Abstract
Carbapenem-resistant Acinetobacter baumannii (CRAb) shelter cohabiting carbapenem-susceptible bacteria from carbapenem killing via extracellular release of carbapenem-hydrolyzing class D β-lactamases, including OXA-58. However, the mechanism of the extracellular release of OXA-58 has not been elucidated. In silico analysis predicted OXA-58 to be translocated to the periplasm via the Sec system. Using cell fractionation and Western blotting, OXA-58 with the signal peptide and C terminus deleted was not detected in the periplasmic and extracellular fractions. Overexpression of enhanced green fluorescent protein fused to the OXA-58 signal peptide led to its periplasmic translocation but not extracellular release, suggesting that OXA-58 is selectively released. The majority of the extracellular OXA-58 was associated with outer membrane vesicles (OMVs). The OMV-associated OXA-58 was detected only in a strain overexpressing OXA-58. The presence of OXA-58 in OMVs was confirmed by a carbapenem inactivation bioassay, proteomic analysis, and transmission electron microscopy. Imipenem treatment increased OMV formation and caused cell lysis, resulting in an increase in the OMV-associated and OMV-independent release of extracellular OXA-58. OMV-independent OXA-58 hydrolyzed nitrocefin more rapidly than OMV-associated OXA-58 but was more susceptible to proteinase K degradation. Rose bengal, an SecA inhibitor, inhibited the periplasmic translocation and OMV-associated release of OXA-58 and abolished the sheltering effect of CRAb. This study demonstrated that the majority of the extracellular OXA-58 is selectively released via OMVs after Sec-dependent periplasmic translocation. Addition of imipenem increased both OMV-associated and OMV-independent OXA-58, which may have different biological roles. SecA inhibitor could abolish the carbapenem-sheltering effect of CRAb.
INTRODUCTION
Acinetobacter baumannii is a major cause of nosocomial infections worldwide. The rapid emergence of carbapenem-resistant isolates has severely reduced therapeutic options (1, 2). Recently, we demonstrated that carbapenem-resistant A. baumannii (CRAb) sheltered coexisting carbapenem-susceptible bacteria, preventing them from being killed by carbapenem and, thereby, leading to polymicrobial infections with enhanced pathogenicity compared to that of monomicrobial infection (3). This sheltering effect is clinically relevant because 20 to 50% of A. baumannii infections have been found to be polymicrobial (4–6).
The primary mechanism of carbapenem resistance in A. baumannii is high-level production of carbapenemases, especially carbapenem-hydrolyzing class D β-lactamases (CHDLs), which include the OXA-23, -40, -51, -58, and -143 classes (7). We demonstrated that the extracellular release of CHDLs contributed to the sheltering effect (3), but this was seen only when CHDLs were expressed at high levels using a strong promoter. At the time of the earlier study, the mechanism for the extracellular release of CHDLs had not been elucidated.
In this study, we determined that extracellular OXA-58 was associated with Sec-dependent periplasmic translocation and that the majority of the extracellular OXA-58 was selectively released via outer membrane vesicles (OMVs) in the absence of a carbapenem. Carbapenem treatment increased OMV formation and caused cell lysis, resulting in an increase in the OMV-associated and OMV-independent release of extracellular OXA-58. Addition of rose bengal, an SecA inhibitor, abolished the periplasmic translocation, reduced the extracellular release of OXA-58, and inhibited the carbapenem-sheltering effect of CRAb in the presence of carbapenem.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1, and primers used in this study are listed in Table S1 in the supplemental material. Bacterial strains were cultured in Luria Bertani broth (LB; Difco, Detroit, MI) at 37°C. Overnight cultures were subcultured in fresh LB broth for 2 h before antibiotics or chemicals were added. Imipenem, ticarcillin, kanamycin, rose bengal, erythrosin B, saponin, and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma-Aldrich (St. Louis, MO). 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) was purchased from USB Corporation (Cleveland, OH). Restriction enzymes and proteinase K were purchased from New England BioLabs (Beverly, MA). Electroporation of A. baumannii cells was performed as previously described (8).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid(s) | Description | Reference or source |
|---|---|---|
| Strains | ||
| Ec1003 | A clinical carbapenem-susceptible E. coli isolate (imipenem MIC, 0.125 μg/ml). This isolate was used to demonstrate the sheltering effect of carbapenem-resistant A. baumannii. | 3 |
| Ab290 | A clinical A. baumannii isolate susceptible to multiple antimicrobials, which was used as the recipient for multiple transformations. | 3 |
| Plasmids | ||
| pEGFP | A commercial plasmid containing enhanced green fluorescent protein. | Clontech Laboratories, Inc. |
| pYMAb-2 | A shuttle vector created by inserting a replicon of a plasmid from A. baumannii ATCC 19606T into pET-28a; Kanra | 3 |
| pYMAb-3 | A shuttle vector created by inserting a replicon of pMAC from A. baumannii ATCC 19606T into pET-28a; Kanr | 8 |
| pOXA-58-2 | IS1008-ΔISAba3-blaOXA-58 with its P2 and P1 promoters was amplified using primers IS1008(XbaI)F and OXA-58(XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. | 3 |
| pOXA-58-3 | IS1008-ΔISAba3-blaOXA-58 was amplified using primers XbaIIS1008 and NcoIOXA58 and cloned into the XbaI and NcoI sites of pYMAb-3. | 8 |
| pOXA-23 | blaOXA-23 and its promoter in ISAba1 were amplified using primers ISAba1(XbaI)F and OXA23-like(XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. OXA-23 was His tagged. | 3 |
| pOXA-72 | blaOXA-72 and its promoter were amplified using primers OXA-24-like(XbaI)F and OXA-24-like(XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. OXA-72 was His-tagged. | 3 |
| pOXA-83 | blaOXA-83 and its promoter in ISAba1 were amplified using primers ISAba1(XbaI)F and OXA-51-like(XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. OXA-83 was His tagged. | 3 |
| pOXA-58ΔSP | Promoters P2 and P1 of IS1008-ΔISAba3-blaOXA-58 were amplified using primers IS1008(XbaI)F and IS1008(M-BamHI)R. blaOXA-58 minus the signal peptide was amplified using primers OXA-58Δ20aaSP(BamHI)F and OXA-58(XhoI)R. These two fragments were BamHI ligated and cloned into the XbaI and XhoI sites of pYMAb-2.b | This study |
| pOXA-58ΔP2-1, pOXA-58ΔP2-2, and pOXA-58ΔP2-3 | IS1008-ΔISAba3-blaOXA-58 with deletions in P2 was amplified using primer IS1008-ΔP2-1(XbaI)F, IS1008-ΔP2-2(XbaI)F, or IS1008-ΔP2-3(XbaI)F and primer OXA-58(XhoI)R and cloned into the XbaI and XhoI sites of pYMAb-2. | 3 |
| pOXA-58Δ5AA-CT, pOXA-58Δ10AA-CT, and pOXA-58Δ15AA-CT | IS1008-ΔISAba3-blaOXA-58 with a deletion of 5, 10, or 15 amino acids at C terminus of OXA-58 was amplified using primers IS1008(XbaI)F and OXA-58-CΔ5aa(XhoI)R, OXA-58-CΔ10aa(XhoI)R, or OXA-58-CΔ15aa(XhoI)R and cloned into the XbaI and XhoI site of pYMAb-2. | This study |
| pEGFP-2 | The P2 and P1 promoter of IS1008-ΔISAba3-blaOXA-58 was amplified using primers IS1008(BamHI)F and IS1008(NcoI)R and cloned into the BamHI and NcoI sites of pEGFP. The promoter-EGFP fragment was cloned into the XbaI and BamHI sites of pYMAb-2. | This study |
| pOXA-58SP-EGFP | The P2 and P1 promoter and signal peptide of IS1008-ΔISAba3-blaOXA-58 was amplified using primers IS1008(BamHI)F and OXA-58-20aaSP(NcoI)R and cloned into the BamHI and NcoI sites of pEGFP. The promoter-signal peptide-EGFP fragment was cloned into the XbaI and BamHI sites of pYMAb-2. | This study |
Kanr, kanamycin resistance.
The signal peptide of OXA-58 was predicted using the program SignalP, version 4.0, server (http://www.cbs.dtu.dk/services/SignalP/).
Cell fractionation.
Overnight A. baumannii cultures were diluted 100-fold in 100 ml of LB broth and grown to logarithmic phase. Cell densities (CFU/milliliter) of the cultures were determined. The cultures were centrifuged at 10,000 × g for 15 min at 4°C. Supernatants (extracellular fractions) were collected, filtered through 0.22-μm-pore-size filters (Millipore, Bedford, MA), and concentrated using 10,000-molecular-weight-cutoff (MWCO) Amicon Ultra centrifugal filters (Millipore). Proteins in the supernatants were fractionated by size using 100,000 -MWCO Amicon Ultra centrifugal concentrators (Millipore). Proteins in the <100-kDa fraction were further concentrated using 10,000-MWCO Amicon Ultra centrifugal concentrators. The periplasmic and cytoplasmic cell fractions were separated as previously described (9). All of the fractions were stored at −20°C.
Western blot analysis.
Proteins in the different cell fractions from ∼2 × 108 CFU of bacteria were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were incubated with primary antibody, followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Millipore) or HRP-conjugated goat anti-mouse secondary antibody (Sigma-Aldrich). Primary polyclonal antibodies against A. baumannii OXA-58 and gyrase were generated in rabbits (3). Antibody for the detection of enhanced green fluorescent protein (EGFP) was purchased from Clontech (Palo Alto, CA), and His-tagged proteins were purchased from Sigma-Aldrich. An ECL Western blotting kit (PerkinElmer, Boston, MA) was used for detection. Densitometry was performed using ImageQuant TL software, version 7.0 (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Sample buffers were included as negative controls (see Fig. S1 in the supplemental material).
Preparation of OMVs.
Overnight cultures of A. baumannii were diluted 100-fold into 1 liter of LB broth. After growing to logarithmic phase, bacteria were centrifuged at 10,000 × g for 15 min at 4°C. Supernatants were filtered through 0.22-μm-pore-size filters and concentrated using 100,000-MWCO Amicon Ultra centrifugal concentrators (Millipore). Precipitates containing OMVs were collected by centrifugation at 100,000 × g for 1 h. The OMV suspensions were cultured on Mueller-Hinton agar to confirm that they were free of bacteria (10).
Bioassay to determine carbapenem inactivation.
Aliquots containing suspended OMV (OMV associated) and the extracellular fraction containing proteins of <100 kDa (OMV independent) were used. The two aliquots were adjusted to have similar concentrations of OXA-58 using densitometry analysis. After treatment with 100 μg/ml proteinase K, 2% saponin, or both for 1 h at 37°C and deactivation of proteinase K with 10 mM PMSF and AEBSF, 10-μl aliquots were treated with 10 μl of 8 μg/ml imipenem for 1 h at 37°C. Each 20-μl mixture was loaded onto a blank disk (Becton Dickinson and Company, Franklin Lakes, NJ) that was placed on an agar plate containing a lawn of Ec1003, a carbapenem-susceptible Escherichia coli. Plates were incubated overnight at 37°C, and zones of inhibition were measured. A decreased zone of inhibition indicated carbapenem inactivation.
Nitrocefin hydrolysis assay.
Samples containing equal amounts of OXA-58 were incubated with 50 μg/ml of nitrocefin (Oxoid, Basingstoke, United Kingdom) in a total volume of 100 μl. Samples were loaded into microtiter plates (Corning, NY), and absorbance at 486 nm was measured at 5-min intervals for 30 min in the dark using a U3300 Pro spectrophotometer (Amersham Biosciences, Freiburg, Germany).
Transmission electron microscopy (TEM).
A. baumannii cells and OMVs were collected during logarithmic-phase growth. Aliquots were immuno-hybridized with anti-OXA-58 primary antibody and 5-nm gold particle-conjugated secondary antibody (Abcam, Cambridge, United Kingdom) before embedding. Samples were embedded in melted agarose and fixed overnight at 4°C with 2.5% glutaraldehyde and 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4). After two washes with PBS, samples were postfixed in 1% osmium tetroxide and dehydrated using a graded acetone series (30%, 50%, 70%, 85%, 95%, and 100%). Samples were embedded in Spurr's resin (Electron Microscopy Science, Hatfield, PA) and incubated at 70°C until polymerized. Ultrathin sections were collected using grids, stained in an oversaturated uranyl acetate solution for 5 min, and examined using a JEM-2000EXII microscope (JEOL, Tokyo, Japan) at 100 kV (11).
Bacterial cocultures.
To determine the effect of an SecA inhibitor on the carbapenem-sheltering effect, overnight cultures of A. baumannii and E. coli were diluted 100-fold and cosubcultured in 5 ml of LB broth in culture tubes (Pyrex; Corning, Manassas, VA) for 2 h before the addition of imipenem and rose bengal. At given time points, cocultures were 10-fold serially diluted, and 100 μl of the dilutions was plated onto MacConkey agar (Difco) to differentiate between A. baumannii and E. coli and to determine counts of CFU/milliliter. Plates were incubated at 37°C overnight (3).
RESULTS
OXA-58 was primarily and selectively released via OMVs after Sec-dependent periplasmic translocation in the absence of carbapenem.
Even in the absence of a carbapenem, we detected OXA-58 in the extracellular fraction of an A. baumannii strain that produced high levels of OXA-58 (Fig. 1A). In this strain, blaOXA-58 is preceded by ISAba3, which is truncated by IS1008 (IS1008-ΔISAba3). The genetic structure of IS1008-ΔISAba3 provides a strong promoter, P2, and a weak promoter, P1, for the expression of blaOXA-58 (3, 8).
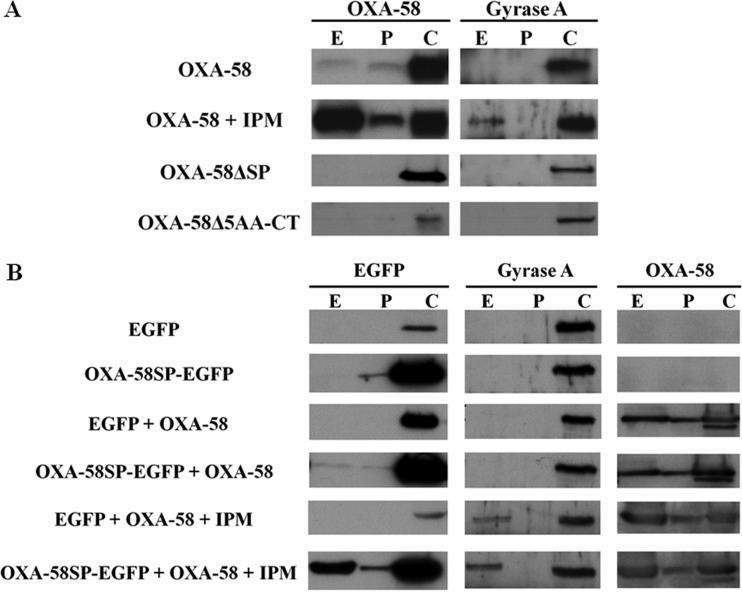
FIG 1.
Periplasmic localization is required for the selective extracellular release of OXA-58. (A) Western blot analysis of OXA-58 in fractions obtained from Acinetobacter baumannii (Ab290) strains expressing wild-type OXA-58, OXA-58 with a deletion of the N-terminal signal peptide (OXA-58ΔSP), or OXA-58 with a deletion of the C-terminal 5 amino acids (OXA-58Δ5AA-CT). (B) Western blot analysis of enhanced green fluorescent protein (EGFP) in fractions obtained from Ab290 expressing EGFP fused with the OXA-58 signal peptide (OXA-58SP-EGFP) or without the OXA-58 signal peptide (EGFP). These two strains were cotransformed with a compatible plasmid (pYMAb-3) carrying blaOXA-58 (pOXA-58-3) to produce both OXA-58 and EGFP or OXA-58SP-EGFP. E, P, and C indicate extracellular, periplasmic, and cytoplasmic fractions, respectively. IPM, imipenem (8 μg/ml). Blots were probed with primary antibodies to OXA-58, EGFP, or gyrase A.
We determined whether OXA-58 was released after translocation to the periplasm or directly from the cytoplasm. Two major export systems, Sec and Tat, are involved in the periplasmic translocation of proteins in Gram-negative bacteria (12, 13). In silico analysis of the OXA-58 signal sequence with the programs SignalP, version 4.0 (14), and TatP, version 1.0 (15), predicted that OXA-58 was preferentially translocated via the Sec system (see Fig. S2 in the supplemental material). After deletion of the putative signal sequence (the first 20 N-terminal amino acids), OXA-58 was not detected in the periplasmic or extracellular fraction (Fig. 1A). Because the C termini of β-lactamases are also required for periplasmic translocation (16), strains with C-terminal deletions were also examined to verify that extracellular release of OXA-58 was associated with periplasmic translocation. OXA-58 was not detected in the periplasmic or extracellular fractions of strains that had high OXA-58 expression and C-terminal deletions of the last 5 (Fig. 1A), 10, or 15 amino acids (see Fig. S3 in the supplemental material). These results indicated that OXA-58 was released after translocation to the periplasm.
We then determined whether periplasmic OXA-58 was selectively or passively released. We used a strain in which EGFP was fused to the signal sequence and the natural promoter of OXA-58 (OXA-58SP-EGFP). OXA-58SP-EGFP was detected in the cytoplasmic and periplasmic fractions but not in the extracellular fraction (Fig. 1B). However, OXA-58SP-EGFP was detected in the extracellular fraction when OXA-58 was coexpressed (Fig. 1B). These results indicated that periplasmic OXA-58 was selectively released.
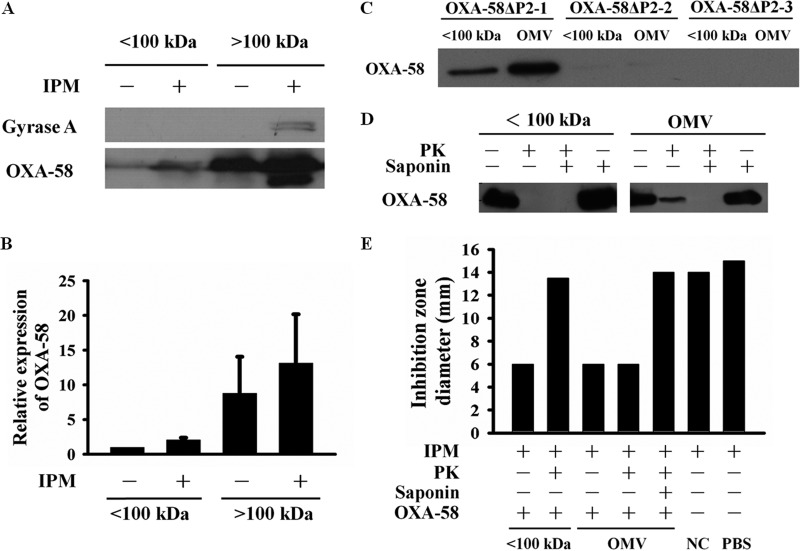
In Gram-negative bacteria, proteins can be secreted across the outer membrane via different transporters (17, 18), OMVs, or both (19). Although the predicted molecular mass of OXA-58 is ∼31 kDa, we found that the majority of the extracellular OXA-58 existed in a >100-kDa protein complex (Fig. 2A and B). OXA-58 (Fig. 2C) and other CHDLs (see Fig. S5C in the supplemental material) were detected in purified OMVs via Western blotting, but OMV-associated OXA-58 was not detected in the strain in which the P2 promoter of OXA-58 was deleted (Fig. 2C). Proteomic analysis (20, 21) of purified OMVs also revealed the presence of OXA-58 (see Table S2 in the supplemental material). OMV-associated OXA-58 was partially protected from proteinase K degradation, and this protection was abolished in the presence of saponin (Fig. 2D). In contrast, OXA-58 collected from the <100-kDa fraction (defined as OMV-independent OXA-58) was susceptible to proteinase K degradation. Carbapenem inactivation by OMV-associated and OMV-independent OXA-58 is demonstrated by the data shown in Fig. 2E. The results were in accordance with those shown in Fig. 2D, indicating that enzyme activity of OMV-independent OXA-58 was susceptible but that of OMV-associated OXA-58 was resistant to proteinase K degradation.
FIG 2.
Overexpressed OXA-58 was primarily released via OMVs. (A and B) Western blot analysis of Acinetobacter baumannii (Ab290) extracellular OXA-58 showed that most of the extracellular OXA-58 was present in a protein complex of >100 kDa, with or without treatment with 8 μg/ml imipenem (IPM). (B) Densitometry results from the experiment shown in panel A. (C) Western blot analysis of OXA-58 in the <100-kDa fraction and in purified OMVs using the following strains: OXA-58 with an intact P2 promoter (OXA-58ΔP2-1), OXA-58 with a deletion at −35 of the P2 promoter (OXA-58ΔP2-2), or OXA-58 with deletions at −35 and −10 of the P2 promoter (OXA-58ΔP2-3). (D) Western blot analysis of OXA-58 in the <100-kDa fraction or associated with OMVs after treatment with proteinase K (PK; 100 μg/ml), 2% saponin, or both. (E) Zones of inhibition resulting from incubation of a carbapenem-susceptible E. coli, Ec1003, with 8 μg/ml imipenem and exposure to OXA-58 in the <100-kDa fraction or associated OMVs, with or without pretreatment with PK, saponin, or both. NC, OMVs from the negative-control strain carrying the shuttle vector. Each bar represents the mean of zone sizes in three independent experiments.
Carbapenem addition increased extracellular levels of OXA-58 by increasing OMV formation and cell lysis.
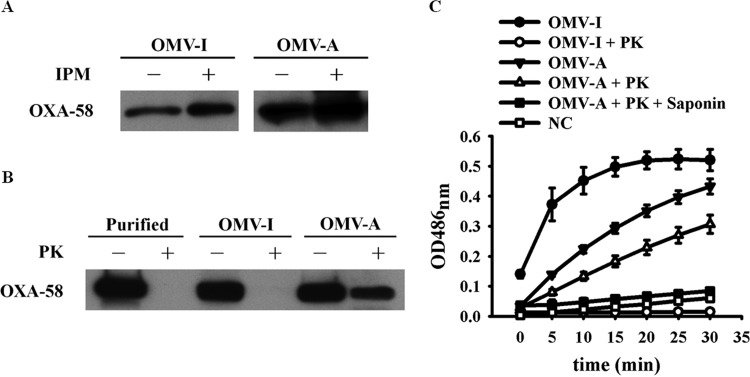
After imipenem treatment, levels of OXA-58 in the periplasmic and extracellular fractions increased (Fig. 1A), and the majority of the extracellular OXA-58 was associated with a >100-kDa protein complex (Fig. 2A and B). We demonstrated that OXA-58 increased in the purified OMVs in the presence of imipenem challenge (Fig. 3A). This OMV-associated OXA-58 was also partially protected from proteinase K degradation (Fig. 3B).
FIG 3.
Outer membrane vesicle-associated (OMV-A) OXA-58 and OMV-independent (OMV-I) OXA-58 increased after challenge with imipenem (IPM). (A) Western blot analysis of OMV-independent or OMV-associated OXA-58 after challenge with 8 μg/ml imipenem. (B) Susceptibility of OMV-independent or OMV-associated OXA-58 to 100 μg/ml proteinase K (PK) degradation. Purified OXA-58 was included as a control. (C) Nitrocefin hydrolysis by OMV-independent or OMV-associated OXA-58 with or without pretreatment with 100 μg/ml proteinase K, 2% saponin, or both. NC, OMVs from the negative-control strain carrying the shuttle vector.
After imipenem treatment, OXA-58 levels in the <100-kDa fractions also increased, although not to the extent that they did in the >100-kDa fractions (Fig. 2A and B). In contrast to OMV-associated OXA-58, this <100-kDa OMV-independent OXA-58 was susceptible to proteinase K degradation (Fig. 3B). In addition, extracellular gyrase was also detected (Fig. 2A). However, gyrase was not found in purified OMVs by proteomic analysis (see Table S2 in the supplemental material) and was susceptible to proteinase K degradation (see Fig. S4 in the supplemental material). The results indicated that OMV-independent OXA-58 and extracellular gyrase were likely released upon cell lysis. The OMV-independent OXA-58 hydrolyzed nitrocefin more rapidly than OMV-associated OXA-58 (Fig. 3C).
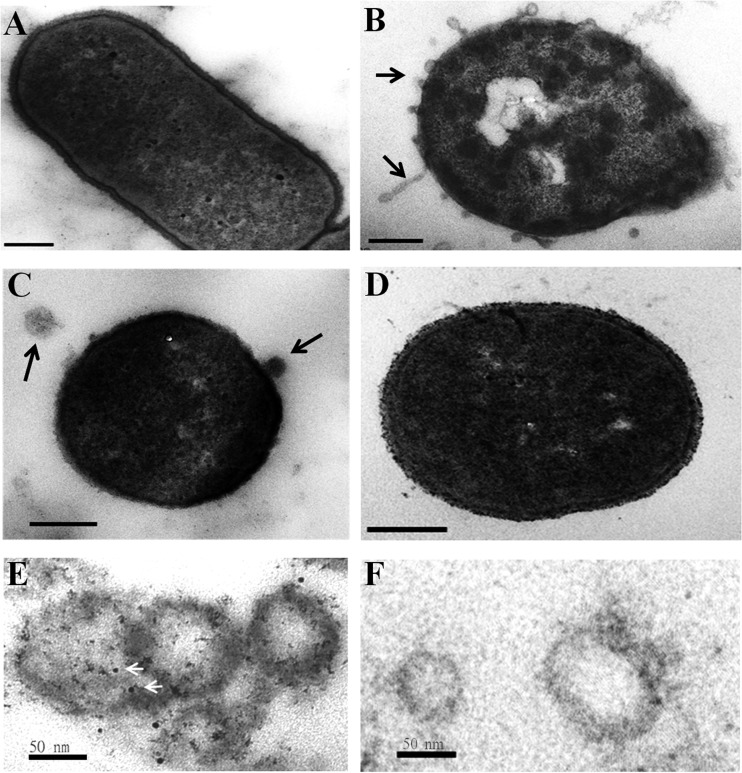
TEM showed that imipenem treatment increased the formation of OMVs on the surface of an A. baumannii strain that expressed OXA-58 at high levels (Fig. 4B). Increased OMV formation on the cell surface was also induced with ceftazidime (Fig. 4C) but not with kanamycin (Fig. 4D). OXA-58 was detected in purified OMVs of a strain highly expressing OXA-58 (Fig. 4E) but not in a strain carrying a shuttle vector (data not shown). OXA-58 was also not detected by using gold-conjugated secondary antibody alone (Fig. 4F).
FIG 4.
OMV formation increased after treatment with β-lactam antibiotics. TEM of an Acinetobacter baumannii (Ab290) strain expressing wild-type OXA-58 without antibiotic treatment (A), with 8 μg/ml imipenem (B), with 4 μg/ml ceftazidime (C), or with 200 μg/ml kanamycin (D). The black arrows indicate OMVs. (E) OMVs were hybridized with anti-OXA-58 and 5-nm gold particle-conjugated secondary antibody (white arrows). (F) OMVs were hybridized with 5-nm gold particle-conjugated secondary antibody as the negative control. Scale bars, 200 nm (A to D) and 50 nm (E and F).
An SecA inhibitor reduced extracellular CHDL levels and abolished the sheltering effect of CRAb.
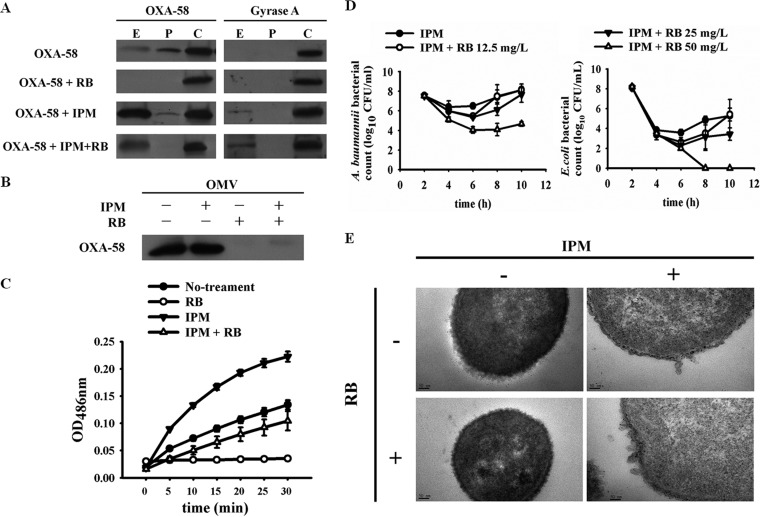
Since the deletion of the OXA-58 signal sequence abolished its periplasmic translocation and extracellular release, we hypothesized that inhibition of the translocation pathway would also reduce the extracellular release of OXA-58. In the Sec-dependent translocation system, polypeptides with signal sequences are recognized by SecA and translocated to the periplasm. Several SecA inhibitors, including rose bengal and erythrosin B (22), have been developed. Addition of rose bengal and erythrosin B at sublethal doses lowered the MIC of imipenem (see Fig. S5A and B in the supplemental material). Because rose bengal had a stronger effect, we used this inhibitor in further studies. Addition of rose bengal at a sublethal dose of 12.5 μg/ml inhibited the periplasmic translocation and extracellular release of OXA-58 in the absence of imipenem (Fig. 5A) and decreased the extracellular OXA-58 in the presence of imipenem (Fig. 5A). The extracellular OXA-58 collected after bacteria was treated with imipenem and rose bengal was susceptible to proteinase K degradation (see Fig. S6 in the supplemental material), indicating that this extracellular OXA-58 was OMV independent.
FIG 5.
Effect of the SecA inhibitor rose bengal on periplasmic translocation and OMV-associated release of OXA-58. Western blot of OXA-58 from an Acinetobacter baumannii (Ab290) strain expressing wild-type OXA-58 in different cell fractions (A) and in OMVs (B) after treatment with 8 μg/ml imipenem (IPM), 12.5 μg/ml rose bengal (RB), or both. (C) Nitrocefin hydrolysis by OMV-associated OXA-58 without treatment, with 8 μg/ml imipenem, with 12.5 μg/ml rose bengal, or with both. (D) Cocultures of Ab290 expressing wild-type OXA-58 and a carbapenem-susceptible E. coli strain, Ec1003, with 8 μg/ml imipenem and rose bengal at various concentrations. Rose bengal at 50 μg/ml eliminated the carbapenem-resistant A. baumannii sheltering of E. coli from imipenem killing (right panel). (E) TEM of Ab290 expressing wild-type OXA-58 after treatment with imipenem, rose bengal, or both. E, P, and C indicate extracellular, periplasmic and cytoplasmic fractions, respectively. Images were taken at a magnification of ×200,000. Scale bar, 50 nm. OD486nm, optical density at 486 nm.
The amounts of OMV-associated OXA-58 (Fig. 5B) and other CHDLs (see Fig. S5C in the supplemental material), as well as the nitrocefin hydrolyzing activity of OMV-associated OXA-58, decreased after rose bengal treatment (Fig. 5C). The inhibitory activity of rose bengal on A. baumannii SecA was verified by inhibition of periplasmic translocation of another Sec-dependent protein, alkaline phosphatase (see Fig. S5D in the supplemental material) (23). Addition of rose bengal at a sublethal dose of 50 μg/ml abolished the CRAb sheltering effect for imipenem-susceptible E. coli (Fig. 5D). Notably, rose bengal did not inhibit imipenem-induced formation of OMVs (Fig. 5E).
DISCUSSION
Previously, we demonstrated that CHDLs were released extracellularly and sheltered cohabiting carbapenem-susceptible bacteria from carbapenem killing. This sheltering effect has been associated with polymicrobial infections, which demonstrate higher pathogenicity than monomicrobial infections in the presence of imipenem treatment (3). In this study, we demonstrated that OXA-58 was primarily and selectively released via OMVs after translocation to the periplasm via the Sec system. OMV-associated OXA-58 was released even in the absence of stressors, but its release was increased upon carbapenem challenge. During carbapenem challenge, cell lysis also played a minor role in the extracellular release of OMV-independent OXA-58. Treatment with an SecA inhibitor diminished extracellular CHDL levels and abolished the carbapenem-sheltering effect.
Deletions of the OXA-58 signal sequence and C terminus suggested that OXA-58 was released via OMVs after translocation to the periplasm rather than being transported directly from the cytoplasm. In contrast, some cytoplasmic proteins identified in our OMVs and in the literature (11, 24) could be directly packaged from the cytoplasm via outer-inner membrane vesicles (25). Another possibility for the identification of proteins annotated to be located in cytoplasm was that these proteins actually had multiple locations and functions, such as translation elongation factor Tu (see Table S2 in the supplemental material) (26).
We demonstrated that, in the absence of carbapenem, OXA-58 was released via OMVs only when it was overexpressed. It has been demonstrated that overexpression of periplasmic proteins triggers OMV formation (27). However, when EGFP was fused to the OXA-58 signal sequence and overexpressed with the strong P2 promoter, EGFP was detected in the periplasmic fraction but not in the extracellular fraction. This result suggests that OXA-58 contains an additional domain(s) that selectively triggers OMV formation. Similarly, the N-terminal signal sequence of Salmonella enterica serovar Typhimurium PagK directs its periplasmic localization but is insufficient for its incorporation into OMVs (28).
In the absence of imipenem, there was a small amount of proteinase K-susceptible OXA-58 in the <100-kDa fraction. This OMV-independent OXA-58 probably did not result from cell lysis because cells were carefully collected during log-phase growth, and no extracellular gyrase was detected. This OMV-independent OXA-58 may be secreted via an unidentified pathway. The partial degradation of OMV-associated OXA-58 by proteinase K indicated that some of the OXA-58 is attached to the outer surface of the OMVs. Although Moraxella catarrhalis β-lactamase was found within OMVs, a Bacteroides cephalosporinase was found on the outer surface of OMVs (29). This proteinase K-susceptible OXA-58 may also arise from the breakdown of OMVs.
In the presence of harmful carbapenem, A. baumannii increased extracellular OXA-58 through increased release of OMV-associated OXA-58 and OMV-independent OXA-58. We did not determine whether the increase in OMV-associated OXA-58 was due to an increase in OMVs, an increase in the amount of OXA-58 in each OMV, or both. But TEM showed that imipenem and ceftazidime enhanced OMV formation. Because the outer membrane is bound to peptidoglycan via several lipoproteins (30), inhibition of peptidoglycan synthesis by these β-lactams may have caused increased vesiculation and OMV formation (25). In contrast, kanamycin, an antibiotic that does not inhibit peptidoglycan synthesis, did not induce OMV formation in A. baumannii or in E. coli (31). Enhanced expression of periplasmic OXA-58 (Fig. 1A) by imipenem may also trigger OMV formation, but inhibition of OXA-58 periplasmic translocation by rose bengal did not abolish the OMV formation induced by imipenem treatment, indicating that multiple mechanisms may be responsible for imipenem-induced OMV formation.
The biological roles of OMV-associated OXA-58 release and OMV-independent OXA-58 release may differ. The OMV-independent OXA-58 hydrolyzed β-lactams more rapidly than OMV-associated OXA-58 but was more susceptible to degradation by proteases, which may be present in the extracellular environment (10). In E. coli, it has been demonstrated that release of OMV-associated hemolysin and release of free hemolysin play different roles in endothelial cell pathogenesis. Free hemolysin caused cell lysis, whereas OMV-associated hemolysin caused apoptosis via cellular internalization of OMVs (32). Further, the activities of free and OMV-associated cytolysin A also differed (33).
OMV-associated β-lactamase release has been observed in Pseudomonas aeruginosa (34), M. catarrhalis (10), and Bacteroides species (29). Previously, an Acinetobacter-derived cephalosporinase was identified in A. baumannii OMVs, but its function was not characterized (11). In addition to OXA-58, we demonstrated that other CHDLs, including OXA-23, OXA-83 (in the OXA-51 family), and OXA-72, are also found in OMVs. In addition to β-lactamases, many other degradative enzymes are also released via OMVs (35–38), and macromolecules that are degraded by OMV-associated enzymes support the growth of other bacteria (39). These data demonstrate that OMV-associated enzyme release is beneficial to members of a bacterial community.
Two major export systems, Sec and Tat, translocate proteins into the periplasm of Gram-negative bacteria. Although most β-lactamases are translocated via the Sec system, some β-lactamases are transported via the Tat system (40). The SignalP program, version 4.0, predicted that the OXA-58 signal sequences belonged to the Sec-dependent system. SecA participates in co- and posttranslational translocation (41, 42) and is a drug target (22) because it is essential and conserved, has no human homolog, and has several ligand-binding pockets (43). The fluorescein analogues rose bengal and erythrosin B were both found to effectively inhibit the ATPase activity of SecA (44), but we found rose bengal to be more effective in our experiments. In the absence of imipenem, treatment with a sublethal dose of rose bengal inhibited the periplasmic translocation and OMV-associated secretion of OXA-58. In the presence of imipenem, addition of rose bengal was also able to diminish the amount of extracellular OXA-58 through inhibition of OMV-associated release of OXA-58 and abolished the carbapenem-sheltering effect.
In conclusion, OXA-58 was translocated to the periplasm via the Sec system, and the majority of the extracellular OXA-58 was selectively released via OMVs. Imipenem treatment increased extracellular OXA-58 levels by increasing OMV formation and cell lysis. Treatment with an SecA inhibitor abolished the OMV-associated release of OXA-58 and the carbapenem-sheltering effect conferred by CRAb.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Science Council (104-2314-B-010-027-MY3) and in part by the Electron Microscopy Facility in National Yang-Ming University.
T.-L.C. is a medical advisor of TTY Biopharm. There are no other conflicts of interest to report.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01343-15.
REFERENCES
- 1.Higgins PG, Dammhayn C, Hackel M, Seifert H. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 2.Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, Ko WC. 2007. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 28:713–719. doi: 10.1086/517954. [DOI] [PubMed] [Google Scholar]
- 3.Liao YT, Kuo SC, Lee YT, Chen CP, Lin SW, Shen LJ, Fung CP, Cho WL, Chen TL. 2014. Sheltering effect and indirect pathogenesis of carbapenem-resistant Acinetobacter baumannii in polymicrobial infection. Antimicrob Agents Chemother 58:3983–3990. doi: 10.1128/AAC.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YT, Kuo SC, Yang SP, Lin YT, Tseng FC, Chen TL, Fung CP. 2012. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis 55:209–215. doi: 10.1093/cid/cis385. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Huang YT, Tan CK, Kuo YW, Liao CH, Lee PI, Hsueh PR. 2011. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J Antimicrob Chemother 66:1839–1846. doi: 10.1093/jac/dkr200. [DOI] [PubMed] [Google Scholar]
- 6.Trottier V, Namias N, Pust DG, Nuwayhid Z, Manning R, Marttos AC Jr, Dunham MB, Schulman CI, McKenney MG. 2007. Outcomes of Acinetobacter baumannii infection in critically ill surgical patients. Surg Infect (Larchmt) 8:437–443. doi: 10.1089/sur.2006.029. [DOI] [PubMed] [Google Scholar]
- 7.Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. 2009. OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother 53:5035–5038. doi: 10.1128/AAC.00856-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL. 2008. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob Agents Chemother 52:2573–2580. doi: 10.1128/AAC.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valenzuela JK, Thomas L, Partridge SR, van der Reijden T, Dijkshoorn L, Iredell J. 2007. Horizontal gene transfer in a polyclonal outbreak of carbapenem-resistant Acinetobacter baumannii. J Clin Microbiol 45:453–460. doi: 10.1128/JCM.01971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaar V, Nordstrom T, Morgelin M, Riesbeck K. 2011. Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother 55:3845–3853. doi: 10.1128/AAC.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon SO, Gho YS, Lee JC, Kim SI. 2009. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol Lett 297:150–156. doi: 10.1111/j.1574-6968.2009.01669.x. [DOI] [PubMed] [Google Scholar]
- 12.Natale P, Bruser T, Driessen AJ. 2008. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane-distinct translocases and mechanisms. Biochim Biophys Acta 1778:1735–1756. doi: 10.1016/j.bbamem.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Pugsley AP. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev 57:50–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 15.Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S. 2005. Prediction of twin-arginine signal peptides. BMC Bioinformatics 6:167. doi: 10.1186/1471-2105-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshland D, Botstein D. 1980. Secretion of β-lactamase requires the carboxy end of the protein. Cell 20:749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- 17.Kostakioti M, Newman CL, Thanassi DG, Stathopoulos C. 2005. Mechanisms of protein export across the bacterial outer membrane. J Bacteriol 187:4306–4314. doi: 10.1128/JB.187.13.4306-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalbey RE, Kuhn A. 2012. Protein traffic in gram-negative bacteria-how exported and secreted proteins find their way. FEMS Microbiol Rev 36:1023–1045. doi: 10.1111/j.1574-6976.2012.00327.x. [DOI] [PubMed] [Google Scholar]
- 19.Bonnington KE, Kuehn MJ. 2014. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta 1843:1612–1619. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X, Zhu H. 2005. Tube-gel digestion: a novel proteomic approach for high-throughput analysis of membrane proteins. Mol Cell Proteomics 4:1948–1958. doi: 10.1074/mcp.M500138-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JH, Chang YW, Yao CW, Chiueh TS, Huang SC, Chien KY, Chen A, Chang FY, Wong CH, Chen YJ. 2004. Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc Natl Acad Sci U S A 101:17039–17044. doi: 10.1073/pnas.0407992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segers K, Anne J. 2011. Traffic jam at the bacterial sec translocase: targeting the SecA nanomotor by small-molecule inhibitors. Chem Biol 18:685–698. doi: 10.1016/j.chembiol.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Tse YM, Yu M, Tsang JS. 2009. Topological analysis of a haloacid permease of a Burkholderia sp. bacterium with a PhoA-LacZ reporter. BMC Microbiol 9:233. doi: 10.1186/1471-2180-9-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EY, Choi DS, Kim KP, Gho YS. 2008. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev 27:535–555. doi: 10.1002/mas.20175. [DOI] [PubMed] [Google Scholar]
- 25.Koning RI, de Breij A, Oostergetel GT, Nibbering PH, Koster AJ, Dijkshoorn L. 2013. Cryo-electron tomography analysis of membrane vesicles from Acinetobacter baumannii ATCC 19606T. Res Microbiol 164:397–405. doi: 10.1016/j.resmic.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Dallo SF, Zhang B, Denno J, Hong S, Tsai A, Haskins W, Ye JY, Weitao T. 2012. Association of Acinetobacter baumannii EF-Tu with cell surface, outer membrane vesicles, and fibronectin. ScientificWorldJournal 2012:128705. doi: 10.1100/2012/128705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBroom AJ, Kuehn MJ. 2007. Release of outer membrane vesicles by gram-negative bacteria is a novel envelope stress response. Mol Microbiol 63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon H, Ansong C, Adkins JN, Heffron F. 2011. Discovery of Salmonella virulence factors translocated via outer membrane vesicles to murine macrophages. Infect Immun 79:2182–2192. doi: 10.1128/IAI.01277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stentz R, Horn N, Cross K, Salt L, Brearley C, Livermore DM, Carding SR. 2015. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics. J Antimicrob Chemother 70:701–709. doi: 10.1093/jac/dku466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S, Cookson BT. 2009. Biogenesis of bacterial membrane vesicles. Mol Microbiol 72:1395–1407. doi: 10.1111/j.1365-2958.2009.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. 2006. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol 188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bielaszewska M, Ruter C, Kunsmann L, Greune L, Bauwens A, Zhang W, Kuczius T, Kim KS, Mellmann A, Schmidt MA, Karch H. 2013. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog 9:e1003797. doi: 10.1371/journal.ppat.1003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25–35. doi: 10.1016/S0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 34.Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Hoiby N. 2000. Chromosomal β-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J Antimicrob Chemother 45:9–13. doi: 10.1093/jac/45.1.9. [DOI] [PubMed] [Google Scholar]
- 35.Bauman SJ, Kuehn MJ. 2006. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect 8:2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson SS, Naidu YM, Pestka JJ. 1985. Ultrastructural localization of an extracellular protease in Pseudomonas fragi by using the peroxidase-antiperoxidase reaction. Appl Environ Microbiol 50:1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasilyeva NV, Tsfasman IM, Suzina NE, Stepnaya OA, Kulaev IS. 2008. Secretion of bacteriolytic endopeptidase L5 of Lysobacter sp. XL1 into the medium by means of outer membrane vesicles. FEBS J 275:3827–3835. doi: 10.1111/j.1742-4658.2008.06530.x. [DOI] [PubMed] [Google Scholar]
- 38.Elhenawy W, Debelyy MO, Feldman MF. 2014. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio 5:e00909-14. doi: 10.1128/mBio.00909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haurat MF, Elhenawy W, Feldman MF. 2015. Prokaryotic membrane vesicles: new insights on biogenesis and biological roles. Biol Chem 396:95–109. doi: 10.1515/hsz-2014-0183. [DOI] [PubMed] [Google Scholar]
- 40.Pradel N, Delmas J, Wu LF, Santini CL, Bonnet R. 2009. Sec- and Tat-dependent translocation of β-lactamases across the Escherichia coli inner membrane. Antimicrob Agents Chemother 53:242–248. doi: 10.1128/AAC.00642-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papanikou E, Karamanou S, Economou A. 2007. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol 5:839–851. doi: 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- 42.Kusters I, Driessen AJ. 2011. SecA, a remarkable nanomachine. Cell Mol Life Sci 68:2053–2066. doi: 10.1007/s00018-011-0681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajduk PJ, Huth JR, Tse C. 2005. Predicting protein druggability. Drug Discov Today 10:1675–1682. doi: 10.1016/S1359-6446(05)03624-X. [DOI] [PubMed] [Google Scholar]
- 44.Huang YJ, Wang H, Gao FB, Li M, Yang H, Wang B, Tai PC. 2012. Fluorescein analogues inhibit SecA ATPase: the first sub-micromolar inhibitor of bacterial protein translocation. ChemMedChem 7:571–577. doi: 10.1002/cmdc.201100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.