Abstract
The formation of the embryonic brain and spinal cord begins as the neural plate bends to form the neural folds, which meet and fuse to close the neural tube. The neural ectoderm and surrounding tissues also coordinate proliferation, differentiation, and patterning. This highly orchestrated process is susceptible to disruption, leading to neural tube defects (NTDs), a common birth defect. Here, we highlight genetic and epigenetic contributions to neural tube closure. We describe an online database we created as a resource for researchers, geneticists, and clinicians. Neural tube closure is sensitive to environmental influences, and we discuss disruptive causes, preventative measures, and possible mechanisms. New technologies will move beyond candidate genes in small cohort studies toward unbiased discoveries in sporadic NTD cases. This will uncover the genetic complexity of NTDs and critical gene-gene interactions. Animal models can reveal the causative nature of genetic variants, the genetic interrelationships, and the mechanisms underlying environmental influences.
Keywords: embryonic brain, embryonic spinal cord, gene-environment interactions, neural tube defects
OVERVIEW OF EMBRYONIC NEURAL TUBE CLOSURE
Neural Tube Closure: The Embryonic Beginning of the Brain and Spinal Cord
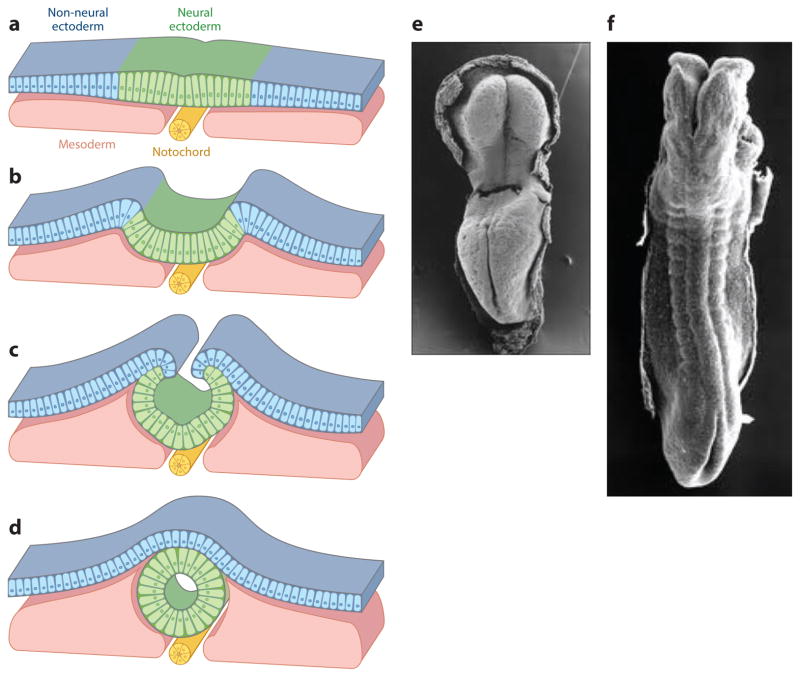
The embryonic brain and spinal cord start with the formation of a simple tube. The neural tube (NT) forms when the flat sheet of neuroepithelial cells, bordered by cells that become the epidermis, rolls up and seals together to form the closed neural tube covered by a layer of surface ectoderm (Figure 1a–d). Closure occurs in a progressive manner, starting at the caudal hindbrain/rostral spinal cord and then proceeding through the head and down along the spinal cord between week 3 and 4 of human gestation (Figure 1e–g) (embryonic day E8.75–10 in mouse).
Figure 1.
Neural tube closure. (a–d) Schematic transverse sections that illustrate the (a) flat neural plate stage, (b) hinge-point formation and neural-fold elevation, (c) apposition of the neural folds with the neural ectoderm covered by the non-neural ectoderm (NNE), and the (d) meeting and remodeling of the neural ectoderm and NNE to form a closed neural tube covered by a single layer of NNE.
(e–f) Carnegie stages 9–11 human embryos (~20–24 days of gestation) just prior to the (e) initiation of neural tube closure and to (f) closure of most of the spinal region. Panels a–d adapted from Reference 133; panels e and f taken from scanning electron micrographs of early human embryos by Dr. K. Sulik, from embryos collected by Dr. Vekemans and T. Attie-Bitacha, and presented on http://embryology.med.unsw.edu.au/embryology/index.php?title=Embryonic_Development.
The deceptively simple tube formation requires highly orchestrated activities, including dramatic tissue movements, especially in the brain region with its large neural folds, tight coordination between numerous cellular and molecular processes, and extensive interactions between the neural ectoderm, mesenchyme, and surface ectoderm across time and space. A few examples of individual activities are presented here. Cell proliferation must be tightly regulated and coordinated with cell differentiation: Failure of NT closure is associated with gene mutations that cause either too little or too much cell division or with alterations in the timing of differentiation [for example, Neurofibromin, Pax3, Phactr4, Jumonji, Notch pathway (36, 69, 70, 171)]. Cell movements and changes in cell shape critically drive the process of NT closure [see planar cell polarity (PCP) pathway below and cytoskeletal proteins such as SHROOM3, VINCULIN, COFILIN, and MENA (36, 69)]. Patterning of the neural tissue occurs during NT closure, and disruption of key patterning pathways are associated with failure of NT closure [e.g., Sonic Hedgehog signaling, bone morphogenetic protein (BMP) signaling, and retinoid signaling (36, 69)]. Movement of the neural folds toward one another requires physical forces generated by the neural, mesenchymal, and surface ectoderm tissues (3, 111, 121). The complexity of these tissue interactions is perhaps best highlighted by the final step in NT closure. As the neural folds rise up and approach each other across the physical gap, the neural ectoderm and surface ectoderm must release their contact with one another and then rapidly reestablish contact with their cognate tissue layer on the other neural fold to seal the neural tube and cover the NT with a single sheet of ectoderm (133). Concomitantly, the neural crest cells, which arise at the border between the neural and surface ectoderm, undergo an epithelial to mesenchymal transition and migrate away, whereas the neighboring neural ectoderm and surface ectoderm must maintain their epithelial character. Overall, the actions of many genes that regulate multiple cell biological and molecular events need to be tightly coordinated in time and space for proper NT closure.
Neural Tube Defects
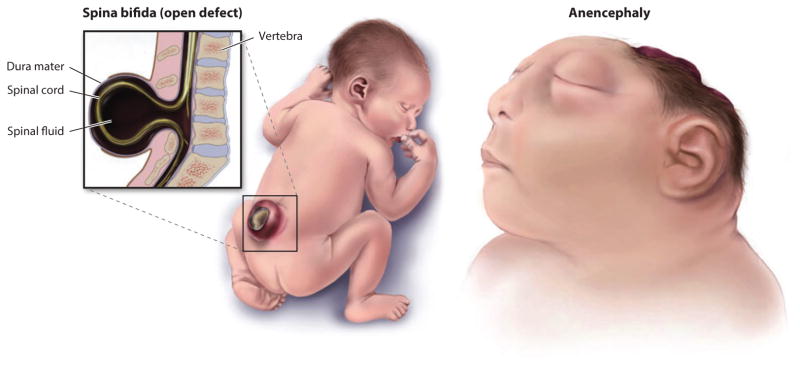
As might be expected for such an exquisitely coordinated and dynamic morphogenetic process, NT closure is highly sensitive to perturbations, and these can result in neural tube defects (NTDs), a common and devastating birth defect (Figure 2). Failure to close the NT in the cranial region (exencephaly or called anencephaly after degradation of the exposed neural tissue) leads to death before or at birth. Infants born with caudal NTDs (e.g., myelomeningocele or spina bifida) have increased risk of mortality, and those that survive often face life-long disabilities and neurologic, cognitive, urologic, and gastrointestinal complications. NTDs occur in ~1 in 1,000 live births in the United States and resulted in 71,000 deaths globally in 2010 (28, 105). Therefore, understanding neural tube development and the causes of NTDs are among the most important health-related studies today.
Figure 2.
Two types of open neural tube defects (NTDs). Spina bifida occurs in the spinal region, whereas failure of cranial neural tube closure is initially called exencephaly but after exposure and degradation of the brain tissue is called anencephaly. Both caudal and cranial NTDs can vary in extent of the opening and the rostral-caudal level. Adapted from Centers for Disease Control and Prevention, National Birth Defects and Developmental Disabilities.
The embryo grows rapidly during the period of NT closure. If the neural folds fail to rise up and come together within the correct time frame, it is likely that continued growth of the embryo will result in neural folds that are too far apart to close. It is possible that even minor changes in the timing of gene function could affect the movement and meeting of the neural folds and result in NTDs. Moreover, the highly orchestrated activities during NT closure indicate the involvement of numerous genes in this critical embryonic process. Indeed, the great majority of NTDs in humans are thought to have a multifactorial and complex etiology in which disturbances in more than one gene affect closure. Moreover, environmental factors can also alter the risk of NTDs, and this is discussed later in the review. Next, we discuss the current state of understanding of the genetic causes of NTDs, which has largely been driven by studies in animal models.
GENETIC REGULATION OF NEURAL TUBE CLOSURE
A few genetic syndromes are associated with NTDs in humans, including trisomy for chromosome 3, 18, and the X chromosome, although the responsible gene or genes that are dosage sensitive on these chromosomes are unknown (57). In the general population, the genetic risk for having a second child with an NTD is only 2–5% (40, 81). This suggests that differences in genetic makeup can affect the penetrance of a phenotype. Indeed, in mouse models, genetic background can strongly affect NTD risk, and the search for modifier loci has been an ongoing effort (69, 70, 86, 93, 95).
This genetic complexity is acknowledged, but as a starting point to understand the genetic contributions to NT closure, the efforts to date have largely focused on single gene mutations. NT closure in the mouse is similar to human neurulation at the cellular, tissue, and genetic levels, and is the favored animal model for extrapolation to humans. Research in the mouse, from classical mouse NTD mutants to targeted mutations and random mutagenesis screens, has provided considerable insights into the genes that are critically required for NT closure. Indeed, several hundred genes have been identified whose functions are required for NT closure in the mouse embryo (61, 69, 70), and this number is expected to increase as research in this field expands. These data have informed human studies, starting from the search for candidate genes in small cohorts of NTD patients to recent whole-genome or exome sequencing of NTD-affected individuals. Some prominent examples are the genes involved in the formation and signaling of cilia and the PCP pathway (see below). We also discuss the folic acid (FA) metabolism pathway, which, despite years of study and because of the action of FA in preventing NTDs, has provided very few genes that have been conclusively associated with human or mouse NTD etiology. As outlined below, it is critical to move between human and animal cell studies to functionally interpret the human data and to move toward an understanding of complex gene interactions.
Cilia and the Hedgehog Signal Transduction Pathway
Cilia are hair-like extensions from the cell surface that regulate signal transduction by mechanical and molecular cues. The evidence for a role of genes involved in cilia formation and function in NT closure converged from both human and mouse studies. In humans, NTDs have been associated with several syndromes, including Meckel-Gruber Syndrome and Joubert Syndrome (94, 103). Mapping and cloning of the genes causative for these syndromes, and the creation of mouse genetic models that recapitulate many of the clinical features, has highlighted the importance of cilia in key embryonic and adult processes, including NT closure. Functional studies in human cells and animal systems also showed the requirement for cilia in key developmental signaling pathways, most prominently the Sonic Hedgehog pathway during NT formation (2, 91, 103, 177). In the mouse, approximately 5% of the known NTD genes are related to cilia formation and function, providing a rich source of gene candidates for analysis in human NTD samples that is only beginning to be explored (34).
Planar Cell Polarity Pathway
Over the past few years, a candidate gene approach has driven the identification of human NTD genes. A prominent focus has been on the PCP or noncanonical WNT pathway. The PCP pathway provides a prime example to illustrate insights gained, as well as the challenges and open questions discovered, from studies of human NTDs and animal models. PCP core proteins, such as VANGL1/2, DISHEVELLED, CELSR1, and SCRIBBLE, and PCP effector proteins, such as FUZZY and INTURNED, have been widely implicated in driving critical cell movements during NT closure. Research in frog, mouse, and chick embryos has shown that the PCP pathway controls convergent extension cell movements, which help to lengthen the embryo along the rostral-caudal axis and narrow the neural plate (170, 185). Disruptions in the PCP pathway result in a wide neural plate, which prevents the neural folds from contacting each other. This results in the most severe NTD, which is called craniorachischisis, wherein the neural tube remains open from the brain to the spinal cord. On the basis of this severe phenotype in animal models, studies have sought to determine whether craniorachischisis in humans is also associated with mutations in the PCP pathway. Interestingly, mutations in PCP genes have been identified in human fetuses with craniorachischisis (82, 137), highlighting the importance of animal models in informing human NTD studies.
Craniorachischisis may be the rare case in which a specific pathway can be implicated in the etiology of an NTD. However, it is becoming apparent that mutations in PCP genes are also associated with a broader range of NTDs, including sporadic cases of cranial, spinal, and open and closed NTDs. Sequencing of PCP genes in a larger cohort of patients with NTDs has identified rare variants in PCP genes (82, 84). This highlights the challenges in predicting genotype/phenotype relationships. In general, if failed NT closure is the ultimate observational consequence and the initiating event is unknown, it is unlikely that strong predictions can be made as to the causative genetic defect. Similarly, NTDs that occur at a specific rostral-caudal level may not mean that the gene has a particular function in that region. There is evidence to argue for this, as well as evidence that argues for a more complex situation, including cases in which the same genetic mutation in the same genetic background may result in cranial NTD in one animal and caudal NTD in another (69, 70). Even the evidence in favor of region-specific function may be overturned as additional NTD samples are analyzed, new alleles are found, and gene-gene interactions are discovered. In reality, it may remain difficult to predict precise genotype/phenotype relationships. Nonetheless, the gene candidates revealed by animal studies provide a solid foundation for human genetic studies, although the penetrance, expressivity, and regional specificity may depend on other genetic and environmental factors.
Unbiased Determination of the Genetics Underlying Neural Tube Defects in Humans
Considering the large spectrum of genes identified in animal models and the multigenic nature of human NTDs, significant advances in understanding the genetics of NTDs will require moving beyond single candidate gene studies to discovery-based approaches, including high-throughput sequencing of large cohorts of patients. With the decreasing costs of exome and whole-genome sequencing, investigators are beginning to explore the genomes of NTD-affected individuals and families. It is not yet known how copy number variants (CNVs) may contribute to NTDs, beyond a few genetic syndromes associated with NTDs (trisomy for chromosome 3, 18, or the X chromosome) and the recent association between CNVs in cilia genes in NTD samples (34). Protein coding mutations and potential regulatory mutations will likely form the first wave of functional analyses. However, as can be expected from studies of other human diseases, numerous gene variants will be identified and the challenge will be to evaluate the data and define possible causative mutations. Notably, the set of genes identified in animal NTD models will provide an invaluable framework to begin to decipher these large genomic data sets. Moreover, it will be important to return to animal models or simpler systems, such as cultured cells, to functionally validate gene variants.
The greater promise of the comprehensive view of the genome is the ability to reveal potential gene-gene interactions and to open avenues to interrogate the combinatorial effects of polymorphisms in NTD pathway genes across the genome. However, the challenges increase almost exponentially because of the potential for combinatorial interactions. Importantly, new technologies such as CRISPR will allow rapid genome editing, such that multiple genetic variants can be created in cells or animals to begin to decipher genetic relationships. Overall, in sporadic cases of NTDs, it is unlikely that the NTD results from a single gene mutation but rather may be attributable to the combinatorial effect of multiple genes, each with small contributions, including changes in gene and/or protein levels in time and space, in conjunction with epigenetic and environmental effects, which are discussed below.
Creation of a Publicly Available Repository of Genes Whose Function Is Required for Neural Tube Closure
One goal of this review is to establish an online, publicly available repository to provide a current and continually updated list of genes implicated in NT closure based on mutations that cause NTDs. The ultimate goal is for the NTD scientific community to contribute their information and insights to the online database to enhance the flow of knowledge between researchers, geneticists, clinicians, and epidemiologists, and to inform ongoing studies of human NTD patients. We gratefully acknowledge Dr. Muriel Harris and Dr. Diana Juriloff for their exceptional reviews (69, 70), which have provided the initial foundation of the online database, and Dr. Claudia Kappen and Dr. Michael Salbaum for providing an extraction of the MGI database related to NTDs (141). The link for the NTD Wiki database is http://ntdwiki.wikispaces.com.
With more than 300 genes already identified as critically required for NT closure, it may seem like a daunting prospect to attempt to understand this complex embryonic process. However, through the use of well-defined molecular markers, histology, and even newly developed live-imaging systems to visualize the dynamic cell and tissue movements of NT closure (111), it is possible to define the function of even novel genes in the process of NT closure. Thus, a framework is established to categorize the functions of new genes, and we predict that the major pathways and processes involved in NT closure have been identified. The field is coming to the point at which it should be possible to take a systems-level approach to help decipher the genomic information that is forthcoming from genome sequencing efforts.
EPIGENETIC REGULATION OF NEURAL TUBE CLOSURE
Genome-Wide Contributions to Human Neural Tube Defects
A prominent mechanism in which gene expression can be modified globally is through the action of epigenetic regulators. The mammalian epigenome consists of all modifications to the genome that confer heritable changes in gene function independently of nucleotide sequence. This includes, but is not limited to, DNA methylation, chromatin modification, and nucleosome repositioning. Mutations in a growing number of epigenetic regulators in mice have been shown to result in NTDs (Table 1), suggesting that coordinate regulation of transcriptional networks is required for proper NT closure. These observations highlight the potential of animal models to uncover pathways that will inform a global view of human NTD etiology. This, in combination with advancing technologies in whole-genome sequencing, gene expression analysis, and epigenetic profiling, provides a means for better understanding of the genetic causes of NTDs in humans. Moreover, epigenetic-related hypotheses extend to the mechanisms of FA-induced NTD prevention (discussed below) and the NTD risk conferred by disease treatments with the histone deacetylase (HDAC)-inhibitor valproic acid (136). Thus, the role of the epigenome in NT closure is of particular interest. Here, we focus on the mechanisms of epigenetic modification as contributing factors to proper closure of the mammalian neural tube.
Table 1.
Epigenetic regulators required for neural tube closure
| Gene | Protein | Function | Observed Neural Tube Defect | Reference |
|---|---|---|---|---|
| Ppm1g | PPM1G | Chromatin remodeling | Exencephaly | (50) |
| Dnmt3a | DNMT3A | DNA methylation | Exencephaly | (123) |
| Dnmt3b | DNMT3B | DNA methylation | Exencephaly | (123) |
| Dnmt3L | DNMT3L | DNA methylation | Exencephaly | (71) |
| CBP | CBP | Histone acetylation | Exencephaly | (128) |
| Kat2a | GCN5 | Histone acetylation | Exencephaly | (22) |
| Ep300 | p300 | Histone acetylation | Exencephaly | (128) |
| Hdac4 | HDAC4 | Histone deacetylation | Exencephaly | (166) |
| Sirt1 | SIRT1 | Histone deacetylation | Exencephaly | (35) |
| Kdm2b | FBXL10 | Histone demethylation | Exencephaly | (52) |
| Kdm6a | UTX | Histone demethylation | Exencephaly | (152, 179) |
| Uty | UTY | Histone demethylation | Exencephaly | (152) |
| Alkbh1 | ALKBH1 | Histone methylation | Exencephaly | (126) |
| Jmj | JARID2/Jumonji | Histone methylation | Exencephaly | (162) |
| Cited2 | CITED2 | Co-regulator of CBP/p300 | Exencephaly | (187) |
| Smarcc1 | BAF155 | Nucleosome remodeling | Exencephaly | (67, 85) |
| Smarca4 | BRG1 | Nucleosome remodeling | Exencephaly | (23) |
| Cecr2 | CECR2 | Nucleosome remodeling | Exencephaly | (10) |
| mIR-124a | N/A | Nucleosome remodeling | Spina bifida | (178) |
| mIR-9* | N/A | Nucleosome remodeling | Spina bifida | (178) |
| Nap1L2 | NAP1L2 | Nucleosome assembly | Exencephaly | (138) |
Ground Level Changes: DNA Methylation
DNA methylation is a direct chemical modification of DNA that alters transcription of target genes via proposed mechanisms involving changes in stabilization of transcription factors and alteration of chromatin structure (115). Methylation occurs at CpG dinucleotides, is mediated by a class of enzymes known as DNA methyltransferases, and is dynamically regulated during mammalian development. Prior to implantation of the embryo, the majority of the embryonic genome, with the exception of imprinted regions, undergoes complete demethylation and is subsequently remethylated by the de novo DNA methylases DNMT3A and DNMT3B, and is maintained by DNMT1 (19). These events result in stable methylation of the majority (>90%) of CpG dinucleotides across the genome in all cell types (161). However, regions with high concentrations of CpG dinucleotides, called CpG islands, remain relatively unmethylated and are the main targets of DNA methylation-mediated transcriptional regulation (115).
Although there is a wealth of data supporting the role of CpG island methylation in transcriptional repression, recent data have painted a more complex picture. In addition to acting as a strong epigenetic suppressor of transcription through destabilization of transcription factors with their target promoters, CpG methylation of distal regulatory elements and distant promoters has been linked to binding of sequence-specific transcription factors (17). These data together support an integral role of CpG methylation in genome-wide transcriptional regulation. Moreover, due to its reversible nature, DNA methylation allows for dynamic tuning of transcriptional programs. Indeed, numerous human diseases have been linked to defects in DNA methylation and its dynamics, including Prader-Willi syndrome [loss of genomic imprinting, which confers monoallelic, parent-of-origin specific expression of genes (26)] and Rett syndrome [loss of Mecp2 which reads the methylation state of DNA (4)]. To date, DNA methylation defects have been identified in developmental disorders, cancer, diabetes, and heart failure, suggesting that they may underlie many common human disorders (16, 63).
With respect to NTDs, mouse models lacking the de novo DNA methyltransferases DNMT3A and DNMT3B exhibit cranial NTDs, demonstrating that proper remethylation of the genome after implantation is essential for NT closure (123). The closely related DNMT3L is also required for NT closure; however, its function in DNA methylation remains poorly understood beyond its involvement in maternal imprinting (71). Other null mouse models for methyltransferases such as DNMT1, however, have been less informative about the role of DNA methylation in NT closure, largely because of early embryonic death (98). Several genes involved in one-carbon metabolism (OCM), which provides methyl groups for DNA methylation, have been studied in human NTDs; however, we only touch on these below, as they have been extensively reviewed by Greene et al. (62). Together, these data, in conjunction with the knowledge that the majority of CpG dinucleotides in the genome outside of CpG islands are constitutively methylated, suggest that methylation-related NTDs may be due to disruption of methylation at specific CpG islands. In future studies of mouse models and human NTD patients, it will be important to utilize next-generation sequencing technologies in conjunction with methylation-specific analysis to uncover the epigenetic code that helps to drive NT closure.
Altering Genomic Topology: Histone Modifications
Within the nucleus, DNA is packaged via its association with histone octamers called nucleosomes. Each nucleosome is wrapped by 147 base pairs of DNA and consists of four homodimers of the core histone proteins H2A, H2B, H3, and H4 that extend N-terminal tails from the core of the nucleosome into the nucleus (9). Histone tails can be modified with eight different covalent post-translational modifications and more than 60 different modification sites on histone tails have been identified, but this underestimates the repertoire of histone modifications, as many residues undergo di- and trivalent modification, adding to the total possible number of alterations (88). The most commonly studied histone modifications, acetylation and methylation, are generally involved in transcriptional activation and repression, respectively. However, the actual effect of any given modification depends upon the modified residue, as well as the state of neighboring residues. The predominant theory as to how these modifications affect downstream transcription is that they alter the way that nucleosomes interact with each other (88). More specifically, they either promote or antagonize interactions between nucleosomes, therefore altering the packaging of DNA and the accessibility of transcriptional machinery to its target genomic regions (9). It is therefore reasonable to surmise that defects in the enzymatic pathways responsible for adding and removing these chemical modifications can lead to severe cellular and developmental phenotypes.
Mouse NTD models have implicated several chromatin-modifying enzymes in NT closure. Most notably, the histone acetyltransferases (HATs) GCN5 and CBP/p300, which play critical roles in transcriptional activation, are each required for NT closure (22, 128). Conversely, inhibition of HDACs with the drugs valproic acid and trichostatin A (TSA) disrupts NT closure in animal models and humans (118, 136). The H3K27me3 histone demethylase UTX is required for NT closure (179), as is the histone demethylase FBXL10 (52), but there is no direct evidence that histone methyltransferases are needed for NT closure (70). However, the polycomb repressive complex 2 (PRC2) component Jumonji, which plays a role in loading PRC2 onto target loci, is required for NT closure (162), and PRC2 has known roles in transcriptional repression through histone methylation (148). Nonetheless, the bias toward mutation of genes involved in histone acetylation and demethylation in NTDs is interesting, as histone acetylation is thought to be far more dynamic than histone methylation. The requirement for histone acetylation and histone demethylation, which often precede transcriptional activation, suggests that regulation of dynamic changes in chromatin structure and transcriptional activation is more important for proper NT closure than stable repression of gene expression through histone methylation. This is consistent with the highly dynamic yet coordinated cellular and molecular programs that drive changes in morphology, patterning, proliferation, and differentiation during NT closure.
It has been difficult to identify the underlying molecular mechanisms by which mutations in the aforementioned enzymes lead to NTDs. In the case of CBP/p300, it is possible that its role in neurulation is, in part, related to regulation of metabolic genes, as the interacting protein CITED2 is also required for NT closure and is thought to act through mechanisms that regulate cellular responses to hypoxia and energy requirements (187). Interestingly, CITED2 can regulate glucose homeostasis through PGC-1α (140), a direct target of acetylation by GCN5 (96). Additionally, p300 has been linked to regulation of hepatic glycogen storage (72). Although the underlying cause of NTDs in mice that lack GCN5 acetyltransferase activity (Gcn5hat) has yet to be uncovered, GCN5 plays important roles in glucose homeostasis and stress response (27, 96, 119) and the convergence of these data with those related to CBP/p300 and CITED2 and NTDs suggests that these complexes may play essential roles in regulating specific metabolic pathways that are required for NT closure. This is especially intriguing considering the NTD risk associated with maternal diabetes, which is discussed below.
Histone acetylation also appears to control the expression of patterning molecules that are essential for NT closure. By treating mouse embryos with the HDAC inhibitor TSA, it was discovered that class I and II HDAC activity is required to inhibit BMP2/4 signaling in the developing forebrain (149). Embryos treated with TSA display a distinct shift in the fate of neural progenitor cells, and defects in neurogenesis can cause NTDs (33). Our unpublished microarray data from Gcn5hat/hat mice also suggest defects in programs of neural differentiation, indicating that HATs and HDACs may play multiple roles within the developing neural tube and that combinatorial fine-tuning of these transcriptional programs is required to coordinate the complex morphological events that are the basis of primary neurulation. The developing NT seems particularly susceptible to changes in chromatin architecture and its dynamic remodeling, as HAT mutants are exencephalic but otherwise largely anatomically normal at the time of NT closure (22, 128).
Moving Roadblocks: Nucleosome Positioning
In addition to regulation of nucleosome aggregation via histone modification, transcriptional output is also modified via deposition and repositioning of nucleosomes along chromosomes. Nucleosome repositioning is carried out by ATP-dependent chromatin-remodeling complexes. These can shift nucleosomes to different chromosomal positions to regulate transcription by protecting the nucleosome-associated DNA from binding with transcriptional machinery (157). Genome-wide studies of nucleosome positioning have revealed that positioning can vary greatly, even within a single cell type (157). Nonetheless, it is clear that nucleosome positioning plays an important role in transcriptional regulation in conjunction with DNA methylation and histone modifications. It is therefore of no surprise that enzymes responsible for nucleosome localization are required for embryonic development and, more specifically, NT closure.
The SWI/SNF-related nucleosome remodeling BAF complex appears to play a specific role in NT closure, as mutations in multiple subunits result in NTDs in mouse. Both the core catalytic component BRG1 and the complex subunit BAF155 are required for NT closure, suggesting that proper nucleosome positioning by this complex is required for neurulation (23, 67). Complete loss of Brg1 causes early embryonic death, highlighting the importance of nucleosome positioning in general development, but loss of a single allele results in, among other defects, exencephaly (23). An allele of BAF155 that forms an assembled but malfunctional BAF chromatin-remodeling complex results in exencephaly and decreased neural cell survival and proliferation, yet a surprisingly small number of genes were dysregulated (67). The function of BAF155 is still not well understood. Although it is thought to be a core component of the BAF complex in all cells, it is unclear whether this BAF155 allele causes defects in nucleosome repositioning. Other studies of BAF155 function in ES cells, however, have demonstrated a direct role in regulating repression of self-renewal genes, which may help explain its roles in regulating neural development, proliferation, and cell survival (146).
Additional components of nucleosome remodeling complexes, as well as miRNAs that regulate such complexes, have also been implicated in NTDs. CECR2, which forms a specific chromatin-remodeling complex with SNF2L, causes exencephaly when mutated (10). Additionally, NAP1L2, a neuronal-specific protein closely related to nucleosome assembly factors, promotes proliferation of neuroepithelial cells and is required for cranial NT closure (138). As is the case with DNA methylation and histone modification, dynamic changes in nucleosome positioning are also important for cellular function and these changes can be mediated by switching of subunits in chromatin-remodeling complexes (66). As such, changes in the activity of miR-9* and miR-124a cause defects in subunit switching of ATP-dependent chromatin-remodeling complexes, resulting in spina bifida in rats (178).
As may be expected, epigenetic regulators do not function independently of one another. NAP1L2, for example, can promote acetylation of H3K9 and H3K14 and loss of NAP1L2 causes overproliferation of neural stem cells, resulting in inadequate neural differentiation that has been attributed to NAP1L2 promotion of histone acetylation at the Cdkn1c promoter (8). Although the exact mechanistic attributes of NAP1L2 have not been discovered, it does associate with nucleosome assembly proteins such as NAP1L1 and NAP1L4 (7), suggesting that nucleosome assembly and positioning are tightly correlated with histone modification at specific loci. As another example, local demethylation of DNA is directed by the acetylation state of specific residues on locally positioned nucleosomes (31). Altogether, these concepts strongly support highly regulated mechanisms for altering genomic structure and function through multiple interconnections between epigenetic regulators.
Distal Regulatory Elements and Their Functions
In addition to gene regulatory elements located in close proximity to gene bodies, transcription is modified by distal regulatory elements. These elements include enhancers, locus control regions, insulators, and silencing elements (73). Recent data from the ENCODE project suggest that these elements are highly promiscuous, with some distal elements interacting with up to 10 different transcriptional start sites (144). Promoters also interact with numerous distal regulatory elements, even within a single cell type (144). It is therefore reasonable to hypothesize that coordinate regulation of genomic architecture is required within each cell type to bring the proper regulatory elements in close proximity to their target promoters. A theme that emerges when examining chromatin-remodeling complexes is the diversity of subunits seen within a given complex. Recent data suggest that these variable subunits may play important roles in recognizing these regulatory elements. For example, ChIP-seq experiments demonstrate that SAGA and ATAC, the two complexes in which the HAT GCN5 is the major enzymatic subunit, have significant overlap in their preferences for promoters, but significant divergence with respect to their regulatory element localization (89). Thus, SAGA and ATAC may function similarly to promote transcription via acetylation of histone residues near active promoters, but the two complexes may have tissue-specific gene regulatory functions mediated by specific preferences for distal regulatory elements. As another example, the HAT PCAF can compensate for GCN5, although these two HATs have differing affinities for their cognate regulatory complexes, and this may partially explain why Gcn5hat/hat mutants show such specific defects in NT closure (22).
The importance of distal regulatory elements in development may also be an underlying reason why it has been difficult to identify causes for human NTDs. The discovery of genomic elements such as super enhancers that control complex traits (78) suggests that sequence variability within distal regulatory elements that control multiple aspects of neurulation could contribute to NTD risk. Unfortunately, the promiscuity of these elements, as well as data showing that distal elements interact with the nearest promoter only 7% of the time (144), makes it extremely difficult to infer the downstream effects of sequence variability within a human regulatory element. Moreover, distal regulatory elements coincide with CpG dinucleotides whose methylation states are crucial for regulation of broad gene regulatory networks and maintenance of cellular health (5). This could help explain how distal element-specific localization of histone modification complexes interfaces with DNA methylation to regulate large transcriptional networks that are required for NT closure.
Transcriptional Variability in Development and Disease
Although the above-mentioned mechanisms are robust and redundant, transcriptional output is still subject to significant noise. This transcriptional noise might be expected to have profound negative effects upon development, but it has been postulated that transcriptional stochasticity actually plays an important role in proper development (130). During the progression from embryonic stem cells to differentiated cells, the mammalian genome undergoes massive rearrangements that require active repression of stemness genes, temporary maintenance of poised epigenetic states for differentiative genes, and activation of genes that give differentiated cells their individual traits. This process has been well-characterized in the developing nervous system, where neural stem cells undergo multiple differentiative events to give rise to more committed progenitors that eventually contribute to the diverse neuronal and glial cell populations (76). It therefore seems necessary for mechanisms that regulate stochastic gene expression within stem and progenitor cells to both allow for differentiation into multiple cell types and to repress unwanted cellular characteristics. It is hypothesized that chromatin modifiers are the main regulatory elements used to maintain order within the required transcriptional noise of development (130).
Variability of gene expression during development poses a significant problem, however, if elements that keep stochasticity in check are disrupted, which can lead to phenotypic changes. This concept was beautifully demonstrated in a paper by Raj et al. (131) that identified increased variability in gene expression as the underlying cause of incomplete phenotypic penetrance. Interestingly, gene expression data sets from the Baf155 (67) and Gcn5hat (J. Wilde & L. Niswander, unpublished results) mouse models of NTDs indicate that individual mutant embryos display significantly different gene expression patterns from one another, suggesting that GCN5 and the BAF complexes play important roles in controlling variability of gene expression during NT closure. As reviewed by Pujadas & Feinberg (130), the epigenome is particularly susceptible to variability, leading to the hypothesis that tight regulation of chromatin structure and dynamics is required during neurulation. This may be especially true in the developing cranial neural tube, as functional loss of almost all epigenetic modifiers identified in animal models of NTDs causes exencephaly (Table 1). This could be due to the fact that the closing cranial NT simultaneously undergoes some of the most complex and dynamic morphogenetic, patterning, and differentiative events during embryogenesis. These processes may necessitate that specific regions of the genome remain extremely plastic, through modulation of chromatin structure and inherent transcriptional variability. Future studies, therefore, may warrant a deeper look into the relationships between transcriptional variability and the regulation of chromatin structure in mouse models of NTDs.
ENVIRONMENTAL CONTRIBUTIONS TO NEURAL TUBE CLOSURE
Environmental Alteration of the Genomic Landscape
Growing evidence now suggests that environmental factors have the ability to alter the epigenetic landscape and, therefore, transcriptional activity. Several lines of study using the agouti viable yellow (Avy) mouse have shown that exposure to endocrine disruptors, dietary changes, and toxic compounds can cause phenotypic changes in offspring owing to disrupted DNA methylation (47). Interestingly, gestation appears to be particularly sensitive to environmental changes, as maternal folate intake during gestation induces differential DNA methylation and phenotypic changes in Avy offspring but not in their mothers (176). Even in normal animals, long-term methyl-enriched diets can lead to profound changes within the epigenome, specifically, increased transcriptional variability (97). These studies have added to a growing amount of literature suggesting that maternal diet is tightly linked to the epigenome of the offspring. This link is not just important for development, however, as rats born from mothers with suboptimal nutrition have a significantly increased risk for developing type II diabetes (143), and mice born from mothers on a low-protein diet show disrupted Ppara expression (102); both phenotypes were shown to be associated with alterations in the epigenome. Together, these data strongly support a significant epigenetic role for maternal nutrition in the proper development of offspring and suggest that epigenetic fidelity is imperative for proper NT closure.
Folic Acid Fortification: Reducing the Incidence of Neural Tube Defects
Although there is clearly a genetic component to NTDs, numerous environmental factors have also been strongly implicated in NTD etiology. One of the earliest records correlating environmental risks with NTDs comes from an eighteenth-century midwife, Catherina Schrader. Her exceptional records showed two clusters of increased NTD incidence: the first following unusually poor crop yields and the second primarily in lower-class, urban families, implicating socioeconomic status, nutrition, and possibly folate deficiency in the incidence of NTDs in eighteenth-century Holland (113). Since then, gene-environment interactions have been implicated in numerous diseases, including diabetes, metabolic syndrome, neurological diseases, and numerous cancers (156). As mentioned above, maternal diet can affect the developing embryo, and one of the best-studied dietary factors relative to NT closure is maternal folate.
Folate deficiency was suggested as a risk factor for early fetal death in the 1950s (163, 164), when the folate antagonist, aminopterin, was used to induce therapeutic abortions. Women who failed to abort with aminopterin treatment gave birth to babies with numerous defects, including NTDs (175). Work by Hibbard & Smithells (75) implicated maternal folate deficiency as a risk for NTD-affected pregnancies in the 1960s, and several landmark human trials in the late 1980s and early 1990s supported these findings (38, 117). The Medical Research Council Vitamin Study, published in 1991, was the first large, randomized trial to show a strong protective effect of FA supplementation. For women who had already had one NTD-affected pregnancy, supplementation with 4 mg/day of FA decreased the risk of a second NTD-affected pregnancy by 72% (6 of 593 NTDs in FA-supplemented group versus 21 of 602 in the unsupplemented group) (117). A second large-scale randomized trial in Hungary provided evidence that FA supplementation could reduce the risk of a first NTD-affected pregnancy. Supplementation with 0.8 mg/day of FA for one month prior to conception and for the first 8 weeks of pregnancy significantly decreased the risk of having a first NTD-affected pregnancy (0 of 2,104 NTD cases in the vitamin-supplement group, compared to 6 of 2,052 NTD cases in the control group) (38).
As a result of these studies, the US Centers for Disease Control and Prevention (CDC) made the official recommendation that “[a]ll women of childbearing age in the United States who are capable of becoming pregnant should consume 0.4 mg of folic acid per day for the purpose of reducing their risk of having a pregnancy affected with spina bifida or other NTDs” (29). This supplementation campaign proved unsuccessful, however, possibly in part because physicians and patients were not sufficiently educated on the importance of supplementation (65), and less than a third of women were reported to be taking a FA supplement in 1997, despite increased public awareness (30). Given the limited effectiveness of the supplementation campaign and the fact that an estimated 50% of pregnancies in the United States are unplanned (87), the United States implemented a fortification campaign in 1998 to ensure that women of childbearing age received the daily recommended dose of 400 μg/day (46). Since fortification, the NTD incidence in the US has decreased by almost 20% overall (31% reduction in spina bifida and 19% reduction in anencephaly) (122). Similar fortification campaigns in other countries have yielded comparable results (41, 42, 104). Overall, more than 75 countries have implemented mandatory FA fortification campaigns, primarily in wheat flour, and it is estimated that FA fortification has prevented 15–25% of FA-responsive NTDs worldwide (188). Interestingly, the incidence of spina bifida did not continue to decline after the initial decrease but rates of anencephaly did (21).
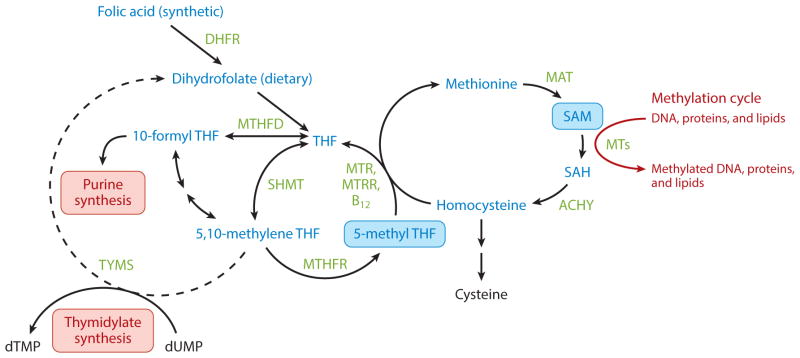
Folate is a water-soluble vitamin that is found naturally in many foods, especially green, leafy vegetables (18). Dietary folates, in the form of tetrahydrofolates (THF), play an important role in OCM (Figure 3). FA is a synthetic form of folate that is more stable than the naturally occurring form. Both FA and folate must be reduced by dihydrofolate reductase (DHFR) and then converted to a biologically active form, 5-methyl-tetrahydrofolate (5-meTHF), by serinehydroxymethyl-transferase (SHMT) and 5,10-methylenetetrahydrofolate reductase (MTHFR) (60). 5-meTHF is then used in the biosynthesis of purines and thymidylate, the synthesis of methionine from homocysteine, and the biosynthesis of S-adenosylmethionine (SAM), the universal methyl donor for cellular methylation reactions, including DNA and protein methylation (14).
Figure 3.
Schematic of folate in one-carbon metabolism. Synthetic and dietary folates are reduced to tetrahydrofolates (THFs), which are converted to the biologically active 5-methyltetrahydrofolate (5-methyl-THF) by SHMT and MTHFR. 5-methyl-THF acts a methyl donor for purine and thymidylate synthesis and generates the major cellular methylation donor S-adenosylmethionine (SAM). Abbreviations: AHCY, S-adenosylhomocysteine hydrolase; DHFR, dihydrofolate reductase; MAT, methionine adenosyltransferase; MTHFD, methylenetetrahydrofolate dehydrogenase; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; MTs, methyltransferases; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine-hydroxymethyltransferase.
Drugs that act as folate antagonists have continued to be implicated as risk factors for NTDs. Women who took folate antagonists in their first trimester were more than sixfold more likely to have an NTD-affected pregnancy. These included DHFR-inhibitors, such as methotrexate, which inhibit the conversion of folate to its active form, and the antiepileptic drug valproic acid (an HDAC inhibitor) (6, 112).
After the Brownsville Cluster, in which the incidence of NTDs was four- to fivefold higher along the Texas-Mexico border than the rest of the United States, attention turned to other potential environmental risk factors. The Mexican-American population in the region was known to consume high levels of corn, and cornmeal samples harvested around the time of the Cluster contained high levels of fumonisin contamination. Fumonisin is a mycotoxin that inhibits the biosynthesis of sphingolipids, ultimately interfering with the cellular uptake of 5-meTHF (159). In mouse studies, FA supplementation partially rescued fumonisin-induced NTDs (55, 139). Furthermore, much of the population affected in the Brownsville cluster was also folate deficient, suggesting that fumonisin is more likely to compound folate deficiency in a human population and less likely to be a significant risk factor in a folate-sufficient population.
Given the strong epidemiological evidence for the requirement of adequate FA during development, and especially at the time of NT closure, attention has focused on genes in the folate OCM pathway. More than 40 genes involved in FA metabolism have been studied in NTD patient samples (reviewed in 61). Numerous studies have focused on a common polymorphism in the MTHFR gene, 677C→T. MTHFR is required to reduce 5,10-methylenetetrahydrofolate (5,10-methylene-THF) to 5-methyl THF, which is the main circulating form of folate and is required to generate SAM (18). The 677C→T mutation generates a more thermolabile enzyme with significantly reduced activity that results in low plasma folate levels and higher levels of homocysteine (51, 165). Several meta-analyses of the epidemiological data indicate that the 677T→C mutation is an overall risk factor, with either the CT or TT allelic combinations conferring a 1.2–2-fold increased risk of an NTD-affected pregnancy (181, 191). Furthermore, the risk associated with the MTHFR 677C→T mutation can be alleviated by increased maternal folate (92), again highlighting the importance of gene-environment interactions. Despite the strong association with the MTHFR polymorphism and the intensive focus on OCM gene variants in cohorts of NTD patients, there is little additional evidence for a major causative association between genetic variants in this pathway and NTDs (61).
Consistent with the human NTD data, very few genes involved in OCM have been implicated in mouse NTDs (68), and folate deficiency in the absence of a genetic insult fails to cause spontaneous murine NTDs. A handful of mouse NTD models have been used for FA supplementation studies, with very mixed results (Table 2). For some mouse models, FA has a preventative effect (12, 13, 68, 116), whereas others are nonresponsive (68, 120), much like in the human population. Surprisingly, several models have shown a detrimental response to FA, including increased NTDs and increased fetal loss (59, 110). These studies raise an important question: In some cases, is the decreased incidence of NTDs due to an increase in early fetal loss rather than a rescue of NTDs, a possibility first raised nearly two decades ago (79)? It will be important to understand which genetic variants are favorably influenced by FA fortification, and it is expected that animal models can provide genetic, experimental, and mechanistic insights into FA-responsive and nonresponsive NTDs. Some experts have raised the idea that the dose of FA should be increased to try to further decrease the incidence of NTDs in the population (168). Indeed, FA fortification in conjunction with an increase in vitamin usage has resulted in a trend toward higher FA levels throughout the US population (80, 129). However, it is important to note that there will likely be certain genotypes that are resistant to FA, and the potential for long-term epigenetic changes remains an important consideration.
Table 2.
Mouse NTD models studied for responsiveness to folic acid
| Gene Name | Folic acid response | Process | Reference | ||
|---|---|---|---|---|---|
| Beneficial | Detrimental | None | |||
| Amt null | x | Involved in glycine cleavage system, part of the mitochondrial folate metabolism; implicated in human and mouse neural tube defects; responsive to methionine | (120) | ||
| Axd mutant | x | Unknown function; axial defects; responsive to methionine | (68) | ||
| Cart1 null | x | Paired-class homeobox domain-containing gene, aka Alx1 | (68) | ||
| Cited2 null | x | Required for heart morphogenesis and left-right axis determination in mouse development | (68) | ||
| Curly tail (Grhl3 mutant) | x | Transcription factor; Ct mutation is responsive to inositol and retinoic acid | (68) | ||
| Fkbp8 null | x | Member of immunophilin protein family; involved in dorsal-ventral patterning of neural tube; inositol-resistant | (68) | ||
| Folr1 null | x | Folate receptor; involved in transport of 5-MTF into the cell | (68) | ||
| Frem2m1Nisw allele | x | Extracellular matrix protein | (110) | ||
| Grhl2m1Nisw allele | x (het) | x (null) | Transcription factor | (110) | |
| Grhl3 null | x | Transcription factor | (68) | ||
| Kat2a hypomorph (GCN5) | x | Histone acetyltransferase | (68) | ||
| L3P | x | Ciliogenesis | (110) | ||
| Lrp6 null | x | Receptor or coreceptor (with Frizzled) of Wnt; canonical Wnt/β-catenin signaling | (59) | ||
| Lrp6Cd allele | x | (68) | |||
| Map3k4 null | x | MAPK pathway | (68) | ||
| Mthfd1lz allele | Untested with folic acid; partial rescue observed upon sodium formate supplementation | (116) | |||
| Nog null | x | Bone morphogenetic protein antagonist; resistant to inositol and pifithrin-α (cell-death inhibitor) | (68) | ||
| SELH/Bc strain | x | Inbred mouse strain with a high frequency of nonsyndromic neural tube defects | (68) | ||
| Shmt null | x | Thymidylate biosynthesis pathway; choline-resistant | (12, 13) | ||
| Shroom3m1Nisw allele | x (short-term) | x (long-term) | Involved in actin remodeling, especially apical constriction and apicobasal elongation | (110) | |
| Slc19a1 null (RFC1) | x | Reduced folate carrier | (68) | ||
| Sp and Sp2H mutant at Pax3 gene | x | Transcription factor involved in ear, eye, and facial development as well as dorsal patterning of the neural tube | (68, 110) | ||
| Zic2m1Nisw allele | x | Transcriptional activator and repressor involved in organogenesis of the central nervous system | (110) | ||
The mechanism by which FA affects NT closure is unknown, but as discussed above, changes in DNA methylation likely play a role, as do alterations in synthesis of nucleotide precursors. Methylation of the insulin-like growth factor gene differentially methylated region (IGF2 DMR) was significantly higher in children whose mothers took FA supplements (400 μg/day) than in children of unsupplemented mothers (154). In mice, genome-wide CpG methylation patterns were found to be significantly different in the cerebral hemispheres in offspring of wild-type dams exposed to a FA-fortified diet compared with nonfortified controls (11). Furthermore, differential methylation of important developmental genes was correlated with altered levels of gene expression. Future studies are needed to determine what the link may be between FA status, changes in DNA methylation, and NTD incidence. Moreover, the OCM cycle also produces nucleotides, which are needed for proliferation, and the rate of proliferation is extremely important in NT closure. Hence, adequate folate levels likely play an important role in maintaining sufficient proliferation levels during NT closure.
Maternal Diabetes and Obesity
Maternal diabetes has long been identified as a risk factor for congenital birth defects, including NTDs (190). SNPs in maternal genes related to glucose metabolism, such as FTO, LEP, TCF7L2, LEPR, GLUT1 and HK1, have been linked to an increased risk for NTDs (39, 107), and potentially informative maternal-fetal SNP interactions have also been correlated to altered NTD risk. For example, SNPs in maternal ENPP1 and fetal SLC2A2 correlate with increased risk of NTD (2–3.5-fold), whereas the same fetal SLC2A2 SNPs interact with maternal SNPs in LEP to provide a protective effect (0.5-fold risk) (108). Fetal SNPs in SLC2A2 alone do not appear to confer altered risks of NTDs (107). It is thought that altered maternal glucose metabolism leads to an altered intrauterine environment, which is not tolerated by the developing embryo, as pancreatic function in human embryos begins at approximately week 7 of pregnancy. Diabetic embryopathies are known to particularly affect the neural tube and heart, both of which form prior to week 7 (107).
Animal models have proven invaluable in elucidating the molecular pathophysiology of diabetic embryopathies. In mice, high glucose levels cause decreased proliferation and increased apoptosis in neural progenitor cells in the developing spinal cord (53), suggesting that a reduction in cell number contributes to diabetic embryopathies. Hyperglycemia can activate several related pathways, including altered lipid metabolism, increased generation of reactive oxygen species (ROS), and activation of apoptotic pathways (reviewed in 135). Briefly, hyperglycemia alters arachidonic acid metabolism, leading to the generation of altered levels of prostaglandins, which have been linked to adverse pregnancy outcomes. Hyperglycemia also leads to increased glucose transport into the cell, which activates the polyol pathway, causing increased accumulation of sorbitol and ultimately increased ROS levels. Increased sorbitol and the hyperglycemia-induced stress response in the endoplasmic reticulum (ER) have both been shown to activate the JNK1/2 pathway, which has been implicated in diabetes-induced NTDs (101, 183). Oxidative and ER stress have been shown to activate the PKC pathway (77), which can also activate the JNK1/2 pathway (24, 25) and induce apoptosis via Caspase-8 (192). Alleviating oxidative stress by overexpression of the antioxidant enzyme superoxide dismutase 1 (SOD1) can rescue diabetes-induced NTDs coincident with decreased activation of the PKC and JNK1/2 pathways (99, 100, 172). Moreover, ROS in mice activates apoptosis signal-regulating kinase 1 (ASK1), which activates the transcription factor FoxO3a and Caspase-8, as well as increased levels of the apoptosis-promoting adaptor protein, TRADD, which is a direct target of FoxO3a. Interestingly, increased ASK1 phosphorylation, decreased phosphorylation of FoxO3a, increased TRADD expression, and increased cleaved Caspase-8 were also observed in neural tissue from human NTD-affected fetuses (182).
Intriguingly, a model of transcriptional variability as the underlying cause of partial penetrance has been proposed, supported by recent data from diabetic mouse models. In both a chemically induced diabetes model and a nonobese diabetic (NOD) strain, NTD-affected embryos had significantly higher variability in gene expression compared with their non-NTD littermates. Furthermore, the pathways enriched in the class of highly variable genes were different than those enriched in the differentially expressed but less-variable gene sets, suggesting a role for environmentally induced variable gene expression as a mechanism of increased NTD risk (83). Mechanistically, this may fit with recent data showing that hyperglycemia alters the epigenetic landscape. Shyamasundar et al. (153) found that neural stem cells isolated from chemically induced diabetic pregnancies had increased global levels of H3K9 trimethylation, increased DNA methylation, and decreased H3K9 acetylation compared with neural stem cells derived from normal pregnancies. As discussed above, disruption of the epigenetic landscape can increase transcriptional variability and may be an underlying factor of NTDs.
Given what is known about the molecular mechanisms involved in diabetes-induced NTDs, several measures have been proposed to protect developing embryos. There is evidence that nutritional supplements can alleviate the risks. Diabetic mice supplemented with trehalose had a much lower incidence of NTD-affected embryos than unsupplemented mice (10% versus 28%) (180). Diabetic rats supplemented with FA showed a decreased incidence of NTDs (supplemented, 6%; unsupplemented, 28%) (54). However, diabetic mice also showed premature neuronal and astrocyte differentiation when given high doses of FA compared with a moderate dose, suggesting that too much FA may cause unintended consequences (189). Human epidemiological data also suggest that FA supplementation may reduce the risk of NTDs in a diabetic pregnancy (37, 127), with a larger protective effect observed against anencephaly than spina bifida (37).
Maternal obesity, defined as a body mass index (BMI) ≥30, has also been linked to an increased risk for NTDs. Meta-analyses showed an overall 1.7–1.8-fold increase in NTD-affected pregnancies for women who are obese and 3.1-fold increase for women who are severely obese (132, 150, 155). Interestingly, a prefortification study in the United States showed that the risk associated with increased BMI was independent of maternal folate status (74, 150), a finding confirmed in a Canadian study spanning pre- and postfortification, which showed that FA fortification did not alleviate the obesity-related risk of NTDs (134, 169).
Maternal Hyperthermia
Maternal hyperthermia can arise from either febrile illnesses or external exposure to heat, such as prolonged periods in a hot tub or sauna. Epidemiological studies have strongly implicated maternal fever in early pregnancy as a risk factor for NTD-affected pregnancies. A recent meta-analysis evaluated 46 studies ranging from 1990–2013 and found that the NTD risk was increased almost threefold in cases of maternal fever during the first trimester The risk for oral clefts and congenital heart defects also increased (43). Interestingly, studies of Mexican-American women along the Texas-Mexico border (160), a more general US population (48), and Hungarian women (1) indicate that use of antifever medication can significantly alleviate this risk. Furthermore, the use of either a multivitamin or FA supplementation also appears to abrogate the risk associated with maternal febrile illness (1, 20). Surprisingly, animal models of maternal hyperthermia show a mixed response to FA supplementation. In the golden hamster, FA supplementation does not appear to rescue heat-induced NTDs (58), whereas studies indicate that FA can rescue heat-induced NTDs in mice (151).
Early studies linking maternal hyperthermia as a result of prolonged external exposure, such as in a hot tub or sauna, to increased risk for NTDs are mixed. An increased risk was found in several studies (114, 142), but another study found no correlation with sauna use and NTD risk in a Finnish cohort (145). Data collected from the large, multisite National Birth Defects Prevention Study from 1997 to 2005 show the risk for anencephaly increased by 1.7-fold for women who reported using hot tubs during early pregnancy. However, only hot tub sessions lasting more than 30 minutes had a significant effect (44). Overall, in animal and human models, the key factor appears to be raising the core temperature more than 2°C above baseline (32).
The mechanism by which heat may affect the developing embryo is unknown, but there appears to be a genetic component. In one study, five different mouse strains were exposed to hyperthermic treatment on embryonic day 8.5, just as the neural tube is closing. One strain had a 44% incidence of heat-induced NTDs in their offspring, whereas the other four strains showed no more than a 14% penetrance of NTDs (49). A follow-up study confirmed these findings and suggested the importance of maternal genotype in susceptibility to heat-induced NTDs (106).
Although it has been proposed that hyperthermia-induced NTDs may arise from changes in metabolism, research in animal models indicates that it is the heat that adversely affects development, likely as a result of increased cell death, decreased proliferation, disruption of gene expression, and damage to the embryonic vasculature (reviewed in 45). Recent work on mouse neural stem cells showed a narrow temperature range of responsiveness to heat shock (124), leading to induction of apoptosis, inhibition of proliferation, and delayed differentiation. These data highlight the importance of maintaining thermal homeostasis during the critical period of NT closure.
Maternal and Embryonic Exposure to Alcohol and Drugs
Although there is no question that exposure to alcohol in utero is detrimental to the developing fetus, the epidemiology linking maternal alcohol consumption to NTDs is less clear. A small Italian study found that mothers of children with spina bifida were 3 times more likely to have consumed 0.5 liters/day or more of alcohol in the 3 months leading up to and immediately following conception (41). Two American studies, however, showed no correlation between periconceptional maternal alcohol consumption and NTDs (109, 158). However, both American studies were retrospective, and the authors suggest that the social stigma against alcohol during pregnancy may have led to under-reporting. Animal models have shown adverse response to ethanol exposure, evidenced by increased NTDs in mice injected with ethanol around the time of NT closure (64). The authors proposed that ethanol interferes with polyamine synthesis in the developing embryo. As polyamines are known to regulate cell growth and proliferation, this may be one mechanism by which alcohol disrupts embryonic development. Alcohol can also interfere with folate in OCM, although the mechanisms are not well understood (reviewed in 90). Ethanol treatment of pigs results in decreased expression of methionine synthase (MS), a key enzyme in OCM (167), and it has been suggested that ethanol-induced inactivation of MS could lead to a pseudofolate deficiency.
Maternal smoking and maternal exposure to environmental tobacco smoke (ETS) have also been studied epidemiologically with respect to risk for an NTD-affected pregnancy, with conflicting conclusions. Some find that smoking shows no correlation and some show an increased risk of NTD-affected pregnancies. Recent meta-analyses indicate that maternal smoking confers a small increased risk for spina bifida, and a moderately increased NTD risk in cases of maternal ETS exposure (173, 174). Interestingly, the data suggest regional differences in susceptibility, with maternal smoking being a higher risk factor in Europe than in the United States (173). Both smoking and ETS are associated with decreased serum folate levels (125), so it is possible that the increased risk in Europe compared with the United States may be related to differences in FA fortification policies. It has been proposed that the risk associated with maternal smoking may be underestimated, as there is evidence that both maternal smoking and ETS are associated with a significantly increased risk of spontaneous abortion (56), and early fetal loss is rarely included in retrospective studies.
Given the prevalence of caffeine use, several studies have looked at whether periconceptional caffeine use is a risk factor for NTDs. One US study found no correlation between maternal caffeine intake and NTD-affected pregnancy (15), whereas another small study suggested that there might be a slightly elevated risk (147). Moreover, the susceptibility to caffeine as a risk factor varied by maternal race/ethnicity, with non-Hispanic whites showing no correlation and Hispanic and all other ethnicities had a 1.5–2-fold increased risk (147). An Italian study, however, showed a strong association between maternal coffee intake (≥3 cups/day) and spina bifida–associated pregnancy (41), but it was a relatively small study, and the overall conclusions from the study focused on folate deficiency as the most important risk factor. Tea has also been implicated in NTDs, as tea contains catechins that can inhibit DHFR activity. In northern China, daily tea consumption was shown to increase the risk of NTD-affected pregnancy by more than threefold (186), although most of these women were also FA deficient, confounding the interpretation. However, in the United States, no correlation was seen between tea consumption and NTDs (184).
CONCLUSIONS AND FUTURE OUTLOOK
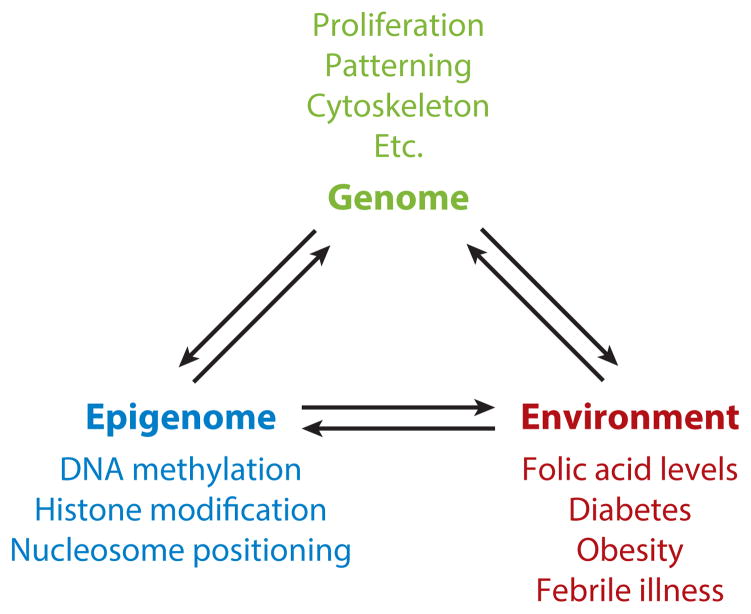
As highlighted in this review, NTDs are a complex disease impacted by genetic susceptibility, epigenetic influences, and environmental insults (Figure 4). The tools are now available to identify the genetic contributions in humans using unbiased methods to evaluate the genome and the epigenome. This, combined with expanded NTD cohorts that include familial and sporadic cases should lead to a much broader understanding of this complex genetic trait. It may be discovered that most sporadic cases of NTD in humans are not due to strong single gene contributions but instead may result from a combination of small genetic perturbations in a number of genes. Animal models will be necessary to provide experimental validation of coding and regulatory polymorphisms, the functional intersections between multiple genetic components, and mechanistic insights into the normal action of these genes and why NT closure fails upon genetic disruption. Finally, there is little insight into the mechanisms that underlie environmental contributions, whether disruptive or preventative. Research has lagged behind in using animal models to decipher how environmental factors impact NT closure and in determining genetic susceptibility to environment influences. Moreover, despite the clear beneficial impact of FA fortification on NTD risk in the general population, there are still a large number of NTD-affected pregnancies. By understanding the cellular and molecular mechanisms of neural tube closure and metabolic factors, it may be possible to identify other measures to help prevent this devastating birth defect.
Figure 4.
Interrelationship between the genome, epigenome, and environment, with respect to neural tube closure.
Acknowledgments
We thank A. Copp, R. Finnell, N. Greene, M. Harris, D. Juriloff, C. Kappen, L. Mitchell, M. Salbaum, and the NTD community for their contributions to the NTD Wiki site and for discussions at the International Conferences on Neural Tube Defects, sponsored by the National Institute of Child Health and Human Development. This work was supported by NIH R01NS058979, and L.N. is an investigator at the Howard Hughes Medical Institute.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Jonathan J. Wilde, Email: jonathan.wilde@ucdenver.edu.
Juliette R. Petersen, Email: juliette.petersen@ucdenver.edu.
Lee Niswander, Email: lee.niswander@ucdenver.edu.
LITERATURE CITED
- 1.Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol. 2005;73(12):989–96. doi: 10.1002/bdra.20195. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar A, Meunier A, Strehl L, Martinovic J, Bonniere M, et al. Analysis of human samples reveals impaired SHH-dependent cerebellar development in Joubert syndrome/Meckel syndrome. Proc Natl Acad Sci USA. 2012;109:16951–56. doi: 10.1073/pnas.1201408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez IS, Schoenwolf GC. Expansion of surface epithelium provides the major extrinsic force for bending of the neural plate. J Exp Zool. 1992;261:340–48. doi: 10.1002/jez.1402610313. [DOI] [PubMed] [Google Scholar]
- 4.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–88. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 5.Aran D, Sabato S, Hellman A. DNA methylation of distal regulatory sites characterizes dysregulation of cancer genes. Genome Biol. 2013;14(3):R21. doi: 10.1186/gb-2013-14-3-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–78. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 7.Attia M, Murko C, Förster A, Lagger S, Rachez C, et al. Interaction between nucleosome assembly protein 1-like family members. J Mol Biol. 2011;407(5):647–60. doi: 10.1016/j.jmb.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Attia M, Rachez C, De Pauw A, Avner P, Rogner UC. Nap1l2 promotes histone acetylation activity during neuronal differentiation. Mol Cell Biol. 2007;27(17):6093–102. doi: 10.1128/MCB.00789-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banting GS. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum Mol Genet. 2004;14(4):513–24. doi: 10.1093/hmg/ddi048. [DOI] [PubMed] [Google Scholar]
- 11.Barua S, Kuizon S, Chadman KK, Flory MJ, Brown WT, Junaid MA. Single-base resolution of mouse offspring brain methylome reveals epigenome modifications caused by gestational folic acid. Epigenet Chromatin. 2014;7(1):1–15. doi: 10.1186/1756-8935-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudin AE, Abarinov EV, Malysheva O, Perry CA, Caudill M, Stover PJ. Dietary folate, but not choline, modifies neural tube defect risk in Shmt1 knockout mice. Am J Clin Nutr. 2011;95:109–14. doi: 10.3945/ajcn.111.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaudin AE, Abarinov EV, Noden DM, Perry CA, Chu S, et al. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am J Clin Nutr. 2011;93(4):789–98. doi: 10.3945/ajcn.110.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaudin AE, Stover PJ. Folate-mediated one-carbon metabolism and neural tube defects: balancing genome synthesis and gene expression. Birth Defects Res C Embryo Today. 2007;81(3):183–203. doi: 10.1002/bdrc.20100. [DOI] [PubMed] [Google Scholar]
- 15.Benedum C, Yazdy M, Mitchell A, Werler M. Risk of spina bifida and maternal cigarette, alcohol, and coffee use during the first month of pregnancy. Int J Environ Res Public Health. 2013;10(8):3263–81. doi: 10.3390/ijerph10083263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat Struct Mol Biol. 2013;20(3):274–81. doi: 10.1038/nsmb.2518. [DOI] [PubMed] [Google Scholar]
- 17.Blattler A, Ronan JL, Farnham PJ, Wu W, Crabtree GR. Cross-talk between site-specific transcription factors and DNA methylation states. J Biol Chem. 2013;288(48):34287–94. doi: 10.1074/jbc.R113.512517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blom HJ, Shaw GM, Heijer den M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7(9):724–31. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borgel J, Guibert S, Li Y, Chiba H, Schübeler D, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nat Genet. 2010;42(12):1093–100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 20.Botto LD, Erickson JD, Mulinare J, Lynberg MC, Liu Y. Maternal fever, multivitamin use, and selected birth defects: evidence of interaction? Epidemiology. 2002;13(4):485–88. doi: 10.1097/00001648-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82(7):527–32. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 22.Bu P, Evrard YA, Lozano G, Dent SYR. Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol Cell Biol. 2007;27(9):3405–16. doi: 10.1128/MCB.00066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bultman SJ, Herschkowitz JI, Godfrey V, Gebuhr TC, Yaniv M, et al. Characterization of mammary tumors from Brg1 heterozygous mice. Oncogene. 2007;27(4):460–68. doi: 10.1038/sj.onc.1210664. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Zhao Z, Eckert RL, Reece EA. Protein kinase Cβ2 inhibition reduces hyperglycemia-induced neural tube defects through suppression of a caspase 8–triggered apoptotic pathway. Am J Obstet. 2011;204:226, e1–5. doi: 10.1016/j.ajog.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Zhao Z, Eckert RL, Reece EA. The essential role of protein kinase Cδ in diabetes-induced neural tube defects. J Matern Fetal Neonatal Med. 2012;25(10):2020–24. doi: 10.3109/14767058.2012.677963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassidy SB, Schwartz S. Prader-Willi and Angelman syndromes: disorders of genomic imprinting. Medicine. 1998;77(2):140–51. doi: 10.1097/00005792-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205(1):97–106. doi: 10.1677/JOE-09-0345. [DOI] [PubMed] [Google Scholar]
- 28.Cent. Dis Control. Prev. CDC Birth Defects Data/Statistics Registry. Atlanta, GA: Cent. Dis. Control Prev; 2011. [Google Scholar]
- 29.Cent. Dis. Control. Prev. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rec. 1992;41:1–77. [PubMed] [Google Scholar]
- 30.Cent. Dis. Control Prev. Use of folic acid–containing supplements among women of childbearing age: United States, 1997. MMWR Morb Mortal Wkly Rep. 1998;47(7):131–34. [PubMed] [Google Scholar]
- 31.Cervoni N. Demethylase activity is directed by histone acetylation. J Biol Chem. 2001;276(44):40778–87. doi: 10.1074/jbc.M103921200. [DOI] [PubMed] [Google Scholar]
- 32.Chambers CD. Risks of hyperthermia associated with hot tub or spa use by pregnant women. Birth Defects Res A Clin Mol Teratol. 2006;76(8):569–73. doi: 10.1002/bdra.20303. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Lai F, Niswander L. The ubiquitin ligase mLin41 temporally promotes neural progenitor cell maintenance through FGF signaling. Genes Dev. 2012;26(8):803–15. doi: 10.1101/gad.187641.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Shen Y, Gao Y, Zhao H, Sheng X, et al. Detection of copy number variants reveals association of cilia genes with neural tube defects. PLoS ONE. 2013;8:e54492. doi: 10.1371/journal.pone.0054492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng H-L, Mostoslavsky R, Saito S, Manis JP, Gu Y, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100(19):10794–99. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Copp AJ, Greene NDE. Genetics and development of neural tube defects. J Pathol. 2010;220(2):217–30. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correa A, Gilboa SM, Botto LD, Moore CA, Hobbs CA, et al. Lack of periconceptional vitamins or supplements that contain folic acid and diabetes mellitus–associated birth defects. Am J Obstet Gynecol. 2012;206(3):218, e1–13. doi: 10.1016/j.ajog.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832–35. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 39.Davidson CM, Northrup H, King TM, Fletcher JM, Townsend I, et al. Genes in glucose metabolism and association with spina bifida. Reprod Sci. 2008;15(1):51–58. doi: 10.1177/1933719107309590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deak KL, Siegel DG, George TM, Gregory S, Ashley-Koch A, et al. Further evidence for a maternal genetic effect and a sex-influenced effect contributing to risk for human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2008;82(10):662–69. doi: 10.1002/bdra.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Marco P, Merello E, Calevo MG, Mascelli S, Pastorino D, et al. Maternal periconceptional factors affect the risk of spina bifida–affected pregnancies: an Italian case-control study. Child’s Nerv Syst. 2011;27(7):1073–81. doi: 10.1007/s00381-010-1372-y. [DOI] [PubMed] [Google Scholar]
- 42.De Wals P, Tairou F, Van Allen MI, Uh S-H, Lowry RB, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007;357(2):135–42. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 43.Dreier JW, Andersen A-MN, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics. 2014;133(3):e674–88. doi: 10.1542/peds.2013-3205. [DOI] [PubMed] [Google Scholar]
- 44.Duong HT, Shahrukh Hashmi S, Ramadhani T, Canfield MA, Scheuerle A, et al. Maternal use of hot tub and major structural birth defects. Birth Defects Res A Clin Mol Teratol. 2011;91(9):836–41. doi: 10.1002/bdra.20831. [DOI] [PubMed] [Google Scholar]
- 45.Edwards MJ, Saunders RD, Shiota K. Effects of heat on embryos and foetuses. Int J Hyperthermia. 2003;19(3):295–324. doi: 10.1080/0265673021000039628. [DOI] [PubMed] [Google Scholar]
- 46.FDA. Food Standards: Amendment of Standards of Identity for Enriched Grain Products to Require Addition of Folic Acid. Vol. 61. Washington, DC: Fed. Regist; 1996. [Google Scholar]
- 47.Feil R, Waterland RA, Fraga MF, Jirtle RL. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13(2):97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 48.Feldkamp ML, Meyer RE, Krikov S, Botto LD. Acetaminophen use in pregnancy and risk of birth defects. Obstet Gynecol. 2010;115(1):109–15. doi: 10.1097/AOG.0b013e3181c52616. [DOI] [PubMed] [Google Scholar]
- 49.Finnell RH, Moon SP, Abbott LC, Golden JA, Chernoff GF. Strain differences in heat-induced neural tube defects in mice. Teratology. 1986;33(2):247–52. doi: 10.1002/tera.1420330213. [DOI] [PubMed] [Google Scholar]
- 50.Foster WH, Langenbacher A, Gao C, Chen J, Wang Y. Nuclear phosphatase PPM1G in cellular survival and neural development. Dev Dyn. 2013;242(9):1101–9. doi: 10.1002/dvdy.23990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–13. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 52.Fukuda T, Li E, Tokunaga A, Bestor TH, Sakamoto R, et al. Fbxl10/Kdm2b deficiency accelerates neural progenitor cell death and leads to exencephaly. Mol Cell Neurosci. 2011;46(3):614–24. doi: 10.1016/j.mcn.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Gao Q, Gao Y-M. Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord. Int J Dev Neurosci. 2007;25(6):349–57. doi: 10.1016/j.ijdevneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Gäreskog M, Eriksson UJ, Wentzel P. Combined supplementation of folic acid and vitamin E diminishes diabetes-induced embryotoxicity in rats. Birth Defects Res A Clin Mol Teratol. 2006;76(6):483–90. doi: 10.1002/bdra.20278. [DOI] [PubMed] [Google Scholar]
- 55.Gelineau-van Waes J, Starr L, Maddox J, Aleman F, Voss KA, et al. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res A Clin Mol Teratol. 2005;73(7):487–97. doi: 10.1002/bdra.20148. [DOI] [PubMed] [Google Scholar]
- 56.George L, Granath F, Johansson ALV, Annerén G, Cnattingius S. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology. 2006;17(5):500–5. doi: 10.1097/01.ede.0000229984.53726.33. [DOI] [PubMed] [Google Scholar]
- 57.Goetzinger KR, Stamilio DM, Dicke JM, Macones GA, Odibo AO. Evaluating the incidence and likelihood ratios for chromosomal abnormalities in fetuses with common central nervous system malformations. Am J Obstet Gynecol. 2008;199(3):285, e1–6. doi: 10.1016/j.ajog.2008.06.100. [DOI] [PubMed] [Google Scholar]
- 58.Graham JM, Ferm VH. Heat- and alcohol-induced neural tube defects: interactions with folate in a golden hamster model. Pediatr Res. 1985;19(2):247–51. doi: 10.1203/00006450-198502000-00022. [DOI] [PubMed] [Google Scholar]
- 59.Gray JD, Nakouzi G, Slowinska-Castaldo B, Dazard J-E, Rao JS, et al. Functional interactions between the LRP6 WNT co-receptor and folate supplementation. Hum Mol Genet. 2010;19(23):4560–72. doi: 10.1093/hmg/ddq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenberg JA, Bell SJ, Guan Y, Yu Y-H. Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. 2011;4(2):52–59. [PMC free article] [PubMed] [Google Scholar]
- 61.Greene NDE, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18(R2):R113–29. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greene NDE, Stanier P, Moore GE. The emerging role of epigenetic mechanisms in the etiology of neural tube defects. Epigenetics. 2011;6(7):875–83. doi: 10.4161/epi.6.7.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haas J, Frese KS, Park YJ, Keller A, Vogel B, et al. Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol Med. 2013;5(3):413–29. doi: 10.1002/emmm.201201553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haghighi Poodeh S, Alhonen L, Salonurmi T, Savolainen MJ. Ethanol-induced impairment of polyamine homeostasis: a potential cause of neural tube defect and intrauterine growth restriction in fetal alcohol syndrome. Biochem Biophys Res Commun. 2014;446:173–78. doi: 10.1016/j.bbrc.2014.02.079. [DOI] [PubMed] [Google Scholar]
- 65.Hall J, Solehdin F. Folic acid for the prevention of congenital anomalies. Eur J Pediatr. 1998;157(6):445–50. doi: 10.1007/s004310050850. [DOI] [PubMed] [Google Scholar]
- 66.Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harmacek L, Watkins-Chow DE, Chen J, Jones KL, Pavan WJ, et al. A unique missense allele of BAF155, a core BAF chromatin remodeling complex protein, causes neural tube closure defects in mice. Dev Neurobiol. 2014;74:483–97. doi: 10.1002/dneu.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris MJ. Insights into prevention of human neural tube defects by folic acid arising from consideration of mouse mutants. Birth Defects Res A Clin Mol Teratol. 2009;85(4):331–39. doi: 10.1002/bdra.20552. [DOI] [PubMed] [Google Scholar]
- 69.Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79(3):187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- 70.Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol. 2010;88(8):653–69. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- 71.Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129(8):1983–93. doi: 10.1242/dev.129.8.1983. [DOI] [PubMed] [Google Scholar]
- 72.He L, Cao J, Meng S, Ma A, Radovick S, Wondisford FE. Activation of basal gluconeogenesis by coactivator p300 maintains hepatic glycogen storage. Mol Endocrinol. 2013;27(8):1322–32. doi: 10.1210/me.2012-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heintzman ND, Ren B. Finding distal regulatory elements in the human genome. Curr Opin Genet Dev. 2009;19(6):541–49. doi: 10.1016/j.gde.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hendricks KA, Nuno OM, Suarez L, Larsen R. Effects of hyperinsulinemia and obesity on risk of neural tube defects among Mexican Americans. Epidemiology. 2001;12(6):630–35. doi: 10.1097/00001648-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Hibbard ED, Smithells RW. Folic acid metabolism and human embryopathy. Lancet. 1965;1(7398):1254. [Google Scholar]
- 76.Hirabayashi Y, Schaniel C, Pujadas E, Banting GS, Gotoh Y, et al. Epigenetic control of neural precursor cell fate during development. Nat Rev Neurosci. 2010;11:377–88. doi: 10.1038/nrn2810. [DOI] [PubMed] [Google Scholar]
- 77.Hiramatsu Y, Sekiguchi N, Hayashi M, Isshiki K, Yokota T, et al. Diacylglycerol production and protein kinase C activity are increased in a mouse model of diabetic embryopathy. Diabetes. 2002;51(9):2804–10. doi: 10.2337/diabetes.51.9.2804. [DOI] [PubMed] [Google Scholar]
- 78.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155(4):934–47. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hook EB, Czeizel AE. Can terathanasia explain the protective effect of folic-acid supplementation on birth defects? Lancet. 1997;350(9076):513–15. doi: 10.1016/S0140-6736(97)01342-1. [DOI] [PubMed] [Google Scholar]
- 80.Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, et al. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST) BMC Public Health. 2011;11(1):46. doi: 10.1186/1471-2458-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joó JG, Beke A, Papp C, Tóth-Pál E, Csaba A, et al. Neural tube defects in the sample of genetic counselling. Prenat Diagn. 2007;27(10):912–21. doi: 10.1002/pd.1801. [DOI] [PubMed] [Google Scholar]
- 82.Juriloff DM, Harris MJ. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2012;94(10):824–40. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- 83.Kappen C, Salbaum JM. Gene expression in teratogenic exposures: a new approach to understanding individual risk. Reprod Toxicol. 2014;45:94–104. doi: 10.1016/j.reprotox.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, et al. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007;356(14):1432–37. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- 85.Kim JK, Huh SO, Choi H, Lee KS, Shin D, et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol. 2001;21(22):7787–95. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korstanje R, Desai J, Lazar G, King B, Rollins J, et al. Quantitative trait loci affecting phenotypic variation in the vacuolated lens mouse mutant, a multigenic mouse model of neural tube defects. Physiol Genomics. 2008;35(3):296–304. doi: 10.1152/physiolgenomics.90260.2008. [DOI] [PubMed] [Google Scholar]
- 87.Kost K. Unintended Pregnancy Rates at the State Level: Estimates For 2002, 2004, 2006 and 2008. New York: Guttmacher Instit; 2013. [Google Scholar]
- 88.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 89.Krebs AR, Karmodiya K, Lindahl-Allen M, Struhl K, Tora L. SAGA and ATAC histone acetyl transferase complexes regulate distinct sets of genes and ATAC defines a class of p300-independent enhancers. Mol Cell. 2011;44(3):410–23. doi: 10.1016/j.molcel.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kruman II, Fowler A-K. Impaired one carbon metabolism and DNA methylation in alcohol toxicity. J Neurochem. 2014;192:770–80. doi: 10.1111/jnc.12677. [DOI] [PubMed] [Google Scholar]
- 91.Kyttala M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38:155–57. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 92.Lacasana M, Blanco-Muñoz J, Borja-Aburto VH, Aguilar-Garduño C, Rodríguez-Barranco M, et al. Effect on risk of anencephaly of gene-nutrient interactions between methylenetetrahydrofolate reductase C677T polymorphism and maternal folate, vitamin B12 and homocysteine profile. Public Health Nutr. 2012;15(8):1419–28. doi: 10.1017/S136898001100334X. [DOI] [PubMed] [Google Scholar]
- 93.Lakkis MM, Golden JA, O’Shea KS, Epstein JA. Neurofibromin deficiency in mice causes exencephaly and is a modifier for Splotch neural tube defects. Dev Biol. 1999;212(1):80–92. doi: 10.1006/dbio.1999.9327. [DOI] [PubMed] [Google Scholar]
- 94.Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lemos MC, Harding B, Reed AAC, Jeyabalan J, Walls GV, et al. Genetic background influences embryonic lethality and the occurrence of neural tube defects in Men1 null mice: relevance to genetic modifiers. J Endocrinol. 2009;203(1):133–42. doi: 10.1677/JOE-09-0124. [DOI] [PubMed] [Google Scholar]
- 96.Lerin C, Rodgers JT, Kalume DE, Kim S-H, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab. 2006;3(6):429–38. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 97.Li CCY, Cropley JE, Cowley MJ, Preiss T, Martin DIK, Suter CM. A sustained dietary change increases epigenetic variation in isogenic mice. PLOS Genet. 2011;7(4):e1001380. doi: 10.1371/journal.pgen.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69(6):915–26. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 99.Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. Am J Obstet Gynecol. 2011;205(1):84, e1–6. doi: 10.1016/j.ajog.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li X, Weng H, Xu C, Reece EA, Yang P. Oxidative stress-induced JNK1/2 activation triggers proapoptotic signaling and apoptosis that leads to diabetic embryopathy. Diabetes. 2012;61(8):2084–92. doi: 10.2337/db11-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Xu C, Yang P. C-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes. 2013;62(2):599–608. doi: 10.2337/db12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135(6):1382–86. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 103.Logan CV, Abdel-Hamed Z, Johnson CA. Molecular genetics and pathogenic mechanisms for the severe ciliopathies: insights into neurodevelopment and pathogenesis of neural tube defects. Mol Neurobiol. 2011;43:12–26. doi: 10.1007/s12035-010-8154-0. [DOI] [PubMed] [Google Scholar]
- 104.López-Camelo JS, Orioli IM, da Graça Dutra M, Nazer-Herrera J, Rivera N, et al. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. Am J Med Genet A. 2005;135(2):120–25. doi: 10.1002/ajmg.a.30651. [DOI] [PubMed] [Google Scholar]
- 105.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lundberg YW, Wing MJ, Xiong W, Zhao J, Finnell RH. Genetic dissection of hyperthermia-induced neural tube defects in mice. Birth Defects Res A Clin Mol Teratol. 2003;67(6):409–13. doi: 10.1002/bdra.10044. [DOI] [PubMed] [Google Scholar]
- 107.Lupo PJ, Canfield MA, Chapa C, Lu W, Agopian AJ, et al. Diabetes and obesity-related genes and the risk of neural tube defects in the national birth defects prevention study. Am J Epidemiol. 2012;176(12):1101–9. doi: 10.1093/aje/kws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lupo PJ, Mitchell LE, Canfield MA, Shaw GM, Olshan AF, et al. Maternal-fetal metabolic gene-gene interactions and risk of neural tube defects. Mol Genet Metab. 2014;111(1):46–51. doi: 10.1016/j.ymgme.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Makelarski JA, Romitti PA, Sun L, Burns TL, Druschel CM, et al. Periconceptional maternal alcohol consumption and neural tube defects. Birth Defects Res A Clin Mol Teratol. 2013;97(3):152–60. doi: 10.1002/bdra.23122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marean A, Graf A, Zhang Y, Niswander L. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Hum Mol Genet. 2011;20(18):3678–83. doi: 10.1093/hmg/ddr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Massarwa R, Niswander L. In toto live imaging of mouse morphogenesis and new insights into neural tube closure. Development. 2013;140(1):226–36. doi: 10.1242/dev.085001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matok I, Gorodischer R, Koren G, Landau D, Wiznitzer A, Levy A. Exposure to folic acid antagonists during the first trimester of pregnancy and the risk of major malformations. Br J Clin Pharmacol. 2009;68(6):956–62. doi: 10.1111/j.1365-2125.2009.03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Michie CA. Neural tube defects in 18th century. Lancet. 1991;337:504. doi: 10.1016/0140-6736(91)93453-g. [DOI] [PubMed] [Google Scholar]
- 114.Milunsky A, Ulcickas M, Rothman KJ, Willett W, Jick SS, Jick H. Maternal heat exposure and neural tube defects. J Am Med Assoc. 1992;268(7):882–85. [PubMed] [Google Scholar]
- 115.Miranda TB, Bassuk AG, Jones PA, Kibar Z. DNA methylation: the nuts and bolts of repression. J Cell Physiol. 2007;213(2):384–90. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 116.Momb J, Lewandowski JP, Bryant JD, Fitch R, Surman DR, et al. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc Natl Acad Sci USA. 2013;110(2):549–54. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MRC Vitam. Study Res. Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–37. [PubMed] [Google Scholar]
- 118.Murko C, Lagger S, Steiner M, Seiser C, Schoefer C, Pusch O. Histone deacetylase inhibitor trichostatin A induces neural tube defects and promotes neural crest specification in the chicken neural tube. Differentiation. 2013;85(1–2):55–66. doi: 10.1016/j.diff.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 119.Nagy Z, Riss A, Romier C, le Guezennec X, Dongre AR, et al. The human SPT20-containing SAGA complex plays a direct role in the regulation of endoplasmic reticulum stress-induced genes. Mol Cell Biol. 2009;29(6):1649–60. doi: 10.1128/MCB.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Narisawa A, Komatsuzaki S, Kikuchi A, Niihori T, Aoki Y, et al. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum Mol Genet. 2012;21(7):1496–503. doi: 10.1093/hmg/ddr585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- 122.Obican SG, Finnell RH, Mills JL, Shaw GM, Scialli AR. Folic acid in early pregnancy: a public health success story. FASEB J. 2010;24(11):4167–74. doi: 10.1096/fj.10-165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 124.Omori H, Otsu M, Suzuki A, Nakayama T, Akama K, et al. Effects of heat shock on survival, proliferation and differentiation of mouse neural stem cells. Neurosci Res. 2014;79:13–21. doi: 10.1016/j.neures.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 125.Ortega RM, Requejo AM, López-Sobaler AM, Navia B, Mena MC, et al. Smoking and passive smoking as conditioners of folate status in young women. J Am Coll Nutr. 2004;23(4):365–71. doi: 10.1080/07315724.2004.10719380. [DOI] [PubMed] [Google Scholar]
- 126.Ougland R, Lando D, Jonson I, Dahl JA, Moen MN, et al. ALKBH1 is a histone H2A dioxygenase involved in neural differentiation. Stem Cells. 2012;30(12):2672–82. doi: 10.1002/stem.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parker SE, Yazdy MM, Tinker SC, Mitchell AA, Werler MM. The impact of folic acid intake on the association among diabetes mellitus, obesity, and spina bifida. Am J Obstet Gynecol. 2013;209(3):239, e1–8. doi: 10.1016/j.ajog.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Partanen M, Motoyama J, Hui CC. Developmentally regulated expression of the transcriptional cofactors/histone acetyltransferases CBP and p300 during mouse embryogenesis. Int J Dev Biol. 1999;43:487–94. [PubMed] [Google Scholar]
- 129.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr. 2012;142(5):886–93. doi: 10.3945/jn.111.156919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pujadas E, Feinberg AP. Regulated noise in the epigenetic landscape of development and disease. Cell. 2012;148(6):1123–31. doi: 10.1016/j.cell.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raj A, Rifkin SA, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature. 2011;463(7283):913–18. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198(6):611–19. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 133.Ray HJ, Niswander L. Mechanisms of tissue fusion during development. Development. 2012;139(10):1701–11. doi: 10.1242/dev.068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ray JG, Wyatt PR, Vermeulen MJ, Meier C, Cole DEC. Greater maternal weight and the ongoing risk of neural tube defects after folic acid flour fortification. Obstet Gynecol. 2005;105(2):261–65. doi: 10.1097/01.AOG.0000151988.84346.3e. [DOI] [PubMed] [Google Scholar]
- 135.Reece EA. Diabetes-induced birth defects: What do we know? What can we do? Curr Diab Rep. 2012;12(1):24–32. doi: 10.1007/s11892-011-0251-6. [DOI] [PubMed] [Google Scholar]
- 136.Robert E, Smith-Roe SL, Guibaud P, Bultman SJ. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2(8304):937. doi: 10.1016/s0140-6736(82)90908-4. [DOI] [PubMed] [Google Scholar]
- 137.Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, et al. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 2012;33(2):440–47. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rogner UC, Spyropoulos DD, Le Novère N, Changeux JP, Avner P. Control of neurulation by the nucleosome assembly protein-1-like 2. Nat Genet. 2000;25(4):431–35. doi: 10.1038/78124. [DOI] [PubMed] [Google Scholar]
- 139.Sadler TW, Merrill AH, Stevens VL, Sullards MC, Wang E, Wang P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology. 2002;66(4):169–76. doi: 10.1002/tera.10089. [DOI] [PubMed] [Google Scholar]
- 140.Sakai M, Tujimura T, Yongheng C, Noguchi T, Inagaki K, et al. CITED2 links hormonal signaling to PGC-1α; acetylation in the regulation of gluconeogenesis. Nat Med. 2012;18:612–17. doi: 10.1038/nm.2691. [DOI] [PubMed] [Google Scholar]
- 141.Salbaum JM, Kappen C. Neural tube defect genes and maternal diabetes during pregnancy. Birth Defects Res A Clin Mol Teratol. 2010;88(8):601–11. doi: 10.1002/bdra.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sandford MK, Kissling GE, Joubert PE. Neural tube defect etiology: new evidence concerning maternal hyperthermia, health and diet. Dev Med Child Neurol. 1992;34(8):661–75. doi: 10.1111/j.1469-8749.1992.tb11502.x. [DOI] [PubMed] [Google Scholar]
- 143.Sandovici I, Harris MJ, Smith NH, Juriloff DM, Nitert MD, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci USA. 2011;108(13):5449–54. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489(7414):109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saxén L, Holmberg PC, Nurminen M, Kuosma E. Sauna and congenital defects. Teratology. 1982;25(3):309–13. doi: 10.1002/tera.1420250307. [DOI] [PubMed] [Google Scholar]
- 146.Schaniel C, Ang Y-S, Ratnakumar K, Cormier C, James T, et al. Smarcc1/Baf155 couples self-renewal gene repression with changes in chromatin structure in mouse embryonic stem cells. Stem Cells. 2009;27:2979–91. doi: 10.1002/stem.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schmidt RJ, Romitti PA, Burns TL, Browne ML, Druschel CM, et al. Maternal caffeine consumption and risk of neural tube defects. Birth Defects Res A Clin Mol Teratol. 2009;85(11):879–89. doi: 10.1002/bdra.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8(1):9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 149.Shakèd M, Weissmüller K, Svoboda H, Hortschansky P, Nishino N, et al. Histone deacetylases control neurogenesis in embryonic brain by inhibition of BMP2/4 signaling. PLOS ONE. 2008;3(7):e2668. doi: 10.1371/journal.pone.0002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shaw GM, Todoroff K, Finnell RH, Lammer EJ. Spina bifida phenotypes in infants or fetuses of obese mothers. Teratology. 2000;61(5):376–81. doi: 10.1002/(SICI)1096-9926(200005)61:5<376::AID-TERA9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 151.Shin J-H, Shiota K. Folic acid supplementation of pregnant mice suppresses heat-induced neural tube defects in the offspring. J Nutr. 1999;129:2070–73. doi: 10.1093/jn/129.11.2070. [DOI] [PubMed] [Google Scholar]
- 152.Shpargel KB, Sengoku T, Yokoyama S, Magnuson T. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet. 2012;8(9):e1002964. doi: 10.1371/journal.pgen.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shyamasundar S, Jadhav SP, Bay BH, Tay SSW, Kumar SD, et al. Analysis of epigenetic factors in mouse embryonic neural stem cells exposed to hyperglycemia. PLOS ONE. 2013;8(6):e65945. doi: 10.1371/journal.pone.0065945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, et al. Periconceptional maternal folic acid use of 400 μg per day is related to increased methylation of the IGF2 gene in the very young child. PLOS ONE. 2009;4(11):e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. J Am Med Assoc. 2009;301(6):636–50. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 156.Stover PJ, Caudill MA. Genetic and epigenetic contributions to human nutrition and health: managing genome-diet interactions. J Am Diet Assoc. 2008;108(9):1480–87. doi: 10.1016/j.jada.2008.06.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Struhl K, Rogner UC, Segal E, Spyropoulos DD, Le Novère N, et al. Determinants of nucleosome positioning. Nat Struct Mol Biol. 2013;20(3):267–73. doi: 10.1038/nsmb.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Suarez L, Felkner M, Brender JD, Canfield M, Hendricks K. Maternal exposures to cigarette smoke, alcohol, and street drugs and neural tube defect occurrence in offspring. Matern Child Health J. 2007;12(3):394–401. doi: 10.1007/s10995-007-0251-y. [DOI] [PubMed] [Google Scholar]
- 159.Suarez L, Felkner M, Brender JD, Canfield M, Zhu H, Hendricks KA. Neural tube defects on the Texas-Mexico border: what we’ve learned in the 20 years since the Brownsville cluster. Birth Defects Res A Clin Mol Teratol. 2012;94(11):882–92. doi: 10.1002/bdra.23070. [DOI] [PubMed] [Google Scholar]
- 160.Suarez L, Felkner M, Hendricks K. The effect of fever, febrile illnesses, and heat exposures on the risk of neural tube defects in a Texas-Mexico border population. Birth Defects Res A Clin Mol Teratol. 2004;70(10):815–19. doi: 10.1002/bdra.20077. [DOI] [PubMed] [Google Scholar]
- 161.Suzuki MM, Zhang Y, Salbaum JM, Bamforth SD, Bird A, et al. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9(6):465–76. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 162.Takeuchi T, Yamazaki Y, Katoh-Fukui Y, Tsuchiya R, Kondo S, et al. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995;9(10):1211–22. doi: 10.1101/gad.9.10.1211. [DOI] [PubMed] [Google Scholar]
- 163.Thiersch JB. Therapeutic abortions with a folic acid antagonist, 4-aminopteroylglutamic acid (4-amino P.G.A) administered by the oral route. Am J Obstet Gynecol. 1952;63(6):1298–304. doi: 10.1016/s0002-9378(16)38924-4. [DOI] [PubMed] [Google Scholar]
- 164.Thiersch JB, Philips FS. Effect of 4-amino-pteroylglutamic acid (aminopterin) on early pregnancy. Proc Soc Exp Biol Med. 1950;74(1):204–8. doi: 10.3181/00379727-74-17855. [DOI] [PubMed] [Google Scholar]
- 165.van der Put NM, Steegers-Theunissen RP, Frosst P, Trijbels FJ, Eskes TK, et al. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet. 1995;346(8982):1070–71. doi: 10.1016/s0140-6736(95)91743-8. [DOI] [PubMed] [Google Scholar]
- 166.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555–66. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 167.Villanueva JA, Halsted CH. Hepatic transmethylation reactions in micropigs with alcoholic liver disease. Hepatology. 2004;39(5):1303–10. doi: 10.1002/hep.20168. [DOI] [PubMed] [Google Scholar]
- 168.Wald NJ, Law MR, Morris JK, Wald DS. Quantifying the effect of folic acid. Lancet. 2001;358(9298):2069–73. doi: 10.1016/s0140-6736(01)07104-5. [DOI] [PubMed] [Google Scholar]
- 169.Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161(8):745–50. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- 170.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–53. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 171.Wallingford JB, Niswander LA, Shaw GM, Finnell RH. The continuing challenge of understanding, preventing, and treating neural tube defects. Science. 2013;339(6123):1222002. doi: 10.1126/science.1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. Am J Obstet Gynecol. 2013;209(4):345, e1–7. doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang M, Wang Z-P, Gong R, Zhao Z-T. Maternal smoking during pregnancy and neural tube defects in offspring: a meta-analysis. Child’s Nerv Syst. 2014;30(1):83–89. doi: 10.1007/s00381-013-2194-5. [DOI] [PubMed] [Google Scholar]
- 174.Wang M, Wang Z-P, Zhang M, Zhao Z-T. Maternal passive smoking during pregnancy and neural tube defects in offspring: a meta-analysis. Arch Gynecol Obstet. 2014;289(3):513–21. doi: 10.1007/s00404-013-2997-3. [DOI] [PubMed] [Google Scholar]
- 175.Warkany J, Beaudry PH, Hornstein S. Attempted abortion with aminopterin (4-amino-pteroylglutamic acid); malformations of the child. Am Med Assoc J Dis Child. 1959;97(3):274–81. doi: 10.1001/archpedi.1959.02070010276003. [DOI] [PubMed] [Google Scholar]
- 176.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and hedgehog signaling. Hum Mol Genet. 2009;18:4565–75. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wei X, Li H, Miao J, Liu B, Zhan Y, et al. miR-9*- and miR-124a-mediated switching of chromatin remodelling complexes is altered in rat spina bifida aperta. Neurochem Res. 2013;38(8):1605–15. doi: 10.1007/s11064-013-1062-8. [DOI] [PubMed] [Google Scholar]
- 179.Welstead GG, Creyghton MP, Bilodeau S, Cheng AW, Markoulaki S, et al. X-linked H3K27me3 demethylase Utx is required for embryonic development in a sex-specific manner. Proc Natl Acad Sci. 2012;109(32):13004–9. doi: 10.1073/pnas.1210787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu C, Li X, Wang F, Weng H, Yang P. Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. Am J Physiol Endocrinol Metab. 2013;305(5):E667–78. doi: 10.1152/ajpendo.00185.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yan L, Zhao L, Long Y, Zou P, Ji G, et al. Association of the maternal MTHFR C677T polymorphism with susceptibility to neural tube defects in offsprings: evidence from 25 case-control studies. PLOS ONE. 2012;7(10):e41689. doi: 10.1371/journal.pone.0041689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang P, Li X, Xu C, Eckert RL, Reece EA, et al. Maternal hyperglycemia activates an ASK1-FoxO3a-caspase 8 pathway that leads to embryonic neural tube defects. Sci Signal. 2013;6(290):ra74. doi: 10.1126/scisignal.2004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochem Biophys Res Commun. 2007;357(3):749–54. doi: 10.1016/j.bbrc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 184.Yazdy MM, Tinker SC, Mitchell AA, Demmer LA, Werler MM. Maternal tea consumption during early pregnancy and the risk of spina bifida. Birth Defects Res A Clin Mol Teratol. 2012;94(10):756–61. doi: 10.1002/bdra.23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134(4):789–99. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye R, Ren A, Zhang L, Li Z, Liu J, et al. Tea drinking as a risk factor for neural tube defects in northern China. Epidemiology. 2011;22(4):491–96. doi: 10.1097/EDE.0b013e31821b4526. [DOI] [PubMed] [Google Scholar]
- 187.Yin Z, Haynie J, Yang X, Han B, Kiatchoosakun S, et al. The essential role of Cited2, a negative regulator for HIF-1α, in heart development and neurulation. Proc Natl Acad Sci USA. 2002;99(16):10488–93. doi: 10.1073/pnas.162371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Youngblood ME, Williamson R, Bell KN, Johnson Q, Kancherla V, Oakley GP., Jr 2012 Update on global prevention of folic acid–preventable spina bifida and anencephaly. Birth Defects Res A Clin Mol Teratol. 2013;97(10):658–63. doi: 10.1002/bdra.23166. [DOI] [PubMed] [Google Scholar]
- 189.Yuan Q, Zhao S, Liu S, Zhang Y, Fu J, et al. Folic acid supplementation changes the fate of neural progenitors in mouse embryos of hyperglycemic and diabetic pregnancy. J Nutr Biochem. 2013;24(7):1202–12. doi: 10.1016/j.jnutbio.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 190.Zabihi S, Loeken MR. Understanding diabetic teratogenesis: Where are we now and where are we going? Birth Defects Res A Clin Mol Teratol. 2010;88(10):779–90. doi: 10.1002/bdra.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang T, Lou J, Zhong R, Wu J, Zou L, et al. Genetic variants in the folate pathway and the risk of neural tube defects: a meta-analysis of the published literature. PLOS ONE. 2013;8(4):e59570. doi: 10.1371/journal.pone.0059570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhao Z, Yang P, Eckert RL, Reece EA. Caspase-8: a key role in the pathogenesis of diabetic embryopathy. Birth Defects Res B. 2009;86(1):72–77. doi: 10.1002/bdrb.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]