Abstract
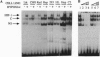
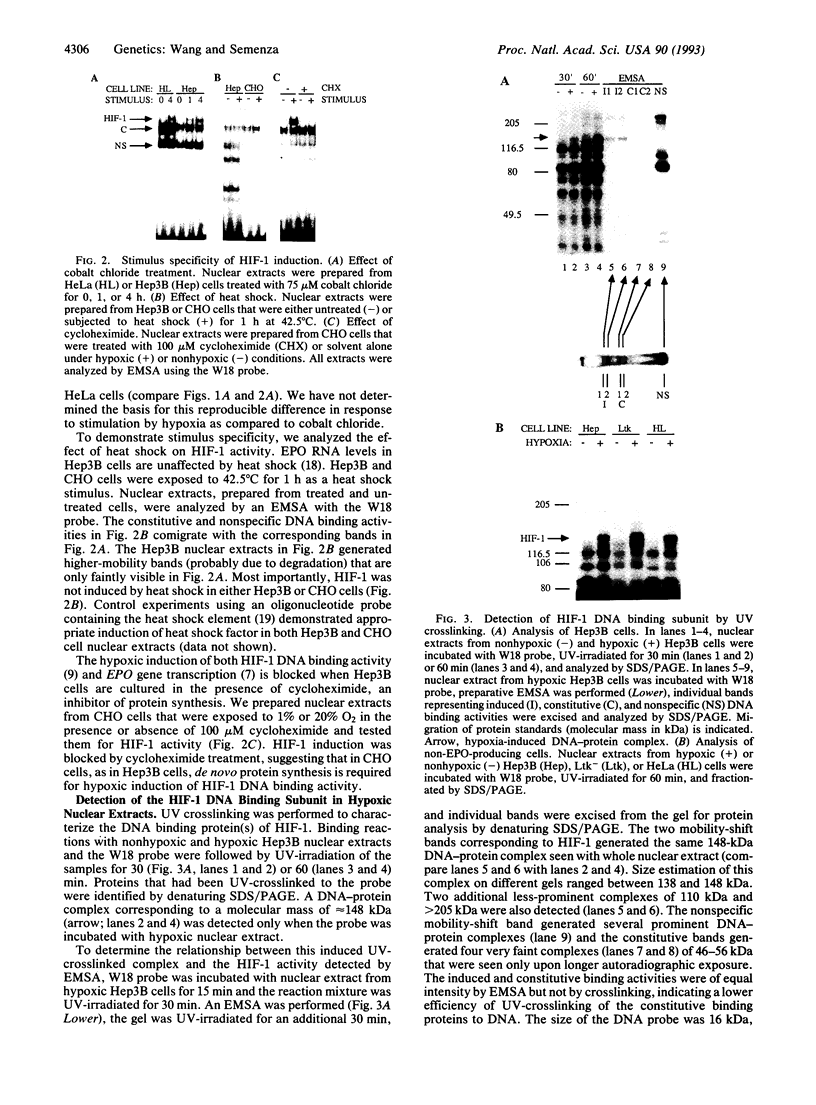
Transcription of the human erythropoietin (EPO) gene is activated in Hep3B cells exposed to hypoxia. Hypoxia-inducible factor 1 (HIF-1) is a nuclear factor whose DNA binding activity is induced by hypoxia in Hep3B cells, and HIF-1 binds at a site in the EPO gene enhancer that is required for hypoxic activation of transcription. In this paper, we demonstrate that HIF-1 DNA binding activity is also induced by hypoxia in a variety of mammalian cell lines in which the EPO gene is not transcribed. The composition of the HIF-1 DNA binding complex and its isolated DNA binding subunit and the mechanism of HIF-1 activation appear to be similar or identical in EPO-producing and non-EPO-producing cells. Transcription of reporter genes containing the EPO gene enhancer is induced by hypoxia in non-EPO-producing cells and mutations that eliminate HIF-1 binding eliminate inducibility. These results provide evidence that HIF-1 and its recognition sequence are common components of a general mammalian cellular response to hypoxia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck I., Ramirez S., Weinmann R., Caro J. Enhancer element at the 3'-flanking region controls transcriptional response to hypoxia in the human erythropoietin gene. J Biol Chem. 1991 Aug 25;266(24):15563–15566. [PubMed] [Google Scholar]
- Beru N., Smith D., Goldwasser E. Evidence suggesting negative regulation of the erythropoietin gene by ribonucleoprotein. J Biol Chem. 1990 Aug 25;265(24):14100–14104. [PubMed] [Google Scholar]
- Blanchard K. L., Acquaviva A. M., Galson D. L., Bunn H. F. Hypoxic induction of the human erythropoietin gene: cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Mol Cell Biol. 1992 Dec;12(12):5373–5385. doi: 10.1128/mcb.12.12.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Daveran M. L., Batut J., Dedieu A., Domergue O., Ghai J., Hertig C., Boistard P., Kahn D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988 Aug 26;54(5):671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez M. A., Ditta G. S., Helinski D. R. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991 Mar 14;350(6314):170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Dunning S. P., Bunn H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988 Dec 9;242(4884):1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Gaut C. C., Bunn H. F. Erythropoietin mRNA levels are governed by both the rate of gene transcription and posttranscriptional events. Blood. 1991 Jan 15;77(2):271–277. [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992 Apr;72(2):449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Kourembanas S., Hannan R. L., Faller D. V. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990 Aug;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991 Sep;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury S. T., Bondurant M. C., Koury M. J., Semenza G. L. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood. 1991 Jun 1;77(11):2497–2503. [PubMed] [Google Scholar]
- Krantz S. B. Erythropoietin. Blood. 1991 Feb 1;77(3):419–434. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxwell P. H., Pugh C. W., Ratcliffe P. J. Inducible operation of the erythropoietin 3' enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson E. K., Weinstein M., Ditta G. S., Helinski D. R. The FixL protein of Rhizobium meliloti can be separated into a heme-binding oxygen-sensing domain and a functional C-terminal kinase domain. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4280–4284. doi: 10.1073/pnas.89.10.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C. W., Tan C. C., Jones R. W., Ratcliffe P. J. Functional analysis of an oxygen-regulated transcriptional enhancer lying 3' to the mouse erythropoietin gene. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10553–10557. doi: 10.1073/pnas.88.23.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Dureza R. C., Traystman M. D., Gearhart J. D., Antonarakis S. E. Human erythropoietin gene expression in transgenic mice: multiple transcription initiation sites and cis-acting regulatory elements. Mol Cell Biol. 1990 Mar;10(3):930–938. doi: 10.1128/mcb.10.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Koury S. T., Nejfelt M. K., Gearhart J. D., Antonarakis S. E. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8725–8729. doi: 10.1073/pnas.88.19.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Nejfelt M. K., Chi S. M., Antonarakis S. E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Traystman M. D., Gearhart J. D., Antonarakis S. E. Polycythemia in transgenic mice expressing the human erythropoietin gene. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2301–2305. doi: 10.1073/pnas.86.7.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Wang G. L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992 Dec;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeniwas R., Koga S., Karakurum M., Pinsky D., Kaiser E., Brett J., Wolitzky B. A., Norton C., Plocinski J., Benjamin W. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992 Dec;90(6):2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Webster K. A. Regulation of glycolytic enzyme RNA transcriptional rates by oxygen availability in skeletal muscle cells. Mol Cell Biochem. 1987 Sep;77(1):19–28. doi: 10.1007/BF00230147. [DOI] [PubMed] [Google Scholar]