Abstract
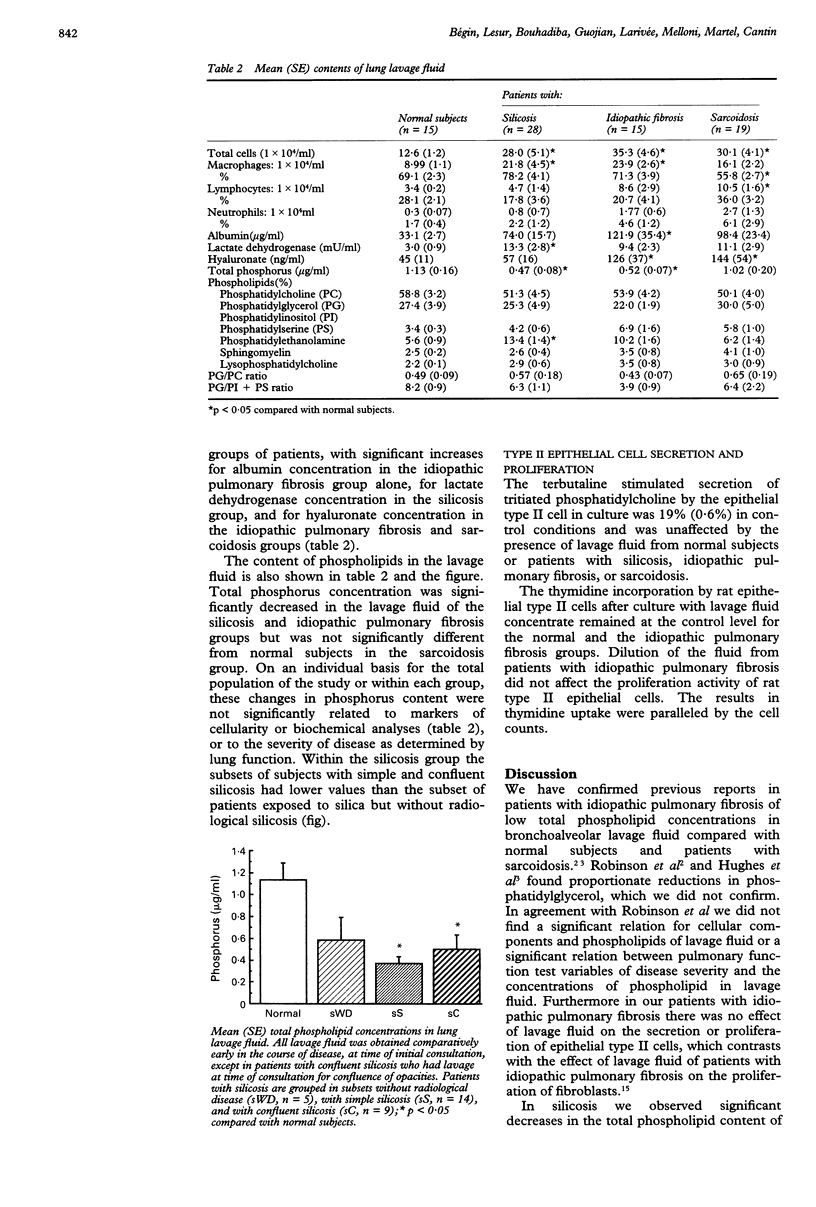
BACKGROUND--Some of the prominent features of silicosis are hyperplasia and hypertrophy of epithelial type II cells, which in experimental animals are often accompanied by accumulation of phospholipids in the lung. METHODS--The total phospholipid content of lung lavage fluid and its composition in 28 granite stone cutters with long term exposure to silica dust (23 with radiological silicosis) was compared with that of lavage fluid in 15 normal volunteers, 15 patients with untreated idiopathic pulmonary fibrosis, and 19 patients with untreated stage 2 or 3 sarcoidosis. All lavage fluid was obtained at the time of first pulmonary investigation, which also included lung function tests. RESULTS--In the normal subjects total phospholipid content was 1.13 (0.16) micrograms phosphorus/ml of lung lavage, in the patients with idiopathic pulmonary fibrosis 0.52 (0.07) microgram/ml (p < 0.05), and in the patients with sarcoidosis 1.02 (0.20) microgram/ml composition being in the range reported in humans. In the patients with silicosis total phospholipid content was significantly decreased to an average of 0.46 (0.08) microgram/ml compared with the findings in normal subjects and patients with sarcoidosis. Within the group exposed to silica changes in total phospholipid content did not correlate with the severity of the radiographic disease, changes in lung function, the cellularity of lung lavage fluid, or hyaluronate concentrations. The secretory capacity of rat epithelial type II cells was not significantly different when cultured with bronchoalveolar lavage fluid from all four groups of subjects. CONCLUSIONS--Total phospholipid content in lung lavage fluid was significantly reduced in granite workers with radiological evidence of lung disease, but showed no correlation with radiological or functional markers of disease severity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Brody A. R., Roe M. W., Evans J. N., Davis G. S. Deposition and translocation of inhaled silica in rats. Quantification of particle distribution, macrophage participation, and function. Lab Invest. 1982 Dec;47(6):533–542. [PubMed] [Google Scholar]
- Bégin R. O., Cantin A. M., Boileau R. D., Bisson G. Y. Spectrum of alveolitis in quartz-exposed human subjects. Chest. 1987 Dec;92(6):1061–1067. doi: 10.1378/chest.92.6.1061. [DOI] [PubMed] [Google Scholar]
- Bégin R., Cantin A., Massé S. Recent advances in the pathogenesis and clinical assessment of mineral dust pneumoconioses: asbestosis, silicosis and coal pneumoconiosis. Eur Respir J. 1989 Nov;2(10):988–1001. [PubMed] [Google Scholar]
- Bégin R., Dufresne A., Cantin A., Possmayer F., Sébastien P., Fabi D., Bilodeau G., Martel M., Bisson D., Pietrowski B. Quartz exposure, retention, and early silicosis in sheep. Exp Lung Res. 1989 May;15(3):409–428. doi: 10.3109/01902148909087868. [DOI] [PubMed] [Google Scholar]
- Bégin R., Possmayer F., Ormseth M. A., Martel M., Cantin A., Massé S. Effect of aluminum inhalation on alveolar phospholipid profiles in experimental silicosis. Lung. 1989;167(2):107–115. doi: 10.1007/BF02714937. [DOI] [PubMed] [Google Scholar]
- Calhoun W. J., Christman J. W., Ershler W. B., Graham W. G., Davis G. S. Raised immunoglobulin concentrations in bronchoalveolar lavage fluid of healthy granite workers. Thorax. 1986 Apr;41(4):266–273. doi: 10.1136/thx.41.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin A. M., Boileau R., Bégin R. Increased procollagen III aminoterminal peptide-related antigens and fibroblast growth signals in the lungs of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1988 Mar;137(3):572–578. doi: 10.1164/ajrccm/137.3.572. [DOI] [PubMed] [Google Scholar]
- Cantin A., Bégin R., Rola-Pleszczynski M., Boileau R. Heterogeneity of bronchoalveolar lavage cellularity in stage III pulmonary sarcoidosis. Chest. 1983 Mar;83(3):485–486. doi: 10.1378/chest.83.3.485. [DOI] [PubMed] [Google Scholar]
- Christman J. W., Emerson R. J., Graham W. G., Davis G. S. Mineral dust and cell recovery from the bronchoalveolar lavage of healthy Vermont granite workers. Am Rev Respir Dis. 1985 Aug;132(2):393–399. doi: 10.1164/arrd.1985.132.2.393. [DOI] [PubMed] [Google Scholar]
- Davis G. S. Pathogenesis of silicosis: current concepts and hypotheses. Lung. 1986;164(3):139–154. doi: 10.1007/BF02713638. [DOI] [PubMed] [Google Scholar]
- Dreisin R. B., Schwarz M. I., Theofilopoulos A. N., Stanford R. E. Circulating immune complexes in the idiopathic interstitial pneumonias. N Engl J Med. 1978 Feb 16;298(7):353–357. doi: 10.1056/NEJM197802162980701. [DOI] [PubMed] [Google Scholar]
- Dubois F., Bégin R., Cantin A., Massé S., Martel M., Bilodeau G., Dufresne A., Perreault G., Sébastien P. Aluminum inhalation reduces silicosis in a sheep model. Am Rev Respir Dis. 1988 May;137(5):1172–1179. doi: 10.1164/ajrccm/137.5.1172. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hallman M., Spragg R., Harrell J. H., Moser K. M., Gluck L. Evidence of lung surfactant abnormality in respiratory failure. Study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J Clin Invest. 1982 Sep;70(3):673–683. doi: 10.1172/JCI110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G., Fletcher K., Wyatt I. Abnormalities of lung lipids following inhalation of quartz. Experientia. 1972 Aug 15;28(8):938–939. doi: 10.1007/BF01924959. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G. The role of surfactant in the pulmonary reaction to mineral particles. Int J Exp Pathol. 1991 Oct;72(5):599–616. [PMC free article] [PubMed] [Google Scholar]
- Hughes D. A., Haslam P. L. Changes in phosphatidylglycerol in bronchoalveolar lavage fluids from patients with cryptogenic fibrosing alveolitis. Chest. 1989 Jan;95(1):82–89. doi: 10.1378/chest.95.1.82. [DOI] [PubMed] [Google Scholar]
- Laurent U. B., Tengblad A. Determination of hyaluronate in biological samples by a specific radioassay technique. Anal Biochem. 1980 Dec;109(2):386–394. doi: 10.1016/0003-2697(80)90665-x. [DOI] [PubMed] [Google Scholar]
- Lesur O., Cantin A. M., Tanswell A. K., Melloni B., Beaulieu J. F., Bégin R. Silica exposure induces cytotoxicity and proliferative activity of type II pneumocytes. Exp Lung Res. 1992 Mar-Apr;18(2):173–190. doi: 10.3109/01902149209031679. [DOI] [PubMed] [Google Scholar]
- Lesur O., Melloni B., Cantin A. M., Bégin R. Silica-exposed lung fluids have a proliferative activity for type II epithelial cells: a study on human and sheep alveolar fluids. Exp Lung Res. 1992 Sep-Oct;18(5):633–654. doi: 10.3109/01902149209031699. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Whitsett J. A. Inhibition of surfactant release from isolated Type II cells by compound 48/80. Biochim Biophys Acta. 1984 Nov 13;805(3):261–267. doi: 10.1016/0167-4889(84)90081-8. [DOI] [PubMed] [Google Scholar]
- Robinson P. C., Watters L. C., King T. E., Mason R. J. Idiopathic pulmonary fibrosis. Abnormalities in bronchoalveolar lavage fluid phospholipids. Am Rev Respir Dis. 1988 Mar;137(3):585–591. doi: 10.1164/ajrccm/137.3.585. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Schuyler M. R., Gaumer H. R., Stankus R. P., Kaimal J., Hoffmann E., Salvaggio J. E. Bronchoalveolar lavage in silicosis. Evidence of type II cell hyperplasia. Lung. 1980;157(2):95–102. [PubMed] [Google Scholar]
- VIGLIANI E. C., PERNIS B. Immunological factors in the pathogenesis of the hyaline tissue of silicosis. Br J Ind Med. 1958 Jan;15(1):8–14. doi: 10.1136/oem.15.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]


