Abstract
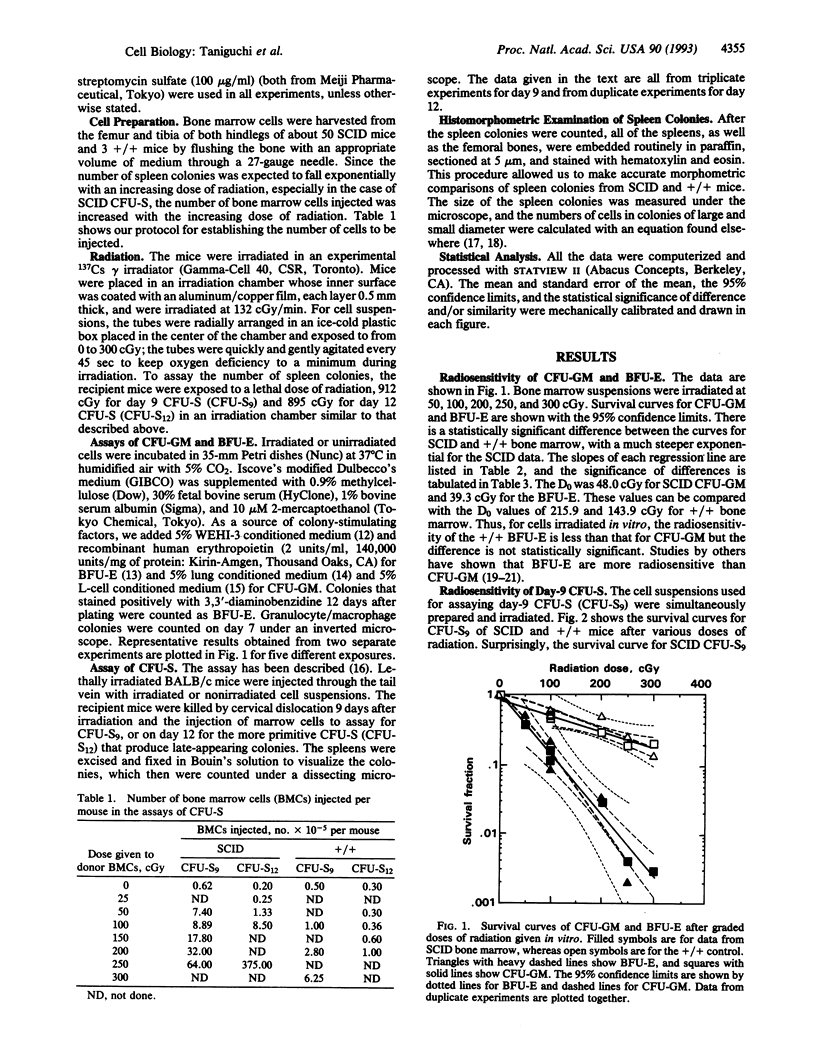
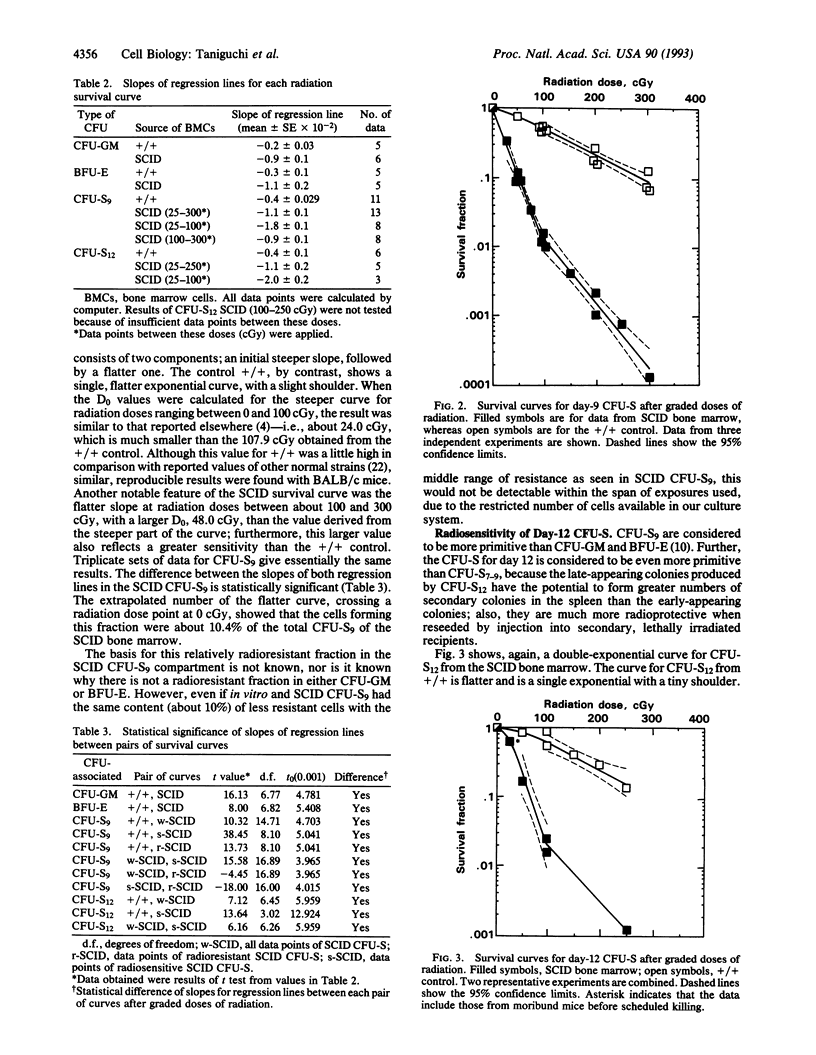
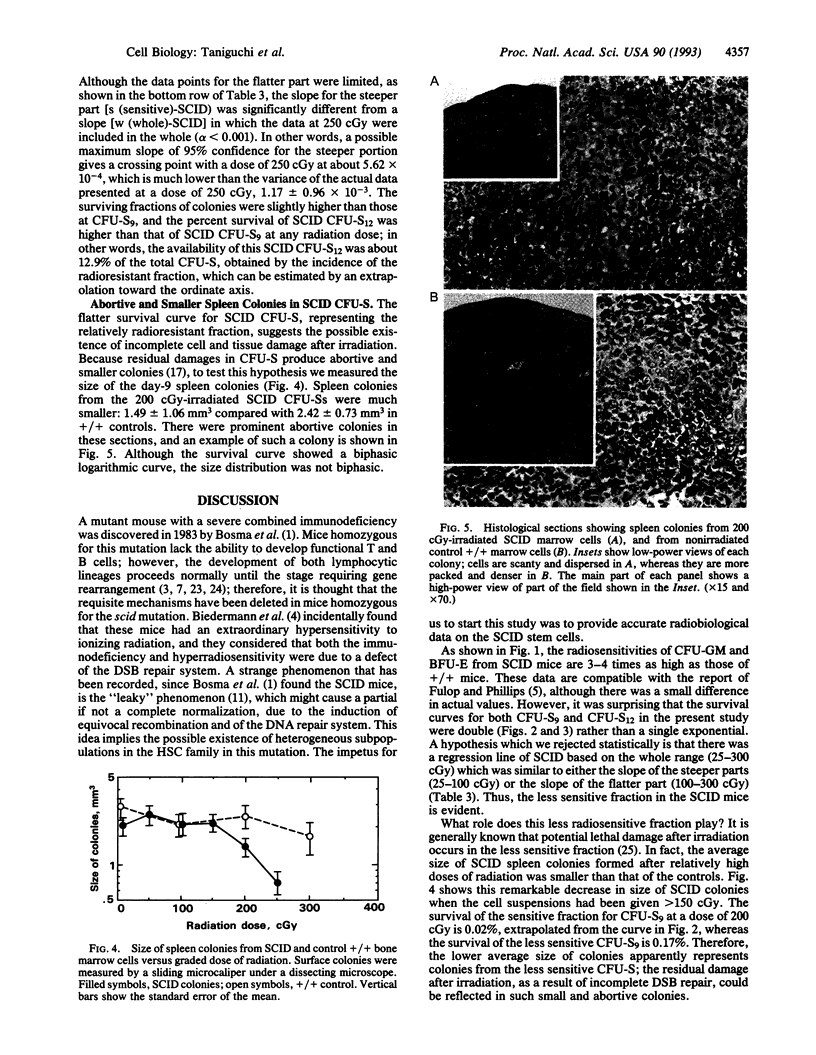
It has been reported that SCID (severe combined immunodeficiency, scid/scid) mice are more radiosensitive than normal mice. In the present studies, graded doses of radiation were given to bone marrow cells from SCID mice, and double-exponential survival curves were observed for day-9 and day-12 colony-forming units in the spleen (CFU-S). Single-exponential curves were found for SCID CFU in in vitro assays for granulocyte/macrophage-CFUs and erythroid burst-forming units, as reported elsewhere. Since the size of this more resistant fraction seems to decrease with stem-cell maturation, the finding implies that this fraction is a primitive subpopulation of the stem-cell compartment. The mean lethal dose (D0), however, of this less sensitive SCID CFU-S is much less than the D0 of regular CFU-S in normal littermates. Spleen colonies produced by SCID bone marrow were relatively small and abortive. The size of these colonies decreased nearly exponentially with increasing doses of radiation. These colonies were believed to be produced by this less sensitive fraction of the stem cells, which carried residual injuries. The colonies produced by the sensitive fraction have disappeared, being killed by a relatively low dose of radiation. This observation might account for the high lymphomagenesis arising from primitive hemopoietic stem cells in SCID mice, because the smallness of the colonies suggests that there is unrepaired or misrepaired damage. Furthermore, this less sensitive fraction might be a source of the "leaky" change of T and B cells, possibly due to the induction of an equivocal repair system which appears in the later stages of life in the SCID mice.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Bosma M. J., Bosma G. C., Unanue E. R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986 Jul 1;137(1):4–9. [PubMed] [Google Scholar]
- Bartelmez S. H., Andrews R. G., Bernstein I. D. Uncovering the heterogeneity of hematopoietic repopulating cells. Exp Hematol. 1991 Oct;19(9):861–862. [PubMed] [Google Scholar]
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Kozka I. J., Simpson R. J., Vairo G., Hamilton J. A., Nice E. C. Purification of two forms of colony-stimulating factor from mouse L-cell-conditioned medium. J Biol Chem. 1985 Dec 15;260(29):16004–16011. [PubMed] [Google Scholar]
- Chu-tse W., Min-pei L. Characteristics of proliferation and differentiation of spleen colony-forming cells from bone marrow. Int J Cell Cloning. 1984 Mar;2(2):69–80. doi: 10.1002/stem.5530020201. [DOI] [PubMed] [Google Scholar]
- Cronkite E. P., Bond V. P., Carsten A. L., Inoue T., Miller M. E., Bullis J. E. Effects of low level radiation upon the hematopoietic stem cell: implications for leukemogenesis. Radiat Environ Biophys. 1987;26(2):103–114. doi: 10.1007/BF01211405. [DOI] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. In vivo kinetic status of hematopoietic stem and progenitor cells as inferred from labeling with bromodeoxyuridine. Exp Hematol. 1984 Oct;12(9):683–687. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Imai Y., Nakao I. In vivo radiosensitivity and recovery pattern of the hematopoietic precursor cells and stem cells in mouse bone marrow. Exp Hematol. 1987 Sep;15(8):890–895. [PubMed] [Google Scholar]
- Inoue T., Bullis J. E., Cronkite E. P., Kubo S. Stem cell proliferation and 125IdUrd incorporation into spleen and whole skeletal tissue. Ann N Y Acad Sci. 1985;459:162–178. doi: 10.1111/j.1749-6632.1985.tb20824.x. [DOI] [PubMed] [Google Scholar]
- Inoue T., Cronkite E. P., Hübner G. E., von Wangenheim K. H., Feinendegen L. E. Stem cell quality following irradiation: comparison of IUdR uptake in the spleen and number and size of splenic colonies in bone marrow transfused, fatally irradiated mice. J Radiat Res. 1984 Dec;25(4):261–273. doi: 10.1269/jrr.25.261. [DOI] [PubMed] [Google Scholar]
- Lerner C., Harrison D. E. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990 Feb;18(2):114–118. [PubMed] [Google Scholar]
- Magli M. C., Iscove N. N., Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982 Feb 11;295(5849):527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Meijne E. I., van der Winden-van Groenewegen R. J., Ploemacher R. E., Vos O., David J. A., Huiskamp R. The effects of x-irradiation on hematopoietic stem cell compartments in the mouse. Exp Hematol. 1991 Aug;19(7):617–623. [PubMed] [Google Scholar]
- Moore M. A. Review: Stratton Lecture 1990. Clinical implications of positive and negative hematopoietic stem cell regulators. Blood. 1991 Jul 1;78(1):1–19. [PubMed] [Google Scholar]
- Mulder A. H., Visser J. W., van den Engh G. J. Thymus regeneration by bone marrow cell suspensions differing in the potential to form early and late spleen colonies. Exp Hematol. 1985 Sep;13(8):768–775. [PubMed] [Google Scholar]
- Petrini J. H., Carroll A. M., Bosma M. J. T-cell receptor gene rearrangements in functional T-cell clones from severe combined immune deficient (scid) mice: reversion of the scid phenotype in individual lymphocyte progenitors. Proc Natl Acad Sci U S A. 1990 May;87(9):3450–3453. doi: 10.1073/pnas.87.9.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. A., Spaner D. E. The scid mouse: mutation in a DNA repair gene creates recipients useful for studies on stem cells, lymphocyte development and graft-versus-host disease. Immunol Rev. 1991 Dec;124:63–74. doi: 10.1111/j.1600-065x.1991.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Van Zant G. Studies of hematopoietic stem cells spared by 5-fluorouracil. J Exp Med. 1984 Mar 1;159(3):679–690. doi: 10.1084/jem.159.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]



