Abstract
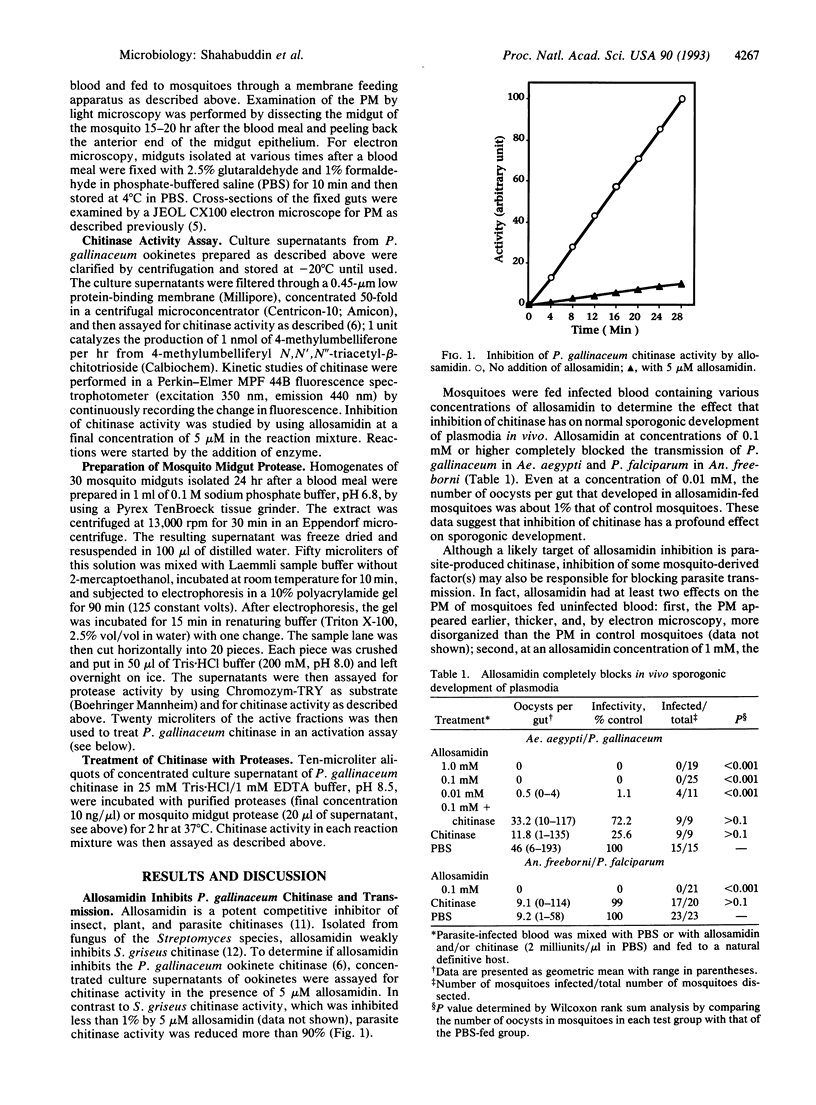
During development in the mosquito midgut, malarial parasites must traverse a chitin-containing peritrophic matrix (PM) that forms around the food bolus. Previously Huber et al. [Huber, M., Cabib, E. & Miller, L. H. (1991) Proc. Natl. Acad. Sci. USA 88, 2807-2810] reported that the parasite secretes a protein with chitinase activity, and they suggested that parasite chitinase (EC 3.2.1.14) plays an important role in the parasite's egress from the blood meal. We found that allosamidin, a specific inhibitor of chitinase, completely blocked oocyst development in vivo and thus blocked malaria parasite transmission. Addition of exogenous chitinase to the blood meal prevented the PM from forming and reversed the transmission-blocking activity of allosamidin. Using exogenous chitinase, we also found that the PM does not limit the number of parasites that develop into oocysts, suggesting that the parasite produces sufficient quantities of chitinase to penetrate this potential barrier. In addition, we found that treatment of parasite chitinase with a diisopropyl fluorophosphate-sensitive trypsinlike protease from the mosquito midgut or endoproteinase Lys-C increased its enzymatic activity. These results suggest that malaria parasite has evolved an intricate mechanism to adapt to the PM and the protease-rich environment of the mosquito midgut.
Full text
PDF
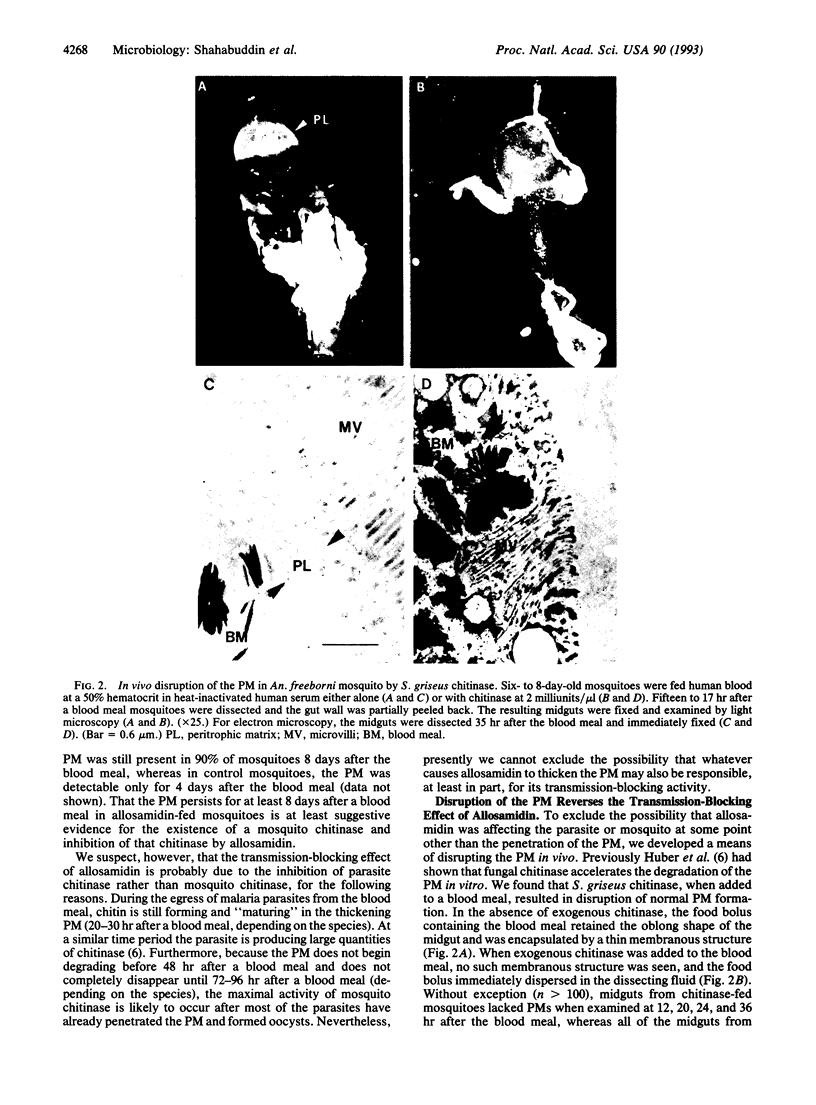

Images in this article
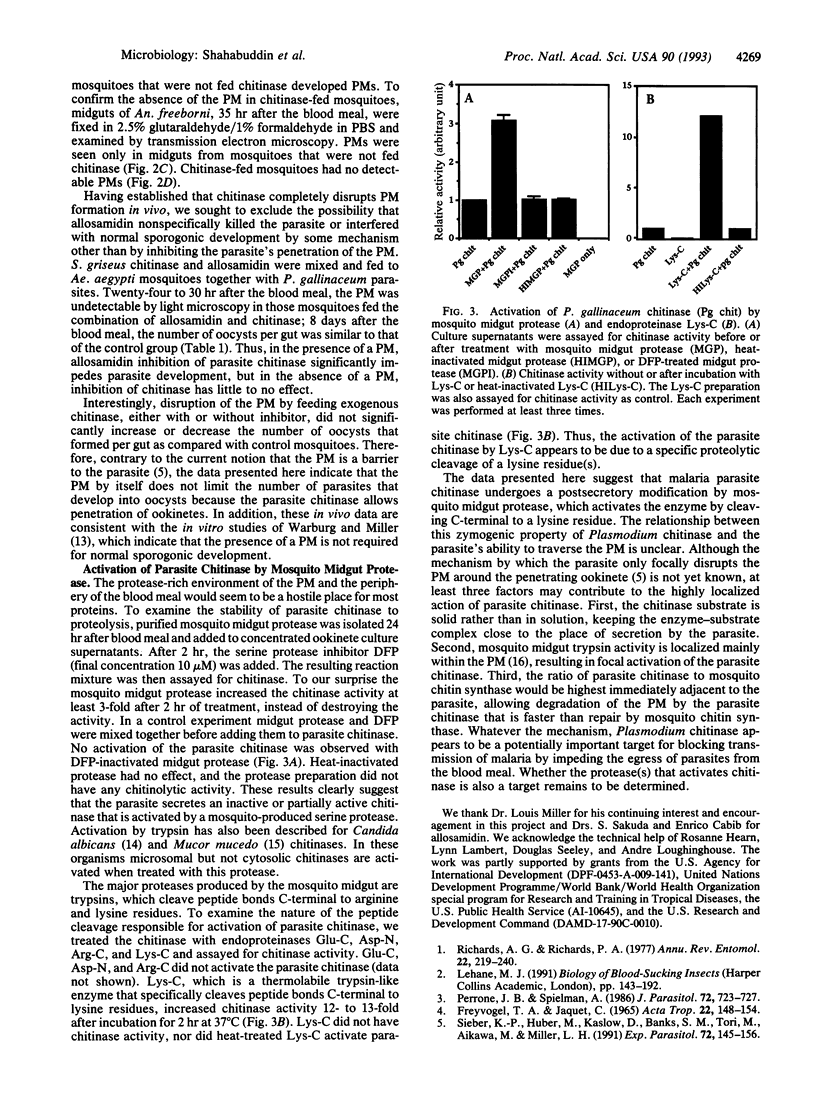
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dickinson K., Keer V., Hitchcock C. A., Adams D. J. Microsomal chitinase activity from Candida albicans. Biochim Biophys Acta. 1991 Jan 23;1073(1):177–182. doi: 10.1016/0304-4165(91)90199-q. [DOI] [PubMed] [Google Scholar]
- FREYVOGEL T. A., JAQUET C. THE PREREQUISITES FOR THE FORMATION OF A PERITROPHIC MEMBRANE IN CULICIDAE FEMALES. Acta Trop. 1965;22:148–154. [PubMed] [Google Scholar]
- Gooday G. W., Brydon L. J., Chappell L. H. Chitinase in female Onchocerca gibsoni and its inhibition by allosamidin. Mol Biochem Parasitol. 1988 Jun;29(2-3):223–225. doi: 10.1016/0166-6851(88)90077-1. [DOI] [PubMed] [Google Scholar]
- Graf R., Raikhel A. S., Brown M. R., Lea A. O., Briegel H. Mosquito trypsin: immunocytochemical localization in the midgut of blood-fed Aedes aegypti (L.). Cell Tissue Res. 1986;245(1):19–27. doi: 10.1007/BF00218082. [DOI] [PubMed] [Google Scholar]
- Huber M., Cabib E., Miller L. H. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2807–2810. doi: 10.1073/pnas.88.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifediba T., Vanderberg J. P. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981 Nov 26;294(5839):364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- Kaushal D. C., Carter R., Rener J., Grotendorst C. A., Miller L. H., Howard R. J. Monoclonal antibodies against surface determinants on gametes of Plasmodium gallinaceum block transmission of malaria parasites to mosquitoes. J Immunol. 1983 Nov;131(5):2557–2562. [PubMed] [Google Scholar]
- Perrone J. B., Spielman A. Microfilarial perforation of the midgut of a mosquito. J Parasitol. 1986 Oct;72(5):723–727. [PubMed] [Google Scholar]
- Richards A. G., Richards P. A. The peritrophic membranes of insects. Annu Rev Entomol. 1977;22:219–240. doi: 10.1146/annurev.en.22.010177.001251. [DOI] [PubMed] [Google Scholar]
- Schlein Y., Jacobson R. L., Shlomai J. Chitinase secreted by Leishmania functions in the sandfly vector. Proc Biol Sci. 1991 Aug 22;245(1313):121–126. doi: 10.1098/rspb.1991.0097. [DOI] [PubMed] [Google Scholar]
- Sieber K. P., Huber M., Kaslow D., Banks S. M., Torii M., Aikawa M., Miller L. H. The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp Parasitol. 1991 Feb;72(2):145–156. doi: 10.1016/0014-4894(91)90132-g. [DOI] [PubMed] [Google Scholar]
- Warburg A., Miller L. H. Sporogonic development of a malaria parasite in vitro. Science. 1992 Jan 24;255(5043):448–450. doi: 10.1126/science.1734521. [DOI] [PubMed] [Google Scholar]