Abstract
Induction of drug-metabolizing enzymes through the antioxidant response element (ARE)-dependent transcription was initially implicated in chemoprevention against cancer by antioxidants. Recent progress in understanding the biology and mechanism of induction revealed a critical role of induction in cellular defense against electrophilic and oxidative stress. Induction is mediated through a novel signaling pathway via two regulatory proteins, the nuclear factor erythroid 2-related factor 2 (Nrf2) and the Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Keap1). Nrf2 binds to Keap1 at a two site-binding interface and is ubiquitinated by the Keap1/cullin 3/ring box protein-1-ubiquitin ligase, resulting in a rapid turnover of Nrf2 protein. Electrophiles and oxidants modify critical cysteine thiols of Keap1 and Nrf2 to inhibit Nrf2 ubiquitination, leading to Nrf2 activation and induction. Induction increases stress resistance critical for cell survival, because knockout of Nrf2 in mice increased susceptibility to a variety of toxicity and disease processes. Collateral to diverse functions of Nrf2, genome-wide search has led to the identification of a plethora of ARE-dependent genes regulated by Nrf2 in an inducer-, tissue-, and disease-dependent manner to control drug metabolism, antioxidant defense, stress response, proteasomal degradation, and cell proliferation. The protective nature of Nrf2 could also be hijacked in a number of pathological conditions by means of somatic mutation, epigenetic alteration, and accumulation of disruptor proteins, promoting drug resistance in cancer and pathologic liver features in autophagy deficiency. The repertoire of ARE inducers has expanded enormously; the therapeutic potential of the inducers has been examined beyond cancer prevention. Developing potent and specific ARE inducers and Nrf2 inhibitors holds certain new promise for the prevention and therapy against cancer, chronic disease, and toxicity.
I. Introduction
Electrophiles and oxidants are ubiquitous to aerobic organisms arising from both xenobiotic challenge and internal metabolism (Raymond and Segrè, 2006; Halliwell and Gutteridge, 2007; Ma, 2010). Although some electrophiles and oxidants have physiological functions participating in enzymatic reactions, cell signaling, and defense against microbes, many are capable of causing harm to the cell as a result of their reactive chemistry. Electrophiles, such as benzo-[a]pyrene arene oxide, could form DNA adducts through their electron-deficient centers to cause mutations. Oxidants, such as reactive oxygen species (ROS1), could damage macromolecules to cause lipid peroxidation, protein amino acid side-chain oxidation, and DNA single- and double-strand breaks. A growing body of evidence indicates that oxidants and electrophiles are among the principal agents responsible for the development of toxicity and diseases, such as cancer, chronic inflammatory disease, arthrosclerosis, neurodegeneration, and aging (Bossy-Wetzel et al., 2004; Finkel, 2005; Storz, 2005). On the other hand, most species have evolved with elaborate intrinsic strategies to cope with electrophilic and oxidative insults (Talalay et al., 1988; Kensler et al., 2007; Ma, 2010).
Drug-metabolizing enzymes (DMEs) metabolize and detoxify electrophiles and oxidants (Talalay et al., 2003; Ma and Lu, 2012). Cytochrome P450s catalyze the mono-oxygenation of chemicals, which often serves as the first step of metabolism. A group of heterogeneous enzymes carry out the “phase II reactions” that include 1) a nucleophilic trapping reaction between an electrophilic substrate and GSH through glutathione transferase (GST), 2) a nucleophilic reaction between an epoxide and water via epoxide hydrolase, 3) a conjugation reaction by UDP-glucuronosyl-transferase (UGT) and sulfotransferase that converts lipophilic chemicals to water-soluble glucuronide and sulfate conjugates to facilitate excretion through the bile and urine, and 4) a reduction reaction, such as the obligatory two-electron reduction of quinones and quinonoids by NAD(P)H:quinone oxidoreductase 1 (NQO1). Chemicals and their metabolites are transported out of cells through drug transporters for distribution and elimination. The antioxidant systems—including superoxide dismutase, catalase, glutathione peroxidase, peroxiredoxin, and thioredoxin—detoxify ROS directly.
Many DMEs are induced transcriptionally. Induction is controlled by a group of xenobiotic-activated receptors (XARs), transcription factors that are evolved from several phylogenetic families to mediate the adaptive response to chemical exposure (Ma, 2008). XARs include the basic helix-loop-helix--PER/ARNT/SIM (periodicity/aryl hydrocarbon receptor nuclear translocator/simple-minded) protein aryl hydrocarbon receptor (AhR) and the zinc finger nuclear receptors pregnane X receptor and constitutive androstane receptor. XARs control the induction of DMEs in response to elevated concentrations of specific chemicals to increase the rate of turnover of the chemicals. Induction subsides as the inducers are metabolized. In this manner, chemical sensing is directly coupled with transcription of the enzymes through an XAR, enabling the body to respond to a xenobiotic challenge quickly and as needed. Early studies revealed that planar aromatic hydrocarbons, such as benzo[a]pyrene (Bp), induce both CYP1A1 and the “phase 2” enzymes GST, NQO1, and UDP glucuronosyltransferase and are called bifunctional inducers, whereas, some other inducers, such as the antioxidant tert-butyl hydroquinone (tBHQ), induce “phase 2” enzymes only and are named monofunctional inducers (Talalay et al., 1978; Prochaska and Talalay, 1988). Induction by bifunctional inducers is mediated through AhR and requires the dioxin response element (DRE) located in the enhancers of the enzymes’ genes (Whitlock, 1999). Induction by monofunctional inducers, however, requires a XAR and DNA regulatory sequence distinct from AhR and DRE (Prochaska and Talalay, 1988; Rushmore and Pickett, 1990).
The DNA regulatory sequence responsible for induction by monofunctional inducers was identified as an antioxidant response element (ARE) (Rushmore and Pickett, 1990; Favreau and Pickett, 1991), also called the electrophile response element (Friling et al., 1990). ARE contains a consensus sequence 5′-TGACnnnGC-3′ and was found in the regulatory regions of rat and mouse Gst and Nqo1 genes. The XAR for induction by monofunctional inducers was identified in late 1990s from an unexpected source. The nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor similar in structure and DNA-binding activity to the nuclear factor erythroid 2 (NFE2), a transcription factor key to the regulation of β-globin expression during erythropoiesis and platelet development (Moi et al., 1994). Unlike NFE2, Nrf2 is expressed broadly and does not seem to be critical for hematopoiesis; instead, it was found to mediate the induction of ARE-dependent DMEs by monofunctional inducers (Venugopal and Jaiswal, 1996; Itoh et al., 1997).
The identification of Nrf2 as the XAR for induction of ARE-dependent DMEs spurred an array of subsequent studies that not only revealed the signaling pathway of induction but also provided significant insights into the molecular mechanism by which cells defend against electrophiles and oxidants. For example, molecular and structural analyses of the degradation of Nrf2 in un-stimulated cells and the activation of Nrf2 by monofunctional inducers shed light into the mechanisms that underlie the specificity, efficacy, and plasticity of chemical sensing and signal transduction toward oxidants and electrophiles by Nrf2. Targeted knockout of Nrf2 and Keap1 (Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1) in mice helped to reveal the involvement of the Nrf2-ARE pathway in the development of a variety of diseases and toxicity. Identification of a plethora of ARE-regulated genes at a genomic scale provided a rationale for the diverse functions of Nrf2. Last, but not least, developing new and potent ARE inducers led to new approaches for disease prevention and therapy. In retrospect, the past 15 years have witnessed a rapid advancement in the study of ARE-dependent gene regulation that has significantly influenced our understanding of the molecular basis of oxidative and electrophilic defense.
In this review, we discuss major issues with regard to transcriptional regulation of ARE-dependent genes through the Nrf2-ARE pathway, the function of Nrf2 in mammalian resistance to oxidants and electrophiles, and the preventive and therapeutic potentials of Nrf2 in the battle against cancer, chronic disease, and toxicity. The possibility that persistent activation of Nrf2 causes more harm than benefit under certain conditions is also discussed. Nrf2 target genes expand rapidly beyond DMEs, but all use ARE as a common regulatory element. In the following sections, we designate the genes induced by monofunctional inducers through Nrf2 as “ARE-dependent genes” and monofunctional inducers as “ARE-dependent inducers.”
II. The Nuclear Factor Erythroid 2-Related Factor 2–Antioxidant Response Element (Nrf2-ARE) Pathway: Finding the Xenobiotic-Activated Receptor
A. Antioxidant Response Element-Dependent Inducers
The interest in ARE-dependent inducers originated from the observation that phenolic antioxidants, such as butylated hydroxyanisole (BHA), a once widely used food additive in the United States, protected animals from the carcinogenic and toxic effects of polycyclic aromatic hydrocarbons, such as Bp, which led to the concept of “chemoprevention” (Wattenberg, 1972, 1985). Subsequently, BHA supplemented in rodent diet was found to inhibit the formation of mutagenic metabolites of Bp, and inhibition was associated with induction of GSTs and NQO1 (but not CYP1A1), paving the road for mechanistic study of chemoprevention (Benson et al., 1978; Pearson et al., 1983).
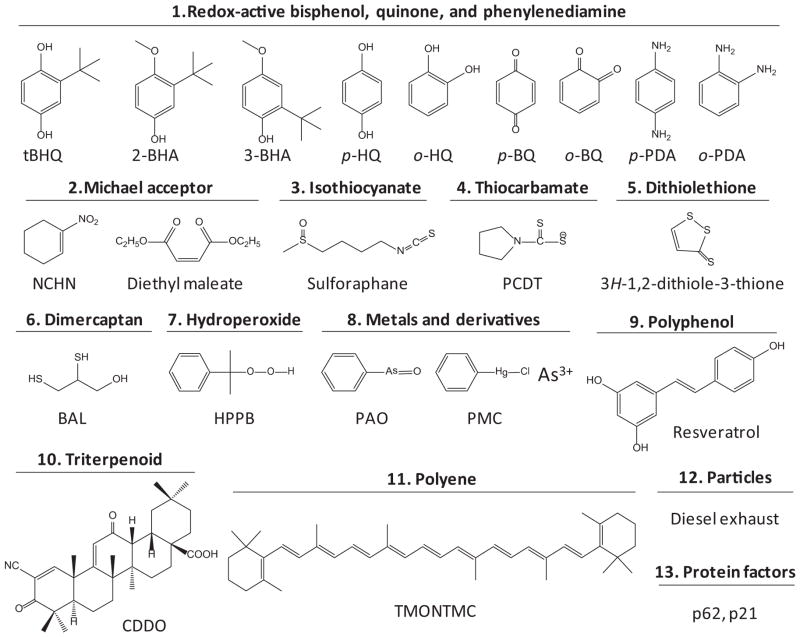
ARE inducers consist of several types of chemicals, including 1) redox-active bisphenols, quinones, and phenylenediamines; 2) Michael acceptors; 3) isothiocyanates; 4) thiocarbamates; 5) dithiolethiones; 6) dimercaptans; 7) hydroperoxides; 8) metals and derivatives; 9) polyphenols; 10) triterpenoids; 11) polyenes; 12) particles and fibers; and 13) protein factors (Fig. 1). The inducers differ considerably in structure but share certain chemical properties. Many inducers are electrophiles or redox-active compounds capable of modifying protein cysteine thiols by oxidation, reduction, alkylation, and metal chelation. This finding suggests that modification of protein cysteine thiols is a key mechanism of chemical sensing for ARE inducers (Talalay et al., 1988; Dinkova-Kostova et al., 2001).
Fig. 1.
Representative chemical types of ARE inducers. HQ, hydroquinone; BQ, benzoquinone; PDA, phenylenediamine; NCHN, 1-nitrocyclohex-1-ene; PCDT, pyrrolidine-1-carbodithioate; BAL, 2,3-dimercaptopropanol; HPPB, (2-hydroperoxypropan-2-yl)benzene; PAO, phenylarsine oxide; PMC, phenylmercury(II) chloride; TMONTMC, 2,2′-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(1,3,3-trimethylcyclohex-1-ene).
Some inducers, such as Michael reaction acceptors and metals, induce ARE-dependent DMEs by binding to the cysteine thiols of Keap1 and Nrf2. Polyphenols and triterpenoids—two large groups of inducers with complex structures—are listed as distinct groups even though they contain diphenol or Michael acceptor structures. Some inducers, such as BHA and β-naphthoflavone (βNF), acquire thiol-reactive properties after oxidative metabolism. BHA is converted to tBHQ through dealkylation to induce ARE-dependent genes. βNF, a bifunctional inducer, is oxidized to a redox-active quinone intermediate; therefore, βNF induces GSTA1 and NQO1 through a dual signaling mechanism: 1) direct binding to AhR to activate DRE-dependent transcription and 2) activating the Nrf2-ARE pathway through its quinone metabolites (Rushmore and Pickett, 1990). Fibers and particles may promote ARE-dependent gene expression via surface-bound chemicals that undergo redox cycling to generate ROS; alternatively, fibers and particles stimulate cells to produce endogenous inducing agents. Some proteins, such as autophagy substrate p62 and p53-regulated protein p21, are called “disruptor” proteins because they activate Nrf2 to induce ARE-dependent genes by disrupting Nrf2-Keap1 binding, comprising a novel group of endogenous inducers (Chen et al., 2009b; Komatsu et al., 2010).
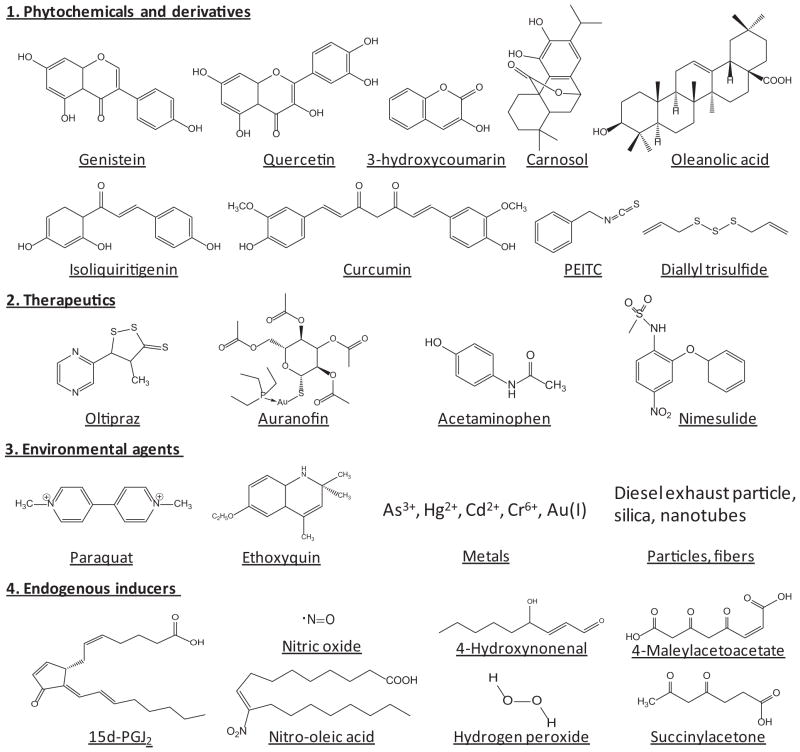
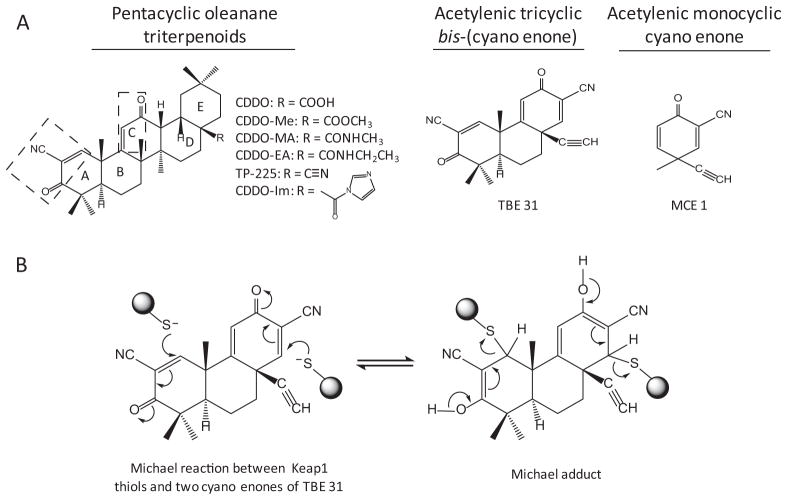
ARE inducers are derived from a variety of sources (Fig. 2). A large number of phytochemicals and derivatives are capable of inducing ARE-dependent genes and are chemopreventive. Oleanolic acid is a natural triterpenoid and is used in Chinese herbal medicine for treatment of liver disease. Synthetic triterpenoids, some of which contain the CDDO [2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid] structure, are among the most potent inducers of ARE-dependent genes examined to date (Dinkova-Kostova et al., 2005, 2010). Oltipraz, a substituted 1,2-dithiole-3-thione originally developed as an antischistosomal drug, induces GSTs and NQO1 and was identified as an effective chemopreventive agent in humans (Kensler et al., 2000). Induction of ARE-dependent genes by auranofin—a gold-containing antirheumatoid arthritis drug—is thought to help reduce drug toxicity in target joints (Kataoka et al., 2001). Many environmental chemicals and substances induce ARE-dependent genes as an adaptive response to toxic stimuli.
Fig. 2.
ARE inducers from different sources. PEITC, phenethyl isothiocyanate; 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2.
A growing list of endogenous inducers has been identified. The cyclopentenone prostaglandins 15-deoxy-Δ12,14-prostaglandin J2 and prostaglandin A2 activate Nrf2 to induce heme oxygenase 1 (HO-1) and peroxiredoxin 1, which contribute to the anti-inflammatory activity of Nrf2 (Itoh et al., 2004). Nitro-fatty acids are electrophilic signaling mediators formed in vivo via nitric oxide (NO)- and nitrite-dependent reactions, some of which are structurally similar to 15-deoxy-Δ12,14-prostaglandin J2 and activate Nrf2 by modifying Keap1 cysteine codes (Kansanen et al., 2011; Tsujita et al., 2011). 4-Hydroxynonenal, acrolein, and nitric oxide modify the alkenal and NO sensors of Keap1 (McMahon et al., 2010). As discussed, p62 and p21 induce ARE-dependent genes by interfering Nrf2-Keap1 binding. Although endogenous inducers are not as potent as synthetic triterpenoids for induction, their presence in target tissues at critical time points makes them important regulators of ARE-dependent genes in physiology and disease. To date, many ARE inducers have been examined for preventive and therapeutic applications in animals and some in humans.
B. Antioxidant Response Element and Antioxidant Response Element-Dependent Genes
1. Antioxidant Response Element
The 5′ regulatory sequence of the rat Gsta2 gene contains a 41-base pair enhancer sequence that was responsive to βNF in the presence of AhR but was different from DRE (Telakowski-Hopkins et al., 1988; Rushmore et al., 1990). The enhancer also mediated induction of the gene by tBHQ and redox-active diphenols independently of AhR, from which the name antioxidant response element was derived (Rushmore and Pickett, 1990; Rushmore et al., 1991). An identical sequence was found in the enhancer of the mouse Gsta1 gene for induction by tBHQ, dimethyl fumerate, and trans-4-phenyl-buten-2-one and was named the electrophile response element (Friling et al., 1990). Similar ARE sequences were found in rat and human NQO1 genes (Favreau and Pickett, 1991; Jaiswal, 1991) and several other DMEs and transporters (Ma, 2012). ARE was also required for the basal expression of these genes.
Deletion and mutation analyses of the 41-bp ARE of rat Gsta2 identified a core sequence, 5′-TGACnnnGC-3′, that is essential for induction by ARE inducers (Rushmore et al., 1991). The core sequence was extended into a 20-bp and later a 16-bp consensus sequence through comparison among AREs from rat, mouse, and human GST and NQO1 genes and mutation studies. The 16-bp consensus is expressed as 5′-TMAnnRTGAYnnnGCR-3′, where M = A or C, R = A or G, Y = C or T, W = A or T, and n = any base (Wasserman and Fahl, 1997; Nioi et al., 2003). The 5′-TMA (i.e., T-A/C-A) bases of the consensus seem to be necessary for induction of some ARE-dependent genes in addition to the core.
The 5′-TGAC-3′ tetranucleotide within the ARE core is similar to the half-site of the TPA (12-O-tetradecanoylphorbol 13-acetate) response element (TRE), 5′-TGAGTCA-3′. AP-1 proteins bind to TRE in response to phorbol esters, growth factors, and a variety of stress signals—such as UV light, metals, and oxidative stress—to induce “immediate response” genes. Although AP-1 proteins are capable of binding ARE in vitro with a low affinity, several lines of evidence distinguished ARE-dependent transcription from TRE-dependent transcription. First, the 3′-GC box of the ARE core sequence was critical for ARE inducibility and could separate the ARE core from TRE for induction by tBHQ or TPA (Nguyen et al., 1994). Second, many ARE inducers failed to activate TRE transcription but induced ARE transcription in cells that lacked endogenous TRE-binding proteins (Prestera and Talalay, 1995). Third, although tBHQ induced protein binding at both ARE and TRE, the major ARE-binding protein(s) activated by tBHQ was not AP-1; on the other hand, tBHQ induced c-Jun, junB, fra-1, and fra-2, which form Fra-containing inhibitory AP-1 complexes to antagonize the transcriptional effects of TPA at TRE by Jun-Fos heterodimer (Yoshioka et al., 1995).
ARE resembles the NFE2-binding motif, 5′-TGCTGAGTCAC-3′, found in the DNase 1 hypersensitive site 2 of the β-globin locus control region (Moi and Kan, 1990) and the enhancer of the porphobilinogen deaminase gene (Mignotte et al., 1989). NFE2 forms a heterodimer with a small Maf (musculoaponeurotic fibrosarcoma) protein to bind the sequence and control the transcription of the genes during hematopoiesis (Andrews et al., 1993a,b; Igarashi et al., 1994). The NFE2-binding motif contains TRE and can be bound by several transcription factors, including Nrf2. Nrf2 is not a natural binding protein of the NFE2-binding motif; however, as discussed below, the similarity between ARE and the NFE2-binding motif provided the initial clue that led to the identification of Nrf2 as a bona fide mediator of ARE transcription.
The core region of ARE also resembles the Maf recognition element (MARE). The palindromic MARE is either 5′-TGCTGAG/CTCAGCA-3′ (13 bp), which has a TRE in the core, or TGCTGAGC/CGTCAGCA (14 bp), which contains a cAMP response element in the core (Kataoka et al., 1994; Kerppola and Curran, 1994). MARE is recognized by a Maf homodimer that binds to the GC dinucleotide of MARE. Nrf2 forms a heterodimer with a small Maf to bind ARE, where Nrf2 recognizes the ARE core and the small Maf binds to the 3′-end GC dinucleotide of ARE (Kurokawa et al., 2009).
These findings suggest that the basic leucine zipper (bZip) proteins—such as NFE2, Nrf1, Nrf2, Nrf3, Bach1 (BTB and CNC homolog 1), Bach 2, small Mafs, AP-1 proteins, and cAMP-response element binding protein/ATF—exhibit both unique and overlapping specificities in DNA recognition and transcriptional regulation by means of dimerization and overlapping DNA recognition, which, in the case of Nrf2, expands its capacity to regulate the cellular defense against electrophilic and oxidative stress under physiological and disease conditions.
2. Antioxidant Response Element Dependent Genes
In recent years, genome-wide search for ARE-dependent genes has expanded the repertoire of ARE-dependent genes far beyond the originally studied DMEs (Kwak et al., 2003; Lee et al., 2003; Rangasamy et al., 2004; Shen et al., 2005; Hayes et al., 2010; Malhotra et al., 2010; He and Ma, 2012). ARE-dependent genes are involved in a variety of cellular functions. For example, Nrf2 controls the transcription of an array of DMEs and transporters, including oxidation enzymes (CYP2A5 and ALDH3A1), reduction enzymes (NQO1 and AKR1B3), conjugation enzymes (UGT1A1 and SULT3A1), enzymes catalyzing nucleophilic trapping reactions (GSTs, mEH, and ES-10), and multidrug resistance-associated proteins 2 and 3. By controlling the basal and inducible expression of DMEs and transporters, Nrf2 regulates the metabolic fate of numerous exogenous and endogenous chemicals. An emerging function of ARE-dependent genes is associated with antioxidant defense and oxidant signaling. In this context, Nrf2 regulates the cellular oxidant level and function by 1) increasing catabolism of oxidants (superoxide dismutase, peroxiredoxin, and glutathione peroxidase); 2) regeneration of oxidized redox factors (thioredoxin and GSSG); 3) synthesis of antioxidants and reducing equivalents (GSH and NADPH); 4) suppression of antioxidant inhibitors (thioredoxin-interacting protein); 5) induction of redox transporter proteins (xCT), metal-binding proteins (metallothioneins), and oxidative stress response proteins (HO-1); and 6) expression of signaling molecules for ROS-stimulated programs (p62 for autophagy deficiency and nuclear respiratory factor-1 for mitochondrial biogenesis) (Ma, 2013). Nrf2 also controls the expression of clusters of genes involved in proteasomal protein degradation, cell proliferation, and stress responses.
A combined analysis of Nrf2 chromatin immunoprecipitation sequencing profiling, basal target gene microarray [Nrf2(−/−) versus wild type], and inducible target gene microarray [Keap1(−/−) versus wild type] provided certain insights into direct and indirect gene regulation by Nrf2 (i.e., genes regulated by Nrf2 through binding to ARE versus genes regulated by Nrf2 but with no apparent Nrf2 binding) (Malhotra et al., 2010). A basal gene expression cluster for “cell proliferation” regulation was segregated from an inducible cluster for “stress response” in the analysis.
Transcriptional regulation of ARE-dependent genes through the ARE pathway has been experimentally demonstrated for some, but not all, of the genes. The identified ARE sequences exhibit certain heterogeneity: some have embedded “TRE” motif; some bear the 5′-TCA motif; some have the ARE core only; and some exist in more than one copy in a target gene. This heterogeneity of ARE sequences contributes to the functional diversity of the Nrf2-ARE pathway in the regulation of the basal and inducible expression of ARE-dependent genes. In addition, Nrf2 cross-interacts with several other signaling pathways—such as NF-κB, AhR, p53, and homeodomain transcription factors—which also expands the scope of Nrf2-regulated genes (Ma et al., 2004; Iida et al., 2007; Shin et al., 2007; Li et al., 2008; Pitha-Rowe et al., 2009; Malhotra et al., 2010).
C. Protein Components
The question in searching for the XAR that controls induction of ARE-dependent genes is 2-fold: 1) what transcription factor (or factors) bind ARE and mediate transcription and 2) what protein (or proteins) control the activation of the transcription factor by ARE inducers?
1. The Antioxidant Response Element-Binding Proteins, Nuclear Factor Erythroid 2-Related Factor 2 and Small Musculoaponeurotic Fibrosarcoma Proteins
Nrf2 was first cloned through expression cloning using a tandem NFE2-binding motif as a screening probe (Moi et al., 1994). On the basis of structure, Nrf2 belongs to the cap ‘n’ collar (CNC) bZip family of transcription factors, which includes NFE2 (Andrews et al., 1993a; Chan et al., 1993b), Nrf1 (Chan et al., 1993a), Nrf2, Nrf3 (Kobayashi et al., 1999), Bach1 (Oyake et al., 1996), and Bach2 (Muto et al., 1998). NFE2, the founding member of the group, controls the developmental expression of hematopoietic genes, such as β globin and porphobilinogen deaminase. Disruption of NFE2 resulted in a severe defect in platelet formation (Shivdasani and Orkin, 1995). Nrf1 was identified by virtue of its binding to NFE2-binding motif and is expressed broadly in mammalian tissues. Knockout of Nrf1 is lethal to embryos in mice as a result of anemia and severe liver damage at midgestation (Chan et al., 1998). Like Nrf1, Nrf2 is expressed in a broad range of tissues, but disruption of Nrf2 in mice did not cause severe phenotypes in the early life of the mice; therefore, Nrf2 was initially thought to be dispensable for mouse development (Chan et al., 1996).
The close resemblance between NFE2-binding motif and human NQO1 ARE, in conjunction with a similar tissue expression pattern among Nrf1, Nrf2, and NQO1, raised the possibility that the Nrf proteins modulate ARE-mediated induction of human NQO1. Indeed, overexpression of Nrf1 or Nrf2 significantly increased induction of ARE-driven reporter expression by βNF and tBHQ (Venugopal and Jaiswal, 1996). This notion was examined genetically in a separate study (Itoh et al., 1997). Compared with wild type, the basal expression and induction of GSTs and NQO1 by phenolic antioxidants in the liver and intestine of Nrf2-null mice was largely eliminated, and Nrf2 was shown to heterodimerize with a small Maf protein to bind to ARE. These and subsequent studies from several laboratories provided evidence to establish Nrf2 as the bona fide transcription factor that mediates ARE-driven basal expression and induction of ARE-dependent genes.
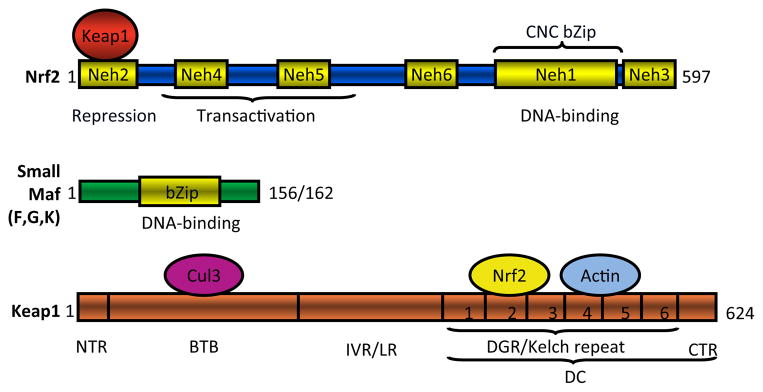
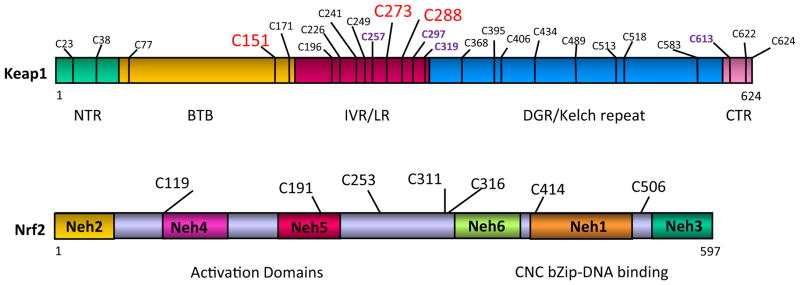
The human Nrf2 protein consists of 605 or 589 amino acids, and mouse Nrf2 protein consists of 597 or 581 amino acids, depending on the starting codon used (Moi et al., 1994) (Fig. 3). Nrf2 protein has a predicted molecular mass of ~66 kDa but shows an apparent molecular mass of ~96 kDa on SDS-polyacrylamide gel electrophoresis, possibly because of the abundance of acidic amino acid residues and/or post-transcriptional modifications. Nrf2 contains a bZip motif in the C-terminal half; the basic region is responsible for DNA recognition, whereas the leucine zipper mediates dimerization with a small Maf protein. Juxtaposed at the N terminus of bZip is a region of approximately 36 amino acids (termed CNC because of its homology with CNC, a Drosophila melanogaster segmentation protein) and other CNC bZip proteins (Mohler et al., 1991; Moi et al., 1994). Comparison between human and chicken Nrf2 amino acid sequences led to the identification of six highly conserved domains designated Neh (Nrf2-ECH homology) domains 1 through 6 (ECH = erythroid cell-derived protein with CNC homology = chicken Nrf2) (Itoh et al., 1995). CNC bZip is within Neh1. Located within the N-terminal half are Neh4 and Neh5, which confer transactivation activities individually and synergistically; transactivation involves cooperative binding of the domains with coactivator CBP (Katoh et al., 2001). Neh3 at the C terminus of Nrf2 also assists transactivation through interaction with chromatin remodeling protein CHD6 (Nioi et al., 2005). Neh6 is localized between Neh5 and Neh1 and may function as a degron to mediate degradation of Nrf2 in the nucleus (McMahon et al., 2004). Neh2 at the N terminus was particularly noted because it is highly conserved across species, and deletion of Neh2 resulted in a marked increase in Nrf2 transactivation activity; moreover, negative regulation by Neh2 could be antagonized by coexpression of a Neh2-GBD fusion protein, suggesting that Neh2 negatively regulates Nrf2 by binding to a repressor protein (Itoh et al., 1999). A bipartite nuclear localization signal was identified between residues 494 and 511 and mediates nuclear import of Nrf2, whereas a nuclear export signal was found between residues 545 and 554 for nuclear export of the protein (Jain et al., 2005).
Fig. 3.
Domain structures of mouse Nrf2, small Mafs, and Keap1. NTR, N-terminal region.
The Maf proteins consist of large Mafs (v-Maf, c-Maf, MafA, MafB, and NRL) and small Mafs (MafF, MafG, MafK, and MafT) (Motohashi et al., 1997; Yang and Cvekl, 2007). The founding member of the group, v-Maf, is an oncogene encoding the transforming component of the avian musculoaponeurotic fibrosarcoma virus AS42 (Nishizawa et al., 1989). Maf proteins contain a bZip domain for DNA binding and dimerization (Fig. 3). Large Mafs have a distinctive acidic transactivation domain in the N-terminal region, whereas small Mafs (~18 kDa) lack such a transactivation domain. Small Mafs mainly form heterodimers with CNC bZip transcription factors in which the CNC bZip protein binds to the core sequence of a DNA response element, such as ARE, and provides the transcriptional activation function, whereas the small Maf binds to the GC dinucleotide of the elements contributing to high affinity and specific DNA-binding (Kurokawa et al., 2009).
2. The Cytoplasmic Repressor Kelch-Like Erythroid Cell-Derived Protein with CNC Homology-Associated Protein 1 (Keap1)
The modular and titratable inhibitory activity of Nrf2 Neh2 domain led to a search for the repressor protein(s) that inhibit(s) Nrf2 through Neh2. From a yeast two-hybrid screening using Neh2 as a bait, a novel cytoplasmic protein was identified and was named Keap1 for Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Itoh et al., 1999), because of its homology with D. melanogaster actin-binding protein Kelch (Xue and Cooley, 1993). Several lines of evidence support that Keap1 is a bona fide regulator of Nrf2 and a sensor of ARE inducers. Keap1 has a broad expression pattern similar to that of Nrf2, binds to Neh2 both in vitro and in vivo, and suppresses Nrf2 transactivation activity in a number of assays. Suppression of Nrf2 by Keap1 is liberated by electrophilic agents. Knockout or knockdown of Keap1 results in constitutive activation of Nrf2 (Itoh et al., 1999; Wakabayashi et al., 2003). Molecular elucidation of Keap1-Nrf2 interaction uncovered a novel mechanism of chemical sensing and signaling for induction of ARE-dependent genes by electrophiles and oxidants.
Both human and mouse Keap1 proteins are polypeptides of 624 amino acid residues with a predicted molecular mass of ~68 kDa (Fig. 3). Keap1 has two readily recognizable canonical protein-protein interacting motifs: a BTB (Bric-a-brac, tramtrack, broad-complex) domain [amino acid (a.a.) residues 61–179] and a DGR (double glycine repeat) domain (a.a. 315–598) (also called Kelch domain because of its six Kelch repeats) (Itoh et al., 1999). In addition, Keap1 contains an N-terminal region (a.a. 1–60) domain at the N terminus, an IVR (intervening region) domain (a.a. 180–314) between BTB and DGR (also called linker region), and a CTR (C-terminal region) domain (a.a. 599–624) at the C terminus. The BTB domain and the N-terminal portion of IVR mediate homodimerization of Keap1 as well as binding with Cul3. The DGR and CTR together form a six-bladed propeller structure and are therefore defined jointly as the DC domain that mediates the interaction with Nrf2 Neh2 (Tong et al., 2006). It is noteworthy that Keap1 is rich in cysteine residues, with 25 cysteines in mouse and rat and 27 in human Keap1 proteins. Some cysteine residues are located adjacent to basic residues and have properties of reactive cysteines that have low pKa values and are excellent targets of electrophiles and oxidants.
3. Other Proteins
Although Nrf1 was found capable of stimulating ARE-driven gene transcription when overexpressed in cells, direct evidence of its role in ARE-driven gene induction is lacking. Nonetheless, Nrf1 and Nrf2 have overlapping functions during early development, because a double knockout of Nrf1 and Nrf2 in mice caused early embryonic lethality and excessive oxidative stress that were distinct from the midgestation lethality found in homozygous Nrf1 knockout and no apparent embryonic death in homozygous Nrf2 knockout mice (Leung et al., 2003).
The Bach proteins are more distantly related among CNC bZip proteins. Bach proteins often act as transcription repressors (Toki et al., 2005; Igarashi and Sun, 2006). Bach1 heterodimerizes with MafK, and Bach1/MafK dimer binds to the E1 and E2 enhancers of human heme oxygenase 1 gene promoter (located at around −4.0 and −9.0 kb) to repress HO-1 expression. Each enhancer contains multiple copies of AREs (also called StRE or MARE by different laboratories). Heme induces HO-1 by releasing Bach1 from the enhancers, whereas ARE inducers induce the gene by stimulating Nrf2 binding to the AREs (Alam et al., 1995, 2000; Sun et al., 2002; Dohi et al., 2006; He et al., 2007; Reichard et al., 2007). In a manner similar to that of HO-1, Bach1/small Maf competes with Nrf2/small Maf to suppress ARE-driven NQO1 basal expression and induction by tBHQ, whereas heme relieves the inhibitory effect of Bach1 to stimulate NQO1 expression (Dhakshinamoorthy et al., 2005).
Although Nrf2-null mice showed substantial reduction in the basal expression and induction of Nqo1 and Gsts (class Alpha and Mu subunits), some Gsts—such as Gsta1 and/or Gsta2, Gsta4, and Gstm1—were still induced in the intestine by BHA, coumarin, and diterpenes (Itoh et al., 1997; McMahon et al., 2001; Higgins et al., 2008). This variability in Nrf2-dependence of intestinal ARE-dependent genes for induction suggests that additional factors other than Nrf2 are activated by ARE inducers to mediate induction of the genes in the tissue in the absence of Nrf2. Given the heterogeneity of ARE sequences and overlapping DNA-binding activities among bZip proteins, it is possible that Nrf1, AP-1, or other bZip transcription factors mediate the induction of some ARE-dependent genes in the intestinal epithelium of Nrf2 knockout mice. It is equally possible that adaptive changes in Nrf2-null cells, such as increased oxidative stress, synergize with ARE inducers to activate an alternative inducing mechanism(s) that is not functional in the presence of Nrf2 otherwise.
III. Mechanism of Nuclear Factor Erythroid 2-Related Factor 2 Regulation: Insights from Structure
A key question raised after Nrf2 and Keap1 were identified was how Nrf2 is regulated for induction of ARE-dependent genes.
A. Nuclear Factor Erythroid 2-Related Factor 2 Turnover through the Ubiquitin-26S Proteasome Pathway
Nrf2 mRNA is readily detectable in a wide range of cells, implying that transcription of Nrf2 is not a major mechanism by which Nrf2 is regulated. A clue of post-transcriptional regulation of Nrf2 was provided when induction of NQO1 was compared with induction of CYP1A1 in the presence of cycloheximide, a potent inhibitor of protein synthesis. Induction of CYP1A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin (a potent AhR ligand) was markedly enhanced by cycloheximide, a phenomenon termed “superinduction.” Superinduction of CYP1A1 results from inhibition of synthesis of a labile inhibitory protein (called AhR degradation-promoting factor), which promotes proteasomal degradation of ligand-activated AhR (Ma and Baldwin, 2000; Ma et al., 2000). In contrast, the basal expression and induction of NQO1 by tBHQ or 2,3,7,8-tetrachlorodibenzo-p-dioxin were totally blocked by cotreatment with cycloheximide, suggesting that a labile protein was required for the basal expression and induction of NQO1 by either inducer (Ma and Kinneer De-Fede, 2001). The labile factor was subsequently found to be Nrf2, which is degraded through the ubiquitin-26S proteasome pathway with a half-life of 20 min (Sekhar et al., 2002; McMahon et al., 2003; Kobayashi et al., 2004; Ma et al., 2004; He et al., 2006). The findings also indicate that the AhR-DRE pathway cross-interacts with the Nrf2-ARE pathway for induction NQO1 by bifunctional inducers (Ma and Kinneer De-Fede, 2001; Ma et al., 2004).
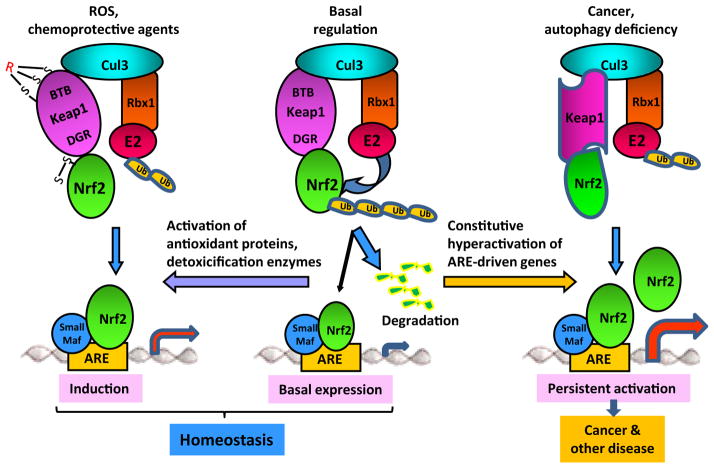
A number of molecular, biochemical, and genetic studies implicated Keap1 in the control of the ubiquitination-proteasomal degradation of Nrf2. In this framework, Keap1 controls the ubiquitination of Nrf2 by acting as an adaptor protein to bring Nrf2 into a Cul3-dependent ubiquitin ligase (E3) complex (Fig. 4) (Sekhar et al., 2002; McMahon et al., 2003; Wakabayashi et al., 2003; Kobayashi et al., 2004; Zhang et al., 2004; Furukawa and Xiong, 2005; He et al., 2006). In the E3, Cul3 functions as a scaffold protein, where its N-terminal domain interacts with the BTB domain of Keap1 and its conserved C-terminal domain binds with RING box protein 1 (Rbx1). Rbx1 recruits the catalytic function of ubiquitin-conjugating enzyme (E2) to the complex. Keap1 binds Nrf2 through its C-terminal DC domain and presents Nrf2 to Rbx1-bound E2 for polyubiquitination within the complex. Polyubiquitinated Nrf2 is then rapidly degraded by the 26S-proteasomes, resulting in a short half-life and a low protein level of Nrf2 in unstimulated cells (Fig. 4). The Keap1/Cul3/Rbx1-E3 complex has an overall structure that is similar to those of the SCF complex and the ElonginC–Cul2–SOCS box complex, which catalyze the ubiquitination of inhibitor of NF-κB α and hypoxia inducible factor 1α, respectively (Ma, 2010).
Fig. 4.
Models of Nrf2 regulation. Under basal conditions, Nrf2 is rapidly polyubiquitinated through the Keap1/Cul3/Rbx1–E3 and degraded by the 26S-proteasomes resulting in a short half-life. A small portion of Nrf2 accumulates in the nucleus to mediate basal expression of ARE-dependent genes to contribute to maintenance of homeostasis. Under stress conditions or in the presence of chemoprotective agents, ARE inducers modify the cysteine codes of Keap1 and Nrf2 to inhibit polyubiquitination of Nrf2 resulting in activation of Nrf2 and induction of ARE-dependent genes to resist stress. Nrf2 plays a more important role in the induction of genes, such as NQO1 and GST, than in their basal expression, because, presumably, other signaling pathways support basal expression of ARE-dependent genes in addition to Nrf2, which may explain why Nrf2 KO mice have a largely normal phenotype in unstressed conditions. In certain pathological scenarios, such as cancer and autophagy deficiency, interaction between Nrf2 and Keap1 or between Keap1 and Cul3 is altered through mutations, epigenetic changes, accumulation of disruptor proteins, leading to inhibition of Nrf2 ubiquitination and persistent hyperactivation of Nrf2, a condition that promotes tumor growth, drug resistance, and toxicity.
The chemical mechanism by which ARE inducers activate Nrf2 was suggested by Dinkova-Kostova et al. (2001). By comparing inducers, they showed that a single and universal property of all inducers examined was reactivity with cysteine thiols at rates closely correlated with their potency for induction of NQO1. Binding of ARE inducers to Keap1 cysteine thiols was demonstrated using tritium-labeled inducer, stoichiometry, UV spectroscopy, and mass spectrometry analyses of purified Keap1 (Dinkova-Kostova et al., 2002). The most reactive residues of Keap1 were identified as Cys-257, Cys-273, Cys-288, and Cys-297, located in the IVR region between BTB and Kelch repeats of Keap1 (Fig. 5). These findings suggest that Keap1 serves as a cellular sensor through its reactive cysteine thiols, which recognize and interact with ARE inducers chemically by alkylation or oxidation, thereby transducing the signal for Nrf2 activation and gene induction.
Fig. 5.
Cysteine residues of Nrf2 and Keap1.
Analyses of inducer-Keap1 interaction by several laboratories using mass spectrometry, mutagenesis, and mouse genetics identified a number of cysteine residues that are highly reactive to inducers and are important for activation of Nrf2 by ARE inducers or for suppression of Nrf2 under basal conditions (Eggler et al., 2005; Hong et al., 2005a,b; He and Ma, 2010). Highly reactive cysteine residues—including Cys-273, Cys-288, and Cys-297—were consistently found in the IVR region (Fig. 5). Mutational studies revealed that Cys-151, Cys-273, and Cys-288 are important for regulation of Nrf2. Expression of the cysteine mutants in Keap1-null mice further confirmed that Cys-273 and Cys-288 are required for suppression of Nrf2 and Cys-151 for activation of Nrf2 by inducers (Yamamoto et al., 2008). In a Zebrafish model, electrophiles were shown to recognize distinct sets of cysteine residues of Keap1, coined as cysteine codes, to activate Nrf2 (Kobayashi et al., 2009).
Unlike Keap1, which contains 25 to 27 cysteine residues, Nrf2 has only six (human Nrf2) or seven (mouse and rat Nrf2) cysteine residues that are highly conserved across species (Fig. 5) (He and Ma, 2009). Two arsenic-based ARE inducers, FlAsH (a fluorophore) and phenylarsine oxide, bind to Nrf2 through interaction with its cysteine thiols. Further analyses revealed that Nrf2 cysteine residues have several functions in Nrf2 signaling, including induction of NQO1 by arsenic.
B. Structures of Nuclear Factor Erythroid 2-Related Factor 2 and Kelch-Like Erythroid Cell-Derived Protein with CNC Homology-Associated Protein 1
Structural studies provided major insights into Nrf2-Keap1 interaction and Nrf2 signaling. The studies focused on two questions: How does Keap1 interact with Nrf2 for ubiquitination of Nrf2? How do ARE inducers inhibit this process?
Although the crystal structures of full-length Nrf2 and Keap1 are not available at the present time, major progress was made in structural resolution of the Nrf2 Neh2 domain, the Keap1 DC domain with or without Nrf2 Neh2, and the full-length Keap1 homodimer structure. Nrf2 Neh2 (a.a. 1–98) is the primary binding structure of Nrf2 for interaction with Keap1. Neh2 contains three evolutionarily conserved motifs among the CNC proteins: an ETGE motif (a.a. 79–82, named after the four key amino acid residues), which is required for interaction with Keap1 (Kobayashi et al., 2002); a DLG motif (a.a. 29–31, named after the three key amino acid residues), which is important for ubiquitination and degradation of Nrf2 (Katoh et al., 2005); and a DIDLID motif (a.a. 16–25, named after the six key amino acid residues), which is N-terminal to DLG and is involved in Nrf2 degradation, but acts as an activation domain in the Caenorhabditis elegans Nrf2 homolog SKN1 (Walker et al., 2000; McMahon et al., 2004). Between DLG and ETGE is a hydrophilic region with seven lysine residues that are indispensable for Keap1-dependent polyubiq-uitination and degradation of Nrf2 (Zhang et al., 2004). NMR spectroscopy revealed that Neh2 is intrinsically disordered with local elements of secondary structures, including a 33-residue central α-helix (a.a. 39–71) followed by a mini antiparallel β-sheet (a.a. 74–76 and 82–85) (Tong et al., 2006). Of the seven lysine residues in the α-helix, six face one side of the helix. ETGE locates in the hydrophilic hairpin loop (Glu-78 to Gly-81) between the two mini β-sheets. A tight β-hairpin conformation composed of Arg-25, Gln-26, Asp-27, Ile-28, and Asp-29 is located N-terminal to DLG (Tong et al., 2007).
Keap1 is one of the BTB-Kelch proteins that are named after the D. melanogaster Kelch protein and consist of more than 50 members encoded by the human genome. The consensus Kelch repeat motif contains several highly conserved residues, including two adjacent glycine residues and a tyrosine/tryptophan pair separated by exactly seven residues (Bork and Doolittle, 1994). The structure of the human Keap1 Kelch domain (a.a. 321–609) was resolved by crystallography at a 1.85 Å resolution. The Kelch domain folds up into a six-bladed β-propeller structure and uses a C-terminal strand mechanism of closure; i.e., residues of the C terminus form the first strand of the first blade (Li et al., 2004c). The conserved tyrosine/tryptophan pairs participate in interblade interactions, and the glycine doublets contribute to a conserved hydrogen-bonding network within each blade. In a separate study, the mouse Keap1 DC domain (a.a. 309–624, consisting of the Kelch/DGR and the CTR domains) was structurally resolved by crystallography at 1.60 Å (Padmanabhan et al., 2006). The overall tertiary structure is a drum-shaped, β propeller structure similar to the Kelch structure described for human Keap1. An inner cavity buried in the central core has a strong hydrophobic character, opening on the top with a diameter of approximately 15 Å and on the bottom, 9.5 Å.
The overall structure of mouse Keap1 was examined by using single-particle electron microscopy and three-dimensional reconstruction at a 24-Å resolution. Keap1 is a dimer consisting of two large globular structures attached by short linker arms to the sides of a small forked-stem structure, resembling a cherry-bob (Ogura et al., 2010). Each globular domain is a vertically elongated sphere with dimensions of 60 × 60 × 78 Å3, and each has a tunnel corresponding to the central core of the β-propeller structure in the crystal structure of Kelch or DC domain. The stem-shaped central region comprises two thin, interacting layers separated by a narrow gap, reflecting the dimerization interface between two Keap1 monomers through the BTB domain; this dimeric interface is in agreement with a previous finding that BTB is required for Keap1 dimerization and function (Zipper and Mulcahy, 2002). Comparison of the three-dimensional Keap1 dimer structure with the theoretical mass ratio of the Keap1 domains deduced from the primary amino acid sequences (i.e., BTB, 28%; IVR, 23%; DC, 49%) suggested that the stem region (13.5% of the total volume) is part of the BTB domain, whereas the globular spheres (86.5% of the total volume) consist mostly of the IVR and DC domains with IVR on the outer surface and DC in the interior of the spheres.
C. A Two-Site Binding Model
Isothermal calorimetry and NMR titration analyses first suggested that one Neh2 domain of Nrf2 interacts with two molecules of Keap1 through its ETGE and DLG motifs (Tong et al., 2006). Both ETGE and DLG bind to similar sites on the bottom surface of the β-propeller structure of Keap1 DC. However, the binding affinity of ETGE is 2 orders of magnitude higher than that of DLG. The central α-helix between ETGE and DLG of Neh2 serves as a bridge connecting the two motifs in the association with two Keap1 DC spheres. This spatial relationship among the ETGD, α helix, DLG of Neh2, and the two DC of Keap1 is in a good agreement with the dimeric cherry-bob structure of Keap1 obtained from single-particle electron microscopy, where the distance between the two binding pockets of Keap1 dimer roughly equals the distance between ETGE and DLG of Neh2 (i.e., ~80 Å). (Ogura et al., 2010).
The crystal structures of human Keap1 Kelch domain bound with Nrf2 ETGE motif and mouse Keap1 DC domain bound with either ETGE or DLG revealed the interaction between Nrf2 and Keap1 at the binding interface in more details (Lo et al., 2006; Padmanabhan et al., 2006; Tong et al., 2007). The binding cleft of Keap1 DC resides at the entrance of the central cavity on the bottom of the β-propeller structure. ETGE and DLG interact with Keap1 in a very similar manner: both adopt a tight β-turn conformation and orient in essentially the same manner with respect to the Keap1 DC structure. However, the ETGE motif has more electrostatic interactions (~13) with Keap1 DC than the DLG motif has (~8), and ETGE is more deeply embedded into the Keap1 DC binding cleft compared with DLG-DC binding, accounting for the large difference between the binding affinities of the motifs for Keap1 DC (Tong et al., 2007).
These studies provided a structural basis for a two-site binding model for the control of Nrf2 ubiquitination by Keap1. Under a basal condition, Keap1 recruits newly synthesized Nrf2 protein to the Keap1/Cul3/Rbx1-E3 complex by binding to Nrf2 through Keap1 DC-Nrf2 Neh2 interaction and to Cul3 through Keap1 BTB-Cul3 N terminus interaction. Nrf2 Neh2 takes a rod-like shape. Because of its higher binding affinity, the ETGE motif binds to one of the dimeric Keap1 globular subunits at the bottom binding cleft of DC as the first step. Binding of ETGE promotes binding of DLG to the other DC sphere at a similar binding surface. This “hinge and latch” mode of binding positions the lysine residues in the α-helix between DLG and ETGE to the E2 enzyme recruited by Rbx1 for polyubiquitination. Ubiquitinated Nrf2 is then rapidly degraded by the 26S proteasomes resulting in a short half-life of Nrf2 protein. It is noteworthy that, although the majority of synthesized Nrf2 proteins are degraded rapidly, a small pool of Nrf2 accumulates in the nucleus to mediate a low, but detectable, basal expression of ARE-controlled NQO1 and GSTs (Fig. 4).
The molecular mode of action for inhibition of Nrf2 ubiquitination by ARE inducers is not well understood. Several mechanisms have been proposed. First, the two-site binding model predicts that modification of critical cysteine thiols of the IVR domain, which are close to the DC domain in the globular structure, provokes dissociation of DLG from DC (the latch) but not ETGE from DC (the hinge) binding, because DLG-DC binding is much weaker than ETGE-DC binding. This conformation of the complex prevents Nrf2 from ubiquitination, even though Nrf2 remains in the complex. The observation supporting this model is that some inducers, such as phenolic antioxidant tBHQ, failed to disrupt Nrf2-Keap1 binding at concentrations at which Nrf2 was activated and ARE-dependent genes were induced (He et al., 2006; Kobayashi et al., 2006). In this scenario, the inducer-modified and Nrf2-bound Keap1 is inactivated and, consequently, newly synthesized Nrf2 proteins bypass Keap1 and translocate into the nucleus to mediate induction of ARE-dependent genes. Second, some inducers, such as metal inducers As3+, Cd2+, and Cr6+, are capable of disrupting Nrf2-Keap1 binding as revealed by loss of coimmunoprecipitation of Nrf2 and Keap1 at inducing concentrations; this finding suggests that the metal inducers release Nrf2 from the Keap1/Cul3/Rbx1-E3 complex to activate Nrf2 (He et al., 2006, 2007, 2008). Third, modification of certain Keap1 cysteines close to the interface between Keap1 and Cul3, such as Cys-151 in the BTB domain, may disrupt the Keap1-Cul3 interaction, thereby preventing Nrf2 from ubiquiti-nation by E3.
The close proximity of BTB Cys-151 to Keap1-Cul3 interaction and IVR Cys-273 and 288 to Keap1 DC-Nrf2 Neh2 interaction supports the above mechanisms. However, it has been difficult to dissect the exact role of individual cysteine residues in the control of Nrf2 activation for several reasons. For example, detailed structural information of the 25 to 27 cysteines of Keap1 and the six to seven cysteines of Nrf2 in the E3 complex is not available at the present time. Multiple cysteine residues are often modified by any given inducer. Cysteine residues of Keap1 and Nrf2 often have multiple and overlapping functions that include maintenance of tertiary structure, DNA-binding, and transcription activation, in addition to chemical sensing and ubiquitination of Nrf2 under basal conditions. Therefore, identification of the cysteine codes for different inducers and resolution of the three-dimensional structure of the quaternary Nrf2-Keap1-Cul3-Rbx1 complex to reveal the spatial arrangement of each component and individual cysteine residues would help elucidate the mechanism by which ARE inducers activate Nrf2 at a molecular level.
D. Other Mechanisms of Nuclear Factor Erythroid 2-Related Factor 2 Signaling
In addition to cysteine thiol modification by inducers, several other mechanisms have been described to regulate Nrf2 signaling in a cell type-, target gene-, and disease-dependent manner.
1. Suppression of Kelch-Like Erythroid Cell-Derived Protein with CNC Homology-Associated Protein 1 by Disrupter Proteins
Autophagy is a conserved protein degradation pathway responsible for the disposal of long-lived proteins, excess or damaged organelles, and aggregation-prone proteins. The p62 protein is a selective substrate for autophagy and a regulator of protein aggregate formation. Loss of autophagy in mice caused marked accumulation of p62 accompanied with liver injury and robust induction of antioxidant proteins including NQO1 and GSTs (Komatsu et al., 2007, 2010). This finding suggests that p62 serves as an endogenous protein inducer of Nrf2 target genes. p62 binds to Keap1 through its KIR motif, which binds to the bottom basic surface pocket of Keap1 overlapping with the binding pocket for Nrf2 ETGE and DLG. The KIR motif (a.a. 345–359) of p62 contains a STGE sequence (a.a. 351–355) that mediates binding of p62 to Keap1 at the “latch” site with a binding affinity similar to that of Nrf2 DLG-Keap1 DC binding. Excessive buildup of p62 leads to suppression of Keap1 by inhibiting Nrf2 DLG-Keap1 binding leading to activation of Nrf2 and induction of ARE-dependent genes (Jain et al., 2010; Komatsu et al., 2010; Lau et al., 2010). Thus, p62 is a disruptor of Nrf2-Keap1 binding. Loss of Nrf2 alleviated, but loss of Keap1 exacerbated the liver injury observed in autophagy-deficient mice, revealing a role of Nrf2-Keap1 dysregulation in autophagy-related pathologic liver conditions.
The cyclin-dependent protein kinase p21 (Cip1/WAF1) is a target gene of p53 and is up-regulated in response to oxidative stress to protect cells from oxidative damage. The antioxidant function of p21 was suggested to be mediated through activation of Nrf2 (Chen et al., 2009b). In this scenario, p21 acts as a disrupter protein of Nrf2-Keap1 binding. The basic tripeptide KRR (a.a. 154–156) of p21 directly interacts with the DLG and ETGE motifs of Nrf2, thereby competing with Keap1 for Nrf2 binding to suppress Nrf2 ubiquitination and degradation.
2. Kelch-Like Erythroid Cell-Derived Protein with CNC Homology-Associated Protein 1-Independent Degradation of Nuclear Factor Erythroid 2-Related Factor 2 Signaling
The Neh6 domain (a.a. 331–381) of Nrf2 was shown to mediate degradation of Nrf2 under redox stress conditions under which Keap1 was inhibited, implying that Nrf2 can be degraded through a Keap1-independent mechanism (McMahon et al., 2004). The redox-insensitive degron contains multiple serine residues at 335, 338, 342, 347, 351, and 355 positions of mNrf2, which can be phosphorylated by glycogen synthase kinase 3 (GSK-3) and, possibly, other kinases (Rada et al., 2011). Phosphorylaton of Ser-335 and Ser-338 in a conserved DSGIS (a.a. 334–338) sequence of Nrf2 by GSK-3 creates a recognition motif DpSGX(1–4)pS, which is recognized by the WD substrate recognition domain of β-transducin repeat-containing protein, an adaptor protein for the Cul1-dependent SCF E3 complex. β-Transducin repeat-containing protein brings Nrf2 into the SCF E3 complex by binding to Skp1 through its F-box domain and promotes the ubiquitination and subsequent proteasomal degradation of Nrf2 in a manner similar to the ubiquitination and degradation of IκBα. It was postulated that this PI3K/Akt-GSK-3-regulated, redox-insensitive degradation pathway of Nrf2 has certain implications in neurodegenerative pathology and, perhaps, the aging process (Rada et al., 2011).
3. Modulation of Nuclear Factor Erythroid 2-Related Factor 2 Signaling-Dependent Transcription
Some bZip proteins—such as Nrf1, Bach proteins, AP-1 proteins, and CCAAT/enhancer-binding protein—may directly interfere with Nrf2-dependent transcription. Nrf1 and Bach proteins can heterodimerize with small Mafs and bind to ARE enhancers, whereas AP-1 proteins and CCAAT/enhancer-binding protein bind to TRE or cAMP response element within composite ARE sequences. A number of other proteins—including ATF3, ERα, peroxisome proliferator-activated receptor γ, RARα, NF-κB, and p53—can antagonize Nrf2 for induction of ARE-dependent genes through various mechanisms. ATF3 is a member of cAMP response element-binding proteins; ATF3 suppresses Nrf2-mediated transcription of ARE-dependent genes by dimerizing with Nrf2 and binding to AREs located in the proximal promoters of Nrf2 target genes, which suppresses recruitment of CBP (Brown et al., 2008). NF-κB may also antagonize Nrf2 by depriving CBP from Nrf2 (Liu et al., 2008a). The estrogen 17β-estradiol suppresses ARE-dependent DMEs; estrogen-bound ERα is coimmunoprecipitated with Nrf2, suggesting a direct interaction of ligand-activated ERα with Nrf2 that suppresses ARE-dependent gene induction (Ansell et al., 2005). All-trans retinoic acid suppresses Nrf2-mediated ARE induction by activating RARα to form a complex with Nrf2, whereas vitamin A deficiency up-regulates ARE-dependent gene expression by inhibiting RARα (Wang et al., 2007).
4. Phosphorylation
A number of protein kinases have been reported to phosphorylate Nrf2 in response to different stressors, including casein kinase 2, PKC, GSK, c-Jun NH2-terminal kinase, protein kinase RNA-like endoplasmic reticulum kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase (Nguyen et al., 2003). The human Nrf2 exhibits two bands on SDS-polyacrylamide gel electrophoresis: the lower band corresponds to the unphosphorylated form, whereas the upper band is due to phosphorylation of Nrf2 by casein kinase 2 in the transcription activation domain, which promotes the nuclear translocation and transcription activation of Nrf2 (Apopa et al., 2008). Phorbol esters stimulate transcription of ARE-dependent genes, suggesting a role of PKC (Nguyen et al., 1994). The PKC-catalytic subunit and PKC immunoprecipitated from cells could directly phosphorylate Nrf2 in vitro at Ser-40; moreover, tBHQ-induced phosphorylation and nuclear localization of Nrf2 were inhibited by PKC inhibitors (Huang et al., 2000, 2002; Bloom and Jaiswal, 2003; Numazawa et al., 2003). The tyrosine kinase Fyn phosphorylates Tyr-568 to allow Nrf2 nuclear export through Crm1 for degradation in the cytoplasm (Jain and Jaiswal, 2006). In addition to promoting degradation of Nrf2 through SCF-E3, phosphorylation of Nrf2 by GSK-3β localizes Nrf2 to the cytoplasm, providing another means of inhibiting ARE transcription by Nrf2 through the phosphatidylinositol 3/Akt pathway (Salazar et al., 2006). The mitogen-activated protein kinase signaling pathways were also implicated in the regulation of Nrf2 (Nguyen et al., 2003). It is generally believed that these signaling pathways modulate Nrf2 activation steps, such as nuclear translocation and transcription activation, in cell type- and gene-specific manner, but do not replace the Keap1-dependent ubiquitination and degradation of Nrf2 as the primary mechanism of Nrf2 regulation.
5. Acetylation/Deacetylation
In addition to acting as a coactivator of Nrf2, CBP promotes acetylation of Nrf2, which is associated with nuclear localization, increased binding to ARE, and increased transcription by Nrf2 (Kawai et al., 2011). On the other hand, sirtuin 1 decreases acetylation of Nrf2, and deacetylation of Nrf2 results in the nuclear export and cytoplasmic localization of Nrf2.
IV. Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Disease and Toxicity: Expecting the Unexpected
Parallel to the remarkable progress in the understanding of the mechanism of Nrf2 signaling during the past decade was the rapid accumulation of a body of knowledge on the function of the Nrf2-ARE pathway. Investigations using knockout mouse models, gene arrays, mutations and polymorphisms, and new inducers have revealed the involvement of Nrf2 in a surprisingly broad range of physiologic and disease processes.
A. Carcinogenesis
The Nrf2 knockout mice served as a genetic model for testing the importance of Nrf2 in chemical carcinogenesis, which was predicted from the chemopreventive effects of ARE inducers. The first carcinogen examined was Bp (Ramos-Gomez et al., 2001). Treatment of mice with Bp induced tumors in the forestomach, but the Nrf2 knockout mice showed a significantly higher burden of gastric tumors than wild type. The number of tumors in the forestomach per mouse was 9.5 ± 1.0 for wild type and 14.1 ± 1.2 for knockout mice (p = 0.003). Consistent with the results, constitutive hepatic and gastric activities of GST and NQO1 were reduced by 50 to 80% in Nrf2-null mice compared with wild type. To evaluate the impact of Nrf2 genotype on Bp bioactivation and disposition, levels of Bp-DNA adducts were measured as tetrols released from DNA isolated from target (forestomach) and nontarget tissues (liver) of wild-type and Nrf2-null mice treated with Bp (Ramos-Gomez et al., 2003). Levels of Bp-DNA adducts in the forestomach, but not in the liver, were significantly higher in Nrf2-null mice than in wild type. Together, these findings provided direct evidence supporting an important role of Nrf2 in protection against Bp carcinogenesis through controlling the expression of ARE-dependent genes and Bp-DNA adduct disposition.
Nrf2-null mice were susceptible to urinary bladder cancer induced by N-nitrosobutyl(4-hydroxybutyl)amine (BBN) (Iida et al., 2004). The incidence of urinary bladder carcinoma by BBN was significantly higher in Nrf2-null mice than in wild type; invasive carcinoma was 24% for the wild type but 38.5% for the knockout mice. Oltipraz effectively inhibited BBN-induced bladder cancer. Crossing Nrf2-null and p53 knockout mice [Nrf2(−/−)::p53(+/−)] significantly increased susceptibility of the mice to BBN-induced urinary bladder cancer compared with wild-type, Nrf2-null, and p53(+/−) phenotypes individually (Iida et al., 2007). BBN increased p53-mediated expression of p21, Mdm2, and Bax, and the inducible expression of p21 was significantly increased in Nrf2-null mice. On the other hand, urinary concentrations of N-nitrosobutyl(3-carboxy-propyl)amine, a proximate carcinogen of BBN, were increased in Nrf2-null, but not p53(+/−), mice. Modest increases in NQO1 and UGT1A6 expression were observed in p53(+/−) mice compared with wild type after BBN exposure. These results demonstrate that tumor susceptibility is synergistically exacerbated in Nrf2- and p53-deficient mice in comparison with either mutation alone as a result of poor detoxification and accelerated proliferation.
Nrf2 deficiency also increased sensitivity of mice to several other cancer models. Treatment with 7,12-dimethylbenz(a)anthracene and TPA resulted in an increase in the incidence of skin tumors as well as tumor numbers per mouse in wild-type and Nrf2 knockout mice; however, both indices were markedly higher in Nrf2-null mice than in wild type (Xu et al., 2006). In a mouse model of azoxymethane/DSS-induced colorectal carcinogenesis, Nrf2 knockout mice showed increased incidence, multiplicity, and size of all colorectal tumors compared with wild type upon treatment; the proportion of adenocarcinoma was much higher in knockout (80%) versus wild-type (29%) mice (Khor et al., 2008). Using the Nrf2-deficient gpt delta mice, which lack Nrf2 but harbor a transgenic guanine phosphoribosyltransferase gene (a target gene for assessing genotoxicity in vivo), Nrf2 was shown to protect against spontaneous and Bp-induced mutations in the lungs of the mice (Aoki et al., 2007).
Nrf2 affects tumor progression and metastasis. Susceptibility of Nrf2-deficient mice to pulmonary cancer metastasis was investigated after implantation of the mouse Lewis lung carcinoma cell line. Nrf2-null mice reproducibly exhibited a higher number of pulmonary metastatic nodules than wild-type mice, indicating that Nrf2 inhibited cancer metastasis in the lungs, presumably by preserving the redox balance in the hematopoietic and immune systems (Satoh et al., 2010). Treatment with 7,12-dimethylbenz(a)anthracene and medroxypro-gesterone acetate induced premalignant lesions and mammary carcinomas in mice. Although no apparent difference in the formation of the premalignant lesions was observed between Nrf2 knockout and wild-type mice, the knockout mice exhibited rapid and aggressive mammary carcinoma progression (Becks et al., 2010).
A few recent studies indicate that Nrf2 promotes tumorigenesis in certain models of cancer, suggesting that the role of Nrf2 in cancer varies depending on the context in which the tumor was induced. Targeted deletion of Nrf2 in mice reduced urethane-induced lung tumor formation compared with wild-type mice, revealing that Nrf2 is activated in wild-type mice to confer resistance to anticancer agents and promote malignancy (Bauer et al., 2011). Expression of endogenous oncogenic alleles of Kras, Braf, and Myc suppressed ROS by activating the Nrf2 antioxidant program, indicating that the Nrf2-mediated antioxidant and cellular detoxification functions are involved in oncogenesis by oncogenes (DeNicola et al., 2011). As discussed later, some cancers overactivate Nrf2 through somatic mutations and epigenetic mechanisms to result in a prosurvival phenotype for tumor growth.
B. Chemical Toxicity
Loss of Nrf2 in mice substantially increases sensitivity of the mice to a wide range of acute and chronic chemical toxicity. The first chemical tested for toxicity in Nrf2-null mice was butylated hydroxytoluene (BHT), a food additive used to prevent lipid peroxidation in meat. BHT treatment by either systemic or oral administration caused transient lung damage with destruction of alveolar type I epithelial cells, massive edema, and hemorrhage that generally recover within 7 days of exposure. However, when treated with BHT at doses that were well tolerated by wild-type mice, the Nrf2-null mice succumbed from a severe acute respiratory distress syndrome with a high rate of mortality (Chan and Kan, 1999). When fed with 1% BHT in diet, 80% of Nrf2-null mice died, whereas the wild-type mice were resilient to the diet with only transient general toxicity. The profound sensitivity to BHT lung toxicity observed in Nrf2 knockout mice was attributed to reduced detoxification of the toxicant in the intestine, liver, and kidneys and type II alveolar epithelial cells in the lungs.
The impact of Nrf2 knockout on acetaminophen hepatotoxicity exemplifies the multiple roles of the Nrf2-ARE pathway on chemical toxicity. At doses well tolerated by wild-type mice, Nrf2 knockout mice developed centrilobular hepatocellular necrosis with elevated levels of plasma alanine aminotransferase activity; homozygous Nrf2-null mice died of liver failure in severe cases (Chan et al., 2001; Enomoto et al., 2001). On the other hand, mice with hepatocyte-specific disruption of Keap1, which caused constitutive activation of Nrf2 and elevated expression of NQO1 and GSTs, showed remarkable resistance to lethality by high doses of acetaminophen (Okawa et al., 2006). In addition, induction of GSTs by ARE inducers were effective in inhibition of acetaminophen toxicity (Ansher et al., 1983; Reisman et al., 2009a). The mechanism by which Nrf2 protected mice from acetaminophen hepatotoxicity was severalfold. Acetaminophen caused depletion of GSH in the liver of both wild-type and knockout mice; however, the wild-type mice were able to compensate and regain the normal GSH level, whereas the knockout mice failed to do so because of decreased synthesis of GSH (Chan et al., 2001). The effect of Nrf2 on the pharmacokinetics of acetaminophen in mice was examined in wild-type, Nrf2-null, and heterozygous Keap1 knockout mice using a subtoxic dose (50 mg/kg) (Reisman et al., 2009b). Loss of Nrf2 caused reduction of glucuronidation of acetaminophen as well as efflux of acetaminophen-glucuronide conjugate via multidrug resistance-associated protein 3. On the other hand, activation of Nrf2 by knocking out Keap1 in the liver resulted in increased elimination of acetaminophen-glucuronide conjugate as a result of increased expression of multidrug resistance-associated protein 3.
Many other chemicals were studied for toxicity using Nrf2 knockout mice, where Nrf2 was shown to be protective against the acute and chronic toxicities of the chemicals. Examples include acute and chronic carbon tetrachloride liver injury (liver necrosis, inflammation, and fibrosis) (Xu et al., 2008), metal toxicity (arsenic, cadmium, and chromium) (He et al., 2006, 2007, 2008; Jiang et al., 2009), diesel exhaust particle-induced inflammatory and oxidative damage in bronchial epidermis (Aoki et al., 2001; Li et al., 2004b), and lipopolysaccharide-induced sepsis (Thimmulappa et al., 2006). Nrf2 also protected against neurotoxicant-induced toxicity to neurons, glial cells, and peripheral nervous tissues, including 3-nitropropionic acid and malonate-induced striatal damage, which involves inhibition of mitochondrial respiratory chain complex II (Calkins et al., 2005), kainic acid-induced hippocampal neuron damage and seizure (Kraft et al., 2006), and 6-hydroxydopamine-induced cell loss in the substantia nigra and striatal dopamine depletion (Jakel et al., 2007).
C. Disease Pathologic Features
1. Spontaneous Lesions
Although the initial characterization of Nrf2 knockout mice showed that the mice developed normally and mature to reproduce, later studies revealed some spontaneous lesions. Female Nrf2-null mice showed a tendency to develop multiorgan autoimmune inflammation and glomerulonephritis, especially in aged mice (Yoh et al., 2001; Li et al., 2004a; Ma et al., 2006). The mice exhibited increased proliferation of CD4+ T cells, elevated ratio of CD4+ to CD8+ cells, and escalated oxidative damage in tissues, linking Nrf2 functions to peripheral lymphocyte homeostasis and autoimmune surveillance. The Nrf2 knockout mice also developed splenomegaly and regenerative immune-mediated hemolytic anemia, in which chronic oxidative stress may sensitize erythrocytes to cause hemolysis (Lee et al., 2004). Nrf2-null mice showed vacuolar (spongiform) type of leukoencephalopathy with widespread astrogliosis in the brain, suggesting a physiological function for Nrf2 in the maintenance of the central nervous system myelin structure and function (Hubbs et al., 2007). It is noteworthy that conditional knockout of Nrf1 in mouse brain using the Cre-Lox technology caused age-dependent neurobehavioral abnormalities and brain atrophy as a result of apoptosis of the cortex (Kobayashi et al., 2011; Lee et al., 2011). Moreover, the mice showed loss of neurons in the hippocampal area and increased accumulation of polyubiquitinated proteins in brain regions, partly because of reduced expression of certain components of the 26S proteasome. The genes encoding the proteasome proteins have ARE enhancers, and Nrf1 up-regulates the expression of the genes by binding to the AREs in a similar fashion to Nrf2 (Lee et al., 2011).
2. Induced and Genetic Disease Models
Nrf2-deficient mice are susceptible to induced or genetic models of diseases involving the respiratory, neuro, immune, reproductive, and metabolic functions. Bleomycin, an antibiotic type of anticancer drug, causes pulmonary fibrosis in susceptible patients with cancer. Bleomycin induced lung lesions and fibrosis with increased hydroxyproline content, collagen deposition, fibrotic score, and cell proliferation more significantly in Nrf2-null mice than in wild type (Cho et al., 2004). Consistent with these findings, bleomycin activated Nrf2 and induced ARE-dependent genes in wild-type but not knockout mice, accounting for a protective role of Nrf2 in lung fibrosis in wild-type mice. Cigarette smoking increases the oxidative burden in the lungs and causes emphysema, a progressive and destructive lung disease associated with chronic obstructive pulmonary disorder. In comparison with wild-type mice, Nrf2-deficient mice developed early onset and extensive emphysema upon exposure to cigarette smoke for 6 months (Rangasamy et al., 2004). Ovalbumin sensitization and challenge caused severe airway inflammation and hyper-responsiveness that was marked with pronounced mucus cell hyperplasia and infiltration of eosinophils in Nrf2-deficient mice compared with wild-type litter-mates (Rangasamy et al., 2005). Hyperoxia (>95% oxygen) causes acute lung injury by generating ROS. Nrf2 was identified as a candidate gene associated with increased susceptibility to hyperoxia in mice through a genome-wide lineage analysis (Cho et al., 2002b). Exposure of Nrf2 knockout mice to hyperoxia resulted in more severe pulmonary injury and inflammation than wild-type control mice, confirming a protective role of Nrf2 against lung toxicity of hyperoxia (Cho et al., 2002a).
Dominant mutations in superoxide dismutase 1 cause familial amyotrophic lateral sclerosis, a progressive and fetal neurodegenerative disease characterized by progressive loss of motor neurons. Overexpressing Nrf2 in astrocytes was protective against mutant human super-oxide dismutase1-induced toxicity to neurons. Protection was also observed by crossing mice that overexpress Nrf2 with two amyotrophic lateral sclerosis mouse models. In both cases, overexpression of Nrf2 significantly delayed the onset of disease and extended survival of the mice (Vargas et al., 2008). MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) causes oxidative damage and Parkinson’s disease-like lesions in mouse brain. Nrf2 deficiency increased the sensitivity of mice to MPTP-induced lesions; on the other hand, overexpression of Nrf2 in astrocytes abolished MPTP toxicity (Chen et al., 2009a).
Multiple sclerosis is an autoimmune disease in which peripherally activated CD4+ T cells migrate into the central nervous system to mount autoimmune attack on myelin and oligodendrocytes. When Nrf2-null mice were immunized with myelin oligodendrocyte glycoprotein (MOG 35–55), the mice developed experimental autoimmune encephalomyelitis, an acute model of multiple sclerosis, which was more severe and more rapidly onset than in wild-type control mice (Johnson et al., 2010). The finding is in agreement with the notion that the Nrf2-ARE pathway modulates immune functions in vivo.
Female mammals are born with primordial follicles in the ovaries. Activation of resting follicles occurs through an unknown triggering mechanism intrinsic to the ovary and independent of pituitary gonadotropins. Depletion of ovarian follicles through atresia during aging causes ovarian failure (menopause). Infertility secondary to early follicular depletion in women younger than 40 is defined as premature ovarian failure. VCD (4-vinylcyclohexene diepoxide) is a metabolite of 4-vinylcyclohexene, an occupational by-product from the dimerization of 1,3-butadiene. VCD selectively damages small follicles in the ovary and causes ovarian cancer in rodents (Hoyer and Sipes, 1996). VCD destroyed small follicles by ~50% in wild-type mice, but depleted the follicles by 95% in Nrf2-deficient mice (Hu et al., 2006). The knockout mice exposed to VCD developed premature ovarian failure by 30 weeks of age compared with 50 weeks for the wild-type control mice. Follicle depletion correlated with diminished expression of microsomal epoxide hydrolase in the liver and Foxo3a in the ovaries as well as elevated oxidative stress and apoptosis in the ovaries of Nrf2-null mice.
V. Genetics and Cancer Mutation: Revealing the Perilous Side of Nuclear Factor Erythroid 2-Related Factor 2 Signaling Activation
A. Genetic Activation of Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Mice
Knockout of the Keap1 gene in mice caused lethality within 3 weeks after birth. The weaning mice died from malnutrition due to hyperkeratinization and occlusion of the esophagus and forestomach (Wakabayashi et al., 2003). Nrf2 was constitutively activated, and Nrf2 activity affected the expression of several keratinocyte-specific genes in the mice. Crossing Keap1-null mice with the Nrf2-null mice reversed Keap1-deficiency phenotypes. These findings imply that persistent activation of Nrf2 has a detrimental effect on the development of the digestive system. Conditional knockout of Keap1 was generated to circumvent the postnatal lethality by generating floxed alleles of the Keap1 gene and hepatocyte-specific deletion of Keap1 by crossing the floxed-Keap1 mice with transgenic mice expressing Cre recombinase under the regulation of the Albumin gene (Alb-Cre mice) (Okawa et al., 2006). These mice showed reduced Keap1 activity but elevated Nrf2 function and increased expression of ARE-dependent genes, and they were resistant to toxicity in several models, including acetaminophen hepatotoxicity (Okawa et al., 2006), concanavalin A inflammatory liver injury (Osburn et al., 2008), and bile duct ligation-induced cholestasis (Okada et al., 2009). Floxation of the Keap1 allele in the mice caused a partial disruption of Keap1 expression, creating Keap1 knockdown. Reduced expression of Keap1 and subsequent activation of Nrf2 in the mice conferred resistance against acute toxicity of acetaminophen, as expected; however, a decrease in Keap1 levels to less than 50% of that in control mice resulted in increased mortality in a study of 2-year-old mice, indicating that the benefits of Nrf2 activation are context-dependent, and persistent activation of Nrf2 exceeding a certain threshold is harmful to survival (Taguchi et al., 2010).
B. NRF2 Mutation in Cancer
Early studies revealed that expression of GSTs (GST P1, GST A2) and NQO1 was highly elevated in preneoplastic nodules compared with surrounding normal tissues in the liver chemical carcinogenesis model. Elevated expression of GSTs and NQO1 were also observed in tumor cell lines and liver, lung, colon, and breast tumors compared with normal tissues of the same origin. These findings led to the concept that cancer cells evolve to develop resistance to cancer-killing agents by overexpressing cytoprotective enzymes (Farber, 1984; Kitahara et al., 1984; Cowan et al., 1986; Belinsky and Jaiswal, 1993). Recent characterization of NRF2 and KEAP1 gene mutations in human cancers provided new insights into Nrf2 function in tumors. In two separate studies, mutations of the NRF2 gene were found in 6 to 25% of the human cancers examined. In one study, mutations were 11 of 103 (11%) in primary lung cancer and 3 of 12 (25%) in primary head and neck cancer (Shibata et al., 2008b); whereas, in the other study, mutations were 10 of 125 (8%) in lung cancer, 3 of 23 (13%) in head and neck cancer, 6 of 70 (11%) in esophageal cancer, and 1 of 17 (6%) in skin cancer (Kim et al., 2010). Most mutations were found within the ETGE (57%) and DLG (43%) motifs, which caused disruption of the low-affinity DLG-DC interaction or the high-affinity ETGE-DC binding, either of which inhibits the ubiquitination of Nrf2 to result in the activation and accumulation of Nrf2. The NRF2 mutations were mostly heterozygous and were associated with squamous cell carcinoma (77%), a smoking history (79%), lower incidence of simultaneous epidermal growth factor receptor mutations, and poor prognosis.
C. KEAP1 Mutation in Cancer
Mutations of the KEAP1 gene in cancer involving missense mutations and amino acid substitutions can lead to impaired Keap1 function (Padmanabhan et al., 2006; Singh et al., 2006; Nioi and Nguyen, 2007; Ohta et al., 2008; Shibata et al., 2008a; Takahashi et al., 2010). The mutations were mostly found in the DC domain (65%), followed by IVR (29%), BTB (3%), and the N-terminal region (3%), and were frequently associated with adenocarcinomas. Lung and prostate cancer cells also have reduced expression of Keap1 as a result of increased DNA methylation at the KEAP1 promoter (Wang et al., 2008; Zhang et al., 2010). These mutations and reduced expression of Keap1 resulted in constitutive stabilization of Nrf2 protein and persistently elevated expression of ARE-dependent cytoprotective genes.
The findings from Keap1 knockout and human cancer mutation studies led to the emerging view that—like any biological defense systems, such as inflammation, immune reaction, and DNA damage repair—the Nrf2-ARE pathway is a double-edged sword (Fig. 4)(Kensler and Wakabayashi, 2010; Taguchi et al., 2011). In normal cells under most conditions, Nrf2 is protective for the whole body, inhibiting both tumor initiation and cancer metastasis by eliminating carcinogens, ROS, and other DNA-damaging agents. On the other hand, persistently elevated activity of Nrf2 may be advantageous to single cancer cells for survival and resistance to ROS, anticancer drugs, and stress. It is believed that increased representation of Nrf2 and Keap1 mutations and elevated expression of ARE-dependent genes in cancer cells are the result of selection during tumor development rather than a cancer-initiating event, because knockdown of Keap1 in mice resulted in constitutive activation of Nrf2 but did not cause cancer over a 2-year period of time (Taguchi et al., 2010).
D. Human Polymorphism
Individual variability in the response to drugs, environmental insults, and disease signals in humans is a major challenge in today’s medicine and risk evaluation. Given the role of the Nrf2-ARE pathway as a major defense system in the body, individual variations of the pathway would contribute to such variability in human populations. Both genetic polymorphisms and epigenetic alterations could cause changes in protein level and/or structure to influence the function of key proteins in individual patients. Although knowledge on individual variability of the Nrf2 pathway remains sparse, a few recent studies provided examples that illustrate potential connections between Nrf2 polymorphisms and development of human diseases.
Position cloning of murine susceptibility genes for oxidant-induced acute lung injury identified Nrf2 as a candidate susceptibility gene for acute lung injury (Cho et al., 2002b). A human single-nucleotide polymorphism (SNP) (−617C/A) was identified in the promoter region of human Nrf2 (Marzec et al., 2007). The SNP significantly diminished the promoter activity in a reporter assay of the promoter. A nested case-control study showed that patients with the −617A polymorphism had a significantly higher risk for developing acute lung injury after major trauma compared with wild type (odds ratio of 6.44; 95% confidence interval of 1.34–30.8; p = 0.021), implicating Nrf2 in the protection against oxidant-induced acute lung injury in humans.
Among Japanese, three SNPs (i.e., −686A/G, −684G/A, and −650C/A) and one triplet repeat polymorphism (i.e., CCG repeats between −20 and −6) were found in the regulatory region of human Nrf2 (Yamamoto et al., 2004). The frequency of each polymorphism was examined in patients with systemic lupus erythematosus or chronic obstructive pulmonary disease. This study did not reveal a close connection between the polymorphisms and the risk of these two diseases. In a study with 209 Japanese patients with gastric cancer and 198 patients with no evidence of gastric malignancies, the −686/−684 A/G allele carriers showed a significantly reduced risk for gastric carcinogenesis (p = 0.022), especially for the diffuse type (p = 0.02), in Helicobacter pylori-negative cases. The inflammation scores in the carriers were significantly lower than those in the non-A/G carriers, suggesting that the −686/−684 haplotype is associated with the development of gastric inflammation and gastric carcinogenesis (Arisawa et al., 2008a). In a separate study with 89 Japanese patients with ulcerative colitis and 141 healthy volunteers, the −686*-684 genotype of Nrf2 was suggested to be associated with the development of ulcerative colitis (Arisawa et al., 2008b).
Nrf2 polymorphisms interact with smoking status to affect annual decline in forced expiratory volume in 1 s (FEV1) (Masuko et al., 2011). The mean annual FEV1 declines in people with rs6726395 (NIH dbSNP database) G/G, G/A, or A/A were significantly different (Pcorr = 0.016). A significant interaction between rs6726395 genotype and smoking status on the FEV1 decline was found (p for interaction = 0.011). The haplotype rs2001350T/rs6726395A/rs1962142A/rs2364722A/rs6721961T was associated with lower annual decline in FEV1 (p = 0.004). Therefore, Nrf2-dependent responses to exogenous stimuli may affect annual FEV1 decline in the general population, which is modified by smoking status, suggesting a gene-environment interaction in accelerated decline of FEV1.
In African Americans, −653G variant allele carriers (rs35652124) had significantly lower forearm blood flow and higher forearm vascular resistance under basal conditions as well as in response to bradykinin or sodium nitroprusside, compared with wild-type individuals (P < 0.05). In whites, although no significant associations were observed with the −653A/G genotype, −617A variant allele carriers (rs6721961) had significantly higher forearm vascular resistance at baseline and in the presence of bradykinin or sodium nitroprusside, compared with wild-type individuals (P < 0.05). These findings suggest that polymorphisms within the NFE2L2 promoter were associated with impaired forearm vasodilator responses in an endothelial-independent manner, supporting a role of Nrf2 in the regulation of vascular function in humans (Marczak et al., 2012).
VI. Targeting the Nuclear Factor Erythroid 2-Related Factor 2 Signaling-Antioxidant Response Element Pathway for Drug Development: Going beyond the Promise
The rapid advancement in understanding the biology and signaling mechanism of the Nrf2-ARE pathway has facilitated the development of ARE inducers as preventive and therapeutic agents in several ways. Dissection of the Nrf2-ARE signaling pathway has provided target molecules for mechanistic analysis of chemoprotection at molecular levels. Because Nrf2 is implicated in an increasing list of disease processes, the spectrum of target disease of ARE inducers has expanded beyond cancer to include many chronic diseases and toxicity. Development of new ARE inducers with potencies of <1 nM opened new possibilities to animal study and clinical trials. The observation that the cytoprotective effect of Nrf2 can be hijacked by cancer cells raised concerns about the potential perilous effects of Nrf2 activation but, at the same time, brought up the possibility of developing Nrf2 inhibitors to treat cancers with elevated Nrf2 functions.
A. New and Potent Antioxidant Response Element Inducers
Because the initial validation of phenolic inducers as effective chemopreventive agents, many new ARE inducers have been found from a variety of sources, including natural products and derivatives, environmental agents, clinical drugs, and endogenous compounds (Figs. 1 and 2). Among the inducers, oltipraz (a dithiolethione antiparasitic drug), sulforaphane (an isothiocyante), and some Michael reaction acceptors (coumarins, curcuminoids, chalcones, cinnamic acid derivatives, flavonoids and related synthetic bis(benzylidene)alkanone deviratives, and triterpenoids) have been examined in animal studies and some in clinical trials.
A series of triterpenoid analogs of oleanolic acid were synthesized as anticancer agents (Fig. 6A) (Liby et al., 2007b). The triterpenoids were identified as highly potent inducers of ARE-dependent genes, and the potency for induction of NQO1 was closely correlated (over 5 orders of magnitude) with the potency for inhibition of iNOS, a marker and mediator of inflammatory response (Dinkova-Kostova et al., 2005). To date, the triterpenoids are found to be among the most potent inducers of ARE-dependent genes. For a comparison, the potency for induction of ARE-dependent genes in mouse hepa1c1c7 cells (expressed as concentration required for doubling the specific activity of NQO1) is 2 nM for CDDO-Im (imidazolide of CDDO), 100 nM for sulforaphane, 10 μM for oltipraz, and 45 mM for BHT (Kensler and Wakabayashi, 2010). Triterpenoid inducers have been shown effective in protection against toxicity, cancer, and chronic diseases in a range of animal models. CDDO-Me (bardoxolone methyl) has successfully completed a phase II clinical trial for the treatment of chronic kidney disease associated with type 2 diabetes with a relatively mild safety profile (Pergola et al., 2011; Crunkhorn, 2012).
Fig. 6.
Triterpenoid inducers and Michael reaction with cysteine thiols. A, structures of selected triterpenoids and tricyclic and monocyclic cyano enones. Two Michael accepter motifs of triterpenoids are shown in boxs with broken lines. B, illustration of Michael adduct formation between Keap1 cysteine thiols and TBE 31 at two cyano enone sites.
Triterpenoid inducers interact with Keap1 cysteine thiols through Michael acceptor addition reaction in which a nucleophilic group, such as a cysteine thiol of Keap1, attacks the α,β-unsaturated carbonyl structures present in the A and C rings of triterpenoids, producing CDDO-Keap1 adducts. The cyano enone moiety in the A ring is more electrophilic than the enone group in the C ring (conjugated alkene and ketone) because of an additional cyano group (–C≡N). TBE 31 is an acetylenic tricyclic bis(cyano enone), which has a cyano enone in both A and C rings (Fig. 6A). The potency of TBE 31 for induction of NQO1 expressed in Dm (i.e., the concentration of inducer to cause 50% effect) is 1.1 nM, which is close to those of TP-225 (Dm = 0.27 nM, the most potent inducer so far) and CDDO (Dm = 2.5 nM) but is 31-fold lower than that of MCE 1 (Dm = 34 nM, a C ring-based, monocyclic cyano enone inducer) and is 264-fold less than that of sulforaphane (Dm = 290 nM) (Dinkova-Kostova et al., 2010). Both cyano enone moieties of TBE 31 react with Keap1 cysteine thiols to activate transcription of ARE-dependent genes, but the cyano enone in the C ring contributes more to inducer potency than that of the A ring, which is influenced by its spatial proximity to an acetylenic group in the C ring. The presence of two cyano enone moieties in A and C rings within a rigid three-ring molecule is likely to facilitate binding of the inducer to vicinal cysteine thiols of Keap1 to result in an exceptionally high inducer potency (Fig. 6B). These structure-activity relationships of triterpenoids and derivatives for induction of ARE-dependent genes are likely applicable to inhibition of inflammation by the inducers because of the close correlation between ARE induction and iNOS inhibition, although the molecular events underlying inhibition of iNOS by the inducers is less well defined (Honda et al., 2011).
Inhibition of Keap1-Nrf2 binding by peptide inhibitors provides another lead way for developing Nrf2 activators. A series of peptide inhibitors were synthesized based on the Nrf2 Neh2 domain and the peptide sequences of p62 and prothymosin-α, which are known to bind Keap1 DC competitively with Nrf2 Neh2. Among the peptide inhibitors, hybrid peptides based on the Nrf2 ETGE motif and the binding sequence of p62 exhibited superior binding activities (Hancock et al., 2012). However, modification of the peptides to increase stability is needed before the inhibitors can be evaluated in cell-and animal-based models.
B. Cancer Prevention and Therapy
Cruciferous vegetables such as broccoli contain anticarcinogens, such as glucoraphanin, the principal glucosinolate in broccoli sprouts. Glucoraphanin can be hydrolyzed by myrosinase in the plant or by gut microflora to sulforaphane (Zhang et al., 1992; Fahey et al., 2001). The effectiveness of broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetrols was examined in a randomized clinical trial in He Zuo township, Qidong, China, where the incidence of hepatocellular carcinoma is high because of the consumption of aflatoxin-contaminated foods (Kensler et al., 2005). An inverse association was observed for excretion of dithiocarbamates and aflatoxin-DNA adducts (p =0.002; r =0.31) in persons receiving broccoli sprout glucosinolate, although striking interindividual differences in bioavailability were noted. In animal studies, sulforaphane induced GSTs and NQO1 in wild-type but not Nrf2-deficient mice (Fahey et al., 2002). In an agreement with the induction activity, sulforaphane prevented Bp-induced tumor formation in the forestomach and inhibited extracellular, intracellular, and antibiotic-resistant strains of H. pylori, a risk factor for stomach cancer.
A placebo-controlled, double blind clinical trial of oltipraz was conducted in residents of Qidong, China, who have increased risks of liver cancer (Kensler et al., 2000). Oltipraz significantly increased excretion of aflatoxin-mercapturic acid, a derivative of the aflatoxin-glutathione conjugate, in the urine of participants receiving 125 mg of oltipraz by mouth daily, whereas administration of 500 mg of oltipraz once a week led to a significant reduction in the excretion of the primary oxidative metabolite of aflatoxin B1 (i.e., aflatoxin M1). In animal studies, oltipraz induced GST and NQO1 in wild-type mice by 2- and 5-fold, respectively, and significantly reduced the multiplicity of Bp-induced gastric neoplasia in the mice by 55% but had no effect on enzyme induction and tumor burden in Nrf2-null mice (Ramos-Gomez et al., 2001). Oltipraz significantly reduced Bp-DNA adduct formation (measured as tetrol) in the forestomach of the wild-type but not Nrf2-null mice (Ramos-Gomez et al., 2003). Oltipraz reduced the incidence of urinary bladder carcinoma by BBN in wild-type mice but had little effect in Nrf2-null mice (Iida et al., 2004). The anti-BBN bladder cancer effect of oltipraz was accompanied with significant induction of BBN glucuronidation, resulting from induction of UGTs, and reduction of urinary excretion of N-nitrosobutyl(3-carboxypropyl)amine, a proximate carcinogen of BBN, in wild-type mice, indicating reduced metabolic activation of BBN.
Triterpenoids potently inhibit tumorigenesis in animal studies. CDDO-Im inhibited aflatoxin-induced tumor formation in rat liver (Yates et al., 2006). CDDO-Im treatment caused an 85% reduction in the hepatic focal burden of preneoplastic lesions (i.e., GST-P positive foci) at 1 μmol/kg body weight and a >99% reduction at 100 μmol/kg body weight. Inhibition of tumorigenesis was accompanied with reduction of aflatoxin-DNA adducts by approximately 40 to 90% over the dose range. ARE-dependent genes were induced in an Nrf2-dependent manner in mouse liver after treatment with CDDO-Im. CDDO-Im was 100-fold more potent than oltipraz in this rat antitumorigenesis model. The methyl ester and ethyl amide of CDDO (CDDO-Me and CDDO-EA) prevented the development of lung cancer induced by vinyl carbamate in A/J mice, markedly reducing the number, size, and severity of lung carcinomas (Liby et al., 2007a). TBE-31 is among the most potent ARE inducer; oral administration of TBE-31 significantly reduced the formation of aflatoxin-DNA adducts and decreased the size and number of aflatoxin-induced preneoplastic hepatic lesions in rats by >90% (Liby et al., 2008).
C. Targeting Chronic Disease and Toxicity
On characterizing phenolic antioxidants for gene regulation, the inducers were found to exhibit potent anti-inflammatory activities, such as inhibition of the NF-κB signaling pathway and production of pro-inflammatory cytokines, including tumor necrosis factor α, interleukin 1β, and interleukin 6 in macrophages (Ma and Kinneer, 2002; Ma et al., 2003). This observation led to the proposal of chemoprotection for both chronic disease and cancer, as opposed to chemoprevention, which is traditionally proposed for cancer prevention. Many ARE inducers, including oltipraz, sulforaphane, and lately triterpenoids, have now been shown capable of both inducing ARE-dependent genes and inhibiting inflammatory response (Dinkova-Kostova et al., 2005; Killeen et al., 2006; Talalay et al., 2007; Liu et al., 2008b; Honda et al., 2011). Accordingly, ARE inducers have been examined for protection against a variety of chronic diseases and toxicity in an Nrf2-dependent manner over the past decade.
UV radiation (UVR) is a complete carcinogen and elicits a range of pathological events, including DNA damage, generation of ROS, inflammation, and immunosuppression. Topical application of sulforaphane-rich extracts of 3-day-old broccoli sprouts up-regulated ARE-dependent genes in mouse and human skin, which correlated with protection against UVR-induced inflammation and edema in mice and reduction of susceptibility of human skin to UVR-induced erythema (Talalay et al., 2007). Sulforaphane also induced ARE-dependent genes in retinal pigment epithelial cells and protected the cells from UVR (UV 320–400 nm) photooxidative damage, suggesting a potential of sulforaphane for prevention of age- and UVR-related macular degeneration (Gao and Talalay, 2004). Oltipraz protected mouse liver from α-naphthylisothiocyanate-induced cholestasis (Tanaka et al., 2009). BHA protected animals in a number of toxicity models of oxidative and electrophilic stress, including methylazoxymethanol acetate-induced liver necrosis and acetaminophen-induced hepatotoxicity (Reddy et al., 1982; Hazelton et al., 1986).
The high potency of the new, synthetic triterpenoids in the induction of ARE-dependent genes and inhibition of inflammation has raised a high interest in their potentials for prevention and therapy against chronic disease and toxicity, such as atherosclerosis, hepatitis/hepatic cirrhosis/hepatotoxicity, asthma/emphysema, inflammatory bowel disease/Crohn’s disease/ulcerative colitis, diabetes, and neurodegeneration, in addition to cancer (Liby et al., 2007b). CDDO-Im protected mouse liver from concanavalin A-induced inflammatory liver injury and acetaminophen hepatotoxicity in wild-type but not Nrf2-null mice (Osburn et al., 2008; Reisman et al., 2009a). CDDO-Im also reduced high fat diet-induced increases of body weight, fat mass, and liver lipid accumulation in wild-type but not Nrf2-null mice (Shin et al., 2009). CDDO prevented ileitis and down-regulated the production of inflammatory cytokines in mice infected with Toxoplasma gondii in a mouse model of inflammatory bowel disease (Minns et al., 2004). CDDO-MA is protective against the loss of dopaminergic neurons and striatal lesions in mouse models of Parkinson’s and Huntington’s diseases (Liby et al., 2007b). Dihydro-CDDO-trifluoroethyl amide, a new CDDO derivative, activated Nrf2 to suppress oxidative stress in cardiomyocytes, suggesting a potential for cardiac diseases (Ichikawa et al., 2009).
CDDO-Me has been examined in phase II clinical trials for the treatment of chronic kidney disease associated with type 2 diabetes, wherein CDDO-Me was associated with improvement in the estimated glomerular filtration rate in patients with the diseases at 24 weeks and improvement persisted at 52 weeks (Pergola et al., 2011). Moreover, the overall safety profile of CDDO-Me during the trial is encouraging, including mild muscle spasms, hypomagnesemia, and mild liver and intestinal effects (Pergola et al., 2011).
D. Nuclear Factor Erythroid 2-Related Factor 2 Inhibitors
Some cancer cells persistently up-regulate the Nrf2-ARE pathway through somatic mutations and epigenetic changes to take advantage of the protective power of the Nrf2 pathway for growth and survival. This observation suggests that Nrf2 inhibitors are potentially effective for anticancer therapy against certain tumors. However, few specific inhibitors of Nrf2 are currently available. High-throughput screening for compounds that inhibit Nrf2 is potentially useful for developing Nrf2 inhibitors. By screening natural products, brusatol, a plant quassinoid with potent antineoplastic activity, was found to inhibit the Nrf2-ARE pathway (IC50 = 40 nM) and enhance the efficacy of chemotherapy by cis-platin in vivo and in vitro (Ren et al., 2011). Structure-based and domain-specific inhibitors of Nrf2 can be developed by analogy with the development of selective inhibitors of the AP1 bZip domain from the three-dimensional structure of the AP1 bZip-DNA complex (Aikawa et al., 2008). This latter approach may help differentiate among the CNC bZip proteins such that only Nrf2, but not other bZip proteins, are inhibited. Mutant Nrf2 or Keap1 may exhibit differential sensitivities to individual inhibitors of Nrf2 from tumor to tumor. Thus, Nrf2 inhibitors should be characterized with respect to specific mutations in tumors, and inhibitor therapy should target persons with tumors bearing mutations that are sensitive to the inhibitor through targeted and individualized cancer therapy.
E. Safety Consideration on Pharmacological Activation of Nuclear Factor Erythroid 2-Related Factor 2
Many small-molecule inducers of ARE-dependent genes are electrophiles, raising the question of whether they cause gene mutation and cancer if used as pharmacological agents. On the other hand, it is known that electrophiles differ in their modes of interaction with macromolecules, in which “hard” and “soft” parameters determine the rates and reversibility of interaction with nucleophiles. The reactivity of chemoprotective agents toward cellular nucleophiles is distinct from those of the genotoxic electrophilic carcinogens. Michael acceptor inducers, such as those of ARE inducers, preferentially react with protein cysteine thiolates, which have low pKa and are “soft” bases, rather than the “hard” nucleophiles that are found in nucleic acids and are targets of electrophilic carcinogens. This notion is supported by the fact that no evidence for direct genotoxicity of ARE inducers was found so far (Kensler and Wakabayashi, 2010). In the clinical trial of CDDO-Me in patients with chronic kidney disease and type 2 diabetes, the overall safety profile during the 52-week treatment is consistent with mild “off target” side effects (Pergola et al., 2011). However, safety evaluation of CDDO-Me in larger patient populations is needed in order to detect rare and unanticipated adverse events.
Another safety concern over chemoprotective agents derives from the findings from genetic models of Nrf2 activation, in particular 1) the mouse model of Keap1 knockout that causes lethality in the early life of mouse and 2) cancers with mutations of Nrf2 and Keap1 that promote tumor growth and drug resistance. In these scenarios, persistent activation of Nrf2 and elevated expression of ARE-dependent genes were the major molecular phenotypes. A direct comparison between pharmacological and genetic activation of the Nrf2 pathway was performed by comparing the expression levels and patterns of ARE-dependent genes induced by CDDO-Im with those of hepatocyte-specific Keap1-knockout mice (Yates et al., 2009). The findings showed that the pathways affected by both means were similar, but genetic disruption caused much stronger and more persistent activation of Nrf2 than CDDO-Im; CDDO-Im induced pronounced but transient induction of ARE-dependent genes. Genetic activation of Nrf2 also resulted in significant induction of more genes within functional families than CDDO-Im. These results reveal that activation of the Nrf2 pathway by small-molecule inducers does not replicate the magnitude and duration of genetic perturbation. Therefore, an optimal activation of the pathway may be achieved in a pharmacological range between the biologically effective dose that minimally activates Nrf2 and a maximal-tolerated dose beyond which “off target” toxicity is induced to avoid any substantial side effects (Kensler and Wakabayashi, 2010).
VII. Conclusion
Induction of ARE-dependent DMEs and cytoprotective proteins has been a useful system for analyzing cellular defense against electrophiles and oxidants, which are common “stressor” molecules of many chemical and pathological insults. Such studies have provided significant new insights into the molecular basis of the stress response, in which the interplay among Nrf2, Keap1, and ARE inducers dictates the specificity, efficacy, and plasticity of chemical sensing and signaling toward electrophiles and oxidants. The functional importance of the Nrf2-ARE pathway was underscored by studies with Nrf2 and Keap1 knockout mouse models that exhibit altered sensitivities to a wide range of diseases and toxicity. Analysis of the pathway was instrumental for the identification of an increasing list of ARE-dependent genes with diverse functions and for the development of new and potent ARE inducers, some of which have shown efficacy against cancer, chronic diseases, and toxicity. The effect of persistent activation of Nrf2, potentially of more harm than benefit, was unveiled; tumor cells were found capable of harnessing the protective power of Nrf2 for their own survival.
A few notable questions remain to be addressed. The chemistry of the cysteine codes of Keap1 and Nrf2 and their interaction with specific ARE inducers remain largely unclear. Presumably, knowledge of the chemical nature of the cysteine codes and structural information of their spatial relationship to Nrf2, Keap1, and Cul3 in the E3 complex would be useful in addressing the question. Significant progress has been made in developing potent and specific ARE inducers as drug leads, whereas the search for Nrf2 inhibitors is just beginning. Future drug development would be facilitated by combining Nrf2 signaling-based high throughput screening, resolution of the three-dimensional structures of target proteins, and elucidation of the chemistry of cysteine thiolelectrophile/oxidant interaction. Increasingly important is the evaluation of potential side effects, especially those that are rare and unanticipated, of Nrf2 activators and inhibitors as preventive and therapeutic agents in large human populations.
Footnotes
Abbreviations: βNF, β-naphthoflavone; a.a., amino acid(s); AhR, aryl hydrocarbon receptor; AP-1, activator protein 1; ARE, antioxidant response element; ATF, activating transcription factor; Bach, BTB and CNC homolog; BBN, N-nitrosobutyl(4-hydroxybutyl)amine; BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene; bp, base pair(s); Bp, benzo[a]pyrene; BTB, Bric-a-brac, tramtrack, broad-complex; bZip, basic region leucine zipper; CBP, cAMP response element-binding protein-binding protein; CDDO, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid; CNC, cap ‘n’ collar; CTR, C-terminal region; Cul3, cullin 3; DC, DGR plus CTR; DGR, double glycine repeat; DME, drug-metabolizing enzyme; DRE, dioxin response element; ECH, erythroid cell-derived protein with CNC homology; ERα, estrogen receptor α; FEV1, forced expiratory volume in 1 s; GSK, glycogen synthase kinase; GST, glutathione transferase; HO-1, heme oxygenase 1; iNOS, inducible nitric-oxide synthase; IVR, intervening region; Keap1, Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1; Maf, musculoaponeurotic fibrosarcoma protein; MARE, Maf recognition element; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NF-κB, nuclear factor-κB; NFE2, nuclear factor erythroid 2; NQO1, NAD(P)H:quinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2-related factor 2; PKC, protein kinase C; RAR, retinoic acid receptor; Rbx1, RING box protein 1; ROS, reactive oxygen species; SCF, Skp1-Cul1-F-box; SNP, single-nucleotide polymorphism; tBHQ, tert-butyl hydroquinone; TPA, 12-O-tetradecanoylphorbol 13-acetate; TRE, TPA response element; UVR, UV radiation; VCD, 4-vinylcyclohexene diepoxide; XAR, xenobiotic-activated receptor.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Ma and He.
References
- Aikawa Y, Morimoto K, Yamamoto T, Chaki H, Hashiramoto A, Narita H, Hirono S, Shiozawa S. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat Biotechnol. 2008;26:817–823. doi: 10.1038/nbt1412. [DOI] [PubMed] [Google Scholar]
- Alam J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem. 1995;270:11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, Choi AM, Burow ME, Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Erdjument-Bromage H, Davidson MB, Tempst P, Orkin SH. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993a;362:722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Kotkow KJ, Ney PA, Erdjument-Bromage H, Tempst P, Orkin SH. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci USA. 1993b;90:11488–11492. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell PJ, Lo SC, Newton LG, Espinosa-Nicholas C, Zhang DD, Liu JH, Hannink M, Lubahn DB. Repression of cancer protective genes by 17beta-estradiol: ligand-dependent interaction between human Nrf2 and estrogen receptor alpha. Mol Cell Endocrinol. 2005;243:27–34. doi: 10.1016/j.mce.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ansher SS, Dolan P, Bueding E. Chemoprotective effects of two dithiol-thiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983;3:932–935. doi: 10.1002/hep.1840030608. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Hashimoto AH, Amanuma K, Matsumoto M, Hiyoshi K, Takano H, Ma-sumura K, Itoh K, Nohmi T, Yamamoto M. Enhanced spontaneous and benzo(a)pyrene-induced mutations in the lung of Nrf2-deficient gpt delta mice. Cancer Res. 2007;67:5643–5648. doi: 10.1158/0008-5472.CAN-06-3355. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- Apopa PL, He X, Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J Biochem Mol Toxicol. 2008;22:63–76. doi: 10.1002/jbt.20212. [DOI] [PubMed] [Google Scholar]
- Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Yoshioka D, Okubo M, Hirata I, et al. Nrf2 gene promoter polymorphism and gastric carcinogenesis. Hepatogastroenterology. 2008a;55:750–754. [PubMed] [Google Scholar]
- Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Yoshioka D, Okubo M, Sakata M, et al. Nrf2 gene promoter polymorphism is associated with ulcerative colitis in a Japanese population. Hepatogastroenterology. 2008b;55:394–397. [PubMed] [Google Scholar]
- Bauer AK, Cho HY, Miller-Degraff L, Walker C, Helms K, Fostel J, Yamamoto M, Kleeberger SR. Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice. PLoS One. 2011;6:e26590. doi: 10.1371/journal.pone.0026590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becks L, Prince M, Burson H, Christophe C, Broadway M, Itoh K, Yamamoto M, Mathis M, Orchard E, Shi R, et al. Aggressive mammary carcinoma progression in Nrf2 knockout mice treated with 7,12-dimethylbenz[a]anthracene. BMC Cancer. 2010;10:540. doi: 10.1186/1471-2407-10-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993;12:103–117. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- Benson AM, Batzinger RP, Ou SY, Bueding E, Cha YN, Talalay P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978;38:4486–4495. [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- Bork P, Doolittle RF. Drosophila kelch motif is derived from a common enzyme fold. J Mol Biol. 1994;236:1277–1282. doi: 10.1016/0022-2836(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10(Suppl):S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- Brown SL, Sekhar KR, Rachakonda G, Sasi S, Freeman ML. Activating transcription factor 3 is a novel repressor of the nuclear factor erythroid-derived 2-related factor 2 (Nrf2)-regulated stress pathway. Cancer Res. 2008;68:364–368. doi: 10.1158/0008-5472.CAN-07-2170. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Han XL, Kan YW. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci USA. 1993a;90:11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Han XL, Kan YW. Isolation of cDNA encoding the human NF-E2 protein. Proc Natl Acad Sci USA. 1993b;90:11366–11370. doi: 10.1073/pnas.90.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Kwong M, Lu R, Chang J, Wang B, Yen TS, Kan YW. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17:1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci USA. 2009a;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009b;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002a;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am J Respir Cell Mol Biol. 2002b;26:42–51. doi: 10.1165/ajrcmb.26.1.4536. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- Cowan KH, Batist G, Tulpule A, Sinha BK, Myers CE. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci USA. 1986;83:9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunkhorn S. Deal watch: Abbott boosts investment in Nrf2 activators for reducing oxidative stress. Nat Rev Drug Discov. 2012;11:96. doi: 10.1038/nrd3655. [DOI] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Talalay P, Sharkey J, Zhang Y, Holtzclaw WD, Wang XJ, David E, Schiavoni KH, Finlayson S, Mierke DF, et al. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J Biol Chem. 2010;285:33747–33755. doi: 10.1074/jbc.M110.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi Y, Alam J, Yoshizumi M, Sun J, Igarashi K. Heme oxygenase-1 gene enhancer manifests silencing activity in a chromatin environment prior to oxidative stress. Antioxid Redox Signal. 2006;8:60–67. doi: 10.1089/ars.2006.8.60. [DOI] [PubMed] [Google Scholar]
- Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984;44:5463–5474. [PubMed] [Google Scholar]
- Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H: quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- Friling RS, Bensimon A, Tichauer Y, Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci USA. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci USA. 2004;101:10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 4. Oxford University Press; New York: 2007. [Google Scholar]
- Hancock R, Bertrand HC, Tsujita T, Naz S, El-Bakry A, Laoruchupong J, Hayes JD, Wells G. Peptide inhibitors of the Keap1-Nrf2 protein-protein interaction. Free Radic Biol Med. 2012;52:444–451. doi: 10.1016/j.freeradbiomed.2011.10.486. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- Hazelton GA, Hjelle JJ, Klaassen CD. Effects of butylated hydroxyanisole on acetaminophen hepatotoxicity and glucuronidation in vivo. Toxicol Appl Pharmacol. 1986;83:474–485. doi: 10.1016/0041-008x(86)90230-9. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Lin GX, Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2·Keap1·Cul3 complex and recruiting Nrf2·Maf to the antioxidant response element enhancer. J Biol Chem. 2006;281:23620–23631. doi: 10.1074/jbc.M604120200. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- He X, Lin GX, Chen MG, Zhang JX, Ma Q. Protection against chromium (VI)-induced oxidative stress and apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association. Toxicol Sci. 2007;98:298–309. doi: 10.1093/toxsci/kfm081. [DOI] [PubMed] [Google Scholar]
- He X, Ma Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol Pharmacol. 2009;76:1265–1278. doi: 10.1124/mol.109.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Ma Q. Critical cysteine residues of Kelch-like ECH-associated protein 1 in arsenic sensing and suppression of nuclear factor erythroid 2-related factor 2. J Pharmacol Exp Ther. 2010;332:66–75. doi: 10.1124/jpet.109.160465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Ma Q. Redox regulation by Nrf2: gate-keeping for the basal and diabetes-induced expression of thioredoxin interacting protein. Mol Pharmacol. 2012 doi: 10.1124/mol.112.081133. http://dx.doi.org/10.1124/mol.112.081133. [DOI] [PMC free article] [PubMed]
- Higgins LG, Cavin C, Itoh K, Yamamoto M, Hayes JD. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicol Appl Pharmacol. 2008;226:328–337. doi: 10.1016/j.taap.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Honda T, Yoshizawa H, Sundararajan C, David E, Lajoie MJ, Favaloro FG, Jr, Janosik T, Su X, Honda Y, Roebuck BD, Gribble GW. Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents. J Med Chem. 2011;54:1762–1778. doi: 10.1021/jm101445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005a;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J Biol Chem. 2005b;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Sipes IG. Assessment of follicle destruction in chemical-induced ovarian toxicity. Annu Rev Pharmacol Toxicol. 1996;36:307–331. doi: 10.1146/annurev.pa.36.040196.001515. [DOI] [PubMed] [Google Scholar]
- Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated ovarian failure induced by 4-vinyl cyclohexene diepoxide in Nrf2 null mice. Mol Cell Biol. 2006;26:940–954. doi: 10.1128/MCB.26.3.940-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci USA. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Benkovic SA, Miller DB, O’Callaghan JP, Battelli L, Schwegler-Berry D, Ma Q. Vacuolar leukoencephalopathy with widespread astrogliosis in mice lacking transcription factor Nrf2. Am J Pathol. 2007;170:2068–2076. doi: 10.2353/ajpath.2007.060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009;4:e8391. doi: 10.1371/journal.pone.0008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Kataoka K, Itoh K, Hayashi N, Nishizawa M, Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994;367:568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- Iida K, Itoh K, Maher JM, Kumagai Y, Oyasu R, Mori Y, Shimazui T, Akaza H, Yamamoto M. Nrf2 and p53 cooperatively protect against BBN-induced urinary bladder carcinogenesis. Carcinogenesis. 2007;28:2398–2403. doi: 10.1093/carcin/bgm146. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2) Mol Cell Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- Jain AK, Jaiswal AK. Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J Biol Chem. 2006;281:12132–12142. doi: 10.1074/jbc.M511198200. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991;30:10647–10653. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain research. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Huang Z, Chan JY, Zhang DD. Nrf2 protects against As(III)-induced damage in mouse liver and bladder. Toxicol Appl Pharmacol. 2009;240:8–14. doi: 10.1016/j.taap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114:237–246. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Ylä-Herttuala S, et al. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Handa H, Nishizawa M. Induction of cellular antioxidative stress genes through heterodimeric transcription factor Nrf2/small Maf by anti-rheumatic gold(I) compounds. J Biol Chem. 2001;276:34074–34081. doi: 10.1074/jbc.M105383200. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994;14:700–712. doi: 10.1128/mcb.14.1.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, McMahon M, Hayes JD, Itoh K, Yamamoto M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys. 2005;433:342–350. doi: 10.1016/j.abb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Curphey TJ, Maxiutenko Y, Roebuck BD. Chemoprotection by organosulfur inducers of phase 2 enzymes: dithiolethiones and dithiins. Drug Metabol Drug Interact. 2000;17:3–22. doi: 10.1515/dmdi.2000.17.1-4.3. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kerppola TK, Curran T. A conserved region adjacent to the basic domain is required for recognition of an extended DNA binding site by Maf/Nrl family proteins. Oncogene. 1994;9:3149–3158. [PubMed] [Google Scholar]
- Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS, et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila) 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen ME, Englert JA, Stolz DB, Song M, Han Y, Delude RL, Kellum JA, Fink MP. The phase 2 enzyme inducers ethacrynic acid, DL-sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by RAW 264.7 cells. J Pharmacol Exp Ther. 2006;316:1070–1079. doi: 10.1124/jpet.105.092841. [DOI] [PubMed] [Google Scholar]
- Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- Kitahara A, Satoh K, Nishimura K, Ishikawa T, Ruike K, Sato K, Tsuda H, Ito N. Changes in molecular forms of rat hepatic glutathione S-transferase during chemical hepatocarcinogenesis. Cancer Res. 1984;44:2698–2703. [PubMed] [Google Scholar]
- Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M. Molecular cloning and functional characterization of a new Cap‘n’ collar family transcription factor Nrf3. J Biol Chem. 1999;274:6443–6452. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Tsukide T, Miyasaka T, Morita T, Mizoroki T, Saito Y, Ihara Y, Takashima A, Noguchi N, Fukamizu A, et al. Central nervous system-specific deletion of transcription factor Nrf1 causes progressive motor neuronal dysfunction. Genes Cells. 2011;16:692–703. doi: 10.1111/j.1365-2443.2011.01522.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Lee JM, Johnson DA, Kan YW, Johnson JA. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J Neurochem. 2006;98:1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Motohashi H, Sueno S, Kimura M, Takagawa H, Kanno Y, Yamamoto M, Tanaka T. Structural basis of alternative DNA recognition by Maf transcription factors. Mol Cell Biol. 2009;29:6232–6244. doi: 10.1128/MCB.00708-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Lee C, Hu T, Nguyen JM, Zhang J, Martin MV, Vawter MP, Huang EJ, Chan JY. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc Natl Acad Sci USA. 2011;108:8408–8413. doi: 10.1073/pnas.1019209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lee JM, Chan K, Kan YW, Johnson JA. Targeted disruption of Nrf2 causes regenerative immune-mediated hemolytic anemia. Proc Natl Acad Sci USA. 2004;101:9751–9756. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003;278:48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004a;18:261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004b;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang D, Hannink M, Beamer LJ. Crystal structure of the Kelch domain of human Keap1. J Biol Chem. 2004c;279:54750–54758. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, Gribble GW, Dmitrovsky E, Sporn TA, Sporn MB. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007a;67:2414–2419. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- Liby K, Yore MM, Roebuck BD, Baumgartner KJ, Honda T, Sundararajan C, Yo-shizawa H, Gribble GW, Williams CR, Risingsong R, et al. A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin. Cancer Res. 2008;68:6727–6733. doi: 10.1158/0008-5472.CAN-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multi-functional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007b;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008a;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci USA. 2008b;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SC, Li X, Henzl MT, Beamer LJ, Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006;25:3605–3617. doi: 10.1038/sj.emboj.7601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Xenobiotic-activated receptors: from transcription to drug metabolism to disease. Chem Res Toxicol. 2008;21:1651–1671. doi: 10.1021/tx800156s. [DOI] [PubMed] [Google Scholar]
- Ma Q. Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther. 2010;125:376–393. doi: 10.1016/j.pharmthera.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ma Q. Overview of AHR functional domains and the classical AHR signaling pathway: induction of drug-metabolizing enzyems. In: Pohjanvirta R, editor. The AH receptor in biology and toxicology. John Wiley & Sons; Hoboken, New Jersey: 2012. pp. 35–45. [Google Scholar]
- Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013 doi: 10.1146/annurev-pharmtox-011112-140320. http://dx.doi.org/10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed]
- Ma Q, Baldwin KT. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activaton and DNA binding of AhR. J Biol Chem. 2000;275:8432–8438. doi: 10.1074/jbc.275.12.8432. [DOI] [PubMed] [Google Scholar]
- Ma Q, Battelli L, Hubbs AF. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am J Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kinneer De-Fede K. A labile factor regulates induction of NQOR by TCDD and phenolic antioxidants (Abstract) Toxicologist. 2001;60:364. [Google Scholar]
- Ma Q, Kinneer K. Chemoprotection by phenolic antioxidants. Inhibition of tumor necrosis factor alpha induction in macrophages. J Biol Chem. 2002;277:2477–2484. doi: 10.1074/jbc.M106685200. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kinneer K, Bi Y, Chan JY, Kan YW. Induction of murine NAD(P)H: quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap ‘n’ collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem J. 2004;377:205–213. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kinneer K, Ye J, Chen BJ. Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol Pharmacol. 2003;64:211–219. doi: 10.1124/mol.64.2.211. [DOI] [PubMed] [Google Scholar]
- Ma Q, Lu AYH. Drug metabolizing enzymes, a group of promiscuous proteins. In: Prakash C, Gan L, Zhong D, Aizawa H, Lee P, editors. Handbook of Metabolic Pathways of Xenobiotics. John Wiley & Sons; New York: 2012. [Google Scholar]
- Ma Q, Renzelli AJ, Baldwin KT, Antonini JM. Superinduction of CYP1A1 gene expression. Regulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced degradation of Ah receptor by cycloheximide. J Biol Chem. 2000;275:12676–12683. doi: 10.1074/jbc.275.17.12676. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczak ED, Marzec J, Zeldin DC, Kleeberger SR, Brown NJ, Pretorius M, Lee CR. Polymorphisms in the transcription factor NRF2 and forearm vasodilator responses in humans. Pharmacogenet Genomics. 2012;22:620–628. doi: 10.1097/FPC.0b013e32835516e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- Masuko H, Sakamoto T, Kaneko Y, Iijima H, Naito T, Noguchi E, Hirota T, Tamari M, Hizawa N. An interaction between Nrf2 polymorphisms and smoking status affects annual decline in FEV1: a longitudinal retrospective cohort study. BMC Med Genet. 2011;12:97. doi: 10.1186/1471-2350-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap‘n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci USA. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- Mignotte V, Wall L, deBoer E, Grosveld F, Romeo PH. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989;17:37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns LA, Buzoni-Gatel D, Ely KH, Rachinel N, Luangsay S, Kasper LH. A novel triterpenoid induces transforming growth factor beta production by intra-epithelial lymphocytes to prevent ileitis. Gastroenterology. 2004;127:119–126. doi: 10.1053/j.gastro.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Mohler J, Vani K, Leung S, Epstein A. Segmentally restricted, cephalic expression of a leucine zipper gene during Drosophila embryogenesis. Mech Dev. 1991;34:3–9. doi: 10.1016/0925-4773(91)90086-l. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moi P, Kan YW. Synergistic enhancement of globin gene expression by activator protein-1-like proteins. Proc Natl Acad Sci USA. 1990;87:9000–9004. doi: 10.1073/pnas.87.22.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD. The world according to Maf. Nucleic Acids Res. 1997;25:2953–2959. doi: 10.1093/nar/25.15.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Hoshino H, Madisen L, Yanai N, Obinata M, Karasuyama H, Hayashi N, Nakauchi H, Yamamoto M, Groudine M, et al. Identification of Bach2 as a B-cell-specific partner for small maf proteins that negatively regulate the immunoglobulin heavy chain gene 3′ enhancer. EMBO J. 1998;17:5734–5743. doi: 10.1093/emboj/17.19.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Rushmore TH, Pickett CB. Transcriptional regulation of a rat liver glutathione S-transferase Ya subunit gene. Analysis of the antioxidant response element and its activation by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. J Biol Chem. 1994;269:13656–13662. [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H: quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioi P, Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun. 2007;362:816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- Nioi P, Nguyen T, Sherratt PJ, Pickett CB. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol Cell Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M, Kataoka K, Goto N, Fujiwara KT, Kawai S. v-maf, a viral oncogene that encodes a “leucine zipper” motif. Proc Natl Acad Sci USA. 1989;86:7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- Ogura T, Tong KI, Mio K, Maruyama Y, Kurokawa H, Sato C, Yamamoto M. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc Natl Acad Sci USA. 2010;107:2842–2847. doi: 10.1073/pnas.0914036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Sugimoto H, Utsunomiya H, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem Biophys Res Commun. 2009;389:431–436. doi: 10.1016/j.bbrc.2009.08.156. [DOI] [PubMed] [Google Scholar]
- Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, Taguchi K, Yamamoto M, Kensler TW. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Pearson WR, Windle JJ, Morrow JF, Benson AM, Talalay P. Increased synthesis of glutathione S-transferases in response to anticarcinogenic antioxidants. Cloning and measurement of messenger RNA. J Biol Chem. 1983;258:2052–2062. [PubMed] [Google Scholar]
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- Pitha-Rowe I, Liby K, Royce D, Sporn M. Synthetic triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci. 2009;50:5339–5347. doi: 10.1167/iovs.09-3648. [DOI] [PubMed] [Google Scholar]
- Prestera T, Talalay P. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci USA. 1995;92:8965–8969. doi: 10.1073/pnas.92.19.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska HJ, Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988;48:4776–4782. [PubMed] [Google Scholar]
- Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J, Segrè D. The effect of oxygen on biochemical networks and the evolution of complex life. Science. 2006;311:1764–1767. doi: 10.1126/science.1118439. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Furuya K, Hanson D, DiBello J, Berke B. Effect of dietary butylated hydroxyanisole on methylazoxymethanol acetate-induced toxicity in mice. Food Chem Toxicol. 1982;20:853–859. doi: 10.1016/s0015-6264(82)80218-6. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic acids research. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman SA, Buckley DB, Tanaka Y, Klaassen CD. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol. 2009a;236:109–114. doi: 10.1016/j.taap.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman SA, Csanaky IL, Aleksunes LM, Klaassen CD. Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol Sci. 2009b;109:31–40. doi: 10.1093/toxsci/kfp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci USA. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- Satoh H, Moriguchi T, Taguchi K, Takai J, Maher JM, Suzuki T, Winnard PT, Jr, Raman V, Ebina M, Nukiwa T, Yamamoto M. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis. 2010;31:1833–1843. doi: 10.1093/carcin/bgq105. [DOI] [PubMed] [Google Scholar]
- Sekhar KR, Yan XX, Freeman ML. Nrf2 degradation by the ubiquitin proteasome pathway is inhibited by KIAA0132, the human homolog to INrf2. Oncogene. 2002;21:6829–6834. doi: 10.1038/sj.onc.1205905. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN. Comparison of (-)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008a;135:1358–1368. 1368.e1–e4. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA. 2008b;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc Natl Acad Sci USA. 1995;92:8690–8694. doi: 10.1073/pnas.92.19.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol. 2010;30:3016–3026. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sonobe M, Menju T, Nakayama E, Mino N, Iwakiri S, Nagai S, Sato K, Miyahara R, Okubo K, et al. Mutations in Keap1 are a potential prognostic factor in resected non-small cell lung cancer. J Surg Oncol. 2010;101:500–506. doi: 10.1002/jso.21520. [DOI] [PubMed] [Google Scholar]
- Talalay P, Batzinger RP, Benson AM, Bueding E, Cha YN. Biochemical studies on the mechanisms by which dietary antioxidants suppress mutagenic activity. Adv Enzyme Regul. 1978;17:23–36. doi: 10.1016/0065-2571(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzyme Regul. 2003;43:121–134. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci USA. 2007;104:17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol Sci. 2009;108:247–257. doi: 10.1093/toxsci/kfp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telakowski-Hopkins CA, King RG, Pickett CB. Glutathione S-transferase Ya subunit gene: identification of regulatory elements required for basal level and inducible expression. Proc Natl Acad Sci USA. 1988;85:1000–1004. doi: 10.1073/pnas.85.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki T, Katsuoka F, Kanezaki R, Xu G, Kurotaki H, Sun J, Kamio T, Watanabe S, Tandai S, Terui K, et al. Transgenic expression of BACH1 transcription factor results in megakaryocytic impairment. Blood. 2005;105:3100–3108. doi: 10.1182/blood-2004-07-2826. [DOI] [PubMed] [Google Scholar]
- Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita T, Li L, Nakajima H, Iwamoto N, Nakajima-Takagi Y, Ohashi K, Kawakami K, Kumagai Y, Freeman BA, Yamamoto M, et al. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes Cells. 2011;16:46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Walker AK, See R, Batchelder C, Kophengnavong T, Gronniger JT, Shi Y, Blackwell TK. A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap’n’Collar-related basic leucine zipper proteins. J Biol Chem. 2000;275:22166–22171. doi: 10.1074/jbc.M001746200. [DOI] [PubMed] [Google Scholar]
- Wang R, An J, Ji F, Jiao H, Sun H, Zhou D. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochem Biophys Res Commun. 2008;373:151–154. doi: 10.1016/j.bbrc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci USA. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg LW. Inhibition of carcinogenic and toxic effects of polycyclic hydrocarbons by phenolic antioxidants and ethoxyquin. J Natl Cancer Inst. 1972;48:1425–1430. [PubMed] [Google Scholar]
- Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, Conney AH, Kong AN. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- Xu W, Hellerbrand C, Köhler UA, Bugnon P, Kan YW, Werner S, Beyer TA. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab Invest. 2008;88:1068–1078. doi: 10.1038/labinvest.2008.75. [DOI] [PubMed] [Google Scholar]
- Xue F, Cooley L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72:681–693. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Yoh K, Kobayashi A, Ishii Y, Kure S, Koyama A, Sakamoto T, Sekizawa K, Motohashi H, Yamamoto M. Identification of polymorphisms in the promoter region of the human NRF2 gene. Biochem Biophys Res Commun. 2004;321:72–79. doi: 10.1016/j.bbrc.2004.06.112. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Large Maf Transcription Factors: Cousins of AP-1 Proteins and Important Regulators of Cellular Differentiation. Einstein J Biol Med. 2007;23:2–11. doi: 10.23861/ejbm20072347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baum-gartner KJ, Roebuck BD, Liby KT, Yore MM, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Deng T, Cavigelli M, Karin M. Antitumor promotion by phenolic antioxidants: inhibition of AP-1 activity through induction of Fra expression. Proc Natl Acad Sci USA. 1995;92:4972–4976. doi: 10.1073/pnas.92.11.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther. 2010;9:336–346. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipper LM, Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]