Abstract
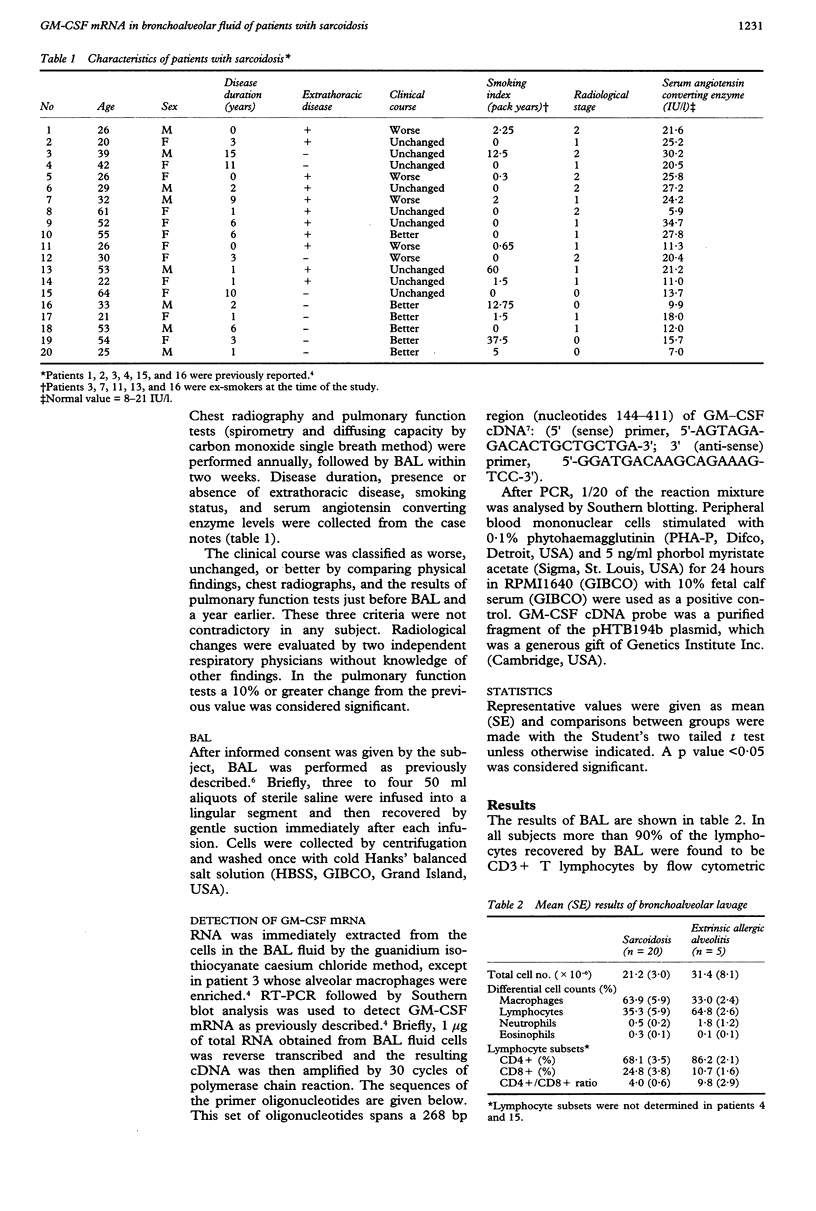
BACKGROUND--Granulocyte-macrophage colony stimulating factor (GM-CSF) has several proinflammatory effects, some of which potentially favour granuloma formation. Its mRNA expression by the inflammatory cells recovered from lungs of patients with pulmonary sarcoidosis has been previously reported. In this study an association between GM-CSF expression and manifestations of the disease was explored. METHODS--GM-CSF mRNA was detected by reverse transcription polymerase chain reaction in the cells of bronchoalveolar lavage (BAL) fluid of 20 patients with pulmonary sarcoidosis. RESULTS--GM-CSF mRNA expression was positive in 15 of 20 patients with sarcoidosis. Fourteen of the 15 patients with positive mRNA expression had worsening or unchanged disease during the year preceding this study, on the basis of radiographic or physical findings, or both, whereas all five "negative" patients were judged to be improving. Similarly, serum levels of angiotensin converting enzyme, the proportion of lymphocytes in BAL fluid, and the CD4+/CD8+ ratio of lymphocytes in BAL fluid were significantly higher in the positive patients. CONCLUSIONS--There was an association between the presence of GM-CSF mRNA in the cells in BAL fluid and other indices of disease activity in sarcoidosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughman R. P., Strohofer S. A., Buchsbaum J., Lower E. E. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J Lab Clin Med. 1990 Jan;115(1):36–42. [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier Y., Bélanger J., Laviolette M. Prognostic significance of bronchoalveolar lymphocytosis in farmer's lung. Am Rev Respir Dis. 1987 Mar;135(3):692–695. doi: 10.1164/arrd.1987.135.3.692. [DOI] [PubMed] [Google Scholar]
- Costabel U., Bross K. J., Guzman J., Nilles A., Rühle K. H., Matthys H. Predictive value of bronchoalveolar T cell subsets for the course of pulmonary sarcoidosis. Ann N Y Acad Sci. 1986;465:418–426. doi: 10.1111/j.1749-6632.1986.tb18518.x. [DOI] [PubMed] [Google Scholar]
- DeRemee R. A., Rohrbach M. S. Serum angiotensin-converting enzyme activity in evaluating the clinical course of sarcoidosis. Ann Intern Med. 1980 Mar;92(3):361–365. doi: 10.7326/0003-4819-92-3-361. [DOI] [PubMed] [Google Scholar]
- Howell C. J., Pujol J. L., Crea A. E., Davidson R., Gearing A. J., Godard P., Lee T. H. Identification of an alveolar macrophage-derived activity in bronchial asthma that enhances leukotriene C4 generation by human eosinophils stimulated by ionophore A23187 as a granulocyte-macrophage colony-stimulating factor. Am Rev Respir Dis. 1989 Nov;140(5):1340–1347. doi: 10.1164/ajrccm/140.5.1340. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Itoh A., Yamaguchi E., Kuzumaki N., Okazaki N., Furuya K., Abe S., Kawakami Y. Expression of granulocyte-macrophage colony-stimulating factor mRNA by inflammatory cells in the sarcoid lung. Am J Respir Cell Mol Biol. 1990 Sep;3(3):245–249. doi: 10.1165/ajrcmb/3.3.245. [DOI] [PubMed] [Google Scholar]
- Kelso A., Metcalf D. T lymphocyte-derived colony-stimulating factors. Adv Immunol. 1990;48:69–105. doi: 10.1016/s0065-2776(08)60752-x. [DOI] [PubMed] [Google Scholar]
- Keogh B. A., Crystal R. G. Alveolitis: the key to the interstitial lung disorders. Thorax. 1982 Jan;37(1):1–10. doi: 10.1136/thx.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreipe H., Radzun H. J., Heidorn K., Barth J., Kiemle-Kallee J., Petermann W., Gerdes J., Parwaresch M. R. Proliferation, macrophage colony-stimulating factor, and macrophage colony-stimulating factor-receptor expression of alveolar macrophages in active sarcoidosis. Lab Invest. 1990 Jun;62(6):697–703. [PubMed] [Google Scholar]
- Lee M. T., Kaushansky K., Ralph P., Ladner M. B. Differential expression of M-CSF, G-CSF, and GM-CSF by human monocytes. J Leukoc Biol. 1990 Mar;47(3):275–282. doi: 10.1002/jlb.47.3.275. [DOI] [PubMed] [Google Scholar]
- Lem V. M., Lipscomb M. F., Weissler J. C., Nunez G., Ball E. J., Stastny P., Toews G. B. Bronchoalveolar cells from sarcoid patients demonstrate enhanced antigen presentation. J Immunol. 1985 Sep;135(3):1766–1771. [PubMed] [Google Scholar]
- Nakata K., Akagawa K. S., Fukayama M., Hayashi Y., Kadokura M., Tokunaga T. Granulocyte-macrophage colony-stimulating factor promotes the proliferation of human alveolar macrophages in vitro. J Immunol. 1991 Aug 15;147(4):1266–1272. [PubMed] [Google Scholar]
- Oster W., Lindemann A., Mertelsmann R., Herrmann F. Production of macrophage-, granulocyte-, granulocyte-macrophage- and multi-colony-stimulating factor by peripheral blood cells. Eur J Immunol. 1989 Mar;19(3):543–547. doi: 10.1002/eji.1830190320. [DOI] [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruef C., Coleman D. L. Granulocyte-macrophage colony-stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis. 1990 Jan-Feb;12(1):41–62. doi: 10.1093/clinids/12.1.41. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Yamaguchi E., Okazaki N., Itoh A., Furuya K., Abe S., Kawakami Y. Enhanced expression of CD2 antigen on lung T cells. Am Rev Respir Dis. 1991 Apr;143(4 Pt 1):829–833. doi: 10.1164/ajrccm/143.4_Pt_1.829. [DOI] [PubMed] [Google Scholar]
- Yamaguchi E., Okazaki N., Tsuneta Y., Abe S., Terai T., Kawakami Y. Interleukins in pulmonary sarcoidosis. Dissociative correlations of lung interleukins 1 and 2 with the intensity of alveolitis. Am Rev Respir Dis. 1988 Sep;138(3):645–651. doi: 10.1164/ajrccm/138.3.645. [DOI] [PubMed] [Google Scholar]



