Abstract
Histotripsy is a non-invasive ablation method that mechanically fractionates tissue by controlling acoustic cavitation. Previous work has shown that tissue mechanical properties play a significant role in the histotripsy process, with stiffer tissues being more resistant to histotripsy-induced tissue damage. In this study, a thermal pretreatment strategy is proposed in order to precondition tissues prior to histotripsy. We hypothesize that a thermal pretreatment can be used to alter tissue stiffness by modulating collagen composition, thus changing tissue susceptibility to histotripsy. More specifically, we hypothesize that tissues will soften and become more susceptible to histotripsy when preheated at ~60°C due to collagen denaturing, but that tissues will rapidly stiffen and become less susceptible to histotripsy when preheated at ~90°C due to collagen contraction. To test this hypothesis, a controlled temperature water bath was used to heat various ex vivo bovine tissues (tongue, artery, liver, kidney medulla, tendon, and urethra). After heating, the Young’s modulus of each tissue sample was measured using a tissue elastometer, and changes in tissue composition (i.e. collagen structure/density) were analyzed histologically. The susceptibility of tissues to histotripsy was investigated by treating the samples using a 750 kHz histotripsy transducer. Results demonstrated a decrease in stiffness and an increase susceptibility to histotripsy for tissues (except urethra) preheated to 58°C. In contrast, preheating to 90°C increased tissue stiffness and reduced susceptibility to histotripsy for all tissues except tendon, which was significantly softened due to collagen hydrolysis into gelatin. Based on these results, a final set of experiments was conducted to demonstrate the feasibility of using high intensity focused ultrasound (HIFU) to provide the thermal pretreatment. Overall, the results of this study demonstrate the initial feasibility of a thermal pretreatment strategy to precondition tissue mechanical properties and alter tissue susceptibility to histotripsy. Future work will aim to optimize this thermal pretreatment strategy in order to determine if this approach can be practically applied for specific clinical applications in vivo without causing unwanted damage to surrounding or overlying tissue.
Keywords: Histotripsy, High Intensity Focused Ultrasound, Thermal therapy, Tissue Stiffness
Introduction
Histotripsy is a non-invasive tissue ablation method that controls acoustic cavitation to mechanically fractionate soft tissue (Xu et al. 2004; Xu et al. 2005; Parsons et al. 2006a; Roberts et al. 2006). With sufficiently high pressure and an adequate number of pulses, histotripsy can completely fractionate soft tissues into a liquefied acellular homogenate resulting in effective tissue removal (Xu et al. 2004; Hall et al. 2007). Histotripsy is currently being studied for many clinical applications where non-invasive tissue removal is desired including tissue debulking to treat benign prostatic hyperplasia (Hempel et al. 2011), clot breakdown to treat deep vein thrombosis (Maxwell et al. 2011), perforation of the atrial septum in the treatment of congenital heart diseases (Xu et al. 2010; Owens et al. 2011), cancer ablation (Styn et al. 2010; Vlaisavljevich et al. 2013), kidney stone removal (Duryea et al. 2011), and fetal interventions (Kim et al. 2013).
Tissue mechanical properties have been observed to affect the histotripsy process (Vlaisavljevich et al. 2014a; Vlaisavljevich et al. 2014b; Vlaisavljevich et al. 2014c). In a previous study, it was demonstrated that stiffer tissues are less susceptible to histotripsy-induced tissue damage (Vlaisavljevich et al. 2014a). In that study, histotripsy was shown capable of completely ablating the majority of soft tissues, while producing no damage for stiffer tissues such as tendon and cartilage (Vlaisavljevich et al. 2014a). The finding that stiffer tissues are more resistant to histotripsy has been utilized to improve histotripsy therapy for specific clinical applications by developing a tissue selective ablation approach in which softer tissues (i.e. liver, kidney) are completely fractionated while stiffer tissues within the focal region (i.e. blood vessels, collecting system) are preserved (Lake et al. 2008; Vlaisavljevich et al. 2013; Vlaisavljevich et al. 2014a). However, the resistance of stiffer tissues to histotripsy may also be problematic in cases where the removal of a stiff tissue is desired, such as in the treatment of fibrous masses (i.e. fibroadenoma, uterine fibroids, or fibrous tumors) or the debulking of stiff tissues (i.e. tendon, ligament, or tongue). As such, a pretreatment strategy that could alter the susceptibility of tissues to histotripsy would be a valuable tool in developing histotripsy for clinical applications in which the removal of stiff tissues is desired.
In this work, we investigate the effects of thermal preconditioning on tissue susceptibility to histotripsy therapy. Previous work has shown that heating can alter tissue stiffness due to changes in the structure of intracellular and extracellular proteins such as actin, myosin, and collagen (Tornberg 2005; Baldwin 2012). Changes in tissue stiffness are dependent upon multiple factors including tissue composition, heating temperature, and heating duration (Tornberg 2005; Baldwin 2012). Since the stiffness of most tissues is largely dictated by collagen composition, we hypothesize that the effectiveness of a thermal pretreatment will be largely determined by the changes in collagen. Previous work has shown that the Young’s modulus of isolated collagen fibers and selective collagenous tissues decreases when heated between 50°C-60°C due to collagen denaturing (unraveling of the collagen triple helix) and breakdown of chemical crosslinks between collagen fibers (Hoermann and Schlebusch 1971; Wall et al. 1999; Wright and Humphrey 2002; Tornberg 2005; Baldwin 2012). However, the Young’s modulus of selective collagenous tissues has been shown to increase when heated at higher temperatures 65°C-90°C due to collagen contraction (shrinking of individual collagen fibers) (Hoermann and Schlebusch 1971; Wall et al. 1999; Wright and Humphrey 2002; Tornberg 2005; Baldwin 2012). Additionally, collagen can also hydrolyze into gelatin (individual fibers cleaved into small pieces), significantly decreasing tissue stiffness (Tornberg 2005; Baldwin 2012). Based on these previous studies, we investigate two different heating temperatures for thermal preconditioning, 58°C and 90°C. We hypothesize that the majority of tissues will soften and become more susceptible to histotripsy after preheating at 58°C due to collagen denaturing, but that tissues will rapidly stiffen and become less susceptible to histotripsy after preheating at 90°C due to collagen contraction, unless collagen hydrolysis into gelatin occurs. Furthermore, in order to provide a more complete understanding of the effects of thermal preconditioning on tissues with different compositions and tissue stiffness’s, we investigate the effects of heating on a variety of tissue types.
In the first part of this study, a constant temperature water bath (58°C and 90°C) was used to provide a controlled heating environment to study the effects of thermal preconditioning on tissue susceptibility to histotripsy for various ex vivo bovine tissues (tongue, artery, liver, kidney medulla, tendon, urethra). After heating, Young’s modulus was measured using a microelastometer, and changes in collagen composition (i.e. collagen structure/density) were analyzed histologically. The effects of preheating on the susceptibility to histotripsy-induced tissue damage was then investigated by applying histotripsy to heated and unheated tissues using a 750 kHz histotripsy transducer. Based on these results, a final set of experiments was conducted to demonstrate the feasibility of using high intensity focused ultrasound (HIFU) to provide the thermal preconditioning treatment, as previous work has demonstrated that HIFU can significantly alter tissue stiffness (Sapin-de Brosses et al. 2010). Overall, the aim of this study is to investigate the feasibility of using thermal preconditioning to decrease tissue stiffness and increase the susceptibility of tissues to histotripsy, which could be a valuable tool in developing histotripsy for clinical applications in which the removal of stiff tissues is desired.
Materials and Methods
Bovine Tissue Processing
To compare the effects of preheating on tissue stiffness and the susceptibility to histotripsy-induced tissue damage, a variety of ex vivo bovine tissues were used. Fresh bovine tongue, artery, liver, kidney, tendon, and urethra were excised at a local slaughterhouse and immediately placed into degassed 0.9% saline solution and stored at 4°C until experiments. Tissue samples were harvested from 5-12 animals for each set of tissue type and experimental conditions (i.e. external heating and HIFU heating at 58°C and 90°C). Tissue samples were sectioned and warmed to room temperature prior to heating experiments. After heating, tissues were either fixed for histology, tested for stiffness using a soft tissue elastometer, or degassed for histotripsy treatments. All samples were used within 48 hours of harvesting.
Constant Temperature Heating
In order to investigate the effects of preheating on tissue stiffness and susceptibility to histotripsy in a controlled environment, ex vivo tissues were heated in a constant temperature water bath, consisting of a slow cooker (Crock-Pot, SCCPVL610-S, Manchester, UK) connected to a sous-vide temperature controller (Dorkfood, DSV, Pensacola, Florida, USA). Ex vivo bovine tongue, artery, liver, kidney medulla, tendon, and urethra were submerged in the water bath and heated for 4, 8, and 12 hours at 58°C and 90°C, with the temperature controller set to maintain a constant water temperature within ±2°C. These temperatures (58°C and 90°C) were chosen based on previous work showing that collagen denaturing (tissue softening) increases with temperature up to ~60°C, at which point the individual collagen fibers start to contract (tissue stiffening) with the majority of collagen fibers contracting rapidly at temperatures above ~85°C (Tornberg 2005; Baldwin 2012). In this study, we expand on this previous work to investigate the effects of thermal preconditioning on a variety of tissue types with different compositions and tissue stiffness’s. Prior to heating, tissues were sectioned into thin pieces <1cm in thickness in order to ensure tissues were more uniformly heated. For each temperature and heating duration, separate tissue samples were heated for Young’s modulus measurements (n=6), histological analysis (n=4), and histotripsy treatment experiments (n=6).
Measurement of Tissue Stiffness
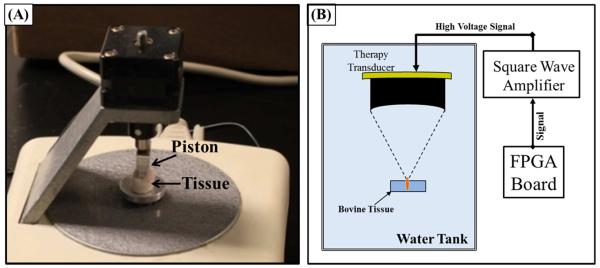
In order to investigate the effects of heating on tissue stiffness, a soft tissue microelastometer (MicroElastometer Model 0301, Artann Laboratories, West Trenton, NJ, USA) was used to measure the Young’s modulus (compression) of tissue samples. The Young’s modulus was measured for tissue samples heated for 4, 8, and 12 hours at 58°C and 90°C as well as unheated controls. Tissues were sectioned into small samples just larger than the microelastometer piston, which was a rectangular stamp of 8.88 mm by 3.98 (Fig.1A). The microelastometer measures tissue stiffness under quasi-static deformation using a small piston that incrementally increases the force applied to the tissue which rests on a pressure sensitive stage. By measuring the vertical tissue displacement and applied force, the stress and strain (compression) can be measured and used to calculate the tissue Young’s modulus. The Young’s Modulus (E) was calculated as the stress divided by the strain. Although tissue is not linearly elastic, an average Young’s modulus was estimated from the microelastometer software as described in previous studies (Xie et al. 2005; Kim et al. 2008), and the results were compared for all samples. Additionally, force vs. strain curves were generated for each sample to provide a more complete analysis of tissue viscoelasticity. For each sample, three measurements were conducted to ensure a consistent measurement was observed. For each experimental condition, 6 tissue samples were analyzed, with the results compared using a Student’s t-test. P-values < 0.05 were considered significant. Tissue samples were kept hydrated in water until immediately prior to the Young’s modulus measurements. As the Young’s modulus measurements of each sample took less than 1 minute, no significant tissue dehydration was expected to occur.
Figure 1. Experimental setup.
(A) After heating, tissue Young’s modulus (compression) was measured using a tissue elastometer.(B) Susceptibility to histotripsy was tested using a 750 kHz therapy transducer with the focused aligned to the tissue surface. Histotripsy was applied for five minutes using 5 cycle pulses, a pulse repetition frequency of 500 Hz, and pressure of 19/60 MPa (p−/P+).
Histological Analysis
To investigate the changes in tissue composition (i.e. collagen structure/density) with heating, histological analysis of tissues was conducted using a Masson’s trichrome stain, which uses three stains to visualized tissue collagen (blue), muscle/cytoplasm (red), and nuclei (black). After heating, tissue samples were fixed in formalin, dissected, processed, and then stained. The changes in tissue composition were examined histologically under a microscope (Nikon Eclipse 50i) using 4x, 10x, 20x, and 40x objective lenses.
Histotripsy Treatment
To compare the susceptibility of tissues to histotripsy-induced damage, histotripsy therapy was applied to the surface of tissue samples using a 750 kHz therapy transducer (Imasonic, SAS, Voray sur l’Ognon, France) with an aperture of 15 cm and a geometric focal length of 12 cm. The transducer was driven by a custom-designed class D amplifier with appropriate electrical matching circuits built in our laboratory and associated low voltage (20V DC power supply; E3630A Hewlett Packard Company, Palo Alto, CA, USA) and high voltage (600V DC power supply; GEN 600, TDK Lambda Americas Inc., San Diego, CA, USA) power supplies. Input signals were provided by a custom built Field-Programmable Gate Array (FPGA) board (Altera Corporation, San Jose, CA, USA) that functioned as a signal generator.
For each set of heating parameters and tissue type, six tissue samples were treated with histotripsy using the experimental set up shown in Figure 1B. The tested samples included tongue, artery, liver, kidney medulla, tendon, and urethra that were heated for 0, 4, 8, and 12 hours at 58°C and 90°C. Prior to histotripsy treatments, tissues were allowed to cool to room temperature under a partial vacuum (~20 kPa, absolute) for 30 minutes to remove any gas trapped within the tissue. Each sample was then embedded onto a 1% w/v agarose tissue phantom to hold tissues inside the water the tissue, and a histotripsy bubble cloud was formed at the tissue-water interface using 5-cycle long ultrasound pulses, a pulse repetition frequency (PRF) of 500 Hz, and peak pressures of 19/60 MPa (P−/P+) as measured by a fiber-optic probe hydrophone (FOPH) built in-house (Parsons et al. 2006b). Bubble cloud formation and alignment were verified using optical and ultrasound imaging. Histotripsy treatments were applied to the tissue surface for 5 minutes (n=6). All samples of a given tissue type were treated in the same orientation as shown in the images in the results section. More specifically, artery and urethra samples were cut open and histotripsy was applied to the internal surface of the lumen (i.e. intima). Kidney samples were sectioned so that histotripsy could be applied to the kidney medulla tissue. Liver samples were not sectioned in any specific orientation. Tongue samples were sectioned transverse to the tongue, with histotripsy applied to the tongue muscle tissue (not the fibrous surface of the tongue). Histotripsy was applied to the side of the tendon samples in the transverse direction as shown. After treatment, tissue samples were examined for damage (lesion formation) by gross morphology. Results were organized into tissues with complete damage (large lesion through entire tissue, i.e. perforation), partial damage (lesion formed into, but not completely through, the tissue), surface damage (slight damage to the tissue surface), and no damage.
HIFU Heating
The feasibility of using thermal HIFU to precondition tissues prior to histotripsy was investigated using ex vivo tongue, liver, and tendon. HIFU was applied to tissues using the same 750 kHz therapy transducer for histotripsy treatments with the temperature at the focus measured using 3 type T hypodermic needle thermocouples (Physitemp Instruments Inc., Clifton, NJ, USA). Thermocouples were superficially inserted into the tissue at the transducer focus, and the temperature was monitored during treatments in real-time. HIFU was applied using 30-35 cycle pulses at a pressure of 8/25 MPa (p−/P+) and a PRF varying from 1300-1750 Hz. During HIFU, the peak temperature measured by the thermocouples was allowed to increase to the desired maximum temperature (60°C or 90°C) and then the PRF was lowered to maintain a nearly constant temperature for the duration of the HIFU exposure. HIFU treatments were applied for 10 minutes in tongue and liver and for 30 minutes in tendon. These treatment durations (10-30 minutes) were chosen in order to investigate if the trends observed for controlled heating experiments could be replicated using HIFU heating in more reasonable treatments times compared to the much longer durations applied in the controlled heating experiments (4-12 hours).
Results
Controlled Preheating at 58°C
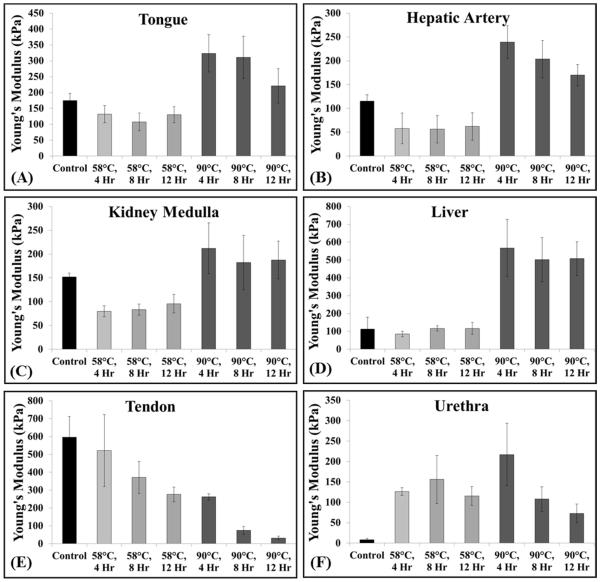
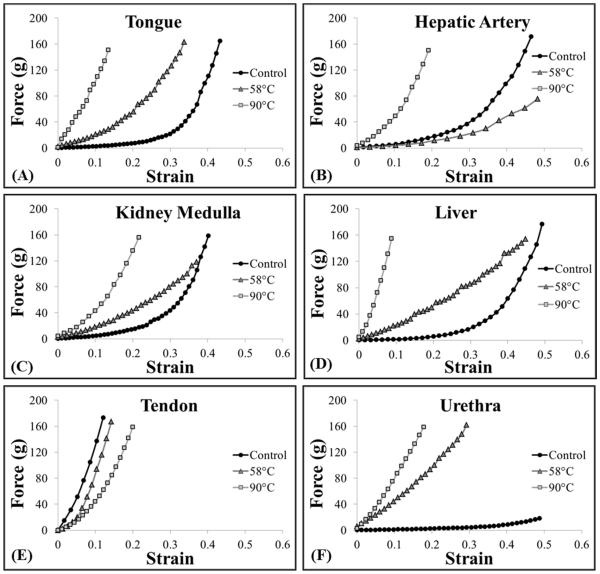
To test the hypothesis that heating at 58°C will soften tissues and increase their susceptibility to histotripsy due to collagen denaturing, bovine tongue, artery, kidney medulla, liver, tendon, and urethra were used. Results demonstrated that heating at 58°C decreased the Young’s modulus of tongue, artery, kidney medulla, liver, and tendon (Fig.2A-E), with the decrease in modulus significant (p<0.05) in all cases compared to controls except for tendon at 4 hours and liver at all-time points (p>0.05). Of all the tissues heated at 58°C, only urethra showed an increase in Young’s modulus (Fig.2F). Analysis of the force vs. strain curves demonstrated that tendon showed a nearly linear elastic behavior, with a decrease in Young’s modulus observed for heated samples (Fig.3E). Further analysis of the force vs. strain curves showed that tissue viscoelasticity was highly nonlinear in unheated samples of tongue, artery, kidney medulla, and liver. In these control samples, the tissues were initially highly compliant (i.e. small load resulting in large strains), but stiffened with increasing strain (Fig.3A-D). The force vs. strain curves for these tissues demonstrated a decrease in initial compliance (i.e. stiffening of tissue under small strain loading) after heating at 58°C (Fig.3A-D). However, these tissues showed a reduction in the slope of the curve for the higher strain regions of heated tissues compared to control tissues (i.e. softening of tissue under large strain loading) (Fig.3A-D). Analysis of the force vs. strain curves for urethra showed nearly linear elastic behavior with an increase in Young’s modulus for heated samples (Fig.3F).
Figure 2. Young’s modulus plots.
Elastometer measurements for tissues heated at 58ºC demonstrated a significant decrease in the Young’s modulus of tongue, artery, kidney medulla, and tendon (A:C, E), no significantly change for liver (D), and a significant increased for urethra (F). Heating at 90ºC significantly increased the Young’s modulus of tongue, artery, kidney medulla, liver, and urethra (A:D, F) and significantly decreased the Young’s modulus of tendon (E).
Figure 3. Representative Force vs. Strain Curves.
Plots show representative Force vs. Strain curves measured by the elastometer for tissues samples comparing unheated control samples with samples that were heated for 4 hours at 58°C and 90°C, respectively.
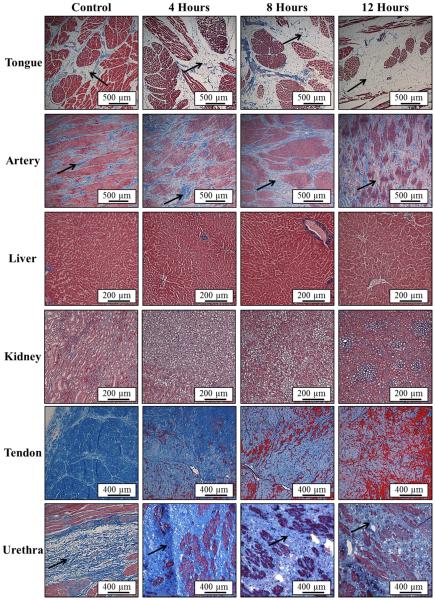
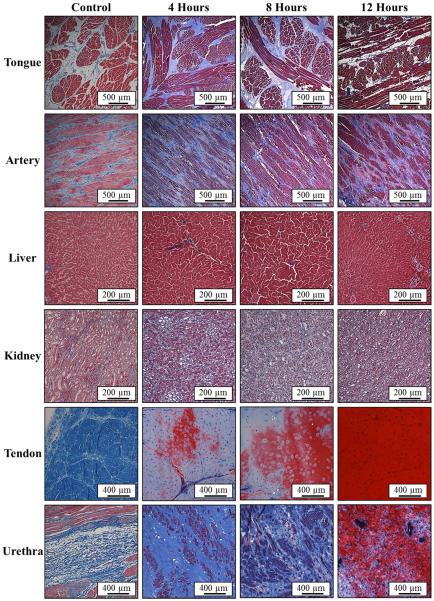
Histological analysis demonstrated a decrease in collagen density for all tissues heated at 58°C, except for urethra. Representative Masson’s trichrome stain slides of the tongue and artery show a significant decrease in collagen (blue) density for heated samples compared to controls (Fig.4). The distance between muscle fibers (red) was observed to increase with heating in these tissues, with this effect most pronounced in the tongue (Fig.4). A similar decrease in collagen density was seen for tendon, where the collagen density decreased continuously as the tissue was heated for 4, 8, and 12 hours (Fig.4). Additionally, an increasing amount of hydrolysis of collagen (blue) into gelatin (dark red) was observed in tendon as the heating duration was increased (Fig.4). Of all the tissues heated at 58°C, only urethra showed an increase in collagen density with heating (Fig.4), matching the Young’s modulus results (Fig.2F). The increase in density and tissue stiffness for urethra was potentially caused by the contraction of elastic fibers inside the highly compliant urethra.
Figure 4. Histology: 58°C Samples.
Images show histology slides stained with a trichrome blue staining for samples heated at 58°C for 4, 8, and 12 hours. Results for tongue, artery, and tendon demonstrated a decrease in collagen (blue) density with heating. Furthermore, some hydrolysis into gelatin (red) was observed in tendon after 8 and 12 hours. Urethra results demonstrated an increase in collagen density after heating compared to control.
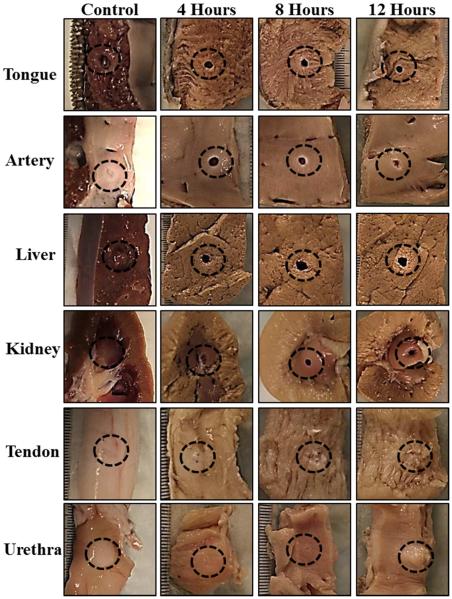
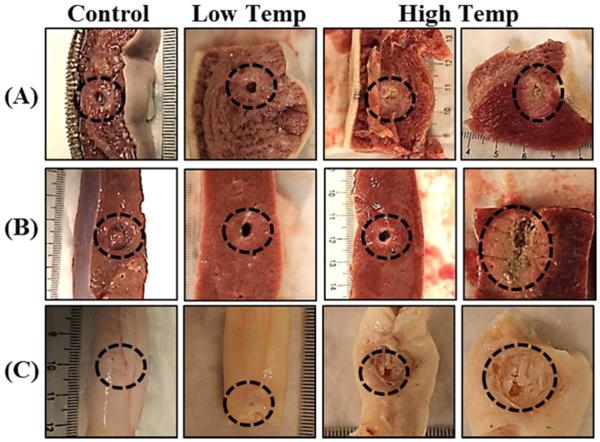
Histotripsy treatment results demonstrated that decreases in tissue stiffness with preheating resulted in an increased susceptibility to histotripsy (Fig.5). For example, histotripsy could only partially damage tongue, artery, and liver prior to heating but could form a lesion completely through the tissue after preheating for 4 hours at 58°C (Fig.5). A similar trend of increasing susceptibility with preheating was observed for kidney medulla and tendon samples. However, even after heating for 12 hours, only superficial damage to the surface of tendon was observed (Fig.5). In contrast to the other tissues, urethra was observed to be less susceptible to histotripsy after heating (Fig.5), matching the tissue stiffness results (Fig.2F). Complete results from the histotripsy treatments of tissues preheated at 58°C are shown in Table 1.
Figure 5. Histotripsy Treatment Pictures: 58°C Samples.
Morphological analysis of bovine tissues after histotripsy demonstrated that tongue, artery, liver, kidney, and tendon were more susceptible to histotripsy-induced tissue damage after heating at 58°C. Urethra was observed to be less susceptible to histotripsy after heating at 58°C.
Table 1.
Histotripsy Treatment Results: 58°C Samples.
| Heated at 58°C | Histotripsy Lesion (# Samples/Total Samples) | ||||
|---|---|---|---|---|---|
|
Tissue
Type |
Heating
Duration |
No Visible
Damage |
Surface Damage
Only |
Partial Damage
(No Perforation) |
Complete Damage
(Perforation) |
| Tongue | |||||
| Control | 0/6 | 0/6 | 6/6 | 0/6 | |
| 4 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| 8 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| 12 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| Artery | |||||
| Control | 0/6 | 3/6 | 3/6 | 0/6 | |
| 4 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| 8 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| 12 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| Liver | |||||
| Control | 0/6 | 0/6 | 6/6 | 0/6 | |
| 4 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| 8 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| 12 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| Kidney | |||||
| Control | 4/6 | 2/6 | 0/6 | 0/6 | |
| 4 Hours | 0/6 | 1/6 | 5/6 | 0/6 | |
| 8 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| 12 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| Tendon | |||||
| Control | 6/6 | 0/6 | 0/6 | 0/6 | |
| 4 Hours | 5/6 | 1/6 | 0/6 | 0/6 | |
| 8 Hours | 0/6 | 6/6 | 0/6 | 0/6 | |
| 12 Hours | 0/6 | 6/6 | 0/6 | 0/6 | |
| Urethra | |||||
| Control | 0/6 | 5/6 | 1/6 | 0/6 | |
| 4 Hours | 6/6 | 0/6 | 0/6 | 0/6 | |
| 8 Hours | 3/6 | 3/6 | 0/6 | 0/6 | |
| 12 Hours | 0/6 | 6/6 | 0/6 | 0/6 | |
Histotripsy treatment results demonstrated that tongue, artery, liver, kidney, and tendon were more susceptible to histotripsy-induced tissue damage after heating at 58°C. Urethra was observed to be less susceptible to histotripsy after heating at 58°C.
Controlled Preheating at 90°C
The hypothesis that preheating tissues at 90°C will stiffen tissues and decrease susceptibility to histotripsy due to collagen contraction was tested using bovine tongue, artery, kidney medulla, liver, tendon, and urethra. Results demonstrated that preheating at 90°C for 4 hours significantly increased the Young’s modulus of tongue, artery, kidney medulla, liver, and urethra compared to controls (p<0.05) (Fig.2A-D,F). After 8 and 12 hours of preheating at 90°C, the Young’s modulus of these tissues decreased compared to the 4 hour samples, but remained significantly higher than the unheated controls (p<0.05) (Fig.2A-D,F). Of all the tissues preheated at 90°C, only tendon demonstrated a decrease in Young’s modulus compared to the unheated controls (Fig.2E). The decreases in Young’s modulus for tendon preheated at 90°C were much larger than the decreases observed at 58°C (Fig.2E). For example, heating tendon for 4 hours at 90°C resulted in a similar decrease in Young’s modulus as seen in samples heated for 12 hours at 58°C (Fig.2E). Results further demonstrated that the Young’s modulus of tendon continually decreased for samples heated for 4, 8, and 12 hours (Fig.2E). Analysis of the force vs. strain curves show tissue stiffening after 4 hours of heating at 90°C for all tissue types except tendon, which showed a decrease in stiffness (Fig.3).
Prior to fixing tissue for histology, tissues were examined using gross morphology. In contrast to tissues preheated at 58°C which were observed to expand slightly, all tissues preheated at 90°C significantly shrunk after heating. Morphological analysis also showed that tongue, artery, kidney medulla, liver, and urethra appeared dried out and felt significantly tougher than tissues preheated at 58°C, likely due to increased density and decreased water content due to collagen contraction. Histological analysis of these tissues showed a significant increase in collagen density observed after 4 hours of preheating at 90°C (Fig.6). For longer duration heating, collagen density was observed to decrease compared to the 4 hour samples, likely due to collagen dissolution or hydrolysis (Fig.6). The trends observed for tendon preheated at 90°C were different than those observed for other tissues. Although tendon was also observed to shrink after heating, morphological analysis demonstrated continual softening of the tendon, which also became more transparent and gel-like as the duration of heating was increased, likely due to collagen hydrolysis into gelatin. This trend was verified by histology which showed a continual decrease in collagen (blue) density as the heating duration was increased, as well as a corresponding increase in gelatin (dark red) with heating (Fig.6). Collagen hydrolysis into gelatin was also directly observed, albeit to a much lesser extent, in urethra samples heated for 12 hours at 90°C (Fig.6). For all samples, the trends observed by gross morphology and histology matched the trends in tissue stiffness measured by the soft tissue elastometer.
Figure 6. Histology: 90°C Samples.
Images show histology slides stained with a trichrome blue staining for samples heated at 90°C for 4, 8, and 12 hours. Results for tongue, artery, and urethra demonstrated an increase in collagen (blue) density with heating. In tendon, collagen was observed to hydrolyze into gelatin (red) with heating. Some hydrolysis was also observed in the urethra after 12 hour heating.
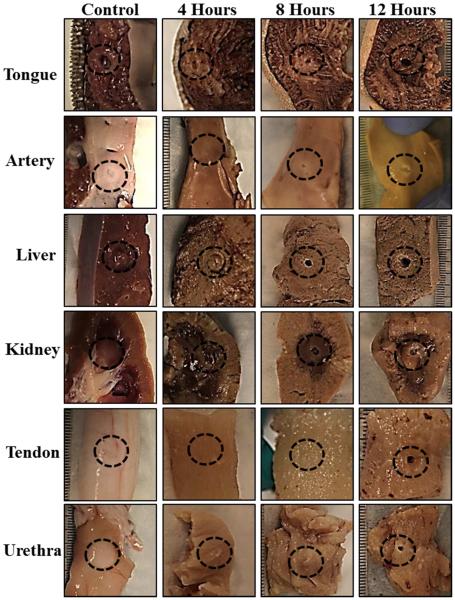
Histotripsy treatment results demonstrated that increases in tissue stiffness, which were observed for all tissues except tendon, resulted in decreased susceptibility to histotripsy for samples preheated at 90°C for 4 hours (Fig.7). Samples preheated at 90°C for 8 and 12 hours were more susceptible to histotripsy than samples heated for 4 hours (Fig.7). For all 90°C heating durations, liver, tongue, artery, and kidney medulla samples were less susceptible to histotripsy than tissues heated for the same duration at 58°C. In contrast, tendon heated at 90°C was more susceptible to histotripsy than tendon heated at 58°C. As the duration of 90°C heating was increased, the susceptibility of tendon to histotripsy continually increased, with complete ablation through the tendon achieved for all 12 hour samples (Fig.7). A similar result was observed for the urethra after 12 hours of heating, with complete ablation in 4 out of 6 samples. Complete results from the histotripsy treatments of tissues heated at 90°C are shown in Table 2.
Figure 7. Histotripsy Treatment Pictures: 90°C Samples.
Morphological analysis of bovine tissues after histotripsy demonstrated tongue, artery, liver, kidney, and urethra were less susceptible to histotripsy-induced tissue damage after heating at 90°C for 4 hours while tendon was more susceptible to histotripsy after heating. All tissues became more susceptible to histotripsy after heating was continued for 8 and 12 hours (compared to 4 hour samples).
Table 2.
Histotripsy Treatment Results: 90°C Samples.
| Heated at 90°C | Histotripsy Lesion (# Samples/Total Samples) | ||||
|---|---|---|---|---|---|
|
Tissue
Type |
Heating
Duration |
No Visible
Damage |
Surface Damage
Only |
Partial Damage
(No Perforation) |
Complete Damage
(Perforation) |
| Tongue | |||||
| Control | 0/6 | 0/6 | 6/6 | 0/6 | |
| 4 Hours | 2/6 | 4/6 | 0/6 | 0/6 | |
| 8 Hours | 0/6 | 3/6 | 3/6 | 0/6 | |
| 12 Hours | 0/6 | 0/6 | 2/6 | 4/6 | |
| Artery | |||||
| Control | 0/6 | 3/6 | 3/6 | 0/6 | |
| 4 Hours | 6/6 | 0/6 | 0/6 | 0/6 | |
| 8 Hours | 1/6 | 5/6 | 0/6 | 0/6 | |
| 12 Hours | 0/6 | 3/6 | 3/6 | 0/6 | |
| Liver | |||||
| Control | 0/6 | 0/6 | 6/6 | 0/6 | |
| 4 Hours | 0/6 | 6/6 | 0/6 | 0/6 | |
| 8 Hours | 0/6 | 0/6 | 2/6 | 4/6 | |
| 12 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| Kidney | |||||
| Control | 4/6 | 2/6 | 0/6 | 0/6 | |
| 4 Hours | 5/6 | 1/6 | 0/6 | 0/6 | |
| 8 Hours | 0/6 | 4/6 | 2/6 | 0/6 | |
| 12 Hours | 0/6 | 0/6 | 1/6 | 5/6 | |
| Tendon | |||||
| Control | 6/6 | 0/6 | 0/6 | 0/6 | |
| 4 Hours | 0/6 | 6/6 | 0/6 | 0/6 | |
| 8 Hours | 0/6 | 1/6 | 4/6 | 1/6 | |
| 12 Hours | 0/6 | 0/6 | 0/6 | 6/6 | |
| Urethra | |||||
| Control | 0/6 | 5/6 | 1/6 | 0/6 | |
| 4 Hours | 2/6 | 3/6 | 1/6 | 0/6 | |
| 8 Hours | 0/6 | 4/6 | 2/6 | 0/6 | |
| 12 Hours | 0/6 | 0/6 | 2/6 | 4/6 | |
Histotripsy treatment results demonstrated that tongue, artery, liver, kidney, and urethra were less susceptible to histotripsy-induced tissue damage after heating at 90°C for 4 hours while tendon was more susceptible to histotripsy after heating. All tissues became more susceptible to histotripsy after heating was continued for 8 and 12 hours (compared to 4 hour samples).
HIFU Heating
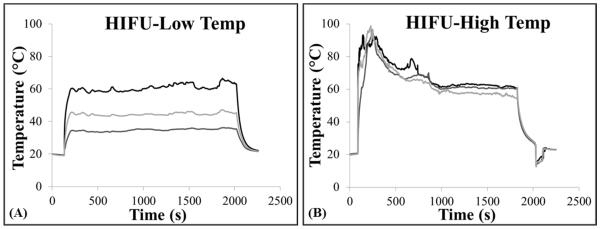
Bovine liver, tongue, and tendon were exposed to thermal HIFU followed by histotripsy therapy to demonstrate the feasibility of using HIFU to alter tissue susceptibility to histotripsy. For HIFU treatments, the temperature at the focus was quickly increased to ~60°C or ~90°C and then the PRF was lowered to hold the temperature constant for the duration of heating (Fig.8). It should be noted that tissues heated to ~90°C significantly deformed during heating due to tissue contraction, causing the thermocouples to move out of the focus and making it difficult to measure a high temperature for the duration of heating. For example, the tendon was observed to bend in half within 30 seconds after the temperature reached ~90°C, resulting in movement of the thermocouples out of the focus and a decrease in the measured temperature (Fig.8B). Similar trends were observed for all tissue types heated to 90°C. The movement of the focus due to tissue contraction made it difficult to measure a sustained peak temperature in the targeted range, but it is expected that the actual peak temperature in the tissue remained near 90°C for the duration of heating. No tissue contraction was observed for the ~60°C HIFU treatments, resulting in a measured peak temperature that remained near ~60°C for the duration of the exposure (Fig.8A). The heating parameters (peak temperature, duration) applied for all samples are listed in Table 3.
Figure 8. HIFU Thermal Treatment.
Plots show example temperature vs. time curves for HIFU heating of tendon measured using hypodermic needle thermocouples. For all experiments, 3 thermocouples were placed near the focal region, and HIFU was applied to rapidly increase the temperature to (A) ~60°C or (B) ~90°C. Once the desired temperature was reached, the PRF was lowered to stabilize the temperature at a near constant level for the duration of the heating. (B) For the higher temperature HIFU treatments, the temperature measured by the thermal couples did not remain above 90°C due to thermocouple movement caused by tissue contraction. Similar curves were obtained for HIFU treatments in all tissue types.
Table 3.
Histotripsy Treatment Results: Ultrasound Heated Samples.
| HIFU Heating | Histotripsy Lesion (# Samples/Total Samples) | |||||
|---|---|---|---|---|---|---|
|
Tissue
Type |
Peak
Temp |
Heating
Duration |
No Visible
Damage |
Surface Damage
Only |
Partial Damage
(No Perforation) |
Complete Damage
(Perforation) |
| Tongue | Control | 0 | 0/6 | 0/6 | 6/6 | 0/6 |
| ~60°C | 10 minutes | 0/6 | 0/6 | 0/6 | 6/6 | |
| ~90°C | 10 minutes | 0/6 | 6/6 | 0/6 | 0/6 | |
| Liver | Control | 0 | 0/6 | 0/6 | 6/6 | 0/6 |
| ~60°C | 10 minutes | 0/6 | 0/6 | 0/6 | 6/6 | |
| ~90°C | 10 minutes | 0/6 | 0/6 | 6/6 | 0/6 | |
| Tendon | Control | 0 | 6/6 | 0/6 | 0/6 | 0/6 |
| ~60°C | 30 minutes | 0/6 | 6/6 | 0/6 | 0/6 | |
| ~90°C | 30 minutes | 0/6 | 0/6 | 6/6 | 0/6 | |
Histotripsy treatment results demonstrated an increase in susceptibility to histotripsy-induced tissue damage for tongue, liver, and tendon heated with ultrasound at a peak temperature of ~60°C. Tongue and liver samples heated with ultrasound at a peak temperature of ~90°C were less susceptible to histotripsy while tendon became more susceptible to histotripsy after heating at ~90°C.
After heating with HIFU, tissues were treated with histotripsy using the same parameters as in the controlled heating experiments, with results showing similar trends (Table 3). All tissues were more susceptible to histotripsy after HIFU was applied at ~60°C (Fig.9). Histotripsy was capable of forming well defined lesions completely through all liver and tongue samples that were preheated with HIFU for 10 minutes at 60°C, whereas only partial damage was observed for control samples (Fig.9). Similarly, histotripsy treatments applied to tendon preheated with HIFU for 30 minutes at 60°C resulted in superficial lesions visible on the tendon surface, demonstrating a slight increase in susceptibility to histotripsy compared to control samples (Fig.9).
Figure 9. Histotripsy Treatment Pictures: Ultrasound Heated Samples.
Morphological analysis of bovine tissues treated with thermal HIFU followed by histotripsy demonstrated similar results to controlled heating experiments. Results showed an increase in susceptibility to histotripsy for tongue, liver, and tendon heated with ultrasound at a peak temperature of ~60°C. Tongue and liver samples heated with ultrasound at a peak temperature of ~90°C were less susceptible to histotripsy while tendon became more susceptible to histotripsy after heating at ~90°C.
For the 90°C HIFU treatments, liver and tongue samples were observed to increase in stiffness, with visible contraction and tissue dehydration observed after heating (Fig.9). Histotripsy results showed that tongue was less susceptibility to histotripsy after 90°C HIFU, with only superficial damage to the surface of the tongue compared to lesions formed partially into the tongue in control samples (Fig.9, Table 3). Partial damage through the tissue was observed in liver samples heated with HIFU at 90°C as well as in control samples (Table 3). However, gross morphology revealed that the histotripsy lesions in these tissues stopped at the center of the focal region where the extent of tissue stiffening was most significant (Fig.9). Furthermore, in some samples, the lesions were observed to form around the dense tissue in the focal region, similar to previous work demonstrating that histotripsy can be self-limiting at the boundaries of stiffer tissues (Lake et al. 2008; Vlaisavljevich et al. 2013; Vlaisavljevich et al. 2014a). Results for tendon samples heated with HIFU at 90°C showed a significant increase in susceptibility to histotripsy, with well-defined lesions formed partially through the tendon in all heated samples compared to no damage in control samples (Fig.9C, Table 3).
Discussion
Tissue mechanical properties play a significant role in the histotripsy process, with stiffer tissues being more resistant to histotripsy (Vlaisavljevich et al. 2014a). In this study, the effects of thermal preconditioning on tissues was investigated, with results demonstrating that increasing temperature can be used to modulate tissue stiffness and susceptibility to histotripsy. In the first part of this study, a constant temperature water bath was used to provide a controlled heating environment to study the effects of preheating (58°C and 90°C) on various ex vivo bovine tissues. Results of these experiments demonstrated that, with the exception of urethra, preheating tissues at 58°C decreased the tissue Young’s modulus due to collagen denaturing and the breakdown of the chemical cross-links between collagen fibers. The decreases in tissue stiffness and initial tissue compliance after heating at 58°C resulted in an increase in susceptibility to histotripsy. In contrast, tissues preheated at 90°C had the opposite response to heating, with an increase in tissue stiffness observed due to collagen contraction. The exception to this was tendon preheated at 90°C, in which the Young’s modulus decreased and the susceptibility to histotripsy increased due to collagen hydrolysis into gelatin.
The results of this study demonstrate that thermal preconditioning of tissues depends on multiple factors including heating temperature, heating duration, and tissue composition. For example, the results suggest that, in order to soften most tissues, heating should be applied at a temperature that can maximize collagen denaturing while minimizing collagen contraction. However, while tissue softening due to collagen denaturing is likely sufficient to improve histotripsy ablation in most tissues, very stiff tissues such as tendon may require substantial collagen hydrolysis into gelatin in order to sufficiently soften the tissue prior to histotripsy. It should be noted, however, that substantial collagen hydrolysis into gelatin was not directly observed in the majority of tissues, likely due to tissue dehydration caused by collagen contraction (Tornberg 2005). While some degree of hydrolysis is likely responsible for the decrease in tissue stiffness and increased susceptibility to histotripsy in all tissues heated at 90°C for the longer durations, the degree of hydrolysis was significantly greater in tendon compared to the other tissue types. Since the hydrolysis process requires water (Tornberg 2005), significant hydrolysis will likely only be achievable in tissues such as tendon or ligament which are composed primarily of collagen and water with minimal ground substance (proteoglycans, glycosaminoglycans, and glycoproteins) (Woodard and White 1986). As observed in this study, the rate and extent of collagen hydrolysis is significantly reduced in tissues containing a larger amount of ground substance, since collagen contraction drives the free water out of the tissue, leaving collagen fibers surrounded by dehydrated ground substance that does not contain enough water to facilitate substantial hydrolysis (Tornberg 2005; Fratzl 2008). These results demonstrate the need for further work investigating the effects of heating on other tissue components that may counteract the changes in collagen (i.e. elastic fibers, ground substance), especially in tissues whose mechanical properties are significantly determined by these components. For example, unlike the other tissues, urethra was shown to increase in stiffness after heating at 58°C, potentially due to the contraction of elastic fibers. Similarly, the effects of heating on tissue stiffness in muscular tissues will also be highly dependent upon the changes in the structure (i.e. denaturation/contraction) of actin and myosin proteins in addition to the changes in collagen (Tornberg 2005; Baldwin 2012). In addition, the extent of chemical cross-linking between collagen fibers further complicates the process, as previous studies have shown that the rate and extent of collagen denaturation and hydrolysis are dependent upon the extent of chemical cross-linking (as well as the direct effects of collagen cross-linking on tissue stiffness) (Wright and Humphrey 2002; Fratzl 2008). Future work will aim to optimize the thermal pretreatment parameters for specific tissues of interest.
In the final part of this study, the feasibility of using HIFU for tissue preconditioning was investigated, with the results supporting our hypothesis that HIFU can be used to alter tissue stiffness and susceptibility to histotripsy. For example, the susceptibility to histotripsy was shown to increase for liver and tongue samples heated with HIFU at ~60°C but decrease when heated at ~90°C. The results for tendon also matched the controlled heating experiments, with a slight increase in susceptibility to histotripsy observed after HIFU heating at ~60°C, and a more substantial increase in susceptibility observed at ~90°C. These results show the initial feasibility of using a purely ultrasonic pretreatment strategy. For example, in this study, the same 750 kHz transducer was used to apply both thermal HIFU and histotripsy to the tissue by modulating the pulse parameters.
The results of this study show the initial feasibility of using thermal HIFU as a pretreatment strategy for histotripsy. However, future work is needed to optimize this approach for specific clinical applications in order to determine if this strategy is practical in vivo. For example, the peak temperatures applied by HIFU in this study (60°C-90°C) may be difficult to sustain for long durations in vivo without causing unwanted damage to surrounding and overlying tissue. Furthermore, the thermal pretreatment strategy may not be a viable option for very large volumes, unless the heating duration can be significantly reduced to avoid causing profound consequences to the surrounding and overlying tissue. As such, future work is needed to optimize the thermal pretreatment approach in order to develop a practical treatment strategy for specific clinical applications. Nonetheless, the results of this initial study, which demonstrated that tissue susceptibility to histotripsy was significantly altered after 10-30 minutes of HIFU heating, are promising. The changes to the tissue for HIFU heating were localized to the focal region of the transducer and seemed to occur much more rapidly than for the external heating experiments. For example, significant contraction of the tendon was observed within ~30 seconds after the temperature reached ~90°C in comparison to a much slower process for controlled heating experiments in which the tendon slowly contracted over the course of many minutes or hours. Furthermore, previous work has shown that small changes in heating temperature can substantially change the rate of tissue softening (<5°C change in temperature resulted in an order of magnitude increase in softening rate) (Sapin-de Brosses et al. 2010), suggesting that our pretreatment approach may be optimized to alter tissue stiffness in much shorter durations than those used in this study.
Thermal pretreatment of tissues not only has the potential to improve the ablation of very stiff tissues, but it may also be used to increase the efficiency of ablation in softer tissues, such as the liver and tongue samples in this study. However, choosing optimal heating parameters will be essential in developing this approach in order to avoid tissue stiffening due to collagen contraction. This possibility should also be considered in ultrasound ablation applications that combine thermal and mechanical effects such as cavitation enhanced HIFU (Takagi et al. 2010) or boiling histotripsy (Wang et al. 2013). For example, the results of this study suggest that the high temperatures used in boiling histotripsy may either increase tissue stiffness (reducing the efficiency of tissue fractionation) or decrease tissue stiffness (increasing the efficiency of tissue fractionation), depending on the treatment parameters. This effect likely explains the results of a previous study investigating volume ablation using boiling histotripsy, which demonstrated that stiff tissues (i.e. blood vessels) inside the focal volume were more likely to remain intact as the extent of thermal damage was decreased (Wang et al. 2014).
In addition to the changes in tissue susceptibility to histotripsy, the thermal pretreatment approach may have other effects on histotripsy treatments applied inside bulk tissue, as previous work has shown that the histotripsy cavitation threshold and bubble dynamics are highly dependent upon tissue properties (Vlaisavljevich et al. 2014a; Vlaisavljevich et al. 2014b; Vlaisavljevich et al. 2015a; Vlaisavljevich et al. 2015b). Stiffer tissues have been shown to impede bubble expansion, thereby reducing the strain applied to the tissue (Vlaisavljevich et al. 2015b). These changes in bubble dynamics would be expected to further enhance the effects seen in this study (i.e. larger bubbles generated in softer tissue would increase fractionation efficiency while smaller bubbles in stiffer tissues would impede tissue fractionation). In addition to the changes in bubble dynamics, previous work has demonstrated that the pressure threshold for generating histotripsy bubble clouds using the shock scattering method of cloud initiation (multi-cycle pulses) increases in stiffer tissues (Vlaisavljevich et al. 2014b), which would also enhance the effects seen in this study. In contrast, previous work studying the intrinsic threshold method of cloud initiation (single cycle pulses) has shown that the intrinsic threshold is independent of tissue stiffness (Vlaisavljevich et al. 2015a). However, the intrinsic threshold would likely be impacted by changes in focal temperature (i.e. decreased threshold at higher temperature). In addition, the cavitation threshold would likely decrease for tissues in which local dehydration occurs due to collagen contraction, as previous work suggests that histotripsy bubbles are generated in the water inside of the tissue (Vlaisavljevich et al. 2015a). Future work will investigate the effects of the thermal pretreatment strategy on the histotripsy cavitation thresholds and the resulting bubble behavior.
The thermal pretreatment strategy investigated in this work has clinically relevant implications for the development of histotripsy therapy. For example, this approach may allow histotripsy to be extended to clinical applications where the removal of very stiff tissues is desired, such as the treatment of tendons, ligaments, fibroadenomas, uterine fibroids, and fibrous tumors. However, since the required time-temperature will likely be above the threshold for tissue necrosis (Sapareto and Dewey 1984), the thermal pretreatment strategy may be limited to applications where the desired clinical outcome is to potentiate tissue removal rather than simply inducing tissue death, such as in the treatment of benign masses such fibroadenomas or uterine fibroids. Using a combined thermal pretreatment and histotripsy approach may also be beneficial for improving the treatment of malignant tumors using thermal ablation, as previous work has shown that thermal ablation often leaves behind a permanent fibrous mass which is often associated with negative side effects such as pain or loss of organ function (Coad et al. 2003; Ding et al. 2013). In contrast to the permanent fibrous mass left after thermal ablation, the fractionated tissue homogenate produced in histotripsy is resorbed over time, which may be beneficial in certain clinical applications (Hall et al. 2007; Hall et al. 2009). Future work will aim to develop the thermal preconditioning strategy for clinical applications in which this approach would be both practical and beneficial to the patient.
Overall, the results of this work demonstrate that thermal preconditioning can alter tissue mechanical properties in order to increase tissue susceptibility to histotripsy. While this work focused on a thermal method, the general pretreatment approach introduced in this study could feasibly be extended to non-thermal methods. For example, the same effect may be achieved by locally administering a drug or enzyme that disrupts tissue collagen prior to histotripsy treatment. It is possible that this “tissue marinating” pretreatment approach may be more feasible than the “tissue cooking” approach in certain clinical applications in which thermal ablation is impractical, such as the treatment of large volumes or in regions containing large blood vessels (Okada et al. 2006; Leslie et al. 2008).
Summary
In this study, the effects of thermal preconditioning on tissue susceptibility to histotripsy were investigated. Results demonstrated that the effects of the thermal preconditioning depend on multiple factors including tissue composition, heating temperature, and heating duration. Controlled heating experiments demonstrated that the Young’s modulus decreased in the majority of tissues when heated to 58°C due to collagen denaturing, resulting in an increase in susceptibility to histotripsy. In contrast, heating to 90°C increased the Young’s modulus of the majority of soft tissues due to collagen contraction. The exception to this was seen in tendon, in which heating at 90°C caused significant softening due to collagen hydrolysis into gelatin, resulting in a significant increase in susceptibility to histotripsy. Based on these results, a final set of experiments was conducted using thermal HIFU to provide the preconditioning treatment, demonstrating that a thermal pretreatment strategy can be implemented non-invasively using a purely ultrasonic method. Overall, the results of this study demonstrate the proof of concept for using a thermal therapy approach to precondition tissue mechanical properties and alter the susceptibility of tissues to histotripsy.
Acknowledgements
This material is based upon work supported by a National Science Foundation Graduate Research Fellowship. This work was supported by grants from National Institute of Biomedical Imaging And Bioengineering (NIBIB) of the National Institutes of Health under Award Number R01EB008998, a Research Scholar Grant from the American Cancer Society (RSG-13-101-01-CCE), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK087871), The Hartwell Foundation, and Focused Ultrasound Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure notice: Drs. Charles Cain, William Roberts, and Zhen Xu have financial interests and/or other relationship with HistoSonics Inc.
References
- Baldwin D. Sous vide cooking: A review. International Journal of Gastronomy and Food Science. 2012;1:15–30. [Google Scholar]
- Coad JE, Kosari K, Humar A, Sielaff TD. Radiofrequency ablation causes ‘thermal fixation’ of hepatocellular carcinoma: a post-liver transplant histopathologic study. Clin Transplant. 2003;17:377–84. doi: 10.1034/j.1399-0012.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- Ding J, Jing X, Liu J, Wang Y, Wang F, Du Z. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379–84. doi: 10.1016/j.ejrad.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Duryea AP, Hall TL, Maxwell AD, Xu Z, Cain CA, Roberts WW. Histotripsy erosion of model urinary calculi. J Endourol. 2011;25:341–4. doi: 10.1089/end.2010.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratzl P. Collagen: Structure and Mechanics. Springer Science+Business Media, LLC; New York, NY: 2008. [Google Scholar]
- Hall TL, Hempel CR, Wojno K, Xu Z, Cain CA, Roberts WW. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009;74:932–7. doi: 10.1016/j.urology.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TL, Kieran K, Ives K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. J Endourol. 2007;21:1159–66. doi: 10.1089/end.2007.9915. [DOI] [PubMed] [Google Scholar]
- Hempel CR, Hall TL, Cain CA, Fowlkes JB, Xu Z, Roberts WW. Histotripsy fractionation of prostate tissue: local effects and systemic response in a canine model. J Urol. 2011;185:1484–9. doi: 10.1016/j.juro.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoermann H, Schlebusch H. Reversible and irreversible denaturation of collagen fibers. Biochemistry. 1971;10:932–7. doi: 10.1021/bi00782a003. [DOI] [PubMed] [Google Scholar]
- Kim K, Johnson LA, Jia C, Joyce JC, Rangwalla S, Higgins PD, Rubin JM. Noninvasive ultrasound elasticity imaging (UEI) of Crohn's disease: animal model. Ultrasound Med Biol. 2008;34:902–12. doi: 10.1016/j.ultrasmedbio.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Fifer CG, Gelehrter SK, Owens GE, Berman DR, Vlaisavljevich E, Allen SP, Ladino-Torres MF, Xu Z. Developmental impact and lesion maturation of histotripsy-mediated non-invasive tissue ablation in a fetal sheep model. Ultrasound Med Biol. 2013;39:1047–55. doi: 10.1016/j.ultrasmedbio.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Lake AM, Xu Z, Wilkinson JE, Cain CA, Roberts WW. Renal ablation by histotripsy--does it spare the collecting system? J Urol. 2008;179:1150–4. doi: 10.1016/j.juro.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Leslie TA, Kennedy JE, Illing RO, Ter Haar GR, Wu F, Phillips RR, Friend PJ, Roberts IS, Cranston DW, Middleton MR. High-intensity focused ultrasound ablation of liver tumours: can radiological assessment predict the histological response? Br J Radiol. 2008;81:564–71. doi: 10.1259/bjr/27118953. [DOI] [PubMed] [Google Scholar]
- Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Jr., Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol. 2011;22:369–77. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Murakami T, Mikami K, Onishi H, Tanigawa N, Marukawa T, Nakamura H. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn Reson Med Sci. 2006;5:167–71. doi: 10.2463/mrms.5.167. [DOI] [PubMed] [Google Scholar]
- Owens GE, Miller RM, Ensing G, Ives K, Gordon D, Ludomirsky A, Xu Z. Therapeutic ultrasound to noninvasively create intracardiac communications in an intact animal model. Catheter Cardiovasc Interv. 2011;77:580–8. doi: 10.1002/ccd.22787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006a;32:115–29. doi: 10.1016/j.ultrasmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Parsons JE, Cain CA, Fowlkes JB. Cost-effective assembly of a basic fiber-optic hydrophone for measurement of high-amplitude therapeutic ultrasound fields. J Acoust Soc Am. 2006b;119:1432–40. doi: 10.1121/1.2166708. [DOI] [PubMed] [Google Scholar]
- Roberts WW, Hall TL, Ives K, Wolf JS, Jr., Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–8. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- Sapin-de Brosses E, Gennisson JL, Pernot M, Fink M, Tanter M. Temperature dependence of the shear modulus of soft tissues assessed by ultrasound. Physics in Medicine and Biology. 2010;55:1701–18. doi: 10.1088/0031-9155/55/6/011. [DOI] [PubMed] [Google Scholar]
- Styn NR, Wheat JC, Hall TL, Roberts WW. Histotripsy of VX-2 tumor implanted in a renal rabbit model. J Endourol. 2010;24:1145–50. doi: 10.1089/end.2010.0123. [DOI] [PubMed] [Google Scholar]
- Takagi R, Yoshizawa S, Umemura S. Enhancement of Localized Heating by Ultrasonically Induced Cavitation in High Intensity Focused Ultrasound Treatment. Japanese Journal of Applied Physics. 2010:49. [Google Scholar]
- Tornberg E. Effects of heat on meat proteins - Implications on structure and quality of meat products. Meat Sci. 2005;70:493–508. doi: 10.1016/j.meatsci.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Vlaisavljevich E, Kim Y, Allen S, Owens G, Pelletier S, Cain C, Ives K, Xu Z. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med Biol. 2013;39:1398–409. doi: 10.1016/j.ultrasmedbio.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Kim Y, Owens G, Roberts W, Cain C, Xu Z. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys Med Biol. 2014a;59:253–70. doi: 10.1088/0031-9155/59/2/253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Lin KW, Maxwell A, Warnez M, Mancia L, Singh R, Putnam A, Fowlkes JB, Johnsen E, Cain C, Xu Z. Effects of Ultrasound Frequency and Tissue Stiffness on the Histotripsy Intrinsic Threshold for Cavitation. Ultrasound Med Biol. 2015a doi: 10.1016/j.ultrasmedbio.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Lin KW, Warnez M, Singh R, Mancia L, Putnam A, Johnsen E, Cain C, Xu Z. Effects of Tissue Stiffness, Ultrasound Frequency, and Pressure on Histotripsy-induced Cavitation Bubble Behavior. Phys Med Biol. 2015b doi: 10.1088/0031-9155/60/6/2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevich E, Maxwell A, Warnez M, Johnsen E, Cain CA, Xu Z. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE Trans Ultrason Ferroelectr Freq Control. 2014b;61:341–52. doi: 10.1109/TUFFC.2014.6722618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MS, Deng XH, Torzilli PA, Doty SB, O’Brien SJ, Warren RF. Thermal modification of collagen. Journal of Shoulder and Elbow Surgery. 1999;8:339–44. doi: 10.1016/s1058-2746(99)90157-x. [DOI] [PubMed] [Google Scholar]
- Wang YN, Khokhlova T, Bailey M, Hwang JH, Khokhlova V. Histological and Biochemical Analysis of Mechanical and Thermal Bioeffects in Boiling Histotripsy Lesions Induced by High Intensity Focused Ultrasound. Ultrasound in Medicine and Biology. 2013;39:424–38. doi: 10.1016/j.ultrasmedbio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard HQ, White DR. The composition of body tissues. Br J Radiol. 1986;59:1209–18. doi: 10.1259/0007-1285-59-708-1209. [DOI] [PubMed] [Google Scholar]
- Wright NT, Humphrey JD. Denaturation of collagen via heating: an irreversible rate process. Annu Rev Biomed Eng. 2002;4:109–28. doi: 10.1146/annurev.bioeng.4.101001.131546. [DOI] [PubMed] [Google Scholar]
- Xie H, Kim K, Aglyamov SR, Emelianov SY, O’Donnell M, Weitzel WF, Wrobleski SK, Myers DD, Wakefield TW, Rubin JM. Correspondence of ultrasound elasticity imaging to direct mechanical measurement in aging DVT in rats. Ultrasound Med Biol. 2005;31:1351–9. doi: 10.1016/j.ultrasmedbio.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: the role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am. 2005;117:424–35. doi: 10.1121/1.1828551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, Cain CA. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:726–36. doi: 10.1109/tuffc.2004.1308731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121:742–9. doi: 10.1161/CIRCULATIONAHA.109.889071. [DOI] [PMC free article] [PubMed] [Google Scholar]