Abstract
The receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain (RAIDD/CRADD) functions as a dual adaptor and is a constituent of different multi-protein complexes implicated in the regulation of inflammation and cell death. Within the PIDDosome complex, RAIDD connects the cell death-related protease, Caspase-2, with the p53-induced protein with a death domain 1 (PIDD1). As such, RAIDD has been implicated in DNA-damage-induced apoptosis as well as in tumorigenesis. As loss of Caspase-2 leads to an acceleration of tumor onset in the Eμ-Myc mouse lymphoma model, whereas loss of Pidd1 actually delays onset of this disease, we set out to interrogate the role of Raidd in cancer in more detail. Our data obtained analyzing Eμ-Myc/Raidd−/− mice indicate that Raidd is unable to protect from c-Myc-driven lymphomagenesis. Similarly, we failed to observe a modulatory effect of Raidd deficiency on DNA-damage-driven cancer. The role of Caspase-2 as a tumor suppressor and that of Pidd1 as a tumor promoter can therefore be uncoupled from their ability to interact with the Raidd scaffold, pointing toward the existence of alternative signaling modules engaging these two proteins in this context.
A number of mechanisms have evolved to trace and remove potentially dangerous cells. Deregulation of the induction of apoptosis upon oncogenic stress, for example, can facilitate the accumulation of cells prone to undergo malignant transformation. Cell death by apoptosis depends on the cascade-like activation of proteases of the Caspase family.1 Among these, the evolutionarily most conserved protease, Caspase-2, turns out to be a potent tumor suppressor in mice2, 3, 4, 5, 6, 7 and correlative expression data support a conserved role in human cancer.8, 9, 10, 11, 12, 13
Early studies suggested that Caspase-2 interacts with other proteins for its activation (e.g., after genotoxic stress), but the protease seems also able to auto-activate cell death on its own when present in sufficiently high concentration.14, 15, 16, 17, 18 The most prominent Caspase-2-containing protein complex was dubbed the ‘PIDDosome' and described to contain the p53-induced protein with a death domain (PIDD1) and receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain (RAIDD, also known as CRADD).19 Although the molecular details of the pro-apoptotic potential of Caspase-2 are still discussed and alternative roles in the DNA-damage response, cell cycle arrest or sensor of metabolic stress are mechanistically poorly understood, Caspase-2 clearly limits tumorigenesis in different settings. These include aberrant expression of c-Myc in B cells3, 4 or deletion of the DNA-damage response regulator, ataxia telangiectasia mutated kinase (ATM), both driving lymphomagenesis6 as well as overexpression of the Her2/ErbB2 oncogene in breast5 or that of mutated KRAS in the lung epithelium, driving carcinoma formation.7 One of these studies, addressing also the role of Pidd1 in c-Myc-driven lymphomagenesis, revealed an unexpected oncogenic role for Pidd1, thereby questioning the physiological relevance of the PIDDosome complex in Caspase-2-mediated cell death and tumor suppression.4, 20 However, the exact role of the scaffold protein Raidd within these processes remains unaddressed so far.
Raidd, a bipartite adapter containing a death domain (DD) and a caspase-recruitment domain (CARD) was first described to bind to the DD-containing kinase RIPK1 and the C. elegans caspase CED-3,21 supporting a role in cell death initiation. Subsequently, the interaction of Caspase-2 and Raidd was biochemically proven22 and proposed to be required for Caspase-2 autoprocessing preceding its activation.19 More recent studies propose an anti-inflammatory role for Raidd through suppression of nuclear factor kappa-light-chain enhancer (NF-κB) activation and cytokine production upon T-cell receptor stimulation by negatively interfering with the Carma1/Malt1/Bcl-10 signaling complex.23, 24
First evidence for a potential role of RAIDD in human cancer was discovered in a biochemical screen using mantle cell lymphomas, which detected a downregulation of RAIDD by microarray analysis,10 whereas others reported on RAIDD-linked multidrug resistance in osteosarcoma cells.25 Furthermore, tumor cell apoptosis induced by inhibitors of histone de-acetylases in treatment-resistant adult T-cell leukemia lines reportedly required Caspase-2 and Raidd.26 It is also reported that the Caspase-2/Raidd axis is necessary after ER stress, for example, in the course of infection with the oncolytic maraba virus.27
Taken together, these studies support a role for RAIDD in drug-induced cancer cell death as well as in tumor suppression, most likely linked to its role as a direct activator of Caspase-2. Alternatively, RAIDD may negatively interfere with PIDD- or BCL10-regulated NF-κB signaling23, 24, 28 and thereby suppress pro-tumorigenic inflammation. To address the role of Raidd in tumorigenesis in more detail, we exploited different mouse models where we induced thymic lymphomas by γ-irradiation, fibrosarcomas by 3-methylcholanthrene (3-MC) injection or B-cell lymphomas by aberrant expression of the c-Myc proto-oncogene. Our results suggest that Raidd is not a suppressor of tumors in the mouse models tested.
Results
Loss of Raidd has no impact on tumor formation after DNA damage
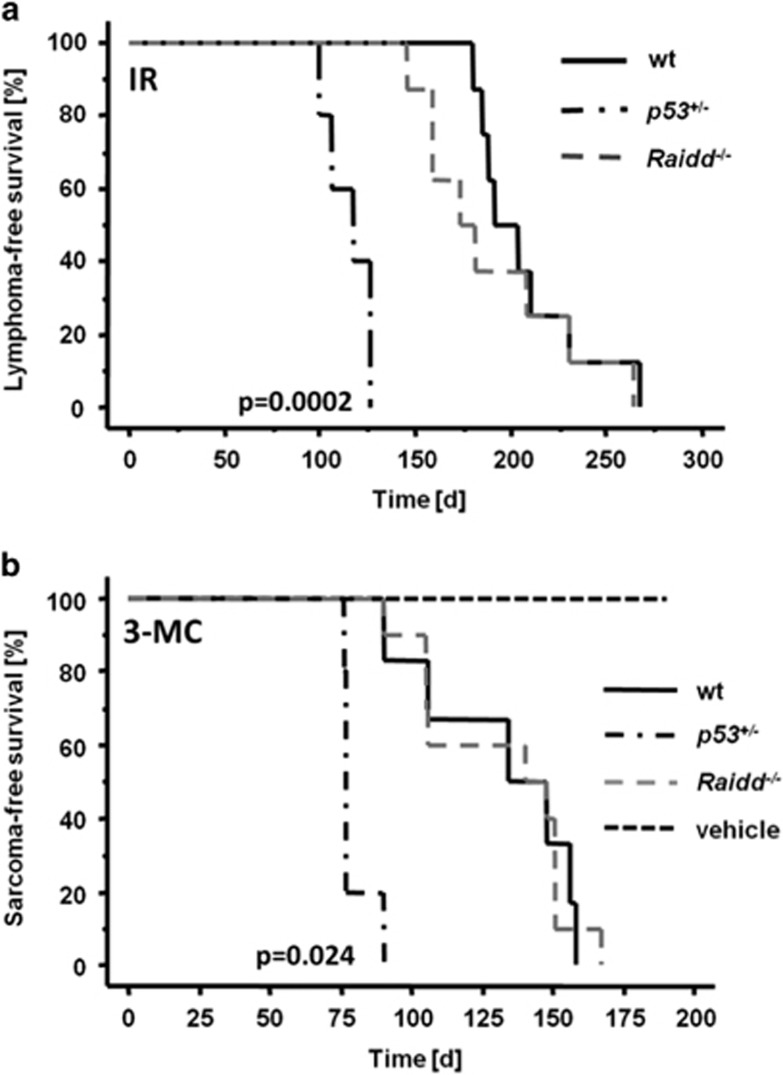
As Raidd was reported to have a role in DNA-damage-induced apoptosis because of its ability to form a complex with Pidd1 and Caspase-2,19 we used different cancer models to evaluate the impact of Raidd in tumorigenesis induced by DNA lesions. Induction of thymic lymphomas was triggered in 4-week-old mice by repeated low-dose irradiation (1.75 Gy). As reported before, tumor formation was significantly accelerated in p53+/− mice when compared with wild-type (wt) controls, showing a mean survival of 115 and 207 days, respectively. Absence of Raidd, however, had no effect on the onset of tumorigenesis (Figure 1a) or tumor immunophenotype (data not shown) and animals developed thymic lymphomas with a mean survival of 190 days, similar to their wt littermates (P=0.37).
Figure 1.
Loss of Raidd does not impact on DNA-damage-induced tumor formation. (a) Kaplan–Meier plot of tumor-free survival in response to repeated low-dose γ−irradiation (4 × 1.75 Gy) of wt (n=8, mean=207 days), Raidd−/− (n=8, mean=190 days) and p53+/− mice (n=5, mean=115 days). (b) Kaplan–Meier analysis of 3-methylcholanthrene (3-MC)-treated mice. Wild type (n=6, median=132 days); Raidd−/− (n=10, mean=131 days); p53+/− (n=5, mean=79 days). Wild-type mice injected with vehicle only (n=3) were used as solvent controls
In another tumor model, we treated adult mice with a single injection of 3-MC causing the formation of bulky adducts in DNA leading to fibrosarcoma formation within 6 months. As reported before,29 p53+/− mice developed 3-MC-induced sarcomas significantly earlier but loss of Raidd failed to accelerate tumor onset when compared with wt controls (Figure 1b; P=0.97).
Together with our previously published data4 this demonstrates that the PIDDosome, and each of its components individually, are unable to prevent DNA-damage-induced tumor formation inflicted by γ-irradiation or bulky DNA-adduct formation.
c-Myc-induced lymphomagenesis is limited by Caspase-2 in a Raidd-independent manner
Caspase-2 and Pidd1 were reported to modulate lymphomagenesis in Eμ-Myc mice, a model somewhat mimicking human Burkitt lymphoma.4 Hence, we introduced Raidd deficiency into Eμ-Myc transgenic animals to explore its impact on tumorigenesis caused by oncogenic stress. Excess proliferation by aberrant c-Myc expression in premalignant mice is usually counterbalanced by increased cell death rates providing a rational why loss of pro-apoptotic proteins, for example, of the BH3-only group, accelerates disease onset.30, 31, 32, 33
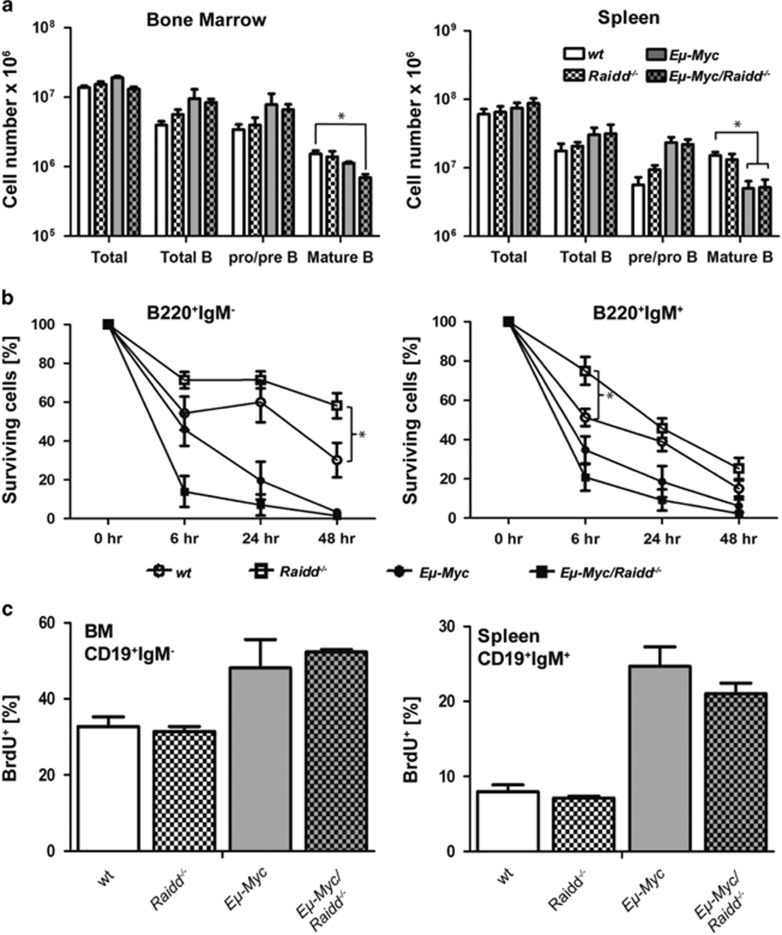
To evaluate the role of Raidd early in disease development, the B-cell subset composition was assessed in premalignant Eμ-Myc/Raidd−/− mice. In line with published data,4, 32, 34, 35 the numbers of B220+IgM− pro/pre-B cells were increased in bone marrow and spleen, whereas numbers of mature B cells were diminished in the periphery of Eμ-Myc mice when compared with wt controls (Figure 2a). However, no other gross alterations within the B-cell composition and distribution in Eμ-Myc mice specifically lacking Raidd were noted.
Figure 2.
B-cell subset composition, proliferation and cell death responsiveness in premalignant Raidd−/− mice. (a) The development and the distribution of B cells in bone marrow and spleen was assessed in 4-week-old Eμ-Myc transgenic mice lacking or expressing Raidd. Single-cell suspensions of the organs were counted and stained with cell surface marker-specific antibodies and analyzed by flow cytometry. Bars represent means (n=3-5 per genotype)±S.E.M. (b) Sorted premalignant B cells (pre-B from bone marrow, CD19+IgM−CD43− or immature B cells CD19+IgMlow from spleen) were analyzed for spontaneous death after 6, 24 and 48 h using Annexin-V plus 7-AAD staining in a flow cytometer. Symbols represent means of n=3-5 per genotype±S.E.M. (c) Proliferation rates were quantified 4 h after a single injection of BrdU in bone-marrow-derived pro/pre-B cells, (CD19+IgM−) or (CD19+IgM+) B cells from spleen. Bars represent means (n=3-5) per genotype±S.E.M.
To monitor B-cell survival upon oncogenic stress in the absence or presence of Raidd, we next sorted pre-B and immature B cells of premalignant mice and put them in culture. Notably, B cells from non-transgenic Raidd−/− mice appeared to be slightly less sensitive to spontaneous cell death when compared with wt (Figure 2b). As reported before,30, 31, 32 B cells derived from Eμ-Myc mice died more rapidly when compared with the non-transgenic counterparts but additional lack of Raidd had no relevant impact (Figure 2b). Similarly, Raidd deficiency had no impact on homeostatic or c-Myc-driven B-cell proliferation, as assessed by BrdU-incorporation analysis in immature CD19+IgM− and CD19+IgM+ B cells from bone marrow or spleen of premalignant mice (Figure 2c).
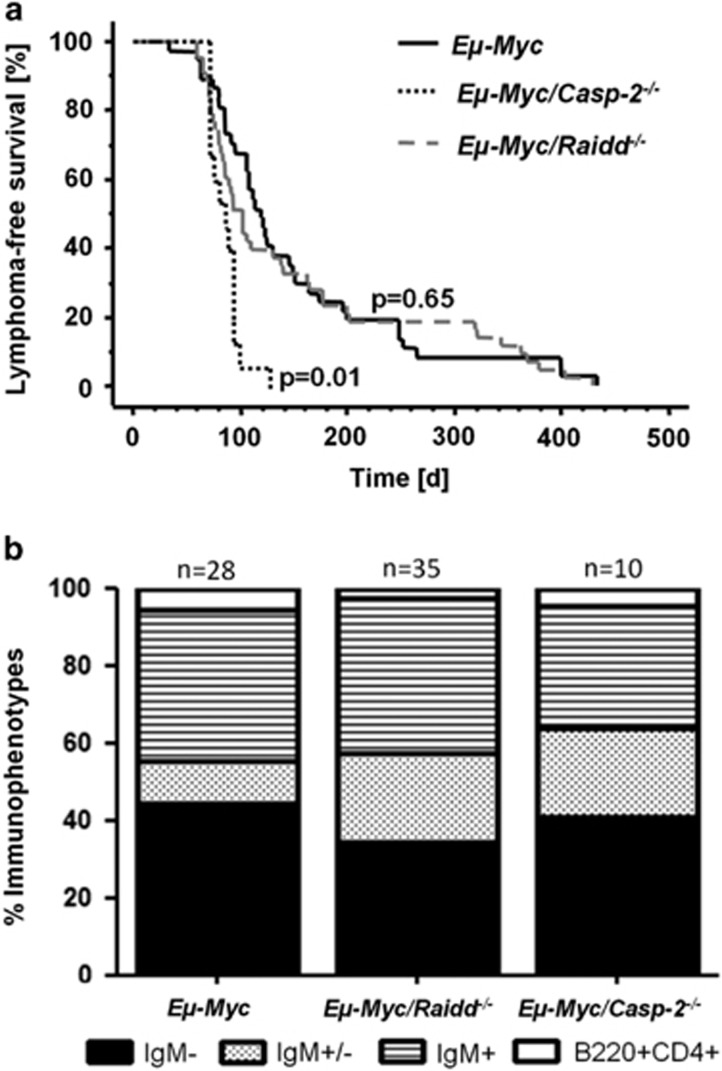
Next, cohorts of Eμ-Myc, Eμ-Myc/Raidd−/− and, for reference, Eμ-Myc/Casp-2−/− mice were monitored for tumor onset. Strikingly, although loss of Caspase-2 leads to accelerated disease onset (mean survival: 101 d), loss of Raidd had no effect, when compared with Raidd-proficient Eμ-Myc mice presenting with a mean survival: 128 versus 124 days (Figure 3a). White blood cell counts and tumor burden were also comparable between genotypes (Supplementary Figures 1a and b).
Figure 3.
Suppression of c-Myc-induced B-cell lymphoma formation by Caspase-2 does not depend on Raidd. (a) Lymphoma-free survival of Eμ-Myc (n=37, mean=128 days), Eμ-Myc/Raidd−/− (n=43, mean=124 days) and Eμ-Myc/Casp-2−/− mice (n=15, mean=101 days). (b) Flow cytometric analysis of tumor immunophenotype. A comparison of the frequency of the different immunophenotype across genotypes by Chi-square test revealed differences between wt and Casp-2−/− tumors (P=0.043), whereas the overall distribution between wt and Raidd−/− tumors was not significantly different (P=0.1)
Tumors were subsequently classified by flow cytometry as either pre-B cell (IgM−), immature B cell (IgM+), mixed (IgM+/−) or as early hematopoietic progenitor-derived lymphomas (B220+CD4+). χ2 analysis confirmed differential distribution between wt and Casp-2−/− tumors (P=0.043), as reported before4 but no significant difference was noted between wt and Raidd−/− tumors (Figure 3b).
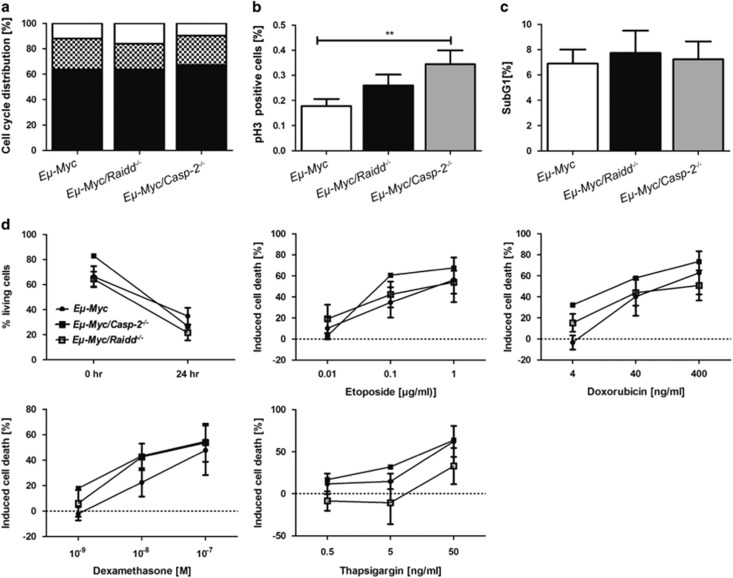
Further, in lymphoma cells derived from Eμ-Myc/Raidd−/− mice, cell cycle distribution (Figures 4a and b), spontaneous (Figure 4c) and drug-induced cell death (Figure 4d) were monitored and found comparable to their wt counterparts. Notably, Caspase-2-deficient tumors showed again an increase in phospho-Histone H3 staining, suggesting perturbed cell cycle control (Figure 4b). In line with a lack of impact on tumor latency, loss of Raidd did not release the pressure to inactivate p53, a known secondary hit, usually observed in about 25% of all Eμ-Myc lymphomas.36 Inactivation of p53, as assessed by western blotting for p53 and p19ARF, was seen at similar rates in Eμ-Myc (26%, n=11) and Eμ-Myc/Raidd−/− (27%, n=19) tumors (Supplementary Figures 1c and d).
Figure 4.
Normal cell cycle distribution and tumor cell apoptosis in Eμ-Myc tumor cells lacking Raidd. (a) Cell cycle phases G1 (black), S (dotted) and G2/M (white) of lymphoma cells were assessed ex vivo by intracellular DNA content analysis in tumors derived from Eμ-Myc (n=21), Eμ-Myc/Raidd−/− (n=22), Eμ-Myc/Casp-2−/− (n=10) mice. (b) To define the percentage of cells in M-phase, ex vivo tumor cells were stained with an antibody specific for the phosphorylated variant of Histone H3 (pH3) and propidium iodide. Bars represent mean values of pH3+ cells±S.E.M. Eμ-Myc (n=17), Eμ-Myc/Raidd−/− (n=11) Eμ-Myc/Casp-2−/− (n=5). (c) Apoptosis in situ was assessed by Sub-G1 analysis of freshly isolated lymph node tumor masses followed flow cytometric analysis (wt n=24, Raidd−/− n=22, Casp-2−/− n=10) mean values±S.E.M. (d) Freshly isolated lymphoma cells were cultivated on feeder cells for 24 h and were either left untreated (upper left graph) or exposed to increasing doses of the indicated chemo-therapeutics. Cell death was assessed by Annexin-V/7-AAD staining combined with anti-CD19 to identify B cells. Symbols represent mean values, n>3–7 for each genotype±S.E.M.
Overall, these data demonstrate that the adaptor protein Raidd is not limiting Myc-driven tumorigenesis thereby uncoupling the tumor-suppressor function of Caspase-2 from Raidd-dependent autoactivation and the oncogenic potential of Pidd1 from Raidd-modulated NF-κB signaling.
Loss of Caspase-2 promotes aneuploidy
As Caspase-2 has been implicated in mediating mitotic catastrophe as a response to DNA-damage after failed cell cycle arrest37, 38 and that c-Myc overexpression drives proliferation stress leading to DNA damage,39 we investigated if lack of Caspase-2 would influence genomic stability of normal, premalignant or transformed cells.
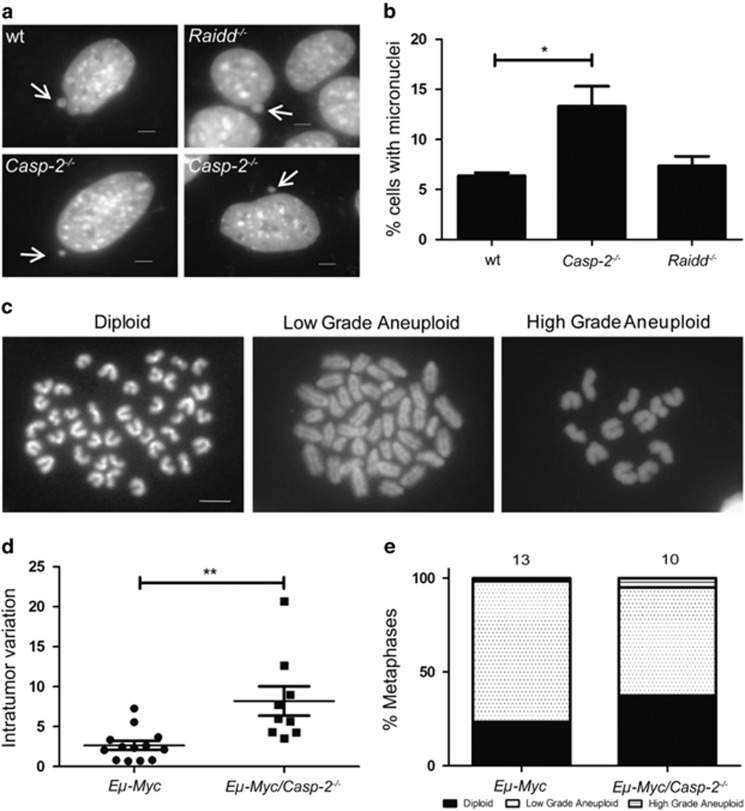
First, we investigated chromosomal stability in SV40-immortalized mouse embryonic fibroblasts (MEFs) from wt and Caspase-2-deficient mice (Figures 5a and b). Numbers of micronuclei, which emerge after errors during mitosis and result in individual chromosomes or fragments outside of the main nucleus, were significantly increased in cells lacking Caspase-2 in comparison to wt cells. Raidd−/− MEFs did not show increased susceptibility to micronuclei formation (Figure 5b). Chromosomal stability was further examined by counting chromosome numbers in metaphase spreads in B-cell lymphomas (Figures 5c–e). Eμ-Myc transgenic B-cell tumors deficient for Caspase-2 did show a higher variation of chromosome numbers within individual tumor samples. Taken together, these data are consistent with published results, indicating that Caspase-2 acts in maintaining genomic stability.37
Figure 5.
Caspase-2-loss leads to increased micronuclei formation and aneuploidy. (a) Representative images of micronuclei formation in SV40 MEFs of the indicated genotypes stained with 4′,6′-diamidino-phenylinodole. Arrows indicate micronuclei formation in wt MEFs. Scale bars=5 μm. (b) Quantification of data assessed in a. A minimum of 300 cells per genotype was evaluated. Wt versus Casp-2−/− (P=0.03; Student's t-test). Bars represent mean of n>4–6 for each genotype±S.E.M. (c) Representative images of chromosome spreads using freshly isolated Eμ-Myc lymphoma cells. Metaphase spreads are showing representative euploid (left panel) or aneuploidy karyogramms ranging from low (middle panel) to high-grade (right panel). Scale bar=5 μm. A minimum of 50 spreads per genotype was evaluated. (d) Variation of counted chromosome numbers within single tumors from Eμ-Myc (n=13) versus Eμ-Myc/Casp-2−/− (n=10) mice (P=0.0029; Student's t-test). (e) Quantification of the variance of chromosome numbers in tumors derived from indicated genotypes. A χ2 test showed a significant difference between wt and Casp-2−/− (P<0.0001)
In an attempt to identify potential effectors of Caspase-2 in this process and based on the reported role of Caspase-2 in p53 activation and target gene expression upon DNA damage,40 we performed qPCR analysis as well as unbiased genome-wide expression analysis comparing mRNA from premalignant splenic IgM+D− B cells of Eμ-Myc transgenic animals deficient or proficient for Caspase-2 or Raidd. We started by comparing expression levels of mRNAs of p21, Noxa and Puma by qRT-PCR, anticipating that c-Myc-driven p53 activation would result in reduced expression of these targets in the absence of Caspase-2.40 This, however, was not the case, or at least the noted differences did not reach statistical significant differences (Supplementary Figure 2). Comparison of gene-chip data revealed only few candidates, including Fbxw10, Fgd6, Hist3h2a, Hmha1, Igf1R, Lyrm7, RhoBTB1, Slc25a13 and Zfp39, that were deregulated more than twofold in the absence of Caspase-2 or RAIDD when compared with wt (Supplementary Figure 2). Only one of these candidates, that is, RhoBTB1, was subsequently confirmed by qPCR analysis. However, as RhoBTB1 is unlikely related to genomic stability this was not followed up in detail during the course of this study. This indicates that there are no significant changes, at least in the premalignant B cells analyzed that may contribute to the genomic instability observed following loss of Caspase-2.
Discussion
In this study, we investigated the relevance of Raidd in tumorigenesis sparked by its function as an adaptor protein for Caspase-2 and Pidd1, both being reported to influence tumorigenesis in vivo. Caspase-2 is well characterized as a tumor-suppressor gene in various mouse cancer models,3, 4, 5, 6, 7 whereas loss of Pidd1 leads to a delayed tumor onset upon c-Myc overexpression.4 Our data demonstrate that loss of Raidd has no influence on the development of radiation-induced thymic lymphomas or chemically induced fibrosarcomas (Figure 1). Although the mean latency in the absence of Raidd appeared to be shortened when compared with wt (190 versus 205 days), statistical significance was not reached, possibly due to the rather small cohort size, requiring more detailed follow up. Yet, these observations are similar to the published results in Caspase-2- and Pidd1-deficient mice4 and exclude a role of any of the PIDDosome components in these DNA-damage-driven models of cancer.
Strikingly, Raidd deficiency also had no influence on tumor latency in c-Myc-driven B-cell lymphomas (Figure 3a), contrasting the findings with Caspase-2- or Pidd1-deficient mice, where Caspase-2 acts as a tumor suppressor and Pidd1 as a tumor promoter in this model. Our data show that loss of Raidd is dispensable for the development of B-cell lymphomas in the Eμ-Myc mouse model and that Raidd deficiency has no impact on the development, proliferation and cell death of premalignant and transformed cells (Figure 2). The results thus indicate that Caspase-2-mediated tumor suppression is independent of both Pidd1 and Raidd. This is in line with the idea that Caspase-2 autoactivation can occur without the need for additional accessory proteins.14, 15, 16 Consistent with our conclusion, it has been shown that Caspase-2 indeed appears to be able to exert biological effects that do not depend on either catalytic activity or interaction with RAIDD or PIDD1, exemplified by translational control of p21 levels.41 As reduced levels of p21 by Caspase-2 knockdown sensitizes Hct116 colon cancer cells to irradiation-induced cell death,41 one may speculate that a similar effect may account for the tumor-suppressive effect noted in Eμ-Myc/Casp-2−/− mice. However, deficiency in Cdkn1a, encoding for p21, has no impact on the tumor latency in Eμ-Myc mice,42 excluding this possibility. However, it remains possible that not all aspects of Caspase-2 biology are conserved between mice and humans, as previously also recognized for the Caspase-2-dependent Chk1-suppressed cell death pathway that appears functional in human cancer cells but not in mouse lymphocytes or MEF.43
It is also not easy to reconcile the findings connecting deregulated expression of RAIDD in human cancer development and drug-resistant phenotypes10, 25, 26, 27 with our data presented here. Although deregulated expression of RAIDD levels in cancer may be a bystander effect, for example, due to de-differentiation of cancer cells or deregulation of multiple signaling pathways ultimately also impacting on RAIDD mRNA levels, the differences in drug resistance, or lack thereof (our study) may simply be due to cell type-specific differences, the oncogenic drivers or the triggers used. Investigating the role of PIDDosome components in other, preferentially, non-hematological tumor models will shed some light.
Prior work linked loss of Caspase-2 to chromosomal and genomic instability as a possible driver of accelerated transformation.37 Consistently, SV40 MEFs and c-Myc transgenic B cells lacking Caspase-2 showed significantly higher levels of micronuclei formation and numbers of chromosomes in metaphase spreads diverted more strongly from the normal number. However, our analysis of chromosome numbers in Eμ-Myc B-cell tumors did not show a direct increase in the frequency of aneuploidy in cells lacking Caspase-2. Instead, we found that the aneuploid cells present in the Caspase-2-deficient tumors showed a greater variation of chromosome numbers in the same tumor sample. Furthermore, our data show that the level of high-grade aneuploidy (i.e., a loss or gain of more than 10 chromosomes) was greater in Caspase-2−/− tumors. Together, these results clearly support a possible role of Caspase-2 in maintaining genomic integrity, whereas loss of the adaptor protein Raidd is dispensable for genomic stability (not shown).
As it is difficult to connect these observations with known substrates of Caspase-2 and the analysis of representative p53-induced genes failed to support impaired murine double minute 2 activity, as reported in cisplatin-treated lung cancer cells,40 we performed whole transcriptome analysis on premalignant Eμ-Myc pre-B cells lacking or expressing Caspase-2 (or Raidd). However, we again failed to identify possible candidates that may be involved in the observed tumor-suppressor phenotype and established p53 targets were also not deregulated (Supplementary Figure 2). Hence, it remains possible that the phenomenon of reduced p53 activation in the absence of Caspase-2 is cell type-specific, for example, in lung epithelium,40 and/or a particular secondary hit, or a fully transformed cellular state.37 Consistent with the latter scenario, we previously reported on a reduced selective pressure to inactivate p53 in Eμ-Myc lymphomas lacking Caspase-2.4
In summary, our data suggest that Raidd does not influence the development of tumors after DNA-damage stress or the overexpression of oncogenes. This is in strong contrast to the opposing effects of its putative interaction partners, Caspase-2 or Pidd1.3, 4 Hence, the function of Raidd as a ‘direct activator of Caspase-2' needs to be re-considered, at least in the context of Eμ-Myc-driven tumorigenesis and justifies the search for additional activators as well as substrates of Caspase-2 to understand its tumor-suppressive function at the molecular level.
Materials and Methods
Mice
All animal experiments were performed in accordance to the Austrian legislation (BGBl. Nr. 501/1988 i.d.F. 162/2005, # BMWF-66.011/0137-II/10b/2009). The generation and genotyping of Casp-2−/−, Raidd−/−, p53+/− and Eμ-Myc transgenic mice have been described elsewhere.34, 44, 45, 46 All mice used for the experiments were on an inbred C57BL/6 background.
Tumorigenesis induced by DNA damage
To induce thymic lymphomas, mice at the age of 4 weeks irradiated with 1.75 Gy in a linear accelerator once per week for 4 weeks. Muscular sarcoma formation was induced by a single 200 μl intra muscular injection of 1 mg 3-MC per mouse (Sigma, Vienna, Austria), dissolved in sesame oil. Mice treated with sesame oil alone (vehicle) served as a control.
Cell culture and reagents
Primary tumor cells derived from Eμ-Myc, Eμ-Myc/Raidd−/−, Eμ-Myc/Casp-2−/− and Eμ-Myc/Pidd1−/− mice were cultured in DMEM (PAA, GE Healthcare, Pasching, Austria), supplemented with 10% FCS (PAA) Pen/Strep (Sigma), 250 μM L-Glutamine (Gibco/Invitrogen, Vienna, Austria) and 50 μM 2-mercaptoethanol (Sigma) and were kept on irradiated Bcl-2-overexpressing NIH-3T3 feeder cells. Tested agents and concentrations were: Etoposide (0.01–1 mg/ml), Dexamethasone (109–107 M), Paclitaxel (0.5–50 mM), Thapsigargin (0.5–50 ng/ml) and Doxorubicin (4–400 ng/ml; all from Sigma). FACS-sorted pre-B, immature and IgM+ B cells were cultured in DMEM with supplements and spontaneous cell death was monitored over time.
Flow cytometric analysis and cell sorting
Tumors were analyzed by flow cytometry using the following cell surface markers for (a) B cells: RA3-6B2, anti-B220; R2/60, anti-CD43; II/41, anti-IgM; 11/26C, anti-IgD; MB19-1, anti-CD19; 53-7.3, anti-CD5 and B3B4, anti-CD23; 7E9, anti-CD21 (BioLegend, Fell, Germany); and (b) T cells: GK1.5, anti-CD4; H57-597, anti-TCRb (all from eBioscience, Vienna, Austria) and 53-6.7, anti-CD8; (from BD Pharmingen, San Diego, CA, USA). Biotinylated antibodies were monitored with streptavidin-RPE (DAKO, Vienna, Austria) or streptavidin-PE-Cy7 (BD Phamingen). Using a FACSVantage cell sorter (Becton Dickinson, Heidelberg, Germany) premalign pre/pro-B cells (B220+/IgM−) or immature (IgM+/IgDlow) and mature (IgM+/IgDhigh) B cells were isolated and sorted from bone marrow and spleen, respectively. Flow cytometry data were analyzed using Cyflogic free ware and FlowJo (Ashland, OR, USA).
Immunoblotting
Proteins were extracted from tumor cells for 1 h on ice in protein lysis-buffer.36 Insoluble debris was cleared by centrifugation for 5 min at 13 000 r.p.m. and 4 °C. For evaluation of the p53 status of tumor cells 30 μg protein/lane was separated by SDS-PAGE, transferred to a nitrocellulose membrane and probed with rat anti-p19/ARF (5-C3-1; Santa Cruz Biotechnology, Szabo-Scandic, Vienna, Austria) or mouse anti-p53 antiserum (1C12; Cell Signaling, New England Biolabs, Frankfurt, Germany). Comparability of protein loading was assessed by re-probing membranes with an antibody recognizing GAPDH (Sigma). Horseradish peroxidase-conjugated sheep anti-rat Ig antibodies (Jackson Research, Vienna, Austria), goat anti-rabbit or rabbit anti-mouse antibodies (DAKO) were used as secondary antigens. Antibody binding was detected using enhanced chemiluminiscence (Amersham, Freiburg, Germany) system.
Cell cycle analysis
Cell cycle analysis was based on fixing lymphoma cells in 70% ethanol and staining with PI (propidium iodide at 40 μg/ml; Sigma). To analyze the percentage of cells in M-phase, ethanol-fixed lymphoma cells were permeabilized using Triton-X (0.25%, Sigma) for 15 min on ice and co-stained with phosphorylated H3 (Ser10; Cell Signaling) and PI. Distribution of cell cycle phases was analyzed using a FACScan cell cytometer (BD, Heidelberg, Germany).
BrdU incorporation
Cell proliferation of immature (CD19+IgM−) and mature (CD19+IgM+) B cells in premalignant 4-week-old mice was assessed by injecting 1 mg BrdU/mouse i.p. Four hours later, primary cells derived from bone marrow and spleen were isolated and stained for BrdU incorporation using the BrdU/APC flow kit (BD, Vienna, Austria) according to the manufacturer's recommendation. Samples were analyzed using FACS-Calibur (BD).
Cell viability assay
Tumor cells were co-stained with Annexin-V-FITC (1 : 1800 in Annexin-V-binding buffer; BD) and 7-AAD (1 μg/ml, Sigma) and spontaneous cell death was analyzed by subsequent flow cytometric analysis.
Cytogenetic analysis
Micronuclei were examined by seeding 2 × 105 MEF on a sterile coverslip treated with cytochalasin (4 μg/ml) for 16 h before fixation in PTEMF fixative47 for 10 min and staining with 4′,6′-diamidino-phenylinodole. A minimum of 300 cells per condition and genotype were screened for micronuclei under a ZEISS IMAGER Z1 Microscope and Zeiss EC Plan-NEOFLUAR 40 × /0,75 Ph2 objective using AxioVision Release 4.8.2 imaging software (Zeiss, Oberkochen, Germany).
Chromosome spreads from freshly isolated tumors were generated by incubating 1 × 106 lymphoma cells in media containing 1 μM nocodazole (Sigma) for 5 h at 37 °C in a CO2 incubator. After collecting and washing the cells in PBS, the pellet was resuspended in 5 ml 0.075 M KCl (Merck) and incubated for 5 min at 37 °C. Then 1 ml Carnoys fixative (Methanol:Acetic Acid=3:1) was added. Cells were spun down (1200 g for 3 min) and fixed in 1 ml Carnoys fixative (repeated three times). Cells were added drop-wise (from ∼50 cm hight) onto a coverslip, air dried and stained with 4′,6′-diamidino-phenylinodole. A minimum of 50 chromosome spreads per tumor sample was analyzed at × 100 magnification.
qRT-PCR analysis
RNA was isolated using TRIzol (Invitrogen) and transcribed into cDNA (Omniscript, Qiagen, Hilden, Germany) using random hexamer primers after DNAse digestion (Promega, Mannheim, Germany). cDNA of interest was amplified using primers listed in Supplementary Table 1 and 2 × DyNAmo Color Flash SYBR Green Master Mix (Thermo Scientific, Vienna, Austria) in an Eppendorf Mastercycler ep realplex2 cycler. Relative expression of target/housekeeper was calculated using the delta-CT method.
Microarray data set generation and analysis
Microarray data for gene expression were obtained using Affymetrix MoGene 1.0 ST v.1 arrays (Affymetrix, Santa Clara, CA, USA). Sample preparation was performed according to the manufacturer's protocol. In brief, 250 ng of high-quality RNA per sample were processed using the Ambion Affymetrix GeneChip WT Expression Kit (Part no. 308 4411974, Ambion/Thermo Scientific, Vienna, Austria) and the Affymetrix GeneChip WT Terminal Labeling Kit (Affymetrix). The resulting biotinylated targets were hybridized in an Affymetrix hybridization oven to a total of 14 Affymetrix MoGene 1.0 ST v.1 microarrays, which were then washed and stained in an Affymetrix fluidic station 450. Raw fluorescence signals were recorded in an Affymetrix scanner 3000 and image analysis was made with the Affymetrix GeneChip Command Console software.
Subsequent analyses have been performed in R (version 3.0.2) using packages from the Bioconductor project48 (version 2.13, BioConductor project: Fred Hutchinson Cancer Research Center, Seattle, WA, USA). The raw microarray data were pre-processed using ‘generalgcrma' package49 and our custom transcript-level ‘CEL definition file' (CDF) as described in Bindreither et al.50 Transcripts for all genes have been defined using Ensembl version 71 (EMBL-EBI, Wellcome Trust Genome Campus, Hinxton, UK). The CDF contained a total of 63 455 probe sets for 22 991 genes. Background adjustment, normalization and summarization of the microarray data were performed using the GCRMA method.51 Further, a single-probe set per gene was selected as following: (a) if there were probe sets with more than 5 probes, only those were considered for further analysis, (b) if any of considered probe sets represented protein coding transcripts, the list of probe sets was limited to such and (c) the probe set which had the highest multiple of average expression and standard deviation across all samples was chosen for expression analysis.
Differential expression analysis was performed using limma package.52 P-values were adjusted for multiple hypotheses testing correction by method of Benjamini and Hochberg.53 Genes with adjusted P-value<0.05 and absolute log2 fold change >1 were considered to be significantly regulated.
The raw and pre-processed microarray data have been submitted to the Gene Expression Omnibus (accession number GSE64920).
Statistical analysis
Statistical analysis was performed using unpaired Student's t-test or ANOVA with Student–Newman–Keuls as post hoc test. Comparison of Kaplan–Meier survival plots was performed using a log-rank test and for evaluation of statistical difference in frequency distributions the χ2 (Fisher's exact) analysis algorithm was used. P-values of <0.05 (*) or P<0.001 (**) indicate significant differences.
Acknowledgments
We thank I. Gaggl and C. Soratroi for excellent technical assistance as well as K. Rossi and B. Rieder for animal care. We further thank Professor P. Lukas and his team (LINAC 1-4), Department of Therapeutic Radiology and Oncology, Medical University Innsbruck, for enabling irradiation experiments, R. Kofler for help with gene-chip analysis and G. Böck for cell sorting. L. Peintner was supported by a DOC Fellowship of the Austrian Academy of Sciences (ÖAW). This study was funded by the ‘Österreichische Krebshilfe', Branch Tyrol; the Austrian Science Fund (Project P 26856) and the MCBO postgraduate program (Project: W1101) as well as the National Health and Medical Research Council grants (1002863 and 1043057).
Glossary
- PIDD
p53-induced protein with a death domain
- RAIDD
receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain
- NF-kB
nuclear factor kappa-light-chain enhancer
- MEF
mouse embryonic fibroblast
- wt
wild type
- 3-MC
3-methylcholanthrene
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by C Borner
Supplementary Material
References
- 1McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harbor Perspect Biol 2013; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Puccini J, Dorstyn L, Kumar S. Caspase-2 as a tumour suppressor. Cell Death Differ 2013; 20: 1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proc Natl Acad Sci USA 2009; 106: 5336–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Manzl C, Peintner L, Krumschnabel G, Bock F, Labi V, Drach M et al. PIDDosome-independent tumor suppression by Caspase-2. Cell Death Differ 2012; 19: 1722–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Parsons MJ, McCormick L, Janke L, Howard A, Bouchier-Hayes L, Green DR. Genetic deletion of caspase-2 accelerates MMTV/c-neu-driven mammary carcinogenesis in mice. Cell Death Differ 2013; 20: 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Puccini J, Shalini S, Voss AK, Gatei M, Wilson CH, Hiwase DK et al. Loss of caspase-2 augments lymphomagenesis and enhances genomic instability in Atm-deficient mice. Proc Natl Acad Sci USA 2013; 110: 19920–19925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Terry MR, Arya R, Mukhopadhyay A, Berrett KC, Clair PM, Witt B et al. Caspase-2 impacts lung tumorigenesis and chemotherapy response in vivo. Cell Death Differ 2014. e-pub ahead of print 10 October 2014; doi:10.1038/cdd.2014.159. [DOI] [PMC free article] [PubMed]
- 8Kumar S, White D, Takai S, Tuczynowicz S, Juttner C, Hughes T. Apoptosis regulatory gene NEDD2 maps to human chromosome segment 7q34-35, a region fequently affected in haematological neoplasms. Hum Genet 1995; 95: 641–644. [DOI] [PubMed] [Google Scholar]
- 9Estrov Z, Thall PF, Talpaz M, Estey EH, Kantarjian HM, Andreeff M et al. Caspase 2 and Caspase 3 protein levels as predictors of survival in acute myelogenous leukemia. Blood 1998; 92: 3090–3097. [PubMed] [Google Scholar]
- 10Hofmann WK, de Vos S, Tsukasaki K, Wachsman W, Pinkus G, Said J et al. Altered apoptosis pathways in mantle cell lymphoma detected by oligonucleotide microarray. Blood 2001; 98: 787–794. [DOI] [PubMed] [Google Scholar]
- 11Holleman A, MLd Boer, Kazemier KM, Beverloo HB, von Bergh ARM, Janka-Schaub GE et al. Decreased PARP and procaspase-2 protein levels are associated with cellular drug resistance in childhood acute lymphoblastic leukemia. Blood 2005; 106: 1817–1823. [DOI] [PubMed] [Google Scholar]
- 12Kim MS, Kim HS, Jeong EG, Soung YH, Yoo NJ, Lee SH. Somatic mutations of caspase-2 gene in gastric and colorectal cancers. Pathol Res Pract 2011; 207: 640–644. [DOI] [PubMed] [Google Scholar]
- 13Ren K, Lu J, Porollo A, Du C. Tumor-suppressing Function of Caspase-2 Requires Catalytic Site Cys-320 and Site Ser-139 in Mice. J Biol Chem 2012; 287: 14792–14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Colussi PA, Harvey NL, Kumar S. Prodomain-dependent Nuclear Localization of the Caspase-2 (Nedd2) Precursor: A NOVEL FUNCTION FOR A CASPASE PRODOMAIN. J Biol Chem 1998; 273: 24535–24542. [DOI] [PubMed] [Google Scholar]
- 15Butt AJ, Harvey NL, Parasivam G, Kumar S. Dimerization and Autoprocessing of the Nedd2 (Caspase-2) Precursor Requires both the Prodomain and the Carboxyl-terminal Regions. J Biol Chem 1998; 273: 6763–6768. [DOI] [PubMed] [Google Scholar]
- 16Baliga BC, Read SH, Kumar S. The biochemical mechanism of caspase-2 activation. Cell Death Differ 2004; 11: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 17Krumschnabel G, Sohm B, Bock F, Manzl C, Villunger A. The enigma of caspase-2: the laymen's view. Cell Death Differ 2009; 16: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Krumschnabel G, Manzl C, Villunger A. Caspase-2: killer, savior and safeguard–emerging versatile roles for an ill-defined caspase. Oncogene 2009; 28: 3093–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 2004; 304: 843–846. [DOI] [PubMed] [Google Scholar]
- 20Bock FJ, Peintner L, Tanzer M, Manzl C, Villunger A. P53-induced protein with a death domain (PIDD): master of puppets. Oncogene 2012; 31: 4733–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Duan H, Dixit V. RAIDD is a new ‘death' adaptor molecule. Nature 1997; 238: 86–89. [DOI] [PubMed] [Google Scholar]
- 22Zhou P, Chou J, Olea R, Yuan J, Wagner G. Solution structure of Apaf-1 CARD and its interaction with caspase-9 CARD- a structural basis for specific adaptor-caspase interaction. Proc Natl Acad Sci USA 1999; 96: 11265–11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Lin Q, Liu Y, Moore DJ, Elizer SK, Veach RA, Hawiger J et al. Cutting edge: the ‘death' adaptor CRADD/RAIDD targets BCL10 and suppresses agonist-induced cytokine expression in T lymphocytes. J Immunol 2012; 188: 2493–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Qiao H, Liu Y, Veach RA, Wylezinski L, Hawiger J. The Adaptor CRADD/RAIDD Controls Activation of Endothelial Cells by Proinflammatory Stimuli. J Biol Chem 2014; 289: 21973–21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Yang C, Hornicek FJ, Wood KB, Schwab JH, Mankin H, Duan Z. RAIDD expression is impaired in multidrug resistant osteosarcoma cell lines. Cancer Chemother Pharmacol 2009; 64: 607–614. [DOI] [PubMed] [Google Scholar]
- 26Hasegawa H, Yamada Y, Tsukasaki K, Mori N, Tsuruda K, Sasaki D et al. LBH589, a deacetylase inhibitor, induces apoptosis in adult T-cell leukemia/lymphoma cells via activation of a novel RAIDD-caspase-2 pathway. Leukemia 2011; 25: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Mahoney D, Lefebvre C, Allan K, Brun J, Sanaei C, Baird S et al. Virus-tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus-triggered Caspase-2 cell death. Cancer Cell 2011; 20: 443–456. [DOI] [PubMed] [Google Scholar]
- 28Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell 2005; 123: 1079–1092. [DOI] [PubMed] [Google Scholar]
- 29Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 2007; 448: 375–379. [DOI] [PubMed] [Google Scholar]
- 30Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med 1988; 167: 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Frenzel A, Labi V, Chmelewskij W, Ploner C, Geley S, Fiegl H et al. Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood 2010; 115: 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Sochalska M, Tuzlak S, Egle A, Villunger A. Lessons from gain- and loss-of-function models of pro-survival Bcl2 family proteins: implications for targeted therapy. FEBS J 2015; 282: 834–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Adams J, Harris A, Pinkert C, Corcoran L, Alexander W, Cory S et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985; 318: 533–538. [DOI] [PubMed] [Google Scholar]
- 35Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA 2004; 101: 6164–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF–Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev 1999; 13: 2658–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Dorstyn L, Puccini J, Wilson CH, Shalini S, Nicola M, Moore S et al. Caspase-2 deficiency promotes aberrant DNA-damage response and genetic instability. Cell Death Differ 2012; 19: 1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Fava LL, Bock FJ, Geley S, Villunger A. Caspase-2 at a glance. J Cell Sci 2012; 125: 5911–5915. [DOI] [PubMed] [Google Scholar]
- 39Bric A, Miething C, Bialucha CU, Scuoppo C, Zender L, Krasnitz A et al. Functional identification of tumor suppressor genes through an in vivo RNA interference screen in a mouse lymphoma model. Cancer Cell 2009; 16: 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Oliver TG, Meylan E, Chang GP, Xue W, Burke JR, Humpton TJ et al. Caspase-2-mediated cleavage of Mdm2 creates a p53-induced positive feedback loop. Mol Cell 2011; 43: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Sohn D, Budach W, Janicke RU. Caspase-2 is required for DNA damage-induced expression of the CDK inhibitor p21(WAF1/CIP1). Cell Death Differ 2011; 18: 1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Newbold A, Salmon JM, Martin BP, Stanley K, Johnstone RW. The role of p21waf1/cip1 and p27Kip1 in HDACi-mediated tumor cell death and cell cycle arrest in the Eμ-myc model of B-cell lymphoma. Oncogene 2014; 33: 5415–5423. [DOI] [PubMed] [Google Scholar]
- 43Manzl C, Fava LL, Krumschnabel G, Peintner L, Tanzer MC, Soratroi C et al. Death of p53-defective cells triggered by forced mitotic entry in the presence of DNA damage is not uniquely dependent on Caspase-2 or the PIDDosome. Cell Death Dis 2013; 4: e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44O'Reilly L, Ekert P, Harvey N, Mardsen V, Cullen L, Vaux D et al. Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9. Cell Death Differ 2002; 9: 832–841. [DOI] [PubMed] [Google Scholar]
- 45Berube C, Boucher LM, Ma W, Wakeham A, Salmena L, Hakem R et al. Apoptosis caused by p53-induced protein with death domain (PIDD) depends on the death adapter protein RAIDD. Proc Natl Acad Sci USA 2005; 102: 14314–14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Cridao LM, Klatt P, Flores JM et al. 'Super p53' mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 2002; 21: 6225–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Elowe S, Dulla K, Uldschmid A, Li X, Dou Z, Nigg EA. Uncoupling of the spindle-checkpoint and chromosome-congression functions of BubR1. J Cell Sci 2010; 123: 84–94. [DOI] [PubMed] [Google Scholar]
- 48Gentleman R, Carey V, Bates D, Bolstad B, Dettling M, Dudoit S et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Rainer J, Lelong J, Bindreither D, Mantinger C, Ploner C, Geley S et al. Research resource: transcriptional response to glucocorticoids in childhood acute lymphoblastic leukemia. Mol Endocrinol 2012; 26: 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Bindreither D, Ecker S, Gschirr B, Kofler A, Kofler R, Rainer J. The synthetic glucocorticoids prednisolone and dexamethasone regulate the same genes in acute lymphoblastic leukemia cells. BMC Genomics 2014; 15: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Association 2004; 99: 909–917. [Google Scholar]
- 52Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004; 3: 29. [DOI] [PubMed] [Google Scholar]
- 53Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodol) 1995; 57: 289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.