Abstract
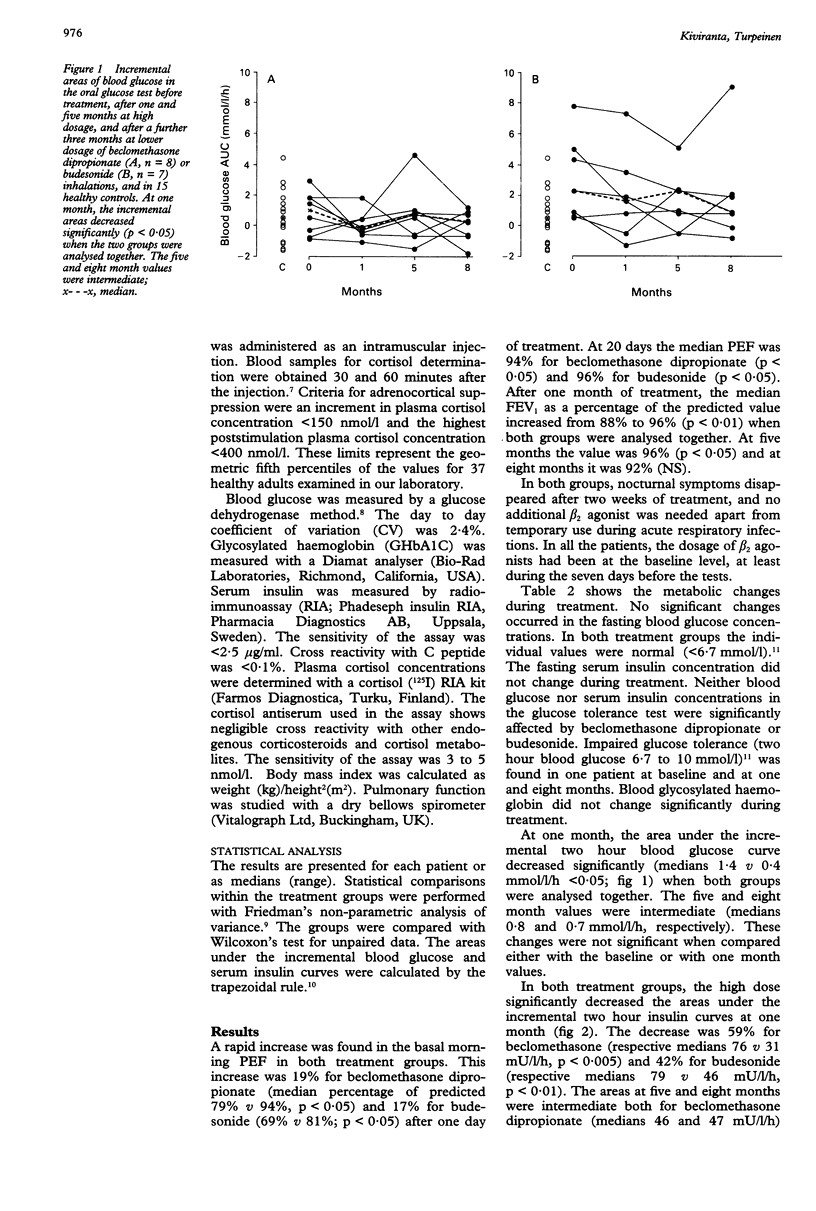
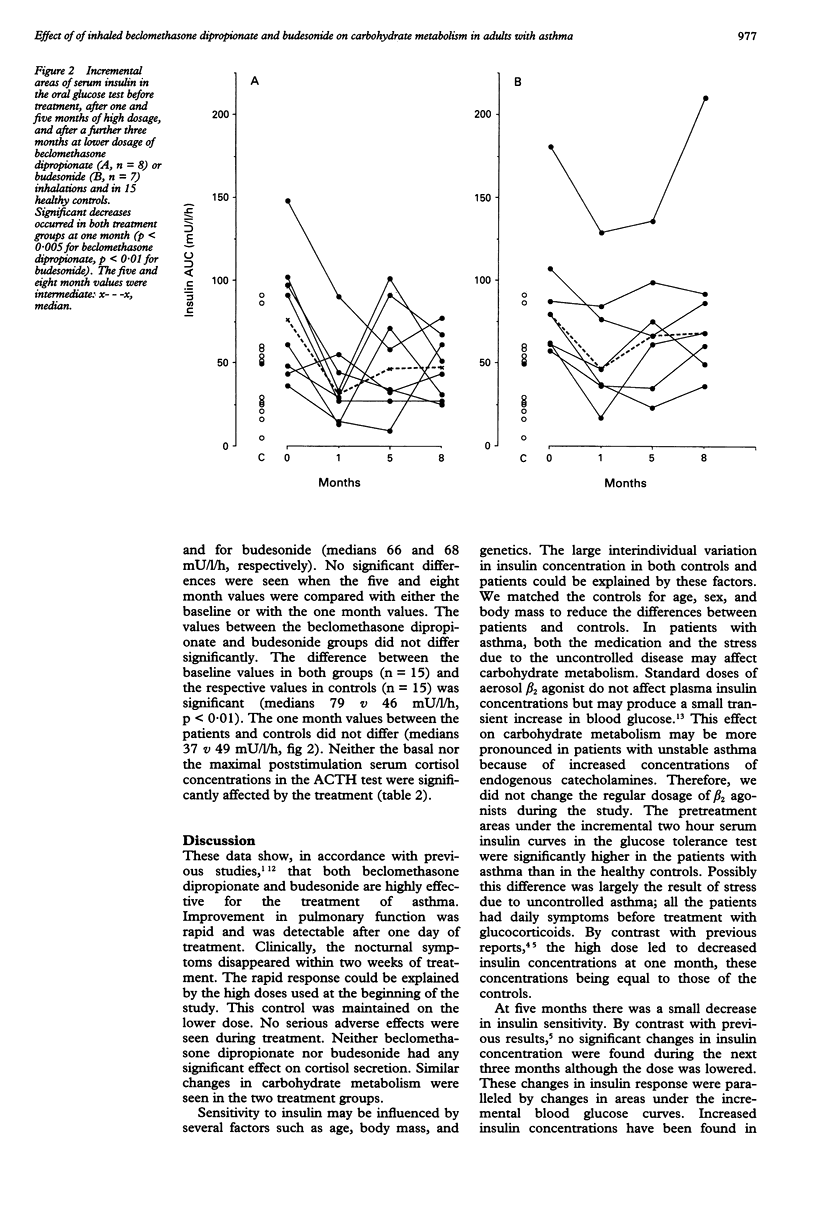
BACKGROUND--The safety of high dose inhaled steroids has been a subject of debate. The efficacy and safety of beclomethasone dipropionate and budesonide inhalations were evaluated by measuring their effects on pulmonary function, on the hypothalamic-pituitary-adrenocortical axis, and on carbohydrate metabolism in adults with unstable asthma. METHODS--Fifteen adults with unstable asthma and 15 healthy controls were studied. Eight patients were treated with beclomethasone dipropionate in initially high (2 mg/day for five months) and subsequently lower (1 mg/day for three months) doses. Seven patients were treated with budesonide at doses of 1.6 mg/day for five months followed by 0.8 mg/day for three months. Blood glucose and serum insulin were measured in an oral glucose tolerance test and plasma cortisol in an adrenocorticotrophic hormone test. The antiasthmatic effect of treatment was evaluated by measuring peak morning expiratory flow rates and forced expiratory volume in one second (FEV1). RESULTS--The FEV1 increased significantly after one month of treatment (medians 88% v 96%, p < 0.01), and nocturnal symptoms disappeared within two weeks of treatment in both groups. At one month, the high dose significantly decreased serum insulin concentrations as calculated from the areas under the incremental two hours curves in the glucose tolerance test. The decrease was 59% for beclomethasone dipropionate (medians 76 v 31 mU/l/h, p < 0.005) and 42% for budesonide (medians 79 v 46 mU/l/h, p < 0.01). The median areas at five and eight months were intermediate for both drugs. No significant differences were found when the five and eight month values were compared either with the baseline or with one month values. The difference between the baseline values of both groups and the respective values in healthy controls was significant (medians 79 v 49 mU/l/h, p < 0.01). The one month values for the patients and control subjects were similar. Paralleling the changes for insulin, the area under the incremental two hour blood glucose curve decreased significantly (medians 1.4 v 0.4 mmol/l/h, p < 0.05) during the first month of treatment. The five and eight month values were intermediate (medians 0.8 and 0.7 mmol/l/h, respectively). These changes were not significant compared with the baseline or the one month areas. Similar changes were seen in both treatment groups. Neither treatment had any significant effect on plasma cortisol in the one hour adrenocorticotrophic hormone test. CONCLUSIONS--In patients stressed by uncontrolled asthma, the antiasthmatic effect of high dose beclomethasone dipropionate and budesonide was accompanied by a significant initial decrease in insulin resistance with a parallel improvement in glucose tolerance. During prolonged treatment a small increase in insulin sensitivity was found. The overall effect of beclomethasone dipropionate and budesonide inhalations on carbohydrate metabolism may be beneficial in patients with uncontrolled asthma.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banauch D., Brümmer W., Ebeling W., Metz H., Rindfrey H., Lang H., Leybold K., Rick W., Staudinger H. J. Eine Glucose-Dehydrogenase für die Glucose-Bestimmung in Körperflüssigkeiten. Z Klin Chem Klin Biochem. 1975 Mar;13(3):101–107. [PubMed] [Google Scholar]
- Haahtela T., Järvinen M., Kava T., Kiviranta K., Koskinen S., Lehtonen K., Nikander K., Persson T., Reinikainen K., Selroos O. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991 Aug 8;325(6):388–392. doi: 10.1056/NEJM199108083250603. [DOI] [PubMed] [Google Scholar]
- Kruszynska Y. T., Greenstone M., Home P. D., Cooke N. J. Effect of high dose inhaled beclomethasone dipropionate on carbohydrate and lipid metabolism in normal subjects. Thorax. 1987 Nov;42(11):881–884. doi: 10.1136/thx.42.11.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. M., Marchant J. L., Honour J. W., Preece M. A., Warner J. O. Nocturnal adrenal suppression in asthmatic children taking inhaled beclomethasone dipropionate. Lancet. 1986 Apr 26;1(8487):942–944. doi: 10.1016/s0140-6736(86)91045-7. [DOI] [PubMed] [Google Scholar]
- Maynard D. E., Folk R. L., Riley T. R., Wieland R. G., Gwinup G., Hamwi G. J. A rapid test for adrenocortical insufficiency. Ann Intern Med. 1966 Mar;64(3):552–556. doi: 10.7326/0003-4819-64-3-552. [DOI] [PubMed] [Google Scholar]
- Meltzer E. O., Kemp J. P., Welch M. J., Orgel H. A. Effect of dosing schedule on efficacy of beclomethasone dipropionate aerosol in chronic asthma. Am Rev Respir Dis. 1985 May;131(5):732–736. doi: 10.1164/arrd.1985.131.5.732. [DOI] [PubMed] [Google Scholar]
- Neville A., Palmer J. B., Gaddie J., May C. S., Palmer K. N., Murchison L. E. Metabolic effects of salbutamol: comparison of aerosol and intravenous administration. Br Med J. 1977 Feb 12;1(6058):413–414. doi: 10.1136/bmj.1.6058.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthers O. D., Pedersen S. Growth of asthmatic children during treatment with budesonide: a double blind trial. BMJ. 1991 Jul 20;303(6795):163–165. doi: 10.1136/bmj.303.6795.163. [DOI] [PMC free article] [PubMed] [Google Scholar]


