Abstract
Early male-killing (MK) bacteria are vertically transmitted reproductive parasites which kill male offspring that inherit them. Whereas their incidence is well documented, characteristics allowing originally non-MK bacteria to gradually evolve MK ability remain unclear. We show that horizontal transmission is a mechanism enabling vertically transmitted bacteria to evolve fully efficient MK under a wide range of host and parasite characteristics, especially when the efficacy of vertical transmission is high. We also show that an almost 100% vertically transmitted and 100% effective male-killer may evolve from a purely horizontally transmitted non-MK ancestor, and that a 100% efficient male-killer can form a stable coexistence only with a non-MK bacterial strain. Our findings are in line with the empirical evidence on current MK bacteria, explain their high efficacy in killing infected male embryos and their variability within and across insect taxa, and suggest that they may have evolved independently in phylogenetically distinct species.
Keywords: adaptive dynamics, horizontal transmission, population dynamics, reproductive parasite, sex-ratio distortion, Wolbachia
1. Introduction
Male-killing (MK) bacteria are vertically transmitted parasites that alter the biology of their hosts by killing the males that inherit them. They are widespread among insects and are passed to the next generation almost exclusively through the female egg cytoplasm [1–3]. Infected males are killed either in their early phase of embryonic development (early MK) or during a late developmental stage (late MK); in the latter case, male death increases the chance of successful horizontal transmission (HT) [4,5]. The MK bacterium thus benefits from vertical transmission (VT) where possible and from HT otherwise. As a direct cost, MK reduces female reproductive output and distorts the host sex ratio in favour of females.
Although the majority of current MK bacteria are transmitted predominantly vertically, additional HT has been documented, e.g. in mosquito-Microsporidia systems or an agent causing late MK in the tea tortrix Homona magnanima [4]. Given the evidence of HT in MK bacteria, we explore whether early MK could gradually evolve in originally non-MK parasites transmitted both vertically and horizontally. For this, we developed an evolutionary model of potentially early MK bacteria whose ancestor is a virulent non-MK parasite transmitted both vertically and horizontally, or even purely horizontally.
Insects demonstrate a variety of mating systems from polygyny where a male tends to mate with many females, through monogamy up to polyandry where a female tends to mate with multiple males. MK bacteria may affect the host mating system in a variety of ways [6–8]. In particular, Jiggins et al. [9] documented that due to the presence of an MK bacterium, a formerly polygynous system became polyandrous. The reverse may also be possible: different mating systems may be variously supportive of the evolution of MK. As this reverse idea has not been assessed yet, we consider the mating system to be established and let the male-killer adapt to it.
We focus on early MK, in which case infected males are killed during embryogenesis and the surviving offspring benefit from reduced competition between siblings and/or extra resources in the form of their dead brothers, enhancing their fitness relative to when no MK occurs. We show that with at least a bit of HT, the evolution of early MK can occur under a variety of conditions. In particular, male-killers must be transmitted vertically with high enough efficacy and enhance fitness of the surviving host offspring sufficiently. Also, we demonstrate that 100% efficient male-killers are the rule, and that they may evolve even from a purely horizontally transmitted non-MK ancestor. Last but not least, we show that a fully efficient male-killer can form a stable coexistence with a non-MK parasite over evolutionary time.
2. Material and methods
We describe the ecological dynamics of uninfected females Fu and males Mu and of infected females Fi and males Mi by the following two-sex model:
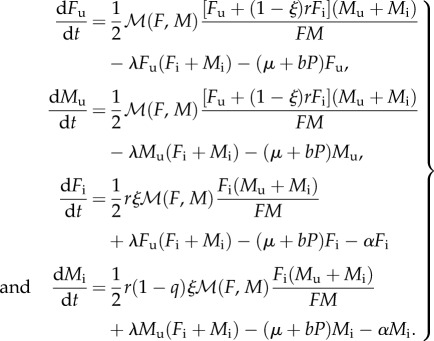 |
2.1 |
We denote by 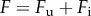 and
and 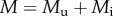 the female and male population density, respectively. Females and males mate to produce progeny with a 1 : 1 sex ratio. Mating (and subsequent reproduction) occurs randomly at rate
the female and male population density, respectively. Females and males mate to produce progeny with a 1 : 1 sex ratio. Mating (and subsequent reproduction) occurs randomly at rate 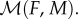 A proportion ξ of the offspring produced by infected females becomes infected. Because of the action of MK bacteria, a proportion q of infected male offspring die. This cost of infection is counterbalanced by a benefit r provided to the surviving offspring. The rate at which the hosts acquire new infections horizontally is
A proportion ξ of the offspring produced by infected females becomes infected. Because of the action of MK bacteria, a proportion q of infected male offspring die. This cost of infection is counterbalanced by a benefit r provided to the surviving offspring. The rate at which the hosts acquire new infections horizontally is  and is assumed to be sex independent. The hosts suffer from density-dependent mortality
and is assumed to be sex independent. The hosts suffer from density-dependent mortality 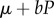 , where μ is the natural intrinsic per capita mortality rate, b is the strength of density dependence and
, where μ is the natural intrinsic per capita mortality rate, b is the strength of density dependence and 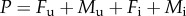 is the total population density. All parameters are listed in the electronic supplementary material, table S1; see also table S2 for details on the MK process. A stability analysis of the model (2.1) is provided in the electronic supplementary material, appendix S1. Alternatively, we consider density dependence acting on births by setting b = 0 in the model (2.1) and multiplying the first, reproductive term in all equations by the Beverton–Holt form 1/(1 + kP).
is the total population density. All parameters are listed in the electronic supplementary material, table S1; see also table S2 for details on the MK process. A stability analysis of the model (2.1) is provided in the electronic supplementary material, appendix S1. Alternatively, we consider density dependence acting on births by setting b = 0 in the model (2.1) and multiplying the first, reproductive term in all equations by the Beverton–Holt form 1/(1 + kP).
The model (2.1) contains several essential components that require a closer look. While we present them here only briefly, all are discussed at large in the electronic supplementary material, appendix S2. Given the VT efficacy ξ, the fitness benefit r conferred to the surviving offspring is expressed as [10,11]
| 2.2 |
where 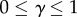 is the fraction of freed resources that can be redistributed among the surviving offspring (further referred to as ‘fitness compensation parameter’). Those freed resources are available to all surviving offspring, including the uninfected progeny. Note that the benefit conferred by MK is only added through fecundity.
is the fraction of freed resources that can be redistributed among the surviving offspring (further referred to as ‘fitness compensation parameter’). Those freed resources are available to all surviving offspring, including the uninfected progeny. Note that the benefit conferred by MK is only added through fecundity.
The next component is the mating rate 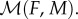 To account for a wide range of mating systems, we consider a frequently used and empirically supported harmonic mean function [12,13]. This function depends on the densities of females (F) and males (M) as:
To account for a wide range of mating systems, we consider a frequently used and empirically supported harmonic mean function [12,13]. This function depends on the densities of females (F) and males (M) as:
| 2.3 |
where c is the per-mating number of offspring (birth rate). The mating system is controlled by the parameter h; h < 1 corresponds to polyandry, h = 1 corresponds to monogamy and h > 1 corresponds to polygyny.
Finally, in line with most theoretical studies on virulence evolution, we assume a positive correlation between the parasite-induced host mortality α (further referred to as ‘virulence’) and HT rate λ [14], which we model as
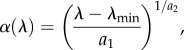 |
2.4 |
where 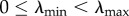 are, respectively, the minimum and maximum HT rates and a1 and a2 are positive constants; α is a convex (or concave) function of λ for 0 < a2 < 1 (or a2 > 1). Also, there might be a trade-off between the VT efficacy ξ and HT rate λ [15,16], which we model as
are, respectively, the minimum and maximum HT rates and a1 and a2 are positive constants; α is a convex (or concave) function of λ for 0 < a2 < 1 (or a2 > 1). Also, there might be a trade-off between the VT efficacy ξ and HT rate λ [15,16], which we model as
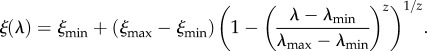 |
2.5 |
Here, 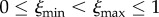 are, respectively, the minimum and maximum VT efficacies. The parameter z > 0 can be viewed to express a cost of VT in the sense of how much the HT rate can increase when small if the VT efficacy drops by a value. With this interpretation, we refer to an increase in z as a decrease in the cost of VT (z < 1, cheap VT), and a decrease in z as an increase in the cost of VT (z > 1, costly VT); ξ is a convex (or concave) function of λ for z < 1 (or z > 1). We note that beyond both trade-offs lies an assumption that the virulence α, HT rate λ and VT efficacy ξ are all related and affected by the parasite's ‘exploitation strategy’ of the host; this relationship is made explicit in the electronic supplementary material, appendix S2.
are, respectively, the minimum and maximum VT efficacies. The parameter z > 0 can be viewed to express a cost of VT in the sense of how much the HT rate can increase when small if the VT efficacy drops by a value. With this interpretation, we refer to an increase in z as a decrease in the cost of VT (z < 1, cheap VT), and a decrease in z as an increase in the cost of VT (z > 1, costly VT); ξ is a convex (or concave) function of λ for z < 1 (or z > 1). We note that beyond both trade-offs lies an assumption that the virulence α, HT rate λ and VT efficacy ξ are all related and affected by the parasite's ‘exploitation strategy’ of the host; this relationship is made explicit in the electronic supplementary material, appendix S2.
3. Results
Purely vertically transmitted bacteria (strains with λ = 0) which kill infected male offspring with 100% efficacy can successfully establish in the host population if the VT efficacy ξ and fitness compensation parameter γ (equation (2.2)) are high enough [10]. However, highly efficient male-killers might have evolved gradually from less-efficient male-killers, and from bacteria that did not possess any MK ability [17]. Our analysis reveals that less efficient male-killers are not viable where highly efficient male-killers can persist (electronic supplementary material, appendix S3). How can a high or even full MK ability evolve gradually? It turns out that a bit of HT allows originally non-MK bacteria to gradually evolve MK.
We assume two scenarios for the evolution of early MK. First, we assume ξ as independent of the HT rate λ and fixed (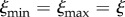 ). In this case, the parasite has no control over how effectively it can be passed to the host offspring (it may be the host who regulates the efficacy of VT). Second, we let the parasite control its VT efficacy and change ξ with λ along the trade-off (2.5). In addition, we consider two types of evolutionary dynamics, each for both scenarios. In sequential trait evolution, we first let the parasite evolve its HT rate λ. Once λ settles at an evolutionary attractor, we let the parasite evolve its MK ability q. In simultaneous trait evolution, we allow both λ and q to coevolve.
). In this case, the parasite has no control over how effectively it can be passed to the host offspring (it may be the host who regulates the efficacy of VT). Second, we let the parasite control its VT efficacy and change ξ with λ along the trade-off (2.5). In addition, we consider two types of evolutionary dynamics, each for both scenarios. In sequential trait evolution, we first let the parasite evolve its HT rate λ. Once λ settles at an evolutionary attractor, we let the parasite evolve its MK ability q. In simultaneous trait evolution, we allow both λ and q to coevolve.
A parasite can invade a fully susceptible host population at its carrying capacity 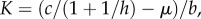 if its basic reproduction number
if its basic reproduction number
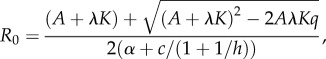 |
3.1 |
where 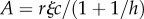 is greater than 1. If R0 < 1, the parasite dies out.
is greater than 1. If R0 < 1, the parasite dies out.
(a). Sequential trait evolution
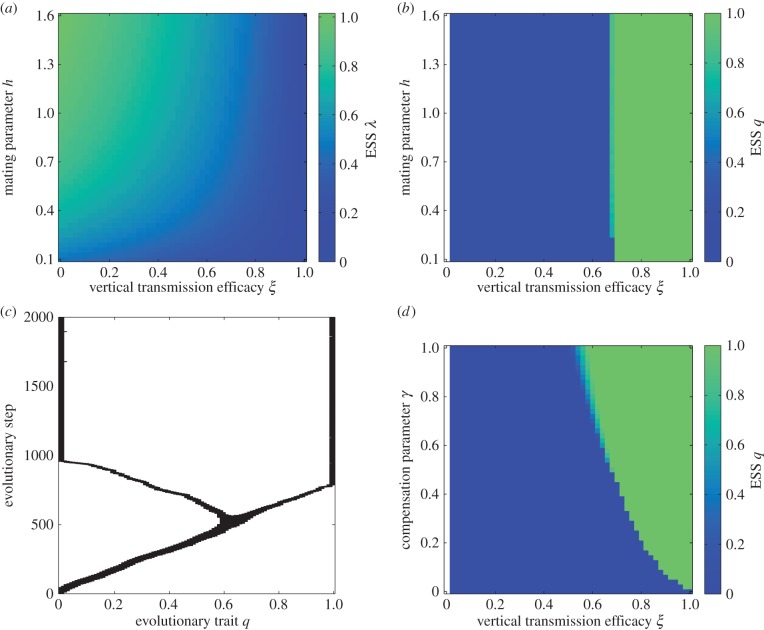
Assume a convex trade-off (2.4) (0 < a2 < 1) and a successful host invasion by a non-MK parasite with a fixed VT efficacy ξ (R0 > 1). Using the techniques of adaptive dynamics [18], we first show that the HT rate λ attains only unique evolutionary attractors λ* (figure 1a; electronic supplementary material, appendix S4). The value of λ* declines with increasing ξ, because the relative number of uninfected hosts and, therefore, the opportunity for HT declines. Conversely, λ* increases when the mating system changes from polyandrous (h < 1) to polygynous (h > 1). In polyandrous populations, a strong competition among females for males lowers the female mating rate by preventing females from finding a mate. Consequently, their contribution to the uninfected population is relatively small, which extorts lower values of λ*. Polygyny relaxes competition among females for males, less females stay unmated and the increased female mating rate allows for increased production of susceptible offspring. As a result, evolution tends to increase the HT rate λ*.
Figure 1.
Sequential trait evolution when the VT efficacy ξ is fixed. (a) Evolutionary attractors λ* when q = 0. (b) Evolutionary endpoints q* provided that the bacterium has the HT rate λ*; the transition zone between q* = 0 and q* = 1 is formed by evolutionary branching points. (c) Simulation of evolutionary branching of an originally non-MK bacterium transmitted horizontally at λ*. (d) Evolutionary endpoints q* provided that the bacterium has the HT rate λ*. White colour = region of non-persistence (R0 < 1). Parameters: (a–c) γ = 0.5, (c) ξ = 0.68, h = 0.52 and (d) h = 1. Common parameters: a1 = 1, a2 = 0.5, c = 3 and μ = b = 0.1. ESS: evolutionary singular strategy. (Online version in colour.)
Once the HT rate of a non-MK bacterium settles at an evolutionary attractor λ*, it is subject to invasion of rare mutants initially with a low MK efficacy 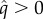 (electronic supplementary material, appendix S5). Surprisingly, the mating system affects the evolution of MK only insignificantly (figure 1b). Rather, it is the VT efficacy ξ which determines whether MK evolves; bacteria transmitted vertically with high enough ξ evolve 100% MK efficacy (q = 1). By contrast, bacteria with low ξ do not evolve any MK ability (q = 0). For a narrow range of intermediate ξ, evolutionary branching occurs and the bacterial strains with 100% MK efficacy and no MK ability coexist (figure 1c). Finally, if the fitness compensation parameter γ is low, it must be overbalanced by enhanced VT efficacy ξ if MK is to evolve (figure 1d). If there is no benefit from killing the infected males (i.e. γ = 0), MK cannot evolve for any value of ξ. We observe qualitatively similar evolutionary results when density dependence acts on births instead of deaths (electronic supplementary material, appendix S6.1; see also appendix S6.2 for the case of concave trade-off (2.4) and appendix S6.3 for the case of sequential trait evolution when VT efficacy ξ and HT rate λ are linked via trade-off (2.5)).
(electronic supplementary material, appendix S5). Surprisingly, the mating system affects the evolution of MK only insignificantly (figure 1b). Rather, it is the VT efficacy ξ which determines whether MK evolves; bacteria transmitted vertically with high enough ξ evolve 100% MK efficacy (q = 1). By contrast, bacteria with low ξ do not evolve any MK ability (q = 0). For a narrow range of intermediate ξ, evolutionary branching occurs and the bacterial strains with 100% MK efficacy and no MK ability coexist (figure 1c). Finally, if the fitness compensation parameter γ is low, it must be overbalanced by enhanced VT efficacy ξ if MK is to evolve (figure 1d). If there is no benefit from killing the infected males (i.e. γ = 0), MK cannot evolve for any value of ξ. We observe qualitatively similar evolutionary results when density dependence acts on births instead of deaths (electronic supplementary material, appendix S6.1; see also appendix S6.2 for the case of concave trade-off (2.4) and appendix S6.3 for the case of sequential trait evolution when VT efficacy ξ and HT rate λ are linked via trade-off (2.5)).
(b). Simultaneous trait evolution
As evolutionary changes in the MK efficacy q may impose selection pressure on the HT rate λ, we also investigated a coevolution of q and λ; the adopted coevolutionary simulation model is described in the electronic supplementary material, appendix S7.
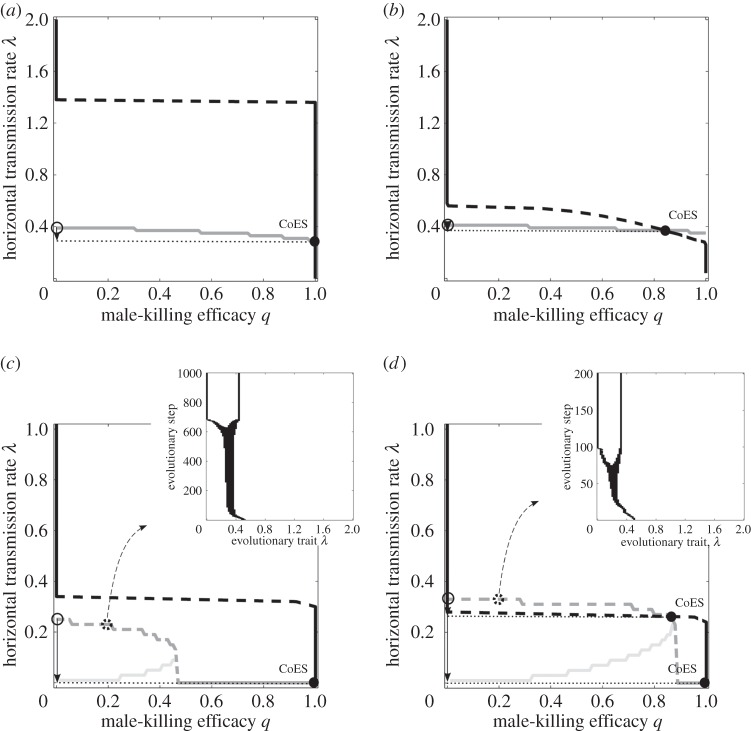
Simulations show that sequential trait evolution is an accurate first approximation of simultaneous trait evolution. Whereas MK does not evolve for fixed, low VT efficacies ξ (results not shown), 100% MK ability evolves for high enough values of ξ (figure 2a). For intermediate values of ξ, the parasite approaches a coevolutionary singularity (CoES) with the HT rate 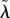 as an attractor and the MK efficacy
as an attractor and the MK efficacy 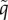 as a branching point (figure 2b). MK then evolves via evolutionary branching and the non-MK and fully efficient MK strains coexist (results not shown). Coadaptation of λ and q generally leads to only a slight decrease in the eventual HT rate (figure 2a–b).
as a branching point (figure 2b). MK then evolves via evolutionary branching and the non-MK and fully efficient MK strains coexist (results not shown). Coadaptation of λ and q generally leads to only a slight decrease in the eventual HT rate (figure 2a–b).
Figure 2.
Simultaneous trait evolution when (a–b) the VT efficacy ξ is fixed, and (c–d) the VT efficacy ξ and HT rate λ are linked via the trade-off (2.5). The grey and black curves represent evolutionary singularities (λ* as a function of q and q* as a function of λ, respectively) once the parasite is allowed to kill the infected males. The evolutionary attracting HT rate in the absence of MK (q = 0) is represented by the open circle. The filled circle then represents the coevolutionary singularity (CoES) of the HT rate 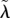 and MK efficacy
and MK efficacy 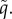 The solid arrows in the bottom left corners show the decrease in the HT rate (from λ* to
The solid arrows in the bottom left corners show the decrease in the HT rate (from λ* to 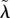 ) following coevolution of λ and q. Legend: solid dark grey, attractor
) following coevolution of λ and q. Legend: solid dark grey, attractor 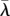 ; dashed dark grey, branching point
; dashed dark grey, branching point 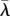 ; light grey, repellor
; light grey, repellor 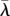 ; solid black, attracting lower
; solid black, attracting lower 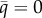 or upper
or upper 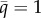 bound; dashed black, branching point
bound; dashed black, branching point 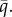 Parameters: (a) ξ = 0.8, h = 1, (b) ξ = 0.68, h = 0.52, (c) z = 0.75, h = 1 and (d) z = 0.7, h = 1. Common parameters: a1 = 1, a2 = 0.5, c = 3 and μ = b = 0.1.
Parameters: (a) ξ = 0.8, h = 1, (b) ξ = 0.68, h = 0.52, (c) z = 0.75, h = 1 and (d) z = 0.7, h = 1. Common parameters: a1 = 1, a2 = 0.5, c = 3 and μ = b = 0.1.
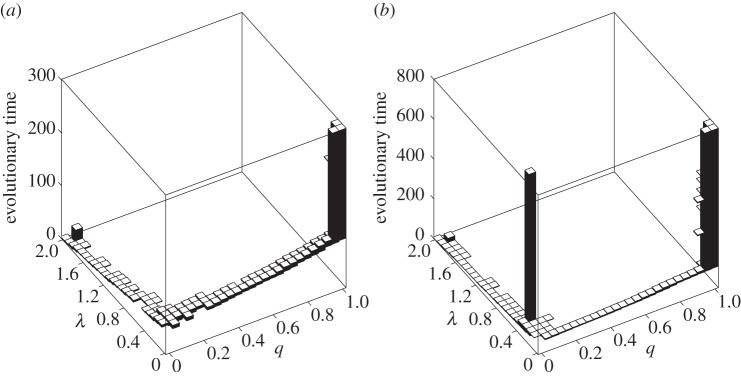
Once the VT efficacy ξ is traded off with the HT rate λ via the formula (2.5), evolutionary suicide occurs if the HT rate is allowed to vanish (λmin = 0; see electronic supplementary material, appendix S1.3). On the contrary, if λmin > 0, bacteria can attain 100% MK either directly for cheap VT (figures 2c, 3a), or via evolutionary branching for moderately costly VT (figure 2d). In the latter case, one branch corresponds to an almost purely horizontally transmitted non-male-killer and the other branch to an almost purely vertically transmitted male-killer (figure 3b). Therefore, if the evolving MK bacterium is to persist, at least a bit of HT needs to be preserved in the host–male-killer association. Coevolution then results in 100% MK, nearly 100% VT, and the lowest possible HT; coadaptation of λ and q thus causes a substantial decrease in the HT rate (figure 2c–d). This outcome emerges not only for initially high λ (and hence low or even no ξ) but also for initially intermediate or low λ (and hence intermediate or high ξ, respectively).
Figure 3.
Simultaneous trait evolution when the VT efficacy ξ is linked to the HT rate λ via the trade-off (2.5) and the parasite is initiated far from its evolutionary attracting HT rate λ* . (a) Coevolutionary attractor. (b) Stable coexistence of two distinct morphs with q = 0 and q = 1 following evolutionary branching. Parameters: (a) z = 0.9, h = 1 and (b) z = 0.75, h = 1. Common parameters: γ = 0.5, a1 = 1, a2 = 0.5, c = 3, μ = b = 0.1, λmin = 0.02, λmax = 2, ξmin = 0 and ξmax = 0.98.
4. Discussion
Early MK bacteria occur in a variety of arthropod species [1,3,5]. Their persistence is thought to be driven by the VT efficacy and a benefit provided to the surviving host offspring by killing the infected male embryos [10], and may also depend on their spatial distribution [11]. We showed that a purely vertically transmitted non-MK bacterium, otherwise able to invade the host as a highly efficient male-killer (electronic supplementary material, appendix S3), cannot persist and evolve MK gradually unless at least a bit of HT is present.
Our simulations revealed that the evolution of MK in a non-MK parasite is possible and may occur under a wide variety of host and parasite characteristics. A horizontally transmitted non-male-killer can evolve full MK efficacy if it is transmitted vertically with high enough efficacy and adequately enhances the fitness of the surviving host offspring. We found this result to be robust: (i) whether the VT efficacy was controlled by the host (fixed VT efficacy) or by the parasite (VT efficacy traded off with HT rate), (ii) whether the HT rate and MK efficacy evolved sequentially or simultaneously and, (iii) whether density dependence affected the host population through births or deaths. In all these cases, we observed adaptation towards 100% MK efficacy in a comparably large portion of the parameter space and speculate that MK ability might have evolved independently in many phylogenetically distinct species.
Direct empirical support for our theoretical observations is hard to obtain. Nevertheless, some studies on MK bacteria can be indicative of the relevance of our results. First, there is ample evidence that early male-killers may achieve full efficacy in killing the infected male embryos [19]. Second, insects are known to be infected by close relatives of MK bacteria, which do not cause MK per se. For example, Kageyama et al. [17] report Spiroplasma infection not causing MK to be prevalent (23–66%) in Japanese populations of Drosophila hydei. The observed spiroplasma is a close relative to MK spiroplasmas from other Drosophila species and, at the same time, the most basal taxon of the clade. Two parsimonious scenarios for the evolution of MK in this spiroplasma clade were suggested [17]. First, a common non-MK ancestor of the clade could evolve MK ability, which was subsequently lost in the spiroplasma lineage of D. hydei, a scenario indicative of the evolutionary branching we observed in our study. Alternatively, a common non-MK ancestor could spontaneously diverge into lineages, some of which might have evolved MK while the others might have stayed as non-male-killers. If those different lineages challenged different VT efficacies and/or fitness compensation intensities, no or full MK ability could evolve, as our model suggests.
Recognizable rates of natural horizontal transfer of male-killers between different host species were shown to exist [20,21]. Moreover, frequent HT from infected to uninfected individuals of another reproductive parasite, a parthenogenesis-inducing Wolbachia, was observed in the parasitoid wasp Trichogramma kaykai [22,23]. Infected and uninfected T. kaykai larvae shared the same host and some of the originally uninfected larvae acquired the infection. The transferred Wolbachia were then maternally transmitted to the offspring of newly infected females. Although hard evidence of horizontally transmitted early male-killers is currently unavailable, one can imagine a similar mechanism for some host–male-killer associations. In fact, the presence of HT in MK bacteria could explain the existence of perfectly vertically transmitted male-killers in the field [24]. In spatial models, a perfectly vertically transmitted male-killer can both invade and persist in a host [11]. In the absence of any spatial component, the male-killer drives the population to extinction [10,25,26]. Our model allows for the persistence of a perfectly vertically transmitted male-killer once it coexists with a non-MK strain, or when some HT is additionally present.
HT also forms an inevitable part of the transmission dynamics of other types of male-killers. For instance, late male-killers replicate as fast as possible to promote their HT via larval cannibalism or spores released from the infected dead bodies [4,5]. Another MK-like bacterium exhibiting HT is Arsenophonus nasoniae; it infects a wasp Nasonia vitripennis, a pupal parasite of flies. Here, A. nasoniae is transmitted to parasitized fly pupae during wasp oviposition, in which developing wasps become infected perorally, that is, horizontally [27].
There are other studies on the evolution of reproductive parasites which emphasize the role of HT. In particular, HT appears to play a prominent role in feminizing endosymbionts, another type of reproductive parasite that changes infected genotypic males into phenotypic females (see also the above-discussed studies on T. kaykai). In this regard, Ironside et al. [28] showed that even a small amount of HT could allow multiple feminizing parasites to coexist within a host population. Further, using a population-genetic model, Yamauchi et al. [29] showed that a bit of HT mediated by males prevented feminizing parasites from evolving the highest levels of fertilization efficiency. Last but not least, Engelstädter & Hurst [30] showed that a sexually transmitted infection can take over an infection by a male-killer either following exposure or when MK is incomplete and sexual transmission is efficient. Both theoretical results and empirical observations point to an important and perhaps unsubstitutable role of HT in the evolution of reproductive parasites.
We showed that the likelihood of MK ability to evolve is not dramatically affected by the host mating system; the critical values of VT efficacy or other host and parasite characteristics are similar for polygynous, monogamous and polyandrous hosts. Interestingly, the reverse need not be valid: in island populations of the butterfly Hypolimnas bolina with varying female sex-ratio bias due to varying prevalence of an MK Wolbachia, increased female bias led to an increase in female mating frequency [7]. In this regard, it has been hypothesized that sex-ratio distorters, such as MK bacteria, may induce reproductive evolution of their hosts, including sex-role reversal [6,7,9]. In this study, we assumed a mating system to be established and let the male-killer adapt to it. A straightforward and certainly useful extension would thus consider coevolution of MK ability in the parasite and the mating system in the host.
The evolutionary dynamics of MK bacteria could be affected by other factors too. Randerson et al. [26] showed that host resistance may generate selection pressure on MK bacteria and reverse the evolution of MK efficacy such that less efficient male-killers outcompete their more efficient relatives. Also, allowing for co-infection would undoubtedly be an interesting extension of our model as multiple infections with vertically transmitted symbionts are common in invertebrates [31]. Nonetheless, in combination with growing evidence on HT in reproductive parasites and encouraging results of our study we conclude that the time has come to recommend that analyses of ecological and evolutionary models should include exploration of the consequences of HT, and that HT should be commonly searched for in empirical studies on reproductive parasites.
Supplementary Material
Acknowledgements
The authors thank S. Alizon and an anonymous reviewer for their detailed and insightful comments that significantly improved the exposition of this paper.
Authors' contributions
V.B. conducted evolutionary analysis and simulations and wrote the manuscript. L.B. edited the manuscript and contributed to writing simulation codes. D.M. conducted ecological analysis. V.B. and L.B. designed the study. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
V.B. acknowledges institutional support MUNI/A/1441/2014 Student Project grant at MU. L.B. acknowledges institutional support RVO:60077344. D.M. acknowledges funding from Wheat Ridge Ministries—O. P. Kretzmann Grant for Research in the Healing Arts and Sciences.
References
- 1.Hurst GDD, Jiggins FM. 2000. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6, 329–336. ( 10.3201/eid0604.000402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiggins FM, Bentley JK, Majerus MEN, Hurst GDD. 2001. How many species are infected with Wolbachia? Cryptic sex ratio distorters revealed to be common by intensive sampling. Proc. R. Soc. B 268, 1123–1126. ( 10.1098/rspb.2001.1632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nature 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi K, Hoshino M, Nakai M, Kunimi Y. 2008. Novel RNA sequences associated with late male killing in Homona magnanima. Proc. R. Soc. B 275, 1249–1254. ( 10.1098/rspb.2008.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelstädter J, Hurst GDD. 2009. The ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. S 40, 127–149. ( 10.1146/annurev.ecolsys.110308.120206) [DOI] [Google Scholar]
- 6.Charlat S, Hurst GDD, Mercot H. 2003. Evolutionary consequences of Wolbachia infections. Trends Genet. 19, 217–223. ( 10.1016/S0168-9525(03)00024-6) [DOI] [PubMed] [Google Scholar]
- 7.Charlat S, Hurst GDD, Mercot H. 2007. Male-killing bacteria trigger a cycle of increasing male fatigue and female promiscuity. Curr. Biol. 17, 273–277. ( 10.1016/j.cub.2006.11.068) [DOI] [PubMed] [Google Scholar]
- 8.Bacelar FS, White A, Boots M. 2011. Life history and mating systems select for male biased parasitism mediated through natural selection and ecological feedbacks. J. Theor. Biol. 269, 131–137. ( 10.1016/j.jtbi.2010.10.004) [DOI] [PubMed] [Google Scholar]
- 9.Jiggins FM, Hurst GDD, Majerus MEN. 2000. Sex-ratio-distorting Wolbachia causes sex-role reversal in its butterfly host. Proc. R. Soc. Lond. B 267, 69–73. ( 10.1098/rspb.2000.0968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst LD. 1991. The incidences and evolution of cytoplasmic male killers. Proc. R. Soc. Lond. B 244, 91–99. ( 10.1098/rspb.1991.0056) [DOI] [Google Scholar]
- 11.Groenenboom MAC, Hogeweg P. 2002. Space and the persistence of male-killing endosymbionts in insect populations. Proc. R. Soc. B 269, 2509–2518. ( 10.1098/rspb.2002.2197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caswell H, Weeks DE. 1986. Two-sex models: chaos, extinction, and other dynamic consequences of sex. Am. Nat. 128, 707–735. ( 10.1086/284598) [DOI] [Google Scholar]
- 13.Miller TEX, Inouye BD. 2011. Confronting two-sex demographic models with data. Ecology 92, 2141–2151. ( 10.1890/11-0028.1) [DOI] [PubMed] [Google Scholar]
- 14.Alizon S, Hurford A, Mideo N, van Baalen M. 2009. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22, 245–259. ( 10.1111/j.1420-9101.2008.01658.x) [DOI] [PubMed] [Google Scholar]
- 15.Turner PE. 2004. Phenotypic plasticity in bacterial plasmids. Genetics 167, 9–20. ( 10.1534/genetics.167.1.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart AD, Logsdon JM Jr, Kelley SE. 2005. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59, 730–739. ( 10.1554/03-330) [DOI] [PubMed] [Google Scholar]
- 17.Kageyama D, Anbutsu H, Watada M, Hosokawa T, Shimada M, Fukatsu T. 2006. Prevalence of a non-male-killing Spiroplasma in natural populations of Drosophila hydei. Appl. Environ. Microbiol. 72, 6667–6673. ( 10.1128/AEM.00803-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geritz SAH, Kisdi E, Meszena G, Metz JAJ. 1998. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. 12, 35–57. ( 10.1023/A:1006554906681) [DOI] [Google Scholar]
- 19.Charlat S, Hornett EA, Dyson EA, Ho PPY, Loc NT, Schilthuizen M, Davies N, Roderick GK, Hurst GDD. 2005. Prevalence and penetrance variation of male-killing Wolbachia across Indo-Pacific populations of the butterfly Hypolimnas bolina. Mol. Ecol. 14, 3525–3530. ( 10.1111/j.1365-294X.2005.02678.x) [DOI] [PubMed] [Google Scholar]
- 20.Dyson EA, Kamath MK, Hurst GDD. 2002. Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): evidence for horizontal transmission of a butterfly male killer. Heredity 88, 166–171. ( 10.1038/sj.hdy.6800021) [DOI] [PubMed] [Google Scholar]
- 21.Majerus TMO, Majerus MEN. 2010. Discovery and identification of a male-killing agent in the Japanese ladybird Propylea japonica (Coleoptera: Coccinellidae). BMC Evol. Biol. 10, 37 ( 10.1186/1471-2148-10-37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huigens ME, Luck RF, Klaassen RHG, Maas MFPM, Timmermans MJTN, Stouthamer R. 2000. Infectious parthenogenesis. Nature 405, 178–179. ( 10.1038/35012066) [DOI] [PubMed] [Google Scholar]
- 23.Huigens ME, de Almeida RP, Boons PAH, Luck RF, Stouthamer R. 2004. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. Lond. B 271, 509–515. ( 10.1098/rspb.2003.2640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majerus MEN, Hinrich J, Schulenburg GVD, Zakharov IA. 2000. Multiple causes of male-killing in a single sample of the two-spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae) from Moscow. Heredity 84, 605–609. ( 10.1046/j.1365-2540.2000.00710.x) [DOI] [PubMed] [Google Scholar]
- 25.Freeland SJ, McCabe BK. 1997. Fitness compensation and the evolution of selfish cytoplasmic elements. Heredity 78, 391–402. ( 10.1038/hdy.1997.62) [DOI] [Google Scholar]
- 26.Randerson JP, Smith NGC, Hurst LD. 2000. The evolutionary dynamics of male-killers and their hosts. Heredity 84, 152–160. ( 10.1046/j.1365-2540.2000.00645.x) [DOI] [PubMed] [Google Scholar]
- 27.Werren JH, Skinner SW, Huger AM. 1986. Male-killing bacteria in a parasitic wasp. Science 231, 990–992. ( 10.1126/science.3945814) [DOI] [PubMed] [Google Scholar]
- 28.Ironside JE, Smith JE, Hatcher MJ, Dunn AM. 2011. Should sex-ratio distorting parasites abandon horizontal transmission? BMC Evol. Biol. 11, 370 ( 10.1186/1471-2148-11-370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamauchi A, Telschow A, Kobayashi Y. 2010. Evolution of cytoplasmic sex ratio distorters: effect of paternal transmission. J. Theor. Biol. 266, 79–87. ( 10.1016/j.jtbi.2010.06.018) [DOI] [PubMed] [Google Scholar]
- 30.Engelstädter J, Hurst GDD. 2009. What use are male hosts? The dynamics of maternally inherited bacteria showing sexual transmission or male killing. Am. Nat. 173, E159–E170. ( 10.1086/597375) [DOI] [PubMed] [Google Scholar]
- 31.Vautrin E, Vavre F. 2009. Interactions between vertically transmitted symbionts: cooperation or conflict? Trends Microbiol. 17, 95–99. ( 10.1016/j.tim.2008.12.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.