Abstract
Objective
Given that people who use illicit drugs (PWUD) often engage in prohibited income generation to support their basic needs, we sought to examine the role of these activities in shaping antiretroviral therapy (ART) adherence and plasma HIV RNA-1 viral load suppression among HIV-infected PWUD.
Design
Longitudinal analyses among HIV-positive, ART exposed PWUD in the AIDS Care Cohort to evaluate Exposure to Survival Services prospective cohort study (2005–2013).
Methods
Generalized linear mixed effects and mediation analyses examined the relationship between prohibited income generation (e.g. sex work, drug dealing, theft, street-based income) and virologic suppression (plasma viral load ≤ 50 copies/mL plasma) adjusting for adherence and potential confounders.
Results
Among 687 HIV-infected PWUD, 391 (56.9%) individuals reported prohibited income generation activity during the study period. In multivariate analyses, prohibited income generation remained independently and negatively associated with virologic suppression (adjusted odds ratio: 0.68, 95% confidence interval: 0.52–0.88) following adjustment for hypothesized confounders, including high-intensity drug use, ART adherence and homelessness. While partially mediated by ART adherence, the relationship between prohibited income generation and virologic suppression was maintained in mediation analyses (Sobel statistic = −1.95, p=0.05).
Conclusions
Involvement in prohibited income generation decreases the likelihood of virologic suppression directly and indirectly through its negative association with ART adherence. These findings suggest that linkages between socio-economic marginalization, the criminalization of illicit drug use and insufficient employment opportunities may produce barriers to access and retention in care. Programmatic and policy interventions that decrease socio-economic vulnerability may therefore reduce HIV-related morbidity, mortality and onward transmission.
Keywords: HIV/AIDS, viral suppression, income generation, sex work, drug dealing, social determinants of health
Introduction
The use of highly active antiretroviral therapy (HAART) to reduce HIV-1 RNA plasma concentrations, increase CD4+ cell counts and prevent onward HIV transmission, referred to as HIV Treatment as Prevention (TasP), is a key component in combination HIV prevention strategies [1–3]. TasP-based strategies have reduced morbidity and mortality among those already living with HIV infection and decreased rates of new HIV infection [4,5]. However, TasP is inhibited by complex challenges [2,3]. These include health system and operational challenges in resource-limited settings [3,6] and the stigmatization of people living with HIV/AIDS (PWH), particularly key affected populations (i.e., people who use illicit drugs [PWUD], sex workers, transgender individuals and men who have sex with men) resulting in suboptimal levels of testing, access to care, antiretroviral therapy (ART) adherence and treatment outcomes [7–9]. Implementation efforts are particularly complex where there is insufficient political will to invest in strategies to promote optimal care [9] and reform legal codes that may entrench impediments to implementing TasP such as the criminalization of HIV non-disclosure, illicit drug use, sex work and same-sex behavior [3,10–12].
Examining determinants of optimal HIV care has been identified as a high priority [13]. A growing body of research has pointed to individual determinants such as illicit drug use, addiction treatment, ART adherence-promoting interventions, physical and mental health comorbidities, housing status, and hunger [14–18]. This work has likewise identified broader social and structural determinants ranging from health care system and policy factors affecting sustained access to care, the availability of social supports, the criminalization of PWH, exposure to correctional environments and access to basic material needs [17,19–21].
An emerging line of inquiry in this area explores employment’s role in HIV treatment and clinical outcomes. Employment promotes engagement and retention in care through improved material security, medical service access, social support, time structure and psychosocial well-being [22]. A recent systematic review identified a 27% increase (Range: 13%–71%) in the likelihood of optimal ART adherence among employed individuals [23]. However, significant employment challenges for PWH persist, including managing episodic illness and comorbidities, maintaining status confidentiality, workplace discrimination, and insufficient workplace accommodation [22,24]. Further, there exist considerable and widespread barriers to employment, particularly for key affected populations, including PWUD [25].
Comparatively poor labor market outcomes among PWH and PWUD engaged in high-intensity or poly-substance use are well documented [24,26]. In the absence of viable employment, PWUD and PWH often rely on prohibited income generation strategies that include drug dealing, sex work, acquisitive crime, and street-based income generation (e.g., panhandling, window washing, informal recycling, etc.) [27]. These activities are linked to elevated risk for HIV transmission, extreme violence, and death [11,28–30]. As such, involvement in prohibited income generation may produce and reinforce the health equity impacts of socio-economic marginalization by embedding vulnerable individuals in drug use scenes and producing institutional disengagement from systems of health treatment and care or a lack of trust in care providers reinforced by stigma or criminalization [7,8].
An increased understanding of the implications of socio-economic marginalization for HIV care may be significant in addressing modifiable individual, social and structural factors that could mitigate disparities in access to, retention in and adherence to HIV treatment. However, very little has been documented about the impact of prohibited forms of income generation on HIV treatment and clinical indicators among PWUD. To address this gap, we undertook this study to examine potential associations between prohibited income generation, ART adherence and virologic non-detectability among HIV-infected PWUD in Vancouver, Canada, a context where all HIV care and treatment is provided universally on a no-cost basis.
Methods
Study population
We used data from the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), a long running prospective cohort of HIV-infected PWUD in Vancouver, Canada. As detailed previously [31], cohort recruitment began in 1996 using community-based methods in Vancouver’s Downtown Eastside (DTES) neighborhood. All individuals in ACCESS must be HIV-infected, be at least 18 years of age, and have used illicit drugs other than cannabis in the month prior to enrollment. After providing written informed consent, at baseline and during semi-annual follow-up study visits thereafter, participants complete an interviewer-administered questionnaire, provide a blood sample for serologic testing and complete a nurse-administered questionnaire and examination. Questionnaires seek participant data on socio-demographic characteristics, drug use, income generation, and other relevant exposures. Participants are provided $30 CAD honoraria for each study visit. Ethics approval for ACCESS has been obtained from the Providence Health Care/University of British Columbia Research Ethics Board (approval H05-50233).
ACCESS data were, with consent, linked to each participant’s HIV care and treatment profile, supplied by the Drug Treatment Program of the British Columbia Centre for Excellence in HIV/AIDS (BC-CfE) [21,31]. As the centralized, province-wide ART dispensary and HIV clinical monitoring laboratory, the BC-CfE possesses complete prospective profiles of CD4+ cell counts, plasma HIV-1 RNA viral load (VL), and ART dispensation records for all individuals who have tested positive for HIV in British Columbia. Individual questionnaire data is linked with all relevant clinical information available during the six months prior to follow up interview, including HIV treatment, CD4 and VL measurements. As all HIV/AIDS care is provided on a universal, no-cost basis in the province of British Columbia, analyses are free from potential confounding from more complex health care arrangements linked to employment contracts or the financial ability to pay.
The analytic sample of the study was restricted to ACCESS participants exposed to HAART. This included all observations from individuals who had received at least one day of HAART prior to enrolment and those from the point of HAART initiation onward from individuals who were HAART-naïve at baseline. Additionally, the study sample included only those individuals who had at least one CD4+ cell count and VL observation within 180 days of study entry. The baseline observation for individuals initiating HAART during the observation period was considered to be the first follow-up interview after their exposure to HAART.
Variable Selection
The primary outcome of interest for this study was virologic suppression. Suppression was defined as having achieved a non-detectable HIV-1 RNA viral load of less than 50 copies/mL plasma in the previous six months [15]. In cases with more than one measure of VL within a six-month follow-up period, we used the median of all observations. For example, if a participant contributed two VL measurements during one observation period, one greater and one lower than 50, the median would be used and if the median was greater than 50, the person would be classified as having detectable viremia during the observation period in question. In cases where there was no VL measurement available during a given observation period, the measurement from the previous observation was carried forward. Our key covariate of interest was prohibited income generation, which was defined, in response to the question, “Over the last six months, what were your sources of income?” as any report of sex work (i.e., exchanging sex for money); illegal income generation such as theft, drug dealing, or other acquisitive criminal activity; as well as street-based income sources, including window washing (i.e., “squeegeeing”), informal recycling (i.e., “binning”) or panhandling. Included as a time-updated measure, this variable was considered a proxy for socio-economic marginalization given these activities’ characteristics as illegal, quasi-legal, sanctioned or socially stigmatized activity [11,32–34].
To estimate the relationship between prohibited income generation and virologic suppression, we additionally considered a series of secondary potential confounding variables based on previous research in this setting [15,20]. We examined demographic characteristics of age (per additional ten years); gender (female vs. male); Caucasian ethnicity (vs. non-Caucasian). We additionally considered time-varying drug-related activities and other relevant exposures. These included high-intensity drug use (at least daily injection of heroin or cocaine or non-injection crack-cocaine use vs. less than daily use); homelessness (yes vs. no), defined as living on the street or having no fixed address; incarceration (yes vs. no), defined as having been held in jail or prison; and enrollment in addiction treatment (yes vs. no), including detox, living in a recovery house, residential house or attending a treatment center, 12-step programs (e.g. Narcotics Anonymous), methadone maintenance therapy or other opioid substitution therapies, out-patient treatment, or drug-treatment court, in which individuals are offered alternatives to prison sentences provided they enroll and maintain enrollment in treatment. We additionally included CD4+ cell counts (CD4) and adherence to ART in the last 180 days, derived from pharmacy refill data. Identified in previous research as a reliable predictor of VL suppression and survival [21,31], this measure was calculated by dividing the number of days for which ART was dispensed in the last 180 days by the number of days since ART initiation, capped at 180. We dichotomized this variable at 95%. All time varying covariates are time-updated measures referring to the six months prior to follow-up interview unless otherwise stated.
Statistical Analysis
First, we longitudinally examined trends in licit and illicit income generation during the study period. Second, we examined the baseline characteristics of our analytic sample stratified by whether participants achieved virologic suppression during the study period using Pearson’s χ2 test for categorical variables and Wilcoxon rank-sum tests for continuous variables. Third, using all available observations (i.e. with participant data from multiple follow up interviews), we estimated longitudinal bivariate relationships between our covariates of interest and virologic suppression using generalized linear mixed-effects regression modeling techniques. This strategy accounts for the correlation of multiple measures from the same participant over time as well as serial correlation across observations collected at the same time point. Fourth, we used generalized linear mixed-effects regression to construct multivariate models using an a priori multivariate manual stepwise model building protocol. Starting with a full model including all covariates of interest, each successive model excluded the covariate from the previous model that produced the smallest relative change in the prohibited income generation coefficient. We continued this process until the maximum change from the full model exceeded 5%. This strategy, used extensively in previous analyses [21,29,35], produced a final model that retains those covariates with the greatest relative influence on the relationship between the outcome of interest and the key covariate of interest.
Fifth, we conducted a mediation analysis following procedures recommended by Baron and Kenney [36] to examine whether the association between prohibited income generation and virologic suppression was mediated by an intermediary relationship between prohibited income generation and adherence to ART. This involved constructing three initial multivariate longitudinal general linear mixed effects regression models to determine relationships: (1) between prohibited income generation and ART adherence (path a); (2) ART adherence and virologic suppression (path b); and (3) prohibited income generation and virologic suppression (path c). We then constructed an additional model including both prohibited income generation and ART adherence as predictors of virologic suppression to determine path c′. Finally, we conducted a Sobel test [37] to determine the statistical significance of the proposed mediation pathway. All analyses were conducted using R v3.1.0 (R Core Team, Vienna, Austria).
Results
From December 2005 to May 2013, 687 eligible participants provided 4713 observations and were under observation for a median of 3.8 years (Interquartile range [IQR] 2.3–6.0) and 4 six-month observation periods (IQR 2–7). Individuals who were ART-naïve throughout the observation period and therefore excluded from analyses (n=129) were, at baseline, more likely to be younger (p<0.001), homeless (p=0.037), and to use injection heroin on a daily basis (p=0.005), and less likely to be enrolled in addiction treatment (p=0.003). Of those included in analyses, 234 (34.1%) self-identified as women and 384 (55.9%) as Caucasian. Among non-Caucasian respondents, 257 (37.4%) reported Aboriginal ancestry (e.g., First Nations, Aboriginal, Inuit or Métis), nine (1.3%) reported Black African or Caribbean ancestry, seven (1.0%) reported East Asian ethnicity (e.g., Chinese, Japanese or Vietnamese), six (0.9%) reported Latin American ethnicity and 24 (3.5%) reported another ethnic ancestry. Table 1 identifies baseline characteristics of the study sample stratified by whether virologic suppression was achieved during the study period. Older individuals, men, and participants who, at baseline, were not homeless, did not inject heroin or use crack on a frequent basis, were ≥ 95% adherent to ART, had higher baseline CD4 T-cell counts and lower baseline VL were significantly more likely to achieve virologic suppression.
TABLE 1.
Baseline characteristics of 687 HIV-seropositive and ART exposed individuals who use illicit drugs, stratified by virologic suppression at any point during the study period, Vancouver, Canada, 2005–2013
| Characteristic | No Virologic Suppression, N=137 (19.9%) | Virologic Suppression, N=550(80.1%) | Odds Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Prohibited income generation* | ||||
| No | 90 (65.7) | 367 (66.7) | ||
| Yes | 47 (34.3) | 183 (33.2) | 0.95 (0.64 – 1.42) | 0.819 |
| Age (per additional 10 years) | ||||
| Median (IQR) | 4.14 (3.46–4.77) | 4.42 (38.1–49.0) | 1.07 (1.03 – 1.11) | <0.001 |
| Gender | ||||
| Female | 56 (40.9) | 178 (32.4) | ||
| Male | 81 (59.1) | 372 (67.6) | 1.11 (1.02 – 1.20) | 0.010 |
| Ethnicity | ||||
| White | 59 (43.1) | 244 (44.4) | ||
| Non-white | 78 (56.9) | 306 (55.6) | 1.05 (0.98 – 1.14) | 0.133 |
| Homelessness* | ||||
| No | 77 (56.2) | 423 (77.3) | ||
| Yes | 60 (43.8) | 124 (22.7) | 0.38 (0.25 – 0.56) | <0.001 |
| Frequent heroin injection* | ||||
| < Daily | 112 (82.4) | 492 (89.5) | ||
| ≥ Daily | 24 (17.6) | 58 (10.5) | 0.55 (0.33 – 0.92) | 0.022 |
| Frequent cocaine injection* | ||||
| < Daily | 126(92.6)) | 509 (92.5) | ||
| ≥ Daily | 10 (7.4) | 41 (7.5) | 1.01 (0.49 – 2.08) | 0.967 |
| Frequent crack use* | ||||
| < Daily | 79 (58.1) | 372 (67.6) | ||
| ≥ Daily | 57 (41.9) | 178 (32.4) | 0.66 (0.45 – 0.97) | 0.036 |
| Incarceration* | ||||
| No | 115(85.2) | 492 (89.6) | ||
| Yes | 20 (14.8) | 57 (10.4) | 0.67 (0.38 – 1.15) | 0.144 |
| Addiction treatment enrolment* | ||||
| No | 69 (51.9) | 240 (43.9) | ||
| Yes | 64 (48.1) | 307 (56.1) | 1.37 (0.94 – 2.02) | 0.096 |
| ART adherence* | ||||
| No | 97 (70.8) | 205 (37.3) | ||
| Yes | 40 (29.2) | 345 (62.7) | 4.08 (2.71 – 6.13) | <0.001 |
| Baseline CD4 T-cell count (per 100 cells) | ||||
| Median (IQR) | 2.9 (1.4 – 4.3) | 3.2 (2.0 – 4.6) | 1.02 (1.00 – 1.03) | 0.015 |
Refers to the 6-month period preceding the baseline interview; ART, highly active antiretroviral therapy; CI, confidence interval
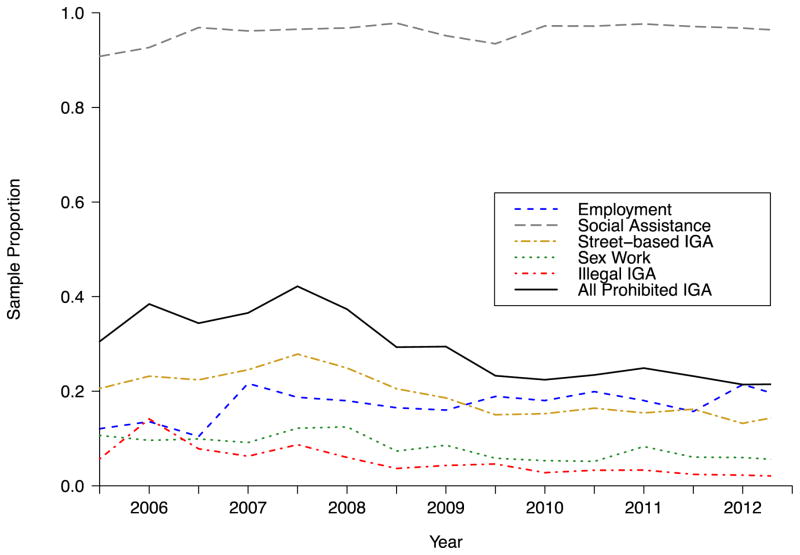
Figure 1 depicts the proportion of the study sample reporting different income generating activities over the course of the study period. Of key relevance, 391 participants (56.9%) reported prohibited income generation at least once. Additionally, the significant majority of individuals received social assistance (median rate 96.8%, IQR 95.6% – 97.2%), followed by relatively low rates of regular, temporary or self-employment (median 18.0%, IQR 15.9% – 18.8%), and declining rates of street-based income generation (median 19.3%, IQR 15.3%–22.8%), sex work (median rate 8.1%, IQR 5.9% – 9.8%), and illegal income generation (median 4.3%, IQR 3.0% – 6.1%). Totals across all forms of income generating activity at each time point are greater than 100% as most individuals in this context generate income from more than one source, for example, by both receiving social assistance and engaging in street-based activities. Over the course of the study period, fully 98.8% of individuals received some form of social assistance, 43.5% engaged in regular, temporary or self-employment, 41.8% undertook street-based income generation, 16.7% were involved in sex work and 16.6% engaged in acquisitive criminal activity.
FIGURE 1.
Income generating activity (IGA) of HIV-positive, ART-exposed individuals who use illicit drugs (N=687) in Vancouver, Canada, 2005–2012
Results from bivariate and multivariate generalized mixed effects analyses of factors associated with HIV-1 RNA levels of <50 copies/mL are displayed in Table 2. Of key importance is the negative association between prohibited income generation and undetectable viremia found in bivariate analysis (odds ratio [OR] = 0.60, 95% confidence interval [CI]: 0.45–0.78) and multivariate analyses (adjusted odds ratio [AOR] = 0.68, 95% CI: 0.56–0.97) following adjustment for hypothesized confounders, including ART adherence, homelessness, age and high intensity drug use.
TABLE 2.
Generalized linear mixed effects analyses of factors associated with plasma HIV-1 RNA viral load <50 copies/mL among 687 ART – exposed, HIV-positive people who use illicit drugs
| Characteristic | Unadjusted Odds Ratio (95% CI)1 | p -value | Adjusted Odds Ratio (95% CI) | p -value |
|---|---|---|---|---|
| Prohibited income generationa,b (yes vs. no) | 0.60 (0.45–0.78) | <0.001 | 0.74 (0.56–0.97) | 0.027 |
| Age (per additional 10 years) | 1.14 (1.11–1.19) | <0.001 | 1.09 (1.07–1.12) | <0.001 |
| Gender (male vs. female) | 2.74 (1.59–4.73) | <0.001 | ||
| Ancestry (Caucasian vs. other) | 1.35 (0.83–2.17) | 0.267 | ||
| Homelessnessa (yes vs. no) | 0.26 (0.19–0.34) | <0.001 | 0.37 (0.27–0.51) | <0.001 |
| High-intensity drug use (yes vs. no) | 0.44 (0.34–0.56) | 0.622 | 0.64 (0.50–0.83) | <0.001 |
| Incarcerationa (yes vs. no) | 0.39 (0.26–0.58) | 0.006 | ||
| Addiction treatment enrolmenta (yes vs. no) | 2.04 (1.54–2.69) | <0.001 | ||
| ART adherencea (≥95% vs. <95%) | 12.10 (9.65 – 15.18) | <0.001 | 6.64 (5.27–8.34) | <0.001 |
| Baseline CD4+ T-cell count (per 100 cells) | 1.00 (1.00–1.00) | 0.671 |
ART, antiretroviral therapy; CI, confidence interval
refers to the six month period preceding the follow-up interview
includes sex work, drug dealing, theft, street-based and other illegal or prohibited sources of income
includes daily or greater heroin injection, cocaine injection or crack-cocaine smoking
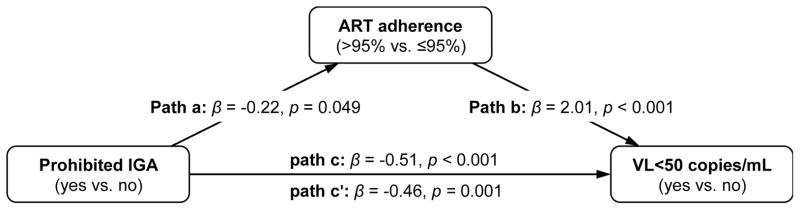
Subsequent mediation analyses, depicted in Figure 2, demonstrated that there was a significant and negative association between prohibited income generation and ART adherence, as well as a strong association between ART adherence and virologic suppression. When the association between prohibited IGA and undetectable viremia was evaluated after controlling for ART adherence, the significant relationship between prohibited income generation and virologic suppression remained, though the strength of this association decreased. These analyses indicate that ART adherence was a partial mediator in the relationship between prohibited income generation and virologic suppression (Sobel test statistic = −1.95, p = 0.05).
FIGURE 2.
Mediation effects for antiretroviral therapy (ART) adherence on the relationship between prohibited income generating activity (IGA) and plasma HIV-1 RNA non-detectable viral load (VL) among 687 ART-exposed people living with HIV/AIDS who use illicit drugs in Vancouver, Canada. Sobel test statistic = −1.95, p=0.05.
Discussion
In this study, we described long-term income generation trends among HAART-exposed, HIV-infected PWUD and the significant involvement of participants in prohibited income generation activities. We further reported a significant negative relationship between prohibited income generation and virologic suppression, as well as partial mediation of this relationship through a negative association between prohibited income generation and adherence to ART. These findings suggest that socio-economic marginalization among PWUD and its attendant impacts decrease the likelihood of retention in HIV care and of subsequently achieving optimal clinical HIV outcomes. This decrease may be attributable to individual, social and structural factors that link engagement in prohibited income generation to decreased stability, exposure to violence, barriers accessing care or detachment from care providers [8,30,38].
Previous research has highlighted how a lack of access to appropriate employment is a key driver of socio-economic and health inequity [39]. This study reinforces these findings by outlining how socio-economic marginalization has direct implications for HIV treatment and clinical outcomes among PWUD through exposure to drug use and sex work scenes, as well as street-based income generation activities [11,27,28]. It further adds to a growing literature identifying social, environmental and structural contributors to suboptimal HIV treatment and virologic outcomes among PWUD [7,8,17,40]. To our knowledge, this is the first study to document prohibited income generation activities among HIV-infected PWUD and their association with HIV clinical indicators. It thereby begins to address our extremely limited understanding of the impacts of economic activity outside the formal labor market on engagement and retention in HIV care.
The current study departs from previous analyses that have framed socio-economic marginalization in terms of barriers to employment or high unemployment rates [24,25]. It instead redirects attention to the role of income generation activity undertaken outside the labour market to meet basic needs and maintain active drug use [27,28]. That socio-economic marginalization pushes people to undertake prohibited income generating activity is of key relevance to issues of health equity. While in some cases these activities provide a low-barrier and flexible means to generate much needed income [33,38], they are also associated with increases in exposure to drug use scenes, violence, increased criminal justice system involvement, unsafe work environments, exploitation by employers and clients, and income insecurity [11,30,33,38]. The current study therefore contributes to a more robust understanding of how health disparities among HIV-infected PWUD are established and entrenched through socio-economic marginalization enacted through legal codes, social sanctions, a lack of viable licit employment opportunities and subsequent exposure to high-risk socio-economic environments. Notably, in the current research context HIV care and treatment is provided on a universal, no-cost basis, and PWH have access to government-provided social assistance provisions. The negative relationship between prohibited income generation and virologic suppression found here may therefore be less pronounced than would be found in other, less supportive environments, reinforcing the potentially critical impact of socio-economic insecurity on HIV outcomes.
This research points to an urgent need to evaluate interventions aimed at mitigating the socio-economic marginalization that relegates HIV-infected PWUD to generate income using strategies that involve illegal, quasi-legal or socially sanctioned activities. Such interventions may include: increases in social assistance levels which, in the current study context are not indexed to inflation and have not been raised since 2007 [41]; legal frameworks that do not disproportionately criminalize and stigmatize HIV-infected PWUD; and appropriate labor market opportunities for HIV-infected PWUD. Such opportunities could include increased access to low-threshold employment that accommodates ongoing health and social service utilization, co-morbid conditions, disability, and episodic absences from labor market participation. Previous research has highlighted the importance of workplace accommodations for PWH [42] and individual willingness to forgo street-based income generation strategies if low-threshold employment opportunities were available [38]. While the impacts of such interventions are thus far unclear, strategies that mitigate the harm experienced as a result of involvement in prohibited income generation could have potentially significant impacts on engagement in HIV care, subsequent clinical and health outcomes, and the risk of onward viral transmission. Finally, strategies to optimize adherence among individuals involved in prohibited income generation activities, including accessible addiction and mental health treatment, should be scaled up to attempt to optimize virological responses among this high-risk group.
Like all observational studies, the current analysis is subject to a number of limitations. First, ACCESS is not representative and may not be generalizable to other groups of HIV-infected PWUD. Second, there is the possibility for social desirability or recall biases due to the self-reported nature of non-clinical measures, which could result in under- or over-reporting prohibited income generation. However, we have no reason to believe individuals would over-report prohibited income generating activities. Any resulting biases would be conservative in nature and therefore underestimate the strength of the relationship between prohibited income generation and virologic suppression. Third, there is the potential for unmeasured confounding from factors not considered here. To mitigate this potential bias we have included as covariates several indicators found to be associated with ART adherence and virologic suppression in the current study context [15,20]. Finally, all available behavioral data were used in analyses to avoid biases associated with listwise or casewise data deletion methods [43].
In sum, alongside scientific evidence supporting the role of TasP-based approaches to the control of the HIV/AIDS pandemic [1], there is growing need to identify factors that affect optimal engagement in HIV care [13]. An emerging focus on employment [23] has thus far neglected to consider prohibited forms of income generation despite the significant proportion of HIV infected PWUD who engage in such activities. The current analysis begins to fill this gap, pointing to key barriers to optimal HIV care among PWUD by identifying a significant negative association between prohibited income generation and virologic suppression, a relationship that is partially mediated through the relationship between these activities and suboptimal adherence to ART. The socio-economic marginalization or HIV-infected PWUD may operate through the criminalization of this vulnerable population, the stigmatization of this key affected populations, and significant barriers to employment among PWH and PWUD [7,9,24,26]. Despite scientific advances in the treatment of HIV, these findings points to a pressing need for the implementation and evaluation of interventions and policy changes that mitigate pressures towards prohibited income generation. The promotion of adequate social assistance income and viable licit labour market opportunities such as low-threshold employment may result in potentially substantial clinical benefits and should therefore be a prominent focus in efforts to promote optimal engagement in HIV care.
Acknowledgments
Sources of support: This research was supported by funding from the National Institutes of Health [R01DA021525] and salary support for the investigators from the National Institutes of Health, the Canadian Institutes of Health Research, the Michael Smith Foundation for Health Research and British Columbia Ministry of Health.
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. LR and MJSM conceptualized the study, LR performed statistical analyses and prepared the first draft of the manuscript. MJSM provided significant advice on statistical analyses and on the writing of the manuscript. MJSM, EW, TK, JSGM, CP, SG, and SD provided critical feedback on initial and revised versions of the draft. The study was supported by the US National Institutes of Health (R01DA021525). This research was undertaken, in part, thanks to funding from the Canada Research Chairs programme through a Tier 1 Canada Research Chair in Inner City Medicine which supports EW. MJSM is supported in part by the United States National Institutes of Health (R01DA021525.) LR is supported by a Michael Smith Foundation for Health Research Scholar Award. JSGM is supported by the British Columbia Ministry of Health; through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse (NIDA) at the US National Institutes of Health (NIH); and through a KT Award from the Canadian Institutes of Health Research (CIHR).
References
- 1.Baggaley RF, White RG, Hollingsworth TD, Boily MC. Heterosexual HIV-1 infectiousness and antiretroviral use: systematic review of prospective studies of discordant couples. Epidemiology. 2013;24:110–21. doi: 10.1097/EDE.0b013e318276cad7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones A, Cremin I, Abdullah F, Idoko J, Cherutich P, Kilonzo N, et al. Transformation of HIV from pandemic to low-endemic levels: a public health approach to combination prevention. Lancet. 2014;384(9939):272–279. doi: 10.1016/S0140-6736(13)62230-8. [DOI] [PubMed] [Google Scholar]
- 3.DeGruttola V, Smith DM, Little SJ, Miller V. Developing and evaluating comprehensive HIV infection control strategies: issues and challenges. Clin Infect Dis. 2010;50 (Suppl 3):S102–107. doi: 10.1086/651480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montaner JS, Lima VD, Harrigan PR, Lourenço L, Yip B, Nosyk B, Wood E, Kerr T, Shannon K, Moore D, Hogg RS, Barrios R, Gilbert M, Krajden M, Gustafson R, Daly P, Kendall P. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” experience in a Canadian setting. PLoS One United States. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermund SH, Tique JA, Cassell HM, Pask ME, Ciampa PJ, Audet CM. Translation of biomedical prevention strategies for HIV: prospects and pitfalls. J Acquir Immune Defic Syndr. 2013;63(Suppl 1):S12–25. doi: 10.1097/QAI.0b013e31829202a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson D, Fraser N. Who pays and why? Costs, effectiveness, and feasibility of HIV treatment as prevention. Clin Infect Dis. 2014;59(Suppl 1):S28–31. doi: 10.1093/cid/ciu300. [DOI] [PubMed] [Google Scholar]
- 7.Mahajan AP, Sayles JN, Patel VA, Remien RH, Sawires SR, Ortiz DJ, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22 (Suppl 2):S67–79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krüsi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Policy. 2010;21(1):4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 9.International AIDS Society. Maximizing the benefits of antiretroviral therapy for key populations: A White Paper by the Key Affected Populations and Treatment as Prevention Working Groups of the International AIDS Society. Geneva: International AIDS Society; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyrer C. Global prevention of HIV infection for neglected populations: men who have sex with men. Clin Infect Dis. 2010;50 (Suppl 3):S108–113. doi: 10.1086/651481. [DOI] [PubMed] [Google Scholar]
- 11.Shannon K, Strathdee SA, Goldenberg SM, Duff P, Mwangi P, Rusakova M, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2014;6736(14):60931–60934. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milloy MJ, Montaner JS, Wood E. Incarceration of People Living with HIV/AIDS: Implications for Treatment-as-Prevention. Curr HIV/AIDS Rep. 2014;11(3):308–316. doi: 10.1007/s11904-014-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis United States. 2013;57(8):1164–71. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 14.Anema A, Kerr T, Milloy MJ, Feng C, Montaner JS, Wood E. Relationship between hunger, adherence to antiretroviral therapy and plasma HIV RNA suppression among HIV-positive illicit drug users in a Canadian setting. AIDS Care. 2014;26(4):459–465. doi: 10.1080/09540121.2013.832724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr T, Marshall BD, Milloy MJ, Zhang R, Guillemi S, Montaner JS, et al. Patterns of heroin and cocaine injection and plasma HIV-1 RNA suppression among a long-term cohort of injection drug users. Drug Alcohol Depend. 2012;124(1–2):108–112. doi: 10.1016/j.drugalcdep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood E, Kerr T, Tyndall MW, Montaner JS. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22(11):1247–1256. doi: 10.1097/QAD.0b013e3282fbd1ed. [DOI] [PubMed] [Google Scholar]
- 18.Milloy MJ, Marshall BD, Montaner J, Wood E. Housing status and the health of people living with HIV/AIDS. Curr HIV/AIDS Rep. 2012;9(4):364–374. doi: 10.1007/s11904-012-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011;52(Suppl 2):S238–246. doi: 10.1093/cid/ciq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milloy MJ, Kerr T, Buxton J, Rhodes T, Krusi A, Guillemi S, et al. Social and environmental predictors of plasma HIV RNA rebound among injection drug users treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59(4):393–399. doi: 10.1097/QAI.0b013e3182433288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milloy MJ, Kerr T, Buxton J, Rhodes T, Guillemi S, Hogg R, et al. Dose-response effect of incarceration events on nonadherence to HIV antiretroviral therapy among injection drug users. J Infect Dis. 2011;203(9):1215–1221. doi: 10.1093/infdis/jir032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Labour Office. The impact of employment on HIV treatment adherence. Geneva: International Labour Office; 2013. [Google Scholar]
- 23.Nachega J, Uthman O, Peltzer K, Richardson L, Mills E, Amekudzi K, Ouédraogo A. The Association of Antiretroviral Therapy Adherence and Employment Status in Men and Women from Low-, Middle- and High-Income Countries: A Systematic Review and Meta-Analysis. Bull World Health Organ. 2015;93:29–41. doi: 10.2471/BLT.14.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worthington C, O’Brien K, Zack E, McKee E, Oliver B. Enhancing Labour Force Participation for People Living with HIV: A Multi-Perspective Summary of the Research Evidence. AIDS Behav. 2012;16(1):231–243. doi: 10.1007/s10461-011-9986-y. [DOI] [PubMed] [Google Scholar]
- 25.Richardson L, Wood E, Kerr T. The impact of social, structural and physical environmental factors on transitions into employment among people who inject drugs. Soc Sci Med. 2013;76(1):126–133. doi: 10.1016/j.socscimed.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henkel D. Unemployment and substance use: a review of the literature (1990–2010) Curr Drug Abuse Rev. 2011;4(1):4–27. doi: 10.2174/1874473711104010004. [DOI] [PubMed] [Google Scholar]
- 27.DeBeck K, Shannon K, Wood E, Li K, Montaner J, Kerr T. Income generating activities of people who inject drugs. Drug Alcohol Depend. 2007;91(1):50–56. doi: 10.1016/j.drugalcdep.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr T, Small W, Johnston C, Li K, Montaner JS, Wood E. Characteristics of injection drug users who participate in drug dealing: implications for drug policy. J Psychoactive Drugs. 2008;40(2):147–152. doi: 10.1080/02791072.2008.10400624. [DOI] [PubMed] [Google Scholar]
- 29.Richardson LA, Milloy MJ, Kerr TH, Parashar S, Montaner JS, Wood E. Employment predicts decreased mortality among HIV-seropositive illicit drug users in a setting of universal HIV care. J Epidemiol Community Health. 2014;68(1):93–96. doi: 10.1136/jech-2013-202918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson L, Long C, Nguyen P, DeBeck K, Milloy MJ, Wood E, Kerr T. Socioeconomic marginalization in the structural production of vulnerability to violence among people who use illicit drugs. J Epidemiol Community Health. 2015 doi: 10.1136/jech-2014-205079. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BDL, Montaner JSG. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300(5):550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 32.Parnaby P. Disaster through Dirty Windshelds: Law, Order and Toronto’s Squeegee Kids. Can J Sociol. 2003;28(3):281–307. [Google Scholar]
- 33.Collins D, Blomley N. Law Commission of Canada, editor. New perspectives on the public-private divide. Vancouver: UBC Press; 2003. Politics, Poverty, and Anti-Panhandling By-Laws in Canadian Cities; pp. 40–67. [Google Scholar]
- 34.Sembiring E, Nitivattananon V. Sustainable solid waste management toward an inclusive society: Integration of the informal sector. Resour Conserv Recy. 2010;54(11):802–809. [Google Scholar]
- 35.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 36.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 37.Sobel ME. Asymptotic intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- 38.Debeck K, Wood E, Qi J, Fu E, McArthur D, Montaner J, et al. Interest in low-threshold employment among people who inject illicit drugs: implications for street disorder. Int J Drug Policy. 2011;22(5):376–384. doi: 10.1016/j.drugpo.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marmot M, Friel S, Bell R, Houweling TAJ, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 41.British Columbia Minsitry of Social Development and Social Innovation. Increases to Income Assistance Rate Tables. 2014 Aug 14; Retrieved from: http://www.eia.gov.bc.ca/factsheets/2007/increase_table.htm.
- 42.O’Brien KK, Bayoumi AM, Strike C, Young NL, Davis AM. Exploring disability from the perspective of adults living with HIV/AIDS: development of a conceptual framework. Health Qual Life Outcomes. 2008;6:76. doi: 10.1186/1477-7525-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beunckens C, Molenberghs G, Thij H, Verbeke G. Incomplete hierarchical data. Stat Methods Med Res. 2007;16:457–492. doi: 10.1177/0962280206075310. [DOI] [PubMed] [Google Scholar]