Abstract
β-Lactam resistance in methicillin-resistant Staphylococcus aureus (MRSA) is mediated by the expression of an alternative penicillin-binding protein 2a (PBP2a) (encoded by mecA) with a low affinity for β-lactam antibiotics. Recently, a novel variant of mecA, known as mecC, was identified in MRSA isolates from both humans and animals. In this study, we demonstrate that mecC-encoded PBP2c does not mediate resistance to penicillin. Rather, broad-spectrum β-lactam resistance in MRSA strains carrying mecC (mecC-MRSA strains) is mediated by a combination of both PBP2c and the distinct β-lactamase encoded by the blaZ gene of strain LGA251 (blaZLGA251), which is part of mecC-encoding staphylococcal cassette chromosome mec (SCCmec) type XI. We further demonstrate that mecC-MRSA strains are susceptible to the combination of penicillin and the β-lactam inhibitor clavulanic acid in vitro and that the same combination is effective in vivo for the treatment of experimental mecC-MRSA infection in wax moth larvae. Thus, we demonstrate how the distinct biological differences between mecA- and mecC-encoded PBP2a and PBP2c have the potential to be exploited as a novel approach for the treatment of mecC-MRSA infections.
INTRODUCTION
Antimicrobial resistance is a global health problem of particular importance, which has led to an urgent need for new antimicrobial drug development. An alternative approach to this problem is to resensitize resistant bacteria to existing antibiotics by using novel inhibitors or synergistic combinations of existing drugs that overcome the mechanism(s) of resistance (1). The classical example of this is combinations of β-lactamase inhibitors such as clavulanic acid or sulbactams and a β-lactam antibiotic. Following the introduction of each generation of β-lactam, resistance rapidly emerged. In the case of Staphylococcus aureus, penicillin resistance mediated by a blaZ-encoded β-lactamase (penicillinase) was followed by the emergence of methicillin-resistant S. aureus (MRSA) shortly after the introduction of methicillin (a β-lactamase-resistant β-lactam) in 1961 (2, 3).
Resistance to β-lactam antibiotics in MRSA is mediated primarily by the acquisition of an alternative penicillin-binding protein 2 (PBP2a) encoded by the mecA gene, which is carried on a mobile element known as staphylococcal cassette chromosome mec (SCCmec) (4). In 2011, a new type of MRSA isolate with a divergent mecA homologue known as mecC was described, which, like some other types of MRSA, is associated with livestock (5, 6). It has been demonstrated that mecC mediates resistance to cefoxitin and oxacillin in a range of strain backgrounds and that mecC expression is inducible with oxacillin (7). mecC-encoded PBP2a (PBP2c) shares only 63% amino acid identity with mecA-encoded PBP2a (PBP2a). The difference in amino acid identity is reflected in the distinct biochemical properties of PBP2c, whereby it shows a greater affinity for oxacillin than for cefoxitin and is less stable at 37°C (8). Furthermore, unlike PBP2a, PBP2c does not require the transglycosylase (TGase) activity of native PBP2 for high-level resistance, suggesting that it may preferentially cooperate with an as-yet-unidentified monofunctional TGase (8).
Here we report that the biological differences between PBP2a and PBP2c extend to the unexpected finding that PBP2c does not mediate resistance to penicillin and that expression of the strain LGA251 β-lactamase gene (blaZLGA251) located adjacent to mecC on the SCCmec type XI element is required for broad-spectrum resistance to β-lactams. We demonstrate that this singular property of PBP2c can be exploited therapeutically for the treatment of infections caused by MRSA strains carrying mecC (mecC-MRSA strains).
MATERIALS AND METHODS
Media and culture conditions.
Bacterial strains and plasmids used in this study are described in Table S5 in the supplemental material. For routine culture, Escherichia coli was grown in lysogeny broth (LB) or on L agar (Oxoid, United Kingdom) at 37°C. S. aureus was grown on tryptone soy agar (TSA), Columbia blood agar, or tryptone soy broth (TSB) (Oxoid, United Kingdom) at 28°C or 37°C, accordingly. E. coli and S. aureus media were supplemented with 10 μg/ml chloramphenicol (Cm10) as appropriate.
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing was performed by using disc diffusion susceptibility testing according to BSAC criteria (9). All antibiotic discs were purchased from Oxoid (United Kingdom). For the clavulanic acid assay, 15 μg/ml potassium clavulanate (Sigma-Aldrich, United Kingdom) was added to Iso-Sensitest agar (ISA) or Mueller-Hinton agar (MHA) (Oxoid, United Kingdom), as appropriate. Test isolates were grown to a 0.5 McFarland standard in Iso-Sensitest broth (Oxoid, United Kingdom) and diluted 1:10 in distilled water before spreading onto agar plates with or without potassium clavulanate. After application of the antibiotic discs to Iso-Sensitest agar or Mueller-Hinton agar with 2% NaCl, as appropriate, all plates were incubated at 35°C for 20 h before inhibition zones were measured. Broth microdilution for MICs was performed according to BSAC guidelines (10). The ranges for MIC determination were 0.015 to 128 μg/ml for penicillin and 1 to 32 μg/ml for cefoxitin. For mecC-blaZ-complemented strains, Iso-Sensitest broth was supplemented with 200 ng/ml anhydrotetracycline (Atc) to induce the expression of mecC and blaZ from pXB01, a modified tetracycline-inducible expression vector derived from pRMC2.
Construction of S. aureus gene deletion mutants.
Oligonucleotide primer sequences are listed in Table S5 in the supplemental material. By using primers de-blaF and de-blaR, inverse PCR was performed on pRMC2 using Kod Hot Start DNA polymerase (Merck, United Kingdom), according to the manufacturer's instructions, to simultaneously remove the resistance gene bla and introduce a NotI restriction site at each end of the PCR product. After NotI digestion and self-ligation, a modified tetracycline-inducible expression vector was obtained and designated pXB01. mecC and blaZ deletion mutants in mecC-MRSA strains were generated by allelic exchange with the temperature-sensitive vector pIMAY, as described previously (11). The upstream sequence (PCR product AB) and downstream sequence (PCR product CD) of the S. aureus gene to be deleted were PCR amplified with primer pairs A/B and C/D, respectively, using Kod Hot Start DNA polymerase (Merck, United Kingdom). PCR products AB and CD were mixed in a single PCR mixture and fused by splicing by overlap extension PCR (SOE PCR) using Kod Hot Start DNA polymerase (Merck, United Kingdom) and primer pair A/D to obtain the deletion construct AD. Product AD was digested with the restriction enzymes KpnI and SacI and ligated into pIMAY digested with the same enzymes. The resulting plasmids were designated pIMAYΔmecC and pIMAYΔblaZ. The plasmids were transformed into E. coli DC10B (a dcm deletion mutant of DH10B, allowing the plasmid to be directly transferred into S. aureus strains [11]). Plasmid DNA extracted from DC10B was then electroporated into recipient strains to create knockout mutants. mecC-blaZ double-deletion mutants were generated by deleting blaZ gene from mecC deletion mutants using plasmid pIMAYΔmecCΔblaZ.
Complementation of mutant strains.
For complement expression of mecC and blaZ, the genes were cloned into expression plasmid pXB01, a derivate of tetracycline-inducible expression vector pRMC2 with the bla gene deleted (12). Both genes, including their ribosome-binding sites, were amplified from LGA251 genome DNA with primer pairs mecC-F-KpnI/mecC-R-SacI and blaZ-F-KpnI/blaZ-R-SacI. PCR products were digested with KpnI and SacI and ligated with the pXB01 vector cleaved with the same enzymes, generating plasmids pXB01-mecC and pXB01-blaZ. The plasmids were transformed into E. coli DC10B, and plasmid DNA was then extracted and electroporated into mutant strains for complementation with expression induced with 200 ng/ml anhydrotetracycline (Sigma-Aldrich, United Kingdom).
Wax moth larva (Galleria mellonella) infection and treatment assay.
The wax moth larva assay was based on a method described previously by Desbois and Coote (13). Galleria mellonella larvae were purchased in bulk from a commercial supplier (Livefood Ltd., United Kingdom). Larvae were stored at 4°C upon arrival and kept at 37°C during the course of the assay. mecC-MRSA strains LGA251, 02.5099.D, and 71277 were selected for evaluation of antimicrobial activities of penicillin and clavulanic acid in combination. Single bacterial colonies were picked for inoculation into 5 ml of TSB, and cultures were grown overnight (∼16 h) at 37°C and at 200 rpm. Cultures were then diluted 1:100 into 5 ml of fresh TSB and grown for a further 4 h at 37°C and at 200 rpm. Cultures were then centrifuged at 2,500 × g for 10 min, and pellets were resuspended in sterile phosphate-buffered saline (PBS) to an optical density at 595 nm (OD595) of 0.2, giving ∼1.3 × 106 CFU (range, 1.1 × 106 to 1.4 × 106 CFU) in 10 μl. For each strain, six groups of G. mellonella larvae (n = 10 in each group) were injected with 10-μl aliquots of the resuspended culture between two posterior thoracic segments by using a Tridak Stepper pipette dispenser (Dymax, United Kingdom). Groups of G. mellonella larvae were treated by injection with 50 mg/kg of body weight vancomycin, 40 mg/kg cefoxitin, 20 mg/kg penicillin sodium salt, 20 mg/kg potassium clavulanate, 20 mg/kg penicillin sodium salt combined with 20 mg/kg potassium clavulanate, or PBS 2, 24, and 48 h after inoculation. The treatments were given blind, and the treatment identities were not revealed until the experiment was completed. Larvae were considered dead when they did not respond to a touch to the head. The experiment was performed twice, with almost identical results; results of one experiment are presented below (see Fig. 5), and the results for the second experiment are presented in Fig. S3 in the supplemental material.
FIG 5.
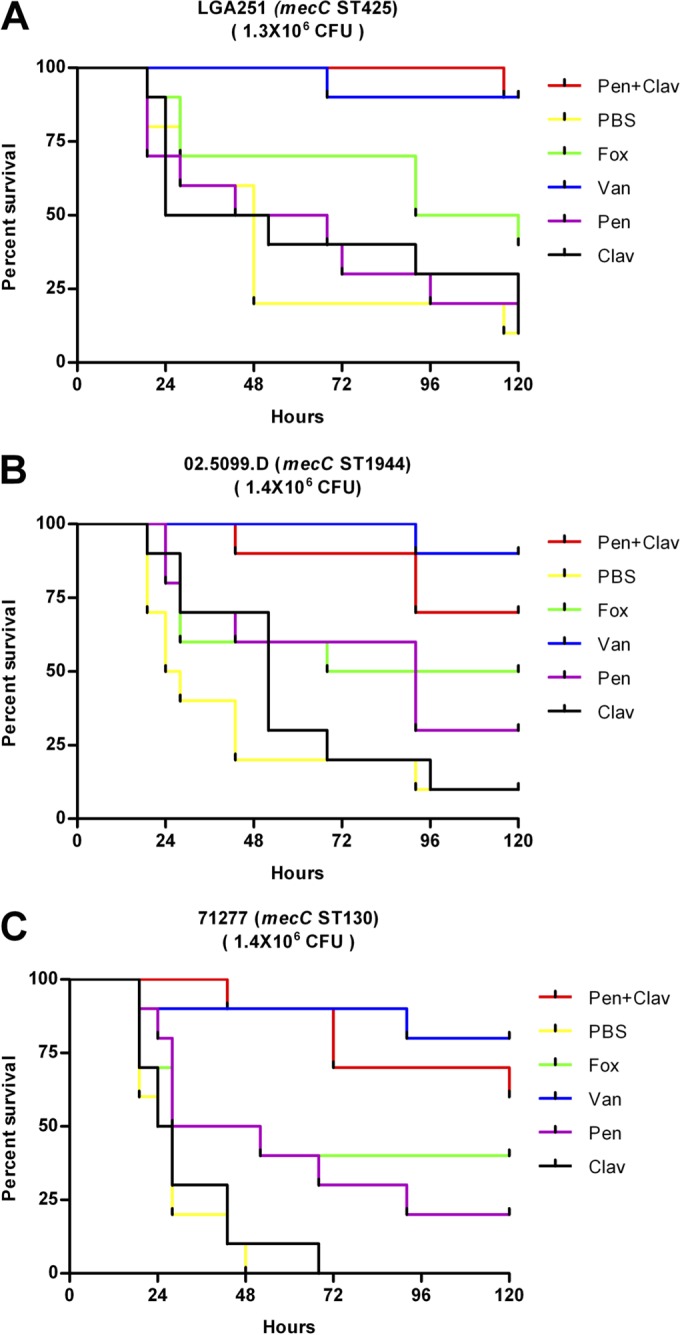
Experimental treatment of Galleria mellonella larvae infected by mecC-MRSA strains LGA251 (A), 02.5099.D (B), and 71277.10 (C). Ten larvae in each group were experimentally infected and then treated at 2, 24, and 48 h with vancomycin (50 mg/kg; 6.73 × 10−9 mol), penicillin (20 mg/kg; 1.12 × 10−8 mol), clavulanic acid (20 mg/kg; 1.69 × 10−8 mol), penicillin-clavulanic acid, cefoxitin (40 mg/kg; 1.78 × 10−8 mol), and PBS alone. Shown are data from a single experiment.
In vitro selection of penicillin resistance.
LGA251 and 02.5099.D were serially passaged for 40 days with subinhibitory concentrations of penicillin in the presence of 15 μg/ml clavulanic acid. Briefly, strains were grown on Columbia blood agar, and four single colonies were used to make a 0.5 McFarland standard in Iso-Sensitest broth. This was diluted to a final 1:200 dilution in 2 ml of Iso-Sensitest broth with a range of 2-fold penicillin concentrations (0.03125 to 4 μg/ml) and a fixed concentration of 15 μg/ml clavulanic acid. After incubation for 24 h at 37°C with shaking at 200 rpm, the culture with the highest antibiotic concentration showing clear visible growth was adjusted back to a 0.5 McFarland standard, used to inoculate a fresh set of tubes as described above, and incubated for another 24 h. Once growth occurred at the 4-μg/ml concentration of penicillin, the increment was changed from 2-fold increases to 2-μg/ml increases. At selected time points, cultures were plated out, 2 to 3 resistant colonies were picked, penicillin and cefoxitin MICs were determined, and the mecC gene was amplified by PCR using primers mecCf, mecCm, and mecCr and sequenced (Source Bioscience Sequencing, Cambridge, United Kingdom) (see Table S5 in the supplemental material). Strains LGA251ΔblaZ and 02.5099.DΔblaZ were also serially passaged for 40 days with subinhibitory concentrations of penicillin in the same manner but in the absence of clavulanic acid.
Bioinformatics analysis.
β-Lactamase sequences of type A (GenBank accession number EVL36279), type B (accession number AGU62029.1), type C (accession number WP_015056218), and type D (accession number Q53699) were downloaded from the NCBI database. Alignments were generated by using Muscle in Seaview (14, 15). For phylogeny, blaZ from Macrococcus caseolyticus (accession number WP_041636568) was included as an outgroup, and blaZ from Staphylococcus xylosus (accession number CCM44120) was included for comparison (16). Maximum likelihood phylogenetic trees were constructed by using PhyML v3.0 in Seaview with a Whelan and Goldman (WAG) substitution model and 100 bootstrap replicates (17).
RESULTS
PBP2c does not mediate resistance to penicillin.
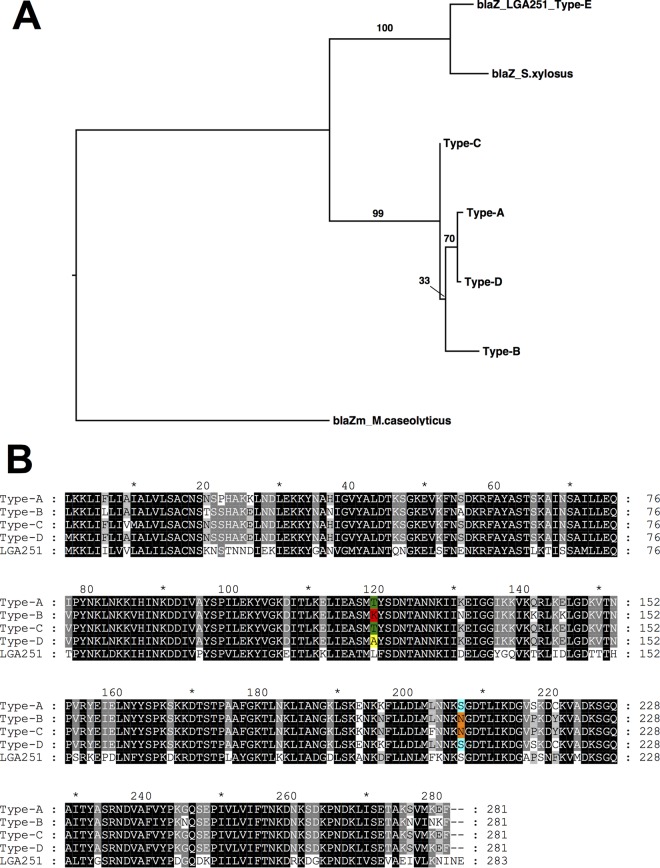
Previously work by Kim et al. identified that PBP2a encoded by mecC (PBP2c) had a higher relative affinity for oxacillin than for cefoxitin, suggesting that PBP2c has a higher affinity for penicillins than for cephalosporins (8). The class E mec complex (mecI-mecR1-mecC-blaZ) present in SCCmec type XI contains a blaZ gene (here blaZLGA251) present downstream of the mecC gene. blaZLGA251 is phylogenetically distinct from other previously reported blaZ genes in S. aureus (Fig. 1A), and mecC-MRSA strains with blaZLGA251 do not harbor any other blaZ genes (data not shown). Currently, there are four types (types A to D) of staphylococcal β-lactamases based on differences of amino acids at positions 128 and 216 (18, 19). At position 216, blaZLGA251 shares a serine with type A and D blaZ genes (Fig. 1B), while at position 128, blaZLGA251 uniquely has a leucine. Therefore, we propose that blaZLGA251 is a new staphylococcal blaZ type, type E blaZ (Fig. 1).
FIG 1.
(A) Maximum likelihood tree generated from amino acid sequences showing relationships of staphylococcal β-lactamases. Values above the branches indicate bootstrap support. (B) Alignment of the amino acid sequences of representative type A to D β-lactamases in comparison with the SCCmec type XI-encoded type E (LGA251) β-lactamase. Highlighted residues indicate amino acids at positions 128 and 216 used to type the β-lactamase.
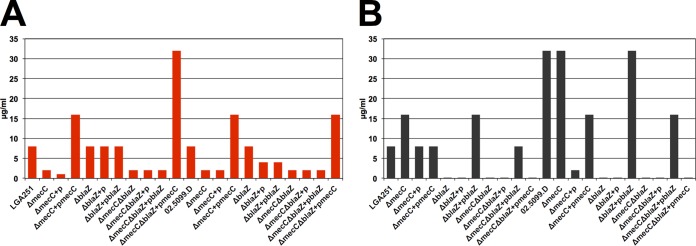
As PBP2c confers low-level resistance to penicillins such as oxacillin, and the SCCmec type XI mec complex included a novel type of blaZ gene, we investigated the relative contribution of mecC and blaZLGA251 to β-lactam resistance. We generated gene deletions of mecC, blaZ, and both mecC and blaZ in two different mecC-MRSA strains: LGA251, a strain of multilocus sequence type 425 (ST425) isolated from cattle in England, and 02.5099.D, a ST1944 (CC130) isolate from human infection in Scotland (5). Deletion of mecC in LGA251 (LGA251ΔmecC) and in 02.5099.D (02.5099.DΔmecC) caused a loss of resistance to cefoxitin, as measured by disc diffusion and MIC assays, while complementation with mecC restored resistance, as previously reported (Fig. 2A; see also Table S1 in the supplemental material) (7). However, deletion of mecC caused no reduction in penicillin resistance in either LGA251 or 02.5099.D, demonstrating that mecC did not mediate resistance to penicillin in the background of either strain (Fig. 2B; see also Table S1 in the supplemental material). In contrast, when the blaZLGA251 gene was deleted, resistance to penicillin was abolished in both strain backgrounds, and the penicillin MIC decreased from 8 to <0.0075 μg/ml and from 32 to 0.0625 μg/ml in LGA251 and 02.5099.D, respectively (Fig. 2B; see also Table S1 in the supplemental material). Complementation of the mutants with blaZ restored penicillin MICs to wild-type levels in both backgrounds (Fig. 2B; see also Table S1 in the supplemental material). These findings were confirmed by the creation of mecC-blaZ double-deletion strains (LGA251ΔmecCΔblaZ and 02.5099.DΔmecCΔblaZ), which were then individually complemented with plasmid-borne copies of mecC and blaZ. In each case, complementation with blaZ and not complementation with mecC restored resistance to penicillin (Fig. 2B; see also Table S1 in the supplemental material).
FIG 2.
Effect of deletion of mecC and blaZ in strains LGA251 and 02.5099.D on the MICs of cefoxitin (A) and penicillin (B). For each strain background (LGA251 and 02.5099.D), the first strain is the wild type, followed to the right by its various mutants and complemented mutants. +p denotes complementation with the empty vector, where +pmecC indicates complementation with a vector-borne copy of mecC and +pblaZ indicates complementation with a vector-borne copy of blaZ.
mecC-MRSA strains are susceptible to the combination of clavulanic acid and penicillin in vitro.
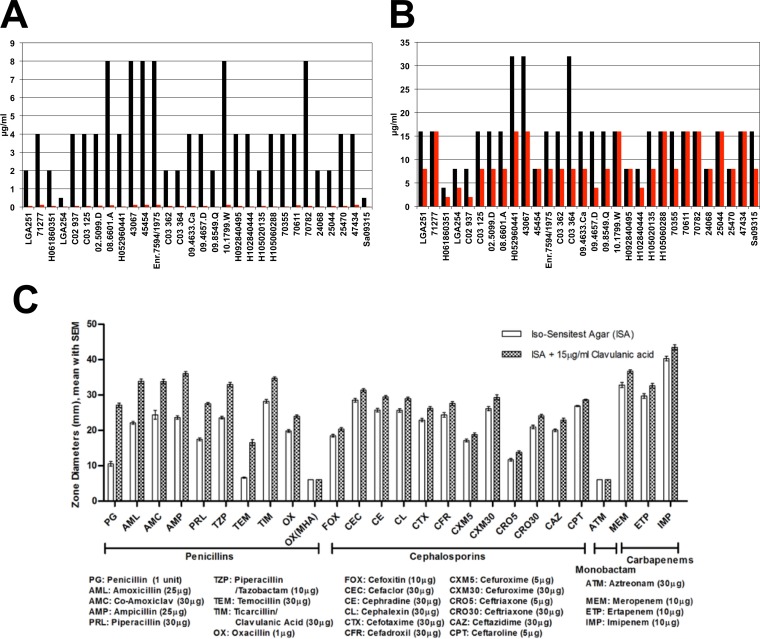
As penicillin resistance in mecC-MRSA strains is mediated by blaZLGA251 alone, we tested whether mecC-MRSA strains were susceptible to the combination of penicillin and clavulanic acid (a β-lactamase inhibitor). We included clavulanic acid at a clinically relevant concentration of 15 μg/ml in bacteriological medium and carried out penicillin and cefoxitin disc diffusion assays, according to BSAC criteria, against a panel of 30 mecC-MRSA isolates (Fig. 3) (20, 21). This analysis showed that clavulanic acid increased susceptibility to penicillin in all strains tested (except for strain Sa09315, which was already susceptible due to a frameshift in blaZ) and more than doubled the mean zone of inhibition (10 versus 27 mm; resistance cutoff of 24 mm) (Fig. 3). Furthermore, clavulanic acid reduced the penicillin MICs by a mean of 65-fold and restored breakpoint susceptibility in 24 of 30 strains tested (Fig. 3A). In the remaining 6 strains, the MIC was reduced to the breakpoint (breakpoint, 0.12 μg/ml) (Fig. 3A). In contrast, only minor reductions were seen for cefoxitin combined with clavulanic acid (Fig. 3B). In view of the relative instability of PBP2c at higher temperatures (the assays were performed at 35°C), we confirmed the effect of clavulanic acid on cefoxitin and penicillin at 25°C, 30°C, and 37°C. The results for cefoxitin showed a clear increase in the zone of inhibition with increasing temperature, as previously reported, with no major effect of clavulanic acid being seen at any temperature (8) (see Fig. S1 in the supplemental material), while for penicillin, there was only a minor effect with increasing temperature and similar zones of inhibition in the presence and absence of clavulanic acid (see Fig. S1 in the supplemental material). Together, these data demonstrate that the effect of clavulanic acid was not due to the temperature-sensitive activity of PBP2c at 35°C.
FIG 3.
Effect of clavulanic acid on mecC-MRSA strains. (A and B) Penicillin MICs (A) and cefoxitin MICs (B) for individual isolates in the presence (red) and absence (black) of clavulanic acid. (C) Mean results for a panel of 30 mecC-MRSA strains tested against a range of β-lactam antibiotics, as measured by disc diffusion assays, in the presence and absence of clavulanic acid. The error bars represent standard errors. Two- and three-letter codes and concentrations of discs are shown below each class of β-lactam antibiotic. Note that results are shown for oxacillin on two different media: Iso-Sensitest agar and Mueller-Hinton agar with 2% NaCl at 30°C (BSAC-recommended media [9]). Resistance breakpoints for penicillin, oxacillin, and cefoxitin are 24 mm, 14 mm, and 21 mm, respectively. The BSAC MIC breakpoints for penicillin (Pen) and cefoxitin (Fox) are 0.12 and 4 μg/ml, respectively. Clavulanic acid was included in the media at 15 μg/ml.
Role of mecC and blaZ in resistance against a broad range of β-lactam antibiotics.
We next tested the effect of clavulanic acid on resistance against a broad range of β-lactam antibiotics (8 penicillins, 12 cephalosporins, 1 monobactam, and 3 carbapenems) for the same panel of 30 mecC-MRSA strains. The results showed that clavulanic acid increased susceptibility to all penicillins tested except for oxacillin (Fig. 3C; see also Table S2 in the supplemental material). Oxacillin was tested on Mueller-Hinton agar as recommended by BSAC (9), EUCAST (34), and CLSI (35) guidelines and also by using Iso-Sensitest agar, where there was a small decrease in the presence of clavulanic acid (mean, 20 versus 24 mm), which was less than that observed for other penicillins. The effect of clavulanic acid was most pronounced for penicillin (mean, 11 versus 27 mm), amoxicillin (mean, 22 versus 34 mm), ampicillin (mean, 24 versus 36 mm), and temocillin (mean, 7 versus 17 mm) (Fig. 3; see also Table S2 in the supplemental material). Only very small increases in susceptibility were seen in the presence of clavulanic acid for the tested cephalosporins and carbapenems, and no effect at all was seen for the one monobactam tested (aztreonam) (Fig. 3; see also Table S2 in the supplemental material).
To further understand the basis for the effect of clavulanic acid, we next tested the mecC and blaZ deletion mutant strains against the same range of β-lactams in disc diffusions assays, alone and in the presence of 15 μg/ml clavulanic acid (see Table S1 in the supplemental material). The results showed that the effect of clavulanic acid on penicillin resistance was negated in both strains LGA251ΔblaZ and 02.5099.DΔblaZ, indicating that, as would be expected, the zone increases mediated by clavulanic acid were dependent on the blaZLGA251-encoded β-lactamase (see Table S1 in the supplemental material). Interestingly, the presence of clavulanic acid alone in the medium prevented the growth of both ΔmecC strains. Furthermore, complementation with mecC on a plasmid failed to reverse this, nor was this effect seen in either of the ΔblaZ strains (LGA251ΔblaZ and 02.5099.DΔblaZ), demonstrating that it was not a by-product of the mutant construction process per se but most likely due to the specific loss of chromosomal mecC (see Table S1 in the supplemental material).
Selection of penicillin resistance in mecC-MRSA isolates.
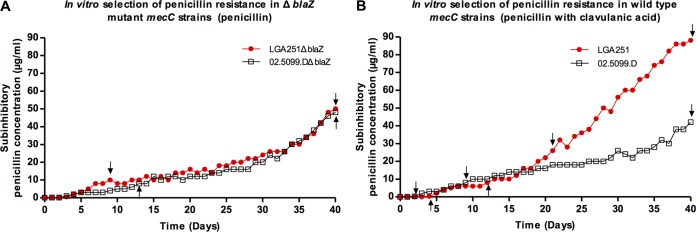
Next, we sought to elucidate the molecular basis for why PBP2c was unable to mediate resistance to penicillin. We first attempted to identify mecC mutations that conferred resistance to penicillin by screening a collection of whole-genome-sequenced mecC-MRSA isolates (data not shown) to identify naturally occurring amino acid substitutions. We identified 10 different amino acid substitutions present in PBP2c and tested representative isolates by penicillin disc diffusion with clavulanic acid to see if there was any effect on penicillin susceptibility (Table 1). None of the isolates tested showed any resistance to the combination of penicillin and clavulanic acid. We next sought to select mutants in vitro with PBP2c mutations conferring penicillin resistance. We grew two wild-type mecC-MRSA strains, LGA251 and 02.5099.D, with gradually increasing concentrations of penicillin supplemented with clavulanic acid at 15 μg/ml for 40 days (Fig. 4). We also grew the corresponding isogenic ΔblaZ mutants (LGA251ΔblaZ and 02.5099.DΔblaZ) in penicillin alone (Fig. 4). At a number of points in the experiment, isolates were plated as single colonies, 2 to 3 individual colonies were tested for penicillin and cefoxitin MICs, and mecC was sequenced to identify potential mutations mediating penicillin resistance (Fig. 4). MIC testing of LGA251ΔblaZ from day 9 and of 02.5099.DΔblaZ from day 13 showed that as well as becoming penicillin resistant, these strains showed substantially increased resistance to cefoxitin (8 versus ≥128 μg/ml), suggesting that the change seen was a general increase in β-lactam resistance and not specific to penicillin (see Table S3 in the supplemental material). Only colonies from wild-type strain 02.5099D grown in penicillin and clavulanic acid at day 40 (02.5099-D40-A) revealed the presence of a G-to-A mutation at position 1636 in mecC causing a Val546Ile substitution in the transpeptidase domain in PBP2c. None of the other strains screened had any mecC mutations, suggesting that resistance was due to mutations elsewhere in the chromosome or an upregulation of genes involved in resistance. Disc diffusion testing of two individual colonies (02.5099-D40-A-C1 and 02.5099-D40-A-C2) with the Val546Ile substitution showed that resistance had increased to all β-lactam antibiotics except for aztreonam, to which the strains were already completely resistant, and to ceftaroline, the new anti-MRSA cephalosporin (see Table S4 in the supplemental material). We cloned the mutated mecC gene (mecCVal546Ile) from 02.5099-D40-A-C1 and 02.5099-D40-A-C2 into RN4220 and into blaZ-mecC-null strains LGA251ΔblaZΔmecC and 02.5099.DΔblaZΔmecC and tested resistance to penicillin and cefoxitin using disc diffusion. We found that the strains with mecCVal546IIe were equally as susceptible to penicillin as wild-type mecC, demonstrating that the Val546Ile substitution alone was not capable of mediating resistance (see Fig. S2A and Table S4 in the supplemental material). Interestingly, however, when we tested the mecCVal546Ile strains for cefoxitin resistance, we found that the strains were not resistant to cefoxitin, as measured by disc diffusion, and had an MIC of 2 μg/ml, in comparison to 16 μg/ml for strains expressing wild-type mecC (see Fig. S2B and Table S4 in the supplemental material). Further disc diffusion testing against the full panel of β-lactams revealed that the Val546Ile substitution affected resistance to cefoxitin only (see Table S4 in the supplemental material). This demonstrates the importance of valine at position 546 for specifically mediating cefoxitin resistance in PBP2c.
TABLE 1.
Locations of amino acid substitutions in PBP2c in wild-type strains and location of the substitution found in strain 02.5099-D40-Aa
| Representative strain | No. of isolates tested | Amino acid substitution at position: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | 130 | 145 | 173 | 185 | 269 | 346 | 349 | 466 | 482 | 546 | ||
| LGA251 | 1 | N | V | K | N | R | Y | A | P | R | D | V |
| 0820 A | 1 | S | ||||||||||
| 52902 | 2 | N | ||||||||||
| ST20120827 | 1 | I | ||||||||||
| 77964 | 9 | K | ||||||||||
| Sa13307 | 4 | K | ||||||||||
| M4A | 1 | A | ||||||||||
| 71957 | 14 | H | ||||||||||
| m-40-71 | 1 | L | ||||||||||
| 1198/2006 | 1 | I | ||||||||||
| 51618 | 1 | C | ||||||||||
| 02.5099-D40-A | 1 | I | ||||||||||
Residues likely to be in the transpeptidase domain (residues 324 to 665) based on alignment with PBP2a are shown in boldface type.
FIG 4.
In vitro selection of penicillin resistance. Graphs show changes in subinhibitory concentrations for mutant mecC-MRSA strains LGA251ΔblaZ and 02.5099.DΔblaZ (A) or wild-type mecC-MRSA strains LGA251 and 02.5099.D (B), during the course of in vitro penicillin resistance selection (A) or penicillin and clavulanic acid selection (B), grown in continuous culture with Iso-Sensitest broth at 37°C. Arrows indicate time points selected for mecC gene sequencing.
The combination of clavulanic acid and penicillin is effective in vivo for treatment of mecC-MRSA infections.
Finally, we sought to determine if the effect of penicillin and clavulanic acid seen in vitro could translate to therapeutic treatment of S. aureus infection in vivo. We used the wax moth larva model of infection with 3 different mecC-MRSA strains belonging to different multilocus sequence types: LGA251 (ST425), 02.5099.D (ST1944), and 71277 (ST130) (13). We compared the effect of penicillin-clavulanic acid (2:1) with penicillin, clavulanic acid, cefoxitin, and PBS against the “gold-standard” treatment of vancomycin (Fig. 5). For both LGA251 and 02.5099.D, 10% of larvae treated with PBS or clavulanic acid survived to 120 h, while for 71277, larvae were all dead by 48 and 68 h, respectively (Fig. 5). Treatment with penicillin alone led to a modest improvement, with survival rates at 120 h of 20% for LGA251, 30% for 02.5099.D, and 20% for 71277 (Fig. 5). Despite the presence of PBP2c, cefoxitin performed moderately better than penicillin, with survival rates at 120 h of up to 40% for LGA251 and 71277 and up to 50% for 02.5099.D. In contrast, survival rates at 120 h for penicillin and clavulanic acid were 90% for LGA251, 65% for 71277, and 70% for 02.5099.D (Fig. 5), while the gold-standard treatment of vancomycin had survival rates at 120 h of 90% for LGA251 and 80% for 02.5099.D and 71277 (Fig. 5). Statistical analysis showed that there was no significant difference between treatment with the combination of penicillin and clavulanic acid and treatment with vancomycin (P = 0.970 for LGA251, P = 0.259 for 02.5099.D, and P = 0.370 for 71277 [as determined by a log rank {Mantel-Cox} test]). Repeat experiments showed broadly identical results (see Fig. S3 in the supplemental material).
DISCUSSION
Antibiotic resistance is a major international problem, with few new antibiotics likely to be available in the immediate future. Novel methods to combat antibiotic resistance are therefore required, including repurposing older antibiotics. Here we present evidence of one such case. We show that the newly described mecC-encoded PBP2c does not mediate resistance to penicillin and that the adjacent type E blaZ gene is required for resistance to penicillin. Our data suggest that this biological difference can be exploited for treatment by combining penicillin and clavulanic acid, a β-lactam inhibitor, to block the action of the blaZLGA251-encoded β-lactamase. Importantly, we show that our in vitro data translate to successful treatment of experimental infections in vivo in a nonvertebrate model. The combination of penicillin and clavulanic acid was as effective as vancomycin in reducing the mortality of wax moth larvae infected with three different mecC-MRSA strains. We also observed in vitro activity of clavulanic acid in combination with amoxicillin, a combination that is already commercially available (Augmentin), suggesting that drugs already in clinical use might be successful in treating mecC-MRSA infections. Additionally, variable resistance to different cephalosporins was reported previously for mecC-MRSA isolates, suggesting that certain cephalosporins might also be also be used for treatment (22). Our data for 30 mecC-MRSA isolates found uniform zones of inhibition (Fig. 3C) for all the cephalosporins tested, including susceptibility to ceftaroline (the new anti-MRSA cephalosporin). However, given the reliance on cefoxitin-oxacillin testing for MRSA detection, there is a lack of clinical breakpoints for most cephalosporins; therefore, it is not clear if these zones of inhibition would translate into clinical efficacy, and further work is required to address this question.
These findings suggest that penicillin can readily bind to PBP2c to prevent cell wall biosynthesis, a notion supported by data reported previously by Kim et al., which showed a higher binding affinity of PBP2c for penicillins than for cephalosporins, in comparison to PBP2a (8). This is consistent with our finding that clavulanic acid was effective at conferring increased susceptibility to all the tested penicillins (except oxacillin) but had no effect against cephalosporins. It remains to be seen if this property is conserved among the variants of mecC identified in different coagulase-negative staphylococci (16, 23–25).
Biologically, the finding that PBP2c has evolved so as not to mediate resistance to penicillin is of interest. The linkage of both mecC and blaZLGA251 on a single genetic element might have enabled PBP2c to evolve properties distinct from those of PBP2a, without the constraint of having to mediate resistance to penicillin. This might suggest that the selective pressure to mediate resistance to cephalosporins or an as-yet-unidentified β-lactam(s) under specific conditions (temperature, pH, and ion concentration, etc.) selected PBP2c to be unable to mediate resistance to penicillin. Indeed, a previous demonstration that PBP2c is unstable at 37°C suggests that selection pressures might have selected for function at lower temperatures (8). Equally, it is known that there are specific fitness costs associated with the expression of PBP2a, including a loss of toxicity and altered biofilm formation, and that high-level β-lactam resistance requires epistatic mutations (26–29). The distinct advantage of PBP2c in comparison to PBP2a or the selective pressures that have driven this remain to be determined.
mecC-MRSA strains are commonly isolated from cattle (30), and it is possible that the routine use of both first- and third-generation cephalosporins for the treatment and prevention of mastitis may have provided the selective pressure that has driven the emergence of mecC-MRSA strains in dairy cows (31). Future studies should be targeted to investigate this question to provide insight for improvements in antibiotic stewardship in veterinary medicine.
We have also identified the first mutation associated with a specific loss of cefoxitin resistance in PBP2c. Comparison of the PBP2c and PBP2a structures suggests that this substitution might affect lobe-lobe positioning; also, V546 is adjacent to a beta strand “cascade” that likely packs differently in PBP2c/PBP2a protein cores; i.e., V546(c)/L549(a) sits adjacent to I354(c)/L357(a), which could propagate all the way up to motif III (KSG) and motif I (SXXK) at the active site (see Fig. S4 in the supplemental material) (32, 33). Further structural insights into the difference between the two proteins are required to shed further light on our understanding of the distinct biological properties of PBP2a and PBP2c.
In conclusion, our findings further highlight how the limited functional sequence space can be exploited for antibiotic drug design and synergistic therapy. This is particularly important given the increasing challenges posed by multidrug resistance and offers a paradigm for tackling an emerging, resistant bacterial pathogen with an old antibiotic.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Medical Research Council (MRC) partnership grant (G1001787/1) held between the Department of Veterinary Medicine, University of Cambridge (M.A.H.), the School of Clinical Medicine, University of Cambridge (S.J.P.), the Moredun Research Institute (R.Z.), and the Wellcome Trust Sanger Institute (J.P. and S.J.P.).
X.B. designed and carried out experimental work, analyzed the data, and contributed to the manuscript. E.M.H. designed and carried out experimental work and bioinformatics analysis, analyzed the data, and wrote the manuscript. A.L.L. contributed to the experimental design and carried out structural analysis. N.G. carried out experimental work. R.Z., J.P., and S.J.P. contributed to the analysis and critically revised the manuscript. M.T.G.H. contributed to the analysis and interpretation of the data and critically revised the manuscript. G.K.P. contributed to the experimental design, analyzed the data, and critically revised the manuscript. M.A.H. coordinated the study and wrote the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01469-15.
REFERENCES
- 1.Tan CM, Therien AG, Lu J, Lee SH, Caron A, Gill CJ, Lebeau-Jacob C, Benton-Perdomo L, Monteiro JM, Pereira PM, Elsen NL, Wu J, Deschamps K, Petcu M, Wong S, Daigneault E, Kramer S, Liang L, Maxwell E, Claveau D, Vaillancourt J, Skorey K, Tam J, Wang H, Meredith TC, Sillaots S, Wang-Jarantow L, Ramtohul Y, Langlois E, Landry F, Reid JC, Parthasarathy G, Sharma S, Baryshnikova A, Lumb KJ, Pinho MG, Soisson SM, Roemer T. 2012. Restoring methicillin-resistant Staphylococcus aureus susceptibility to beta-lactam antibiotics. Sci Transl Med 4:126ra35. doi: 10.1126/scitranslmed.3003592. [DOI] [PubMed] [Google Scholar]
- 2.Kirby WM. 1944. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science 99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 3.Jevons MP. 1961. “Celbenin”-resistant staphylococci. BMJ i:124–125. [Google Scholar]
- 4.Peacock SJ, Paterson GK. 2015. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu Rev Biochem 84:577–601. doi: 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Alvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RL, Larsen AR, Skov RL, Peacock SJ, Maskell DJ, Holmes MA. 2011. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis 11:595–603. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson GK, Harrison EM, Holmes MA. 2014. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol 22:42–47. doi: 10.1016/j.tim.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballhausen B, Kriegeskorte A, Schleimer N, Peters G, Becker K. 2014. The mecA homolog mecC confers resistance against beta-lactams in Staphylococcus aureus irrespective of the genetic strain background. Antimicrob Agents Chemother 58:3791–3798. doi: 10.1128/AAC.02731-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C, Milheirico C, Gardete S, Holmes MA, Holden MT, de Lencastre H, Tomasz A. 2012. Properties of a novel PBP2A protein homolog from Staphylococcus aureus strain LGA251 and its contribution to the beta-lactam-resistant phenotype. J Biol Chem 287:36854–36863. doi: 10.1074/jbc.M112.395962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.British Society for Antimicrobial Chemotherapy. 2014. BSAC methods for antimicrobial susceptibility testing, version 13. British Society for Antimicrobial Chemotherapy, Birmingham, United Kingdom. [Google Scholar]
- 10.Andrews JM. 2001. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 11.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. doi: 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan RM, Foster TJ. 2009. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid 61:126–129. doi: 10.1016/j.plasmid.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Desbois AP, Coote PJ. 2011. Wax moth larva (Galleria mellonella): an in vivo model for assessing the efficacy of antistaphylococcal agents. J Antimicrob Chemother 66:1785–1790. doi: 10.1093/jac/dkr198. [DOI] [PubMed] [Google Scholar]
- 14.Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 15.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison EM, Paterson GK, Holden MT, Morgan FJ, Larsen AR, Petersen A, Leroy S, De Vliegher S, Perreten V, Fox LK, Lam TJ, Sampimon OC, Zadoks RN, Peacock SJ, Parkhill J, Holmes MA. 2013. A Staphylococcus xylosus isolate with a new mecC allotype. Antimicrob Agents Chemother 57:1524–1528. doi: 10.1128/AAC.01882-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 18.Ambler RP, Coulson AF, Frere JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem J 276(Part 1):269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voladri RK, Kernodle DS. 1998. Characterization of a chromosomal gene encoding type B beta-lactamase in phage group II isolates of Staphylococcus aureus. Antimicrob Agents Chemother 42:3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin C, Mallet MN, Sastre B, Viviand X, Martin A, De Micco P, Gouin F. 1995. Comparison of concentrations of two doses of clavulanic acid (200 and 400 milligrams) administered with amoxicillin (2,000 milligrams) in tissues of patients undergoing colorectal surgery. Antimicrob Agents Chemother 39:94–98. doi: 10.1128/AAC.39.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earnshaw JJ, Slack RC, Makin GS, Hopkinson BR. 1987. Tissue and serum concentrations of amoxycillin and clavulanic acid in patients having reconstructive vascular surgery. J Int Med Res 15:205–211. [DOI] [PubMed] [Google Scholar]
- 22.Kriegeskorte A, Ballhausen B, Idelevich EA, Kock R, Friedrich AW, Karch H, Peters G, Becker K. 2012. Human MRSA isolates with novel genetic homolog, Germany. Emerg Infect Dis 18:1016–1018. doi: 10.3201/eid1806.110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison EM, Paterson GK, Holden MT, Ba X, Rolo J, Morgan FJ, Pichon B, Kearns A, Zadoks RN, Peacock SJ, Parkhill J, Holmes MA. 2014. A novel hybrid SCCmec-mecC region in Staphylococcus sciuri. J Antimicrob Chemother 69:911–918. doi: 10.1093/jac/dkt452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Małyszko I, Schwarz S, Hauschild T. 2014. Detection of a new mecC allotype, mecC2, in methicillin-resistant Staphylococcus saprophyticus. J Antimicrob Chemother 69:2003–2005. doi: 10.1093/jac/dku043. [DOI] [PubMed] [Google Scholar]
- 25.Loncaric I, Kubber-Heiss A, Posautz A, Stalder GL, Hoffmann D, Rosengarten R, Walzer C. 2013. Characterization of methicillin-resistant Staphylococcus spp. carrying the mecC gene, isolated from wildlife. J Antimicrob Chemother 68:2222–2225. doi: 10.1093/jac/dkt186. [DOI] [PubMed] [Google Scholar]
- 26.Rudkin JK, Edwards AM, Bowden MG, Brown EL, Pozzi C, Waters EM, Chan WC, Williams P, O'Gara JP, Massey RC. 2012. Methicillin resistance reduces the virulence of healthcare-associated methicillin-resistant Staphylococcus aureus by interfering with the agr quorum sensing system. J Infect Dis 205:798–806. doi: 10.1093/infdis/jir845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins J, Rudkin J, Recker M, Pozzi C, O'Gara JP, Massey RC. 2010. Offsetting virulence and antibiotic resistance costs by MRSA. ISME J 4:577–584. doi: 10.1038/ismej.2009.151. [DOI] [PubMed] [Google Scholar]
- 28.Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. 2012. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 8:e1002626. doi: 10.1371/journal.ppat.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dordel J, Kim C, Chung M, Pardos de la Gandara M, Holden MT, Parkhill J, de Lencastre H, Bentley SD, Tomasz A. 2014. Novel determinants of antibiotic resistance: identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. mBio 5:e01000-13. doi: 10.1128/mBio.01000-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paterson GK, Morgan FJ, Harrison EM, Peacock SJ, Parkhill J, Zadoks RN, Holmes MA. 2014. Prevalence and properties of mecC methicillin-resistant Staphylococcus aureus (MRSA) in bovine bulk tank milk in Great Britain. J Antimicrob Chemother 69:598–602. doi: 10.1093/jac/dkt417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Briyne N, Atkinson J, Pokludova L, Borriello SP. 2014. Antibiotics used most commonly to treat animals in Europe. Vet Rec 175:325. doi: 10.1136/vr.102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim D, Strynadka NC. 2002. Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat Struct Biol 9:870–876. [DOI] [PubMed] [Google Scholar]
- 33.Otero LH, Rojas-Altuve A, Llarrull LI, Carrasco-Lopez C, Kumarasiri M, Lastochkin E, Fishovitz J, Dawley M, Hesek D, Lee M, Johnson JW, Fisher JF, Chang M, Mobashery S, Hermoso JA. 2013. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc Natl Acad Sci U S A 110:16808–16813. doi: 10.1073/pnas.1300118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matuschek E, Brown DF, Kahlmeter G. 2014. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect 20:O255–O66. doi: 10.1111/1469-0691. [DOI] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; approved standard. CLSI, Wayne, PA: . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.