Background: Ca2+ signaling is important for the lytic cycle of T. gondii.
Results: Genetically encoded Ca2+ indicators revealed cytosolic Ca2+ changes in real time.
Conclusion: New approach highlights important features of the lytic cycle.
Significance: Ca2+ influx leads to signaling that results in enhancement of important lytic cycle features.
Keywords: calcium, calcium imaging, host-pathogen interaction, signaling, Toxoplasma gondii, GCaMP, gliding, lytic cycle
Abstract
Toxoplasma gondii is an obligate intracellular parasite that invades host cells, creating a parasitophorous vacuole where it communicates with the host cell cytosol through the parasitophorous vacuole membrane. The lytic cycle of the parasite starts with its exit from the host cell followed by gliding motility, conoid extrusion, attachment, and invasion of another host cell. Here, we report that Ca2+ oscillations occur in the cytosol of the parasite during egress, gliding, and invasion, which are critical steps of the lytic cycle. Extracellular Ca2+ enhances each one of these processes. We used tachyzoite clonal lines expressing genetically encoded calcium indicators combined with host cells expressing transiently expressed calcium indicators of different colors, and we measured Ca2+ changes in both parasites and host simultaneously during egress. We demonstrated a link between cytosolic Ca2+ oscillations in the host and in the parasite. Our approach also allowed us to measure two new features of motile parasites, which were enhanced by Ca2+ influx. This is the first study showing, in real time, Ca2+ signals preceding egress and their direct link with motility, an essential virulence trait.
Introduction
Fluctuations of the cytosolic calcium ion (Ca2+) concentration regulate a variety of cellular functions in all eukaryotes, such as secretion, contraction, cell division, and differentiation (1). Cells contain a sophisticated set of mechanisms to balance their cytosolic Ca2+ levels, and the signals that elevate cytosolic Ca2+ are compensated by mechanisms that reduce it. Ca2+ is sequestered into different organelles, where it is released via calcium release channels in response to specific signals (2). Ca2+ signaling is universal, and it is utilized by prokaryotic and eukaryotic unicellular organisms as well as by multicellular eukaryotes (3). Ca2+ signals decoded by a specific cell may lead to specific physiological responses mediated either through gene expression changes or independent of changes in gene expression (3, 4).
Apicomplexan parasites include a number of pathogens of medical and veterinary relevance, such as Toxoplasma gondii, the agent of toxoplasmosis, and Plasmodium spp., the agents of malaria. During its lytic cycle, T. gondii actively invades host cells, creating a parasitophorous vacuole (PV),9 where it divides to finally exit in search of a new host cell. Parasite invasion is a highly coordinated and active process involving several discrete steps (5). In Toxoplasma, indirect evidence showed that Ca2+ signals are decoded to result in stimulation of gliding motility, microneme secretion, conoid extrusion, and invasion. However, information on the mechanisms and the molecules involved is fragmented or missing. Our previous studies have shown that a nifedipine-sensitive Ca2+ channel is involved in Ca2+ entry (6), although the molecular components involved have not been identified. We also demonstrated that Ca2+ influx enhances lytic cycle traits of T. gondii, such as microneme secretion, conoid extrusion, motility, and invasion (6). Our work highlighted the significance of Ca2+ entry, resulting in Ca2+ signals that affect virulence traits of an intracellular pathogen.
Previous studies on the role of Ca2+ fluctuations in gliding motility (7), conoid extrusion (8), microneme secretion (9–11), host cell invasion (7, 12), and egress (13) were done using indirect methods, such as labeling extracellular parasites with fluorescent dyes and following Ca2+ changes during their gliding motility (7) and using Ca2+ ionophores and other exogenous agents to elevate Ca2+ in extracellular parasites stimulating conoid extrusion (8) or microneme secretion (9–11). Another indirect strategy was the use of intracellular or extracellular Ca2+ chelators to prevent host cell invasion (7, 12) or egress (13). These methods have serious limitations. Loading with fluorescent Ca2+ indicators is highly invasive and can be damaging to cells. These dyes can compartmentalize during extended incubations and are incompatible with prolonged in vivo measurements; additionally, dyes cannot be used for studies involving intracellular parasites because parasite-specific loading cannot be accomplished because host cells will also be loaded.
Of all the Apicomplexan parasites, T. gondii is the most genetically tractable, and in this study we took advantage of this property to use genetically encoded calcium indicators (GECIs) to investigate the role of Ca2+ in motility, invasion, and egress. GECIs are powerful tools, and recent efforts in protein engineering have significantly increased their performance (14). GECIs have the advantage that they enable noninvasive imaging of defined cells and compartments. State-of-the-art GECIs include the single-wavelength sensor GCaMPs, which are based on circularly permuted green fluorescent protein, calmodulin, and the Ca2+/calmodulin-binding “M13” peptide (M13pep) (14). “GECO” sensors, created from GCaMP3 by random mutagenesis (15), are also available in a variety of colors. Our experiments using T. gondii tachyzoites expressing GCaMP3 or GCaMP6f combined with host cells expressing Red-GECO or Blue-GECO (B-GECO) allowed us to directly follow changes in real time in cytosolic Ca2+ in the parasites, while they are inside their host cell and simultaneously in both the parasites and host cells during invasion and egress. The use of these tools provided exciting new information about communication between the cytosolic Ca2+ in the host and the parasite. Our approach has also allowed the measurement of two new features of motile parasites, which were enhanced by Ca2+ influx. This is the first study to show directly that Ca2+ signals precede egress and to establish a direct correlation between Ca2+ signals and motility, an essential virulence trait, throughout the T. gondii lytic cycle.
Experimental Procedures
Cell Cultures
T. gondii tachyzoites (RH strain) were maintained as described (16) using Dulbecco's modified minimal essential media (DMEM) with 1% FBS. HeLa cells (ATCC) were maintained in DMEM supplemented with 10% FBS, 1 mm sodium pyruvate, and 2 mm l-glutamine. hTERT fibroblasts (originally from BD Biosciences) were used as host cells for growth of parasites and also for the invasion experiments described below. These cells were maintained in high glucose DMEM with 10% FCS. GCaMP3-transfected tachyzoites were maintained in hTERT fibroblasts in the presence of 1 μm pyrimethamine. GCaMP6f-transfected tachyzoites were maintained under similar conditions but in the presence of 20 μm chloramphenicol. Parasites were purified as described (16).
Chemicals and Reagents
Fluo-4/AM and Lipofectamine were from Invitrogen (Life Technologies, Inc.). GCaMP3, GCaMP6f (fast version of GCaMP6), R-GECO, and B-GECO were obtained from Addgene, and the plasmids were used for HeLa cells transfections or the respective genes were cloned into T. gondii expression vectors for stable expression in tachyzoites. Thapsigargin, ionomycin, saponin, dithiothreitol (DTT), histamine, and all other chemicals were obtained from Sigma.
Preparation of GECI-expressing Tachyzoites and HeLa Cells
The GCaMP3 gene (17) was amplified from the commercial plasmid and cloned into the T. gondii expression vector pDHFRTubGFP (18) using BglII and AvrII restriction sites and adding a stop codon in front of the GFP sequence. The primers used were forward 5′-AGGCGTGTACGGTGGGAGGTC-3′ and reverse 5′-CTTCCTAGGTTACTTCGCTGTCATCATTTG-3′. The plasmid pDTGCaMP3 was transfected into tachyzoites of the RH strain for pyrimethamine selection. We then isolated cells with low fluorescence by cell sorting to eliminate those highly fluorescent cells in which the GCaMP3 could be buffering Ca2+ and preventing visualization of physiological changes in Ca2+ levels. Sixty nine clones were isolated and analyzed for their response to ionomycin. We incubated these cells in either buffer A (116 mm NaCl, 5.4 mm KCl, 0.8 mm MgSO4, 5.5 mm d-glucose, and 50 mm Hepes, pH 7.2) or buffer A plus 1 μm ionomycin in the presence of 1 mm external CaCl2. We selected the 10 subclones that gave maximal fluorescence response to the ionophore, and we used two of these subclones in our experiments.
Red GECO-coding sequence, cloned into a vector for protein expression into mammalian cells (pCMV) (15), was amplified with the forward primer 5′-CCCGGGTAATGGTCGACTCTTCACGTCGTAAG-3′ and reverse primer 5′-TACGTACTACTTCGCTGTCATCATTTGTAC-3′. SmaI and SnaBI restriction sites were added to the forward and reverse primers, respectively (underlined). R-GECO was cloned in the vector ptub_SAG1-IEα-DsRed_dhfr_sag1CATsag1 (kindly provided by Vern B. Carruthers) between the SmaI (5′)/SnaBI (3′) restriction sites, substituting the DsRed gene, previously shown to label the PV (19). T. gondii parasites (RH strain) were transfected, and selection was done with 20 μm chloramphenicol. This PV version of Red GECO was also transfected in a T. gondii clone (RH strain) expressing the cytosolic GCaMP3 calcium sensor. These parasites were selected in the presence of both 1 μm pyrimethamine and 20 μm chloramphenicol.
Plasmids for expression of GCaMP6 in T. gondii were kindly provided by Kevin Brown and David Sibley, Washington University.10 The coding DNA sequence for GCaMP6f (Addgene) was amplified by PCR and cloned into a T. gondii vector for expression behind the tubulin promoter. Plasmids were electroporated into RH strain parasites and clones selected by chloramphenicol resistance.
HeLa cells (5 × 105) were grown on coverslips (high glucose DMEM with 10% FCS). After 24 h, cells were transfected with 1.25 μg of plasmid DNA encoding R-GECO or B-GECO using Lipofectamine 2000 (Invitrogen) following the instructions of the manufacturer. 6–8 h later, HeLa cells were infected with 106 tachyzoites expressing GCaMP3 or GCaMP6f and were grown for 15–20 h. Rosettes containing 4–8 parasites were used in all the experiments. Variations to the protocol are explained in the legends to the figures.
Cytosolic Ca2+ Measurements
Fluorometric measurements were done with GCaMP3- and GCaMP6f-expressing tachyzoites. The parasites were washed twice in Ringer buffer (155 mm NaCl, 3 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 3 mm NaH2PO4, 10 mm Hepes, pH 7.3, and glucose 10 mm), resuspended in the same buffer to a final density of 1 × 109 cells/ml, and kept in ice. For fluorescence measurements, 50-μl portions of the cell suspension was diluted in 2.5 ml of Ringer (2 × 107 cells/ml final density) in a cuvette placed in a thermostatically controlled Hitachi 4500 spectrofluorometer. Excitation was at 485 nm and emission at 520 nm. Traces shown are representative of three independent experiments conducted on separate cell preparations. Calcium-defined conditions were determined by using EGTA or 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and calcium chloride to arrive at calculated concentrations of free calcium. Calcium-EGTA combinations were determined using Maxchelator software. Fura-2/AM loading and measurements were done as described previously (6).
Invasion Assays
To monitor invasion, extracellular GCaMP3- or GCaMP6f-expressing parasites were collected and resuspended in Ringer buffer with 1 mm EGTA or 1 mm CaCl2. The parasites were then added to a confluent monolayer of hTERT cells plated on MatTek microscopy dishes. The parasites were semi-synchronized by incubating them on ice for 20 min before imaging. The parasites were then imaged at 37 °C. Fluorescence images were captured using an Olympus IX-71 inverted fluorescence microscope with a Photometrix CoolSnapHQ charge-coupled device (CCD) camera driven by DeltaVision software (Applied Precision). Images were collected every 1 s for 10 min, totaling 603 frames, and were transformed in videos using SoftWorx suite 2.0 software from Applied Precision. All experiments were done at 37 °C, and the results were obtained from at least three independent experiments.
Egress Assays
8–12 h after transfection, HeLa cells were infected with 1 × 106 GCaMP6f-expressing tachyzoites. Thirty hours after infection, parasitophorous vacuoles containing 4–8 parasites were observed by microscopy after washing them with Ringer buffer. Drugs were added in Ringer buffer at the concentrations indicated: ionomycin (1 μm), histamine (5 and 100 μm), thapsigargin (1 μm), DTT (5 mm), saponin (0.01%), nifedipine (10 μm). Ringer buffer was used as extracellular buffer (EB). CaCl2 was omitted for experiments in the absence of extracellular calcium, and the media were supplemented with 100 μm or 1 mm EGTA. For experiments using intracellular conditions, an intracellular buffer was used considering the ionic composition of the cytosol as follows: potassium gluconate 140 mm, NaCl 10 mm, MgSO4 2.7 mm, ATP (sodium salt) 2 mm, glucose 1 mm, EGTA 200 μm, CaCl2 65 μm (90 nm free Ca2+), and 10 mm Tris/Hepes, pH 7.3. Images were acquired as for the invasion assays (time-lapse mode with an acquisition rate of at least 2–3 s during 20–30 min).
Motility Assays
35-mm glass bottom cover dishes (MATTEK) were treated with 2 ml of 10% fetal bovine serum (FBS) in phosphate-buffered saline (PBS), pH 7.4, overnight at 4 °C. The following morning the excess FBS was washed with PBS. Freshly egressed tachyzoites expressing GCaMP6f were collected, purified, and resuspended in 1 ml of Ringer buffer without calcium. ∼2.5 × 107 parasites were loaded onto cover dishes using a cell culture cylinder and incubated for ∼15 min on ice to allow for cells to adhere. After removing the cell culture cylinder, Ringer buffer was added to a 2-ml final volume. Cells were imaged using a Zeiss LSM 710 confocal microscope set at 37 °C. Before image acquisition, cells were allowed to equilibrate at 37 °C for 5 min. A 488-nm laser power was set at 4%, and cells were imaged for ∼10 min. After establishment of a baseline for image acquisition, thapsigargin (2 μm final) and CaCl2 solution (2 mm final) were added, and motility was recorded. Ca2+ was added 1 min after thapsigargin addition. For analysis of the data, Trackmate, a plugin available in Fiji, was used to measure fluorescence changes in moving cells (20).
Results
Ca2+ Signaling and Egress
It has been shown that calcium ionophores and other agents induce egress of tachyzoites from their host cells. However, it is difficult to study Ca2+ changes directly in the parasite because any perturbation caused by either Ca2+ ionophores, (21), dithiols (22), Ca2+ chelators (23), or permeabilizing agents such as saponin (24) will affect both the host and parasite. In addition, chemical Ca2+ indicators are not useful because they label both the host and parasite. To investigate whether there is a correlation between Ca2+ signals in the host cell and in the parasite during parasite egress, we created T. gondii tachyzoites expressing GCaMP3 or GCaMP6f and monitored Ca2+ dynamics while they were intracellular. HeLa cells transiently transfected with the Ca2+ indicator, R-GECO, were infected with GECI-expressing tachyzoites and subjected to various treatments previously shown to stimulate egress.
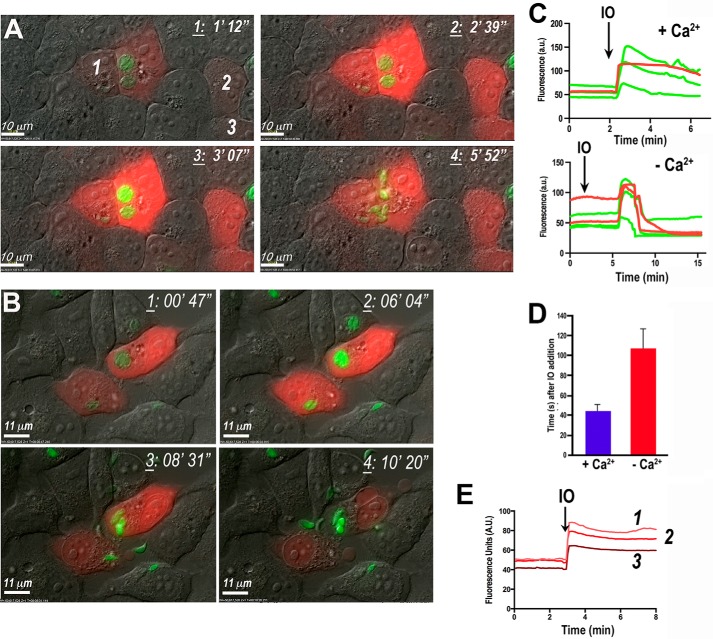
We first tested the effect of ionomycin (1 μm) on HeLa cells transiently transfected with R-GECO and infected with GCaMP3-expressing tachyzoites. Parasite egress was preceded by a Ca2+ increase in the parasite cytosol and increased motility (Fig. 1A and supplemental Video S1). A dramatic Ca2+ increase always immediately preceded the increase in motility and active egress. The first parasites to leave the host cells were able to actively traverse their intact plasma membrane, as shown by the appearance of a moving junction (supplemental Video S1). Only after several parasites had left the host cell was the plasma membrane permeabilized and the remaining tachyzoites egressed passively. The effect of ionomycin is the result of the ionophore inserting in membranes of both host and parasite, leading to simultaneous Ca2+ increase in both compartments (Fig. 1C). This simultaneous increase in Ca2+ was followed by parasite egress (supplemental Video S1). It was not possible to discriminate whether host cell Ca2+ increase was necessary for parasite Ca2+ increase and egress.
FIGURE 1.
Ionomycin stimulates parasite Ca2+ increase and egress in the presence and absence of extracellular Ca2+. Egress of GCaMP3-expressing tachyzoites from R-GECO-expressing HeLa cells in medium with or without extracellular Ca2+ is shown. A, time frames show increases in intracellular Ca2+ in both tachyzoites (green) and host cells (red) upon addition of 1 μm ionomycin (frame 2). Once tachyzoites egressed, the R-GECO fluorescence decreases due to cell permeabilization (frame 4). Frames were obtained from supplemental Video S1 at the indicated times. B, similar experiment to the one shown in A. Cells were incubated in Ringer buffer without CaCl2 and supplemented with 1 mm EGTA. Addition of ionomycin is able to increase Ca2+ in both parasites (green) and host cells (red) (frame 2) and induce parasite egress (frames 3 and 4). Images were taken from a video not shown. C, changes in GCaMP3 (green) and R-GECO (red) fluorescence over time in medium with (+ Ca2+) or without (− Ca2+) extracellular Ca2+. Ionomycin (IO) was added at 2 min (arrow) leading to abrupt increases in both parasites (green) and the host cell (red) intracellular Ca2+, even in the absence of extracellular Ca2+, but it was more sustained and faster in the presence of extracellular Ca2+. Fluorescence change of a region of interest from supplemental Video S1 (plus Ca2+) and not shown (no Ca2+) was done using ImageJ. D, quantification and statistical analysis of a minimum of three independent experiments of stimulation of egress with 1 μm ionomycin in the presence of Ringer buffer with 2 mm Ca2+ (blue bar) versus the same buffer with 1 mm EGTA (red bar). Ionomycin was added 1 min after the start of video recording. The time recorded is the time that parasites started to exit and lysed the PV. HeLa cell growth was the same as for the other egress experiments, i.e. grown to confluence in ∼24 h. E, fluorescence recording of non-infected host cells showing increase of Geco fluorescence in response to the addition of ionomycin. A.U., arbitrary units. The numbers indicate the fluorescence of the cells labeled in A.
Similar experiments with ionomycin in the absence of extracellular Ca2+ (Fig. 1B) led to an increase in cytosolic Ca2+ in both host cells and tachyzoites (Fig. 1C), albeit with a consistent time delay, and also resulted in parasite egress (Fig. 1B), suggesting that Ca2+ release from the parasite intracellular stores caused by ionomycin was sufficient to stimulate Ca2+ increase and parasite egress. We observed that parasites egressed sooner in the presence of extracellular Ca2+, suggesting that Ca2+ influx enhances Ca2+ in the parasite and egress. Fig. 1D shows the quantification of at least three independent experiments for the time to egress after adding ionomycin. In all experiments Ca2+ oscillations preceded egress. Fig. 1E shows the response to ionomycin of non-infected HeLa cells expressing GECO.
We next tested thapsigargin (TG), which inhibits the SERCA-type Ca2+-ATPase at very low concentrations (25) resulting in an increase of cytosolic Ca2+ due to its continual leakage from the endoplasmic reticulum and blockage of its reuptake. Addition of TG in the presence of extracellular Ca2+ stimulates store-operated Ca2+ entry (SOCE) in mammalian cells (26, 27). We recently demonstrated that Ca2+ entry in T. gondii occurs through a pathway that does not involve SOCE (6). Addition of 1 μm TG to HeLa cells transiently transfected with R-GECO and infected with GCaMP3-expressing tachyzoites resulted in cytosolic Ca2+ increases in both the host cell and parasite without egress of the parasites during the time period examined (15 min) (Fig. 2, A and B, and supplemental Video S2). Higher concentrations of TG (10 μm) were toxic to HeLa cells, resulting in plasma membrane blebbing (data not shown). These results are in agreement with previous reports, which demonstrated the toxic effect of TG in eukaryotic cells at high concentrations (28) that can lead to apoptosis (29). However, parasites did not egress even under these conditions (data not shown) suggesting that Ca2+ changes in HeLa cells induced by SOCE are not sufficient to cause parasite egress. The amount of Ca2+ released by ionomycin into the cytoplasm of the cell was 5–6 times larger than the amount released by TG (Fig. 2C), which could explain why ionomycin is highly efficient at inducing Ca2+ increase and egress. These results suggest a threshold for the cytosolic Ca2+ concentration needed for stimulation of motility and egress.
FIGURE 2.
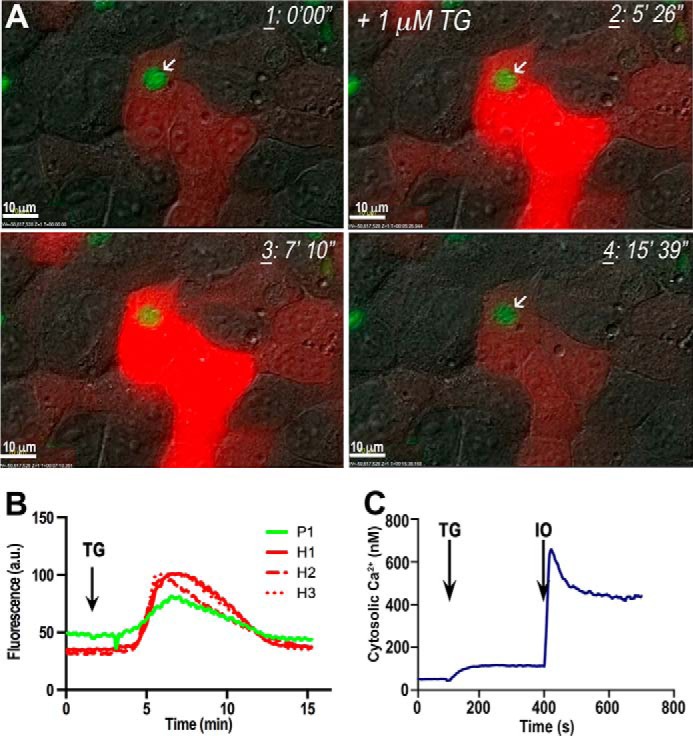
Pharmacological increase of intracellular Ca2+ with TG is unable to trigger tachyzoite egress. A, lack of egress of GCaMP3-expressing tachyzoites from R-GECO-expressing HeLa cells after addition of 1 μm thapsigargin (frame 2). TG increases host cell (red) and parasite (green) cytosolic Ca2+, but the parasites do not egress. Frames were taken from supplemental Video S2 at the times indicated at the top right corner. B, changes in GCaMP3 (green) and R-GECO (red) fluorescence in one group of parasites (P1) and three HeLa cells (H1, H2, and H3) upon addition of thapsigargin (arrow). Ringer buffer was used containing 2 mm CaCl2. More experimental conditions are indicated under “Experimental Procedures.” Fluorescence graph represents the changes in host cells and the PV fluorescence. Analysis was done with ImageJ. C, cytosolic Ca2+ changes of Fura-2/AM-loaded tachyzoites upon addition of TG and ionomycin (IO), both at 1 μm. The effect of ionomycin is noticeably larger than the effect of TG. This is a representative tracing out of at least three identical experiments.
It has been shown previously (22) that dithiothreitol (DTT) stimulates tachyzoite egress. Therefore, we investigated whether this effect was linked to cytosolic Ca2+ increase in the parasite. We tested 5 mm DTT on R-GECO-expressing HeLa cells infected with GCaMP3-expressing parasites. We first observed a Ca2+ increase in tachyzoites, followed by parasite egress, and finally a Ca2+ increase in the infected host cells (Fig. 3, A and B, and supplemental Video S3). Cytosolic Ca2+ in the parasite increased (Fig. 3B, trace e) before host cytosolic Ca2+ (Fig. 3B, peak in red trace a, followed by b and c). This is supported by control experiments showing that there was no effect of DTT when added directly to non-infected HeLa cells expressing R-GECO (Fig. 3D). It has been shown that DTT stimulates a secreted parasite nucleotidase (22), resulting in depletion of host cell ATP levels and exit of parasites (30). ATP is used by both the SERCA pump and the plasma membrane ATPase to pump Ca2+ out of the cytosol, and if it is not available both pumps would stop functioning resulting in an increase of host cytosolic Ca2+. We also observed that the uninfected cells shown in the supplemental Video S3 do not respond to DTT (cells b and c in Fig. 3A and supplemental Video S3) until after tachyzoite egress, suggesting that this secondary Ca2+ increase is the consequence of Ca2+ waves from cell to cell probably through gap junctions (31). Interestingly, we did not observe a direct effect of DTT on the cytosolic Ca2+ of tachyzoites, as tested in Fura-2/AM-loaded extracellular tachyzoites (Fig. 3C), suggesting that the DTT effect on the cytosolic Ca2+ of tachyzoites is indirect and involves a mechanism that is functional or expressed only in intracellular parasites. The traces shown in Fig. 3C compare the response to DTT in the absence of extracellular Ca2+ (red, EGTA) and in its presence (blue). Addition of TG leads to an increase in cytosolic Ca2+ resulting from endoplasmic reticulum leakage under both conditions. Further addition of Ca2+ shows Ca2+ entry (Fig. 3C, red trace).
FIGURE 3.
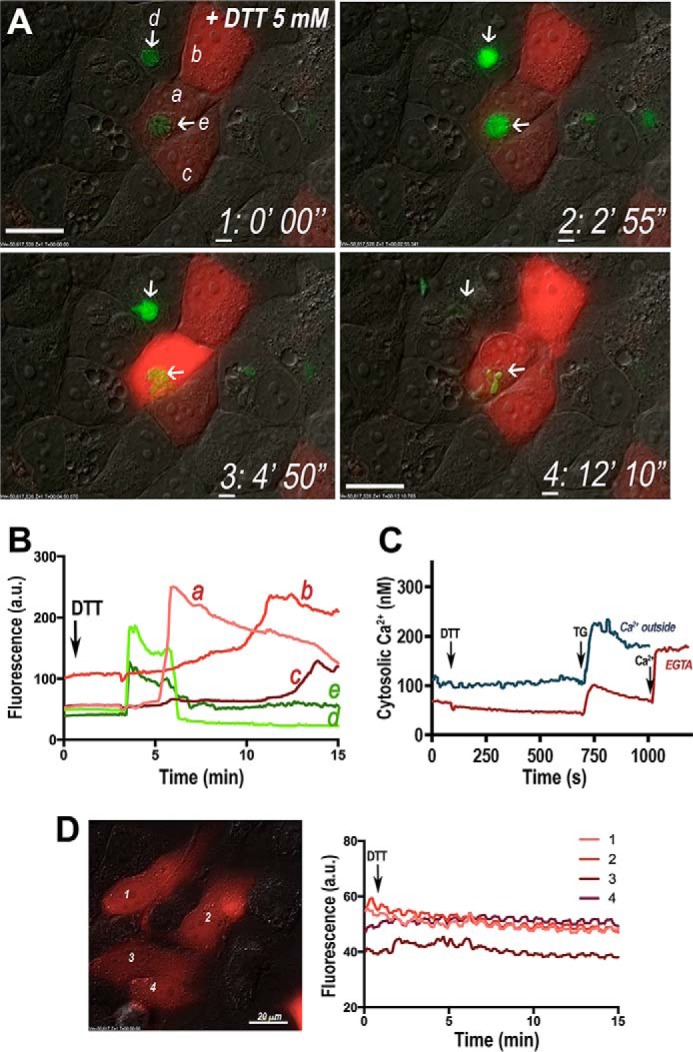
DTT increases intracellular Ca2+ and tachyzoite egress. GCaMP3-expressing tachyzoites egress from HeLa cells upon addition of DTT. A, frames from supplemental Video S3 at four different times after adding 5 mm DTT. The first frame shows cells before addition of DTT. Each frame was taken at the time indicated in the figure. Addition of 5 mm DTT increases intracellular Ca2+ of the tachyzoites (arrows show increase in green fluorescence). Tachyzoites egress, and this is followed by an increase in Ca2+ in the host cell (cell a). Cells b and c (uninfected) show a late increase in intracellular Ca2+. Frames were taken from supplemental Video S3 at the times indicated in the lower right corner. B, graph shows the increase in Ca2+ in the two PVs with tachyzoites (green tracings e and d, which correspond to the areas indicated with arrows in frames 1–4), in an infected cell expressing R-GECO (a) and in uninfected cells (b and c) after addition of DTT (arrow). Two peaks of Ca2+ increase are recorded in GCaMP3-expressing tachyzoites (d), the second being accompanied by Ca2+ increase also in the R-GECO-expressing host cell (a). Fluorescence graph represents the changes in host cells and the PV fluorescence. Analysis was done with ImageJ. C, T. gondii tachyzoites were loaded with Fura-2/AM as described under “Experimental Procedures.” 5 mm DTT was added where indicated with the arrow for both experiments (blue and red tracings). The experiment shown by the red trace was done in Ringer buffer without Ca2+ and with 100 μm EGTA from the beginning, and 1 mm Ca2+ was added at 1000 s. The experiment shown by the blue trace was performed in Ringer buffer with 2 mm Ca2+ from the beginning. In both cases, 1 μm TG was added at 750 s. This is representative from at least three experiments showing the lack of effect of 5 mm DTT in T. gondii intracellular Ca2+. D, confluent HeLa cells were transfected with a plasmid containing the R-GECO Ca2+ indicator gene, and monolayers were stimulated with 5 mm DTT. The fluorescence was recorded at each one of the indicated positions (1–4). Right panel shows fluorescence changes at the areas of interest indicated in the image. A.U., arbitrary units.
Differential Effects of Intracellular and Extracellular Ionic Conditions on Parasite Egress
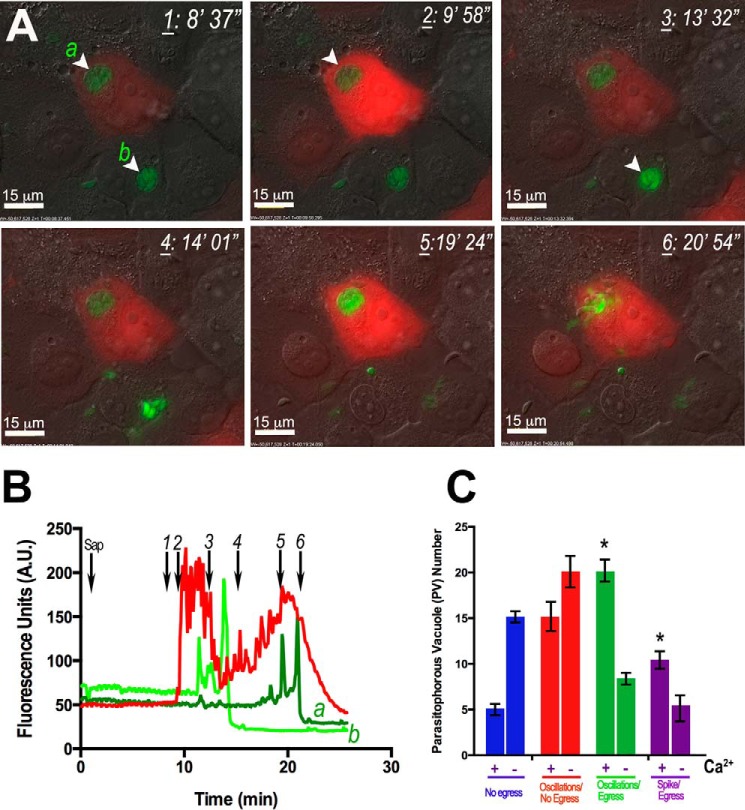
Previous studies have used mild detergents to permeabilize the host cell plasma membrane allowing the intracellular parasites to become exposed to buffers with varying ionic compositions. Using this strategy, it was found that potassium-poor extracellular environment would lead to parasite egress (24). For our experiments, we infected HeLa cells expressing the Ca2+ indicator R-GECO with GCaMP3-expressing tachyzoites (see under “Experimental Procedures” for details) and permeabilized them with saponin (Fig. 4A). Fig. 4 shows Ca2+ oscillations in HeLa cells (Fig. 4B, red trace), followed by Ca2+ oscillations in the parasites (Fig. 4B, light green trace) and, minutes later, egress of the parasites (Fig. 4, A and B, and supplemental Video S4). The oscillations in the parasites are probably the result of Ca2+ entry from the host cytosol as saponin showed no direct effect on the parasite cytosolic Ca2+ (data not shown). However, it appears that the host plasma membrane is not initially permeabilized by this treatment as Ca2+ oscillations continue to occur, a phenomenon incompatible with permeabilization of the plasma membrane. Interestingly, it has been described that saponin treatment of rat myocytes (32) also resulted in Ca2+ oscillations suggesting that saponin stimulates IP3 release by activation of a phospholipase C (33).
FIGURE 4.
Saponin increases intracellular Ca2+ oscillations and tachyzoite egress. GCaMP3-expressing tachyzoites egress from R-GECO-expressing HeLa cells upon saponin addition. A, saponin (0.01%) addition resulted in Ca2+ oscillations in the host cell (red) and in the parasite (green). Ca2+ oscillations in the parasite led to egress and rupture of the PV and the host cell (frame 6). Frames were taken from supplemental Video S4 at the points indicated in B (black arrows). B, graph showing GCaMP3 (green) and R-GECO (red) fluorescence changes after addition of saponin. The numbers indicated are the times at which the frame was taken. Graph was obtained using ImageJ. C, quantification and statistical analysis of a minimum of three experiments of egress events in the presence of an extracellular buffer with (+Ca2+) and without Ca2+ (−Ca2+). There is an increase in the percentage of parasites that experience oscillations (green bars) and spikes (purple bars) in cytosolic Ca2+ and subsequently egress in the presence of Ca2+ (+Ca2+). Red bars represent the parasites that oscillate and do not egress. There is a larger percentage of parasites that do not egress in the absence of Ca2+ (blue). Bars are means ± S.E. Results are from three independent experiments with at least 100 total parasites counted in the presence of each condition. * Student's t test p < 0.05. A.U., arbitrary units.
Prolonged incubation with saponin could lead to permeabilization of the plasma membrane of the host, exposure of tachyzoites to the extracellular medium, and parasite egress. However, when these experiments were done in the same extracellular medium in the absence of extracellular Ca2+, we observed a decrease in the number of parasites showing Ca2+ oscillations and Ca2+ spikes followed by egress (Fig. 4C, green and purple bars). Furthermore, when extracellular Ca2+ was absent, there was an increase in the number of parasites that did not change their intracellular Ca2+ levels or egress (Fig. 4C, blue bars).
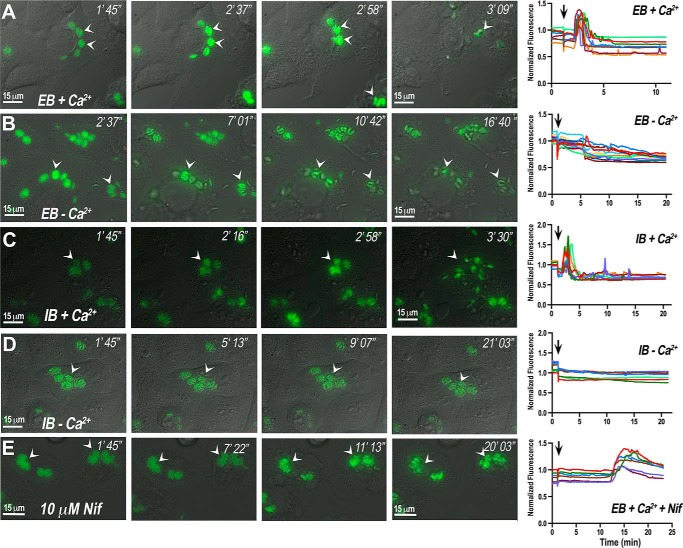
We next investigated further the role of Ca2+ influx during egress, and for this, we used HeLa cells infected with GCaMP6f-expressing tachyzoites in buffers with extracellular or intracellular ionic compositions (EB and IB, see “Experimental Procedures” for a detailed description) (Fig. 5, A–D, and supplemental Video S5, A and B). Addition of saponin to infected HeLa cells in extracellular buffer in the presence of extracellular Ca2+ resulted in tachyzoite Ca2+ increases of high peak amplitude, followed by parasite egress, high motility of the extracellular parasites, and immediate invasion of neighboring cells (Fig. 5A). In the absence of Ca2+, parasite Ca2+ increase was small; egress was delayed; parasites were less motile and were less likely to invade neighboring cells (Fig. 5B). We next tested an intracellular buffer containing high K+, low Na+, and also low Cl− simulating the intracellular composition of these ions. We find that Ca2+ influx still enhances motility and egress under these conditions (Fig. 5C and supplemental Video S5A). In the presence of 90 nm Ca2+ (Fig. 5D), Ca2+ oscillations are not evident; egress is delayed, and the parasites take longer to seek another cell to invade (Fig. 5D and supplemental Video S5 B). The graphics in the right panels of Fig. 5 show intracellular Ca2+ changes in intracellular tachyzoites exposed to these different conditions. Each fluorescence tracing tracks a single PV. These results highlight the enhancing effect of Ca2+ influx in tachyzoite motility and egress.
FIGURE 5.
Buffer composition and inhibitors of Ca2+ entry affect tachyzoite egress. Frames from videos obtained during egress of GCaMP6f-expressing tachyzoites from infected HeLa cells. Saponin (0.01%) was added 1 min after video recording started under different conditions. A, EB plus 2 mm Ca2+. Frames were taken from a video not shown. B, extracellular buffer plus 1 mm EGTA. Frames were taken from a video not shown. C, intracellular buffer plus 1 mm Ca2+. Frames were taken from supplemental Video S5A. D, intracellular buffer plus 90 nm Ca2+. Frames were taken from supplemental Video S5B. These are representative videos of at least three independent biological experiments. E, extracellular buffer plus 1 mm Ca2+ in the presence of 10 μm nifedipine. Frames were taken from a video not shown. The graphs in the right panels show Ca2+ changes associated with tachyzoites present in separate parasitophorous vacuoles. Each tracing in each graph represents the fluorescence change of one PV obtained with ImageJ.
We have previously shown that Ca2+ influx in T. gondii tachyzoites occurs through a nifedipine-sensitive permeation pathway (6). We therefore tested the effect of nifedipine on parasite intracellular Ca2+ levels and egress under extracellular ionic conditions (Fig. 5E) with saponin-permeabilized host cells. The parasites showed a delayed Ca2+ increase and did not egress, even after a 20-min incubation (Fig. 5E, right fluorescence panel). This experiment supports the role of a Ca2+ channel involved in Ca2+ influx needed to trigger a signaling pathway leading to parasite egress. Quantification of egress in incubations in IB or EB with and without extracellular Ca2+ or with nifedipine is shown in Fig. 6. The number of ruptured PVs as a result of parasite egress is significantly lower in the absence of Ca2+. As the host cells were permeabilized by prolonged incubations with saponin, these effects could not be due to changes in host cytosolic Ca2+.
FIGURE 6.
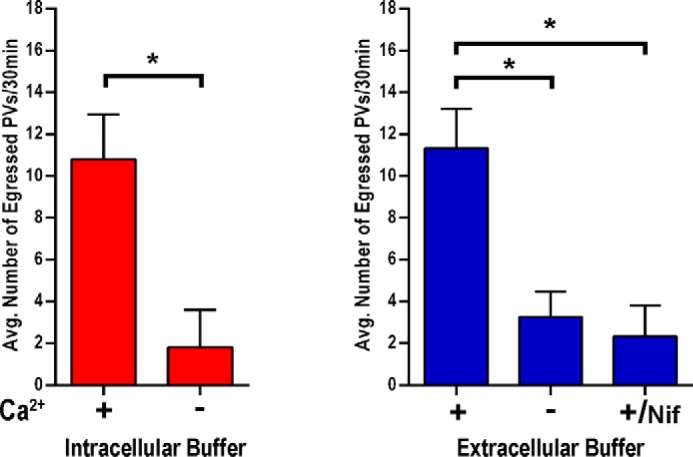
Quantification and statistical analysis of egress events. Egress experiments performed as for Fig. 5 were quantified by counting the number of PVs that fully egressed from host cells within 30 min. Red bars show the quantification of three independent experiments in IB conditions (described under “Experimental Procedures”) with and without 1 mm Ca2+. Blue bars show quantifications of three independent experiments in Ringer buffer with and without Ca2+. +Ca2+/Nif shows the data for egress in the presence of 10 μm nifedipine in the presence of Ca2+. Bars are means ± S.E. Results are from three independent experiments with at least 20 total PVs counted for each condition. Asterisks indicate p < 0.05.
Role of the Host Cytosolic Ca2+
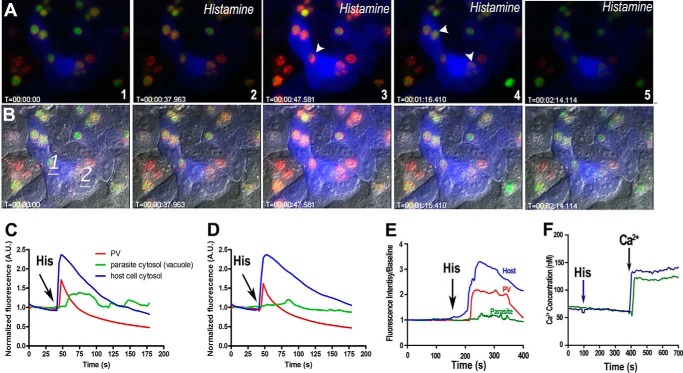
An interesting observation in our experiments with saponin was that the parasites inside HeLa cells without R-GECO appeared to egress sooner (Fig. 4B, trace b). Our interpretation was that R-GECO chelates Ca2+ in the host cell and buffers the free Ca2+ available to parasites delaying oscillations and egress (Fig. 4B, compare green tracing a with b). We next investigated whether the host cytosolic Ca2+ was readily available to the parasites. It has been proposed that the PV membrane is a sieve that allows tachyzoites to freely exchange ions and small molecules with the host cell cytosol (34). We therefore investigated whether physiological Ca2+ signals could result in Ca2+ increases in the PV and intracellular parasites by infecting the HeLa cells transiently expressing B-GECO with tachyzoites expressing GCaMP3 in their cytosol and R-GECO directed to the PV (19). We stimulated the cells with histamine, which activates the plasma membrane H1 receptors in HeLa cells leading to intracellular Ca2+ increase through the gating of the IP3 receptor by IP3 (15). Histamine stimulation of the IP3 signaling pathway in HeLa cells led to Ca2+ increase in the host cytosol (Fig. 7, A–D, blue), and this led to a simultaneous Ca2+ increase in the PV (red) and later increases in the parasites (green) (Fig. 7, C and D, and supplemental Video S6), but we could not detect parasite egress, suggesting that physiological increases in intracellular Ca2+ by histamine are not sufficient to stimulate parasite egress. Fig. 7E shows the average fluorescence changes for host cytosol (blue), parasite cytosol (green), and PV (red) of a minimum of three experiments after stimulation of the infected cells with 100 μm histamine. A similar concentration of histamine was tested against Fura-2/AM-loaded tachyzoites, but these cells were unresponsive, and no effect on cytosolic Ca2+ was observed (Fig. 7F) supporting our interpretation that Ca2+ oscillations in the parasite are the result of Ca2+ influx from the host and not a direct effect of histamine on the parasite.
FIGURE 7.
Histamine increases intracellular Ca2+ in host cells, PV, and tachyzoites, without egress. Changes in intracellular Ca2+ in tachyzoites expressing cytosolic GCaMP3 and parasitophorous vacuole-targeted R-GECO in B-GECO-expressing HeLa cells. A, stimulation of an IP3 signaling pathway in HeLa cells with histamine (frame 2) led to host cytosolic Ca2+ increase (blue, frames 3–4), and immediate increase in Ca2+ in the PV (red, arrowhead in frame 3), followed by a cytosolic Ca2+ increase in tachyzoites (green, arrowheads in frame 4). Ca2+ increase in tachyzoites (green) appears to be a response to the Ca2+ increase in the PV. B, differential interference contrast merged with fluorescence of the same frames shown in A. C and D, graphics show fluorescence changes at two different points (1 and 2 in B, respectively). Blue indicates host cytosolic Ca2+; red indicates PV Ca2+ fluctuations, and green indicates Ca2+ increase in the parasite cytosol. Frames were taken from supplemental Video S6. E, average histamine response in incubations of B-GECO-expressing HeLa cells infected with GCaAMP3-expressing tachyzoites. Changes in Ca2+ in the host cell cytosol (blue), tachyzoite cytosol (green), and PV (red) after addition of histamine (arrow) are shown. At least three experiments were used to calculate the average response of 20 cells for each experiment. F, Fura-2/AM-loaded tachyzoites show no response to 100 μm histamine. Histamine was added where indicated (arrow), and CaCl2 was added at 400 s (arrow). The green tracing show control cells without any previous addition, and the blue tracing shows the response to Ca2+ after adding histamine. This experiment was repeated at least three times with similar results. A.U., arbitrary units.
Ca2+ Signaling and Motility and Invasion
T. gondii invasion of host cells is a highly coordinated process essential for the successful propagation and expansion of the parasite. Ca2+ signaling is essential for the stimulation and enhancement of this virulence trait. It was previously shown that tachyzoites labeled with the fluorescent calcium indicator Fluo-4 (7) underwent Ca2+ oscillations during motility and invasion. Extracellular Ca2+ enhances invasion events (6), and we therefore investigated whether this enhancement occurs at the single cell level.
By using T. gondii tachyzoites expressing GCaMP6f, we were able to demonstrate Ca2+ dynamics in the parasites during invasion. We carried out real time measurements of cytosolic Ca2+ fluctuations in GCaMP6f-expressing tachyzoites in the presence of hTERT cells and acquired images by time-lapse microscopy. To increase the probability of observing actively penetrating parasites, we allowed the parasites to settle onto host cells on ice, thereby synchronizing their invasion. After allowing the parasites to settle for 20 min, tissue culture dishes were transferred to 37 °C resulting in activation of motility and subsequent invasion over the next 10 min. A dramatic Ca2+ elevation was immediately followed by stimulation of motility and invasion of host cells (Fig. 8 and supplemental Video 7). Quantification of these assays showed an enhancement in the number of cytosolic Ca2+ spikes associated with invasion events in the presence of extracellular Ca2+ (Fig. 8B, green bars). Interestingly, when we analyzed the average peak fluorescence for each of the groups (no oscillations/invasion, oscillations/invasion, and spike/invasion), we observed that spikes were larger with Ca2+ outside and that the parasites must fluoresce around 1.5-fold above background (∼75 arbitrary units versus resting 50 arbitrary units) to invade (Fig. 8C). Once intracellular, tachyzoites ceased to show Ca2+ oscillations (Fig. 8A, frame 6, and supplemental Video S7). In the absence of extracellular Ca2+, cytosolic Ca2+ oscillations and invasion events still occurred, but the presence of extracellular Ca2+ had an enhancing effect on the number of invasion events. The results from Fig. 8 and supplemental Video S7 show that there is a correlation between the increase in Ca2+ spikes and invasion in the presence of extracellular Ca2+, indicating that Ca2+ influx increases invasion (Fig. 8B).
FIGURE 8.
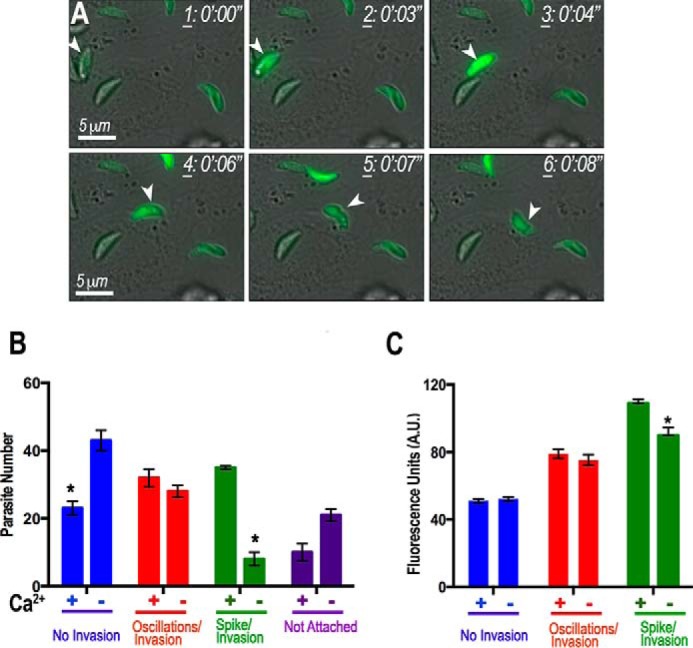
Extracellular calcium enhances invasion. A, GCaMP6f-expressing tachyzoite is highlighted with an arrowhead before (frames 1–3), during (frames 4 and 5), and after (frame 6) invasion. A Ca2+ peak (frame 2) precedes the initiation of motility (frame 3) and invasion (frames 4 and 5). The arrowhead in frame 5 denotes the moving junction of the parasite during invasion. Ca2+ decreases and remains low after invasion (frame 6). Frames were taken from supplemental Video S7. B, quantification and statistical analysis of invasion events and Ca2+ peaks and oscillations. There is an increase in the percentage of parasites that experience a spike in cytosolic Ca2+ and subsequently invade host cells (hTERT) in the presence of extracellular Ca2+ (+Ca2+, green). The percentage of parasites that oscillate and do not invade is higher in buffer without Ca2+ and with 1 mm EGTA (−Ca2+, blue). There is no difference in parasites that oscillate and eventually invade (red). There is a larger percentage of parasites that do not attach or invade in a buffer without Ca2+ and with 1 mm EGTA (purple). C, quantification and statistical analysis of peak fluorescence values associated with invasion events. There is an increase in peak fluorescence of parasites that exhibit a spike in cytosolic Ca2+ and invade in the presence of extracellular Ca2+ (green). There is no difference in average peak fluorescence values calculated for both parasites that oscillate but do not invade (blue) and parasites that oscillate and eventually invade (red). Bars are means ± S.E. Results are from three independent experiments with at least 100 total parasites counted under each condition. *, Student's t test p < 0.05. A.U., arbitrary units.
The invasion experiments showed that a Ca2+ peak leads to parasites invading host cells, and extracellular Ca2+ enhanced these events. It has been shown that gliding movement is associated with Ca2+ oscillations and is stimulated by drugs that cause Ca2+ release (35).
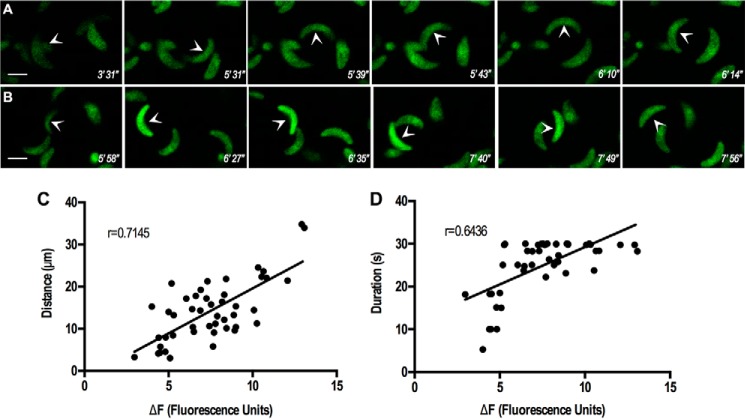
We observed previously that Ca2+ influx enhances gliding motility (6). We wanted to investigate more in depth this phenomenon, and so we looked into the dynamics and the motility features that would be influenced by Ca2+ entry. GCaMP6f-expressing parasites provided optimal conditions for Ca2+ influx (addition of TG in the presence of extracellular Ca2+) (supplemental Videos 8, A and B). We previously observed that cytosolic Ca2+ regulates Ca2+ entry, and TG pre-treatment leads to higher Ca2+ influx. We now show that under those conditions there is an increase in gliding motility (Fig. 9A and supplemental Video 8B). We also looked at Ca2+ entry after adding Ca2+ (supplemental Video 8A). We observed that under basal conditions parasites drifted and oscillated in a disorganized manner. However, upon Ca2+ addition, there was a very consistent increase in cytosolic Ca2+, and a large number of parasites stopped drifting and then attached and started gliding. This suggests that the elicited increase in cytosolic Ca2+ leads to a significant change in parasite behavior, in which they become increasingly motile and invasive. We looked at two essential features associated with invasion and egress, the duration of motility, and the distance traveled (Fig. 9, C and D). Interestingly we observed that extracellular Ca2+ increases the peak fluorescence intensity and that there is a correlation between Ca2+ influx and both duration of motility and distance traveled. These two features are essential for a successful invasion.
FIGURE 9.
Extracellular Ca2+ enhances motility. A, GCaMP6f-expressing tachyzoites (arrowhead) show stimulation of gliding after adding Ca2+. Notice that parasites drift and oscillate, but after addition of Ca2+ to the buffer (from supplemental Video S8A), fluorescence increases, parasites halt, attach, and start gliding. Ca2+ was added at 3 min and 39 s after starting the video. B, GCaMP6f-expressing tachyzoite (arrow) shows stimulation of motility after addition of thapsigargin (3 min and 4 s) and Ca2+ (4 min and 43 s) (from supplemental Video S8B). C, relationship between average peak fluorescence and distance traveled by extracellular tachyzoites was plotted and showed significant correlation by Pearson correlation coefficient (r = 0.7145). D, relationship between average peak fluorescence and duration of motility was significant (Pearson correlation coefficient, r = 0.6436). Each dot indicates a single parasite that was analyzed from at least four different videos.
Discussion
We measured Ca2+ oscillations in intracellular tachyzoites and found that an increase in cytosolic Ca2+ precedes the increase in motility needed for egress. We utilized different types of GECIs so that we could simultaneously monitor calcium fluxes in both the host and the parasite during egress. We demonstrated that there is significant communication between the host and the parasite, although not always resulting in parasite egress. Additionally, we demonstrate that Ca2+ entry plays a relevant role in parasite egress, and this entry occurs through a Ca2+ channel that is sensitive to nifedipine. Physiological (histamine) or pharmacological (thapsigargin) agents, known to increase cytosolic Ca2+ in mammalian cells, were able to stimulate Ca2+ increase in the infected cells and only weakly in the parasites, but this increase did not lead to parasite egress, suggesting that the parasite is able to buffer these physiological or pharmacological changes in host cell Ca2+.
T. gondii has been reported to grow within vacuoles surrounded by host mitochondria (36), but the biological consequences of this association have remained enigmatic. It has been demonstrated that in some cells, such as pancreatic acinar cells, mitochondria could form a belt that acts as a “firewall” confining Ca2+ signals to the secretory pole (37). Ca2+ sequestration by mitochondria is also crucial in defining cellular subdomains in neurons, acting as Ca2+ buffers (38, 39). It is tempting to speculate that host mitochondria could also form a firewall close to the parasitophorous vacuole protecting tachyzoites from physiological or pharmacological cytosolic Ca2+ increases in the host cell thus preventing their premature release during the normal Ca2+ signaling activity of the host cell. Disruption of the structure of the PV during a natural egress event would expose the parasite to Ca2+ fluctuations or ionic changes surrounding the PV.
We also tested the effect of other agents such as ionomycin, DTT, and saponin. Ionomycin increases cytosolic Ca2+ in both parasite and host cell leading to egress, and this effect occurs with and without adding Ca2+ to the buffer, suggesting that Ca2+ release from the parasite or host intracellular stores is sufficient to account for the increased motility and egress of the parasites. Ionomycin releases Ca2+ from all neutral stores and results in a much higher level of intracellular Ca2+ than those obtained by adding other ionophores or inhibitors like thapsigargin (40), thereby explaining its powerful effect on egress.
The information on intracellular Ca2+ release pathways in Apicomplexan parasites is fragmented and poorly defined. There is pharmacological evidence for the presence of channels responsive to IP3 (7, 41, 42) and cyclic ADP-ribose (43), although there are no orthologous genes to the mammalian IP3 receptor or ryanodine receptor (in any of the Apicomplexan genomes). This is despite evidence for the presence of enzymes involved in the generation of some of these second messengers, such as a phosphoinositide phospholipase C (44) and cyclic ADP-ribose cyclase and hydrolase activities (43).
Addition of DTT led to a Ca2+ increase and motility in the parasite, resulting in egress. These results revealed that the increase in cytosolic Ca2+ levels in the host cells is secondary to parasite egress, supporting previous proposals (22) that this compound acts through the activation of a parasite nucleotidase that depletes the host cell of ATP (30). Interestingly, adjacent uninfected cells also show Ca2+ increases after the release of parasites from infected cells, which could be the result of intercellular Ca2+ wave propagation (31). Intercellular Ca2+ waves could result from the diffusion of second messengers, such as IP3, through gap junctions between adjacent cells. In addition or alternatively, cell rupture due to parasite egress could release agonists, which could activate the production of IP3, in the adjacent cells generating a Ca2+ signal. Each mechanism can occur in isolation or synergistically (31). Interestingly, DTT did not have a direct effect on cytosolic Ca2+ of Fura-2/AM-loaded isolated parasites or on cytosolic Ca2+ of non-infected cells. The dramatic increase of cytosolic Ca2+, which only occurs when the parasite is intracellular, is intriguing and likely involves either 1) other unknown factors of host origin or 2) that the parasite nucleotidase in question is only expressed during intracellular stages of development.
Previous experiments using saponin suggested that the ionic composition of the buffer used, and in particular low potassium levels, was important to facilitate parasite egress by this permeabilizing agent (24). We then designed an intracellular buffer with low sodium and chloride and high potassium to compare with extracellular buffer conditions (high sodium and chloride and low potassium). Parasites were triggered to egress with saponin, and in both cases, egress was faster and more efficient in the presence of Ca2+, highlighting the role of Ca2+ entry in the stimulation of motility and lessening the role of other ions under these conditions. We did not observe a significant difference in egress between intracellular and extracellular ionic conditions when Ca2+ was present. Previous data from our laboratory supported the participation of a nifedipine-sensitive permeation pathway in Ca2+ entry in T. gondii (6). Our intracellular buffer ionic composition is high in potassium but also low in chloride (10 mm), resembling the ionic composition of the intracellular milieu. Previous observations have shown that T. gondii becomes depolarized in low chloride (45), a condition that would favor Ca2+ entry.
Our results using R-GECO targeted to the parasitophorous vacuole demonstrated that Ca2+ is freely exchanged between the host cytosol and the PV, supporting the suggestions that the PV membrane acts as a sieve for small ions and molecules.
Several studies have proposed a role for Ca2+ signaling during the lytic cycle of T. gondii. Using Ca2+ ionophores, Ca2+-chelating agents, and ethanol, a link between Ca2+ and conoid extrusion was postulated (8, 46), although no direct parallel Ca2+ measurements were reported. The effect of Ca2+ on gliding motility was studied on trypsin-permeabilized tachyzoites (47) or by measuring Ca2+ oscillations in extracellular tachyzoites loaded with the Ca2+ dye Fluo-4/AM (7, 35). A role for Ca2+ signaling in invasion was postulated on the basis of the analysis of changes in Ca2+ occurring in parasites loaded with Fura-2/AM upon their attachment to host cells (12) or by detecting the cessation of Ca2+ oscillations in extracellular tachyzoites loaded with Fluo-4/AM (7, 48). These and more recent studies have provided indirect evidence that Ca2+ signaling is part of the pathways that result in the stimulation of conoid extrusion, gliding motility, microneme secretion, and invasion. Ca2+ signaling was also proposed to be involved in the pathway leading to parasite egress from the host cells based on the effect of Ca2+ ionophores, which stimulate egress, although it was never shown that cytosolic Ca2+ increased in tachyzoites before egress (13, 21). Most of the studies on Ca2+ signaling were done with extracellular tachyzoites (conoid extrusion, gliding motility, microneme secretion, and invasion) measuring indirectly the involvement of Ca2+ (using Ca2+ chelators or ionophores) or detecting Ca2+ changes with fluorescent dyes independently of the phenomena examined. We describe the use of tools to measure directly cytosolic Ca2+ changes during these processes. In this study we were able to perform real time measurements of Ca2+ changes in live intracellular and extracellular parasites. The use of GECIs provided the means to demonstrate that the parasite cytosolic Ca2+ increases and oscillates preceding egress. GECIs allowed measurements of Ca2+ separately and simultaneously in the parasite cytosol, PV, and host cytosol. These tools will prove to be very useful in future genetic studies for the phenotypic characterization of mutants lacking putative virulence-related genes.
Analysis of gliding motility and the fluorescence of the genetic Ca2+ indicator showed that Ca2+ influx correlated with the peak intensity, which correlated with the distance traveled by the parasites and the time that they are in motion. These two features are essential for a successful completion of the lytic cycle and agree with our results showing that invasion and egress events are enhanced under conditions that favored Ca2+ influx. The link between these two features to Ca2+ oscillations and peak intensity was possible because of the use of a clonal cell line expressing GCaAMP6, which is uniformly expressed in the cytosol. Chemical indicators would not have worked because of uneven loading, toxicity, and organellar compartmentalization.
In our model shown in Fig. 10, Ca2+ influx would stimulate the intensity of Ca2+ oscillations leading to initiation of gliding motility and exit from the PV shortly thereafter. Intracellular stores are likely very important for releasing Ca2+ into the cytosol, but they would not necessarily lead to activation of motility and egress. Ca2+ influx through a nifedipine-sensitive pathway will further stimulate cytosolic Ca2+ increase, motility, and egress of the parasite (6). Careful analysis of the nifedipine experiments showed that the PV still ruptured, but parasites did not show high motility. A perforin-like protein has been shown to be involved in the initial rupture of the PV (19), and the release of Ca2+ from intracellular stores could stimulate this release in the natural egress process. Our model proposes that this rupture could result in a downstream stimulation of Ca2+ influx, which would lead to stimulation of gliding motility and egress. We believe that the tools described here will help in the dissection of the factors/signaling molecules involved in the interaction of host and parasite resulting in egress, an essential part of the lytic cycle of T. gondii. In addition, the phylogenetic position of these eukaryotic unicellular microbes will advance our understanding of the evolution of these complex pathways.
FIGURE 10.
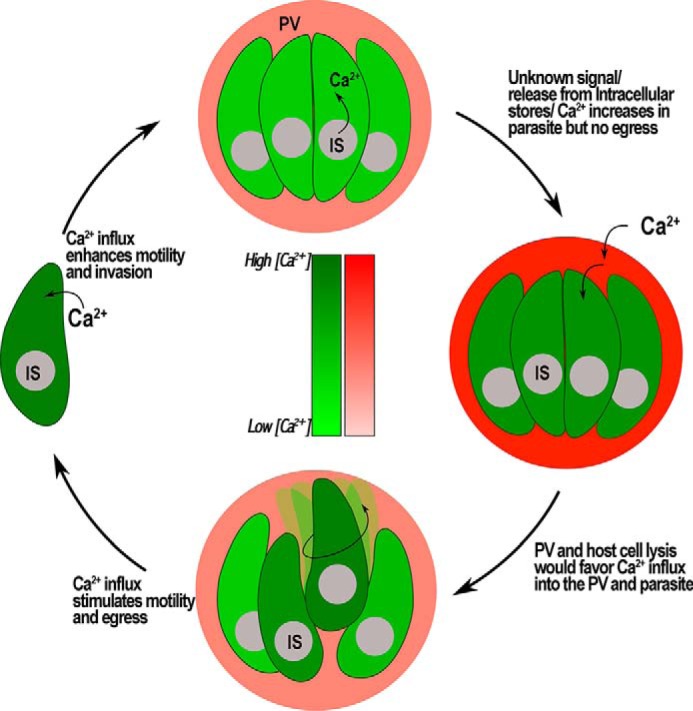
Proposed model for the role of Ca2+ during egress. Ca2+ enters the PV and the parasite when cells are treated with different agents. Intracellular Ca2+ stores (IS) of the parasite release Ca2+ into the cytosol, but no egress is triggered. However, this increase in Ca2+ could stimulate secretion of microneme proteins like perforin-like protein causing lysis of cell and Ca2+ influx from the extracellular media. Influx of Ca2+ leads to an activation of motility and egress. Parasites that egress seek other host cells to invade to continue with its lytic cycle. Ca2+ influx in extracellular parasites stimulates gliding motility and results in an increase of invasion events.
Author Contributions
L. B. P. performed and designed the experiments in Figs. 1–4 and also contributed to the writing of the paper and interpretation of the data. A. B. performed and designed the experiment shown in Fig. 7, performed the experiments used for the statistical analysis presented in Figs. 4C and 8B, constructed the PV-expressing plasmid for visualization of calcium in the PV, and also contributed to the writing of the paper and interpretation of the data. C. A. McK. designed and performed the analysis of motility presented in Fig. 9 and also contributed to the writing of the paper and interpretation of the data. C. A. M. performed the experiments presented in Figs. 5 and 6, designed the model presented in Fig. 10, and contributed to writing of the paper and interpretation of the data. S. A. V. performed the motility assays presented in Fig. 9 and contributed to the analysis and interpretation of the motility data. M. A. H. T. performed the Fura-2/AM experiments shown in Figs. 3C and 7F and also helped with the writing and interpretation of the data. J. L. constructed the plasmid used for expression of GCAMP3 and performed initial experiments on the characterization of the GCAMP3 expressing parasites. C. R. S. G. contributed to the design of some experiments and interpretation of results. D. A. P. contributed to the design of some experiments and the initial isolation and characterization of the GCAMP3-expressing parasites. S. N. J. M. is the principal investigator who managed the project.
Supplementary Material
Acknowledgments
We thank Kevin Brown from David Sibley's laboratory for sharing reagents and useful discussions; Loren L. Looger (Howard Hughes Institute, Janelia Farm Research Campus, Ashburn, VA) for advice on the use of GECIs;, Vern Carruthers for the PV expression vector; and Thayer King and Beejan Asady for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AI-110027 and AI-096836 (to S. N. J. M.). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Videos 1–8.
K. Brown and D. Sibley, manuscript in preparation.
- PV
- parasitophorous vacuole
- TG
- thapsigargin
- SOCE
- store-operated Ca2+ entry
- GECI
- genetically encoded calcium indicator
- IP3
- inositol 1,4,5-trisphosphate
- EB
- extracellular buffer
- IB
- intracellular buffer
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- hTERT
- human telomerase reverse transcriptase.
References
- 1.Bootman M. D., and Berridge M. J. (1995) The elemental principles of calcium signaling. Cell 83, 675–678 [DOI] [PubMed] [Google Scholar]
- 2.Berridge M. J., Bootman M. D., and Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 3.Case R. M., Eisner D., Gurney A., Jones O., Muallem S., and Verkhratsky A. (2007) Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium 42, 345–350 [DOI] [PubMed] [Google Scholar]
- 4.Clapham D. E. (2007) Calcium signaling. Cell 131, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 5.Black M. W., and Boothroyd J. C. (2000) Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64, 607–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace D. A., McKnight C. A., Liu J., Jimenez V., and Moreno S. N. (2014) Calcium entry in Toxoplasma gondii and its enhancing effect of invasion-linked traits. J. Biol. Chem. 289, 19637–19647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovett J. L., and Sibley L. D. (2003) Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J. Cell Sci. 116, 3009–3016 [DOI] [PubMed] [Google Scholar]
- 8.Del Carmen M. G., Mondragón M., González S., and Mondragón R. (2009) Induction and regulation of conoid extrusion in Toxoplasma gondii. Cell Microbiol. 11, 967–982 [DOI] [PubMed] [Google Scholar]
- 9.Carruthers V. B., Moreno S. N., and Sibley L. D. (1999) Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem. J. 342, 379–386 [PMC free article] [PubMed] [Google Scholar]
- 10.Carruthers V. B., and Sibley L. D. (1999) Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31, 421–428 [DOI] [PubMed] [Google Scholar]
- 11.Lovett J. L., Marchesini N., Moreno S. N., and Sibley L. D. (2002) Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from inositol 1,4,5-triphosphate (IP3)/ryanodine-sensitive stores. J. Biol. Chem. 277, 25870–25876 [DOI] [PubMed] [Google Scholar]
- 12.Vieira M. C., and Moreno S. N. (2000) Mobilization of intracellular calcium upon attachment of Toxoplasma gondii tachyzoites to human fibroblasts is required for invasion. Mol. Biochem. Parasitol. 106, 157–162 [DOI] [PubMed] [Google Scholar]
- 13.Garrison E., Treeck M., Ehret E., Butz H., Garbuz T., Oswald B. P., Settles M., Boothroyd J., and Arrizabalaga G. (2012) A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 8, e1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian L., Hires S. A., and Looger L. L. (2012) Imaging neuronal activity with genetically encoded calcium indicators. Cold Spring Harbor Protocols 2012, 647–656 [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y., Araki S., Wu J., Teramoto T., Chang Y. F., Nakano M., Abdelfattah A. S., Fujiwara M., Ishihara T., Nagai T., and Campbell R. E. (2011) An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranda K., Pace D. A., Cintron R., Rodrigues J. C., Fang J., Smith A., Rohloff P., Coelho E., de Haas F., de Souza W., Coppens I., Sibley L. D., and Moreno S. N. (2010) Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol. Microbiol. 76, 1358–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian L., Hires S. A., Mao T., Huber D., Chiappe M. E., Chalasani S. H., Petreanu L., Akerboom J., McKinney S. A., Schreiter E. R., Bargmann C. I., Jayaraman V., Svoboda K., and Looger L. L. (2009) Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Striepen B., He C. Y., Matrajt M., Soldati D., and Roos D. S. (1998) Expression, selection, and organellar targeting of the green fluorescent protein in Toxoplasma gondii. Mol. Biochem. Parasitol. 92, 325–338 [DOI] [PubMed] [Google Scholar]
- 19.Kafsack B. F., Pena J. D., Coppens I., Ravindran S., Boothroyd J. C., and Carruthers V. B. (2009) Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323, 530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J. Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo T., Sethi K. K., and Piekarski G. (1982) Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp. Parasitol. 53, 179–188 [DOI] [PubMed] [Google Scholar]
- 22.Stommel E. W., Ely K. H., Schwartzman J. D., and Kasper L. H. (1997) Toxoplasma gondii: dithiol-induced Ca2+ flux causes egress of parasites from the parasitophorous vacuole. Exp. Parasitol. 87, 88–97 [DOI] [PubMed] [Google Scholar]
- 23.Black M. W., Arrizabalaga G., and Boothroyd J. C. (2000) Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol. Cell Biol. 20, 9399–9408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moudy R., Manning T. J., and Beckers C. J. (2001) The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J. Biol. Chem. 276, 41492–41501 [DOI] [PubMed] [Google Scholar]
- 25.Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., and Dawson A. P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird G. S., DeHaven W. I., Smyth J. T., and Putney J. W. Jr. (2008) Methods for studying store-operated calcium entry. Methods 46, 204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putney J. W. (2009) Capacitative calcium entry: from concept to molecules. Immunol. Rev. 231, 10–22 [DOI] [PubMed] [Google Scholar]
- 28.Vercesi A. E., Moreno S. N., Bernardes C. F., Meinicke A. R., Fernandes E. C., and Docampo R. (1993) Thapsigargin causes Ca2+ release and collapse of the membrane potential of Trypanosoma brucei mitochondria in situ and of isolated rat liver mitochondria. J. Biol. Chem. 268, 8564–8568 [PubMed] [Google Scholar]
- 29.Tsukamoto A., and Kaneko Y. (1993) Thapsigargin, a Ca2+-ATPase inhibitor, depletes the intracellular Ca2+ pool and induces apoptosis in human hepatoma cells. Cell Biol. Int. 17, 969–970 [DOI] [PubMed] [Google Scholar]
- 30.Silverman J. A., Qi H., Riehl A., Beckers C., Nakaar V., and Joiner K. A. (1998) Induced activation of the Toxoplasma gondii nucleoside triphosphate hydrolase leads to depletion of host cell ATP levels and rapid exit of intracellular parasites from infected cells. J. Biol. Chem. 273, 12352–12359 [DOI] [PubMed] [Google Scholar]
- 31.Leybaert L., and Sanderson M. J. (2012) Intercellular Ca2+ waves: mechanisms and function. Physiol. Rev. 92, 1359–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukyanenko V., and Gyorke S. (1999) Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J. Physiol. 521, 575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi S., Rho S. H., Jung S. Y., Kim S. C., Park C. S., and Nah S. Y. (2001) A novel activation of Ca2+-activated Cl− channel in Xenopus oocytes by Ginseng saponins: evidence for the involvement of phospholipase C and intracellular Ca2+ mobilization. Br. J. Pharmacol. 132, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab J. C., Beckers C. J., and Joiner K. A. (1994) The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. U.S.A. 91, 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wetzel D. M., Chen L. A., Ruiz F. A., Moreno S. N., and Sibley L. D. (2004) Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J. Cell Sci. 117, 5739–5748 [DOI] [PubMed] [Google Scholar]
- 36.Pernas L., Adomako-Ankomah Y., Shastri A. J., Ewald S. E., Treeck M., Boyle J. P., and Boothroyd J. C. (2014) Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol. 12, e1001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tinel H., Cancela J. M., Mogami H., Gerasimenko J. V., Gerasimenko O. V., Tepikin A. V., and Petersen O. H. (1999) Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J. 18, 4999–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billups B., and Forsythe I. D. (2002) Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 22, 5840–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzuto R., De Stefani D., Raffaello A., and Mammucari C. (2012) Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 [DOI] [PubMed] [Google Scholar]
- 40.Moreno S. N., and Zhong L. (1996) Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem. J. 313, 655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passos A. P., and Garcia C. R. (1998) Inositol 1,4,5-trisphosphate induced Ca2+ release from chloroquine-sensitive and -insensitive intracellular stores in the intraerythrocytic stage of the malaria parasite P. chabaudi. Biochem. Biophys. Res. Commun. 245, 155–160 [DOI] [PubMed] [Google Scholar]
- 42.Alves E., Bartlett P. J., Garcia C. R., and Thomas A. P. (2011) Melatonin and IP3-induced Ca2+ release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J. Biol. Chem. 286, 5905–5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chini E. N., Nagamune K., Wetzel D. M., and Sibley L. D. (2005) Evidence that the cADPR signalling pathway controls calcium-mediated microneme secretion in Toxoplasma gondii. Biochem. J. 389, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang J., Marchesini N., and Moreno S. N. (2006) A Toxoplasma gondii phosphoinositide phospholipase C (TgPI-PLC) with high affinity for phosphatidylinositol. Biochem. J. 394, 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno S. N., Zhong L., Lu H. G., Souza W. D., and Benchimol M. (1998) Vacuolar-type H+-ATPase regulates cytoplasmic pH in Toxoplasma gondii tachyzoites. Biochem. J. 330, 853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mondragon R., and Frixione E. (1996) Ca2+ dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J. Eukaryot. Microbiol. 43, 120–127 [DOI] [PubMed] [Google Scholar]
- 47.Mondragon R., Meza I., and Frixione E. (1994) Divalent cation and ATP dependent motility of Toxoplasma gondii tachyzoites after mild treatment with trypsin. J. Eukaryot. Microbiol. 41, 330–337 [DOI] [PubMed] [Google Scholar]
- 48.Nagamune K., Beatty W. L., and Sibley L. D. (2007) Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell 6, 2147–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.