Abstract
Disseminated candidiasis is associated with 30–40% mortality in severely immunocompromised patients. Among the causal agents, Candida albicans is the dominant one. Various animal models have been developed for investigating gene functions in C. albicans. Zebrafish injection models have increasingly been applied in elucidating C. albicans pathogenesis because of the conserved immunity, prolific fecundity of the zebrafish and the low costs of care systems. In this study, we established a simple, noninvasive zebrafish egg bath infection model, defined its optimal conditions, and evaluated the model with various C. albicans mutant strains. The deletion of SAP6 did not have significant effect on the virulence. By contrast, the deletion of BCR1, CPH1, EFG1, or TEC1 significantly reduced the virulence under current conditions. Furthermore, all embryos survived when co-incubated with bcr1/bcr1, cph1/cph1 efg1/efg1, efg1/efg1, or tec1/tec1 mutant cells. The results indicated that our novel zebrafish model is time-saving and cost effective.
Introduction
The prevalence of invasive fungal infections has increased substantially because the size of populations at risk have increased [1, 2]. The use of antifungal drugs and the incidence of drug resistance have increased concurrently [3, 4]. The limited variety of antifungal drugs and the emergence of drug resistance emphasize the importance of novel antifungal agents. Disseminated candidiasis is associated with 30–40% mortality in severely immunocompromised patients and Candida albicans is one of frequently isolated fungal pathogenic species in humans [5–7].
Mouse models are predominantly used for studying C. albicans pathogenesis, but are limited by difficulties in performing large-scale studies, high costs, and being time-consuming. To overcome these limitations, investigators have developed several invertebrate models, such as fruit flies (Drosophila melanogaster), nematodes (Caenorhabditis elegans), and larvae of wax moths (Galleria mellonella) [8–13] models. They feature conserved innate immunity and inexpensive care systems and enable experiments to be performed on a large scale [14].
Zebrafish (Danio rerio) are used in biomedical research because manual experimentation and drug administration are easy and the fish exhibits prolific fecundity. In addition, the optical transparency of zebrafish embryos enables real-time visualization of host-pathogen interactions. Chao et al. demonstrated that C. albicans could colonize and invade zebrafish at multiple anatomical sites, and caused mortality after being injected into the peritoneal cavities of adult fish or the yolks of embryos [15]. Brothers et al. subsequently observed that bath infections of zebrafish were not associated with mortality or fungal invasion, and developed a hindbrain ventricle infection model by using larvae [16]. The disadvantages of an injection model include unavoidable damage caused by injection, stringent technical requirements, and the time-consuming procedures. Gratacap et al. developed a noninvasive model of mucosal candidiasis of the swimbladder, natural infection site for C. albicans. [17]. However, this model did not cause mortality, which is a limitation for investigating virulence even though it is an appropriate model for studying immunity.
In this study, we established a zebrafish egg bath infection model as a simple noninvasive model for investigating C. albicans pathogenesis. Candida albicans switches between the unicellular yeast and the filamentous forms to adapt to various conditions. This switch is induced by many environmental cues. The induction by serum or by macrophages may be the most critical ones for the pathogenicity of C. albicans [18, 19]. The cph1/cph1 efg1/efg1 double mutant fails to form filaments in vitro and does not cause lethal infections in a mouse model [20, 21]. These findings suggest that C. albicans strains possessing the ability to switch between the yeast and filament forms are those capable of penetrating vital organs and proliferating sufficiently to cause lethal infections. Even though the null mutation of EFG1 but not CPH1 affected the virulence of C. albicans in a mouse systemic infection model [21], the cph1/cph1 mutant cells exhibited decreased virulence in a fly infection model [22]. In addition to cph1/cph1, efg1/efg1, cph1/cph1 efg1/efg1 mutants, several other mutants were applied in the present study to evaluate this newly established model. Sap6p is a member of the secreted aspartyl protease family [23] and is highly up-regulated in biofilms [24]. However, the deletion of SAP6 exerted no significant effects on germ tube and hyphal formation as well as on virulence in a mouse model [25]. Hyphal formation was defective in tec1/tec1 mutant cells in liquid media but not solid ones [21, 26]. BCR1 deletion caused defects in adhesion and biofilm formation but not in hyphal growth [27–29]. Our results show that this model enables the observation of hyphal/biofilm formation on the chorion and identification of the genes involved in adhesion.
Materials and Methods
Strains and media
The C. albicans strains used were the SC5314 wild type (WT) [30]; JKC19, cph1/cph1 [31]; HLC52, efg1/efg1 [21]; HLC54, cph1/cph1 efg1/efg1 [21]; CAY3672, bcr1/bcr1 [32]; DSY346, sap6/sap6 [33]; CAY2504, tec1/tec1 [32]; CAF2-dTomato [34] and OG1[15]. Yeast peptone dextrose (1% yeast extract, 2% peptone, and 2% dextrose), Roswell Park Memorial Institute 1640 medium (RPMI) (31800–022, GibcoBRL), and 10% fetal bovine serum (FBS) (GibcoBRL, US-628531) were prepared as described previously [35]. The compounds supplementing the media were obtained from Difco unless otherwise stated. Candida albicans cells were grown on YPD agar medium at 30°C for 1 day. Then, C. albicans were grown overnight with shaking in YPD broth at 30°C to stationary phase and the cell density of the inoculum of each strain was determined by OD600 and confirmed by plating.
Zebrafish egg bath infection model
Wild-type zebrafish (Danio rerio), aged approximately 8–15 months were maintained in the zebrafish core facility at National Health Research Institutes (NHRI) (http://www.zebrafish-nthu-nhri.org/tzcf/) at 28°C in a 10-h dark 14-h light cycle. The fish were maintained according to maintenance and culturing procedures described previously [36]. Embryos were obtained from natural mating and staged according to the procedure described by Kimmel et al. [37]. One day post-fertilization, the embryos were sterilized using 0.028% chlorine bleach containing 0.0017% sodium hypochlorite to reduce the possibility of contamination. Data were derived from ≥ 2 repeated experiments unless otherwise stated.
The effect of 0.028% chlorine bleach to chorion structure was determined by adding fluorescein (CAS No. 2321-07-5) to a final concentration of 0.1 mg/mL after the bleach treatment. After one additional day of incubation, representative embryos were anesthetized in 0.2 mg/mL Tris-buffered tricaine methane sulfonate (Fluka A5040, China) and further immobilized in a mixture of 1% low-melting point agarose (Sigma A9414, St. Louis, MO, USA) in egg water. A Leica TCS SP5 II inverted microscope was used for confocal imaging. The green fluorescein was detected by optical filters for excitation/emission at 500 nm/550 nm. The distance between two slices was approximately 5 μm.
In general, after co-incubation, non-adhered C. albicans cells were removed from embryos by washing with egg water 3 times. Embryos incubated in 1 mL of egg water in 24-well plates at 30°C were imaged daily under an inverted microscope, with a beating heart used to indicate viability.
For the investigation of optimal conditions for conducting the egg infection model, embryos were placed in a 125 mL flask with 10 mL media containing 1 × 106 or 1 × 107 cells/mL of SC5314. The media used in the evaluations were egg water (0.03% sea salt), egg water containing 10% FBS (egg water/serum), RPMI, and RPMI containing 10% FBS (RPMI/serum). The embryos and C. albicans cells were co-incubated at 30°C for 1 h or 4 h with or without shaking at 80 or 180 repetitions per minute (rpm). After non-adhered C. albicans cells were removed, the embryos were incubated in egg water for additional 2 days. Since the laboratory standard wild-type SC5314 strain was used for identifying the conditions for conducting the infection model, we used approximately 10 embryos for each treatment.
To determine whether C. albicans hyphae were inside larvae, we co-incubated embryos with 1 × 106 cells/mL of OG1 C. albicans cells in 6-well plates containing 4 mL of RPMI/serum with shaking at 80 rpm and 30°C for 4 h. After non-adhered C. albicans cells were removed, the embryos were incubated in egg water for additional 1 day. Representative embryos were anesthetized in 0.2 mg/mL Tris-buffered tricaine methane sulfonate and further immobilized in a mixture of 1% low-melting point agarose in egg water. The remaining embryos were used for the survival rate determination after 2 days additional incubation. A Leica TCS SP5 II inverted microscope was used for confocal imaging to determine the localization of C. albicans cells. The green OG1 C. albicans cells were detected by optical filters for excitation/emission at 500 nm/550 nm after 1 day additional incubation. The distance between two slices was approximately 5 μm.
To determine the lowest inoculum for conducting this model, embryos were co-incubated with 1 × 105, 5 × 105 or 1 × 106 cells/mL of wild-type SC5314 or CAF2-dTomato C. albicans cells in 6-well plates containing 4 mL of medium with shaking at 80 rpm and 30°C for 4 h. Representative embryos co-incubated with CAF2-dTomato C. albicans cells were anesthetized in 0.2 mg/mL Tris-buffered tricaine methane sulfonate and further immobilized in a mixture of 1% low-melting point agarose in egg water. The remaining embryos were used for the survival rate determination after 2 days additional incubation. A Leica TCS SP5 II inverted microscope was used for confocal imaging to determine the localization of CAF2-dTomato C. albicans cells. The red CAF2-dTomato C. albicans cells were detected by optical filters for excitation/emission at 556 nm/656 nm after 1 day additional incubation. The distance between two slices was approximately 2 μm.
For evaluations of virulence, embryos were co-incubated with 5 × 105 cells/mL of C. albicans in 6-well plates containing 4 mL of RPMI/serum and were shaken at 80 rpm and 30°C for 4 h. After non-adhered C. albicans cells were removed, the embryos were incubated in egg water at 30°C for additional 2 days. To make sure there were enough embryos to distinguish the level of virulence of various mutant strains, we used approximately 20 embryos for each treatment.
Ethics Statement
The zebrafish protocol entitled “Evaluation of the functions of genes in Candida species using zebrafish models” (NHRI-IACUC-101071-A) was reviewed and approved by the Institutional Animal Care and Use Committee of the NHRI.
Statistical analysis
The statistical significance of the differences in frequencies and proportions was determined by the log-rank test. A p value < 0.05 was considered significant.
Results
Optimal conditions for zebrafish egg bath infection
To determine the optimal conditions for zebrafish egg bath infection, we co-incubated wild-type C. albicans cells, SC5314, with 1-day post-fertilization embryos for various periods of time, at various shaking speeds, and in various media (Table 1). The embryos were imaged daily under an inverted microscope. We found that all embryos eventually hatched if they were not killed by C. albicans cells after an additional 2 days of incubation. Thus, the survival rates after an additional 2 days of incubation were discussed mainly in the present study.
Table 1. Conditions for the zebrafish egg bath infection model (mean of survival rate ± standard deviation).
| 1-h co-incubation | 4-h co-incubation | ||||||
|---|---|---|---|---|---|---|---|
| Shaking speed (rpm) | |||||||
| Medium/inoculum | 0 | 80 | 180 | 0 | 80 | 180 | |
| Additional 1 day of incubation | |||||||
| EW/107 | 100 | 100 | 100 | 100 | 100 | 100 | |
| EW+S/107 | 90 ± 17.3* | 57 ± 27.6 | 97 ± 5.8 | 77 ± 32.2 | 43 ± 51.3 | 67 ± 57.7 | |
| R/107 | 96 ± 6.9 | 79 ± 20.1 | 93 ± 11.6 | 58 ± 45.2 | 7 ± 11.6 | 32 ± 33.1 | |
| R+S/107 | 80 ± 34.6 | 73 ± 23.1 | 100 | 67 ± 49.3 | 33 ± 15.3 | 51 ± 39.1 | |
| R/106 | 97 ± 5.8 | 92 ± 14.4 | 100 | 41 ± 31.0 | 14 ± 16.9 | 54 ± 40.8 | |
| R+S/106 | 93 ± 11.6 | 97 ± 5.8 | 100 | 77 ± 40.4 | 29 ± 24.7 | 100 | |
| Additional 2 days of incubation | |||||||
| EW/107 | 92 ± 13.3 | 100 | 100 | 100 | 100 | 100 | |
| EW+S/107 | 49 ± 19 | 11 ± 19.1 | 67 ± 57.7 | 62 ± 8.7 | 0 | 45 ± 50.7 | |
| R/107 | 63 ± 55.1 | 17 ± 20.7 | 100 | 0 | 0 | 0 | |
| R+S/107 | 60 ± 52.9 | 47 ± 21.6 | 83 ± 15.3 | 3 ± 5.8 | 0 | 29 ± 41.9 | |
| R/106 | 57 ± 37.9 | 33 ± 57.7 | 100 | 0 | 0 | 37 ± 50.1 | |
| R+S/106 | 53 ± 32.6 | 55 ± 50.7 | 72 ± 30.1 | 15 ± 13.8 | 0 | 91 ± 16.2 | |
EW: egg water; R: RPMI; S: 10% FBS; 106:1 × 106 cells/mL;107:1 × 107 cells/mL
This data are from 3 repeat experiments. Approximately 30 embryos were tested for each treatment.
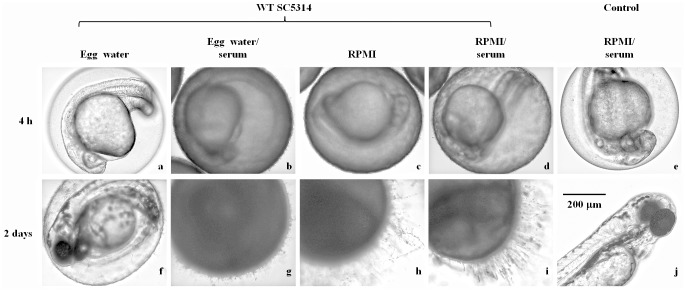
To determine whether bleach treatment affects the chorion membrane, like Dimethyl sulfoxide (DMSO) [38], we used fluorescein entrance as an indicator for chorion integrity. Even though bleach treatment affected chorion membranes and allowed fluorescein enter embryos, it did not kill embryos without the presence of C. albicans (Fig 1e and 1j). This result suggests that bleach-treated embryos can be used for establishing an animal model. All embryos in 11 of the 12 treatments survived when they were co-incubated with SC5314 in egg water alone irrespective of the shaking speed. SC5314 cells are arrested in egg water alone and adding serum to egg water allows the fungal cells to grow and form hyphae. After co-incubated embryos with SC5314 in egg water/serum for 4 h and applied shaking at 0, 80, and 180 rpm, we observed that the survival rates decreased to 62%, 0%, and 45%, respectively after an additional 2 days of incubation. Thus, addition of serum to egg water promotes the growth and/or adhesion capability of C. albicans cells.
Fig 1. Zebrafish egg bath infection model in various media.
Representative embryos were co-incubated with 1 × 106 cells/mL of SC5314 (a-d, f-i) or without C. albicans (control, e, j) in egg water (a, f), egg water/serum (b, g), RPMI medium (c, h), RPMI/serum (d, i) with shaking at 80 rpm and 30°C for 4 h. The embryos were photographed immediately after non-adhered C. albicans cells were removed through washing (a-e) or after an additional 2 days of incubation (f-j). Scale bar = 200 μm. This data are from 3 repeat experiments. Approximately 30 embryos were tested for each treatment.
The survival rates of the embryos co-incubated with SC5314 in RPMI or RPMI/serum without shaking for 1 h prior to an additional 2 days of incubation ranged from 53% to 63%. Under the same conditions but with longer co-incubation (4 h), the survival rates were from 0% to 15% (Table 1). Therefore, we observed higher mortality after 4 h co-incubation than after 1 h. With shaking to 80 rpm for 4 h, all embryos died in all treatments in RPMI or RPMI/serum (Table 1). When we increased the shaking speed to 180 rpm, the embryo survival rates increased from 0% to 29% (1 × 107 inoculum in RPMI/serum), 37% (1 × 106 inoculum in RPMI), and 91% (1 × 106 inoculum in RPMI/serum) (Table 1). Hence, the sharking speed at 80 rpm appears to be suitable for conducting the experiment. Thus, the mixed embryos and C. albicans cells were shaken at 80 rpm for the initial infection in subsequent analyses. Since C. albicans cells formed hyphae and adhered on chorion in RPMI alone, adding serum did not affect mortality levels in RPMI.
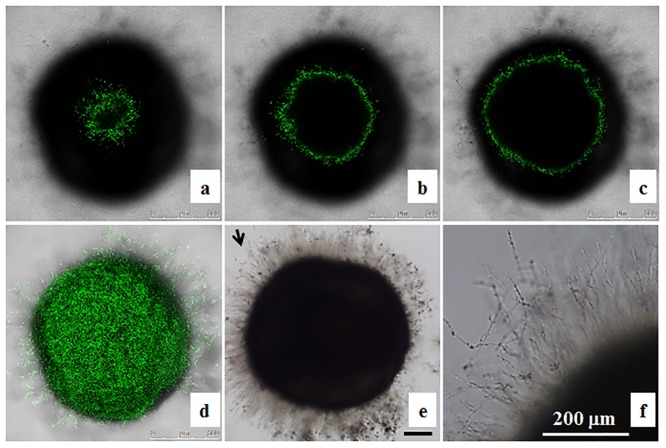
The embryos co-incubated with C. albicans were examined under a microscope. We observed that abundant SC5314 cells were on the chorion of embryos immediately after 4 h co-incubation in egg water/serum (Fig 1b), RPMI (Fig 1c) or RPMI/serum (Fig 1d), but not in egg water alone (Fig 1a). After an additional 2 days of incubation in egg water, C. albicans cells co-incubated with embryos initially in RPMI (Fig 1h), or RPMI/serum (Fig 1i) formed more hyphae than those in egg water/serum (Fig 1g). Few C. albicans cells were detected on the chorion of embryos when co-incubated in egg water (Fig 1f), resulting in no mortality. Furthermore, we found that majority of C. albicans cells grew on the chorion and did not reach the embryo (Fig 2).
Fig 2. Localization of OG1 Candida albicans cells in zebrafish egg bath infection model.
Embryos were co-incubated with 1 × 106 cells/mL of OG1 C. albicans. The representative slices of confocal images (a-c) are shown. The distance between two slices was approximately 55 μm. The whole merged images are presented (d). The phase contrast photos showing C. albicans hyphae were taken by an inverted microscope (e, f). f is the enlargement of the arrow area in e. Scale bars = 200 μm.
Effects of gene deletion on Candida albicans virulence
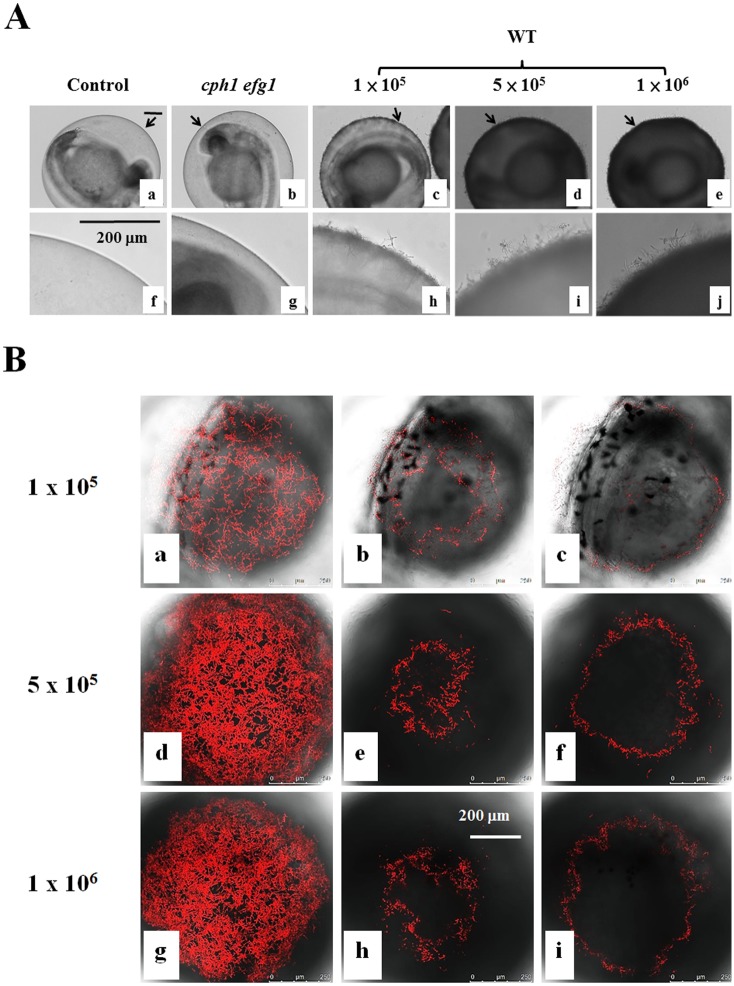
The inocula of 1 × 106 or 1 × 107 cells/mL did not appear to have significantly different effects on the killing activity (Table 1). It is likely that the amount of C. albicans cells used has saturated the activity. Our preliminary data showed that abundant C. albicans cells were on the chorion and kill embryos in the inoculum equal to or greater than 5 × 105 cells/mL, whereas, fewer C. albicans cells were detected on the chorion after 4 h co-incubation (Fig 3A) and a high proportion of embryos survived in the inoculum of 1 × 105 cells/mL. Thus, the inoculum of 5 × 105 cells/mL was chosen to conduct subsequent experiments. Like OG1 cells, we found that majority of CAF2-dTomato C. albicans cells (Fig 3B) grew on the chorion and did not reach the embryo.
Fig 3. Zebrafish egg bath infection model with different inocula.
(A) Embryos were co-incubated in the absence of C. albicans (a, f) or in the presence of 1 × 105 (c, h), 5 × 105 (d-i), or 1 × 106 (e-j) cells/mL of wild-type SC531cells, 1 × 106 (e-j) cells/mL of cph1/cph1 efg1/efg1 mutant cells (b, g) for 4 h. f-j are the enlargement of the arrow areas in a-e. (B) Embryos were co-incubated with 1 × 105 (a-c), 5 × 105 (d-f), or 1 × 106 (g-i) cells/mL of CAF2-dTomato C. albicans. The representative slices (b-c, e-f, h-i) are shown. The distance between two slices was approximately 16 μm. The whole merged images for 1 × 105 (a), 5 × 105 (d) or 1 × 106 (g) cells/mL are presented. Scale bars = 200 μm.
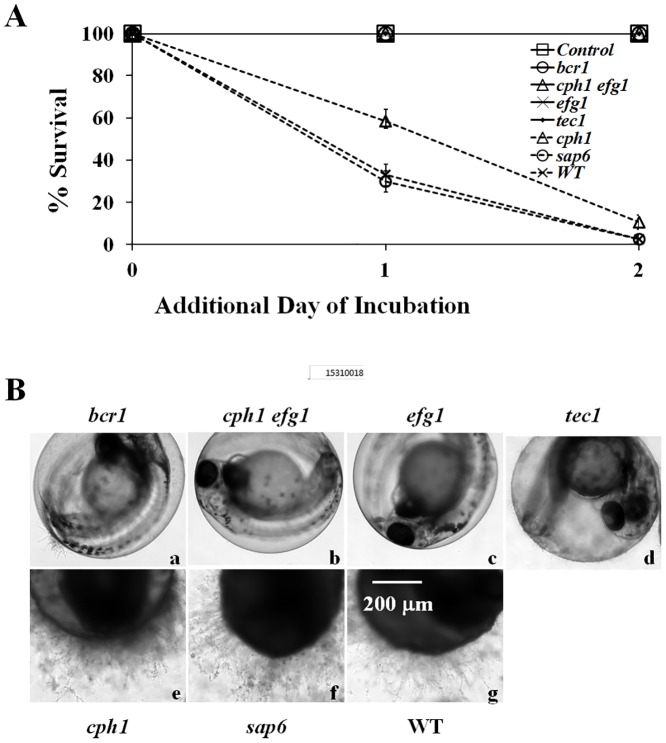
To evaluate our infection model, we examined the virulence of several C. albicans strains. Embryos co-incubated with various mutant strains in RPMI/serum instead of RPMI to mimic the bloodstream of human were shaken at 80 rpm at 30°C for 4 h. The results are summarized in Table 2 and Fig 4. Few embryos survived after co-incubated with the wild-type cells (Fig 4A, crosses with dot line) or sap6/sap6 mutant cells (Fig 4A, circles with dot line). In contrast, the deletion of BCR1, CPH1, EFG1, and TEC1 significantly reduced C. albicans virulence. More embryos survived when co-incubated with cph1/cph1 mutant cells than those with wild-type SC5314 cells after an additional 1 day and 2 days of incubation. Thus, cph1/cph1 mutant cells exhibited decreased level of virulence in this model (p = 0.0006). Furthermore, all embryos survived when co-incubated with the adhesion and biofilm deficient mutants, bcr1/bcr1, cph1/cph1 efg1/efg1, efg1/efg1, or tec1/tec1 (p < 0.0001). Hence, the virulence levels of those strains in this model are Wild-type = sap6/sap6 > cph1/cph1 >> bcr1/bcr1 = cph1/cph1 efg1/efg1 = efg1/efg1 = tec1/tec1.
Table 2. Mean of survival rates of embryos after co-incubated with various mutant strains.
| Control (n = 76) | bcr1 (n = 71) | cph1 efg1(n = 72) | efg1 (n = 73) | cph1 (n = 77) | sap6 (n = 81) | WT (n = 82) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| hours | Mean | Mean | Mean | Mean | Mean | SEM | Mean | SEM | Mean | SEM |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||
| 1 day | 100 | 100 | 100 | 100 | 58.4 | 5.6 | 29.7 | 5.1 | 32.9 | 5.2 |
| 2 days | 100 | 100 | 100 | 100 | 10.4 | 3.5 | 2.5 | 1.7 | 2.4 | 1.7 |
Fig 4. Virulence of C. albicans mutant strains in the infection model.
(A) Survival rates of embryos. Embryos alone (Control) or embryos with 5 × 105 cells/mL of bcr1/bcr1, cph1/cph1 efg1/efg1, efg1/efg1, tec1/tec1, cph1/cph1, sap6/sap6, or WT (SC5314) cells in RPMI/serum were incubated at 30°C for 4 h. Survival rates were determined after an additional 1 day and 2 days of incubation. (B) Representative embryos were co-incubated with (a) bcr1/bcr1, (b) cph1/cph1 efg1/efg1, (c) efg1/efg1, (d) tec1/tec1, (e) cph1/cph1, (f) sap6/sap6, or (g) WT (SC5314) cells, and photographed after an additional 2 days of incubation. Scale bar = 200 μm. The data are from 4 repeat experiments. Approximately 70 embryos were tested for each strain.
Like the wild-type SC5314 cells (Fig 4Bg), there were abundant cph1/cph1 (Fig 4Be), and sap6/sap6 (Fig 4Bf) hyphal cells on the chorion after an additional 2 days of incubation. Few or no bcr1/bcr1 (Fig 4Ba), cph1/cph1 efg1/efg1 (Fig 4Bb), efg1/efg1 (Fig 4Bc), or tec1/tec1 (Fig 4Bd) mutant cells were observed on the chorion. These mutant cells were not associated with mortality.
Discussion
In this study, we identified the conditions (bleached treated 1 day old embryos co-incubated with 5 × 105 cell/mL C. albicans at stationary phase in 4 mL RPMI or RPMI/serum at 80 rpm at 30°C for 4 h) for conducting zebrafish egg bath infection model. We also evaluated this model with various strains known to be defective in virulence in other models. To mimic the bloodstream of human, RPMI/serum was used in the present study. We observed an appropriate shaking speed not only mimic the condition of blood flow but also enable embryos to mix evenly with the C. albicans cells, as suggested previously [17]. This novel zebrafish model is time-saving and cost effective.
To establish an infection, pathogenic cells adhere to the surfaces of host epidermal or endothelial cells, invade host cells, evade the immune system, survive and propagate in the host environment, and then spread to new tissues [39]. However, whether adhesion and hyphal formation are essential for the lethality of C. albicans cells remains unclear. Our observation that bcr1/bcr1 mutant cells were not lethal to the embryos suggests that the adhesion and biofilm formation capabilities during the co-incubation period are critical. The mouse systemic infection model is commonly used for investigating the functions of interested genes related to the virulence of C. albicans. However, when C. albicans cells are injected directly into the mouse tail vein, some of the genes involved in adhesion may not be detected. Our zebrafish egg bath infection model provides an alternative model to identify virulence genes, particularly those involved in adhesion. Since C. albicans hyphal cells did not reach the embryo, the potential cause(s) for the death of the embryos, including failure of transporting toxicities, either secreted by C. albicans or generated by embryos, and/or lack of oxygen are under investigation.
The morphological transition between yeasts and hyphae by C. albicans, generally considered essential for full virulence of this fungus [40], is induced by growth at 37°C and other stimuli, including serum, in vitro. Interestingly, we found that C. albicans can form hyphae at lower temperature, consistent with previous report [16]. Furthermore, we observed that C. albicans adhering to the chorion of embryos formed long hyphae/biofilms (Fig 4Be–4Bg) in egg water at 30°C, indicating that additional host-related factors from embryos are crucial for superseding the need for increased temperature and other stimuli, such as serum. Compared to other in vitro systems, such as polystyrene microplates, mammalian cell lines, and reconstituted human epithelium [41–43], the zebrafish egg bath model can be applied to identify the receptors on the chorion of the embryos which can be beneficial for future study of C. albicans-host interaction.
Acknowledgments
We would also like to thank Dr. Y. C. Chen for kindly providing us with the sap6/sap6 strain, Dr. C. H. Lin for the bcr1/bcr1 and tec1/tec1 strains, and Drs. C. Y. Lan and R. T. Wheeler for the OG1 strain and the CAF2-dTomato strain, respectively. We also thank Drs. F. Fang and C. H. Lin for their helpful suggestions and comments on the manuscript. We thank Mr. M. Swofford for editing the manuscript. We would also thank the NHRI zebrafish core facility for its help in establishing the bath infection model.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in parts by grants from the Ministry of Science and Technology, Taiwan (99-2320-B-400-006-MY3 and 102-2311-B-400-001 to HJL, as well as 99-2320-B-009-001-MY3 and 102-2320-B-009-001 to YLY) and the National Health Research Institutes, Taiwan, (01A1-IV-PP09-014 and 02A1-IVPP08-014 to HJL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chen YC, Chang SC, Sun CC, Yang LS, Hsieh WC, Luh KT. Secular trends in the epidemiology of nosocomial fungal infections at a teaching hospital in Taiwan, 1981 to 1993. Infect Control Hosp Epidemiol. 1997;18: 369–375. [DOI] [PubMed] [Google Scholar]
- 2. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39: 309–317. [DOI] [PubMed] [Google Scholar]
- 3. White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans . Antimicrob Agents Chemother. 2002;46: 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang YL, Lo HJ. Mechanisms of antifungal agent resistance. J Microbiol Immunol Infect. 2001;34: 79–86. [PubMed] [Google Scholar]
- 5. Cheng MF, Yu KW, Tang RB, Fan YH, Yang YL, Hsieh KS, et al. Distribution and antifungal susceptibility of Candida species causing candidemia from 1996 to 1999. Diagn Microbiol Infect Dis. 2004;48: 33–37. [DOI] [PubMed] [Google Scholar]
- 6. Pfaller MA, Diekema DJ, Jones RN, Sader HS, Fluit AC, Hollis RJ, et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001;39: 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang YL, Cheng MF, Wang CW, Wang AH, Cheng WT, Lo HJ, et al. The distribution of species and susceptibility of amphotericin B and fluconazole of yeast pathogens isolated from sterile sites in Taiwan. Med Mycol. 2010;48: 328–334. 10.3109/13693780903154070 [DOI] [PubMed] [Google Scholar]
- 8. Alarco AM, Marcil A, Chen J, Suter B, Thomas D, Whiteway M. Immune-deficient Drosophila melanogaster: a model for the innate immune response to human fungal pathogens. J Immunol. 2004;172: 5622–5628. [DOI] [PubMed] [Google Scholar]
- 9. Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3: e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamilos G, Lionakis MS, Lewis RE, Lopez-Ribot JL, Saville SP, Albert ND, et al. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193: 1014–1022. [DOI] [PubMed] [Google Scholar]
- 11. Chamilos G, Nobile CJ, Bruno VM, Lewis RE, Mitchell AP, Kontoyiannis DP. Candida albicans Cas5, a regulator of cell wall integrity, is required for virulence in murine and toll mutant fly models. J Infect Dis. 2009;200: 152–157. 10.1086/599363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol. 2000;27: 163–169. [DOI] [PubMed] [Google Scholar]
- 13. Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell. 2009;8: 1750–1758. 10.1128/EC.00163-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mylonakis E, Casadevall A, Ausubel FM. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 2007;3: e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao CC, Hsu PC, Jen CF, Chen IH, Wang CH, Chan HC, et al. Zebrafish as a model host for Candida albicans infection. Infect Immun. 2010;78: 2512–2521. 10.1128/IAI.01293-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brothers KM, Newman ZR, Wheeler RT. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell. 2011;10: 932–944. 10.1128/EC.05005-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gratacap RL, Rawls JF, Wheeler RT. Mucosal candidiasis elicits NF-kappaB activation, proinflammatory gene expression and localized neutrophilia in zebrafish. Disease models & mechanisms. 2013;6: 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dabrowa N, Howard DH. Proline uptake in Candida albicans . J Gen Microbiol. 1981;127: 391–397. [DOI] [PubMed] [Google Scholar]
- 19. Shepherd MG, Yin CY, Ram SP, Sullivan PA. Germ tube induction in Candida albicans . Can J Microbiol. 1980;26: 21–26. [DOI] [PubMed] [Google Scholar]
- 20. Chen CG, Yang YL, Cheng HH, Su CL, Huang SF, Chen CT, et al. Non-lethal Candida albicans cph1/cph1 efg1/efg1 transcription factor mutant establishing restricted zone of infection in a mouse model of systemic infection. Int J Immunopathol Pharmacol. 2006;19: 561–565. [DOI] [PubMed] [Google Scholar]
- 21. Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90: 939–949. [DOI] [PubMed] [Google Scholar]
- 22. Chamilos G, Lionakis MS, Lewis RE, Lopez-Ribot JL, Saville SP, Albert ND, et al. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193: 1014–1022. [DOI] [PubMed] [Google Scholar]
- 23. Monod M, Togni G, Hube B, Sanglard D. Multiplicity of genes encoding secreted aspartic proteinases in Candida species. Mol Microbiol. 1994;13: 357–368. [DOI] [PubMed] [Google Scholar]
- 24. Nailis H, Kucharikova S, Ricicova M, Van Dijck P, Deforce D, Nelis H, et al. Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and -independent gene expression. BMC Microbiol. 2010;10: 114 10.1186/1471-2180-10-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Correia A, Lermann U, Teixeira L, Cerca F, Botelho S, da Costa RM, et al. Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect Immun. 2010;78: 4839–4849. 10.1128/IAI.00248-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schweizer A, Rupp S, Taylor BN, Rollinghoff M, Schroppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans . Mol Microbiol. 2000;38: 435–445. [DOI] [PubMed] [Google Scholar]
- 27. Harriott MM, Lilly EA, Rodriguez TE, Fidel PL Jr., Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156: 3635–3644. 10.1099/mic.0.039354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006;2: e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15: 1150–1155. [DOI] [PubMed] [Google Scholar]
- 30. Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198: 179–182. [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266: 1723–1726. [DOI] [PubMed] [Google Scholar]
- 32. Lin CH, Kabrawala S, Fox EP, Nobile CJ, Johnson AD, Bennett RJ. Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans . PLoS Pathog. 2013;9: e1003305 10.1371/journal.ppat.1003305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buu LM, Chen YC. Sap6, a secreted aspartyl proteinase, participates in maintenance the cell surface integrity of Candida albicans . J Biomed Sci. 2013;20: 101 10.1186/1423-0127-20-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brothers KM, Gratacap RL, Barker SE, Newman ZR, Norum A, Wheeler RT. NADPH Oxidase-Driven Phagocyte Recruitment Controls Candida albicans Filamentous Growth and Prevents Mortality. PLoS Pathog. 2013;9: e1003634 10.1371/journal.ppat.1003634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sherman F. Getting started with yeast. Methods Enzymol. 2002;350: 3–41. [DOI] [PubMed] [Google Scholar]
- 36. Westerfield M. The Zebrafish Book A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press; 1993. [Google Scholar]
- 37. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203: 253–310. [DOI] [PubMed] [Google Scholar]
- 38. Kais B, Schneider KE, Keiter S, Henn K, Ackermann C, Braunbeck T. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET). Aquatic toxicology (Amsterdam, Netherlands). 2013;140–141: 229–238. [DOI] [PubMed] [Google Scholar]
- 39. Yang YL. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36: 223–228. [PubMed] [Google Scholar]
- 40. Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther. 2012;10: 85–93. 10.1586/eri.11.152 [DOI] [PubMed] [Google Scholar]
- 41. Lewis RE, Lo HJ, Raad II, Kontoyiannis DP. Lack of Catheter Infection by the efg1/efg1 cph1/cph1 Double-Null Mutant, a Candida albicans Strain That Is Defective in Filamentous Growth. Antimicrob Agents Chemother. 2002;46: 1153–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008;3: 1909–1924. 10.1038/nprot.2008.192 [DOI] [PubMed] [Google Scholar]
- 43. Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72: 157–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.