Abstract
We used bone marrow/liver/thymus (BLT) humanized mice to establish the effect of semen on vaginal HIV infection and on the efficacy of topically applied maraviroc. Our results demonstrate that vaginal transmission of cell-free HIV occurs efficiently in the presence of semen and that topically applied maraviroc efficiently prevents HIV transmission in the presence of semen. We also show that semen has no significant effect on the transmission of transmitted/founder viruses or cell-associated viruses.
TEXT
Most new HIV infections are transmitted heterosexually (1). Women represent about half of all people living with HIV worldwide and more than half (58%) of HIV-positive individuals in sub-Saharan Africa (2). HIV is one of the leading causes of death among women of reproductive age. Gender inequalities, differential access to services, and sexual violence increase women's vulnerability to HIV, and women, especially younger women, are biologically more susceptible to HIV (2). Therefore, there is an urgent need for effective interventions to prevent HIV transmission. Semen is second only to blood in the concentration of virus present per unit volume (3). Sexual intercourse (vaginal and anal) is a high-risk practice for HIV transmission. Thus, there is an urgent need to better understand the potential role semen plays in HIV transmission. This study sought to clarify this role through the use of in vitro as well as extensive in vivo experimentation in the bone marrow/liver/thymus (BLT) humanized mouse model.
Semen does not affect HIV-1 infectivity in vitro.
The majority of sexual exposures to HIV occur in the presence of semen. In order to begin to assess the potential impact of semen on HIV-1 mucosal transmission, we used a well-established quantitative in vitro assay based on the indicator cell line TZM-bl. Cells were exposed to either virus alone or virus in the presence of semen. The semen was obtained from Lee Biosolutions and is a pool of 17 repeat and characterized donors (kindly provided by the Comprehensive Resources for HIV Microbicides and Biomedical Prevention). Infection was then determined as the amount of luciferase activity produced by the infected cells 48 h after exposure. Our results indicate that under our in vitro experimental conditions, semen has no discernible effect on HIV infection (see Fig. S1 in the supplemental material).
BLT humanized mice for the in vivo evaluation of the effect of semen on vaginal HIV-1 transmission.
Currently, humanized mice represent the only in vivo model in which vaginal HIV-1 transmission can be studied. BLT humanized mice have been shown to recapitulate key aspects of vaginal HIV-1 infection and as such represent an excellent model to evaluate the effect of human semen on HIV-1 transmission (4–10). In order to establish the effect of semen on HIV transmission, BLT humanized mice were constructed, and the levels of human cells in their peripheral blood were determined prior to exposure. The average level of human (CD45+) cells in the peripheral blood of mice used for the experiments described in this article was 72% ± 12.5% (mean ± standard deviation [SD]). The majority of the human cells in these mice were CD3+ (60.8% ± 16.3%) and expressed CD4 on their cell surface (80.6% ± 7.6%). Mice were maintained under specific-pathogen-free conditions by the Division of Laboratory Animal Medicine, and all experiments were conducted after review and approval by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Effect of semen on vaginal cell-free HIV-1 transmission and on the protective effect of topically administered maraviroc.
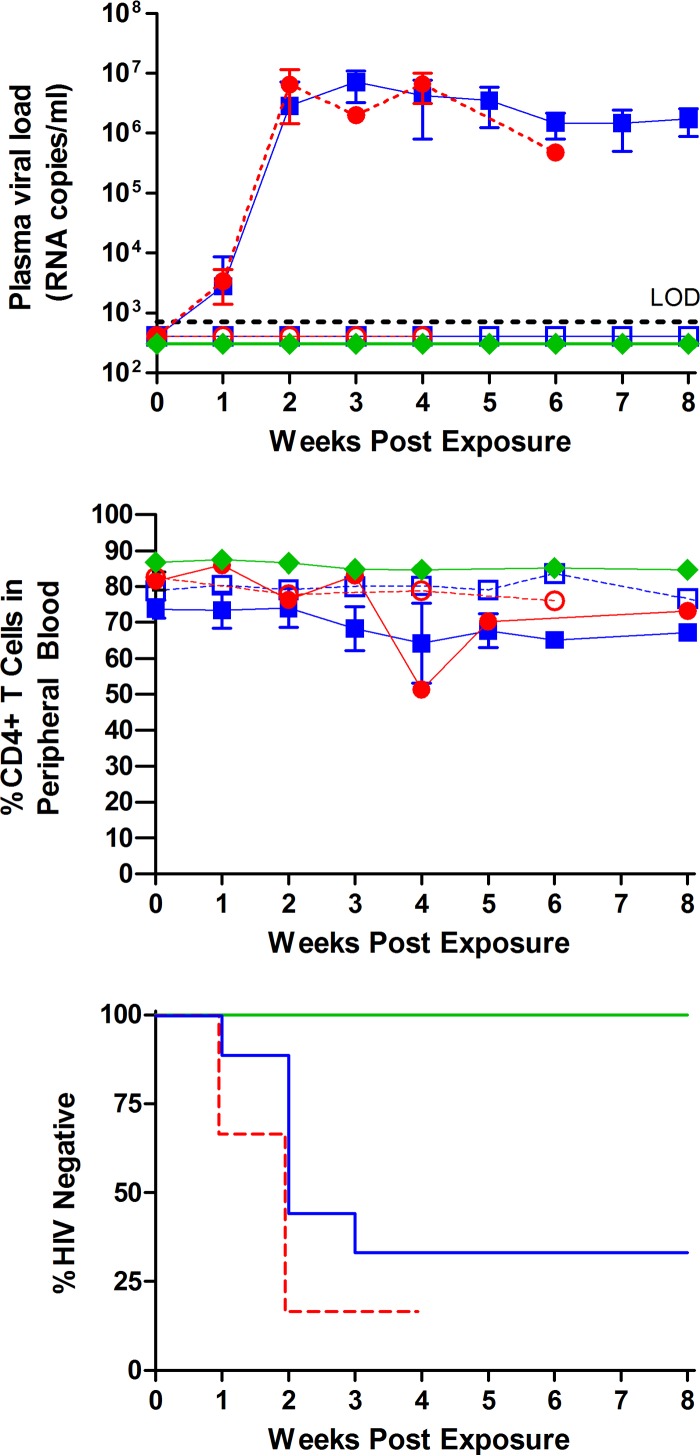
Having established that mixing virus with semen has no effect on its infectivity in vitro, we proceeded to evaluate the in vivo effect of semen on HIV transmission and on the protective effect of a topical microbicide using BLT humanized mice. For this purpose, BLT humanized mice (n = 9) were exposed to HIV-1JR-CSF (7 × 105 tissue culture infectious units [TCIU]) in the presence of semen (50%). Control mice (n = 6) were exposed to virus in the absence of semen (vehicle). In addition, BLT humanized mice (n = 6) were treated with a single dose of topical maraviroc (10 μl of a 5 mM solution) and exposed to virus in semen (10 μl). Mice were monitored longitudinally for the presence of plasma viral RNA as an indication of HIV-1 transmission. In the presence of semen, 6/9 mice became infected after a single exposure to virus, as determined by plasma viral load analysis (Fig. 1). In the absence of semen, 5/6 mice became infected, as determined by the presence of viral RNA in plasma (Fig. 1). Therefore, there was no statistical difference between the rates of transmission observed in the presence or absence of human semen (P = 0.29). Consistent with previous reports using a different humanized mouse model indicating the effectiveness of maraviroc at preventing HIV-1 transmission in humanized mice (although in the absence of human semen) (11), none of the animals treated with maraviroc prior to exposure to virus in the presence of semen showed evidence of viral RNA in plasma (P = 0.01) (Fig. 1). This is especially striking in light of a recent study published by Zirafi et al. that showed a reduced efficacy of many microbicides in the presence of semen, with the exception of maraviroc (12). The sustained efficacy of maraviroc in the presence of semen could be due in part to the fact that maraviroc's mode of action is different from those of the majority of other microbicides, which act on the virus instead of a host protein. Lack of transmission was documented by the absence of DNA in the tissues analyzed from all of the treated animals analyzed (Table 1). These results demonstrate that the presence of human semen does not influence the effectiveness of topically administered maraviroc at preventing vaginal HIV-1 infection. Together these results also demonstrate the utility of the BLT humanized mouse model for the evaluation of the effect of semen on HIV transmission.
FIG 1.
Analysis of the effect of semen on vaginal cell-free HIV-1 transmission and on the protective effect of topically administered maraviroc. Symbols in the top and middle panels: open blue squares, semen, infection negative (n = 3); solid blue squares, semen, infection positive (n = 6); open red circles, vehicle, infection negative (n = 1); solid red circles, vehicle, infection positive (n = 5); green diamonds, top panel, maraviroc plus semen (n = 6), and bottom panel, maraviroc treated (n = 6). BLT humanized mice were exposed vaginally to HIV-1JR-CSF (7.0 × 105 TCIU) in the presence (n = 9) or absence (n = 6) of human semen and in the presence of semen and maraviroc (5 mM). (Top panel) Infection was determined based on the presence or absence of viral RNA in plasma over the course of the experiment. Transmission in the vehicle-treated mice was 5/6, in the mice receiving virus resuspended in semen, 6/9 were positive, and in the mice receiving virus resuspended in semen after maraviroc administration, 0/6 were positive for viral RNA. LOD, limit of detection. (Middle panel) Longitudinal analysis of peripheral blood CD4+ T cell levels of infected and noninfected mice. Symbols in the bottom panel: solid green line, maraviroc plus semen (n = 6); solid blue line, semen (n = 9); dashed red line, vehicle (n = 6). Statistical analysis was performed using the Mantel-Cox log-rank test. Over the course of the experiment, all six maraviroc-treated mice remained HIV negative (P = 0.01). The indicated error bars represent the standard deviations.
TABLE 1.
Description of BLT humanized mice utilized to evaluate the effect of semen on vaginal HIV-1JR-CSF transmission
| Vehicle and mouse no. | PB humanization at time of exposurea |
Plasma viral loadb | Presence of cell-associated viral DNA inc: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % CD45+ | % CD4+ | SPL | LN | BM | ORG | LIV | LNG | PB | ||
| Semen | ||||||||||
| 1 | 78.8 | 76.6 | Positive | + | + | + | + | + | + | ND |
| 2 | 83.9 | 69.6 | Positive | − | ND | + | − | + | + | ND |
| 3 | 74 | 73.9 | Positive | + | + | + | + | + | + | ND |
| 4 | 60.8 | 72.3 | Positive | ND | ND | ND | ND | ND | ND | ND |
| 5 | 73.1 | 75.8 | Positive | − | + | + | + | + | + | + |
| 6 | 77.7 | 74.4 | Positive | − | + | + | + | + | + | + |
| 7 | 46.9 | 74.3 | Negative | − | ND | − | − | ND | − | ND |
| 8 | 80.4 | 74.8 | Negative | − | − | − | − | − | − | ND |
| 9 | 89.1 | 87.3 | Negative | ND | ND | ND | ND | ND | ND | ND |
| Mean ± SD | 73.8 ± 12.8 | 75.4 ± 4.9 | ||||||||
| Vehicle | ||||||||||
| 10 | 84.2 | 88.9 | Positive | + | + | + | + | + | + | ND |
| 11 | 51.6 | 75.1 | Positive | ND | ND | ND | ND | ND | ND | ND |
| 12 | 69.9 | 82.5 | Negative | − | − | − | − | − | − | − |
| 13d | 67.3 | 85.2 | Positive | − | − | − | − | ND | ND | − |
| 14 | 60.8 | 76.6 | Positive | − | + | + | + | + | + | ND |
| 15 | 46.8 | 82.1 | Positive | − | + | + | + | − | − | ND |
| Mean ± SD | 63.4 ± 13.5 | 81.7 ± 5.2 | ||||||||
| Semen with 5 mM maraviroc pretreatment | ||||||||||
| 30 | 86.6 | 87.1 | Negative | ND | ND | ND | ND | ND | ND | ND |
| 31 | 84.8 | 86.6 | Negative | − | − | − | − | − | − | − |
| 32 | 75 | 84.8 | Negative | ND | ND | ND | ND | ND | ND | ND |
| 33 | 85.5 | 85.6 | Negative | − | − | − | − | − | − | − |
| 34 | 89 | 87.8 | Negative | − | − | − | − | − | − | − |
| 35 | 80.5 | 88.8 | Negative | ND | ND | ND | ND | ND | ND | ND |
| Mean ± SD | 83.6 ± 5 | 86.8 ± 1.5 | ||||||||
Gating strategy: live→human CD45 (% CD45+)→human CD3→human CD4 (% CD4+). PB, peripheral blood.
The limit of detection was 800 HIV RNA copies/ml plasma.
Shown are real-time PCR results representative of DNA extracted from 3.6 × 104 to 1 × 106 cells or 15 μl blood at the time of harvest. The assay limit of detection is 10 copies. +, positive for HIV DNA; −, negative for HIV DNA. ND, not done; SPL, spleen; LN, lymph node; BM, bone marrow; ORG, thymic organoid; LIV, liver; LNG, lung.
Mouse 13 was harvested 5 days postexposure and was HIV positive in plasma (11,000 RNA copies/ml). The mouse was negative for HIV DNA in the tissues at this early time point.
Human semen does not affect vaginal infection of BLT humanized mice with HIV-1CH040, a T/F virus.
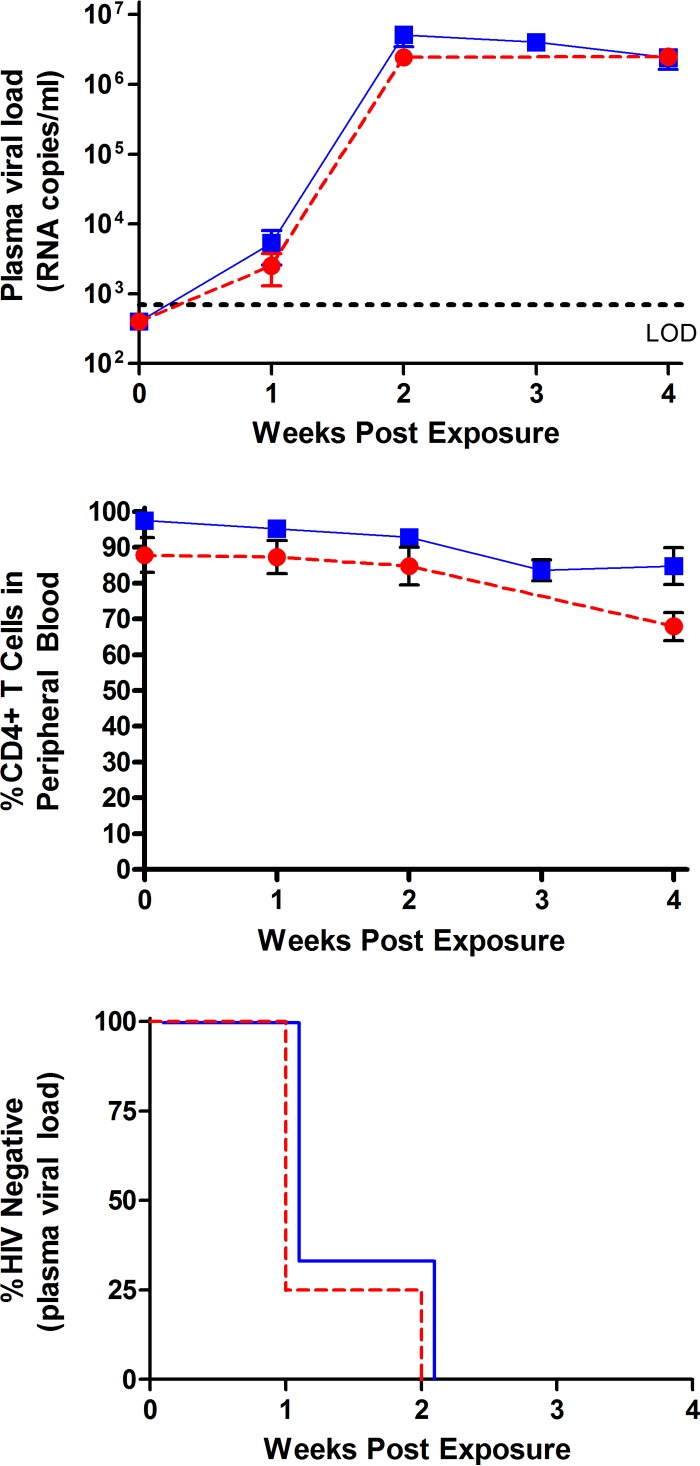
HIV infection after vaginal exposure is established by one or a few viruses that have been designated transmitted/founder (T/F) viruses (13–16). In order to determine if semen had an effect on vaginal infection of BLT humanized mice by a T/F virus, animals were vaginally exposed to HIV-1CH040 (3.5 × 105 TCIU) in the presence (50%) (n = 6) or in the absence (n = 4) of semen (vehicle). In both cases, all of the exposed mice showed readily detectable levels of viral RNA in plasma after a single exposure to virus (Fig. 2). Infection with HIV-1CH040 resulted in a slight drop in CD4+ T cell levels (Fig. 2). Log-rank (Mantel-Cox) analysis of these data indicated that the presence of semen had no deleterious effect on the transmission of HIV-1CH040 in BLT humanized mice (Fig. 2).
FIG 2.
Vaginal infection of BLT humanized mice with HIV-1CH040, a transmitted/founder virus, in the presence of semen. Symbols in the top and middle panels: solid blue squares, semen (n = 6); solid red circles, vehicle (n = 4). BLT humanized mice were exposed vaginally to the transmitted/founder CCR5-tropic HIV-1 isolate CH040 in the presence (50%) (n = 6) or absence (n = 4) of human semen. Plasma viral loads (top) and circulating CD4+ T cell levels (middle) were monitored longitudinally. (Bottom panel) Symbols: solid blue line, semen (n = 6); dashed red line, vehicle (n = 4). There was no statistical difference in the transmission of HIV-1CH040 in the presence or absence of semen (P = 0.8). All mice in both groups became infected 2 weeks postexposure (bottom). Statistical analysis was performed using a Mantel-Cox log-rank test. The indicated error bars represent the standard deviation.
Effect of semen on vaginal transmission of cell-associated HIV-1JR-CSF.
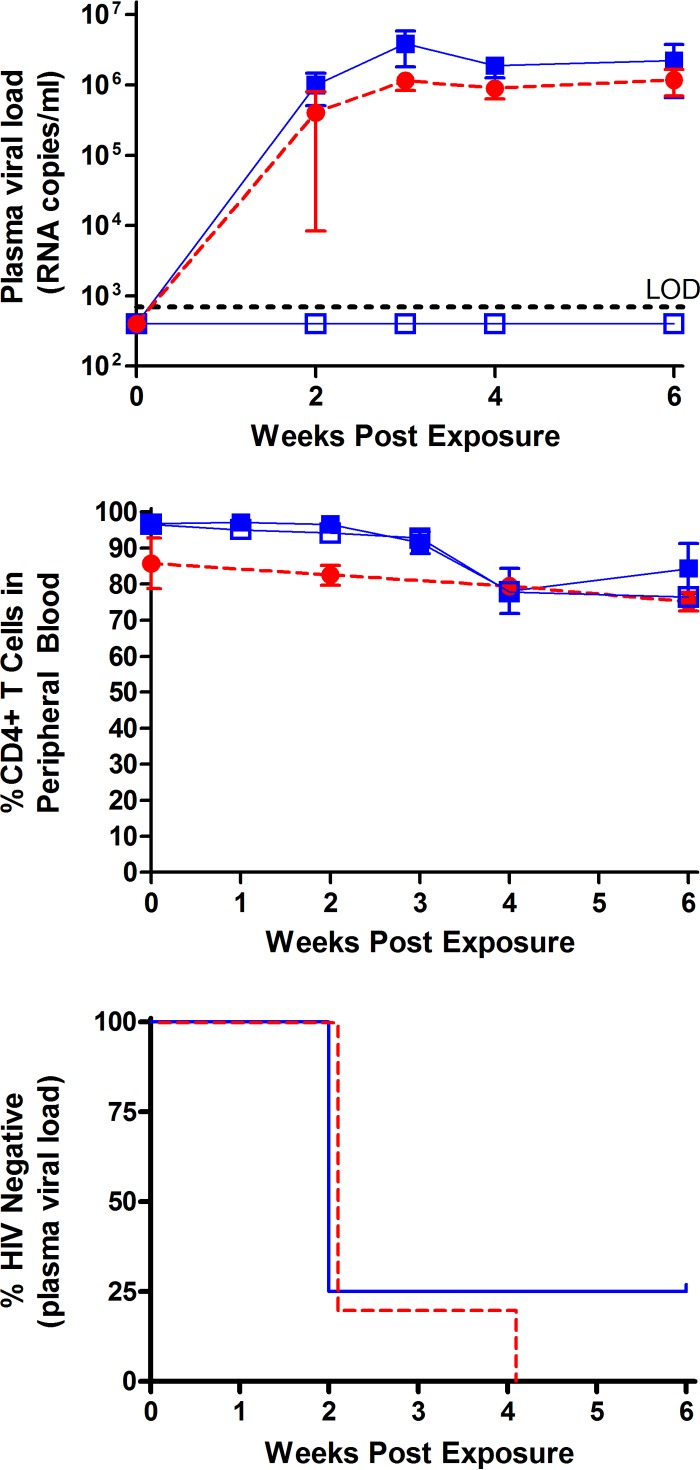
Since human semen contains both cell-free and cell-associated HIV, we sought to determine if semen had an effect on the transmission of cell-associated HIV-1JR-CSF. For this purpose, we exposed BLT humanized mice to human peripheral blood mononuclear cells (PBMCs) infected with HIV-1JR-CSF (1 × 105 p24-positive cells by flow cytometry) in the presence (n = 4) or in the absence (n = 4) of human semen (50%). In the presence of semen, viral RNA was readily detectable in 3/4 exposed mice (Fig. 3). In the absence of semen, viral RNA was readily detected in the plasma of 4/4 of exposed BLT humanized mice (Fig. 3). Log rank (Mantel-Cox) analysis of these data indicates that the presence of semen had no effect on the transmission of cell-associated HIV-1 (Fig. 3).
FIG 3.
Efficient transmission of cell-associated HIV-1JR-CSF in the presence of semen. Symbols in the top and middle panels: solid blue squares, semen, infection positive (n = 3); solid red circles, vehicle, infection positive (n = 4); open blue squares, semen, infection negative (n = 1). BLT humanized mice were exposed vaginally to 1.0 × 105 HIV-1JR-CSF Gag p24+ cells in the presence (n = 4) or absence (n = 4) of human semen. Infection was determined by the presence or absence of plasma viral RNA. Plasma viral load and circulating CD4+ T cell levels were monitored longitudinally (top and middle panels, respectively). Symbols in the bottom panel: solid blue line, semen (n = 4); dashed red line, vehicle (n = 4). Similar levels of vaginal transmission were observed in the presence or absence of human semen (P = 0.5) (bottom). Statistical analysis was performed using a Mantel-Cox log-rank test. The indicated error bars represent the standard deviation.
Even though most sexually acquired cases of HIV-1 occur in the presence of semen, the role semen plays in HIV-1 transmission has been controversial. Many in vitro studies have shown that semen enhances infection (17, 18). Most commonly, the enhancing effect observed with exposure to semen is attributed to cationic polypeptides capable of forming amyloid fibrils; also known as semen-derived enhancer of virus infection (SEVI). A study published by Münch et al. showed that peptide fragments of prostatic acid phosphatase form amyloid fibrils augment a virion's ability to bind to target cells (18). However, this effect has only been observed in vitro (19). Conversely, seminal plasma has been shown to interfere with a variety of mechanisms that facilitate viral uptake and dissemination (20). To date, the role of semen in HIV-1 transmission remains the subject of much debate. As the majority of studies aimed at elucidating its possible role have been conducted in vitro, it is evident that there is a need for comprehensive in vivo analysis. Furthermore, as the majority of sexual exposures to HIV occur in the presence of semen, in vivo analyses of the efficacy of preexposure prophylaxis approaches should be tested in the presence of semen.
In this article, we present an evaluation of the effect of semen on vaginal HIV-1 transmission using BLT humanized mice, a validated model for the study of mucosal HIV-1 transmission and prevention. The BLT humanized mouse model has been previously characterized as an animal model that recapitulates key aspects of HIV infection in humans, including mucosal HIV-1 infection (21–24). While the semen and immune cells in BLT mice are human, the remainder of the female reproductive tract (FRT) is murine. In studies of conception, semen interacts with cells in the FRT to invoke inflammation and then tolerance in preparation for implantation. Some of these interactions take place between semen and the cervicovaginal epithelium. In this transmission model, some of cells exposed to semen would be murine epithelial in origin. It is therefore possible that interactions between the human semen and murine epithelium might not fully emulate the effects of semen in the human FRT. However, even though most mouse cytokines do not act on human receptors, it should be noted that most human cytokines do act on the mouse receptors (25).
In this article, we took advantage of the utility of the BLT model to investigate the possible effect of semen in vaginal HIV transmission. Vaginal HIV transmission in BLT mice has been extensively documented (5–7, 26). BLT humanized mice can be efficiently infected by HIV after a single vaginal inoculation, without the need for any hormonal treatment (5–7, 26), and mucosal HIV transmission in this model can be efficiently prevented by systemic or topically applied antivirals and microbicides (5–7). It is important to note that none of these previous reports using any type of humanized mouse model has used human semen in their experimental design. This study, to our knowledge represents the first comprehensive in vivo analysis of human semen on vaginal HIV-1 transmission. Furthermore, both cell-free, cell-associated, and transmitted/founder viruses were evaluated in the presence of semen, lending breadth to the impact of this study on future investigations. Second, this study impacts continuing efforts to develop topically applied microbicides. In showing that a topically administered 5 mM maraviroc solution is able to completely prevent transmission in this model, we are able to present maraviroc as an attractive candidate for further in vivo testing.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grant AI073146, the UNC Next Generation Pre-Exposure Prophylaxis Program AI096113 (J.V.G.), the UNC Center for AIDS Research P30 AI50410, T32CA009156, F32AI100775 (M.D.S.), and 5T32AI007273-27 (A.W.).
We thank I. Chen and John Kappes for providing pJR-CSF and pCHO40, respectively, via the AIDS Research and Reagent Program. Human semen for these experiments was kindly provided by the Comprehensive Resources for HIV Microbicides and Biomedical Prevention (contract HHSN272201000001C, via James Turpin). We thank former and current lab members and veterinary technicians at UNC Division of Laboratory Animal Medicine for their assistance with various technical aspects of this work.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01496-15.
REFERENCES
- 1.UNAIDS. 2015. Global AIDS response progress reporting 2015. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 2.Buve A, Bishikwabo-Nsarhaza K, Mutangadura G. 2002. The spread and effect of HIV-1 infection in sub-Saharan Africa. Lancet 359:2011–2017. doi: 10.1016/S0140-6736(02)08823-2. [DOI] [PubMed] [Google Scholar]
- 3.Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, Powers KA, Galvin S, Chilongozi D, Gama S, Price MA, Fiscus SA, Cohen MS. 2007. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. 2013. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 8:e60024. doi: 10.1371/journal.pone.0060024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, Powell DA, Payne D, Haase AT, Garcia JV. 2008. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denton PW, Krisko JF, Powell DA, Mathias M, Kwak YT, Martinez-Torres F, Zou W, Payne DA, Estes JD, Garcia JV. 2010. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS One 5:e8829. doi: 10.1371/journal.pone.0008829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, Zein S, Powell DA, Wahl A, Kwak YT, Welch BD, Kay MS, Payne DA, Gallay P, Appella E, Estes JD, Lu M, Garcia JV. 2011. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 85:7582–7593. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nochi T, Denton PW, Wahl A, Garcia JV. 2013. Cryptopatches are essential for the development of human GALT. Cell Rep 3:1874–1884. doi: 10.1016/j.celrep.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, Melkus MW, Padgett-Thomas A, Zupancic M, Haase AT, Garcia JV. 2007. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med 204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahl A, Swanson MD, Nochi T, Olesen R, Denton PW, Chateau M, Garcia JV. 2012. Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog 8:e1002732. doi: 10.1371/journal.ppat.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neff CP, Kurisu T, Ndolo T, Fox K, Akkina R. 2011. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS One 6:e20209. doi: 10.1371/journal.pone.0020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zirafi O, Kim KA, Roan NR, Kluge SF, Muller JA, Jiang S, Mayer B, Greene WC, Kirchhoff F, Munch J. 2014. Semen enhances HIV infectivity and impairs the antiviral efficacy of microbicides. Sci Transl Med 6:262ra157. doi: 10.1126/scitranslmed.3009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baalwa J, Wang S, Parrish NF, Decker JM, Keele BF, Learn GH, Yue L, Ruzagira E, Ssemwanga D, Kamali A, Amornkul PN, Price MA, Kappes JC, Karita E, Kaleebu P, Sanders E, Gilmour J, Allen S, Hunter E, Montefiori DC, Haynes BF, Cormier E, Hahn BH, Shaw GM. 2013. Molecular identification, cloning and characterization of transmitted/founder HIV-1 subtype A, D and A/D infectious molecular clones. Virology 436:33–48. doi: 10.1016/j.virol.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. 2012. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 18.Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Gimenez-Gallego G, Sanchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Southern PJ. 2013. Missing out on the biology of heterosexual HIV-1 transmission. Trends Microbiol 21:245–252. doi: 10.1016/j.tim.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Sabatte J, Ceballos A, Raiden S, Vermeulen M, Nahmod K, Maggini J, Salamone G, Salomon H, Amigorena S, Geffner J. 2007. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J Virol 81:13723–13734. doi: 10.1128/JVI.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olesen R, Wahl A, Denton PW, Garcia JV. 2011. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol 88:195–203. doi: 10.1016/j.jri.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denton PW, Garcia JV. 2009. Novel humanized murine models for HIV research. Curr HIV/AIDS Rep 6:13–19. doi: 10.1007/s11904-009-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denton PW, Garcia JV. 2011. Humanized mouse models of HIV infection. AIDS Rev 13:135–148. [PMC free article] [PubMed] [Google Scholar]
- 24.Denton PW, Garcia JV. 2012. Mucosal HIV-1 transmission and prevention strategies in BLT humanized mice. Trends Microbiol 20:268–274. doi: 10.1016/j.tim.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manz MG. 2007. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26:537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Chateau M, Swanson MD, Garcia JV. 2013. Inefficient vaginal transmission of tenofovir resistant HIV-1. J Virol 87:1274–1277. doi: 10.1128/JVI.01777-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.