) Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature 399, 276–279 [DOI] [PubMed] [Google Scholar]
- 8.Stevens J. L., Cantin G. T., Wang G., Shevchenko A., Shevchenko A., and Berk A. J. (2002) Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit. Science 296, 755–758 [DOI] [PubMed] [Google Scholar]
- 9.Conkright M. D., Canettieri G., Screaton R., Guzman E., Miraglia L., Hogenesch J. B., and Montminy M. (2003) TORCs: transducers of regulated CREB activity. Mol. Cell 12, 413–423 [DOI] [PubMed] [Google Scholar]
- 10.Fukuda A., Nakadai T., Shimada M., and Hisatake K. (2009) Heterogeneous nuclear ribonucleoprotein R enhances transcription from the naturally configured c-fos promoter in vitro. J. Biol. Chem. 284, 23472–23480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao H. J., Kobayashi R., and Mathews M. B. (1998) Activities of adenovirus virus-associated RNAs: purification and characterization of RNA binding proteins. Proc. Natl. Acad. Sci. U.S.A. 95, 8514–8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langland J. O., Kao P. N., and Jacobs B. L. (1999) Nuclear factor-90 of activated T-cells: A double-stranded RNA-binding protein and substrate for the double-stranded RNA-dependent protein kinase, PKR. Biochemistry 38, 6361–6368 [DOI] [PubMed] [Google Scholar]
- 13.Patel R. C., Vestal D. J., Xu Z., Bandyopadhyay S., Guo W., Erme S. M., Williams B. R., and Sen G. C. (1999) DRBP76, a double-stranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR. J. Biol. Chem. 274, 20432–20437 [DOI] [PubMed] [Google Scholar]
- 14.Shim J., Lim H., R Yates J., and Karin M. (2002) Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol. Cell 10, 1331–1344 [DOI] [PubMed] [Google Scholar]
- 15.Pullmann R. Jr., Kim H. H., Abdelmohsen K., Lal A., Martindale J. L., Yang X., and Gorospe M. (2007) Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol. Cell. Biol. 27, 6265–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vumbaca F., Phoenix K. N., Rodriguez-Pinto D., Han D. K., and Claffey K. P. (2008) Double-stranded RNA-binding protein regulates vascular endothelial growth factor mRNA stability, translation, and breast cancer angiogenesis. Mol. Cell. Biol. 28, 772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwano Y., Kim H. H., Abdelmohsen K., Pullmann R. Jr., Martindale J. L., Yang X., and Gorospe M. (2008) MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol. Cell. Biol. 28, 4562–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunimov N., and Laneuville O. (2010) Characterization of proteins associating with 5′ terminus of PGHS-1 mRNA. Cell. Mol. Biol. Lett. 15, 196–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L., Zhao G., Qiu D., Godfrey W. R., Vogel H., Rando T. A., Hu H., and Kao P. N. (2005) NF90 regulates cell cycle exit and terminal myogenic differentiation by direct binding to the 3′-untranslated region of MyoD and p21WAF1/CIP1 mRNAs. J. Biol. Chem. 280, 18981–18989 [DOI] [PubMed] [Google Scholar]
- 20.Pfeifer I., Elsby R., Fernandez M., Faria P. A., Nussenzveig D. R., Lossos I. S., Fontoura B. M., Martin W. D., and Barber G. N. (2008) NFAR-1 and -2 modulate translation and are required for efficient host defense. Proc. Natl. Acad. Sci. U.S.A. 105, 4173–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brzostowski J., Robinson C., Orford R., Elgar S., Scarlett G., Peterkin T., Malartre M., Kneale G., Wormington M., and Guille M. (2000) RNA-dependent cytoplasmic anchoring of a transcription factor subunit during Xenopus development. EMBO J. 19, 3683–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larcher J. C., Gasmi L., Viranaïcken W., Eddé B., Bernard R., Ginzburg I., and Denoulet P. (2004) Ilf3 and NF90 associate with the axonal targeting element of Tau mRNA. FASEB J. 18, 1761–1763 [DOI] [PubMed] [Google Scholar]
- 23.Xu Y. H., and Grabowski G. A. (1999) Molecular cloning and characterization of a translational inhibitory protein that binds to coding sequences of human acid β-glucosidase and other mRNAs. Mol. Genet. Metab. 68, 441–454 [DOI] [PubMed] [Google Scholar]
- 24.Isken O., Baroth M., Grassmann C. W., Weinlich S., Ostareck D. H., Ostareck-Lederer A., and Behrens S. E. (2007) Nuclear factors are involved in hepatitis C virus RNA replication. RNA 13, 1675–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isken O., Grassmann C. W., Sarisky R. T., Kann M., Zhang S., Grosse F., Kao P. N., and Behrens S. E. (2003) Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 22, 5655–5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corthésy B., and Kao P. N. (1994) Purification by DNA affinity chromatography of two polypeptides that contact the NF-AT DNA binding site in the interleukin 2 promoter. J. Biol. Chem. 269, 20682–20690 [PubMed] [Google Scholar]
- 27.Kao P. N., Chen L., Brock G., Ng J., Kenny J., Smith A. J., and Corthésy B. (1994) Cloning and expression of cyclosporin A- and FK506-sensitive nuclear factor of activated T-cells: NF45 and NF90. J. Biol. Chem. 269, 20691–20699 [PubMed] [Google Scholar]
- 28.Orford R. L., Robinson C., Haydon J. M., Patient R. K., and Guille M. J. (1998) The maternal CCAAT box transcription factor which controls GATA-2 expression is novel and developmentally regulated and contains a double-stranded-RNA-binding subunit. Mol. Cell. Biol. 18, 5557–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto S., Morisawa K., Ota K., Nie J., and Taniguchi T. (1999) A binding protein to the DNase I hypersensitive site II in HLA-DR α gene was identified as NF90. Biochemistry 38, 3355–3361 [DOI] [PubMed] [Google Scholar]
- 30.Saunders L. R., Perkins D. J., Balachandran S., Michaels R., Ford R., Mayeda A., and Barber G. N. (2001) Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 276, 32300–32312 [DOI] [PubMed] [Google Scholar]
- 31.Reichman T. W., and Mathews M. B. (2003) RNA binding and intramolecular interactions modulate the regulation of gene expression by nuclear factor 110. RNA 9, 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichman T. W., Muñiz L. C., and Mathews M. B. (2002) The RNA binding protein nuclear factor 90 functions as both a positive and negative regulator of gene expression in mammalian cells. Mol. Cell. Biol. 22, 343–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichman T. W., Parrott A. M., Fierro-Monti I., Caron D. J., Kao P. N., Lee C. G., Li H., and Mathews M. B. (2003) Selective regulation of gene expression by nuclear factor 110, a member of the NF90 family of double-stranded RNA-binding proteins. J. Mol. Biol. 332, 85–98 [DOI] [PubMed] [Google Scholar]
- 34.Krasnoselskaya-Riz I., Spruill A., Chen Y. W., Schuster D., Teslovich T., Baker C., Kumar A., and Stephan D. A. (2002) Nuclear factor 90 mediates activation of the cellular antiviral expression cascade. AIDS Res. Hum. Retroviruses 18, 591–604 [DOI] [PubMed] [Google Scholar]
- 35.Nie Y., Ding L., Kao P. N., Braun R., and Yang J. H. (2005) ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing. Mol. Cell. Biol. 25, 6956–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda A., Yamauchi J., Wu S. Y., Chiang C. M., Muramatsu M., and Hisatake K. (2001) Reconstitution of recombinant TFIIH that can mediate activator-dependent transcription. Genes Cells 6, 707–719 [DOI] [PubMed] [Google Scholar]
- 37.Fukuda A., Nakadai T., Shimada M., Tsukui T., Matsumoto M., Nogi Y., Meisterernst M., and Hisatake K. (2004) Transcriptional coactivator PC4 stimulates promoter escape and facilitates transcriptional synergy by GAL4-VP16. Mol. Cell. Biol. 24, 6525–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada M., Nakadai T., Fukuda A., and Hisatake K. (2010) cAMP-response element-binding protein (CREB) controls MSK1-mediated phosphorylation of histone H3 at the c-fos promoter in vitro. J. Biol. Chem. 285, 9390–9401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber G. N. (2009) The NFAR's (nuclear factors associated with dsRNA): evolutionarily conserved members of the dsRNA binding protein family. RNA Biol. 6, 35–39 [DOI] [PubMed] [Google Scholar]
- 40.Parrott A. M., and Mathews M. B. (2007) Novel rapidly evolving hominid RNAs bind nuclear factor 90 and display tissue-restricted distribution. Nucleic Acids Res. 35, 6249–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders L. R., and Barber G. N. (2003) The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 17, 961–983 [DOI] [PubMed] [Google Scholar]
- 42.Buaas F. W., Lee K., Edelhoff S., Disteche C., and Braun R. E. (1999) Cloning and characterization of the mouse interleukin enhancer binding factor 3 (Ilf3) homolog in a screen for RNA binding proteins. Mamm. Genome 10, 451–456 [DOI] [PubMed] [Google Scholar]
- 43.Ting N. S., Kao P. N., Chan D. W., Lintott L. G., and Lees-Miller S. P. (1998) DNA-dependent protein kinase interacts with antigen receptor response element binding proteins NF90 and NF45. J. Biol. Chem. 273, 2136–2145 [DOI] [PubMed] [Google Scholar]
- 44.Bass B. L., Hurst S. R., and Singer J. D. (1994) Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr. Biol. 4, 301–314 [DOI] [PubMed] [Google Scholar]
- 45.Rao A., Luo C., and Hogan P. G. (1997) Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747 [DOI] [PubMed] [Google Scholar]
- 46.Kornblihtt A. R., de la Mata M., Fededa J. P., Munoz M. J., and Nogues G. (2004) Multiple links between transcription and splicing. RNA 10, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Auboeuf D., Batsché E., Dutertre M., Muchardt C., and O'Malley B. W. (2007) Coregulators: transducing signal from transcription to alternative splicing. Trends Endocrinol. Metab. 18, 122–129 [DOI] [PubMed] [Google Scholar]
- 48.Monsalve M., Wu Z., Adelmant G., Puigserver P., Fan M., and Spiegelman B. M. (2000) Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell 6, 307–316 [DOI] [PubMed] [Google Scholar]
- 49.Auboeuf D., Dowhan D. H., Li X., Larkin K., Ko L., Berget S. M., and O'Malley B. W. (2004) CoAA, a nuclear receptor coactivator protein at the interface of transcriptional coactivation and RNA splicing. Mol. Cell. Biol. 24, 442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwasaki T., Chin W. W., and Ko L. (2001) Identification and characterization of RRM-containing coactivator activator (CoAA) as TRBP-interacting protein, and its splice variant as a coactivator modulator (CoAM). J. Biol. Chem. 276, 33375–33383 [DOI] [PubMed] [Google Scholar]
- 51.Nakajima T., Uchida C., Anderson S. F., Lee C. G., Hurwitz J., Parvin J. D., and Montminy M. (1997) RNA helicase A mediates association of CBP with RNA polymerase II. Cell 90, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 52.Xu Y. H., Leonova T., and Grabowski G. A. (2003) Cell cycle dependent intracellular distribution of two spliced isoforms of TCP/ILF3 proteins. Mol. Genet. Metab. 80, 426–436 [DOI] [PubMed] [Google Scholar]
- 53.Rezai-Zadeh N., Zhang X., Namour F., Fejer G., Wen Y. D., Yao Y. L., Gyory I., Wright K., and Seto E. (2003) Targeted recruitment of a histone H4-specific methyltransferase by the transcription factor YY1. Genes Dev. 17, 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker L. M., Fierro-Monti I., and Mathews M. B. (2001) Nuclear factor 90 is a substrate and regulator of the eukaryotic initiation factor 2 kinase double-stranded RNA-activated protein kinase. J. Biol. Chem. 276, 32522–32530 [DOI] [PubMed] [Google Scholar]
- 55.Coolidge C. J., and Patton J. G. (2000) A new double-stranded RNA-binding protein that interacts with PKR. Nucleic Acids Res. 28, 1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang J., Kao P. N., and Herschman H. R. (2000) Protein-arginine methyltransferase I, the predominant protein-arginine methyltransferase in cells, interacts with and is regulated by interleukin enhancer-binding factor 3. J. Biol. Chem. 275, 19866–19876 [DOI] [PubMed] [Google Scholar]
- 57.Bennett M., Piñol-Roma S., Staknis D., Dreyfuss G., and Reed R. (1992) Differential binding of heterogeneous nuclear ribonucleoproteins to mRNA precursors prior to spliceosome assembly in vitro. Mol. Cell. Biol. 12, 3165–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michaud S., and Reed R. (1991) An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev. 5, 2534–2546 [DOI] [PubMed] [Google Scholar]
- 59.Shi L., Godfrey W. R., Lin J., Zhao G., and Kao P. N. (2007) NF90 regulates inducible IL-2 gene expression in T cells. J. Exp. Med. 204, 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parrott A. M., Walsh M. R., Reichman T. W., and Mathews M. B. (2005) RNA binding and phosphorylation determine the intracellular distribution of nuclear factors 90 and 110. J. Mol. Biol. 348, 281–293 [DOI] [PubMed] [Google Scholar]
- 61.Brownawell A. M., and Macara I. G. (2002) Exportin-5, a novel karyopherin, mediates nuclear export of double-stranded RNA binding proteins. J. Cell Biol. 156, 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi L., Qiu D., Zhao G., Corthesy B., Lees-Miller S., Reeves W. H., and Kao P. N. (2007) Dynamic binding of Ku80, Ku70 and NF90 to the IL-2 promoter in vivo in activated T-cells. Nucleic Acids Res. 35, 2302–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Z., Licklider L. J., Gygi S. P., and Reed R. (2002) Comprehensive proteomic analysis of the human spliceosome. Nature 419, 182–185 [DOI] [PubMed] [Google Scholar]
- 64.Das R., Yu J., Zhang Z., Gygi M. P., Krainer A. R., Gygi S. P., and Reed R. (2007) SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol. Cell 26, 867–881 [DOI] [PubMed] [Google Scholar]
- 65.Sims R. J. 3rd, Millhouse S., Chen C. F., Lewis B. A., Erdjument-Bromage H., Tempst P., Manley J. L., and Reinberg D. (2007) Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol. Cell 28, 665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flanagan J. F., Mi L. Z., Chruszcz M., Cymborowski M., Clines K. L., Kim Y., Minor W., Rastinejad F., and Khorasanizadeh S. (2005) Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature 438, 1181–1185 [DOI] [PubMed] [Google Scholar]
- 67.Sims R. J. 3rd, Chen C. F., Santos-Rosa H., Kouzarides T., Patel S. S., and Reinberg D. (2005) Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J. Biol. Chem. 280, 41789–41792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernstein B. E., Kamal M., Lindblad-Toh K., Bekiranov S., Bailey D. K., Huebert D. J., McMahon S., Karlsson E. K., Kulbokas E. J. 3rd, Gingeras T. R., Schreiber S. L., and Lander E. S. (2005) Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 [DOI] [PubMed] [Google Scholar]
- 69.Schneider R., Bannister A. J., Myers F. A., Thorne A. W., Crane-Robinson C., and Kouzarides T. (2004) Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat. Cell Biol. 6, 73–77 [DOI] [PubMed] [Google Scholar]