ABSTRACT
The severe diarrheal disease cholera is endemic in over 50 countries. Current therapies for cholera patients involve oral and/or intravenous rehydration, often combined with the use of antibiotics to shorten the duration and intensity of the disease. However, as antibiotic resistance increases, treatment options will become limited. Linoleic acid has been shown to be a potent negative effector of V. cholerae virulence that acts on the major virulence transcription regulator protein, ToxT, to inhibit virulence gene expression. ToxT activates transcription of the two major virulence factors required for disease, cholera toxin (CT) and toxin-coregulated pilus (TCP). A conjugated form of linoleic acid (CLA) is currently sold over the counter as a dietary supplement and is generally recognized as safe by the U.S. Food and Drug Administration. This study examined whether CLA could be used as a new therapy to reduce CT production, which, in turn, would decrease disease duration and intensity in cholera patients. CLA could be used in place of traditional antibiotics and would be very unlikely to generate resistance, as it affects only virulence factor production and not bacterial growth or survival.
INTRODUCTION
Cholera is a devastating diarrheal disease that affects between 1.4 and 4.3 million people each year and causes an estimated 28,000 to 142,000 deaths annually (1, 2). The disease is characterized by voluminous, watery diarrhea that induces severe dehydration and can lead to hypovolemic shock and eventual death if not treated rapidly. Cholera is caused by oral ingestion of the Gram-negative bacterium Vibrio cholerae, which is an aquatic organism found in coastal areas worldwide (3). Although over 200 V. cholerae serogroups have been identified, only the O1 and O139 serogroups have been implicated in epidemic and pandemic cholera.
Cholera patients are clinically treated to prevent dehydration by the administration of oral rehydration solution (ORS), containing various salts and glucose (4). In severe cholera cases, intravenous rehydration is also used. Without treatment, the cholera survival rate can be as low as 50%, but rehydration with ORS raises the survival rate to more than 99% (5). Antibiotics are a secondary treatment option; however their use is typically limited to severe cases and used to shorten the duration of severe disease symptoms.
To initiate disease, the production of two virulence factors is necessary: cholera toxin (CT) and toxin-coregulated pilus (TCP). CT is an A-B5 family toxin and is directly responsible for inducing the profuse watery cholera diarrhea (6), while TCP is necessary for host intestinal colonization (7). The expression of the genes encoding both of these virulence factors is under the control of the major virulence transcription activator, ToxT (8). ToxT is a member of the AraC/XylS family of transcription factors and consists of two domains, a C-terminal DNA binding domain that contains the AraC/XylS homology and an N-terminal domain that has been implicated in effector binding and, possibly, association of ToxT monomers (9). ToxT activity is modulated by both positive and negative effectors. The positive ToxT effector, bicarbonate, acts to enhance ToxT binding to its cognate DNA sites, known as toxboxes (10, 11). The negative ToxT effectors, unsaturated fatty acids present in bile, act to diminish ToxT binding to its cognate DNA sites (12, 13). Thus, these two effectors have opposing roles in transcription by simply altering ToxT binding affinity, likely by inducing structural alterations in the N-terminal domain (9, 11, 14). V. cholerae encounters high concentrations of bile in the small intestine before entering the mucosal layer, where it colonizes the epithelial surface (15–17). However, unsaturated fatty acids are a relatively small component of the complex mixture that constitutes bile. In the presence of unsaturated fatty acids in vitro, V. cholerae expresses its motility genes but does not express its major virulence genes encoding CT and TCP, as ToxT activity is strongly inhibited (18, 19).
Because of the strong inhibiting effect of linoleic acid that was observed in vitro (13), we investigated whether linoleic acid could potentially act as a cholera therapeutic, reducing the production of CT and subsequently reducing the volume of diarrhea induced by CT in cholera. A conjugated form of linoleic acid, CLA, is sold over the counter in the United States as a weight loss supplement aimed at inhibiting fat absorption. As CLA is relatively inexpensive and easily accessible, we explored whether CLA could potentially be used as a supplemental cholera therapy to reduce disease duration and intensity in conjunction with oral rehydration. As antibiotic resistance becomes a more and more pressing problem, therapies that can inhibit pathogenesis, but not bacterial survival, are becoming much more attractive. Here, we show that CLA inhibits virulence gene expression in vitro by acting on ToxT. We also show that CLA strongly inhibits CT production and fluid accumulation in a rabbit ileal loop model. These findings suggest that CLA could, in fact, be a novel therapy for cholera to be used in lieu of broad-spectrum antibiotics.
MATERIALS AND METHODS
V. cholerae strains and growth conditions.
All V. cholerae strains were maintained in Luria broth (LB) containing 20% glycerol and stored at −70°C. Cultures were grown overnight at 37°C in LB and then subcultured at a dilution of 1:40 into LB, pH 6.5, at 30°C for 3 h for virulence-inducing conditions in the presence or absence of 32 or 128 μM CLA dissolved in dimethyl sulfoxide (DMSO) or, in later experiments, 640 μM CLE transesterified with methanol (ME-CLA), or one of the three common isoforms of CLA, 9E,11E, 9Z,11E, or 10E,12Z, at a concentration of 32 μM (Sigma-Aldrich). V. cholerae strains were selected by growth in 100 μg/ml streptomycin.
β-Galactosidase and CT assays.
β-Galactosidase activity was measured using the basic procedure of Miller (20). Briefly, for β-galactosidase assays, bacteria were grown with or without CLA, ME-CLA, or one of the CLA isoforms for 3 h under virulence-inducing conditions and then analyzed. DMSO was used as a solvent control. CT was detected in the culture supernatant by a GM1 enzyme-linked immunosorbent assay (ELISA) (21), using polyclonal anti-CT primary antibody (Sigma) and goat anti-rabbit alkaline phosphatase conjugate secondary antibody (Southern Biotech). For CT assays, bacteria were grown for 18 h under virulence-inducing conditions in tubes, with or without the addition of the indicated concentration of CLA, followed by centrifugation to retrieve the supernatant. Fifty microliters of supernatant was used to measure CT levels by ELISA. A positive-control assay for quantification of the level of CT in the samples was performed using purified CT (List Biological Laboratories).
EMSAs and binding curve analysis.
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (22). Purified maltose binding protein (MBP)-ToxT was incubated with DNA probes made from the promoter sequence of interest that had previously been inserted into the plasmid pTL61T and labeled with γ-32P (Perkin-Elmer) by T4 polynucleotide kinase (New England BioLabs). The binding reaction mixtures contained various amounts of MBP-ToxT with a constant 10 μg/ml salmon sperm DNA, 10 mM Tris-acetate (pH 7.4), 1 mM potassium EDTA (pH 7.0), 100 mM KCl, 1 mM dithiothreitol (DTT), 0.3 mg/ml bovine serum albumin (BSA), and 10% glycerol in a volume of 30 μl. To each reaction mixture, a constant concentration of the labeled DNA probe was added. In reaction mixtures containing CLA, the final concentration was 32 μM for each reaction mixture. All other reaction mixtures contained 3.33% (1 μl in 30 μl) DMSO as a solvent control. Binding reaction mixtures were incubated for 30 min at 37°C and then loaded into a 6% polyacrylamide gel to be run at 4°C. Gels were dried for 1 h and then analyzed by autoradiography.
Binding curve analysis.
Autoradiographs were analyzed using ImageJ software (NIH) as previously described. Kd (dissociation constant) values were determined for each binding curve, and then the Kd for each condition was compared using the extra sum of squares F test to determine whether the two values were statistically different.
Rabbit ileal loop assays.
Assays of fluid accumulation in rabbit ileal loops were performed as previously described (23). Briefly, 10-cm loops of small intestine were injected with approximately 106 V. cholerae strain C6706 (El Tor) in a 1-ml total volume and, separately, with various concentrations of CLA in 10% Kollidon. A negative-control loop was injected with 1 ml phosphate-buffered saline (PBS). After 16 h, the rabbits were sacrificed and their intestines dissected. The fluid volume from each loop was measured, and CT ELISA was performed to assess the mount of CT produced under each condition. Approval for animal experiments was granted by the Institutional Ethical Animal Committee (IEAC) at NICED, license no. PRO/108/June 2014-July 2016.
RESULTS
CLA inhibits TCP and CT production.
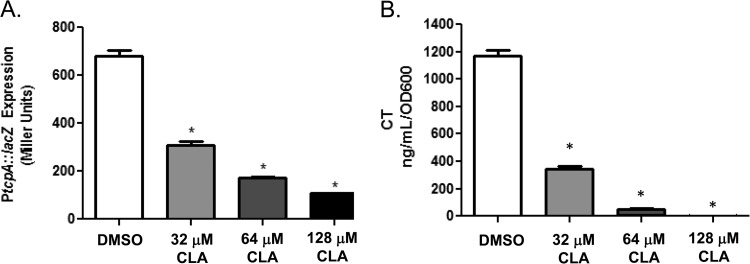
Previous work showed that purified linoleic acid decreases ToxT activity, leading to a decrease in virulence gene expression (13). In the present study, our first aim was to assess whether CLA would also strongly inhibit ToxT activity, as measured by tcp expression using a PtcpA-lacZ fusion, and CT production, as measured by ELISA. To begin, we determined that the highest CLA concentration that did not affect bacterial growth was 128 μM (data not shown). When classical V. cholerae strain O395 was grown under virulence-inducing conditions in the presence of three different CLA concentrations, we observed a significant, dose-dependent reduction in PtcpA-lacZ expression compared to its expression in cultures grown under the same conditions but in the absence of CLA (Fig. 1A). Similarly, CT production was significantly reduced in the presence of the three CLA concentrations tested (Fig. 1B). These results indicate that CLA works as expected to attenuate V. cholerae virulence gene expression in vitro.
FIG 1.
Cultures grown under virulence-inducing conditions in the presence of CLA exhibit reduced virulence gene expression. (A) tcpA::lacZ expression was significantly lowered upon the addition of 32, 64, and 128 μM CLA. (B) CT expression was significantly lowered upon the addition of 32, 64, and 128 μM CLA. Statistical significance was determined using Student's t test. *, P < 0.001.
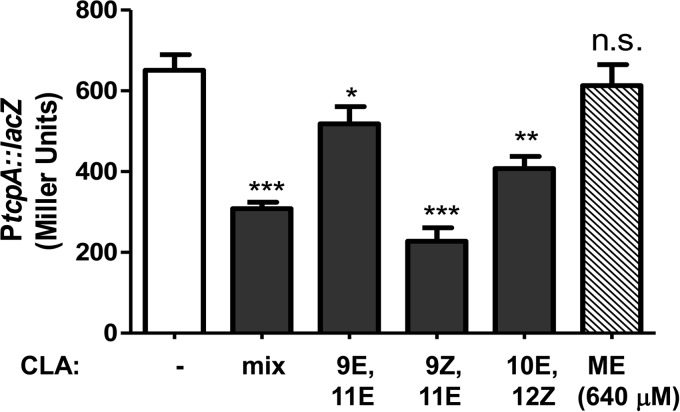
The commercially acquired CLA used in the experiment described above is a mixture of cis- and trans-9,11- and trans-10,12-linoleic acid. To determine whether the CLA inhibitory effect was isoform specific, we compared the results for individual isoforms to the results for the lowest concentration of the multi-isoform CLA that significantly inhibited PtcpA-lacZ expression, 32 μM. First, we examined ME-CLA, which is made by the transesterification of CLA with methanol. This compound had no effect on promoter expression, even at a concentration 20 times higher than the effective concentration of other CLA isoforms, indicating that it is the fatty acid and not only its aliphatic chain that inhibits ToxT activity (Fig. 2). Next, we examined the individual effects of three common CLA isoforms (9E,11E-, 9Z,11E-, and 10E,12Z-CLA) on tcp promoter activity. Although all three isoforms caused a significant decrease in virulence gene expression, variations were observed (Fig. 2). Of the three isoforms, 9Z,11E-CLA was the most effective at reducing PtcpA-lacZ expression and was slightly more effective than the commercial CLA mixture, but this difference was not statistically significant. 9E,11E-CLA affected promoter expression the least. Therefore, the different isoforms of CLA do have differential effects on V. cholerae virulence gene expression but the CLA mixture was as effective as any individual isoform.
FIG 2.
Effects of different CLA isoforms on ToxT activity. V. cholerae was grown under virulence-inducing conditions with the indicated isoforms of CLA present at 32 μM, except for ME-CLA, which was present at 640 μM. β-Galactosidase assays were performed to examine PtcpA activity. Statistical significance of results compared to the results for the DMSO-only control (white bar) was determined by Student's t test. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; n.s., not significant.
CLA reduces the DNA binding affinity of ToxT.
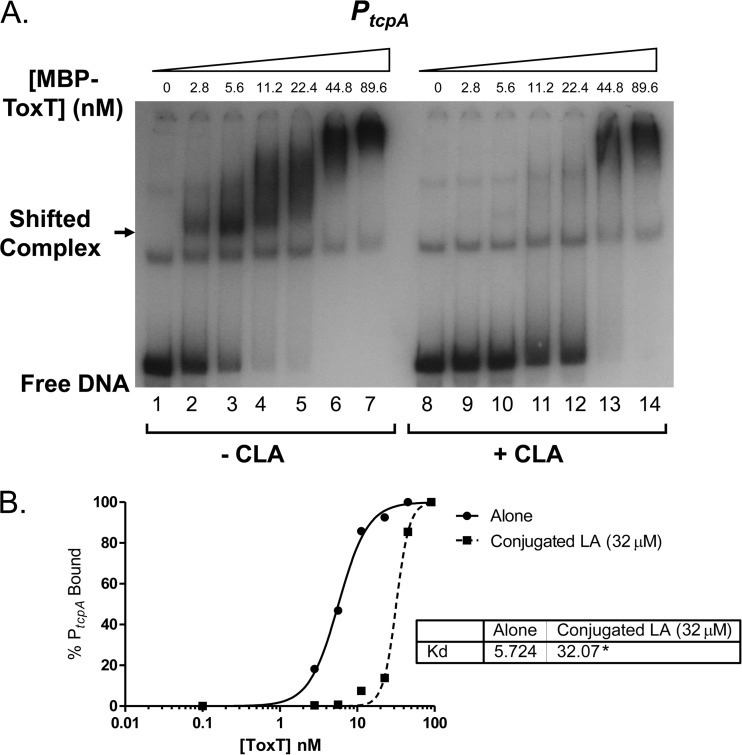
Previous work suggested that linoleic acid directly inhibits ToxT binding to DNA (13). Equilibrium electrophoretic mobility shift assays (EMSAs) were used to assess whether CLA also inhibits ToxT binding to DNA. Increasing amounts of purified MBP-ToxT were added to individual binding reaction mixtures containing 32P-labeled PtcpA DNA probe. Either DMSO (Fig. 3A, left) or 32 μM CLA dissolved in DMSO (Fig. 3A, right) was added, and binding reactions were given 30 min to reach equilibrium. Densitometry analysis of the resulting autoradiographs was used to determine the percentage of DNA probe bound at each MBP-ToxT concentration, leading to Kd calculation (Fig. 3B). The Kd in the binding reaction mixture with no CLA was 5.724 nM, and the Kd in the mixture with CLA was 32.07 nM. These values are significantly different and illustrate that CLA reduces the DNA binding affinity of ToxT, which correlates with the observed effects on virulence gene expression described above.
FIG 3.
CLA inhibits ToxT binding to PtcpA DNA. (A) Autoradiograph of the results of MBP-ToxT binding reactions with PtcpA DNA. Lanes 1 to 7 include DMSO, and lanes 8 to 14 include 32 μM CLA in DMSO. The autoradiograph of EMSAs presented is representative of three or more independent experiments. (B) Binding curve using densitometry of the experiment whose results are shown in panel A. GraphPad software analysis of the Kd indicates that CLA induces a significant reduction in the binding affinity of ToxT for its cognate DNA. Kd values are shown in the inset, with significant difference between the best-fit values indicated by an asterisk: *, P < 0.0001.
Assessment of the in vivo effects of CLA on CT activity.
The results described so far indicate that CLA has a strong negative effect on ToxT activity in vitro, as assessed by both gene expression (CT production and PtcpA activity) and DNA binding (EMSA). The next step was to determine whether the administration of CLA in an animal model for cholera would reduce virulence factor production, leading to a decrease in the fluid accumulation induced by CT. The two most common animal models for cholera are the 3- to 5-day-old infant mouse model and the adult rabbit ileal loop model. The former is an excellent model for assessing colonization but does not produce measurable diarrheal disease in the mice. The latter is an excellent model for assessing CT production, which is exhibited by fluid accumulation in the ileal loops. Given that CT is directly responsible for producing diarrhea in human cholera patients, the latter model was used to determine whether CLA could reduce V. cholerae CT production and subsequent fluid accumulation.
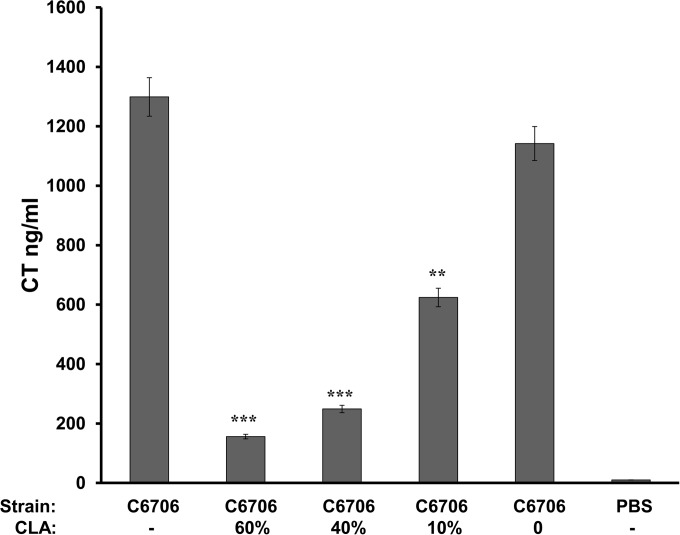
First, the effects of CLA on CT production were assessed using the rabbit ileal loop model. Ileal loops were injected with approximately 106 CFU of V. cholerae El Tor strain C6706. CLA was diluted in either 10% Kollidon emulsion or corn oil (data not shown), both of which are nontoxic carriers for the very hydrophobic CLA. One hundred-, 400-, and 600-μl volumes of CLA in a total volume of 1 ml were independently tested. The administration of CLA, by separate injection into the ileal loop at the time of bacterial inoculation, resulted in CT levels that were significantly reduced at 16 h postadministration for each of the CLA concentrations tested. At the two highest CLA concentrations, CT was reduced by more than 7-fold compared to the levels in loops that received only the 10% Kollidon emulsion (Fig. 4). At the lowest CLA concentration, CT was still reduced by more than 50% compared to the levels in the control loops (Fig. 4). These data demonstrate that the presence of CLA in the rabbit small intestine successfully inhibited CT production.
FIG 4.
CT ELISA of rabbit ileal loop fluid produced in the presence and absence of CLA. Ten-centimeter ileal loops were injected with approximately 106 CFU V. cholerae C6706, followed by a separate injection containing either the indicated percentage of CLA in 10% Kollidon or control carrier 10% Kollidon (denoted by a zero in the CLA row) in a 1-ml volume. Analyzed data are presented as the mean results ± standard deviations. Statistical significance was determined by using the χ2 and Student t tests. P values of less than 0.05 were considered statistically significant. **, P < 0.005; ***, P < 0.0005.
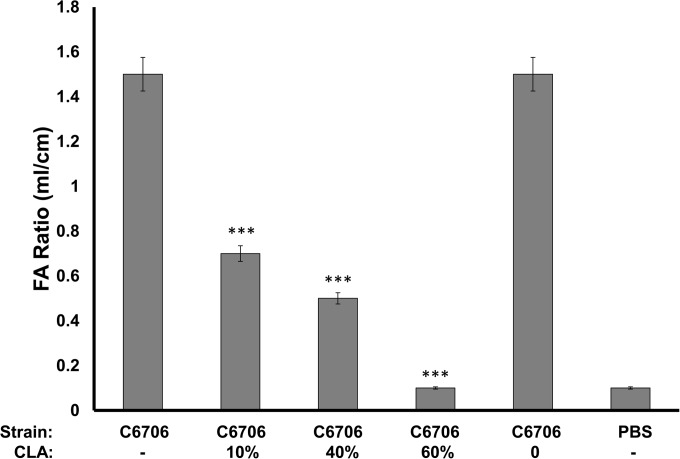
Next, the effects of CLA on fluid accumulation were assessed. Because CT alone induces most of the secretory diarrhea in cholera patients and since we observed reduced CT levels in the ileal loops, this experiment would be the litmus test for whether CLA could reduce fluid loss. Rabbit ileal loops were inoculated with approximately 106 V. cholerae C6706, and the volumes of fluid that accumulated were measured after 16 h. The fluid volume was markedly reduced in loops that received any of the three CLA concentrations tested (Fig. 5). At the lowest CLA concentration, fluid production was less than 50% of that observed in control loops. At the highest CLA concentration, fluid production was less than 7% of that in the controls without CLA. These results strongly suggest that the administration of CLA in vivo could significantly reduce CT production and secretory diarrhea, which should reduce the duration and intensity of disease in human cholera patients.
FIG 5.
Ileal loop fluid volumes produced by V. cholerae C6706 in the presence or absence of CLA. Ten-centimeter ileal loops were injected with approximately 106 CFU V. cholerae C6706, followed by a separate injection containing either the indicated percentage of CLA in 10% Kollidon or control carrier 10% Kollidon (noted by a zero in the CLA row) in a 1-ml volume. Values are presented as the ratio of fluid volume in milliliters to the length of the loop in centimeters. ***, P < 0.0005. FA, fluid accumulation.
DISCUSSION
Based on our previous observations that linoleic acid has a strong inhibitory effect on ToxT activity (13), we explored whether the related compound, CLA, could be used as a cholera therapeutic to supplement rehydration therapy. Here, we describe a strong inhibitory effect of CLA on virulence gene expression, as shown by both PtcpA-lacZ activity and CT expression, as well as a significant decrease in ToxT binding to the PtcpA toxboxes. This suggests that CLA inhibits ToxT binding to DNA, as previously demonstrated with linoleic acid (13). Different isoforms of CLA exhibited some minor differences in potency but no major differences, suggesting that currently available over-the-counter CLA mixtures could be used for therapy. However, methylated CLA was ineffective.
The real test of whether CLA could be an effective cholera therapy required the use of animal models. The rabbit ileal loop model, where fluid accumulation is measured in the ileal loops, is the best model for assessing CT production and secretory diarrhea and, therefore, was chosen for these studies. The results of the rabbit ileal loop experiments indicated that CLA was also effective in reducing both CT production and fluid accumulation in vivo. This suggests that CLA was accessible to colonizing V. cholerae along the small intestinal mucosa.
We have previously proposed models for the control of V. cholerae virulence gene expression that are mediated by two opposing ToxT effectors in vivo (10, 24). Bicarbonate enhances ToxT activity, whereas the unsaturated fatty acid components of bile, such as linoleic acid, inhibit ToxT activity. The concentrations of these two effectors differ between the luminal fluid and within the mucus layer that protects the epithelium. Bile and its components are at high concentrations in the lumen but, due to their relatively large size, are at very low concentrations within the mucus layer. Bicarbonate is present both in the lumen, where it buffers stomach acid, and within the mucus layer, via secretion by the epithelial cells. V. cholerae ToxT is inactivated by bile in the lumen, which permits the bacteria to retain motility and enter the mucus layer so that they can colonize the epithelial surface. The production of TCP and CT prior to entry into the mucus layer would be deleterious, as the bacteria would aggregate in the lumen and be unable to colonize. Thus, these two signals that act inversely on the binding affinity of ToxT for its DNA binding sites are able to direct V. cholerae to the optimal site for colonization.
The hypothesis behind using CLA as a therapeutic was that the very low levels of unsaturated fatty acids present in bile in the intestinal lumen could not penetrate the mucus layer at a sufficient concentration to affect ToxT activity. However, if excess CLA were introduced, perhaps a concentration sufficient to inhibit ToxT activity could be achieved. ToxT is sensitive to concentrations of CLA as low as 3.2 μM (unpublished data). Another factor that adds to the possibility of CLA being effective in vivo is the observation that colonizing V. cholerae bacteria disrupt the mucus layer before exiting the host as part of the mucosal escape response (25, 26). Thus, colonizing bacteria could potentially be even more exposed to higher levels of CLA as the disease progresses. Although V. cholerae that are preparing to exit the host downregulate virulence gene expression, including CT production, CLA could act on the many V. cholerae bacteria still attached to the epithelium and actively producing CT. Our results from this study indicate that CLA is effective in vivo in reducing CT production and fluid accumulation, consistent with this hypothesis.
The phenomenon of antibiotic resistance is a growing problem in the treatment of infectious disease. Currently, effective antibiotic treatments are still available for cholera patients. However, V. cholerae isolates resistant to multiple antibiotics are becoming more prevalent (27). An advantage of using CLA to inhibit CT production is that this treatment does not affect bacterial growth, and thus, selection for resistant bacteria should be greatly reduced compared to selection under antibiotic treatment. CLA treatment in human cholera patients would ostensibly have the same effect as antibiotic treatment in shortening the duration and severity of cholera symptoms. CLA should also have the advantage of not grossly disrupting the intestinal microbiota. Disruption of the microbiota by antibiotic treatment can lead to sensitivity to other pathogens, such as Clostridium difficile. CLA is an essential fatty acid, considered to be safe for use as a dietary supplement by the U.S. FDA, and thus, should be completely safe for human consumption without the necessity for clinical safety trials. Because cholera is a problem in developing countries, cost of treatment must also be a consideration. In the United States, retail costs for softgels containing 1,000 mg CLA are as low as 8 cents per capsule. The costs would likely be considerably lower if CLA is produced in low-income countries.
In summary, we report here that the essential fatty acid CLA is effective both in vitro and in vivo in reducing CT production and subsequent secretory diarrhea. Future research will determine whether CLA administration will be a feasible and effective addition to current cholera rehydration therapy.
ACKNOWLEDGMENTS
This work was funded by a grant from the Bill and Melinda Gates Foundation (OPP1068124, to J.H.W.) and by funds from the Indian Council for Medical Research (to H.K.).
Footnotes
[This article was published on 20 November 2015 with the second author's name misspelled. This has been corrected in the current version, posted on 7 December 2018.]
REFERENCES
- 1.World Health Organization. 2013. Cholera, 2012. Wkly Epidemiol Rec 88:321–334. [PubMed] [Google Scholar]
- 2.Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, Clemens J. 2012. The global burden of cholera. Bull World Health Organ 90:209–218A. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipp EK, Huq A, Colwell RR. 2002. Effects of global climate on infectious disease: the cholera model. Clin Microbiol Rev 15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2006. Oral rehydration salts: production of the new ORS. WHO, Geneva, Switzerland. [Google Scholar]
- 5.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 6.Pierce NF, Greenough WB III, Carpenter CC Jr. 1971. Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev 35:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thelin KH, Taylor RK. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun 64:2853–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci U S A 88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowden MJ, Skorupski K, Pellegrini M, Chiorazzo MG, Taylor RK, Kull FJ. 2010. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci U S A 107:2860–2865. doi: 10.1073/pnas.0915021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abuaita BH, Withey JH. 2009. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun 77:4111–4120. doi: 10.1128/IAI.00409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson JJ, Plecha SC, Withey JH. 2015. A small unstructured region in Vibrio cholerae ToxT mediates the response to positive and negative effectors and ToxT proteolysis. J Bacteriol 197:654–668. doi: 10.1128/JB.02068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee A, Dutta PK, Chowdhury R. 2007. Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect Immun 75:1946–1953. doi: 10.1128/IAI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plecha SC, Withey JH. 2015. The mechanism for inhibition of Vibrio cholerae ToxT activity by the unsaturated fatty acid components of bile. J Bacteriol 197:1716–1725. doi: 10.1128/JB.02409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prouty MG, Osorio CR, Klose KE. 2005. Characterization of functional domains of the Vibrio cholerae virulence regulator ToxT. Mol Microbiol 58:1143–1156. doi: 10.1111/j.1365-2958.2005.04897.x. [DOI] [PubMed] [Google Scholar]
- 15.Guentzel MN, Field LH, Eubanks ER, Berry LJ. 1977. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect Immun 15:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrank GD, Verwey WF. 1976. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun 13:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren J, Svennerholm AM. 1977. Mechanisms of disease and immunity in cholera: a review. J Infect Dis 136(Suppl):S105–S112. doi: 10.1093/infdis/136.Supplement.S105. [DOI] [PubMed] [Google Scholar]
- 18.Butler SM, Camilli A. 2005. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat Rev Microbiol 3:611–620. doi: 10.1038/nrmicro1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krukonis ES, DiRita VJ. 2003. From motility to virulence: sensing and responding to environmental signals in Vibrio cholerae. Curr Opin Microbiol 6:186–190. doi: 10.1016/S1369-5274(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 20.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 21.Sack DA, Huda S, Neogi PK, Daniel RR, Spira WM. 1980. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol 11:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dittmer JB, Withey JH. 2012. Identification and characterization of the functional toxboxes in the Vibrio cholerae cholera toxin promoter. J Bacteriol 194:5255–5263. doi: 10.1128/JB.00952-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajpara N, Vinothkumar K, Mohanty P, Singh AK, Singh R, Sinha R, Nag D, Koley H, Kushwaha Bhardwaj A. 2013. Synergistic effect of various virulence factors leading to high toxicity of environmental V. cholerae non-O1/non-O139 isolates lacking ctx gene: comparative study with clinical strains. PLoS One 8:e76200. doi: 10.1371/journal.pone.0076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson JJ, Withey JH. 2014. Bicarbonate increases binding affinity of Vibrio cholerae ToxT to virulence gene promoters. J Bacteriol 196:3872–3880. doi: 10.1128/JB.01824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen AT, Dolganov NA, Otto G, Miller MC, Wu CY, Schoolnik GK. 2006. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog 2:e109. doi: 10.1371/journal.ppat.0020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schild S, Tamayo R, Nelson EJ, Qadri F, Calderwood SB, Camilli A. 2007. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe 2:264–277. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitaoka M, Miyata ST, Unterweger D, Pukatzki S. 2011. Antibiotic resistance mechanisms of Vibrio cholerae. J Med Microbiol 60:397–407. doi: 10.1099/jmm.0.023051-0. [DOI] [PubMed] [Google Scholar]