Abstract
Transposable elements (TEs) constitute a substantial fraction of the eukaryotic genome and, as a result, have a complex relationship with their host that is both adversarial and dependent. To minimize damage to cellular genes, TEs possess mechanisms that target integration to sequences of low importance. However, the retrotransposon Tf1 of Schizosaccharomyces pombe integrates with a surprising bias for promoter sequences of stress-response genes. The clustering of integration in specific promoters suggests that Tf1 possesses a targeting mechanism that is important for evolutionary adaptation to changes in environment. We report here that Sap1, an essential DNA-binding protein, plays an important role in Tf1 integration. A mutation in Sap1 resulted in a 10-fold drop in Tf1 transposition, and measures of transposon intermediates support the argument that the defect occurred in the process of integration. Published ChIP-Seq data on Sap1 binding combined with high-density maps of Tf1 integration that measure independent insertions at single-nucleotide positions show that 73.4% of all integration occurs at genomic sequences bound by Sap1. This represents high selectivity because Sap1 binds just 6.8% of the genome. A genome-wide analysis of promoter sequences revealed that Sap1 binding and amounts of integration correlate strongly. More important, an alignment of the DNA-binding motif of Sap1 revealed integration clustered on both sides of the motif and showed high levels specifically at positions +19 and −9. These data indicate that Sap1 contributes to the efficiency and position of Tf1 integration.
Keywords: Sap1, Tf1, integration, transposition, Schizosaccharomyces pombe
RETROTRANSPOSONS are pervasive among eukaryotes and, in many cases, account for a substantial portion of the host genome (Moore et al. 2004; Scheifele et al. 2009; Levin and Moran 2011). The ability of these elements to selectively integrate into specific target sequences has been paramount to their success because the employment of specific targeting mechanisms has allowed these retrotransposons to propagate within host genomes without disrupting genes and compromising the host’s survival (Levin and Moran 2011). The long terminal repeat (LTR) retrotransposons Ty1, Ty3, and Ty5 of Saccharomyces cerevisiae avoid causing damage to the host by targeting noncoding genomic regions; Ty1 and Ty3 integrate upstream of RNA polemerase III–transcribed genes, while Ty5 integrates into heterochromatin (Chalker and Sandmeyer 1990; 1992; Ji et al. 1993; Kirchner et al. 1995; Devine and Boeke 1996; Zou et al. 1996; Zou and Voytas 1997; Yieh et al. 2000; Sandmeyer 2003; Lesage and Todeschini 2005).
In Schizosaccharomyces pombe, the LTR retrotransposon Tf1 has a unique targeting mechanism that directs integration to promoters of RNA polymerase II–transcribed genes with a bias for the promoters of stress-response genes (Behrens et al. 2000; Singleton and Levin 2002; Bowen et al. 2003; Leem et al. 2008; Guo and Levin 2010). Surprisingly, insertion of Tf1 into promoters rarely reduces the expression of their downstream genes, and in approximately 40% of cases, it enhances it (Feng et al. 2013).
The LTR elements Ty1, Ty3, and Ty5 in S. cerevisiae rely on host factors to tether integrase (IN) to insertion sites. Ty1 IN binds the AC40 subunit of RNA Pol III to direct integration to sites upstream of transfer RNA (tRNA) genes (Bridier-Nahmias et al. 2015). Ty3 IN binds transcription factors TFIIIB and TFIIIC to position insertions upstream of tRNA genes, while phosphorylated Ty5 IN interacts with the heterochromatin protein Sir4 to direct Ty5 insertion into heterochromatin (Kirchner et al. 1995; Gai and Voytas 1998; Zhu et al. 1999; Yieh et al. 2000, 2002; Xie et al. 2001; Qi and Sandmeyer 2012). Retroviruses such as human immunodeficiency virus 1 (HIV-1) and murine leukemia virus (MLV), which share significant similarities with LTR retrotransposons in their genetic structures and mechanisms of propagation (Levin and Moran 2011), depend on host factors for targeting integration. HIV-1 insertion is directed to the body of RNA polemerase II–transcribed genes by host factor LEDGF, while MLV IN interacts with BET proteins to direct its integration into enhancer sequences of RNA polemerase II–transcribed genes (Ciuffi et al. 2005; Llano et al. 2006; Shun et al. 2007; Gupta et al. 2013; Sharma et al. 2013). The mechanism by which Tf1 accomplishes targeting appears to be significantly different from the preceding examples except perhaps for MLV, which does integrate into promoter sequences. While the BET proteins are transcription coactivators, none of the transcription factors of stress-responce genes in S. pombe appear to play a role in Tf1 integration (Majumdar et al. 2011). As a result, there is still little understanding about how Tf1 integration is positioned.
Switch-activating protein 1 (Sap1), an essential DNA-binding protein in S. pombe (Arcangioli et al. 1994), binds sequences in the LTR of Tf1, as well as genomic regions where Tf1 insertion occurs (Zaratiegui et al. 2011). Sap1 has multiple reported functions, including facilitating mating type switching and causing replication fork arrest at places of genomic instability (Arcangioli and Klar 1991; Krings and Bastia 2005, 2006; Mejia-Ramirez et al. 2005; Noguchi and Noguchi 2007). To determine whether Sap1 plays a role in Tf1 retrotransposition, we studied S. pombe harboring the temperature-sensitive mutant sap1-1 (Noguchi and Noguchi 2007). We found that Tf1 transposition is reduced 10-fold in the sap1-1 mutant strain compared to wild-type sap1+, and this defect was not due to decreases in levels of Tf1 proteins or complementary DNA (cDNA). Together with results of a recombination assay indicating that the sap1-1 mutant did not inhibit transport of Tf1 cDNA to the nucleus, these data argue that Sap1 contributes to the integration of Tf1. Analysis of ChIP-Seq data reveals that ∼6.85% of the S. pombe genome is bound by Sap1. Genome-wide profiles of Tf1 integration with measures of independent insertions at single-nucleotide positions revealed that 73.4% of Tf1 insertions occurred within these Sap1-bound sequences. A strong correlation was observed between positions with high numbers of repeated integration and locations where Sap1 binding was greatest. In addition, analysis of promoter sequences identified strong binding of Sap1 at the nucleosome-free regions (NFRs), and this binding correlated not only with peaks of integration but also with the size of the NFRs. We identified a Sap1 DNA-binding motif and found that Tf1 insertions clustered at two specific nucleotide positions adjacent to the motif, providing additional evidence that Sap1 promotes Tf1 integration.
Materials and Methods
Strains used in this work are listed in Table 1, and the plasmids are listed in Table 2. The media used in this study included yeast extract medium with supplements (YES) and essential minimal medium (EMM), which were prepared as described previously (Forsburg and Rhind 2006) with the following modifications: YES was supplemented with 2 g complete dropout powder, while EMM was supplemented with 2 g dropout powder lacking leucine and uracil. Dropout stock powder was prepared by mixing 5 g adenine SO4 with 2 g each of the remaining 19 amino acids and uracil.
Table 1. Yeast strains.
| Strain number | Genotype | Proteins expressed | Source |
|---|---|---|---|
| YHL912 | h− ura4-294 leu1-32 | Boeke et al. 1987, 21X5A | |
| YHL9752 | h+ leu1-32 ura4 D-18 Sap1-1ts -3X::NAT | This study | |
| YHL5661 | Diploid ura4 D-18/ura4 D-18 ade6-m210/ade6-m216 leu1-32/leu1-32::nmt1-lacZ-leu1 | Singleton and Levin 2002 | |
| YHL9716 | CTY10-5d MATa ade2 trp1-901 leu2-3,112 his3-200 gal4 gal80 ura3::lexAop-lacZ ura3-52 | Studamire and Goff 2008 | |
| YHL9777 | YHL9716/pHL2781, pHL2793 | LexA-Tf1-IN, Gal4-Tf1-IN | This study |
| YHL9774 | YHL9716/pHL2778, pHL2783 | LexA, Gal4 | This study |
| YHL9775 | YHL9716/pHL2778, pHL2793 | LexA, Gal4-Tf1-IN | This study |
| YHL9776 | YHL9716/pHL2781, pHL2783 | LexA-Tf1-IN, Gal4 | This study |
| YHL10822 | YHL9716/pHL2936, pHL2780 | LexA-Sap1, Gal4 | This study |
| YHL10798-10800 | YHL9716/pHL2936 | LexA-Sap1 | This study |
| YHL10804-10815 | YHL9716/pHL2936, pHL2793 | LexA-Sap1, Gal4-Tf1-IN | This study |
| YHL10823 | YHL9716/pHL2936, pHL2938 | LexA-Sap1, Gal4-Sap1 | This study |
| YHL10801-10803 | YHL9716/pHL2938 | Gal4-Sap1 | This study |
| YHL10820-10821 | YHL9716/pHL2938, pHL2778 | LexA, Gal4-Sap1 | This study |
| YHL10816-10819 | YHL9716/pHL2938, pHL2781 | LexA-Tf1-IN, Gal4-Sap1 | This study |
| YHL10014 | YHL912/pHL449-1 | Tf1-NeoAI | This study |
| YHL10015 | YHL912/pHL490-80 | Tf1-NeoAI (PRfs) | This study |
| YHL10016 | YHL912/pHL472-3 | Tf1-NeoAI (Infs) | This study |
| YHL10017 | YHL9752/pHL449-1 | Tf1-NeoAI | This study |
| YHL10018 | YHL9752/pHL490-80 | Tf1-NeoAI (PRfs) | This study |
| YHL10019 | YHL9752/pHL476-3 | Tf1-NeoAI (INfs) | This study |
Table 2. Plasmids used in this study.
| Plasmid number | Plasmid | Description | Source |
|---|---|---|---|
| pHL2778 | pSH2-1 | Expresses LexA DBD for yeast two-hybrid analysis | Studamire and Goff 2008 |
| pHL2780 | pACT2 | Expresses Gal4 AD for yeast two-hybrid analysis | Studamire and Goff 2008 |
| pHL2783 | pACT1 | Expresses Gal4 AD for yeast two-hybrid analysis | Durfee et al. 1993 |
| pHL2781 | pSH2-1-Tf1-IN | Expresses Tf1-intergrase fused to the LexA DBD | This study |
| pHL2793 | pACT1-Tf1-IN | Expresses Tf1-intergrase fused to the Gal4 AD | This study |
| pHL2938 | pACT2-Sap1 | Expresses Sap1 fused to the Gal4 AD | This study |
| pHL2936 | pSH-Sap1 | Expresses Sap1 fused to the LexA-DBD | This study |
| pHL2944 | Serial number library | Introduces serial number into wild-type Tf1-LTR | Chatterjee et al. 2014 |
| pHL449-1 | Tf-neoAI | Expresses Tf1 containing the neo-tagged artificial intron | Levin 1995 |
| pHL490-80 | Tf1-neoAI (PRfs) | Expresses Tf1 PRfs containing the neo-tagged artificial intron | Atwood et al. 1996 |
| pHL472-3 | Tf1-neoAI (INfs) | Expresses Tf1 INfs containing the neo-tagged artificial intron | Levin 1995 |
Drop assays
sap1+ and sap1-1 cells with pHL449-1 (wild-type Tf1) were collected from EMM-uracil plates and resuspended in liquid EMM at a starting OD600 of 0.5. From these initial resuspensions, four fivefold serial dilutions were prepared, and 10 μl of each resuspension and dilution was spotted onto EMM-uracil or YES plates and grown at either 25° or 32° for 5 days. Three independent transformants of each genotype were assessed.
Transposition assays and homologous recombination assays
Assays to determine Tf1 transposition and homologous recombination frequencies were conducted as described previously with the following modification: all assays performed with Tf1-neoAI in the sap1-1 mutant S. pombe and the wild-type controls were incubated at 25° (Levin 1995, 1996; Teysset et al. 2003). Briefly, Tf1 transposition was monitored by placing a neo-marked Tf1 element (Tf1-neoAI) under the control of an inducible nmt1 promoter in a donor plasmid. After the artificial intron (AI) is spliced out, the neo gene allows cells to grow in the presence of 500 μg/ml G418. Patches of S. pombe strains containing donor plasmids were grown on EMM-uracil dropout agar plates in the absence of thiamine to induce transcription of the nmt1 promoter and were further incubated for 4 days. The plates then were replica printed to EMM containing 1 mg/ml 5-fluoroorotic acid (5-FOA) to counterselect against the donor plasmid (Boeke et al. 1987). As a final step, patches were replica printed to plates containing YES, G418, and 5-FOA and incubated 2 additional days to detect strains with integration of Tf1. Wild-type Tf1 produced confluent patches of G418 resistance, while protease frameshift (PRfs) and IN frameshift (INfs) mutations in the Tf1 element reduced cellular growth on the G418-containing plates.
Homologous recombination between cDNA and plasmid sequences was assayed using a protocol similar to the transposition assay with the following modification (Atwood et al. 1996): strains harboring Tf1-neoAI donor plasmids were first grown as patches on agar plates that contained EMM-uracil (plus 10 μM thiamine) and then replica printed to EMM-uracil plates that lacked thiamine. After 4 days of incubation, the patches were replica printed directly to YES containing 500 μg/ml G418.
Transposition assays conducted to identify the location and frequency per position of Tf1 integration were performed using the Tf1 serial number library, as described previously (Chatterjee et al. 2014). S. pombe strains YHL9752 (sap1-1) and YHL5661 (wild type) were transformed with the serial number Tf1-neo plasmid library, and serial number insertion libraries were constructed for each individual strain by pooling approximately 55,000 and 60,000 independent wild-type and sap1-1 transformants, respectively, from EMM plates lacking uracil. To repress expression of neo-marked Tf1 from the nmt1 promoter, thiamine was added to plated medium at a concentration of 10 µM, which was removed prior to induction by mixing the pooled cells for 1 hr at 25° and washing them four times with 225 ml EMM lacking both uracil and thiamine. Transposition was induced by growing cells at 25° in EMM in the absence of uracil and thiamine. The wild-type and sap1-1 mutant cultures were passaged with repeated dilutions to an OD600 of 0.05 until they reached 53 generations; then the cultures were diluted to an OD600 of 0.25 in EMM containing 5-FOA and regrown to an OD600 of 5.0. This selected against cells retaining the Tf1-containing plasmids. The cultures were diluted 10-fold to an OD600 of 0.5 in YES containing both 5-FOA and G418 and grown to an OD600 of 5.0 to isolate both wild-type and sap1-1 mutant cells containing integrated copies of Tf1s-neo.
Quantitative transposition assay
Quantitative transposition assays were performed as described previously (Majumdar et al. 2011) to measure the frequencies of transposition of wild-type and sap1-1 mutant S. pombe strains. Briefly, strains were grown on solid EMM without thiamine and after 4 days were resuspended in liquid medium to an OD600 of 5.0. A series of five 10-fold dilutions were generated from each resuspension starting with 108 cells/ml and ending with 104 cells/ml, and 100 μl of cells from the three lowest dilutions was spread onto YES plates containing 5-FOA, while 100 μl of the three highest dilutions were spread onto YES plates containing both 5-FOA and G418. The transposition frequency is reported as the percentage of 5-FOA/G418-doubly-resistant colonies relative to the total number of 5-FOA-resistant colonies. The data presented in this work were compiled from three independent transformants.
Quantitative recombination assay
Cells were resuspended in liquid EMM without thiamine to an OD600 of 0.05 and were grown for 6 days at 25°. A series of five 10-fold dilutions was generated from each culture starting with 2 × 107 cells/ml and ending with 2 × 103 cells/ml, and 100 µl of cells from the three lowest dilutions was spread onto YES plates, while 100 µl of the three highest dilutions were spread onto YES plates containing G418. The transposition frequency is reported as the percentage of G418-resistant colonies relative to the total number of colonies on nonselective YES plates. The data presented in this work were generated from four to five independent transformants.
Two-hybrid analysis
The yeast two-hybrid system was used as described previously (Studamire and Goff 2008) with the following modifications: in brief, DNA segments encoding IN and Sap1 were amplified via PCR using EcoRI- and BamHI-tailed primers in the case of Sap1 and EcoRI and SalI in the case of Tf1 IN, which were designed to create the respective restriction sites on the 5′ and 3′ ends of the amplified products. These fragments were subsequently both cloned into the LexA DNA-binding domain (DBD) expression vector pSH2-1 and into the GAL4 activation domain (AD) expression vector pACT. The plasmids were transformed into S. cerevisiae strain YHL9716, which contains a copy of the LacZ gene downstream of a LexA operator. Transformants were patched onto synthetic complete (SC) plates lacking histidine and leucine. After 3 days of growth, the patches were transferred to a nitrocellulose membrane and frozen overnight at −80°. Potential interactions were identified by incubating the nitrocellulose in Z-buffer (Miller 1972) and identifying blue patches producing β-galactosidase.
Comparison of Sap1+ and Sap1-1 integration profiles
Methods for sequencing insertion sites are included in the Supplemental Methods (Supporting Information) and the oligos used are listed in Figure S1. The integration site data are provided in File S1, File S2, File S3, File S4, File S5, File S6, File S7, File S8, and File S9. PERL scripts were used to identify the number of insertions per intergenic region that were sequenced from six independent cultures: three independent cultures of wild-type S. pombe and three independent cultures of the Sap1-1 mutant (Table S1). Additional PERL scripts were used to generate matrices reflecting the change in the number of insertions per intergenic region and per nucleotide position for the three combined integration profiles from each genotype (Table S1). Positions were discarded if both strains had fewer than three independent insertions. Because 512,312 insertions were mapped in the sap1-1 strain and 1,086,402 insertions were mapped in the sap1+ strain, the data from the sap1-1 strain were normalized by multiplying each value in the sap1-1 data set by 2.12. Graphs were generated of the normalized data using the software R, and linear regression and R2 analyses were performed using Graphpad Prism. PERL scripts were used to identify insertion positions exhibiting a greater than twofold difference between the sap1+ and sap1-1 strains (Table S1).
Detection of Tf1 glycosaminoglycan, integrase, and reverse transcriptase by immunoblot analysis
Total proteins were extracted from cells grown under Tf1-inducing conditions using a previously published protocol (Atwood et al. 1996). Briefly, cultures were grown to an OD600 of 10 and were harvested by resuspension in 400 μl extraction buffer (15 mM NaCl, 10 mM HEPES-KOH, pH 7.8, and 5 mM EDTA). Cells were then vortexed in the presence of 0.4-mm acid-washed glass beads (Sigma), and the resulting crude protein extracts were recovered and mixed with an equal volume of 2× sample buffer and then boiled for 10 min. Then 10 μg of total protein from each sample was collected and loaded on SDS–10% polyacrylamide gels for immunoblot analysis. Standard electrophoresis and transfer techniques were used with Immobilon-P membranes (Millipore) in conjunction with the ECL System for detection as described by the manufacturer (Amersham). To visualize that equal amounts of protein were loaded in each lane and to determine that the transfer occurred evenly, we stained the membranes with 0.1% Coomassie Brilliant Blue G250 in 50% methanol. After 5 min, the filters were rinsed with water and destained in a solution of 50% methanol and 10% acetic acid. Horseradish peroxidase–conjugated donkey anti-rabbit immunoglobulin was used at a 1:10,000 dilution. The polyclonal antisera used to detect Gag, integrase (IN), and reverse transcriptase (RT) have been described previously (Levin et al. 1993; Hoff et al. 1998).
Preparation and analysis of nucleic acid
cDNA preparations were performed as described previously (Atwood-Moore et al. 2006). In brief, total DNA was extracted from cells grown for 36 hr using glass beads and phenol. The DNA was digested with BstXI prior to analysis by DNA blotting. The probe consisted of a 1-kb BamHI fragment with the sequence of neo (Atwood et al. 1996). The radiolabeled probe was generated using 32P random primer labeling (Roche).
Data availability
All strains, plasmids, and computer programs/scripts are available upon request. Sequence reads were submitted to the Short Read Archive (SRA) at National Center for Biotechnology Information (NCBI) under the accession number PRJNA279274.
Results
Sap1 promotes Tf1 integration
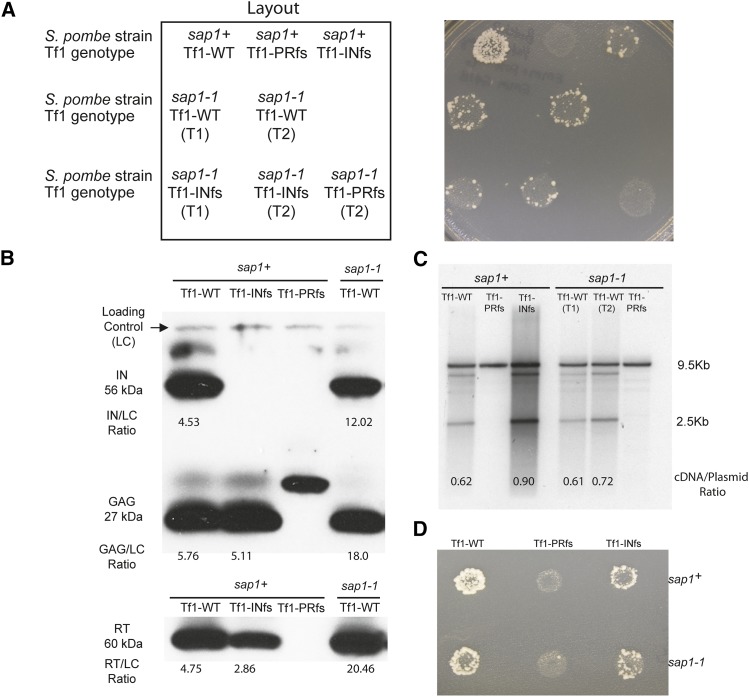
To test whether Sap1 is a host factor that plays a role in Tf1 transposition, assays were conducted in mutant S. pombe harboring the temperature-sensitive allele sap1-1. The sap1-1 mutant showed no apparent growth defects on EMM or YES at 25° compared to the sap1+ control; however, as reported previously, it was unable to grow at 37° (Figure S2). Transposition was measured using an assay that detects the insertion of neo-marked Tf1 (Tf1-neoAI) elements into the S. pombe genome (Levin 1996; Atwood et al. 1998; Teysset et al. 2003). This assay relies on the expression of a plasmid-encoded Tf1-neoAI to generate integration events, which are detected by the ability of cells to grow in the presence of 5-FOA to select against cells containing the original URA3-tagged donor plasmid and G418 to select for insertions. At the permissive temperature of 25°, cells containing the sap1-1 mutant allele had substantially less transposition activity than the wild-type sap1+ cells (Figure 1A, sap1+, Tf1-WT vs. sap1-1, Tf1-WT). The transposition frequency of the sap1-1 strain expressing wild-type Tf1-neoAI was only slightly higher than that of the sap1+ strain expressing Tf1-neoAI with the INfs, indicating that the sap1-1 mutant exhibited low-level residual Tf1 activity. INfs is used as a baseline for transposition assays because no IN is expressed, and the low level of G418R is due to homologous recombination between cDNA and LTR sequences in the genome (Levin 1996). Quantitative transposition assays (see Materials and Methods) revealed that Tf1 transposition was reduced by 10-fold in the sap1-1 mutant (Table 3).
Figure 1.
(A) Tf1 retrotransposition is reduced in S. pombe with a temperature-sensitive sap1-1 allele. The results of transposition patch assays of wild-type S. pombe (sap1+) and of S. pombe harboring a temperature sensitive allele of sap1 (sap1-1). Tf1-PRfs, protease frameshift Tf1 mutant; Tf1-INfs, IN frameshift Tf1 mutant. Patches in which the expression of Tf1 was induced were first replica printed to plates containing 5-FOA and then printed to plates with 5-FOA and G418. T1 and T2 indicate two individual transformants. (B) Immunoblot analysis of Tf1 Gag, IN, and RT in wild-type (sap1+) and sap1-1 mutant S. pombe extracts. Polyclonal antibodies specific for IN, Gag, and RT detected the mature 56-kDa IN, 27-kDa Gag, and 60-kDa RT species. “Loading control” indicates a nonspecific band used for the normalization of IN, Gag, and RT with ImageJ. The numbers represent the ratio of the intensity of the indicated band to the loading control. The frameshift transposons used in this experiment are the same as those listed in A. (C) DNA blot analysis of total DNA extracted from wild-type (sap1+) and sap1-1 mutant S. pombe cells and digested with BstXI. Blots were probed with a randomly labeled 1-kb BamHI fragment containing the neo sequence. The large 9.5-kb fragment resulting from the digestion of the Tf1 donor plasmid served as the loading control for comparing levels of the 2.5-kb cDNA. Numbers indicate the ratio of the intensities of the indicated cDNA band to the loading control. (D) To measure recombination, cells were replica printed to plates with 5-FOA and then printed to plates with 5-FOA and G418.
Table 3. Results of quantitative Tf1 transposition assay with wild-type and Sap1-1 mutant S. pombe.
| S. pombe genotype | Tf1 genotype | Averagea | SD |
|---|---|---|---|
| sap1+ (25°) | Wild type | 0.17 | 3.5 × 10−3 |
| sap1+ (25°) | INfs | 0.010 | 3.5 × 10−3 |
| sap1+ (25°) | PRFs | 0 | 1 × 10−4 |
| sap1-1 (25°) | Wild type | 0.019 | 9.5 × 10−3 |
Percentage of cells with Tf1 integrations from three independent transformants.
To determine whether the sap1-1 mutation reduced Tf1 transposition by lowering expression, immunoblot analyses were performed using lysates of sap1+ and sap1-1 cells expressing wild-type and various mutant versions of Tf1. To quantitate the levels of Tf1 Gag, IN, and RT, ImageJ software was used to normalize each of the bands representing these proteins to a nonspecific band on the blot that was present in each lane. None of the Tf1 proteins analyzed were reduced in the sap1-1 strain relative to sap1+ but actually appeared to be increased by three- to fourfold (Figure 1B). While this apparent increase could simply be the result of a weak signal given by the nonspecific species in the sap1-1 lane, these data argue that Tf1 Gag, IN, or RT expression was not reduced by the sap1-1 mutation. To determine whether Tf1 cDNA production was reduced by the sap1-1 mutation, DNA blot analysis was performed using a probe specific for the neo gene. To differentiate between the Tf1 cDNA and the original donor plasmid, the samples were digested with BstXI, which results in a 2.1-kb band from the Tf1 cDNA and a 9.5-kb band from the donor plasmid (Atwood et al. 1996). We used ImageJ to quantitate the amount of Tf1 cDNA present in the lysates relative to the plasmid, and we found that the sap1-1 mutation did not result in a defect in Tf1 cDNA production (Figure 1C).
Having determined that levels of Tf1 proteins and cDNA were not reduced in sap1-1 cells, we next considered whether their reduced transposition was due to a defect in the nuclear import of Tf1 cDNA. This was tested using the homologous recombination assay, as described previously (Atwood-Moore et al. 2006), which measures the amount of Tf1 cDNA in the nucleus by detecting homologous recombination between Tf1 cDNA and the Tf1 donor plasmid. In this assay, the neo gene within the Tf1 transposon is disrupted by an AI that renders it inactive until the intron is removed by splicing during transcription. An active copy of the neo gene is generated during reverse transcription that is then able to convey resistance to G418 either by integration into the genome or, in this case, by homologous recombination with the donor plasmid. Unlike the transposition assay, the donor plasmid remains in the cells throughout the assay, allowing efficient homologous recombination to occur between the cDNA and the plasmid. The levels of G418 resistance produced by the INfs provided the measure of cDNA present in the nucleus. The sap1-1 mutation did not reduce the level of homologous recombination in patch assays (Figure 1D; compare top-right to bottom-right patches), indicating that the nuclear import of the Tf1 cDNA is not significantly inhibited in S. pombe harboring the sap1-1 mutant allele. To measure more precisely whether Sap1 makes a contribution to homologous recombination, we performed quantitative recombination assays in liquid cultures. The results of these assays revealed that homologous recombination was reduced in the sap1-1 mutant by approximately 2.5-fold when comparing the INfs strains (Table 4). The contribution of Sap1 to recombination could be the result of reduced nuclear import. It is also possible that the Sap1-binding LTR sequence could stimulate homologous recombination. This would not affect transposition measures. Regardless, the 2.5-fold contribution of Sap1 to recombination is substantially less than the 10-fold contribution Sap1 makes to transposition, indicating that the bulk of the defect is in integration. Together with the observations that the levels of Tf1 protein and cDNA were not reduced in the sap1-1 mutant, these data indicate that the sap1-1 mutant significantly affected the process of integration.
Table 4. Quantitative Tf1 homologous recombination assay with sap1+ and sap1-1 strains.
| Tf1 genotype | S. pombe Genotype | Averagea | SD | sap1+/sap1-1 ratio |
|---|---|---|---|---|
| Wild type | sap1+ (25°) | 1.34 | 2.90 × 10−1 | 2.48 |
| Wild type | sap1-1 (25°) | 5.4 × 10−1 | 6.56 × 10−2 | |
| INfs | sap1+ (25°) | 6.9 × 10−1 | 2.09 × 10−1 | 2.48b |
| INfs | sap1-1 (25°) | 2.8 × 10−1 | 1.17 × 10−1 | |
| PRfs | sap1+ (25°) | 0.0 | 1.34 × 10−4 | 0.0 |
| PRfs | sap1-1 (25°) | 1 × 10−3 | 8.00 × 10−4 |
Percentage of cells with G418 resistance from four or five independent transformants.
Student’s t-test P = 0.0083.
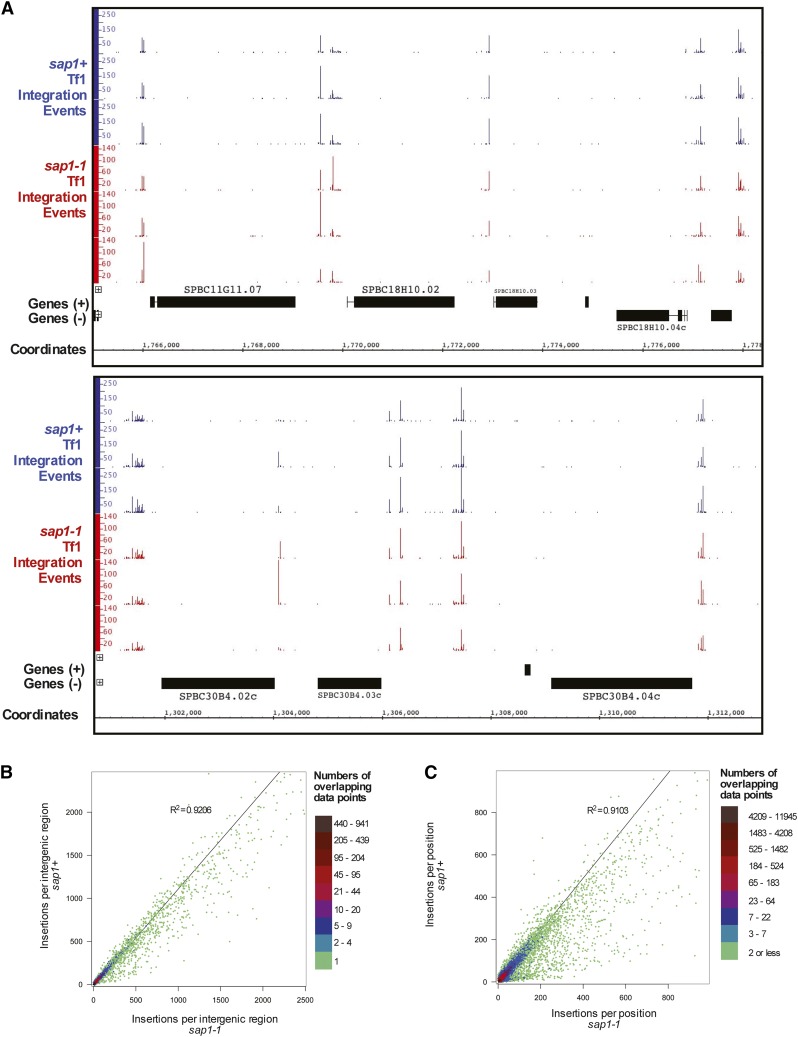
If Sap1 contributes directly to Tf1 integration, the sap1-1 mutation would have the potential to alter the positions of integration. We tested this possibility by generating dense profiles of integration sites by inducing plasmid-encoded copies of Tf1s-neo carrying serial number tags of eight random base pairs that allowed us to measure independent insertions at single-nucleotide positions (Chatterjee et al. 2014). As explained previously (Chatterjee et al. 2014), we used rate-distortion analysis to compensate for errors in serial number measures that result from sequence misreads (Supplementary Methods, File S10 and Supplementary Figure S5, Figure S6, Figure S7, and Figure S8). We isolated genomic DNA from cells with Tf1 insertions, and by ligation-mediated PCR and high-throughput sequencing, we determined dense profiles of integration that included independent insertions at single-nucleotide positions. Three independent cultures of wild-type (sap1+) cells produced integration profiles with an average of 92.3% of events located in intergenic sequences, a level similar to previous profiles and that results from the targeting of specific promoter sequences (Guo and Levin 2010; Chatterjee et al. 2014).
Inspection of integration in individual regions of the genome suggested that the numbers of insertions between replicas were highly reproducible (Figure 2A). Also, in these examples, the sap1-1 mutation did not alter the integration pattern. To evaluate the integration patterns genome-wide, the numbers of insertions per intergenic region were tabulated. The amount of integration within intergenic regions was highly reproducible between the three independent replicas for both the sap1+ and sap1-1 experiments (Table 5, R2 > 0.8). More important, the levels of integration in the intergenic regions correlated strongly between the sap1+ and sap1-1 experiments, indicating that the sap1-1 mutation did not significantly change the integration pattern (Table 5, R2 between 0.75 and 0.91). The combined integration in intergenic sequences of all three wild-type cultures when compared by linear regression with the combined integration of all three sap-1-1 cultures showed a high level of correlation, with an R2 of 0.92 (Figure 2B).
Figure 2.
Integration positions are not substantially altered in sap1-1 mutant cells. (A) Integration levels from sap1+ and sap1-1 cells are shown in two sample regions of the genome. The data shown are from three independent experiments. (B) Density scatter plot and linear regression analysis comparing the number of Tf1 insertions occurring within the intergenic regions of wild-type (sap1+) (y-axis) and mutant (sap1-1) cells (x-axis). The boxes to the left indicate ranges of the number of data points that overlap at any given plot in the graph (i.e., the number of intergenic regions having the same x- and y-axis values). The data shown are combined from three independent experiments. (C) Density scatter plot and linear regression analysis of the number of Tf1 insertions occurring at specific nucleotide positions within wild-type (sap1+) and mutant (sap1-1) cells. The boxes to the left indicate ranges of the number of data points that overlap at any given plot in the graph (i.e., the number of positions with the same x- and y-axis values). The data shown are combined from three independent experiments. For B and C, density scatter plots were created using the function hexbin of the program R. Linear regression analyses were performed, and R2 values were calculated using Graphpad Prism.
Table 5. Correlation coefficients (R2) of the integration levels within the intergenic regions of wild-type and Sap1-1 mutant S. pombe collected from three independent experiments.
| sap1+ (25°) #1 | sap1+ (25°) #2 | sap1+ (25°) #3 | sap1-1 (25°) #1 | sap1-1 (25°) #2 | sap1-1 (25°) #3 | |
|---|---|---|---|---|---|---|
| sap1+ (25°) #1 | 0.96 | 0.91 | 0.77 | 0.81 | 0.76 | |
| sap1+ (25°) #2 | 0.97 | 0.87 | 0.91 | 0.86 | ||
| sap1+ (25°) #3 | 0.92 | 0.90 | 0.91 | |||
| sap1-1 (25°) #1 | 0.84 | 0.86 | ||||
| sap1-1 (25°) #2 | 0.84 |
To test whether the sap1-1 mutation altered the integration patterns within intergenic sequences, we conducted a more thorough examination of integrations at single-nucleotide positions. The tabulation of serial number measures of integration from the sap1+ and sap1-1 strains revealed a total of 153,848 independent insertion sites. Most of these sites had fewer than three insertions in both strains and thus were eliminated from the subsequent analysis. The analysis was conducted with the remaining 34,418 sites. The number of insertions mapped in the sap1-1 strain was normalized to account for differences in the total number of insertions mapped in each strain (512,312 vs. 1,086,402 insertions in the sap1-1 and sap1+ strains, respectively). Analysis of the 34,418 insertion sites revealed that the integration patterns of sap1+ cells correlated highly with those of sap1-1 cells (Figure 2C, R2 = 0.91), indicating that although the sap1-1 mutation lowered integration frequencies substantially, it did not cause a substantial change in the targeting of integration. On closer examination, we identified a total of 12,172 positions (35.37% of the 34,418 positions analyzed) that exhibited a greater than twofold change in the number of integrations between the two strains (Table 6). Of these positions, 8755 exhibited greater than twofold decreased numbers of Tf1 integrations in the sap1-1 strain. These contained 8.09% of all integration events in the sap1+ strain and only 2.14% of all events in the sap1-1 mutant. Thus, at the positions that decreased more than twofold in the sap1-1 mutation, there were 5.96% fewer integration events. Overall, comparing the 5.96% decrease in integration at positions with reduced integration to the 9.60% increase in integration that occurred at positions with greater than a twofold increase in integration, the sap1-1 mutation caused a total increase of 3.64% at these positions.
Table 6. Change in the distribution of Tf1 integrations resulting from sap1-1 mutation.
| No. of positions | Percent of total positions | No. of integrations in sap1+ strain | Percent of integrations in sap1+ strain | No. of integrations in sap1-1 strain | Percent of integrations in sap1-1 strain | Percent of integrations that change positions | |
|---|---|---|---|---|---|---|---|
| Positions with a greater than twofold change in sap1-1 relative to sap1+ | 12,172 | 35.37 | 132,379 | 12.18 | 81,095 | 15.83 | 3.64 |
| Positions with a greater than twofold decrease in integrations in sap1-1 relative to sap1+ | 8,755 | 25.44 | 87,944 | 8.09 | 10,947 | 2.14 | −5.96 |
| Positions with fewer than 11 integrations in sap1+ | 7,260 | 21.09 | 34,429 | 3.17 | 2,438 | 0.48 | −2.69 |
| Positions with between 11 and 100 integrations in sap1+ | 1,458 | 4.24 | 31,897 | 2.94 | 5,435 | 1.06 | −1.88 |
| Positions with between 101 and 500 integrations in sap1+ | 33 | 0.10 | 5,449 | 0.50 | 1,102 | 0.22 | −0.29 |
| Positions with between 501 and 1000 integrations in sap1+ | 3 | 0.01 | 1,761 | 0.16 | 256 | 0.05 | −0.11 |
| Positions with over 1000 integrations in sap1+ | 1 | 0.00 | 14,408 | 1.33 | 1,716 | 0.33 | −0.99 |
| Positions with a greater than twofold increase in Tf1 integrations in sap1-1 relative to sap1+ | 3,417 | 9.93 | 44,435 | 4.09 | 70,148 | 13.69 | 9.60 |
| Positions with fewer than 11 integrations in sap1+ | 2,878 | 8.36 | 8,947 | 0.82 | 13,793 | 2.69 | 1.87 |
| Positions with between 11 and 100 integrations in sap1+ | 440 | 1.28 | 14,910 | 1.37 | 27,712 | 5.41 | 4.04 |
| Positions with between 101 and 500 integrations in sap1+ | 94 | 0.27 | 17,249 | 1.59 | 23,583 | 4.60 | 3.02 |
| Positions with between 501 and 1000 integrations in sap1+ | 5 | 0.01 | 3,329 | 0.31 | 5,060 | 0.99 | 0.68 |
| Positions with over 1000 integrations in sap1+ | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0.00 |
| Positions with a less than twofold change in sap1-1 relative to sap1+ | 22,246 | 64.63 | 853,947 | 78.60 | 382,001 | 74.56 | −4.04 |
Note: Numbers are nonnormalized values, and percentages are derived from nonnormalized values.
To gain a better understanding of how the sap1-1 mutation influenced integration at individual sites, all 12,172 of the positions with more than a twofold change were sorted into groups based on the number of integrations each position holds within the sap1+ strain. This analysis revealed that while these 12,172 positions accounted for 12.18 and 15.83% of all insertions in the sap1+ and sap1-1 strains, respectively, the vast majority of these positions (7260 + 2878 = 10,138, or ∼82%) contained fewer than 11 integration events within the sap1+ strain, showing that the sap1-1 mutation has the most impact on integration in positions that are weak targets for Tf1 integration. While a few of these altered positions were found to be integration hotspots (positions with over 100 integrations) in the reference sap1+ strain, most of these hotspots were located in sites whose Tf1 activity increased in the sap1-1 mutant rather than decreased (99 vs. 37 positions, respectively), further suggesting that the few strong integration targets that are affected by the sap1-1 mutation generally become stronger targets in the sap1-1 strain.
Despite identification of the preceding positions where Tf1 activity is altered by more than twofold by the sap1-1 mutation, most of the Tf1 integrations in each strain (78.60 and 74.56% in the sap1+ and sap1-1 strains, respectively) were at positions that did not change more than twofold in Tf1 activity. This is consistent with our initial conclusion from our linear regression analysis: Tf1 integration-site preference was not grossly altered by the sap1-1 mutation. While the sap1-1 mutation does appear to result in minor changes in integration patterns, it is also possible that these differences resulted from selection because the sap1-1 strain was haploid and the sap1+ cells were diploid.
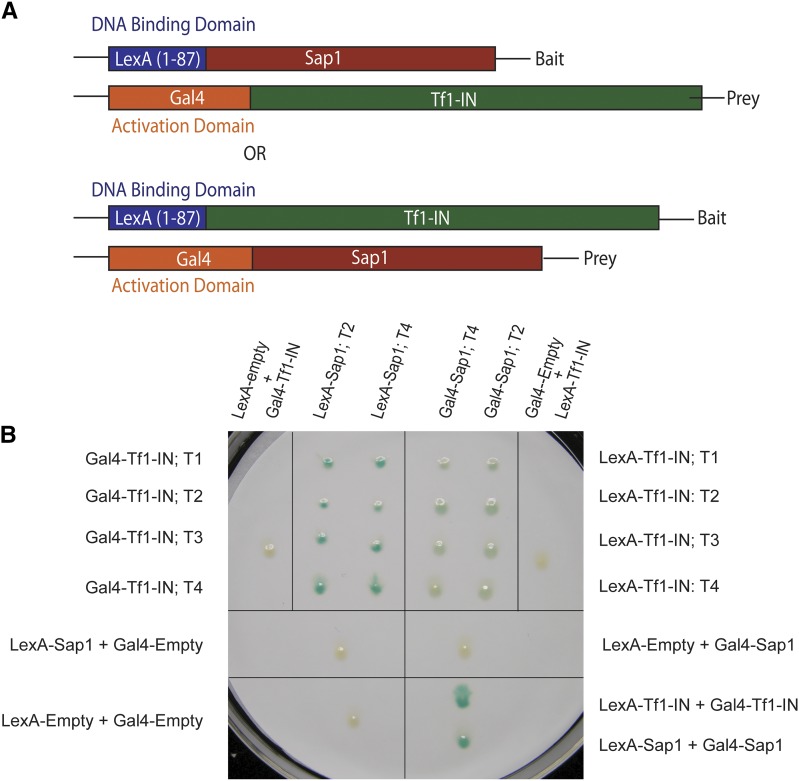
The strong contribution Sap1 makes to integration frequency suggests that it may play a role in integration. Such a role could be mediated through direct interaction. To test for direct interaction between Sap1 and Tf1 IN, we performed a series of pull-down experiments. We tested Sap1 and IN as purified recombinant proteins as well as proteins expressed in S. pombe. Despite testing many configurations of cell extracts and purified proteins, we were unable to obtain evidence that Sap1 interacts with IN (data not shown). We also tested for in vivo interaction between Sap1 and IN using the two-hybrid assay of S. cerevisiae. Fusion of IN to the C termini of the LexA DBD and the Gal4 AD detected IN:IN interaction, as indicated by high expression of a LexA operator–lacZ reporter (Figure 3, A and B, bottom-right panel). Similarly, LexA DBD and Gal4 AD fusions to Sap1 detected Sap1:Sap1 interactions (Figure 3B, bottom-right panel). Importantly, when Sap1 was fused to the C terminus of the LexA DBD and IN was fused to the C terminus of the Gal4 AD, a strong interaction was detected (Figure 3B, top-left panel). Interactions were not observed if the LexA DBD lacked Sap1 or if the Gal4 AD lacked IN. Also, interaction was not observed when IN was fused to the LexA DBD and Sap1 was fused to the Gal4 AD (Figure 3B, top-right panel).
Figure 3.
(A) Sap1 interacts with Tf1 IN in yeast two-hybrid assays. Diagram of the fusion proteins used in the two-hybrid assay. (B) Results of yeast two-hybrid assay. The fusion proteins expressed by each S. cerevisiae patch are indicated. Independent transformants are indicated by T followed by a number. “Gal4-Empty” indicates patches that express the Gal4 portion of the fusion protein only. “LexA-Empty” indicates patches that express the LexA portion of the fusion protein only. An interaction between the two fusion proteins is indicated by the color blue.
Tf1 insertion sites align with genomic regions of enriched Sap1 binding
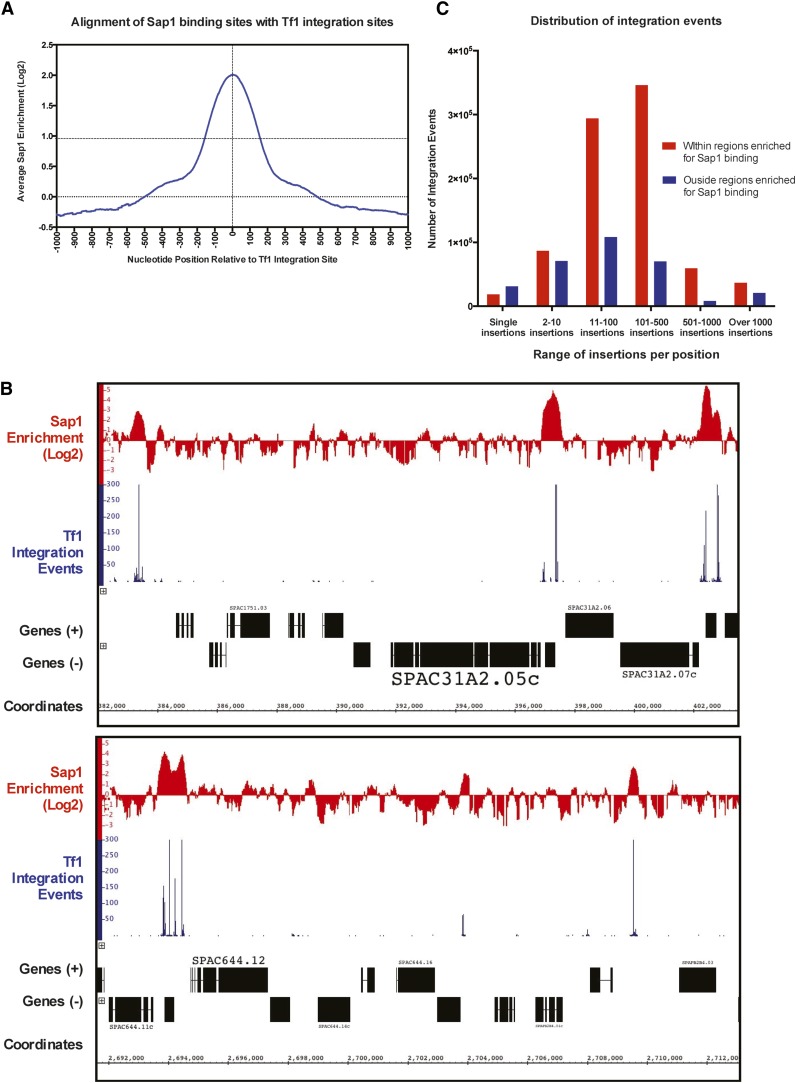
Tf1 insertion data lacking serial number measures of independent insertion at single-nucleotide positions (Guo and Levin 2010) were aligned with positions of Sap1 binding determined by ChIP-Seq (Zaratiegui et al. 2011). The alignment of the insertion sites revealed a peak of Sap1 binding approximately 60 bp from the integration sites (Zaratiegui et al. 2011). The correspondence between insertion sites and Sap1 binding in the genome suggested that Sap1 participates in integration. Here we performed a similar alignment using the previously reported positions of Sap1 binding (Zaratiegui et al. 2011) with Tf1 integration sites recently determined with the serial number system (Chatterjee et al. 2014). Sap1 enrichment throughout the S. pombe genome was determined by calculating the log2 ratio of Sap1 binding signal to that of the whole-cell-extract (WCE) control and was then tabulated relative to aligned Tf1 insertion positions. This analysis confirmed that Tf1 has a strong preference for integration into genomic positions of increased Sap1 binding (Figure 4A). No 60-bp offset between the insertion sites and Sap1 binding was observed.
Figure 4.
Tf1 prefers to integrate in Sap1-bound regions of the genome. (A) Graph showing the alignment of all genomic Tf1 insertion sites with the tabulated average of Sap1 enrichment (log2 ratio of Sap1 binding to WCE signal) at single-nucleotide positions within 1000 bp of the aligned insertion sites. (B) Representative regions of the genome showing that high integration sites align with regions of the genome enriched for Sap1 binding. (C) Graph showing the number of Tf1 insertions that occur within the indicated ranges of insertions per positions, as well as their occurrence in and outside Sap1-enriched regions in the genome.
The relationship between Sap1 binding and integration at individual genomic sequences revealed strong correlation between positions with high numbers of insertions and peaks of Sap1 enrichment (Figure 4B). To determine what fraction of integration events occurred at areas of Sap1 binding genome-wide, we identified all the regions of the S. pombe genome that were enriched twofold or greater for Sap1 binding (receiving a log2 score of 1) and then calculated the number of Tf1 insertions present within and outside these regions. While only 6.85% of the S. pombe genome was enriched above this twofold threshold for Sap1 binding, the vast majority of all integration events (73.1%) were found to lie within this fraction of the genome (Table 7).
Table 7. Tf1 integration positions are predominantly in regions of Sap1 binding.
| No. of nucleotide positions | Percentge of genome | No. of Tf1 integrations | Percentage of Tf1 integrations | |
|---|---|---|---|---|
| Within Sap1-enriched regions | 861,300 | 6.84 | 841,476 | 73.11 |
| Outside Sap1-enriched regions | 11,729,955 | 93.16 | 309,516 | 26.89 |
| Total | 12,591,255 | 100 | 1,150,992 | 100 |
In a detailed analysis that relied specifically on the serial number integration data, all Tf1 integration positions were sorted and grouped based on the number of independent insertions that occurred per position. Integration sites inside regions of Sap1 enrichment were analyzed separately from those outside Sap1-enriched regions. The number of insertions within each group and their location relative to regions of Sap1 enrichment were assessed (Figure 4C). Positions with single insertions are the most evenly distributed between regions with and without Sap1 binding, with most of them lying outside areas of Sap1 enrichment. However, the sites with single insertions constitute just 4.0% of all integration events. Positions with 2–10 independent insertions had more integration in regions of Sap1 binding than outside. This bias becomes significantly more pronounced as the number of insertions per positions increases, with almost no positions containing over 500 insertions found outside Sap1-enriched regions. These analyses argue that there is a very strong correlation between sites with high levels of integration and positions where Sap1 binding is enriched.
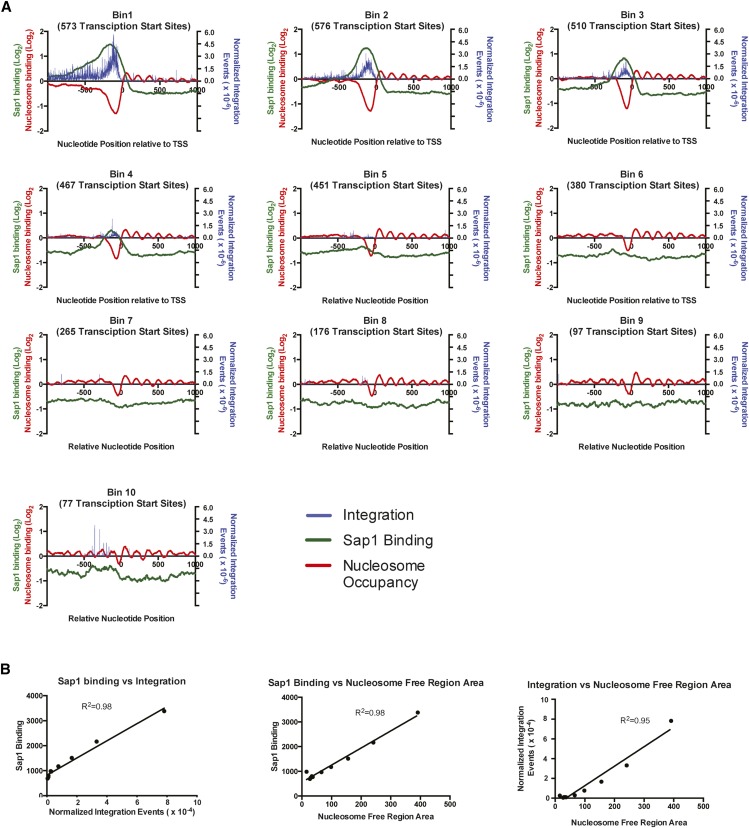
Sap1 binding has been demonstrated previously to align with NFRs upstream of RNA polemerase II transcription start sites (Tsankov et al. 2011). The association of regions of Sap1 binding with integration sites led us to ask whether high levels of Tf1 integration were associated with large NFRs. To address this question, we sorted all the intergenic sequences within the S. pombe genome based on the number of Tf1 insertions within them and binned these sequences into groups of 500. The transcription start sites (TSSs) in each bin were aligned. Relying on previously published data, the average nucleosome occupancy (de Castro et al. 2012), Sap1-binding (Zaratiegui et al. 2011), and the normalized average number of Tf1 insertions (Chatterjee et al. 2014) at each nucleotide position within 1 kb of these TSSs were tabulated. Most Tf1 integration events lie upstream of the TSSs and form a peak that closely matches the binding of Sap1 (Figure 5A). This pattern is most apparent in bins 1–5, which had the greatest number of integration events. Importantly, bin 1 with the highest number of integration events contained the greatest enrichment for Sap1 binding, which peaked upstream of the TSS and aligned well with the Tf1 integration positions containing the greatest number of insertions. Bin 1 also had the largest average NFR. Although similar patterns were observed in bins 2–4, the peaks of Sap1 binding were significantly diminished in each subsequent bin, corresponding with decreases in insertion events relative to the number of TSSs and decreases in the average NFR. Bin 5 had almost no enrichment for Sap1 binding and very little integration. Linear regression analysis performed on the sums for the nucleotides lying within 1000 bp upstream of the TSS for each data set in each bin demonstrated that there were particularly strong correlations between Tf1 integration, Sap1 binding (R2 = 0.9748), and the area of the NRFs (R2 = 0.9510) (Figure 5B).
Figure 5.
Tf1-insertion and Sap1-binding regions both align with NFRs at TSSs. (A) Graphs showing the alignment of all TSSs within 10 bins of intergenic regions from the S. pombe genome. The bins are sorted based on the number of Tf1 insertions occurring within the intergenic regions and are arranged in descending order. For each bin, the average number of Tf1 insertions and average value of Sap1 enrichment (log2 ratio of Sap1 binding to WCE signal) and nucleosome occupancy are plotted on the y-axis at each nucleotide position within 1000 bp of the aligned TSSs. For these analyses, the number of Tf1 insertions was normalized by dividing the amount of integration of each position by the total number of Tf1 insertions. (B) Graphs showing the pairwise comparisons and linear regression analysis of the sum of Tf1 integration, Sap1 enrichment, and area of NFRs that occur 1000 bp upstream of all TSSs within each bin. For these analyses, Sap1 enrichment values were back-transformed into their original non-log2 number, and only the negative values of nucleosome occupancy data were used to sum the total area of NFRs.
Tf1 insertions cluster around the Ter1 sequence in ribosomal DNA (rDNA) repeats but not around switch-activating site 1 (SAS1) in the mating locus
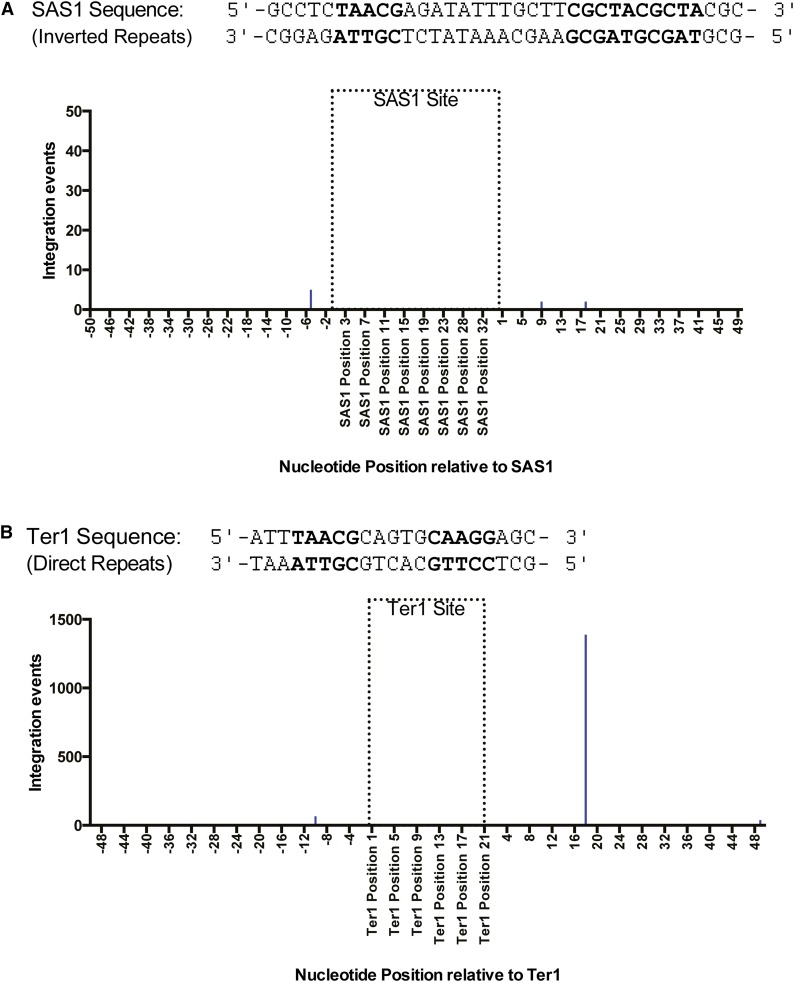
Sap1 has been previously reported to bind specific sequences in both the mating locus and the rDNA repeats in S. pombe. In the rDNA repeats, Sap1 binds a direct repeat known as Ter1, where it initiates replication fork arrest and acts as a fork barrier (Krings and Bastia 2005, 2006; Mejia-Ramirez et al. 2005). In the mating locus, Sap1 binds SAS1, an inverted repeat, where it facilitates mating type switching (Arcangioli and Klar 1991, Krings and Bastia 2006). Examination of the Ter1 loci was challenging because these sites are within repetitive DNA located inside the rDNA genes (Mejia-Ramirez et al. 2005). While this makes it impossible to differentiate integration positions within specific Ter1 loci, the combined integration of all Ter1 sequences can be analyzed. As expected, both the SAS1 sequence in the mating loci and the Ter1 sequences in the rDNA repeats, as well as the surrounding regions, are enriched for Sap1 binding. However, very few Tf1 insertions were found in or near the SAS1 site, with the greatest number of insertions per position amounting to only 5, which is small considering that single-nucleotide positions elsewhere can have well over 1000 insertions (Figure 6A). By contrast, regions surrounding the Ter1 sequence contained high numbers of Tf1 insertions (Figure 6B). While relatively few insertions were within the Ter1 sequence itself, a single-nucleotide position containing over 1300 insertion events was found to be located 17 bp downstream of the Ter1 sequence 3′ end (Figure 6).
Figure 6.
Tf1 insertions cluster around the Ter1 motif in rDNA repeats but not the SAS1 site in the mating locus. (A) Top: Sequence of both strands of the SAS1 element. Bottom: Graph showing the number of Tf1 insertions at single-nucleotide positions within 1000 pb of the SAS1 sequence. (B) Top: Sequence of both strands of the Ter1 element. Bottom: Graph showing the alignment of the three published Ter1 motifs (Pombase.com) and the tabulated number of Tf1 insertions present at single-nucleotide positions within 1000 bp of the aligned Ter1 motifs. The nucleotides in bold indicate nucleotides within core motifs of the Ter1 and Sas1 sequence, as identified by Krings and Bastia (2006).
Tf1 insertion sites cluster at the Sap1-binding motif
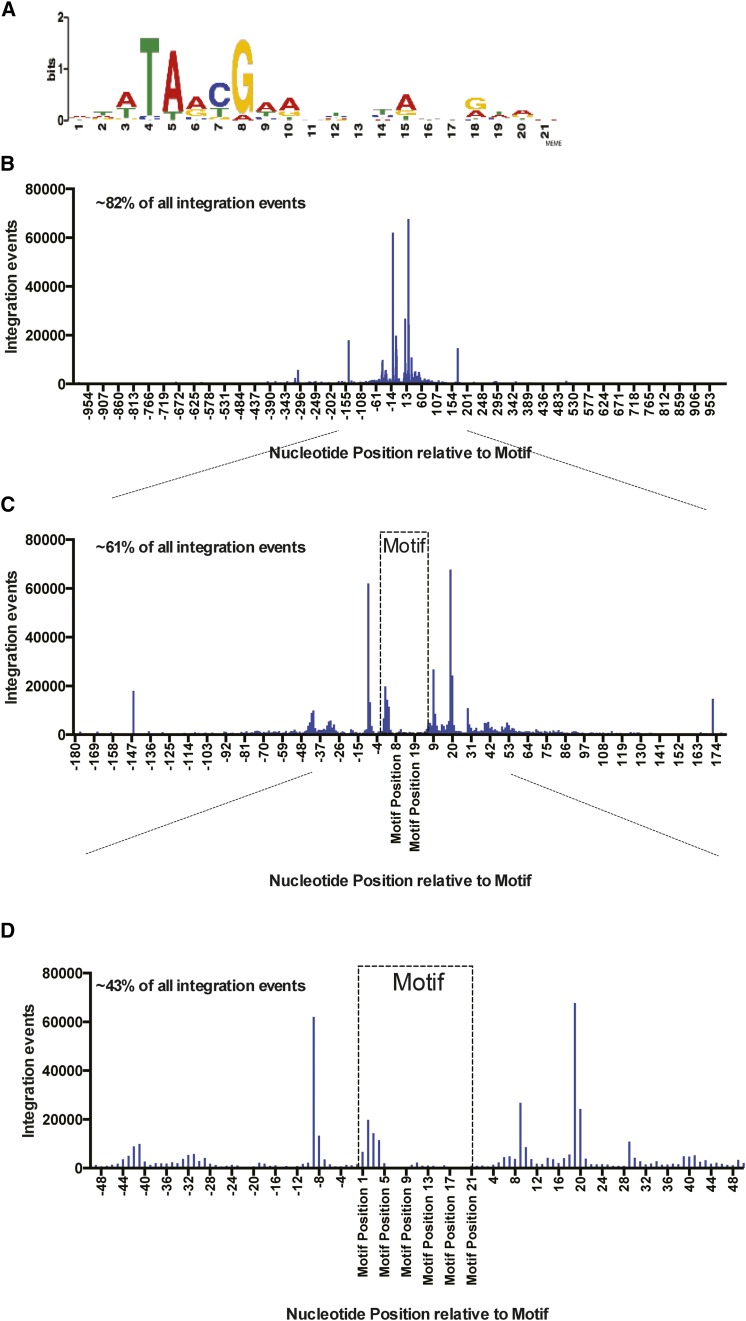
Most Tf1 integration is located within genomic regions enriched for Sap1 binding. If Sap1 were directly responsible for positioning Tf1 integration, we would expect that integration would take place at specific nucleotide positions relative to the nucleotides bound by Sap1. The resolution of Sap1-binding sites provided by ChIP-Seq is not sufficient to determine the specific nucleotides bound by Sap1. To identify precise sites of Sap1 binding, we determined a motif sequence by processing Sap1 ChIP-Seq reads with the MEME Suite (Bailey et al. 2009). The resulting motif presented in Logo form was 21 bp and shared similarity with the Ter1 sequence (Figure 7A) (Krings and Bastia 2005, 2006) and also shares strong similarity with previously published Sap1-binding motifs (Zaratiegui et al. 2011). To identify positions within the S. pombe genome containing the Sap1-binding motif, the FIMO program of the MEME Suite (Grant et al. 2011) was used to perform genomic searches, and these identified 5013 locations that matched this motif. The alignment of all these motifs revealed that 82% of all integration events cluster within 1 kb of this motif (Figure 7B). Importantly, a large fraction of the integration events mapped to four positions, all of which occurred within 20 bp of the motif (Figure 7C). The two largest single-nucleotide hotspots of integration occur either 9 bp upstream or 19 bp downstream of the motif, demonstrating an asymmetrical pattern of integration around the motif (Figure 7D). In addition, the sites with fewer insertions form a sine wave pattern, which is more clearly defined downstream of the motif, with single-nucleotide peaks appearing at approximate 10-bp intervals. Of the insertions that do occur within the motif, most are located asymmetrically toward the 5′ end of the motif.
Figure 7.
Tf1 insertions cluster at specific nucleotide positions around Sap1-binding motifs. (A) Logo of a Sap1 DNA-binding motif identified using the MEME Suite. (B) Graph showing the alignment of ∼5000 genomic Sap1 motifs that were identified using FIMO of the MEME Suite. The tabulated numbers of Tf1 insertions that occur at single-nucleotide positions within 1000 bp of the aligned motifs are plotted on the y-axis. Approximately 82% of all Tf1 insertion events occur within 1000 bp of a Sap1 motif. (C and D) Zoomed-in regions of the graph displayed in B showing the tabulated number of Tf1 insertions that occur at single-nucleotide positions within 180 (C) and 50 bp (D) of the aligned Sap1 motifs. Approximately 61% of all Tf1 insertions occur with 180 bp of a Sap1 motif, and approximately 43% of insertions occur within 50 bp of a Sap1 motif.
The serial number measures of integration analyzed earlier were assayed at 32° (Chatterjee et al. 2014). We also mapped Tf1 integration relative to the Sap1 motif using serial number data measured at 25° in the profiles reported here. In integration relative to the Sap1 motif, we saw no changes in the pattern from assays conducted at 32° with sap1+ cells, at 25° with sap1+ cells, or at 25° with sap1-1 cells (Figure S4).
Sap1 binding to chromosomal DNA is not sufficient to mediate Tf1 integration
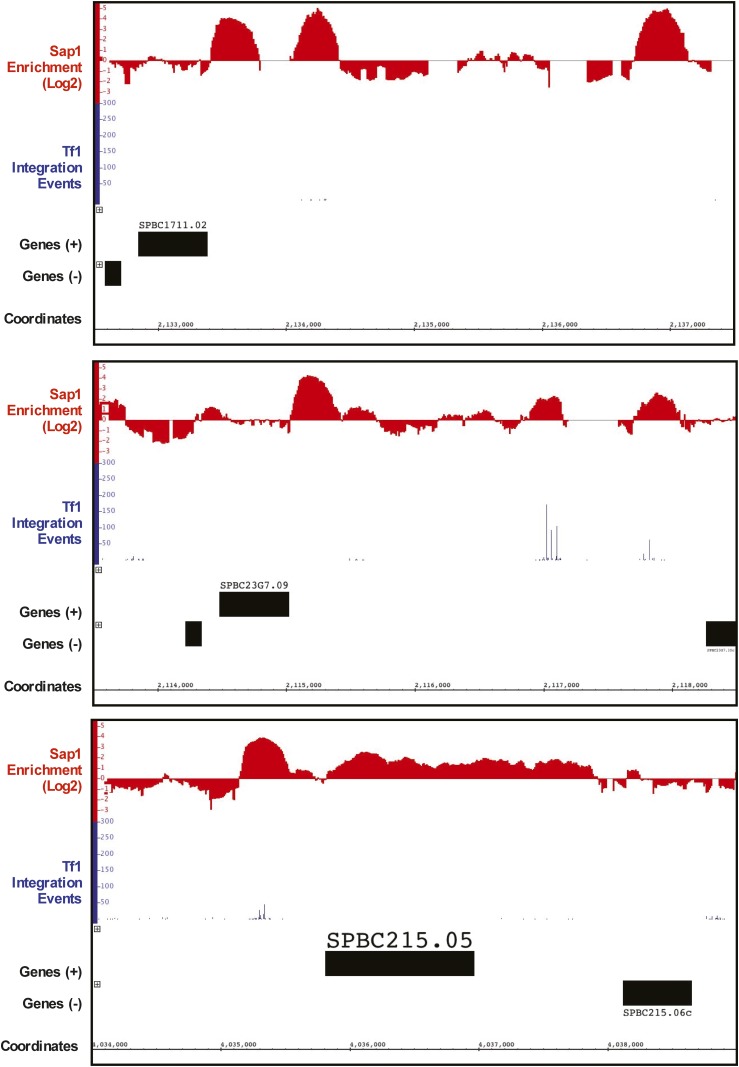
The finding that 73.1% of insertion events occurred at sites where Sap1 binding is enriched together with the substantial reduction in integration caused by the sap1-1 mutation indicates that Sap1 promotes integration. We also evaluated whether Sap1 binding to DNA was sufficient to mediate integration. Inspection of the ChIP-Seq data of Sap1 identified some genomic sequences bound by high levels of Sap1 but that had little Tf1 integration (Figure 8). To quantitate what fraction of Sap1-enriched sequences contained Tf1 insertions, all peaks of Sap1 binding that were enriched two-fold or more were grouped based on the numbers of inserts they contained. Surprisingly, of a total of 7819 peaks of Sap1 enrichment, 5153 (66.0%) contained no integration events (Table 8), and 812 (10.4%) Sap1 peaks contained between 1 and 10 inserts, 483 (6.2%) peaks contained between 11 and 100 inserts, 1152 (14.7%) Sap1 peaks contained between 101 and 1000 inserts, and 219 (2.8%) Sap1 peaks contained 1001 or more Tf1 integration events. Combining the Sap1 peaks containing between 0 and 10 inserts constitutes a full 76.3% of all peaks, but these include just 0.2% of the integration events. The remaining 23.2% of the Sap1 peaks with 11 or more insertions contained 99.7% of all inserts present in Sap1-enriched sequences.
Figure 8.
Sap1 binding is necessary but not sufficient for Tf1 integration. These regions of the S. pombe genome show large Sap1 peaks containing few to no Tf1 insertions. Note that the top two panels shows tall Sap1-binding peaks that have no insertion events, while the bottom panels shows a broad Sap1-binding peak with no insertion events.
Table 8. Sorting of Sap1 peaks based on the number of Tf1 integrations per peak.
| Part A | ||||||
|---|---|---|---|---|---|---|
| Integrations per Sap1 peak | No. of Sap1 peaks | Percentage of Sap1 peaks | No. of integrations | Percent of integrations in Sap1-enriched regions | Percent of total integrations in the genome | Average number of integrations per peak |
| 0 | 5153 | 65.90 | 0 | 0.00 | 0.00 | 0.00 |
| 1–10 | 812 | 10.38 | 2,311 | 0.27 | 0.20 | 2.85 |
| 11–100 | 483 | 6.18 | 22,048 | 2.62 | 1.92 | 45.79 |
| 101–1000 | 1152 | 14.73 | 450,977 | 53.59 | 39.18 | 391.47 |
| 1001 and up | 219 | 2.80 | 366,140 | 43.51 | 31.81 | 1671.87 |
| Total | 7819 | 100.00 | 841476 | 100.00 | 73.11 | NA |
| Part B | ||||||
|---|---|---|---|---|---|---|
| Integrations per Sap1 peak | Average peak length (nt) | Total peak length (nt) | Percentage of peak length | Average peak area | Total peak area | Percentage of peak area |
| 0 | 41.97 | 216,270 | 25.11 | 55.44 | 285,685.52 | 16.36 |
| 1–10 | 93.12 | 75,370 | 8.75 | 136.56 | 110,887.00 | 6.35 |
| 11–100 | 178.43 | 86,180 | 10.01 | 332.44 | 160,568.53 | 9.20 |
| 101–1000 | 315.58 | 361,940 | 42.02 | 733.69 | 840,509.96 | 48.15 |
| 1001 and up | 554.98 | 121,540 | 14.11 | 1589.67 | 348,137.61 | 19.94 |
| Total | NA | 861,300 | 100 | NA | 1,745,788.616 | 100 |
To determine what features distinguish the Sap1 peaks with high numbers of insertion events, we analyzed the size of the peaks. The Sap1-binding peaks with zero inserts averaged 42 nt in length and 55.4 (arbitrary units) in area, measures that are approximately two-fold smaller than the averages for peaks containing 1 to 10 insertions, suggesting that a threshold level of Sap1 binding is required to induce integration (Table 8). Similarly, the Sap1 peaks containing 11 to 100 inserts averaged 45.8 inserts per peak, a level 16-fold greater than Sap1 peaks with 1 to 10 insertions. Importantly, the Sap1 peaks with 11 to 100 inserts had average lengths and areas approximately two-fold greater than the peaks with 1 to 10 insertions. This trend of increasing integration events with larger Sap1 peaks continued through the Sap1 peaks containing 1001 or more insertions, which averaged 1672 inserts per peak and had average lengths of 545 nt. These numbers indicate that Tf1 integration was associated with Sap1 peaks that had greater than a threshold length and area.
Discussion
Our data demonstrate that Sap1 is important for Tf1 transposition and indicate that Sap1 plays a role in the integration of the element. This conclusion is based on the substantial decrease in Tf1 transposition in sap1-1 mutant cells and the presence of transposition intermediates in these cells (Figure 1).
The alignment of Sap1-binding sites with Tf1 inserts is consistent with a role of Sap1 in integration (Zaratiegui et al. 2011) (Figure 4A). Further, the highly quantitative measures of integration provided by the serial number system reveal that Sap1-enriched DNA sequences accounted for 73.4% of all integration events. Importantly, serial number data showed that integration positions with high numbers of independent insertions were much more likely to coincide with Sap1-binding sites than positions with low numbers of inserts, indicating that Sap1 contributes to the efficiency of integration (Figure 4C). Our finding that the sap1-1 mutation greatly reduced integration frequency without causing significant changes in the location of integration also argues that Sap1 promotes the efficiency of integration. One possible contribution Sap1 may make to integration efficiency is nucleosome occlusion. The striking correlation between Sap1 binding, NFR size, and integration at promoters is consistent with a function of Sap1 that excludes nucleosomes and allows the integration complex access to target sites. This model is supported by the finding that a mutation in sap1 causes increased nucleosome occupancies at the promoters that Sap1 binds (Tsankov et al. 2011).
Sap1 also may be playing a direct role in integration by positioning insertion sites. In this case, the sap1-1 mutation would be expected to alter the profile of integration sites. However, the sap1-1 mutation did not cause substantial alterations in the profile. The sap1-1 mutation is in domain IV, a region important for dimerization (Noguchi and Noguchi 2007; Ghazvini et al. 1995). Therefore, the mutation may reduce levels of Sap1 dimer bound to DNA and not the positions where it binds. This would explain why the mutation did not cause large changes in the integration profile. The highly specific clustering of integration events at positions +19 and −9 bp relative to the Sap1-binding motif provides strong support for the model that Sap1 is responsible for positioning integration events (Figure 7C).
The clustering of inserts at single-nucleotide sites flanking the Sap1 motif would be expected to occur if Sap1 covers its binding site on the DNA and directs integration to sites on either side of the protein. Sap1 binds as a head-to-tail dimer to two direct repeats in the Ter1 sequence. On binding this sequence, the Sap1 dimer bends the DNA and distorts the DNA helix, a process that is required for Sap1-mediated replication fork arrest (Bada et al. 2000; Krings and Bastia 2005; 2006). If Sap1 molecules were to bind the motif sequence near insertion sites in the same manner, IN interaction with equivalent regions of each head-to-tail dimer could explain the asymmetry in the single-nucleotide clusters flanking the Sap1 motif. Thus far we have been unable to demonstrate an interaction between Sap1 and Tf1 IN with pull-down assays. This could be because post-translational modifications of IN or Sap1 are required. But the in vivo two-hybrid assay provided support for a Sap1:IN interaction that could directly position integration by a tethering mechanism similar to those of HIV-1, Ty1, Ty3, and Ty5. Finally, a third possibility that must be considered is that an additional host factor interacts with both Sap1 and Tf1 IN/cDNA and forms a bridge in the complex that mediates integration.
The sine wave pattern of integration near the Sap1 motif could be indicative of integration occurring as a result of helical distortion induced by Sap1 binding. The peaks of integration within these sine waves occur in ∼10-bp intervals, the approximate length of a DNA helical turn (Wang et al. 1979). Perhaps the binding of a Sap1 dimer to the DNA results in conformational changes at regular intervals along the DNA helix, which makes certain regions of each helical turn more amenable to Tf1 insertion.
Not all Sap1-binding sites appear to be equal in terms of efficiency of Tf1 insertion, which suggests that Sap1 binding to genomic target sites is important but not sufficient for integration. Fully 25.1% of the genomic sequences enriched for Sap1 binding (total peak length) have no integration events (Table 8). These Sap1-enriched peaks lacking integration indicate that Sap1 binding is not the only feature needed to mediate integration. Conversely, not all integration occurs in Sap1-enriched peaks. Twenty-six percent of the Tf1 insertions occur outside the sequences enriched twofold for Sap1 binding. Even if all sequences with any level of detectable Sap1 enrichment are considered, 13% of the insertions fall outside Sap1-bound peaks (Figure S3 and Table S2). While these data argue for a Sap1-independent mechanism of integration, it is formally possible that the differences in the culture conditions used for the ChIP-Seq experiments compared to the transposition cultures could account for the integration that occurred outside Sap1-binding sites. Additionally, insertion sites with single integration events were not enriched in Sap1-bound sequences, suggesting that these single insertion sites may represent random events that also use a Sap1-independent mechanism.
The integration of Tf1 is known to recognize the promoters of stress-response genes (Guo and Levin 2010; Chatterjee et al. 2014). The binding of Sap1 to targets of Tf1 integration raises the possibility that Sap1 may play a role in responding to environmental stress. Sap1 is known to be able to interrupt the migration of replication forks (Arcangioli and Klar 1991; Krings and Bastia 2005, 2006; Mejia-Ramirez et al. 2005; Noguchi and Noguchi 2007). Future studies of Sap1 will be needed to determine whether its ability to block replication forks functions in response to stress and whether its role in Tf1 integration is regulated by stress.
Supplementary Material
Acknowledgments
We thank Amnon Hizi for purifying Sap1 protein, conducting pull-down experiments, and providing helpful discussions. We thank Yabin Guo for the use of his computer programs and scripts in the bioinformatic analysis of our data. We thank Chanan Rubin for his helpful contributions. This research was supported by the Intramural Research Programs of the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (H.L.L.), and the National Cancer Institute (S.I.S.G.). P.G.M. was supported by the Intramural Research Program of the National Institutes of Health, Center for Information Technology. This study used the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD (http://biowulf.nih.gov).
Footnotes
Communicating editor: D. Voytas
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.181602/-/DC1
Literature Cited
- Arcangioli B., Copeland T. D., Klar A. J., 1994. Sap1, a protein that binds to sequences required for mating-type switching, is essential for viability in Schizosaccharomyces pombe. Mol. Cell. Biol. 14: 2058–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B., Klar A. J., 1991. A novel switch-activating site (SAS1) and its cognate binding factor (SAP1) required for efficient mat1 switching in Schizosaccharomyces pombe. EMBO J. 10: 3025–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood A., Choi J., Levin H. L., 1998. The application of a homologous recombination assay revealed amino acid residues in an LTR-retrotransposon that were critical for integration. J. Virol. 72: 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood A., Lin J. H., Levin H. L., 1996. The retrotransposon Tf1 assembles virus-like particles that contain excess Gag relative to integrase because of a regulated degradation process. Mol. Cell. Biol. 16: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood-Moore A., Yan K., Judson R. L., Levin H. L., 2006. The self primer of the long terminal repeat retrotransposon Tf1 is not removed during reverse transcription. J. Virol. 80: 8267–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada M., Walther D., Arcangioli B., Doniach S., Delarue M., 2000. Solution structural studies and low-resolution model of the Schizosaccharomyces pombe Sap1 protein. J. Mol. Biol. 300: 563–574. [DOI] [PubMed] [Google Scholar]
- Bailey T. L., Boden M., Buske F. A., Frith M., Grant C. E., et al. , 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37: W202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens R., Hayles J., Nurse P., 2000. Fission yeast retrotransposon Tf1 integration is targeted to 5’ ends of open reading frames. Nucleic Acids Res. 28: 4709–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Trueheart J., Natsoulis G., Fink G. R., 1987. 5-Fluoro-orotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175. [DOI] [PubMed] [Google Scholar]
- Bowen N. J., Jordan I., Epstein J., Wood V., Levin H. L., 2003. Retrotransposons and their recognition of pol II promoters: a comprehensive survey of the transposable elements derived from the complete genome sequence of Schizosaccharomyces pombe. Genome Res. 13: 1984–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridier-Nahmias A., Tchalikian-Cosson A., Baller J. A., Menouni R., Fayol H., et al. , 2015. Science 348(6234): 585–8. [DOI] [PubMed] [Google Scholar]
- Chalker D. L., Sandmeyer S. B., 1990. Transfer RNA genes are genomic targets for de novo transposition of Ty3. Genetics 126: 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker D. L., Sandmeyer S. B., 1992. Ty3 integrates within the region of RNA polymerase III transcription initiation. Genes Dev. 6: 117–128. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. G., Esnault C., Guo Y., Hung S., McQueen P. G., et al. , 2014. Serial number tagging reveals a prominent sequence preference of retrotransposon integration. Nucleic Acids Res. 42: 8449–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffi A., Llano M., Poeschla E., Hoffmann C., Leipzig J. et al, 2005. A role for LEDGF/p75 in targeting HIV DNA integration. Nat Med. 11: 1287–1289. [DOI] [PubMed] [Google Scholar]
- de Castro E., Soriano I., Marin L., Serrano R., Quintales L., et al. , 2012. Nucleosomal organization of replication origins and meiotic recombination hotspots in fission yeast. EMBO J. 31: 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine S. E., Boeke J. D., 1996. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes Dev. 10: 620–633. [DOI] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., et al. , 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7(4): 555–69. [DOI] [PubMed] [Google Scholar]
- Feng G., Leem Y. E., Levin H. L., 2013. Transposon integration enhances expression of stress response genes. Nucleic Acids Res. 41: 775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Rhind N., 2006. Basic methods for fission yeast. Yeast 23: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai X., Voytas D. F., 1998. A single amino acid change in the yeast retrotransposon Ty5 abolishes targeting to silent chromatin. Mol. Cell 1: 1051–1055. [DOI] [PubMed] [Google Scholar]
- Ghazvini M., Ribes V., Arcangioli B., 1995. The essential DNA-binding protein sap1 of Schizosccharomyces pombe contains two independent oligomerization interfaces that dictate the relative orientation of the DNA-binding domain. Mol. Cell. Biol. 15: 4939–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. E., Bailey T. L., Noble W. S., 2011. FIMO: scanning for occurrences of a given motif. Bioinformatics 27: 1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Levin H. L., 2010. High-throughput sequencing of retrotransposon integration provides a saturated profile of target activity in Schizosaccharomyces pombe. Genome Res. 20: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. S., Maetzig T., Maertens G. N., Sharif A., Rothe M., et al. , 2013. Bromo and ET domain (BET) chromatin regulators serve as co-factors for murine leukemia virus integration. J. Virol. 87: 12721–12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff E. F., Levin H. L., Boeke J. D., 1998. Schizosaccharomyces pombe retrotransposon Tf2 mobilizes primarily through homologous cDNA recombination. Mol. Cell. Biol. 18: 6839–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Moore D. P., Blomberg M. A., Braiterman L. T., Voytas D. F., et al. , 1993. Hotspots for unselected Ty1 transposition events on yeast chromosome III are near tRNA genes and LTR sequences. Cell 73: 1007–1018. [DOI] [PubMed] [Google Scholar]
- Kirchner J., Connolly C. M., Sandmeyer S. B., 1995. Requirement of RNA polymerase III transcription factors for in vitro position-specific integration of a retroviruslike element [see comments]. Science 267: 1488–1491. [DOI] [PubMed] [Google Scholar]
- Krings G., Bastia D., 2005. Sap1p binds to Ter1 at the ribosomal DNA of Schizosaccharomyces pombe and causes polar replication fork arrest. J. Biol. Chem. 280: 39135–39142. [DOI] [PubMed] [Google Scholar]
- Krings G., Bastia D., 2006. Molecular architecture of a eukaryotic DNA replication terminus-terminator protein complex. Mol. Cell. Biol. 26: 8061–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem Y. E., Ripmaster T. L., Kelly F. D., Ebina H., Heincelman M. E., et al. , 2008. Retrotransposon Tf1 is targeted to pol II promoters by transcription activators. Mol. Cell 30: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage P., Todeschini A. L., 2005. Happy together: the life and times of Ty retrotransposons and their hosts. Cytogenet. Genome Res. 110: 70–90. [DOI] [PubMed] [Google Scholar]
- Levin H. L., 1995. A novel mechanism of self-primed reverse transcription defines a new family of retroelements. Mol. Cell. Biol. 15: 3310–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H. L., 1996. An unusual mechanism of self-primed reverse transcription requires the RNase H domain of reverse transcriptase to cleave an RNA duplex. Mol. Cell. Biol. 16: 5645–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H. L., Moran J. V., 2011. Dynamic interactions between transposable elements and their hosts. Nat. Rev. Genet. 12: 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H. L., Weaver D. C., Boeke J. D., 1993. Novel gene expression mechanism in a fission yeast retroelement: Tf1 proteins are derived from a single primary translation product. EMBO J. 12: 4885–4895 [erratum: EMBO J 13: 1494 (1994)]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano M., Vanegas M., Hutchins N., Thompson D., Delgado S., et al. , 2006. Identification and characterization of the chromatin-binding domains of the HIV-1 integrase interactor LEDGF/p75. J. Mol. Biol. 360: 760–773. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Chatterjee A. G., Ripmaster T. L., Levin H. L., 2011. The determinants that specify the integration pattern of retrotransposon Tf1 in the fbp1 promoter of Schizosaccharomyces pombe. J. Virol. 85: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Ramirez E., Sanchez-Gorostiaga A., Krimer D. B., Schvartzman J. B., Hernandez P., 2005. The mating type switch-activating protein Sap1 Is required for replication fork arrest at the rRNA genes of fission yeast. Mol. Cell. Biol. 25: 8755–8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., 1972. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Moore S. P., Liti G., Stefanisko K. M., Nyswaner K. M., Chang C., et al. , 2004. Analysis of a Ty1-less variant of Saccharomyces paradoxus: the gain and loss of Ty1 elements. Yeast 21: 649–660. [DOI] [PubMed] [Google Scholar]
- Noguchi C., Noguchi E., 2007. Sap1 promotes the association of the replication fork protection complex with chromatin and is involved in the replication checkpoint in Schizosaccharomyces pombe. Genetics 175: 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Sandmeyer S., 2012. In vitro targeting of strand transfer by the Ty3 retroelement integrase. J. Biol. Chem. 287: 18589–18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S., 2003. Integration by design. Proc. Natl. Acad. Sci. USA 100: 5586–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifele L. Z., Cost G. J., Zupancic M. L., Caputo E. M., Boeke J. D., 2009. Retrotransposon overdose and genome integrity. Proc. Natl. Acad. Sci. USA 106: 13927–13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Larue R. C., Plumb M. R., Malani N., Male F., et al. , 2013. BET proteins promote efficient murine leukemia virus integration at transcription start sites. Proc. Natl. Acad. Sci. USA 110: 12036–12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun M. C., Raghavendra N. K., Vandegraaff N., Daigle J. E., Hughes S., et al. , 2007. LEDGF/p75 functions downstream from preintegration complex formation to effect gene-specific HIV-1 integration. Genes Dev. 21: 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton T. L., Levin H. L., 2002. A long terminal repeat retrotransposon of fission yeast has strong preferences for specific sites of insertion. Eukaryot. Cell 1: 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studamire B., Goff S. P., 2008. Host proteins interacting with the Moloney murine leukemia virus integrase: multiple transcriptional regulators and chromatin binding factors. Retrovirology 5: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teysset L., Dang V. D., Kim M. K., Levin H. L., 2003. A long terminal repeat-containing retrotransposon of Schizosaccharomyces pombe expresses a Gag-like protein that assembles into virus-like particles which mediate reverse transcription. J. Virol. 77: 5451–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankov A., Yanagisawa Y., Rhind N., Regev A., Rando O. J., 2011. Evolutionary divergence of intrinsic and trans-regulated nucleosome positioning sequences reveals plastic rules for chromatin organization. Genome Res. 21: 1851–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., 1979. Helical repeat of DNA in solution. Proc. Natl. Acad. Sci. USA. 76: 200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Gai X., Zhu Y., Zappulla D. C., Sternglanz R., et al. , 2001. Targeting of the yeast Ty5 retrotransposon to silent chromatin is mediated by interactions between integrase and Sir4p. Mol. Cell. Biol. 21: 6606–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yieh L., Hatzis H., Kassavetis G., Sandmeyer S. B., 2002. Mutational analysis of the transcription factor IIIB-DNA target of Ty3 retroelement integration. J. Biol. Chem. 277: 25920–25928. [DOI] [PubMed] [Google Scholar]
- Yieh L., Kassavetis G., Geiduschek E. P., Sandmeyer S. B., 2000. The Brf and TATA-binding protein subunits of the RNA polymerase III transcription factor IIIB mediate position-specific integration of the gypsy-like element, Ty3. J. Biol. Chem. 275: 29800–29807. [DOI] [PubMed] [Google Scholar]
- Zaratiegui M., Vaughn M. W., Irvine D. V., Goto D., Watt S., et al. , 2011. CENP-B preserves genome integrity at replication forks paused by retrotransposon LTR. Nature 469: 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. X., Zou S. G., Wright D. A., Voytas D. F., 1999. Tagging chromatin with retrotransposons: target specificity of the Saccharomyces Ty5 retrotransposon changes with the chromosomal localization of Sir3p and Sir4p. Genes Dev. 13: 2738–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S., Ke N., Kim J. M., Voytas D. F., 1996. The Saccharomyces retrotransposon Ty5 integrates preferentially into regions of silent chromatin at the telomeres and mating loci. Genes Dev. 10: 634–645. [DOI] [PubMed] [Google Scholar]
- Zou S., Voytas D. F., 1997. Silent chromatin determines target preference of the Saccharomyces retrotransposon Ty5. Proc. Natl. Acad. Sci. USA 94: 7412–7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All strains, plasmids, and computer programs/scripts are available upon request. Sequence reads were submitted to the Short Read Archive (SRA) at National Center for Biotechnology Information (NCBI) under the accession number PRJNA279274.