Staphylococcus aureus is a Gram-positive microorganism responsible for a wide array of human pathologies, such as impetigo, pneumonia, meningitis, osteomyelitis, and toxic shock syndrome1-2. The severity of S. aureus infections is compounded by the high rate of resistance to individual as well as combinations of front line antibiotics, such as methicillin and vancomycin. One of the primary contributors to the virulence of S. aureus and many Gram-positive pathogens is the extracellular display of virulence and adhesion-associated proteins and enzymes. In Gram-positive bacteria, the sortase enzyme is responsible for covalently linking these proteins to the cell wall peptidoglycan1-3.
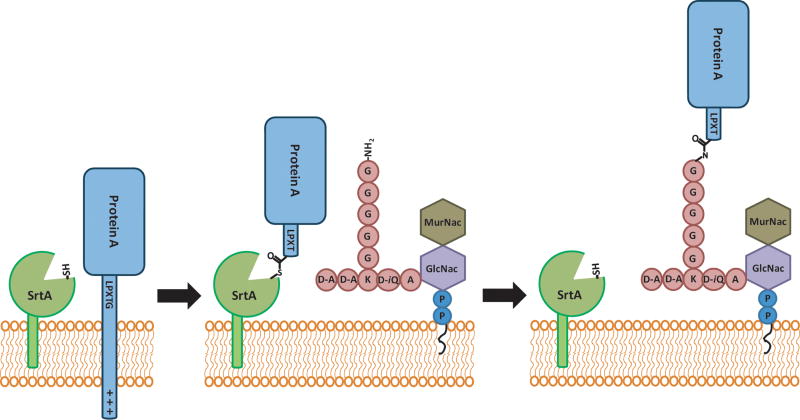
The pathogenesis and infectivity of Gram-positive bacteria are mediated by a multitude of surface protein virulence factors such as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) which facilitate adhesion to host endothelial tissues and evasion of host complement proteins and immunoglobulins3. Despite a variety of functions, these virulence factors are characterized by a common C-terminal pentapeptide sequence which acts as a cell wall sorting signal (CWSS) that enables their covalent attachment to the peptidoglycan layer of Gram-positive bacteria through a reaction catalyzed by the sortase family of transpeptidases (Figure 1)4. These cell wall sorting signals are composed of a tripartite recognition sequence5 including a pentapeptide motif (LPXTG, NPQTN and constantly emerging variants6) followed by a less well conserved hydrophobic domain ending in a basic region. The latter two regions are believed to contribute to membrane localization. The pentapeptide sorting signal is essential for cell wall anchoring to substrates such as the peptidoglycan intermediate Lipid II7-8, partially polymerized peptidoglycan9, and pilin subunits10- 16. This review will focus on the current understanding of the role of sortase in virulence, the specificity and molecular mechanism of the sortase-catalyzed transpeptidation reaction, and the present standing of the attempt to design inhibitors against sortase activity.
Figure 1.
The S. aureus SrtA reaction. S. aureus SrtA recognizes protein A by the conserved LPXTG motif. The reaction proceeds through a thioester acyl-enzyme intermediate. This intermediate is resolved by the attack of an amine nucleophile from pentaglycine branched Lipid II, covalently linking Lipid II to a protein substrate (e.g. Protein A) and regenerating SrtA.
Sortase Classification
Sortase transpeptidases can be grouped into general categories based on sequence alignment and predicted substrate preference (Table 1)5. Common to the sortase superfamily is an active site TLXTC motif, as well as several conserved charged residues. Many Gram-positive bacteria possess multiple sortases within their genome. For example, in S. aureus, two isoforms are found within the genome that are the protein products of the srtA and srtB genes.
Table 1.
General Sortase Isoforms. There are three characterized isoforms of sortase. SrtA is conserved across all Gram-positive bacteria, while SrtB and SrtC have more specialized functions and are only found in select species.
| Sortase Isoform | Recognition Sequence | Function | Pathogenic Species |
|---|---|---|---|
| A | LPXTG | General, “housekeeping” | All Gram-positive bacteria |
| B | NPQTN | Iron deprivation response | Bacilli, Listeria, S. aureus |
| C | QVPTG | Pilin polymerase | Streptococcus, C. diphtheriae, E.faecalis, B. cereus |
Sortase A (SrtA) is known as the ‘housekeeping sortase’ and is responsible for anchoring LPXTG-containing proteins to Lipid II, many of which are involved in pathogenesis. This includes a wide variety of proteins that are encoded across the genome. With the exception of srtA, which occurs as a monocistronic operon in most Gram-positive bacteria, other srt genes occur in operons that also encode their substrates. In S. aureus, sortase B (SrtB) is encoded in an iron deprivation response operon that includes isdA, isdB, and isdCDEF-srtB-isdG and is responsible for attaching the iron acquisition protein IsdC to the cell wall17. Specialized sortases of the recently discovered SrtC family are responsible for catalyzing transpeptidation reactions linking pilin subunits in bacteria such as group A streptococcus (GAS, Table 1) and related bacteria10,16. Pili (also called fimbriae) are filamentous protein structures protruding from the surface of bacteria, commonly composed of multiple subunits of a single major shaft or backbone protein (pilin). They often serve to adhere the bacteria to their environmental niche, which, in the case of pathogenic bacteria, is within the human host. These more specialized sortases have varied representation across species and even strains.
Sortase Biology
Sortase superfamily members have been shown to be integral to the virulence of Gram- positive bacteria. S. aureus SrtA knockouts lose their adherence to mammalian tissues through a loss of binding activity for IgG, fibronectin, and fibrinogen18. This results in a reduced ability to infect the host, as evidenced by lowered bacterial loads and vastly reduced mortality rates in a mouse model of infection19. Additionally, the SrtA knockout has a greatly diminished occurrence of septic arthritis in animal models of infection20. Loss of SrtB has a similar effect on virulence for numerous bacteria, although SrtB exhibits the greatest contribution to virulence during later stages of infection where iron may be a limited environmental resource21-22. SrtA and SrtC knockouts in pili-producing species attenuate the strains for infection and often impair biofilm formation20. In multiple Gram-positive species including Listeria monocytogenes23, Enterococcus faecalis24, Streptococcus pneumoniae25, and Streptococcus suis26-27, loss of sortase activity leads to significantly reduced infection potential in animal models of infection. The ability of the bacteria to persist after initial infection is also reduced in sortase knockouts. This is partially due to the loss of survival in macrophages following phagocytocis28-29. This poorly understood phenomenon may be due to the reduced surface protein presentation in sortase deletion mutants, as similar effects have been reproduced by knocking out individual MSCRAMMs.
Due to the prevalence of sortase enzymes in Gram-positive bacteria and the importance of their substrates to successful infection, sortases make an attractive antivirulence target. Pharmaceutical industry efforts to target anti-infectives against intracellular enzyme targets are often hampered by poor transport across the bacterial cell envelope. However, sortases reside within the cell membrane, making the enzyme potentially accessible to inhibitors. Sortase knockouts have no growth defects, suggesting that inhibition of these enzymes may place less selective pressure on bacterial survival as compared to enzymes essential for life processes, lowering the potential for resistance. Instead, sortase knockouts exhibit greatly attenuated virulence potential. The purported mechanism and sequence homology among sortases is significant, raising the hope for the discovery of class specific inhibitors for sortase activities. This possibility may be further enhanced by the lack of related sortase homologues in eukaryotes.
Sortase Structure
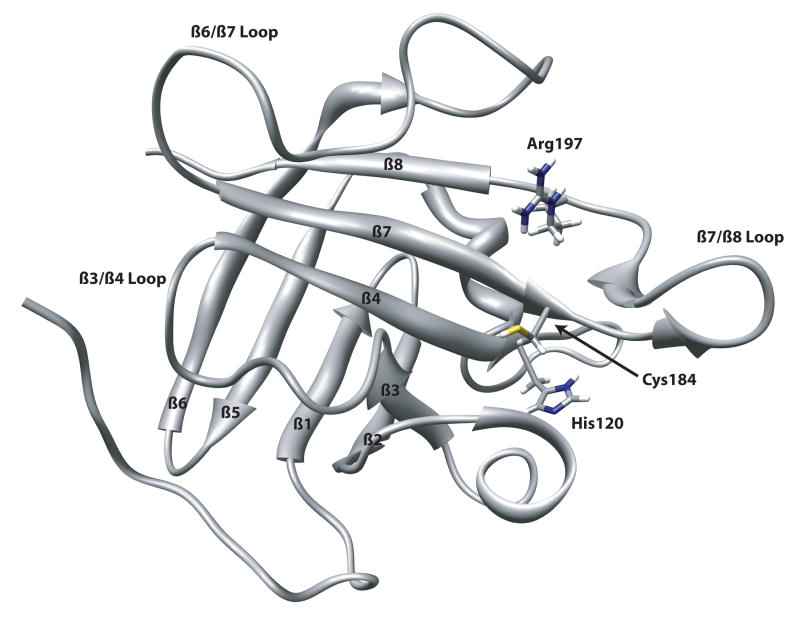
The first structure of a sortase transpeptidase was determined by Clubb and coworkers using NMR methods30-31 (Figure 2). The S. aureus SrtAΔN59 NMR structure (PDB ID 1IJA) reveals a novel protein fold common to all sortases for which structures have been deduced (Figure 3). The polypeptide backbone consists of an uneven eight stranded β barrel connected by random coil loops. The active site containing the catalytic residues H120, C184, and R197 sits at the end of a long groove along one side of the β barrel, but is still moderately solvent exposed. The walls of the groove are formed by the loops connecting the β2/β3, β3/β4, β6/β7, β7/β8 strands.
Figure 2.
NMR structure of S. aureus SrtA (PDB ID 1IJA) indicating the strand and loop numbering.
Figure 3.
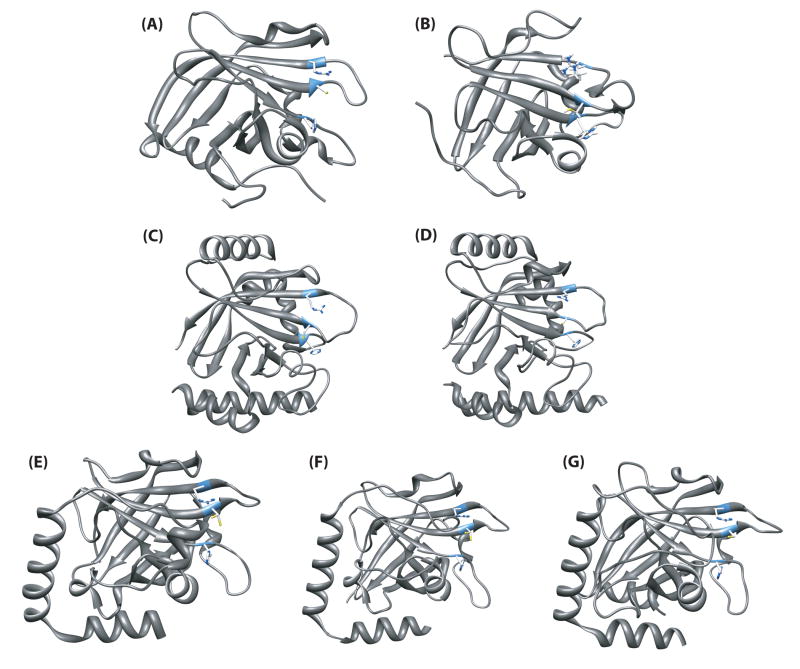
Representative structures of sortase transpeptidases solved to date. The microorganism, enzyme name, and PDB ID are as follows: (A) S. aureus SrtA (1IJA); (B) S. pyogenes SrtA (3FN5); (C) S. aureus SrtB (1NG5); (D) B. anthracis SrtB (1QXA); (E) S. pneumoniae SrtC-1 (2W1J); (F) S. pneumoniae SrtC-2 (3G66); (G) S. pneumoniae SrtC-3 (2W1K).
The structure was solved in the presence of the metal ion activator Ca2+, and thus a significant population of calculated structures positioned E171 (in the β6/β7 loop) oriented towards the putative Ca2+ binding site on the β3/β4 loop. This binding site is located at the rear of the groove, away from C184. The β6/β7 loop was found to be highly mobile, but dynamics were slowed upon Ca2+ binding, indicating metal ion induced stabilization of this loop. A crystal structure (PDB ID 1T2W) of a C184A mutant was solved shortly after which displayed a similar structure32. The crystal was soaked with a sorting signal peptide and one of the enzymes in the repeating trimer unit displayed electron density due to its binding. However, the structure remained virtually unchanged upon substrate binding and was crystallized in the absence of Ca2+. Based on the shift in dynamics upon binding of Ca2+ and substrate seen in the NMR studies, the lack of rearrangement of SrtA in the crystal structure precluded definitive determination of the mechanism of substrate binding. While this helped to localize the active site and important substrate recognition regions, functional predictions based on this structure did not agree with biochemical and genetic studies.
Structures of the S. aureus and B. anthracis SrtB enzymes (PDB ID 1NG5 and 2OQW, respectively) were subsequently determined33-34 (Figure 3). The “sortase fold” is conserved between isoforms, with a few prominent alterations (Figure 4). The SrtB enzymes contain far more α-helical secondary structures than the NMR or crystal structures of SrtA. However, both the core β barrel and configuration of the active site remain mostly intact. As SrtB is not activated by Ca2+ ions, the β6/β7 loop is preset in a “closed” conformation reminiscent of the β6/β7 loop in the presence of Ca2+ in the NMR SrtA structure (Figure 4). The loop is also much longer and contains an α-helix, possibly stabilizing the competent binding configuration without the aid of Ca2+. Analysis of the surfaces of the SrtB enzymes reveals that they are more polar than SrtA, paralleling their recognition of the more polar NPQTN sorting signal versus LPXTG in SrtA. Also, in the SrtB enzymes it is possible that the Cys and His active site residues are joined by an Asp to form a more conventional catalytic triad. To date, detailed mechanistic studies have not been performed on SrtB.
Figure 4.
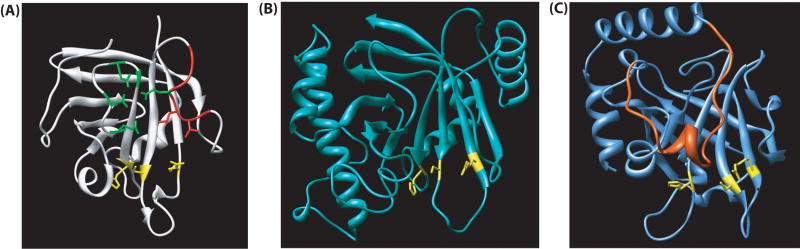
Sortase structure overview. (A) The conserved sortase-fold was first seen in S. aureus SrtA. Some essential features are color coded. The active site is displayed in yellow, Ca2+-binding residues are shown in green, and the β6/β7 loop is in red. (B) S. aureus SrtB contains the same overall sortase fold and arrangement of catalytic residues, with additional helices. (C) S. pneumoniae SrtC-1 also contains the sortase fold, but has an additional loop that lies across the substrate binding site (in orange).
Three pilin polymerase sortase crystal structures, SrtC-1, SrtC-2, and SrtC-3 from S pneumoniae, have been recently determined (PDB ID 2W1J, 3G66, 2W1K, respectively)14,35. The overall fold is very similar to all other known sortases, but it more closely resembles S. aureus SrtB with several conserved α-helical regions (Figures 3,4). However, there is one notable difference in the crystal structure of these enzymes. The N-terminal region has a long random coil that is positioned over the active site before forming the first β strand in the unliganded structure. The sequence of this coil somewhat resembles the LPXTG motif found in S. pneumoniae pilins and lies in the groove of the active site. This appears to act as a lid restricting access to the active site in the absence of some signal or activator (Figure 4C). The function of this lid is still under investigation, but this segment likely interacts with the substrate and may play a role in the temporal regulation of SrtA and SrtC that must be balanced to allow SrtC to polymerize pili before SrtA anchors them. It is not clear if this lid is positioned similarly in all pilin polymerase sortases.
Basis of Sortase Substrate Specificity
Sortases form peptide bonds between two different proteins, and thus they must recognize two different substrates in each reaction. The donor substrate protein is cleaved by the sortase within a specific sequence motif in the CWSS to provide a C-terminal carboxy group. An amine group of the second substrate serves as the acceptor nucleophile in the sortase-catalyzed transpeptidation reaction. Although there is significant structural and sequence homology between SrtA and SrtB of S. aureus, they do not exhibit cross-specificity for either of their substrates in vivo or in vitro2. The best-studied sortase, S. aureus SrtA, exhibits extraordinary specificity for Gly5-branched versus unbranched Lipid II as the acceptor7-8, and even as little as a single branching Gly addition to the Lys epsilon amino group of this molecule is sufficient to promote transpeptidation7. These S. aureus sortases also differ in specificity of the donor substrate: SrtA anchors proteins with an LPXTG motif in their CWSS, while SrtB anchors IsdC, an NPQTN-containing protein. Furthermore, IsdC cannot be anchored by SrtA17. In addition, neither of these types of sortases seems able to polymerize pilin proteins. It has been suggested that the lack of cross-specificity, coupled with the low expression of srtB under iron- deficient conditions, might indicate a highly specialized role for SrtB, which may be able to anchor only isd encoded proteins.
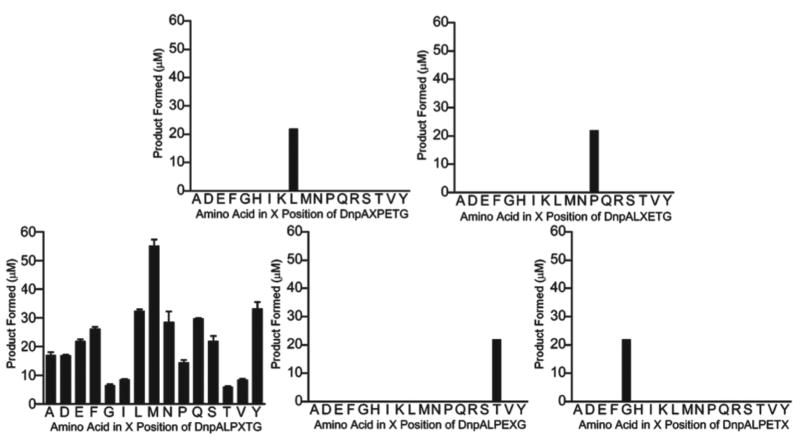
The CWSS resides within a tripartite motif containing a less conserved downstream hydrophobic domain and a highly basic sequence. Examination of individual microbial genomes indicates that even within the CWSS motifs of surface anchored proteins, significant variability is also observed. Seeking to address how positional variants within the LPXTG sequence motif were coupled to kinetic preferences for certain sequences, a positional scanning combinatorial approach36 was devised to examine the sorting signal sequence preference as an in vitro means of probing CWSS residue requirements (Figure 5). It was determined that SrtA kinetically preferred Leu, Pro, Thr, and Gly in positions 1, 2, 4, and 5, respectively, and had no amino acid preference in position. Increasing the incubation time revealed that more than one amino acid may be tolerated in several positions. In many cases these preferences match the identity of the sorting sequences of the 16 putative sortase substrates in the S. aureus genome. These data suggested that peptide libraries can be effectively used to probe the substrate specificity of sortases and both validate and compliment genetic approaches.
Figure 5.
Kinetic preference of SrtA for peptide substrate libraries where amino acids were varied by position within the LPXTG sequence. Results from initial rate experiments indicate a preference for residues of the consensus sorting motif.
The known structures of sortases indicate a very similar β-barrel overall fold, yet there are significant structural differences among the β2/β3, β6/β7 and β7/β8 loops (Figures 2-4). However, these enzymes only differ by minor sequence variations within these substrate recognition loops that flank the active sites. Multiple lines of evidence suggested that loops surrounding the active site contribute to the sortase specificity. Chemical shifts corresponding to the residues in the loop connecting the β6 strand to the β7 strand (161-176) were altered by NMR when interacting with a covalently attached peptide, supporting this theory37. Marked changes in the specificity profile of S. aureus SrtA were obtained by replacing the β6/β7 loop in SrtA with the corresponding domain from S. aureus SrtB. The chimeric β6/β7 loop swap enzyme (SrtLS) conferred the ability to acylate NPQTN-containing substrates, with a kcat/KMapp of 0.0062+/- 0.003 M−1s−1. This enzyme was unable to perform the transpeptidation stage of the reaction, suggesting that additional domains are required for transpeptidation to occur. The overall catalytic specificity profile (kcat/KMapp NPQTN/kcat/KMapp LPETG) of SrtLS was altered 700,000- fold from wild-type SrtA. Alanine scanning mutagenesis of this loop showed that V168 and L169 are essential for SrtA activity towards LPXTG38. This provides the basis for a model of substrate binding in which the protein contacts the sortase active site at Pro in LPXTG, redirecting the polypeptide chain towards C184. The residues immediately upstream of Pro contact the β6/β7 loop at positions 168 and 169. In SrtA, the hydrophobic residues V168 and L169 have affinity for the Leu in the LPXTG sequence. The corresponding polar residues N180 and R183 in S. aureus SrtB bind the upstream Asn in the NPQTN sequence of IsdC. E171 within the β6/β7 loop was also identified as a contributor to sortase activity, but its role is not in direct substrate contact39.
Clubb's S. aureus SrtA NMR structure postulated a Ca2+ binding site at the back of the active site30 (Figure 6). Indeed SrtA activity is stimulated 8-fold in vitro under Ca2+ concentrations equivalent to physiological levels. Biophysical analyses of SrtA Ca2+ complexation revealed that initial substrate binding was affected by, but did not absolutely require, Ca2+ binding39. A single Ca2+ molecule binds in a pocket formed by the β3/β4 loop (Figure 6). Contacts are provided by E105, E108, D112, and N114. The lone coordinating residue outside the β3/β4 loop is E171, which resides in the β6/β7 loop. The involvement of E171 further implicates the role of the β6/β7 loop in substrate recognition. The dynamics of the β6/β7 loop are significantly altered at physiological concentrations of Ca2+. The loop becomes much more stable in the presence of Ca2+, promoting substrate binding and correctly orienting it for attack by C184 through its contacts with the upstream portion of the CWSS39.
Figure 6.
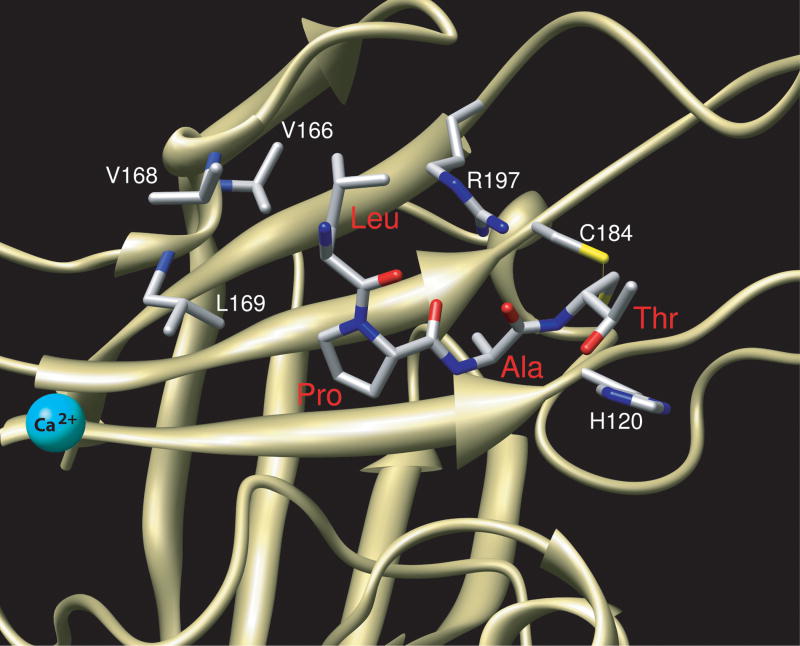
LPAT peptide bound to S. aureus SrtA. Leu and Pro of LPAT bind tightly to the β6/β7 loop, making contacts with V166, V168, and L169 of SrtA. The arrangement of H120, C184, and R197 in the active site is in agreement with functions predicted from biochemical analyses.
This overall model of substrate binding applies to all sortases yet studied, with a few caveats. Ca2+ is not necessarily required for full activity for other sortase enzymes. In fact, SrtA from S. pyogenes is slightly inhibited by Ca2+ 40. Also, SrtB does not require Ca2+ binding for activity. It is unknown if SrtC requires divalent cations for catalysis, as no in vitro kinetic analysis has been performed for this enzyme, although pilin polymerization activity has been recently demonstrated using a recombinant form of the enzyme from S. pneumoniae41. Additionally, the β7/β8 loop has not yet been analyzed in detail by mutagenesis or biophysical analysis for a contribution to substrate recognition. NMR studies have shown this loop to reorient itself in response to substrate binding39.
The recent S. aureus SrtAΔN59-LPAT* enzyme-substrate complex (PDB ID 2KID) structure solved by NMR currently provides the best view of the molecular basis for substrate recognition by the housekeeping sortases (Figure 6). As opposed to the earlier crystal structure with a bound peptide, this complex structure was solved in the presence of calcium ions42. Correspondingly, E171 is oriented towards the Ca2+ binding pocket. The H120 and C184 side chains are close enough to interact appropriately with the substrate to aid in catalysis. The R197 sidechain has a hydrogen-bonding interaction with the backbone carbonyl oxygen atoms at the Leu and Pro positions of the substrate, thereby stabilizing its binding and formation of the tetrahedral intermediate during catalysis. The β6/β7 loop is in the “closed” position, contacting the substrate at the Pro and Ala positions (Figure 6). In addition to the previously identified contacts with V168 and L169, V166 is also important for binding. V166 and V168 contact Leu of the substrate, while V166 and L169 contact the adjacent Pro residue of the substrate. Due to the highly dynamic nature of the β6/β7 loop, it is likely that an induced-fit mechanism of binding is at play in S. aureus SrtA. Ca2+ binding locks the β6/β7 loop in a more constrained conformation, but substrate binding further restricts the movement of this loop. A short 310 helix is induced from residues D165 to L169, providing the essential contacts for the substrate. However, other sortase structures seem to contain a preformed binding site, indicated by the lack of Ca2+-dependence and the presence of a helix in the β6/β7 loop in the absence of bound substrate.
Mechanistic Studies
Recent structural characterization of staphylococcal SrtA and related transpeptidases SrtB from S. aureus and B. anthracis provide many details regarding the active site environment, yet raise questions with regard to the nature of catalysis. To date, only the S. aureus SrtA, and to a lesser degree SrtB, enzyme mechanisms have been studied. Using a combination of pulse chase labeling and mass spectrometry, Schneewind and coworkers deduced that the S. aureus SrtA sorting reaction resulted in Thr-Gly cleavage within the LPXTG pentapeptide sorting motif and pentultimate covalent attachment of the Thr C-terminal residue to the terminal Gly of the pentaglycine peptidoglycan crossbridge43. Subsequent hydroxylamine nucleophile trapping experiments for S. aureus SrtA indicated a possible enzyme acyl intermediate, suggesting an enzymatic reaction mechanism exists akin to the reaction catalyzed by penicillin sensitive transpeptidases in bacterial cell wall peptidoglycan biosynthesis. The sole Cys residue (C184, S. aureus SrtA nomenclature) within the SrtA sequence was shown to be essential for transpeptidation based on inhibition by thiol-reactive electrophiles and mutagenesis44-46. Subsequent mutagenesis experiments of conserved residues within the sortase family members confirmed an essential role for H120 and R197 (S. aureus SrtA nomenclature), but insight into the specific functions of conserved residues to catalysis and substrate recognition proved more complex in light of the discrepancies in the position of key active site residues within the available sortase structures solved by NMR or X-ray crystallography methods38,40,47-48.
To lay the foundation for evaluation of the specificity and kinetic/catalytic mechanism of sortases, a discontinuous high-performance liquid chromatography (HPLC)-based assay was devised that can monitor both hydrolysis and transpeptidation49. Our laboratory examined the kinetic mechanism of SrtA to shed light on aspects of its catalytic mechanism50 (Figure 7). Using steady state, pre-steady state, and bi-substrate kinetic studies, and high-resolution electrospray mass spectrometry, a Ping-Pong hydrolytic shunt kinetic mechanism was determined for recombinant SrtA49-50 (Figure 7). The direct observation of an enzyme-acyl intermediate by mass spectrometry coupled with a pre-steady state burst kinetic profile for product formation and global kinetic fitting yielded accurate acylation, transpeptidation and hydrolysis rates as well as kinetic parameters that agreed well with values we previously obtained by steady-state methods 49.
Figure 7.
A Ping-Pong Bi Bi hydrolytic shunt kinetic mechanism for S. aureus SrtA.
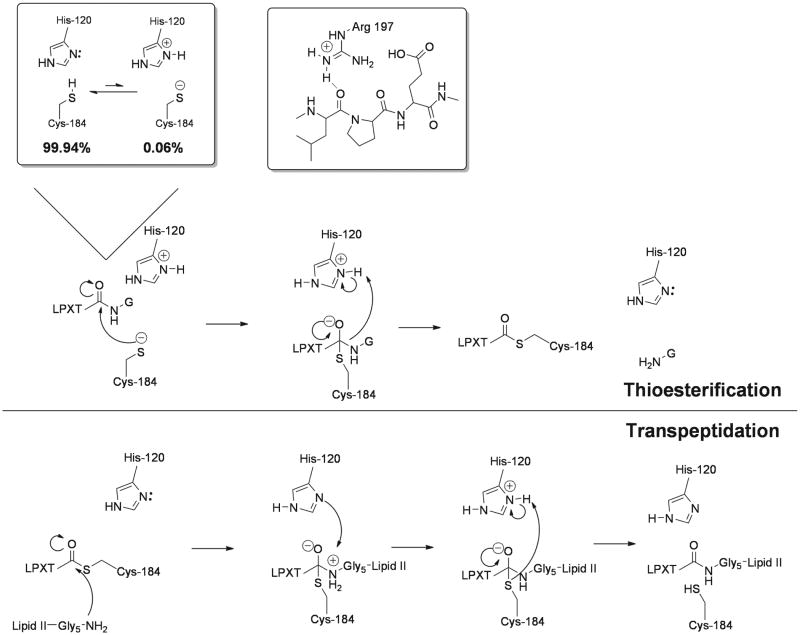
The pH dependencies of kinetic parameters kcat/KMapp and kcatapp for the substrate Abz-LPETG-Dap(Dnp)-NH2 were bell-shaped with pKa values of 6.3 ± 0.2 and 9.4 ± 0.2 for kcatapp and 6.2 ± 0.2 and 9.4 ± 0.2 for kcat/KMapp. Solvent kinetic isotope effect (SKIE) measurements revealed inverse behavior, with D2Okcatapp = 0.89 ± 0.01 and D2O(kcat/KMapp) = 0.57 ± 0.03 reflecting an equilibrium solvent kinetic isotope effect. In addition, SKIE measurements strongly implicated C184 participation in the isotope-sensitive rate-determining chemical step when considered in conjunction with an inverse linear proton inventory for kcatapp. Lastly, the pH dependence of SrtA inactivation by iodoacetamide revealed a single ionization for inactivation. These studies, combined with mutagenesis studies collectively provide compelling evidence for a novel mechanism for SrtA, a reverse protonation mechanism50-53 (Figure 8). In this mechanism, C184 thiolate is the active site nucleophile, H120 imidazolium is a general acid, and R197 is a transition state stabilizer (Figure 8). His and Cys are in equilibrium between thiol-imidazole and tholate-imidazolium forms, with the latter enzymatically active and believed to possess a significantly higher affinity for the substrate than the oppositely protonated enzyme form51-53. Collectively these studies provide compelling evidence for a reverse protonation mechanism where a small fraction (ca. 0.06%) of SrtA is competent for catalysis at physiological pH, yet is highly active with an estimated kcat/KM of >105 M−1s−1.
Figure 8.
S. aureus SrtA sortase transpeptidase employs a reverse protonation chemical mechanism for catalysis.
Reverse protonation takes into account a role for H120 and/or R197 without either residue directly affecting the thiol group of C184. This offers a plausible explanation for the slow apparent kinetic parameters observed. In this model, only ∼0.1% of SrtA is catalytically active at any given point, in a thiolate-imidazolium state. However, due to the extremely low population of this form, the majority of the cysteine residues are protonated. Rather than a classical role for H120 in a thiolate-imidazolium ion pair, the imidazolium is responsible for protonating the amine leaving group from the tetrahedral intermediate, facilitating the formation of the thioester acyl-enzyme intermediate (Figure 8).
To better understand the mechanisms of catalysis by the housekeeping sortase produced by the important human pathogen Streptococcus pyogenes, the crystal structure of this protein was determined40 (Figure 3). The structure reveals a novel arrangement of key catalytic residues in the active site of a sortase, the first that was consistent with kinetic analysis and is supportive of a reverse protonation chemical mechanism. The structure also provides a complete description of residue positions surrounding the active site, overcoming the limitation of localized disorder in previous structures of SrtA-type proteins.
In a separate study, to better define the role of R197 in S. aureus SrtA, the residue was replaced by citrulline (Cit), a steric homolog of Arg lacking a formal positive charge at physiological pH but still capable of hydrogen bonding. This SrtA mutant was synthesized by expressed protein native chemical ligation in which the N-terminal portion of the recombinant enzyme SrtA25-183 was expressed as a thioester homolog and the remaining 23 residues were made synthetically by solid phase peptide synthesis, producing Arg197Cit SrtA. It was observed that SrtA containing the Arg197Cit mutation was indeed fully active, with only a small decrease in kcat, a negligible increase in Km and a decrease in kcat/Km resulting in a 2.8-fold decrease in activity. R197 therefore does not function as a general base, but instead interacts with the substrate through hydrogen bonding as seen in Clubb's SrtA-substrate complex NMR structure (Figure 8).
The significant lifetime and solvent accessibility of sortase thioester intermediates combined with the high specificity of cleavage within the short pentapeptide CWSS has stimulated use of the enzyme as a reagent for chemoselective ligation and recombinant protein expression. In short, thioester intermediates formed upon the hydrolysis half reaction can be resolved by capture with exogenous nucleophiles. Applications for ‘sortagging’ or sortase-mediated chemoselective ligation include incorporation of non-native peptides and non-peptidic molecules into proteins, the generation of nucleic acid-peptide conjugates and neoglycoconjugates, protein cyclization, and labeling of cell surface proteins on living cells. Although discussion of biotechnology applications are beyond the scope of this review, this is a fertile research area that has recently been reviewed54-68.
Sortase Inhibitors
Due to the great interest in sortases as a target for anti-infective therapy, many studies toward finding a potent inhibitor have been conducted over the past decade. While the overall goal of these studies is to produce a novel antibiotic, attempts to find potential leads are in their infancy. For example, compounds that are potent inhibitors through non specific reaction with thiols can be used to study sortase, but are unlikely to be applicable to further drug design. Discovery of classes of molecules able to inhibit sortase is but the first step in the development of chemotherapeutics to be used in the clinic.
The discovery of a catalytically important Cys nucleophile and the inhibition of SrtA by general thiol affinity inactivators paved the way for the rational development of sortase inhibitors44. For example, peptidyl-diazomethanes, peptidyl-chloromethanes 69 and peptidyl-cyanoalkanes37 utilized the LPXTG conserved sequence containing a C-terminal thiol-reactive electrophile for affinity capture of the enzyme. Similarly, non-hydrolyzable phosphinate peptides70 as mimics of the scissile Thr-Gly bond of the LPXTG CWSS were shown to inhibit SrtA and also helped define the kinetic mechanism of the enzyme. Several structural classes of sortase inhibitors are depicted in Figure 9.
Figure 9.
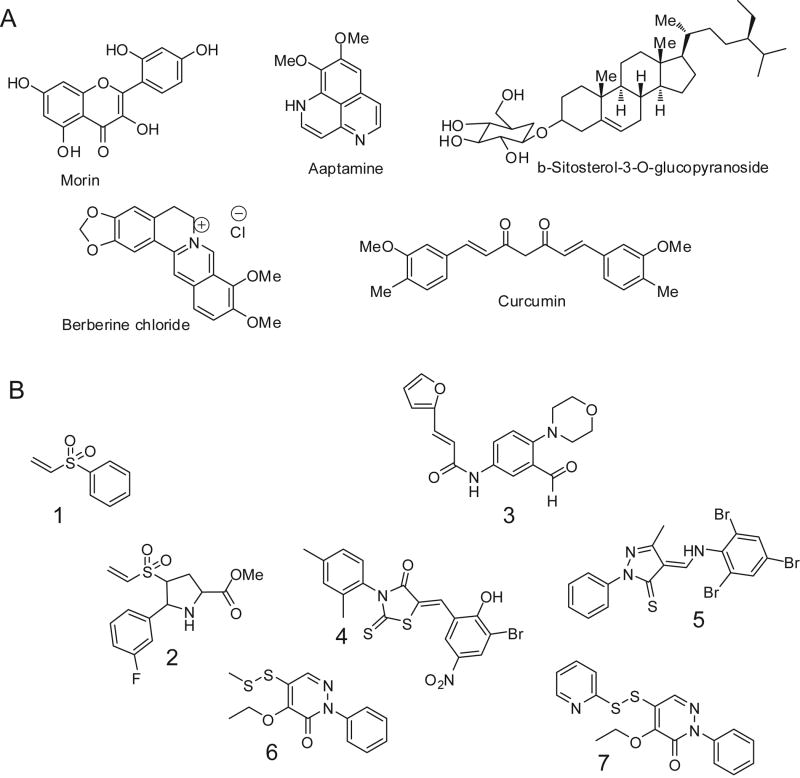
Representative small molecule inhibitors of SrtA: (A) Natural product inhibitor structures; (B) Inhibitors derived from rational design or screening.
Similarly, electrophilic small molecule vinyl sulfones, such as phenyl vinyl sulfone, 1, have been shown to act as irreversible time-dependent inhibitors of S. aureus SrtA in vitro71 (Figure 9). This class of small molecular SrtA inhibitors is the most well studied kinetically. Vinyl sulfones impair the ability of S. aureus bacteria to bind fibronectin-coated surfaces through in vivo inhibition of SrtA-mediated linkage of fibronectin to the cell surface. Vinyl sulfones also prevent Protein A anchoring, as evidenced by immunoflourescence methods. cis-5-Phenyl prolinates with electrophilic substituents at the fourth position of a pyrrolidine ring, such as 4-vinyl sulfonyl 5-phenyl prolinates72, inhibit S. aureus SrtA irreversibly with values of kinact/KI ranging from 1.5 to 2.2 × 104 M−1 min−1. In a physiologically-relevant setting, Sudeesh and coworkers recently showed that the sortase inhibitor phenyl vinyl sulfone inhibits Renibacterium salmoninarum adherence and invasion of host cells73. Vinyl sulfones are particularly effective at eliminating sortase activity in vivo, yet exhibit relatively poor apparent rate constants for inactivation or IC50 values. The explanation for this phenomenon lies in the reverse protonation mechanism of S. aureus SrtA where there is a very low population of the thiolate form of C184 (determined previously to be 0.06%)24. Thiolates react immediately upon collision with activated Michael acceptors, such as vinyl sulfones, whereas thiols are not activated towards alkylation by Michael addition. To better understand the efficiency of inhibition by this class of molecules, true kinact/KI values were determined from the apparent values by correcting for the fraction of enzyme in the enzymatically active thiolate-imidazolium form. The corrected values are more in synch with observed in vivo activity of these inhibitors.
An intriguing new class of mechanism-based inhibitors has recently been described by Schneewind and colleagues74. By screening 135,625 small molecules for inhibition, it was reported that aryl (beta-amino)ethyl ketones inhibit sortase enzymes from staphylococci and bacilli. Inhibition of sortases occurred through an irreversible, covalent modification of their active site cysteine. Sortases specifically activate this class of molecules via beta-elimination, generating a reactive olefin intermediate that covalently modifies the C184 thiol. Analysis of the three-dimensional structure of Bacillus anthracis sortase B with and without inhibitor provides insights into the mechanism of inhibition and reveals binding pockets that may be exploited for drug discovery. Similarly, in silico virtual screening of S. aureus SrtA against commercial compound libraries using FlexX software has led to the identification of novel inhibitors75 (Figure 9). Preliminary structure activity relationship studies on the lead compound resulted in the identification of compounds with improved activity. The most active compound, 3, exhibited an IC50 value of 58 μM against the enzyme.
In addition, natural product screening efforts have yielded interesting sortase inhibitors75-86 (Figure 9A). Many of these compounds have been isolated in the last five years from aquatic species. These compounds possess modest IC50 values, and also act well as general antimicrobials against S. aureus strains. Docking studies and subsequent high-throughput screens have delivered some promising inhibitors75. Rapid screening methods such as FRET assays are generally used in these cases rather than the more definitive HPLC assay which gives reliable concentrations of the substrate and product needed for detailed kinetic analysis.
In a recent study by Clubb and Jung87 based on a high-throughput screen, three lead compounds, along with many analogues, were assayed for SrtA inhibitory activity. The investigators discovered submicromolar inhibitors for SrtA based on pyridazinone and pyrazolethione scaffolds (4, 5, 6, and 7). In addition to sortase inhibition, these compounds were also tested for antimicrobial activity. Molecular docking studies with the more potent inhibitors gave insight into the electronics of the active site. Inhibitors with hydrophobic phenyl moieties were well tolerated. This is most likely due to the hydrophobic pocket created by the side chains of I199, V166 and V168, which is also seen in the substrate-bound NMR structure of SrtA42. Upon the addition of steric bulk (methyl groups or halogens), the rate of inhibition was decreased. This is presumably due to the unfavorable binding in the active site of SrtA. Other observations about the stereoelectronics of the active site were observed, including a potential electrostatic interaction between the carbonyl oxygen of compound 4 with R197. The most potent inhibitor, 5, has an IC50 of 200 nM87.
Conclusions
The sortase family of enzymes plays a pivotal role in the establishment and persistence of infections by Gram-positive microorganisms. As such, this class of transpeptidases may prove attractive targets for the development of inhibitors of cell wall protein attachment and pilin polymerization, processes critical for bacterial virulence. Although insight into the structure, mechanism, specificity and inhibition of sortases is significant at present, several pressing issues remain. Among others, these include: (1) establishing the molecular determinants of sortase substrate recognition with physiologically relevant protein substrates, especially in membrane environments, (2) deducing critical structural and mechanistic features of sortases that are full length, as opposed to truncated catalytic domains, (3) establishing the mechanism and timing of events in sortase-catalyzed pilin polymerization, (4) developing potent, isoform or class selective sortase inhibitors, (5) identifying critical complexes that interact with sortase, such as peptidoglycan synthesis and recycling machinery and the secretion apparatus, and (6) improving upon existing inhibitor leads for the development of next generation anti-virulence chemotherapeutics.
Acknowledgments
We gratefully acknowledge Prof. June R. Scott for her many contributions to our collaboration and the members of the McCafferty laboratory for critical and insightful discussions concerning this manuscript. This work was generously supported by research grant AI46611 from the National Institutes of Health Allergy and Infectious Disease Institute.
Footnotes
This paper is dedicated to Professor Stephen B. H. Kent on the occasion of his receipt of the 2009 Merrifield Award.
References
- 1.Marraffini LA, Dedent AC, Schneewind O. Microbiol Mol Biol Rev. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazmanian SK, Ton-That H, Schneewind O. Mol Microbiol. 2001;40:1049–1057. doi: 10.1046/j.1365-2958.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 3.Scott JR, Barnett TC. Annu Rev Microbiol. 2006;60:397–423. doi: 10.1146/annurev.micro.60.080805.142256. [DOI] [PubMed] [Google Scholar]
- 4.Fischetti VA, Pancholi V, Schneewind O. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 5.Comfort D, Clubb RT. Infect Immun. 2004;72:2710–2722. doi: 10.1128/IAI.72.5.2710-2722.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan SA, Kurian D, Ward PN, Hunt L, Leigh JA. J Proteome Res. 9:1088–1095. doi: 10.1021/pr901025w. [DOI] [PubMed] [Google Scholar]
- 7.Ruzin A, Severin A, Ritacco F, Tabei K, Singh G, Bradford PA, Siegel MM, Projan SJ, Shlaes DM. J Bacteriol. 2002;184:2141–2147. doi: 10.1128/JB.184.8.2141-2147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry AM, Ton-That H, Mazmanian SK, Schneewind O. J Biol Chem. 2002;277:16241–16248. doi: 10.1074/jbc.M109194200. [DOI] [PubMed] [Google Scholar]
- 9.Mazmanian SK, Ton-That H, Su K, Schneewind O. Proc Natl Acad Sci U S A. 2002;99:2293–2298. doi: 10.1073/pnas.032523999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ton-That H, Marraffini LA, Schneewind O. Mol Microbiol. 2004;53:251–261. doi: 10.1111/j.1365-2958.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- 11.Dramsi S, Caliot E, Bonne I, Guadagnini S, Prevost MC, Kojadinovic M, Lalioui L, Poyart C, Trieu-Cuot P. Mol Microbiol. 2006;60:1401–1413. doi: 10.1111/j.1365-2958.2006.05190.x. [DOI] [PubMed] [Google Scholar]
- 12.Swaminathan A, Mandlik A, Swierczynski A, Gaspar A, Das A, Ton-That H. Mol Microbiol. 2007;66:961–974. doi: 10.1111/j.1365-2958.2007.05968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falker S, Nelson AL, Morfeldt E, Jonas K, Hultenby K, Ries J, Melefors O, Normark S, Henriques-Normark B. Mol Microbiol. 2008;70:595–607. doi: 10.1111/j.1365-2958.2008.06396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzano C, Contreras-Martel C, El Mortaji L, Izore T, Fenel D, Vernet T, Schoehn G, Di Guilmi AM, Dessen A. Structure. 2008;16:1838–1848. doi: 10.1016/j.str.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Scott JR, Zahner D. Mol Microbiol. 2006;62:320–330. doi: 10.1111/j.1365-2958.2006.05279.x. [DOI] [PubMed] [Google Scholar]
- 16.Barnett TC, Patel AR, Scott JR. J Bacteriol. 2004;186:5865–5875. doi: 10.1128/JB.186.17.5865-5875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 18.Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O. Proc Natl Acad Sci U S A. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss WJ, Lenoy E, Murphy T, Tardio L, Burgio P, Projan SJ, Schneewind O, Alksne L. J Antimicrob Chemother. 2004;53:480–486. doi: 10.1093/jac/dkh078. [DOI] [PubMed] [Google Scholar]
- 20.Trotonda MP, Tamber S, Memmi G, Cheung AL. Infect Immun. 2008;76:5645–5654. doi: 10.1128/IAI.00735-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonsson IM, Mazmanian SK, Schneewind O, Verdrengh M, Bremell T, Tarko`wski A. J Infect Dis. 2002;185:1417–1424. doi: 10.1086/340503. [DOI] [PubMed] [Google Scholar]
- 22.Manetti AG, Zingaretti C, Falugi F, Capo S, Bombaci M, Bagnoli F, Gambellini G, Bensi G, Mora M, Edwards AM, Musser JM, Graviss EA, Telford JL, Grandi G, Margarit I. Mol Microbiol. 2007;64:968–983. doi: 10.1111/j.1365-2958.2007.05704.x. [DOI] [PubMed] [Google Scholar]
- 23.Bierne H, Mazmanian SK, Trost M, Pucciarelli MG, Liu G, Dehoux P, Jansch L, Garciadel Portillo F, Schneewind O, Cossart P. Mol Microbiol. 2002;43:869–881. doi: 10.1046/j.1365-2958.2002.02798.x. [DOI] [PubMed] [Google Scholar]
- 24.Kemp KD, Singh KV, Nallapareddy SR, Murray BE. Infect Immun. 2007;75:5399–5404. doi: 10.1128/IAI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paterson GK, Mitchell TJ. Microbes Infect. 2006;8:145–153. doi: 10.1016/j.micinf.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Vanier G, Sekizaki T, Dominguez-Punaro MC, Esgleas M, Osaki M, Takamatsu D, Segura M, Gottschalk M. Vet Microbiol. 2008;127:417–424. doi: 10.1016/j.vetmic.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Li M, Feng Y, Zheng F, Dong Y, Pan X, Cheng G, Dong R, Hu D, Feng X, Ge J, Liu D, Wang J, Cao M, Hu F, Tang J. Arch Microbiol. 2009;191:23–33. doi: 10.1007/s00203-008-0425-z. [DOI] [PubMed] [Google Scholar]
- 28.Zink SD, Burns DL. Infect Immun. 2005;73:5222–5228. doi: 10.1128/IAI.73.8.5222-5228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, Foster T, Potempa J. PLoS One. 2008;3:e1409. doi: 10.1371/journal.pone.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilangovan U, Ton-That H, Iwahara J, Schneewind O, Clubb RT. Proc Natl Acad Sci U S A. 2001;98:6056–6061. doi: 10.1073/pnas.101064198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilangovan U, Iwahara J, Ton-That H, Schneewind O, Clubb RT. J Biomol NMR. 2001;19:379–380. doi: 10.1023/a:1011299500628. [DOI] [PubMed] [Google Scholar]
- 32.Zong Y, Bice TW, Ton-That H, Schneewind O, Narayana SV. J Biol Chem. 2004;279:31383–31389. doi: 10.1074/jbc.M401374200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Wu R, Joachimiak G, Mazmanian SK, Missiakas DM, Gornicki P, Schneewind O, Joachimiak A. Structure. 2004;12:1147–1156. doi: 10.1016/j.str.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zong Y, Mazmanian SK, Schneewind O, Narayana SV. Structure. 2004;12:105–112. doi: 10.1016/j.str.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Neiers F, Madhurantakam C, Falker S, Manzano C, Dessen A, Normark S, Henriques-Normark B, Achour A. J Mol Biol. 2009 doi: 10.1107/S1744309108040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruger RG, Otvos B, Frankel BA, Bentley M, Dostal P, McCafferty DG. Biochemistry. 2004;43:1541–1551. doi: 10.1021/bi035920j. [DOI] [PubMed] [Google Scholar]
- 37.Liew CK, Smith BT, Pilpa R, Suree N, Ilangovan U, Connolly KM, Jung ME, Clubb RT. FEBS Lett. 2004;571:221–226. doi: 10.1016/j.febslet.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 38.Bentley ML, Lamb EC, McCafferty DG. J Biol Chem. 2008;283:14762–14771. doi: 10.1074/jbc.M800974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naik MT, Suree N, Ilangovan U, Liew CK, Thieu W, Campbell DO, Clemens JJ, Jung ME, Clubb RT. J Biol Chem. 2006;281:1817–1826. doi: 10.1074/jbc.M506123200. [DOI] [PubMed] [Google Scholar]
- 40.Race PR, Bentley ML, Melvin JA, Crow A, Hughes RK, Smith WD, Sessions RB, Kehoe MA, McCafferty DG, Banfield MJ. J Biol Chem. 2009;284:6924–6933. doi: 10.1074/jbc.M805406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neiers F, Madhurantakam C, Falker S, Normark S, Henriques-Normark B, Achour A. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:55–58. doi: 10.1107/S1744309108040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suree N, Liew CK, Villareal VA, Thieu W, Fadeev EA, Clemens JJ, Jung ME, Clubb RT. J Biol Chem. 2009 doi: 10.1074/jbc.M109.022624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazmanian SK, Liu G, Ton-That H, Schneewind O. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 44.Ton-That H, Liu G, Mazmanian SK, Faull KF, Schneewind O. Proc Natl Acad Sci U S A. 1999;96:12424–12429. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ton-That H, Schneewind O. J Biol Chem. 1999;274:24316–24320. doi: 10.1074/jbc.274.34.24316. [DOI] [PubMed] [Google Scholar]
- 46.Ton-That H, Mazmanian SK, Faull KF, Schneewind O. J Biol Chem. 2000;275:9876–9881. doi: 10.1074/jbc.275.13.9876. [DOI] [PubMed] [Google Scholar]
- 47.Frankel BA, Tong Y, Bentley ML, Fitzgerald MC, McCafferty DG. Biochemistry. 2007;46:7269–7278. doi: 10.1021/bi700448e. [DOI] [PubMed] [Google Scholar]
- 48.Connolly KM, Smith BT, Pilpa R, Ilangovan U, Jung ME, Clubb RT. J Biol Chem. 2003;278:34061–34065. doi: 10.1074/jbc.M305245200. [DOI] [PubMed] [Google Scholar]
- 49.Kruger RG, Dostal P, McCafferty DG. Anal Biochem. 2004;326:42–48. doi: 10.1016/j.ab.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Frankel BA, Kruger RG, Robinson DE, Kelleher NL, McCafferty DG. Biochemistry. 2005;44:11188–11200. doi: 10.1021/bi050141j. [DOI] [PubMed] [Google Scholar]
- 51.Sims PA, Larsen TM, Poyner RR, Cleland WW, Reed GH. Biochemistry. 2003;42:8298–8306. doi: 10.1021/bi0346345. [DOI] [PubMed] [Google Scholar]
- 52.Vocadlo DJ, Wicki J, Rupitz K, Withers SG. Biochemistry. 2002;41:9736–9746. doi: 10.1021/bi020078n. [DOI] [PubMed] [Google Scholar]
- 53.Mock WL, Stanford DJ. Biochemistry. 1996;35:7369–7377. doi: 10.1021/bi952827p. [DOI] [PubMed] [Google Scholar]
- 54.Proft T. Biotechnol Lett. 2009 [Google Scholar]
- 55.Pritz S, Wolf Y, Kraetke O, Klose J, Bienert M, Beyermann M. Adv Exp Med Biol. 2009;611:107–108. doi: 10.1007/978-0-387-73657-0_47. [DOI] [PubMed] [Google Scholar]
- 56.Popp MW, Antos JM, Ploegh HL. Curr Protoc Protein Sci. 2009;Chapter 15(Unit 15):13. doi: 10.1002/0471140864.ps1503s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobashigawa Y, Kumeta H, Ogura K, Inagaki F. J Biomol NMR. 2009;43:145–150. doi: 10.1007/s10858-008-9296-5. [DOI] [PubMed] [Google Scholar]
- 58.Guo X, Wang Q, Swarts BM, Guo Z. J Am Chem Soc. 2009 doi: 10.1021/ja903231v. [DOI] [PubMed] [Google Scholar]
- 59.Antos JM, Popp MW, Ernst R, Chew GL, Spooner E, Ploegh HL. J Biol Chem. 2009;284:16028–16036. doi: 10.1074/jbc.M901752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antos JM, Chew GL, Guimaraes CP, Yoder NC, Grotenbreg GM, Popp MW, Ploegh HL. J Am Chem Soc. 2009;131:10800–10801. doi: 10.1021/ja902681k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka T, Yamamoto T, Tsukiji S, Nagamune T. Chembiochem. 2008;9:802–807. doi: 10.1002/cbic.200700614. [DOI] [PubMed] [Google Scholar]
- 62.Samantaray S, Marathe U, Dasgupta S, Nandicoori VK, Roy RP. J Am Chem Soc. 2008;130:2132–2133. doi: 10.1021/ja077358g. [DOI] [PubMed] [Google Scholar]
- 63.Sadilkova L, Osicka R, Sulc M, Linhartova I, Novak P, Sebo P. Protein Sci. 2008;17:1834–1843. doi: 10.1110/ps.035733.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clow F, Fraser JD, Proft T. Biotechnol Lett. 2008;30:1603–1607. doi: 10.1007/s10529-008-9718-1. [DOI] [PubMed] [Google Scholar]
- 65.Antos JM, Miller GM, Grotenbreg GM, Ploegh HL. J Am Chem Soc. 2008;130:16338–16343. doi: 10.1021/ja806779e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popp MW, Antos JM, Grotenbreg GM, Spooner E, Ploegh HL. Nat Chem Biol. 2007;3:707–708. doi: 10.1038/nchembio.2007.31. [DOI] [PubMed] [Google Scholar]
- 67.Parthasarathy R, Subramanian S, Boder ET. Bioconjug Chem. 2007;18:469–476. doi: 10.1021/bc060339w. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen HD, Schumann W. J Biotechnol. 2006;122:473–482. doi: 10.1016/j.jbiotec.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Scott CJ, McDowell A, Martin SL, Lynas JF, Vandenbroeck K, Walker B. Biochem J. 2002;366:953–958. doi: 10.1042/BJ20020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kruger RG, Barkallah S, Frankel BA, McCafferty DG. Bioorg Med Chem. 2004;12:3723–3729. doi: 10.1016/j.bmc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 71.Frankel BA, Bentley M, Kruger RG, McCafferty DG. J Am Chem Soc. 2004;126:3404–3405. doi: 10.1021/ja0390294. [DOI] [PubMed] [Google Scholar]
- 72.Kudryavtsev KV, Bentley ML, McCafferty DG. Bioorg Med Chem. 2009;17:2886–2893. doi: 10.1016/j.bmc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sudheesh PS, Crane S, Cain KD, Strom MS. Dis Aquat Organ. 2007;78:115–127. doi: 10.3354/dao01859. [DOI] [PubMed] [Google Scholar]
- 74.Maresso AW, Wu R, Kern JW, Zhang R, Janik D, Missiakas DM, Duban ME, Joachimiak A, Schneewind O. J Biol Chem. 2007;282:23129–23139. doi: 10.1074/jbc.M701857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chenna BC, Shinkre BA, King JR, Lucius AL, Narayana SV, Velu SE. Bioorg Med Chem Lett. 2008;18:380–385. doi: 10.1016/j.bmcl.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 76.Maresso AW, Schneewind O. Pharmacol Rev. 2008;60:128–141. doi: 10.1124/pr.107.07110. [DOI] [PubMed] [Google Scholar]
- 77.Jung M, Jang KH, Kim B, Lee BH, Choi BW, Oh KB, Shin J. J Nat Prod. 2008;71:1714–1719. doi: 10.1021/np800321y. [DOI] [PubMed] [Google Scholar]
- 78.Suree N, Jung ME, Clubb RT. Mini Rev Med Chem. 2007;7:991–1000. doi: 10.2174/138955707782110097. [DOI] [PubMed] [Google Scholar]
- 79.Jang KH, Chung SC, Shin J, Lee SH, Kim TI, Lee HS, Oh KB. Bioorg Med Chem Lett. 2007;17:5366–5369. doi: 10.1016/j.bmcl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Oh KB, Oh MN, Kim JG, Shin DS, Shin J. Appl Microbiol Biotechnol. 2006;70:102–106. doi: 10.1007/s00253-005-0040-8. [DOI] [PubMed] [Google Scholar]
- 81.Kang SS, Kim JG, Lee TH, Oh KB. Biol Pharm Bull. 2006;29:1751–1755. doi: 10.1248/bpb.29.1751. [DOI] [PubMed] [Google Scholar]
- 82.Park BS, Kim JG, Kim MR, Lee SE, Takeoka GR, Oh KB, Kim JH. J Agric Food Chem. 2005;53:9005–9009. doi: 10.1021/jf051765z. [DOI] [PubMed] [Google Scholar]
- 83.Lee HS, Shin HJ, Jang KH, Kim TS, Oh KB, Shin J. J Nat Prod. 2005;68:623–625. doi: 10.1021/np040220g. [DOI] [PubMed] [Google Scholar]
- 84.Oh KB, Kim SH, Lee J, Cho WJ, Lee T, Kim S. J Med Chem. 2004;47:2418–2421. doi: 10.1021/jm0498708. [DOI] [PubMed] [Google Scholar]
- 85.Kim SH, Shin DS, Oh MN, Chung SC, Lee JS, Oh KB. Biosci Biotechnol Biochem. 2004;68:421–424. doi: 10.1271/bbb.68.421. [DOI] [PubMed] [Google Scholar]
- 86.Kim SH, Shin DS, Oh MN, Chung SC, Lee JS, Chang IM, Oh KB. Biosci Biotechnol Biochem. 2003;67:2477–2479. doi: 10.1271/bbb.67.2477. [DOI] [PubMed] [Google Scholar]
- 87.Suree N, Yi SW, Thieu W, Marohn M, Damoiseaux R, Chan A, Jung ME, Clubb RT. Bioorg Med Chem. 2009;17:7174–7185. doi: 10.1016/j.bmc.2009.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]