Abstract
Myoblasts can be differentiated into multinucleated myotubes, which provide a well-established and reproducible muscle cell model for skeletal myogenesis in vitro. However, under conventional differentiation conditions, each myotube rarely exhibits robust contraction as well as sarcomere arrangement. Here, we applied trains of optical stimulation (OS) to C2C12 myotubes, which were genetically engineered to express a channelrhodopsin variant, channelrhodopsin-green receiver (ChRGR), to investigate whether membrane depolarization facilitates the maturation of myotubes. We found that light pulses induced membrane depolarization and evoked action potentials in ChRGR-expressing myotubes. Regular alignments of sarcomeric proteins were patterned periodically after OS training. In contrast, untrained control myotubes rarely exhibited the striated patterns. OS-trained and untrained myotubes also differed in terms of their resting potential. OS training significantly increased the number of contractile myotubes. Treatment with nifedipine during OS training significantly decreased the fraction of contractile myotubes, whereas tetrodotoxin was less effective. These results suggest that oscillations of membrane potential and intracellular Ca2+ accompanied by OS promoted sarcomere assembly and the development of contractility during the myogenic process. These results also suggest that optogenetic techniques could be used to manipulate the activity-dependent process during myogenic development.
Repeated exercise with adequate physical activity is important for health by preventing the development of many chronic diseases such as obesity, type 2 diabetes, sarcopenia, neurodegeneration, and osteoporosis1,2,3. The expression of muscle functionality relies on the correct assembly of myofibrils, which comprise of tandem arrays of minimum contractile units, i.e., sarcomeres. Muscle activity such as excitation and contraction is known to regulate the expression and modification of a number of molecules such as acetylcholinesterase4, L-type Ca2+ channels5, and glucose transporters5,6. Previously, it was reported that muscle fiber stimulation with electrical7,8,9, mechanical10,11, or pharmacological12,13 methods, which mimic motor neuron inputs, facilitate the maturation of developing muscles as well as the maintenance of contractility. Cultured myogenic cells subjected to electrical stimuli with a given temporal pattern in vitro exhibited accelerated sarcomere assembly, and the contractile ability was induced by facilitating the production of elongation factors and muscle proteins14,15. Electrical field stimulation using electrodes placed in the extracellular space is a simple method for controlling the temporal pattern of muscle activation. However, it is difficult to identify the activated cells because the electric field is generally widespread and nonuniform. In addition, the placement of metal electrodes in the extracellular space has invasive effects on cells because they generate toxic gases such as H2 and Cl2 and change pH due to a Faradaic reaction. Therefore, the magnitude and duration of electrical field is limited within the range of a few volts/mm and milliseconds to minimize the side effects. However, optogenetic approaches using light-sensitive ion channels, channelrhodopsins (ChRs), have attracted attention as a new method for stimulating cells and tissues16,17,18,19, and their application is expanding in the field of neuroscience20,21,22,23. We have previously demonstrated that muscular contraction was optically controlled in a synchronous manner using a given train of light pulses when a myotube was generated from C2C12 clonal myoblasts, which were genetically engineered to express channelrhodopsin-2 (ChR2)24. However, previous studies have not demonstrated whether it is possible to manipulate the developmental process in any biological tissues25.
Thus, we hypothesized that oscillatory changes of membrane potential after manipulation by optogenetic stimulation would induce the morphological and functional acquisition of the contractile ability in immature muscle. To test this hypothesis, optical stimulation (OS) training was applied to the ChR-expressing myotubes derived from C2C12, a myoblast cell line. We found that flashing a light with a specific rhythm facilitated both sarcomere assembly and the development of contractility in the ChR-expressing myotubes. Our results suggest that the morphological maturation and functional development of C2C12 myotubes is accelerated by rhythmic changes of membrane potential, which is manipulated by light using an optogenetic technique.
Results and Discussion
Formation of the ChR-expressing myotubes
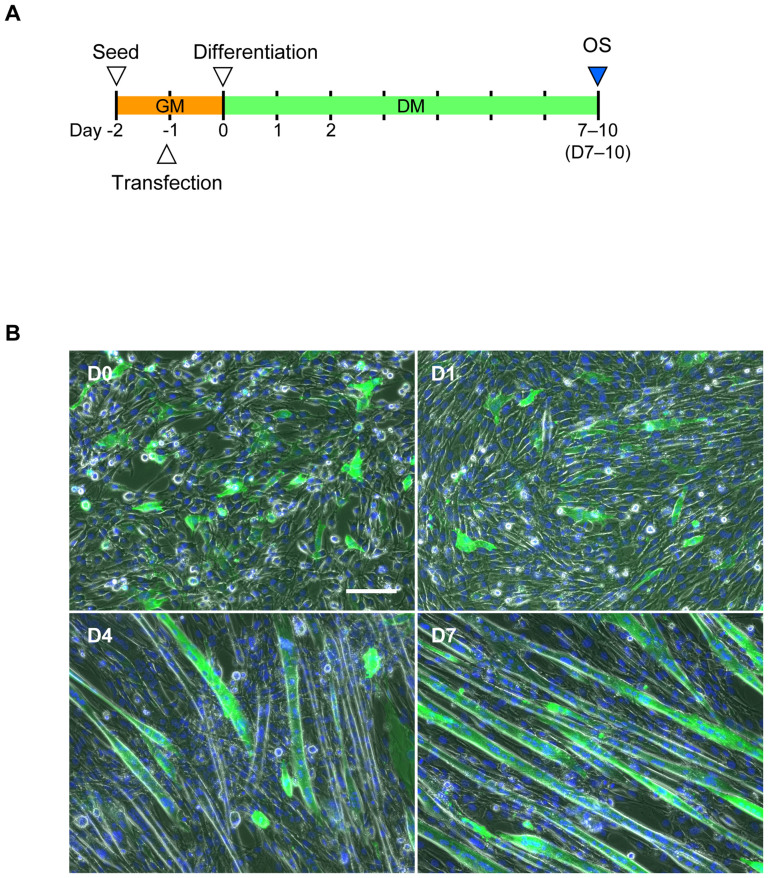
To apply optogenetics, we selected ChR-green receiver (ChRGR), a chimera of ChR1 and 2, because26 it evokes a relatively large photocurrent with minimal desensitization, it is expressed efficiently in the membrane, and it exhibits rapid photocurrent kinetics in both on-off states and during recovery from desensitization. Twenty-four hours after transfection with ChRGR (Fig. 1A; Day 0, D0), some C2C12 myoblasts were found to express ChRGR with bright Venus fluorescence (ChRGR-Ve), whereas others exhibited no fluorescence (Fig. 1B, D0). Subsequently, the mixture of ChRGR-Ve-positive and -negative C2C12 cells was allowed to differentiate in standard differentiation conditions27,28. The C2C12 cells, either fluorescent or non-fluorescent, began to fuse each other to form multinucleated myotubes29,30 after several days (Fig. 1B, D1–D7). Some of these multinucleated myotubes expressed ChRGR-Ve, whereas others did not. The ChRGR-Ve-positive myotubes were easily discriminated from the non-fluorescent ones and they comprised approximately 50%–60% of the multinucleated myotubes at D7, although the expression level was variable.
Figure 1. Generation of photosensitive C2C12 myotubes.
(A) Time schedule for the OS training assay with C2C12 myotubes. One day after seeding (Day −1), cells were transfected with ChRGR-Ve in the growth medium (GM). Twenty-four hours later (Day 0, D0), the medium was replaced with differentiation medium (DM) to promote the formation of multinuclear myotubes. The OS training assay was performed on Days 7–10. (B) Generation of ChRGR-Ve-expressing multinucleated myotubes on day 0 (D0), day 1 (D1), day 4 (D4), and day 7 (D7) after switching to DM. Multinucleated myotubes expressing ChRGR were detected based on Venus-fluorescence (green), and the cell nuclei were stained with Hoechst 33258 (blue). Scale bar, 50 μm.
Light sensitivity of multinucleated myotubes
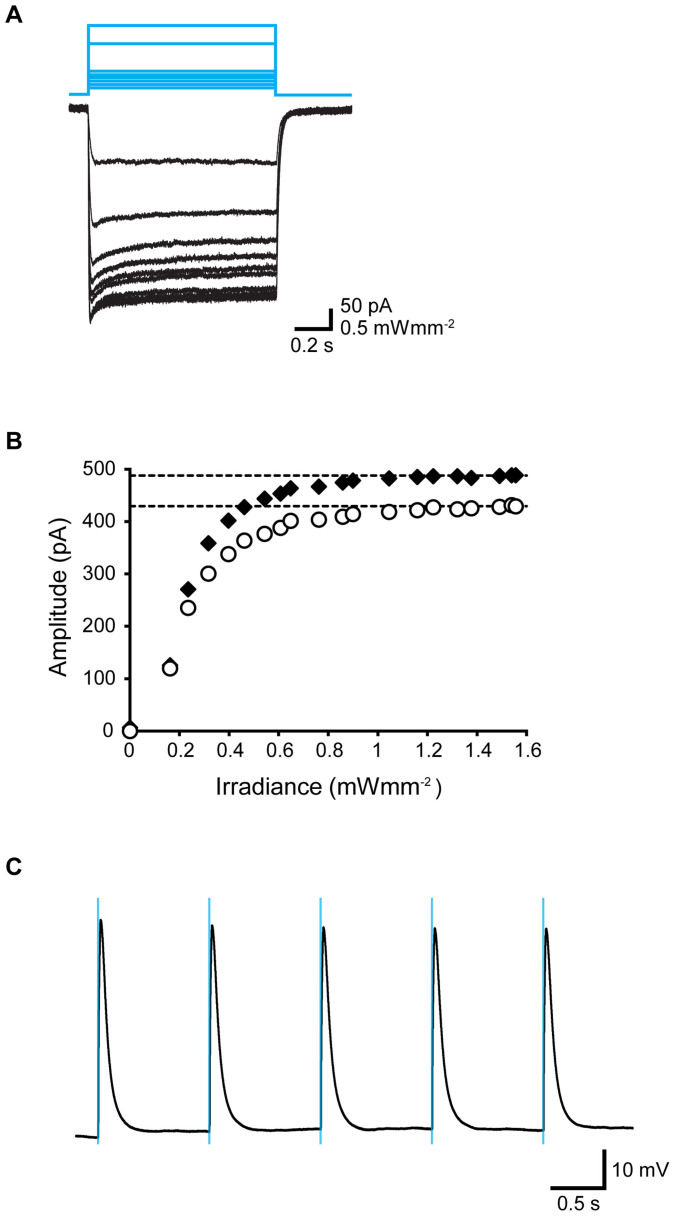
The photosensitivity of fluorescent multinucleated myotubes was investigated using the whole-cell patch-clamp method at −60 mV. Irradiation with a cyan LED light evoked an inward photocurrent in a power-dependent manner (Fig. 2A). The photocurrent consisted of a peak and a steady state with minimal desensitization, as reported previously26. Each photocurrent, peak, or steady-state reached its maximum approximately at 0.8–0.9 mWmm−2 of cyan LED light (Fig. 2B). In the current-clamp mode, pulsative irradiation (duration, 20 ms) of cyan LED light induced rapid membrane depolarization and it evoked an action potential, which was robustly phase-locked to the flashing light (Fig. 2C). The same irradiation produced neither photocurrent nor depolarization in nonfluorescent cells, which was consistent with previous studies31,32.
Figure 2. Optical responses of ChRGR-expressing C2C12 myotubes.
(A) Typical photocurrent traces (black lines) from a photosensitive multinucleated myotube induced by cyan LED light at various intensities (blue lines; 0.16–1.58 mWmm−2). (B) Power dependency of the peak current (filled diamonds) and steady-state current amplitudes (open circles). (C) Rhythmic action potentials generated by repetitive cyan LED flashes (blue lines; duration, 20 ms; frequency, 1 Hz) with the current clamp.
Sarcomere assembly by OS training
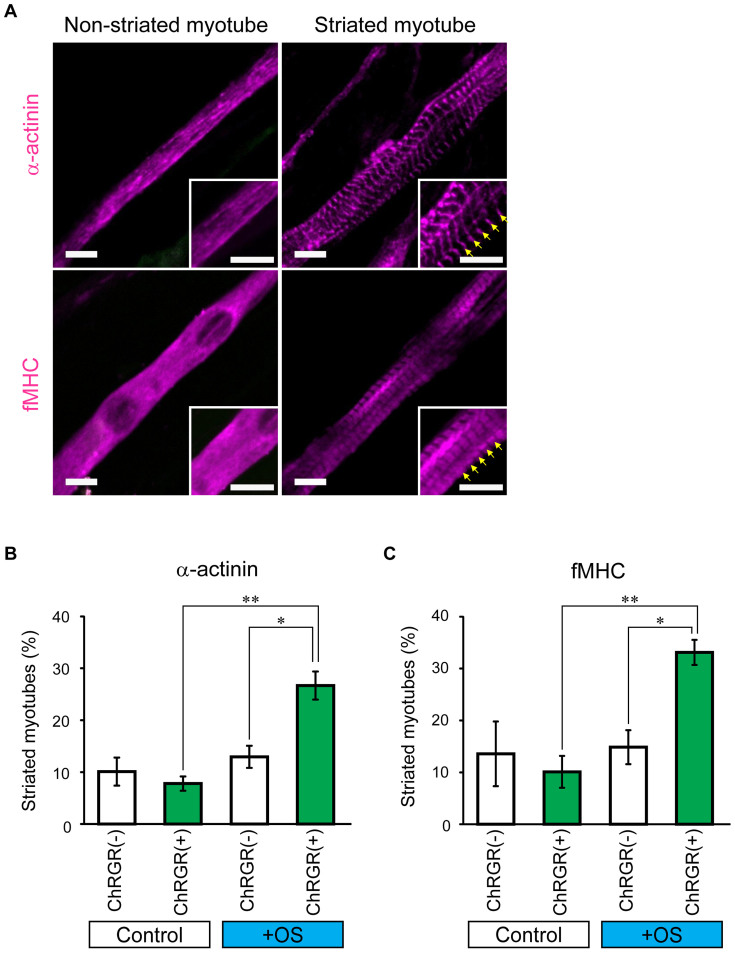
Under conventional myogenic culture conditions, C2C12 myotubes generally remained immature with minimal development of sarcomeric structure and contractility27,33. Extracellular electrical stimulation of multinucleated myotubes has been reported to accelerate the organization of sarcomeric proteins to assemble to form sarcomeres, which are the smallest contractile units in muscle8,15. We hypothesized that a certain OS pattern would facilitate sarcomere assembly in ChRGR-Ve-positive myotubes. To test this hypothesis, the contribution of OS training was evaluated based on the distributions of the Z-line protein, α-actinin, and the A-band protein, skeletal fast myosin heavy chain (fMHC), shortly after OS training. The cyan LED light was irradiated in a certain pattern as the OS training with the irradiance larger than 0.67 mWmm−2, which generated a near maximal photocurrent (Fig. 2B). The ChRGR-Ve-positive or negative myotubes generally exhibited a nonstriated morphology before OS training (Supplementary Figure S1A). Thus, both α-actinin and fMHC were distributed with punctate patterns throughout the cytoplasm or in a continuous pattern along filamentous structures in the myotubes (Fig. 3A). On the other hand, the mature striation pattern of α-actinin and fMHC appeared after the 1-Hz OS training protocol (Fig. 3A, arrows and Supplementary Figure S1B). Because the striations of α-actinin and fMHC were both aligned regularly along the longitudinal axis of the multinucleated myotubes, they were organized to form sarcomeric structures that possessed the Z-line and A-band. The fraction of ChRGR-Ve-positive myotubes that were striated with α-actinin was 27 ± 10% (range, 7.7%–44%, n = 16 regions) after OS training (1 Hz). This was significantly larger than that of nonfluorescent myotubes in the same regions as well as that of either fluorescent or nonfluorescent myotubes at the same stage without OS (Fig. 3B). Similarly, the fraction of ChRGR-Ve-positive myotubes that were striated with fMHC was 33 ± 9.6% (range, 19%–50%, n = 16 regions) after OS training (1 Hz), which was significantly larger than that of nonfluorescent myotubes in the same regions, as well as that of fluorescent or nonfluorescent myotubes at the same stage without OS training (Fig. 3C). These results suggest that OS training induced efficient sarcomere assembly specifically in ChRGR-expressing myotubes.
Figure 3. Effects of OS training on sarcomere assembly in ChRGR-expressing C2C12 myotubes.
(A) Images of typical multinucleated myotubes 7 days differentiation induction. Cells were fixed and stained with anti-sarcomeric α-actinin (upper panel) or fMHC (lower panel). The insets show the magnified images. The sarcomeric structures are indicated by arrows. Scale bar, 10 μm. (B) Fractions of myotubes with mature striation patterns. Assembly of α-actinin (from left to right): control ChRGR-negative myotubes without OS training (white column, n = 12 regions), ChRGR-positive myotubes without OS training (green column, n = 12 regions), ChRGR-negative myotubes with OS training (white column, n = 16 regions), and ChRGR-positive myotubes with OS training (green column, n = 16 regions). (C) Similar to (B), but showing the assembly of fMHC in ChRGR-negative (white bar, each, n = 16 regions) and –positive cells (green bar, each, n = 16 regions). Each experiment consists of at least three independent replicates. *, P < 0.05; **, P < 0.01; Kruskal–Wallis test.
Membrane properties after OS training
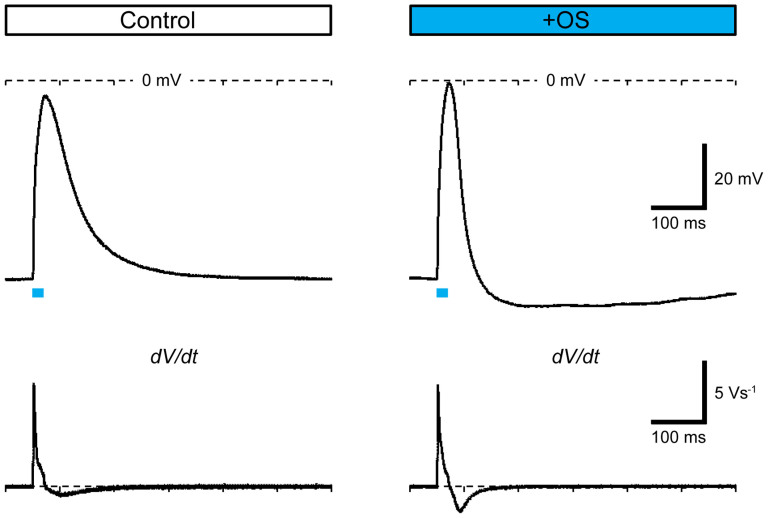
C2C12 myotubes also have immature membrane properties such as small amplitudes in Erest and VAP. Therefore, we investigated the electrophysiological properties of the C2C12 myotube membranes using the whole-cell patch-clamp method after the continuous application of OS training at 1 Hz for 2 h. The OS training protocol improved the membrane properties of fluorescent myotubes to some extent (Table 1). Rin and Ccell remained relatively unchanged compared with that of control myotubes. In OS-trained myotubes, Erest was −42.6 ± 3.18 mV (range = −60 to −33 mV, n = 8 cells), which was significantly more negative than that in the untrained myotubes (range = −39 to −21 mV, n = 10 cells), and this was consistent with previous studies34. The active membrane properties were examined while holding the resting potential at −60 mV by injecting DC current (Fig. 4). However, OS training did not improve the amplitude of VAP or [dV/dt]max, which is an indicator of Na+ channel activity. It has a tendency to increase the negativity of [dV/dt]min, which is an indicator of K+ channel activity, from −0.95 ± 0.18 V/s (range = −0.12 to −1.92 V/s, n = 10 cells) to −1.32 ± 0.29 V/s (range = −0.30 to −2.66 V/s, n = 8 cells) with insignificant difference (Table 1). These results suggest that OS training minimally influence the active membrane properties. There is also no evidence that OS training enhanced the development of the tubular membrane system because it did not affect Rin or Ccell.
Table 1. Summary of the electrophysiological properties of the membranes of ChRGR-expressing C2C12 myotubes without (control) or with OS training.
| Rin (MΩ) | Ccell (pF) | Erest (mV) | VAP (mV) | [dV/dt]max (Vs−1) | [dV/dt]min (Vs−1) | |
|---|---|---|---|---|---|---|
| Control (n = 10) | 131 ± 18.3 | 383 ± 61.1 | −32.5 ± 1.74 | 44.2 ± 6.29 | 6.11 ± 1.36 | −0.95 ± 0.18 |
| OS training (n = 8) | 131 ± 28.5 | 406 ± 74.1 | −42.0 ± 2.81* | 45.7 ± 5.40 | 6.93 ± 1.55 | −1.32 ± 0.29 |
The values represent the mean ± S.E.M. (n = 8–10). *, P < 0.05; Mann–Whitney U–test.
Figure 4. Electrical properties of membranes.
Typical action potentials (upper traces) and their time-derivatives (dV/dt data, lower traces) generated by cyan LED flashes (20 ms, indicated by blue lines) in ChRGR-expressing myotubes without (left) or with OS training (right).
Induction of the contractile ability by OS training
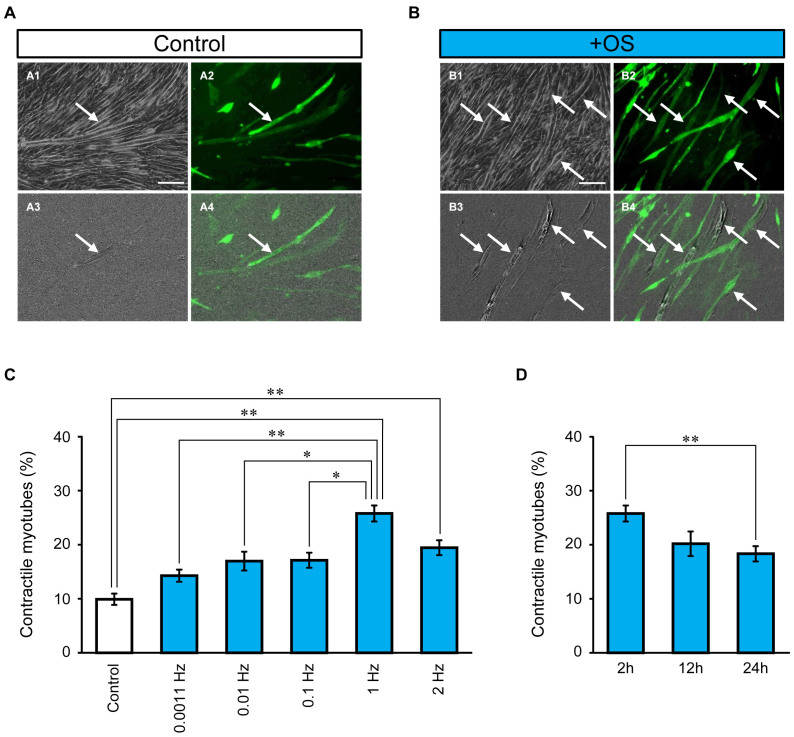
In general, C2C12 myotubes hardly exhibit the robust contraction spontaneously under conventional culture conditions. It is likely that this contractility depends on the assembly of sarcomeres in multinucleated myotubes8,9,15. To investigate the effect of OS training on the induction of the contractile ability, OS training was applied to C2C12 myotubes using the 1 Hz protocol. The control groups without OS training were unresponsive to the cyan LED flash at any frequency or intensity (Fig. 5A and Supplementary Movie S1). In contrast, contractions appeared in fluorescent myotubes in response to a cyan LED flash after OS training (Fig. 5B and Supplementary Movie S2). The fraction of contractile myotubes comprised 26 ± 8.4% (range = 9.5%–50%, n = 32 regions) of ChRGR-Ve-positive myotubes trained by 1 Hz OS-protocol (Fig. 5C), which was significantly larger than that without OS training (9.9 ± 5.1%; range = 0%–21%, n = 32 regions). The induction of the contractile ability also depended on the OS protocol, which was applied for 2 h. Low-frequency protocols such as 0.0011, 0.01, and 0.1 Hz were significantly less effective than the standard protocol of 1 Hz. However, the 2 Hz protocol was less effective than the standard protocol. The effectiveness of OS training also depended on the treatment time (Fig. 5D) where longer OS protocols such as 1 Hz for 24 h were significantly less effective than the standard protocol (1 Hz for 2 h). Based on these results, we concluded that rhythmical OS at 1 Hz for 2 h was suitable for developing contractility in ChRGR-Ve-positive multinucleated myotubes and it was used as the standard.
Figure 5. Induction of contractile ability.
(A) Image difference extraction analysis of myotubes without OS training: phase-contrast image (A1), ChRGR-Ve-fluorescence image (A2), difference image (A3), and merged image of A2 and A3 (A4). Contracted myotubes are indicated by arrows. (B) Myotubes after OS training. Scale bar, 100 μm. (C) Effects of OS protocols on the fraction of contractile fluorescent myotubes divided by the total fluorescent myotubes (from left to right): control without OS (n = 24 regions), 0.0011 Hz (n = 20 regions), 0.01 Hz (n = 20 regions), 0.1 Hz (n = 28 regions), 1 Hz (n = 32 regions), and 2 Hz (n = 16 regions). (D) Fraction of contractile myotubes with OS protocols at 1 Hz for (from left to right): 2 h (n = 32 regions), 12 h (n = 12 regions), and 24 h (n = 16 regions). Each experiment consists of at least four independent replicates. *, P < 0.05; **, P < 0.01; Kruskal–Wallis test.
Involvement of intracellular Ca2+
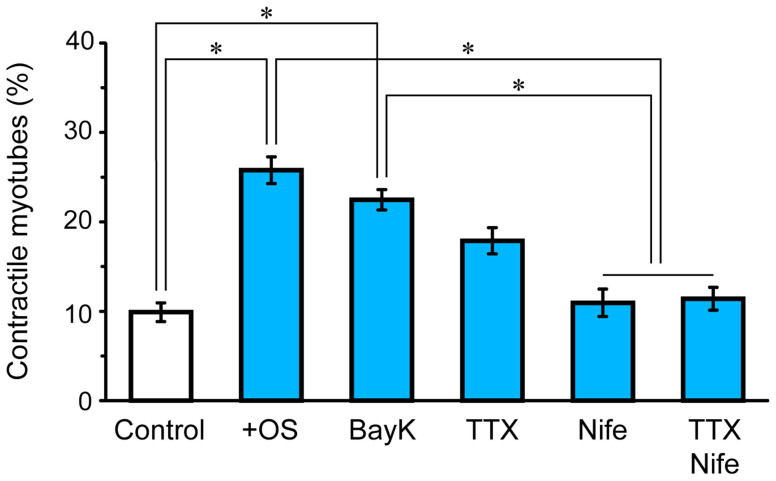
It is hypothesized that the OS training protocol induced a rhythmic change of membrane potential and the subsequent intracellular Ca2+ oscillation, which contributed to the induction of the contractile ability. To test this hypothesis, we examined the effect of various pharmacological treatments on the acquisition of the contractile ability. Bay K 8644 (BayK; an agonist of L-type Ca2+ channels, LTCCs), nifedipine (Nife; an antagonist of LTCCs and excitation-contraction (EC) coupling), and tetrodotoxin (TTX, a blocker of voltage-dependent Na+ channels) were added to the medium during OS training in separate trials. As summarized in Fig. 6, BayK or TTX had no significant effects on the fraction of contractile myotubes, whereas Nife reduced the fraction to the level without training. These results suggest that the rhythmical oscillation of membrane potential and the accompanying intracellular Ca2+ changes may facilitate the development of contractility.
Figure 6. Effects of pharmacological treatments on the induction of the contractile ability.
From left to right: without OS training as a reference (n = 24 regions), OS training without any treatment (n = 32 regions), BayK (n = 20 regions), TTX (n = 20 regions), Nife (n = 24 regions), and TTX and Nife (n = 16 regions) in ChRGR-expressing myotubes. Each experiment consists of at least four independent replicates. *, P < 0.01; Kruskal–Wallis test.
In skeletal muscle cells, the action potential evoked by membrane depolarization is generally conducted along the surface membrane into an invaginated tubular system network. This in turn activates LTCCs/dihydropyridine receptors (DHPRs), which undergoes conformational changes to allow Ca2+ release from ryanodine receptors (RyRs) in the sarcoplasmic reticulum (SR)35,36. At present, we do not have any evidences that the ChRGR-expressing myotubes either develop the tubular system, as previously reported37, or the EC coupling molecular complex. However, we could presume that the whole plasma membrane of a ChRGR-expressing myotube would be depolarized at once directly by light. The lower sensitivity to TTX occurred because the LTCCs/DHPRs were activated directly by light-induced membrane depolarization but without the propagation of action potentials. This increased the intracellular Ca2+ level by Ca2+ influx and/or via RyRs. The rhythmic oscillation of intracellular Ca2+ subsequently facilitated sarcomere assembly. Thus, it is possible that sarcomere assembly may be a prerequisite for light-evoked contraction.
Thus, in this study, we demonstrated that OS training using a flash light with a rhythmical frequency facilitated sarcomere assembly to form the specific structural alignment of sarcomeric proteins, which are involved in muscle contraction. OS training also induced the contractile ability in C2C12 myotubes, which were genetically engineered to express ChRGR. Our results suggest that optogenetic techniques are useful for manipulating the morphological maturation and functional development of C2C12 myotubes.
Methods
Cell cultures and transfection
C2C12 cells are a myoblast line derived from mouse satellite cells (RIKEN Cell Bank), which have been used as models in studies of skeletal muscle development. The cells were maintained for no more than six passages at 37°C with a 5% CO2 atmosphere in Dulbecco's Modified Eagle's Medium (DMEM, Wako Pure Chemical Industries, Osaka, Japan), which was supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 100 units/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich, St Louis, MO). Fragments of cDNA that encoded the codon-humanized ChRGR-Venus conjugate (ChRGR-Ve) were amplified by PCR and subcloned into EcoRI sites in pCAGGS by In-Fusion cloning (Takara Bio, Shiga, Japan), as described previously38. C2C12 myoblasts were grown to 80%–90% confluence on 35 mm-diameter tissue culture dishes coated with rat tail collagen and subsequently transfected with ChRGR-Ve expression vector plasmids using Lipofectamine 2000 transfection reagent (Invitrogen), according to the manufacturer's instructions. Twenty-four hours post-transfection (Day 0), differentiation into multinuclear myotubes was facilitated by switching to differentiation medium (DM), i.e., DMEM containing 2% horse serum (Invitrogen), 1 nM insulin (Invitrogen), 100 units/mL penicillin, 100 μg/mL streptomycin, 200 μM L-ascorbic acid, and Mg-phosphate salt n-hydrate (Wako Pure Chemical Industries). The DM was replenished every day.
OS training
To perform OS training, we used a custom-built irradiation system, which consisted of an array of seven LEDs arranged in series on a board (281MCPCB, Polymer Optics Ltd., England). Cyan LEDs (505 ± 15 nm, LXML-PE01-0070, Philips Lumileds Lighting Co., San Jose, CA) were regulated using a pulse generator (SEN-7203, Nihon Kohden, Tokyo, Japan) with a current booster (SEG-3104, Nihon Kohden). The power density of the LED arrays through a diffuser lens (Polymer Optics Ltd) was ≧ 0.67 mWmm−2 at the bottom of the culture dish, which was measured directly using a visible light-sensitive thermopile (MIR-100Q, Mitsubishi Oil Chemicals, Tokyo, Japan). OS training was conducted with C2C12 myotubes for 7–10 days (D7–10) after switching to DM. During the application of an OS training protocol for 2 h at 37°C in a 5% CO2 atmosphere, the medium was replaced temporarily with OS medium, which consisted of phenol red-free DMEM (Invitrogen) supplemented with 2% horse serum, 2% MEM amino acids solution (Invitrogen), 1% MEM nonessential amino acids solution (Invitrogen), 100 units/mL penicillin, and 100 μg/μL streptomycin. The stimulation protocols were applied at the following frequencies: 0.0011 Hz (duration, 18 s; 8 pulses), 0.01 Hz (duration, 2 s; 72 pulses), 0.1 Hz (duration, 200 ms; 720 pulses), 1 Hz (duration, 20 ms; 7,200 pulses), and 2 Hz (duration, 10 ms; 14,400 pulses). In each protocol, the total irradiation time was 144 s in 2 h. In the control experiments, the C2C12 myotubes were exposed to the same OS medium as the stimulation groups but without any OS training protocols.
Electrophysiology
ChRGR-Ve-positive myotubes were identified by Venus-fluorescence using a conventional epifluorescence microscope (BX51WI, Olympus, Tokyo, Japan), which was equipped with a 60× water-immersion objective lens (LUMplanPl/IR60x, Olympus) and a filter cube (excitation, 495 nm; dichroic mirror, 505 nm; barrier filter, 515 nm). Electrophysiological recordings were performed in the whole-cell patch-clamp mode using a conventional system (Axopatch 200A plus Digidata1200, Molecular Devices, Sunnyvale, CA) and with pCLAMP 10.2 computer software (Molecular Devices). The standard extracellular Tyrode's solution contained (in mM): 138 NaCl, 3 KCl, 2.5 CaCl2, 1.25 MgCl2, 10 HEPES, 4 NaOH, and 11 glucose (pH 7.4 adjusted with HCl). The standard patch pipette solution contained (in mM): 120 CsOH, 100 glutamic acid, 0.2 EGTA, 10 HEPES, 2.5 MgCl2, 3 MgATP, 0.3 Na2GTP, and 0.1 leupeptin (pH 7.4 adjusted with CsOH) for the voltage clamp, or 125 K-gluconate, 10 KCl, 0.2 EGTA, 10 HEPES, 1 MgCl2, 3 MgATP, 0.3 Na2GTP, and 0.1 leupeptin (pH 7.4 adjusted with KOH) for the current clamp. To test the optical responses, we used a cyan LED (505 ± 15 nm, LXHL-NE98, Philips Lumileds Lighting Co.), which was regulated by a pulse generator (SEN-7203) and pCLAMP 10.2 computer software. The maximum power density of the LED light through an objective lens was 1.58 mWmm−2 at the point of focus on the specimen. The photocurrent response induced by the LED light was recorded using a CsCl-based internal solution with the whole-cell voltage clamp. The electrophysiological properties were characterized using a K-gluconate-based internal solution to determine the input resistance (Rin), whole cell capacitance (Ccell), resting membrane potential (Erest). Rin and Ccell were calculated from a current response to the 50 ms, 10 mV hyperpolarizing pulse. To evaluate the cell's excitability, the membrane potential responses to the light pulses (20 ms) were recorded while holding the Erest at −60 mV under current clamp. They were digitally time-differentiated and digitally smoothed by a low-pass filter at 200 Hz (dV/dt data), and action potential amplitude (VAP), the maximal and the minimal value of dV/dt data, [dV/dt]max and [dV/dt]min were characterized. All of the raw data were filtered at 2 kHz and sampled at 10 kHz. All of the experiments were performed at room temperature.
Immunohistochemistry
Cells were fixed with 4% paraformaldehyde/PBS immediately after the OS training protocol and permeabilized with 0.1% Triton X-100/PBS for 15 min. The control myotubes without OS training were processed in a similar manner at the same stage. The samples were blocked in PBS containing 5% goat serum for 1 h and treated with the primary antibodies, i.e., rat monoclonal anti-GFP IgG2a (1:1000, GF090R, Nacalai Tesque, Kyoto, Japan), mouse monoclonal anti-sarcomeric α-actinin IgG1 (1:500, EA-53, Sigma-Aldrich), and mouse monoclonal anti-myosin heavy chain IgG1 (fMHC; 1:500, MY-32, Sigma-Aldrich), diluted in PBS containing 1% goat serum for 1 h, and then they were incubated for 1 h with the secondary antibodies, i.e., Alexa Fluor 488-conjugated goat anti-rat IgG (Invitrogen) and Alexa Fluor 546-conjugated goat anti-mouse IgG (Invitrogen), diluted at 1:200 in PBS containing 1% goat serum. The samples were washed three times with PBS between treatments. The specimens were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and coverslipped. All of the above reactions were performed at room temperature. The fluorescent images were captured with a conventional confocal laser microscope (LSM510META, Carl Zeiss, Oberkochen, Germany), which was equipped with a 40× water immersion objective and corrected for brightness and contrast using conventional software (LSM Image Browser).
Analysis of contractility
The contractility of myotubes was monitored sequentially using a CCD camera (VB-7010, Keyence Co., Osaka, Japan; 7.5 frames/s) by phase contrast/epifluorescence microscopy with a 20× objective lens and a GFP filter set (excitation, 485 nm; dichroic mirror, 510 nm; bandpass filter, 515–565 nm) to observe Ve-fluorescence. The myotubes were photostimulated using a cyan LED flash (peak, 490–520 nm; 0.55 mWmm−2 at the focus; 1 Hz for 100 ms) through the objective lens with a filter set (dichroic mirror, 550 nm; long pass filter, 565 nm), which was regulated by a pulse generator (SEN-7203). Contractile myotubes were identified using image difference extraction analysis; the phase-contrast image before a cyan LED flash was digitally subtracted from the image immediately after the flash and overlaid on the fluorescence image. Thus, the difference extraction images indicated the contractility of fluorescent myotubes. Each 35 mm dish was divided into four regions and images were recorded from several visual fields unintentionally located in each region. The numbers of fluorescent myotubes and contractile fluorescent myotubes were then counted. The fraction of contractile myotubes was calculated as the total number of contractile fluorescent myotubes divided by the total fluorescent myotubes in each region. All of the image analysis was performed using ImageJ software (NIH, Bethesda, MD).
Pharmacological treatments
In the pharmacological experiments, 0.3 μM BayK ((S)-(-)-Bay K 8644, Sigma-Aldrich), an agonist of L-type Ca2+ channels (LTCCs), 2 μM TTX (Tocris Bioscience, Ellisville, MO), a blocker of voltage-gated Na+ channels, 10 μM Nife (Sigma-Aldrich), an antagonist of LTCCs and an inhibitor of EC coupling were added to the medium at these final concentrations. The cells were incubated in OS medium containing each reagent for at least 15 min before the stimulation protocol. During OS training (20 ms duration, 1 Hz frequency) for 2 h, cell detachment and morphological changes were not observed in these conditions. After OS training with pharmacological treatments, myotubes were washed five times with Tyrode's solution and incubated for 5 min, and the contractility was evaluated as described previously.
Statistical analysis
All data in the text and figures were expressed as the mean ± SEM and evaluated using one-way Kruskal–Wallis and Mann–Whitney U–test to determine statistically significant differences, unless stated otherwise. P < 0.05 indicated a statistically significant difference.
Supplementary Material
Supplementary information
Supplementary Movie S1
Supplementary Movie S2
Acknowledgments
This research was supported by a Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows from JSPS, scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (Numbers, 10J07170, 26870336, 25290002, 21676002, 23111705, 26249027, 25670103, 25250001, 25115701), the Global COE Program (Basic & Translational Research Center for Global Brain Science), MEXT, the Moritani Scholarship Foundation, and a grant from the Inochinoiro ALS Research Foundation.
Footnotes
Author Contributions T.A., T.I. and H.Y. proposed and designed the experiments. T.A. and T.I. performed the experiments. T.A. and H.Y. analyzed the data. T.A., T.I., K.M. and H.Y. contributed reagents, materials, and analysis tools. T.A., T.I. and H.Y. wrote the manuscript. All authors participated in the discussion and commented on the paper.
References
- Booth F. W., Chakravarthy M. V., Gordon S. E. & Spangenburg E. E. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 93, 3–30 (2002). [DOI] [PubMed] [Google Scholar]
- Handschin C. & Spiegelman B. M. The role of exercise and PGC1α in inflammation and chronic disease. Nature 454, 463–469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flück M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J. Exp. Biol. 209, 2239–2248 (2006). [DOI] [PubMed] [Google Scholar]
- Sketelj J. et al. Specific impulse patterns regulate acetylcholinesterase activity in skeletal muscles of rats and rabbits. J. Neurosci. Res. 47, 49–57 (1997). [PubMed] [Google Scholar]
- Freud-Silverberg M. & Shainberg A. Electric stimulation regulates the level of Ca-channels in chick muscle culture. Neurosci. Lett. 151, 104–106 (1993). [DOI] [PubMed] [Google Scholar]
- Huang S. & Czech M. P. The GLUT4 Glucose Transporter. Cell Metab. 5, 237–252 (2007). [DOI] [PubMed] [Google Scholar]
- Hofmann S. & Pette D. Low-frequency stimulation of rat fast-twitch muscle enhances the expression of hexokinase II and both the translocation and expression of glucose transporter 4 (GLUT-4). Eur. J. Biochem. 219, 307–315 (1994). [DOI] [PubMed] [Google Scholar]
- Park H. et al. Effects of electrical stimulation in C2C12 muscle constructs. J. Tissue. Eng. Regen. Med. 2, 279–287 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M. H., Simonides W. S. & van Hardeveld C. Electrical stimulation of C2C12 myotubes induces contractions and represses thyroid-hormone-dependent transcription of the fast-type sarcoplasmic-reticulum Ca2+-ATPase gene. Biochem. J. 321, 845–848 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C. A., Smiley B. L., Mills J. & Vandenburgh H. H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol. Cell Physiol. 283, C1557–C1565 (2002). [DOI] [PubMed] [Google Scholar]
- De Deyne P. G. Formation of sarcomeres in developing myotubes: role of mechanical stretch and contractile activation. Am. J. Physiol. Cell Physiol. 279, C1801–C1811 (2000). [DOI] [PubMed] [Google Scholar]
- Hupkes M. et al. Epigenetics: DNA demethylation promotes skeletal myotube maturation. FASEB J. 25, 3861–3872 (2011). [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Dohmae N. & Morishima N. Endoplasmic reticulum stress increases myofiber formation in vitro. FASEB J. 21, 2994–3003 (2007). [DOI] [PubMed] [Google Scholar]
- Burch N. et al. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. Plos One 5, e10970 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H., Nedachi T. & Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp. Cell Res. 313, 1853–1865 (2007). [DOI] [PubMed] [Google Scholar]
- Nagel G. et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395–2398 (2002). [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Kakuda M., Araki R. & Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci. Res. 54, 85–94 (2006). [DOI] [PubMed] [Google Scholar]
- Boyden E. S. et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005). [DOI] [PubMed] [Google Scholar]
- Nagel G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100, 13940–13945 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T. et al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J. Neurosci. 34, 6896–6909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007). [DOI] [PubMed] [Google Scholar]
- Li X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 102, 17816–17821 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Ishizua T. & Yawo H. Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnol. Bioeng. 109, 199–204 (2012). [DOI] [PubMed] [Google Scholar]
- Yawo H., Asano T., Sakai S. & Ishizuka T. Optogenetic manipulation of neural and non-neural functions. Dev. Growth Differ. 55, 474–490 (2013). [DOI] [PubMed] [Google Scholar]
- Wen L. et al. Opto-current-clamp actuation of cortical neurons using a strategically designed channelrhodopsin. Plos One 5, e12893 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J. et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, 877–887 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis R. G., Kosnik P. E., Gilbert M. E. & Faulkner J. A. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am. J. Physiol. Cell Physiol. 280, C288–C295 (2001). [DOI] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P. & Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32, 1171–1180 (1983). [DOI] [PubMed] [Google Scholar]
- Yaffe D. & Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727 (1977). [DOI] [PubMed] [Google Scholar]
- Nagel G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid Behavioral responses. Curr. Biol. 15, 2279–2284 (2005). [DOI] [PubMed] [Google Scholar]
- Ji Z.-G. et al. Light-evoked somatosensory perception of transgenic rats that express channelrhodopsin-2 in dorsal root ganglion cells. Plos One 7, e32699 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Catino D. H., Strong J. C. & Bloch R. J. De novo myofibrillogenesis in C2C12 cells: evidence for the independent assembly of M bands and Z disks. Am. J. Physiol. Cell Physiol. 290, C626–C637 (2006). [DOI] [PubMed] [Google Scholar]
- Curci R. et al. Surface and inner cell behaviour along skeletal muscle cell in vitro differentiation. Micron 39, 843–851 (2008). [DOI] [PubMed] [Google Scholar]
- Ríos E. & Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol. Rev. 71, 849–908 (1991). [DOI] [PubMed] [Google Scholar]
- Lamb G. Excitation–contraction coupling in skeletal muscle: comparisons with cardiac muscle. Clin. Exp. Pharmacol. Physiol. 27, 216–224 (2000). [DOI] [PubMed] [Google Scholar]
- Parton R. G., Way M., Zorzi N. & Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J. Cell Biol. 136, 137–154 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Yamamura K. & Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary Movie S1
Supplementary Movie S2