Abstract
The aim of this study was to perform a meta-analysis of the effects of sanitizing treatments of fresh produce on Salmonella spp., Escherichia coli O157:H7, and Listeria monocytogenes. From 55 primary studies found to report on such effects, 40 were selected based on specific criteria, leading to more than 1,000 data on mean log reductions of these three bacterial pathogens impairing the safety of fresh produce. Data were partitioned to build three meta-analytical models that could allow the assessment of differences in mean log reductions among pathogens, fresh produce, and sanitizers. Moderating variables assessed in the meta-analytical models included type of fresh produce, type of sanitizer, concentration, and treatment time and temperature. Further, a proposal was done to classify the sanitizers according to bactericidal efficacy by means of a meta-analytical dendrogram. The results indicated that both time and temperature significantly affected the mean log reductions of the sanitizing treatment (P < 0.0001). In general, sanitizer treatments led to lower mean log reductions when applied to leafy greens (for example, 0.68 log reductions [0.00 to 1.37] achieved in lettuce) compared to other, nonleafy vegetables (for example, 3.04 mean log reductions [2.32 to 3.76] obtained for carrots). Among the pathogens, E. coli O157:H7 was more resistant to ozone (1.6 mean log reductions), while L. monocytogenes and Salmonella presented high resistance to organic acids, such as citric acid, acetic acid, and lactic acid (∼3.0 mean log reductions). With regard to the sanitizers, it has been found that slightly acidic electrolyzed water, acidified sodium chlorite, and the gaseous chlorine dioxide clustered together, indicating that they possessed the strongest bactericidal effect. The results reported seem to be an important achievement for advancing the global understanding of the effectiveness of sanitizers for microbial safety of fresh produce.
INTRODUCTION
The consumption of fresh fruits and vegetables comprises an essential element of a healthy diet and a protective factor against several chronic diseases (1, 2). Even though the ingestion of these products is highly recommended by health authorities, guaranteeing fresh, safe, and high-quality fruits and vegetables remains an enormous challenge for fresh-produce industries.
In order to deliver the health benefits (2), fruits and vegetables must be safe. One of the chief concerns related to the safety of these products is their recurrent and increased association with disease outbreaks (3–6). Epidemiological investigations indicate that Salmonella, pathogenic Escherichia coli, and Listeria monocytogenes stand out as the most important bacterial agents linked to fresh-produce disease outbreaks (3, 5–7). Recent studies have reported the occurrence and high diversity of these microorganisms in the environment or in close areas of produce farming areas (8–13).
Given the above, fresh-produce industries have been implementing measures at pre- and postharvest steps to reduce or avoid the contamination of these products and, consequently, to diminish the burden of disease outbreaks. At postharvest steps, disinfection is the critical step for reduction of microbial contamination (14, 15). During disinfection, fresh produce is allowed to stay in contact with sanitizers added to the washing tanks aiming to reduce their microbial load. Finally, yet importantly, during disinfection, washing water should not become a point of cross-contamination (15, 16). The phenomenon of cross-contamination during produce washing has been indicated as the potential cause of the spinach E. coli O157:H7 outbreak that resulted in 205 illnesses and three deaths in the fall of 2006 in the United States (17, 18). As disinfection of fresh produce constitutes a critical control point, several reports quantify the pathogens' concentrations in these foods before and after disinfection with different sanitizers (19–26). However, because the log reductions of pathogens attained by the sanitizers are affected by the particular conditions or study characteristics (protocols for washing, type of fruit and vegetable, whole or cut fresh produce, type of sanitizer and concentration, washing time and temperature, pathogenic strains, microbiological essays, etc.) under which the measurements were obtained, variability in the effect size reported in the primary studies is expected to occur. This variability can be observed even among studies investigating the same type of fresh produce and sanitizer. Nonetheless, by means of a posteriori analysis and identification of the sources of variability likely to affect the log reduction of the pathogenic flora due to disinfection, it may be possible to explain, at least to some extent, the differences found among the study outcomes. Most importantly, it may be realistic to build a model that can be generalized to different types of fresh produce.
Meta-analysis has been defined as a statistical analysis of a collection of analytic results for the purpose of integrating the findings from a large amount of primary studies (27). Meta-analysis allows the explanation of the divergences in the study outcomes by the codification of moderating variables representing study characteristics related to research design features, data collection procedures, type of samples, etc., aiming to reduce the between-study heterogeneity or variability (28). Through meta-analysis, it may also be possible to accurately estimate the overall outcome measure, with increased statistical power, than is possible using only a single study (29).
Considering the capabilities of the meta-analysis and the significance of Salmonella, pathogenic E. coli, and L. monocytogenes for the microbial safety of fresh produce and for the public health (3, 5–7), the objective of this study is 4-fold: (i) first, to compile all publicly accessible published findings on the effects of sanitizers on the mean reduction in the log counts of Salmonella spp., E. coli O157:H7, and L. monocytogenes on fresh produce and quantitatively summarize the outcomes by means of meta-analytical models based on mixed-effects linear regressions; (ii) second, to explain a proportion of the total between-study heterogeneity in the reduction of the pathogens' populations by incorporating available study characteristics, such as type of fresh produce, type of sanitizer, concentration, and treatment time and temperature, into the basic meta-analysis model; (iii) third, to assess possible differences in the susceptibilities of Salmonella spp., E. coli O157:H7, and L. monocytogenes to selected sanitizers, as well as differences in the overall microbial log reduction among fresh produce for a given disinfectant treatment; and (iv) finally, to evaluate the effectiveness of the sanitizers to reduce each of the pathogen's populations using a common disinfectant treatment and to propose a classification of sanitizers according to their bactericidal efficacies by means of a meta-analytical dendrogram. The resulting meta-analysis models have the capability to provide overall log reduction estimates for a particular pathogen when using a given sanitizer and sanitizing treatment.
MATERIALS AND METHODS
Data collection and effect size parameterization.
Before commencing any meta-analysis study, the research problem must be stated and three important facets should be defined: population, intervention or treatment, and measured outcome. In this meta-analysis, the problem statement was the estimation of the overall effect of disinfecting fresh and minimally processed fruits and vegetables with aqueous and gaseous chemicals on the final microbial concentration (number of log reductions) of three pathogens (i.e., Salmonella spp., L. monocytogenes, and E. coli O157:H7). The population was specified as fresh produce, fruits and vegetables, prior to the sanitizing treatment, while the intervention or treatment was represented by the disinfection unit operation using aqueous or gaseous sanitizers. The measured outcome is derived from the pathogen's concentration on the fruits or vegetables before and after treatment, giving the number of log reductions.
The next step of literature identification was conducted using electronic search through Google with key terms, both in English and in Portuguese, encompassing the following: “Salmonella,” “Escherichia coli O157:H7,” “Listeria monocytogenes,” “pathogens,” “sanitizers,” “chemicals,” “solutions,” “organic acids,” “detergents,” “washing,” “inactivation,” “antibacterial effect,” “reduction,” “fruits,” “vegetables,” and “produce.” Electronic searches were conducted using sensible combinations of at least three of the above-listed terms. Also, literature for inclusion in the study was identified from bibliographic databases such as PubMed, Science Direct, and Scopus using the same key words. Data included considered studies available in scientific journals and electronically from 1990 to 2014. After an initial screening for research quality, a total of 55 studies were found to report on the effect of sanitizers on the concentration of pathogens in fresh produce. However, these encompassed also studies using ultrasound and irradiation treatments, which were disregarded, as only conventional washing with sanitizers was considered for inclusion in the meta-analysis. A second criterion used in the screening was the need for the primary study to report the concentration of either of the pathogens (Salmonella spp., E. coli O157:H7, and L. monocytogenes) in fresh produce both before (i.e., control) and after the disinfection treatment or, alternatively, the microbial log reduction attained by the disinfection treatment. As a third criterion for inclusion, the primary study had to clearly specify the type of sanitizer and its concentration, washing or exposure time and temperature, and sample size and/or standard deviations. As a fourth criterion, an approved microbiological method for pathogen enumeration had to be employed. Considering all of those requirements, 40 primary studies were regarded as appropriate for inclusion in the present meta-analysis study (19–26, 30–62).
As a next step, a parameterization of the intervention's effect size should be determined. The effect size (θ) refers to the degree to which the hypothetical phenomenon (i.e., reduction in the concentration of pathogens on fresh produce due to disinfection treatment) is present in the population. For the studies' outcomes to be compatible and meaningful for analysis, such effect size should be converted to a common scale that permits direct comparison and summation of the primary studies. Because the data generated by the primary studies are the means of a continuous variable (i.e., microbial concentration in or on fresh produce), the possible parameters to measure effect size or treatment difference are raw (unstandardized) mean difference, standardized mean difference, and response ratios (63). The most suitable parameter to measure effect size was the raw mean difference between control and treatment means, because all the primary studies reported in the same log CFU scale and it is an intuitively meaningful parameter. That is, referring in terms of, say, a 2- or 3-log microbial reduction is of widespread use among food microbiologists.
Consider a primary study j that reports means for two groups (control, or before disinfection treatment, and treated, or after disinfection treatment). Let x̄C and x̄T be the sample means of the two independent groups; hence, the effect size estimate θ̂, which, in our case, is the difference in sample means or mean log reduction R, is defined as
| (1) |
Now, let sC and sT be the sample standard deviations of the two groups and nC and nT be the sample sizes in the two groups, control and treated, respectively. If we assume that the two population standard deviations are different, then the SE of the mean log reduction R can be estimated as
| (2) |
Mean log reductions (R) and their standard errors (SE) for the three pathogens were estimated from the primary studies whose results were reported separately for the control and treated groups. Nonetheless, in some primary studies, mean log reductions and their standard errors were provided as such, so none of the above formulas needed to be applied, and their values were extracted directly from tables or charts.
Description of meta-analytical data set.
The microbial mean log reduction values for the three pathogens, whether estimated using equations 1 and 2 or directly extracted from the primary studies, were the outcomes of experiments carried out with a specific fresh produce under a certain disinfection treatment. Hence, all this additional information was also annotated from the primary studies in the form of study characteristics or moderating variables. The study characteristics considered were bacteria (a categorical variable), type of sanitizer (a categorical variable), sanitizer concentration (a continuous variable), type of produce (a categorical variable), treatment or washing time (a continuous variable), and treatment or washing temperature (a continuous variable). As explained above, sample sizes (nC and nT) and standard deviations (sC and sT) of the control (predisinfection) and treated (postdisinfection) groups were also extracted. Depending upon the sanitizer, a specific concentration unit was used in a primary study. For instance, for gaseous chlorine dioxide, the concentration was often expressed in parts per million, while for sodium chlorite, it was in grams per liter. In order to facilitate comparisons among sanitizers, all concentrations were converted to grams per 100 ml. For the 27 sanitizers recovered (namely, acetic acid [AA], acidified sodium chlorite [ASC], benzalkonium chloride [Bzc], citric acid [CA], calcined oyster shell [Ca-Oy], calcined Sakhalin surf clam [Ca-SS], calcium hypochlorite [CH], Citrox, chlorine dioxide gas [CD], dodecyl-benzenesulfonic acid [DA], sodium 2-ethylhexyl-sulfate [EHS], hydrogen peroxide [HP], lactic acid [LA], malic acid [MA], nisin, ozonized water [OW], ozone gas [Oz], pediocin, peroxyacetic acid [PAA], phytic acid [Phy], slightly acidic electrolysed water [SAEW], sodium chlorite [SC], sodium-dodecyl sulfate [SDS], sodium hypochlorite [SH], tartaric acid [TA], trisodium phosphate [TSP], and Tsunami 100), the concentrations ranged from 0.0001 to 4.8 g/100 ml, although it is important to bear in mind that every sanitizer is associated with a specific concentration range. For instance, in the primary studies, fresh fruits and vegetables are treated with chlorine dioxide gas in concentrations from 0.00015 to 0.00030 g/100 ml, while they are washed with lactic acid in higher concentrations, from 0.003 to 2.0 g/100 ml. In addition to the 27 sanitizers identified, primary studies also provided microbial mean log reductions from washing using only water (i.e., a blank treatment). The water types were tap water (W), distilled water (DisW), and deionized water (DioW), which were categorized within sanitizers, although a solute concentration of 0 g/100 ml was assigned to all water types. Washing times were in the range of 0.15 to 180 min, yet the longer times belonged to chlorine dioxide gas treatments. Temperatures for washing were mostly ambient, although overall they were in the interval from 4 to 55°C.
To get some insight into the spread of the microbial log reduction data among the categorical study characteristics, Table 1 compiles the number of mean log reduction observations partitioned by sanitizer, pathogen, and fresh produce. It should be noticed that the meta-analytical data are highly sparse, meaning that for some sanitizers, fewer data are available. For instance, for SAEW, microbial mean log reduction observations were reported for the three pathogens, while for ozone gas, data were limited to E. coli O17:H7 only. Moreover, the heterogeneity in the distribution of fresh produce across pathogens and sanitizers caused further sparseness. Said otherwise, for a given sanitizer, the types of produce studied did not coincide for all pathogens. From the 27 sanitizers whose microbial mean log reduction information was available in the literature, 9 were excluded from the meta-analyses (i.e., Bzc, Ca-Oy, Ca-SS, DA, EHS, nisin, pediocin, Phy, and SC) because too few observations were available (the threshold was set as equal to or less than four observations per sanitizer) (Table 1).
TABLE 1.
Microbial mean log reduction observations found in the literature according to type of fresh produce, pathogen, and sanitizer, extracted from published studies
| Sanitizera | Type of produce (no. of observations of mean log reduction of indicated organism[s]) |
||
|---|---|---|---|
| E. coli O157:H7 | L. monocytogenes | Salmonella spp. | |
| AA | Baby spinach (6) | Blueberry (4), rocket leaves (8), spring onion (8) | |
| ASC | Carrot (6), cilantro (4), tatsoi (9) | Carrot (7), cherry tomato (1), cucumber (1) | Carrot (3), cherry tomato (1), cucumber (1), tomato (6) |
| Bzc | Lettuce (2), tomato (2) | ||
| CA | Baby spinach (9), cilantro (1), lettuce (20), spinach (1) | Lettuce (20), spinach (1) | Blueberry (2), lettuce (20), rocket leaves (8), spring onion (8) |
| Ca-Oy | Tomato (4) | ||
| Ca-SS | Tomato (4) | ||
| CH | Broccoli (11), lettuce (12), spinach (1) | Spinach (1) | |
| Citrox | Lettuce (9) | ||
| CD | Cabbage (3), cantaloupe (10), carrot (3), lettuce (6), spinach (2), strawberry (6) | Cabbage (3), cantaloupe (10), carrot (3), lettuce (6), strawberry (5) | Apple (3), cabbage (3), carrot (3), cantaloupe (9), lettuce (6), onion (3), peach (3), spinach (2), strawberry (6), tomato (29) |
| DA | Romaine lettuce (2) | ||
| DioW | Baby spinach (6), broccoli (3), lettuce (6), mung bean (4), mung bean sprouts (4), romaine lettuce (2), spinach (1) | Lettuce (2), spinach (1) | Blueberry (2), lettuce (2), mung bean (4), mung bean sprouts (4), pepper (2) |
| DisW | Buckwheat (1), Chinese cabbage (3), lettuce (5), sesame (1), spinach (4), tomato (6), cabbage (2), apple (3), mung bean sprouts (2) | Broccoli (1), cabbage (1), mung bean sprouts (3), Chinese cabbage (1), lettuce (4), sesame leaf (1), spinach (1), cucumber (1) | Buckwheat (1), apple (3), blueberry (3), spinach (1), mung bean sprouts (2), lettuce (1), cucumber (3) |
| EHS | Romaine lettuce (2) | ||
| HP | Baby spinach (9) | Cucumber (1) | Blueberry (5), cantaloupe (2), honeydew (2) |
| LA | Apple (2), baby spinach (9), lettuce (33), spinach (1), tomato (4) | Lettuce (20) | Apple (2), blueberry (2), lettuce (20), spinach (1) |
| MA | Baby spinach (9), lettuce (20) | Lettuce (20) | Lettuce (20) |
| Nisin | Broccoli (1), cabbage (1), mung bean sprouts (1) | ||
| OW | Cabbage (2), Chinese cabbage (2), lettuce (2), spinach (5) | Spinach (1) | Spinach (2) |
| Oz | Cabbage (2), Chinese cabbage (2), lettuce (2), spinach (2) | ||
| PAA | Carrot (4), lettuce (4), mung bean sprouts (7), rocket leaves (1), spinach (1), tomato (3) | Carrot (4), mung bean sprouts (7) | Carrot (4), green onion (4), lettuce (4), mung bean sprouts (7), spinach (1), tomato (8) |
| Pediocin | Broccoli (1), cabbage (1), mung bean sprouts (1) | ||
| Phy | Tomato (4) | ||
| SAEW | Chinese cabbage (2), lettuce (2), daikon lettuce (2), mung bean (16), mung bean sprouts (16), sesame leaf (2), spinach (4) | Chinese cabagge (2), lettuce (2), sesame leaf (2), spinach (4) | Chinese celery (1), daikon lettuce (1), lettuce (1), mung bean (16), mung bean sprouts (16) |
| SC | Cilantro (1) | ||
| SDS | Blueberry (11) | ||
| SH | Baby spinach (3), cabbage (2), carrot (4), Chinese cabbage (2), Chinese celery (1), cilantro (1), daikon lettuce (1), lettuce (12), mung bean sprouts (7), romaine lettuce (4), rocket leaves (1), spinach (3), tatsoi (1), tomato (9) | Carrot (6), cherry tomato (2), cucumber (3), lettuce (2), mung bean sprouts (7), spinach (1) | Blueberry (7), carrot (6), cherry tomato (2), Chinese celery (1), cucumber (2), daikon lettuce (1), green onion (4), lettuce (7), mung bean sprouts (7), pepper (2), tomato (20) |
| TA | Baby spinach (6) | ||
| TSP | Lettuce (4) | Lettuce (4), pepper (4) | |
| Tsunami 100 | Lettuce (6) | ||
| Water | Carrot (4), daikon lettuce (1), lettuce (3), tatsoi (1), tomato (1) | Carrot (4), cucumber (1) | Cantaloupe (1), carrot (4), Chinese celery (1), daikon honeydew (1), lettuce (2), tomato (8) |
Abbreviations: AA, acetic acid; ASC, acidified sodium chlorite; Bzc, benzalkonium chloride; CA, citric acid; Ca-Oy, calcined oyster shell; Ca-SS, calcined Sakhalin surf clam; CH, calcium hypochlorite; CD, chlorine dioxide gas; DA, dodecyl-benzenesulfonic acid; DioW, deionized water; DisW, distilled water; EHS, sodium 2-ethylhexyl-sulfate; HP, hydrogen peroxide; LA, lactic acid; MA, malic acid; OW, ozonized water; Oz, ozone gas; PAA, peroxyacetic acid; Phy, phytic acid; SAEW, slightly acidic electrolyzed water; SC, sodium chlorite; SDS, sodium dodecyl sulfate; SH, sodium hypochlorite; TA, tartaric acid; TSP, trisodium phosphate.
Thus, for the 18 sanitizers remaining for the meta-analyses plus the three water types (i.e., 21 sanitizers), a total of 1,025 microbial mean log reduction values were brought together. In the primary studies, those values were obtained by measuring the effects of disinfectant treatment in 30 types of fresh produce: apple, baby spinach, blueberry, broccoli, buckwheat, cabbage, cantaloupe, carrot, cherry tomato, Chinese cabbage, Chinese celery, cilantro, cucumber, daikon, green onion, honeydew, lettuce, mung bean, mung bean sprouts, onion, peach, pepper, rocket leaves, romaine lettuce, sesame leaf, spinach, spring onion, strawberry, tatsoi, and tomato. For space reasons, the meta-analytical raw data are not presented here, but they can be found in the supplemental material in Tables S1, S2, and S3, separately for E. coli O157:H7, L. monocytogenes, and Salmonella spp., respectively. Such tables compile the microbial mean log reductions and standard errors when available, sanitizer agents, sanitizer concentrations, treatment times and temperatures, types of fresh produce, sample size, and primary studies.
The sparseness of the data has some implications in the choice and the design of the meta-analysis mixed-effect models. Because of the considerable dispersion in the number of microbial mean log reduction values among the sanitizer-pathogen combinations (Table 1), a general meta-analysis model encompassing all data could not be adjusted. Hence, separate meta-analysis studies were conducted, first on data partitioned by sanitizer—in order to make comparisons among pathogens and fresh produce—and, subsequently, on data partitioned by pathogen—in order to make comparisons among the bactericidal efficacies of sanitizers. This is explained in detail in the following subsections.
Meta-analysis models by sanitizer.
When constructing separate meta-analysis models by sanitizer, it is possible to assess both whether there are differences in the levels of resistance to the sanitizer agent among the three pathogens and whether there are differences among produce in the microbial log-reduction attained by a disinfection treatment.
Assessing differences among pathogens.
To assess the bactericidal efficacy of fresh produce disinfection among pathogens, nine sanitizing agents with the least sparseness in the number of observations across pathogens were selected. These were acetic acid, acidified sodium chlorite, chlorine dioxide gas, citric acid, hydrogen peroxide, malic acid, peroxyacetic acid, slightly acidic electrolyzed water, and sodium hypochlorite. Tap water was also selected for comparison, as it can be regarded as a blank treatment for washing (i.e., washing without sanitizer).
A meta-analysis model can be considered a special case of a multilevel analysis using hierarchical linear models, with subjects between studies at the first level and studies at the second level. In a multilevel meta-analysis, one usually starts from the random-effects model, and if the between-study variance is shown to be noteworthy, study characteristics can be added to the model to account for at least part of the heterogeneity in the true effect size θ (in our case, the mean log reduction R). Thus, for each of the 10 selected sanitizers (including tap water), the microbial mean log reduction R was modeled as
| (3) |
where β0 is the fixed effect of the pathogen i, β1 the mean effect of the increment in the logarithm of the sanitizer concentration C, β2 the mean effect of a 1°C increment in disinfection temperature T, and β3 the mean effect of a 1-min increment in disinfection time t. The wide range (0.00005 to 2.0 g/100 ml) of the sanitizer concentration was reduced by log transformation in order to stabilize the parameter estimation of the regression models.
Because of the sparse nature of the data structure, whereby in many cases one primary study reported results for only one or two types of fresh produce, for this meta-analysis it was not feasible either to separate the between-produce variability from the between-study variability or to build a nested covariance of primary studies within a type of fresh produce. To overcome this problem and still be able to account for the evident variability due to the different primary studies j and the different fresh produce k, both variables were merged into an interaction variable (jk). Such interaction was assumed to be the subject of variation of the intercept random effects ujk placed in equation 3. The random effects ujk are assumed to be normally distributed with mean zero and variance s2u. The errors or residuals εijk are also assumed to follow a normal distribution with mean zero and variance s2. Using this model design, the estimated value of Rijk represents therefore the overall mean microbial log reduction for the pathogen i attained by a particular sanitizing treatment (C, T, and t), applicable to the entire population of fresh produce and primary studies. Nonetheless, if we wished to estimate the mean microbial log reduction for a particular fresh produce, it can still be done extracting its corresponding random effect ujk and replacing in equation 3.
Since primary studies are expected to differ from each other in the reliability of estimating the true effect of disinfection on the pathogens' numbers on fresh produce, for instance, due to differences in study sizes, analytical methods or experimental designs, a weighted linear mixed model was preferred, with weights representing the precision in estimating the true microbial log reduction. In meta-analysis, it is common practice to use the standard error of the effect size as a measure of precision to assign weights to each of the primary studies. However, in the present meta-analysis, it was not possible to obtain the standard errors of the log reductions R for all primary studies; and consequently, the precision was instead redefined as some measure proportional to the sample size N used in every primary study. Hence, the weight—the level of confidence on each of the measured log reductions R—was given by the sample size. A weighted mixed-effects linear model (equation 3) was adjusted to each of the 10 selected sanitizers.
Assessing differences among fresh produce.
By partitioning data by sanitizer, it is also possible to appraise whether the same disinfection treatment would achieve variable effects depending upon the type of fresh produce. However, to carry out this assessment, we need to choose sanitizers that have been tested in a wide range of fresh produce, and we needed to ensure that these types of fresh produce are roughly the same at least across two pathogens. For instance, observing the data dispersion shown in Table 1, using the data from the gaseous chlorine dioxide (CD) is a good option because it was tested on cabbage, cantaloupe, lettuce, and strawberry for the three pathogens and tested on spinach for E. coli O157:H7 and Salmonella. Following this reasoning, the sanitizers ASC, CD, SAEW, and SH were considered suitable for this analysis, and the following meta-analysis model was adjusted to each of the four data sets:
| (4) |
Now, β0 is the fixed effect of the type of fresh produce k, and ui(j) are the intercept random effects with subject of variation pathogen i nested in the primary study j. The nested random effects ui(j) are assumed to be normally distributed with means zero and variances s2i and s2j. With such a model design, the variability due to pathogens is extracted, and the response variable Rijk can be thought of the overall mean log reduction in the entire population of pathogens, from treating a fresh produce k by a particular sanitizing treatment (C, T, and t). In a similar fashion, a weighted regression was opted for, in order to account for the differences in precision among primary studies. The sample size N was used as the weight of each log reduction observation.
Meta-analysis models by pathogen.
The microbial data were also partitioned by pathogen, producing three data sets for E. coli O157:H7, L. monocytogenes, and Salmonella spp. Separate meta-analyses were then performed by pathogen, so as to compare the bactericidal efficacies of sanitizers for a common treatment (C, T, and t). For each of the pathogen's data sets, a mixed-effects linear model of the type
| (5) |
was fitted, where β0 now represents the fixed effect of the type of sanitizer l and ujk are intercept random effects, whose subject of variation is the interaction study × fresh produce, which account for the variability due to both the different primary studies j and the different fresh produce k. The regression models were fitted using the sample size N as the weight of each of the observations.
For each of the models explained above (equations 3 to 5), the regression's basic assumption of data normality was verified: the normality of residuals was first assessed, and then the studentized residuals were examined for identifying spurious data points lower than −3.0 and higher than 3.0 (i.e., outliers). In the case that outliers were present, they were removed from the data and regression models refitted. In addition, for the regression models, a measure of between-study heterogeneity, I2, was calculated. The I2 statistics, or intraclass correlation, estimates the proportion of between-study variance from the total variance. If the intraclass variance is higher than 25% of the total variance, the variance between studies can be deemed large enough to attempt to model it using available study characteristics. Measures of goodness of fit such as Akaike information criterion (AIC) and Bayesian information criterion (BIC) were also computed for each of the meta-analysis models.
The mixed-effects linear models were also used to construct meta-analytical forest plots (63), in order to allow a better visualization of the difference in the effect of a given sanitizer and disinfection treatment among fresh produce (from equation 4) and the difference among sanitizers for a given disinfection treatment (from equation 5). The weighted mixed-effects linear models were fitted in R version 2.14.2 (R Development Core Team) using the “lme” function from the “nlme” package (64). Forest plots were built using the “metafor” package (65).
Cluster analysis of sanitizers.
In order to examine similarities and dissimilarities among sanitizers in their bactericidal effects, so that clusters of sanitizers could be identified and separated from others, a hierarchical cluster analysis was performed on the microbial data from selected sanitizers. Since cluster analysis is a multivariate data analysis technique—hence, it requires continuous variables as inputs—it is necessary to first obtain some measurements of the characteristics of each sanitizer in the form of a continuous variable. In a regression analysis of the type
| (6) |
adjusted to the whole data from a given sanitizer, the parameter estimates β0, β1, β2, and β3 can be thought of as the continuous variables characterizing the disinfectant capacity of the sanitizer. This is because for a sanitizer, the higher the intercept β0 (representing the mean log reduction R at the mean log concentration C, the mean temperature T, and the mean time t), the higher the microbial mean log reduction R. Similarly, a sanitizer with higher slopes β1, β2, and β3 will produce a greater mean log reduction R for a given log concentration C, temperature T, and time t, respectively. Thus, for the cluster analysis, the sanitizers selected needed to be those presenting microbial log reduction observations measured over a wide range of temperatures, concentrations, and times so that precise slope estimates could be computed. Suitable sanitizers for analysis were AA, ASC, CA, CD, CH, HP, LA, MA, PAA, SAEW, and SH. The water types, W, DisW and DioW, were also included in the list of sanitizers as a mechanism for testing the performance of the clustering algorithm to build meaningful groups (said otherwise, because it is known a priori that the water types are not sanitizers and their bactericidal effect is the lowest of all, they should be grouped together by the clustering method chosen).
Equation 6 was then fitted to each of the sanitizers l, and the parameter estimates β0l, β1l, β2l, and β3l for l = {1, 2, …14} were organized in an 14-by-4 matrix, where the rows corresponded to the sanitizers and the columns to the four parameter estimates. As the next step, the Euclidean distance between each pair of sanitizers was computed and arranged in a distance matrix. The clustering was performed using a hierarchical algorithm whereby the partition with the cluster in which k = 1 (all sanitizers are together in the same cluster) is part of the output, and also the situation with k = j (each sanitizer forms a separate cluster with only a single element). In between, all values of k = 2, 3, … j − 1 are covered in a kind of gradual transition: the only difference between k = r and k = r + 1 is that one of the r clusters splits up in order to obtain r + 1 clusters (i.e., two of the r + 1 clusters combine to yield r clusters). The clustering method chosen was Ward's method, which is a minimum-variance method aiming at finding compact and spherical clusters (for further information on hierarchical clustering and clustering methods, see reference 66). The distance matrix was computed using the “dist” function, and the agglomerative hierarchical cluster analysis producing the dendrogram was using the “hclus” function, both from the R “stats” package.
RESULTS
Meta-analysis models by sanitizer.
Table 2 shows the results from fitting equation 3 to nine sanitizers plus water (n = 10) studied. These are the overall mean log reductions for the specific pathogens caused by each sanitizer treatment applied to fruits and vegetables. From Table 2, it can be seen that for most sanitizers, concentration, temperature, and time have direct effects on the microbial log reduction, even though for water and ASC a quadratic effect of temperature on mean log reduction was also identified. The covariate was not included for temperature when the treatment was SAEW, as data were available only for ambient temperature. The concentration covariate was not included when treatment was water, as it had no meaning for this treatment.
TABLE 2.
Parameter estimates of the individual meta-analysis mixed-effects linear models by sanitizer, predicting the microbial mean log reduction in fresh produce as a function of microorganism, sanitizer concentration, and washing time and temperaturea
| Sanitizer | Parameters | Mean | SE | Pr > |t| | AIC/BIC |
|---|---|---|---|---|---|
| AA | Predictors of R | ||||
| E. coli | 1.234 X | 0.909 | 0.307 | 27/31 | |
| Listeriab | — | — | — | ||
| Salmonella | 1.325 Y | 0.565 | 0.143 | ||
| Concn | 0.103 | 0.072 | 0.181 | ||
| Temp | 0.022 | 0.004 | <0.0001 | ||
| Time | 0.029 | 0.007 | <0.0001 | ||
| Variances | |||||
| s2u (intercept) | 0.705 | I2 = 99% | |||
| s2 (residual) | 0.004 | ||||
| CA | Predictors of R | ||||
| E. coli | 2.576 Y | 0.429 | <0.0001 | 102/121 | |
| Listeria | 2.337 X | 0.430 | <0.0001 | ||
| Salmonella | 2.868 Z | 0.425 | <0.0001 | ||
| Concn | 0.500 | 0.057 | <0.0001 | ||
| Temp | 0.032 | 0.008 | <0.0001 | ||
| Time | 0.043 | 0.003 | <0.0001 | ||
| Variances | |||||
| s2u (intercept) | 0.436 | I2 = 82% | |||
| s2 (residual) | 0.095 | ||||
| MA | Predictors of R | ||||
| E. coli | 4.223 X | 0.207 | <0.0001 | 5/18 | |
| Listeria | 4.538 Z | 0.212 | <0.0001 | ||
| Salmonella | 4.444 Y | 0.207 | <0.0001 | ||
| Concn | 0.692 | 0.041 | <0.0001 | ||
| Tempc | — | — | — | ||
| Time | 0.051 | 0.003 | <0.0001 | ||
| Variances | |||||
| s2u (intercept) | 0.0007 | I2 = 12% | |||
| s2 (residual) | 0.0053 | ||||
| SAEW | Predictors of R | ||||
| E. coli | 11.34 X | 0.340 | <.00001 | 32/49 | |
| Listeria | 11.45 X | 0.359 | <0.0001 | ||
| Salmonella | 11.31 X | 0.341 | <0.0001 | ||
| Concn | 1.573 | 0.039 | <0.0001 | ||
| Tempc | — | — | — | ||
| Time | 0.019 | 0.005 | <0.0001 | ||
| Variances | |||||
| s2u (intercept) | 0.591 | I2 = 94% | |||
| s2 (residual) | 0.037 | ||||
| PAA | Predictors of R | ||||
| E. coli | 3.843 Z | 0.655 | <0.0001 | 62/74 | |
| Listeria | 3.295 X | 0.658 | <0.0001 | ||
| Salmonella | 3.535 Y | 0.653 | <0.0001 | ||
| Concn | 0.491 | 0.116 | <0.0001 | ||
| Temp | NS | — | |||
| Time | 0.057 | 0.030 | 0.067 | ||
| Variances | |||||
| s2u (intercept) | 0.568 | I2 = 89% | |||
| s2 (residual) | 0.067 | ||||
| SH | Predictors of R | ||||
| E. coli | 3.143 X | 0.712 | <0.0001 | 289/307 | |
| Listeria | 3.233 X | 0.719 | <0.0001 | ||
| Salmonella | 3.861 Y | 0.719 | <0.0001 | ||
| Concn | 0.383 | 0.148 | 0.012 | ||
| Temp | NS | ||||
| Time | 0.097 | 0.047 | 0.043 | ||
| Variances | |||||
| s2u (intercept) | 1.040 | I2 = 94% | |||
| s2 (residual) | 0.061 | ||||
| CD | Predictors of R | ||||
| E. coli | 8.450 X | 1.085 | <0.0001 | 359/378 | |
| Listeria | 8.914 Y | 1.084 | <0.0001 | ||
| Salmonella | 8.855 Y | 1.066 | <0.0001 | ||
| Concn | 0.854 | 0.115 | <0.0001 | ||
| Temp | NS | ||||
| Time | 0.054 | 0.010 | <0.0001 | ||
| Variances | |||||
| s2u (intercept) | 1.413 | I2 = 92% | |||
| s2 (residual) | 0.119 | ||||
| Water | Predictors of R | ||||
| E. coli | 1.765 Y | 0.318 | <0.0001 | 27/40 | |
| Listeria | 1.754 Y | 0.331 | <0.0001 | ||
| Salmonella | 1.653 X | 0.322 | <0.0001 | ||
| Concnd | — | — | — | ||
| Temp | −0.080 | 0.022 | 0.001 | ||
| Temp (quadratic effect)c | 0.001 | 0.000 | <0.0001 | ||
| Time | −0.003 | 0.005 | 0.600 | ||
| Variances | |||||
| s2u (intercept) | 0.181 | I2 = 98% | |||
| s2 (residual) | 0.003 | ||||
| ASC | Predictors of R | ||||
| E. coli | 2.053 Y | 0.525 | <0.0001 | 88/100 | |
| Listeria | 1.625 X | 0.628 | 0.015 | ||
| Salmonella | 2.686 Z | 0.682 | <0.0001 | ||
| Concn | 0.493 | 0.119 | <0.0001 | ||
| Temp | 0.472 | 0.059 | 0.001 | ||
| Temp (quadratic effect)c | −0.011 | 0.002 | 0.001 | ||
| Time | −0.254 | 0.046 | <0.0001 | ||
| Variances | |||||
| s2u (intercept) | 0.052 | I2 = 48% | |||
| s2 (residual) | 0.056 | ||||
| HP | Predictors of R | ||||
| E. coli | 1.739 X | 0.751 | 0.146 | 8/14 | |
| Listeriab | — | ||||
| Salmonella | 2.906 Y | 0.601 | 0.040 | ||
| Concn | 0.348 | 0.137 | 0.027 | ||
| Temp | 0.031 | 0.007 | 0.001 | ||
| Time | 0.059 | 0.043 | 0.197 | ||
| Variances | |||||
| s2u (intercept) | 0.052 | I2 = 66% | |||
| s2 (residual) | 0.026 |
Different letters (X, Y, and Z) indicate significant differences in log reduction among microorganisms. NS, not significant. —, parameter not calculated.
No data for L. monocytogenes were available.
As data were available only for ambient temperature, a temperature covariate could not be included.
The concentration covariate was not included as it has no meaning for water.
Through the meta-analytical model, it was possible to find that the pathogens studied may differ in terms of their resistance depending on the sanitizers. L. monocytogenes presented the lower intercept, meaning that it may be more resistant to CA, PA, and ASC treatments. On the other hand, pathogenic E. coli seems to be more resistant to MA, CD, AA, and HP treatments (Table 2). Salmonella presented the lowest intercepts (higher resistance) only when the treatment was done with water, while L. monocytogenes and pathogenic E. coli presented similar resistances to this treatment (Table 2). SH had a similar impact on L. monocytogenes and pathogenic E. coli inactivation, whereas L. monocytogenes and Salmonella were equally resistant to CD. SAEW was the only sanitizer for which no differences in inactivation resistance were found for the three pathogens studied (Table 2). It should be highlighted that for some sanitizers, such as AA and HP, no data on L. monocytogenes inactivation was available. Therefore, for these sanitizers, only Salmonella and pathogenic E. coli were considered, with the latter being more resistant than the former (Table 2).
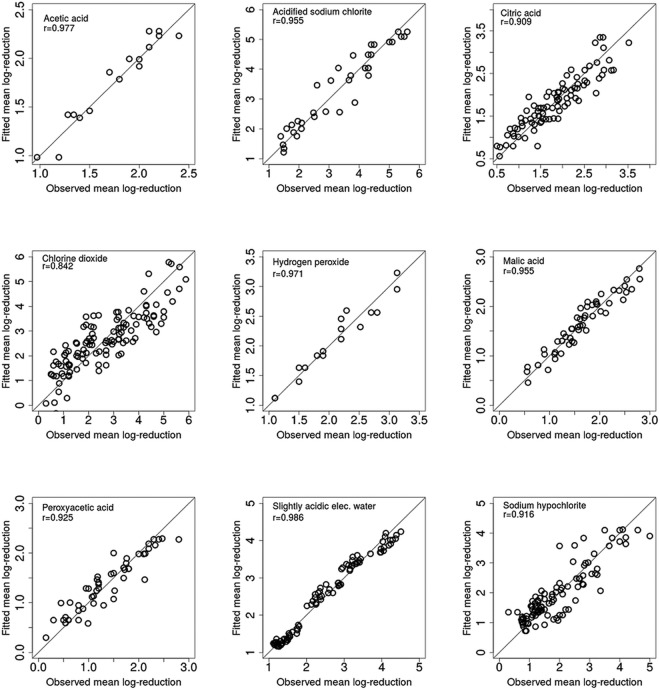
The I2 intraclass correlation values obtained were generally >48%, except for the treatment with MA (12%) (Table 2), which suggest that for most sanitizers, there may be other moderating variables explaining the remaining between-study variability that were not codified in the present meta-analysis study. Despite the above, for each of the sanitizers, a reasonable agreement was shown between the observed mean log reduction values extracted from the primary studies, and those fitted by the models from Table 2, with coefficients of correlation ranging from 0.842 to 0.986 (Fig. 1).
FIG 1.
Scatter plots of mean microbial log reduction values fitted by the independent meta-analysis linear mixed models by sanitizer (from Table 2) in comparison with the observed data.
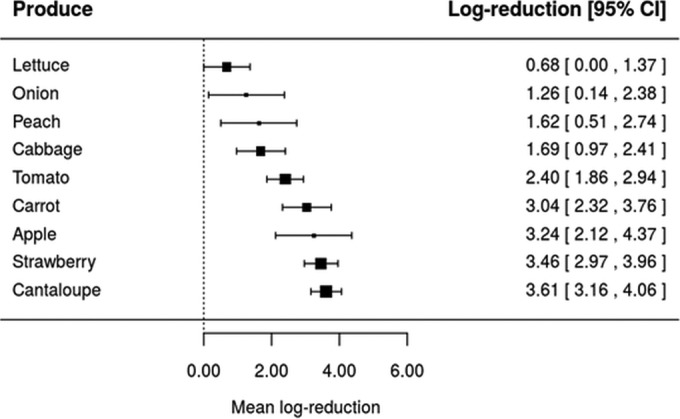
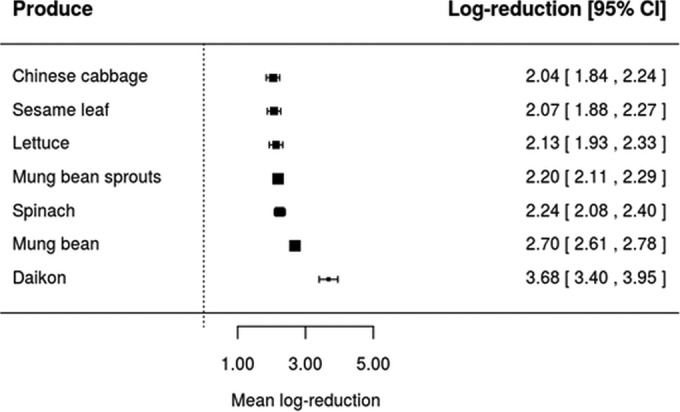
Taking into account that the inactivation of L. monocytogenes, Salmonella, and pathogenic E. coli by four sanitizers (ASC, CD, SAEW, and SH) was assessed in at least four different types of produce (Table 1), equation 4 has been used to assess whether the inactivation effectiveness of the same washing treatment would be affected by the type of fresh produce. Forest plots were constructed for each of the four sanitizing treatments using realistic sanitizer concentration and washing time as shown in Fig. 2, 3, 4, and 5. Also, it should be highlighted that these forest plots were constructed based on the meta-analytical model (equation 4) fitted to ASC, CD, SAEW, and SH. For example, data on L. monocytogenes, Salmonella, and pathogenic E. coli inactivation by ASC were available only for six types of fresh produce, namely, cucumber, cherry tomato, tatsoi, cilantro, tomato, and carrots (Fig. 2). Based on the model represented by equation 4, it can be seen that different mean log reductions were achieved according to the type of fresh produce studied. When ASC was the sanitizer used, mean log reductions varied from 1.68 for cucumber to 5.38 for carrots (Fig. 2), while log reductions varied from 0.68 to 3.61, 2.04 to 3.68, and 0.91 to 3.38 for CD, SAEW, and SH treatments, respectively (Fig. 3 to 5). The data obtained suggest, in general, that sanitizing treatments were less effective (achieved lower log reductions of pathogens) when applied in leafy vegetables than for other fresh produce (Fig. 2, 3, 4, and 5).
FIG 2.
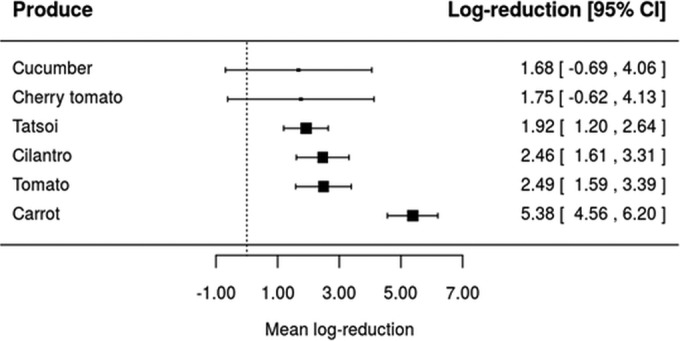
Forest plot of the overall mean log reduction of pathogens (E. coli O157:H7, L. monocytogenes, and Salmonella spp.) on different fresh produce achieved by sanitizing washing with 0.04 g/100 ml of acidified sodium chloride (ASC) at a time and temperature of 3 min and 25°C. CI, confidence interval.
FIG 3.
Forest plot of the overall mean log reduction of pathogens (E. coli O157:H7, L. monocytogenes, and Salmonella spp.) on different fresh produce achieved by sanitizing treatment with 0.00033 g/100 ml of gaseous chlorine dioxide (CD) at a time and temperature of 10 min and 25°C.
FIG 4.
Forest plot of the overall mean log reduction of pathogens (E. coli O157:H7, L. monocytogenes, and Salmonella spp.) on different fresh produce achieved by sanitizing washing with 0.005 g/100 ml of slightly acidic electrolyzed water (SAEW) at a time and temperature of 3 min and 25°C. CI, confidence interval.
FIG 5.
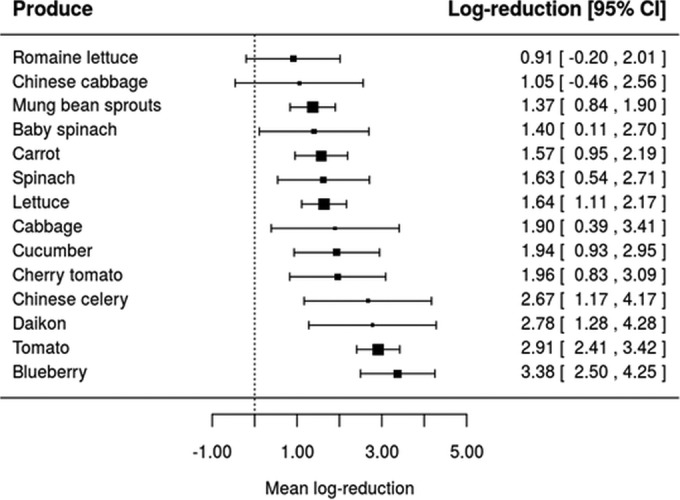
Forest plot of the overall mean log reduction of pathogens (E. coli O157:H7, L. monocytogenes, and Salmonella spp.) on different fresh produce achieved by sanitizing washing with 0.012 g/100 ml of sodium hypochlorite (SH) at a time and temperature of 3 min and 25°C.
Meta-analysis models by pathogen.
A further approach taken was to build separate meta-analytical inactivation models for each of the pathogens studied, i.e., L. monocytogenes, Salmonella, and pathogenic E. coli. This was done with the aim to comparing the bactericidal effects of sanitizers for a common treatment (C, T, and t). Tables 3, 4 and 5 show the parameter estimates obtained using equation 5 fitted to each of the pathogens predicting their log reductions. The I2 values were >60% for the three models predicting the inactivation of E. coli O157:H7, L. monocytogenes, and Salmonella, suggesting significant remaining heterogeneity in the outcomes from the primary studies (Tables 3, 4, and 5). Considering that the meta-analysis models were fitted by pathogen, it is now possible to see the differences among sanitizers and rank them from the lowest to the highest effects on pathogens inactivation (log reduction) (Tables 3, 4, and 5). It should be underlined that the sanitizers listed in these tables are not the same for the three pathogens because of the data sparseness. Totals of 15, 12, and 8 different sanitizers were used for E. coli O157:H7, L. monocytogenes, and Salmonella, respectively. Despite this, it was possible to find some similarity regarding the log reductions caused by the sanitizers over the three pathogens studied. Comparing the sanitizers' intercept values, SAEW, ASC, and CD appeared as the most effective sanitizers against E. coli O157:H7, L. monocytogenes, and Salmonella (Tables 3, 4, and 5). On the other hand, Oz, HP, and AA, CA, SH, and LA caused lower log reductions in E. coli O157:H7 and Salmonella (Tables 3 and 5), while, AA, LA, and SDS caused lower log reductions in L. monocytogenes (Table 4). The fitted intercepts presented in Tables 3, 4, and 5 also suggest that E. coli O157:H7 seems to be more resistant to the most effective sanitizers. While SAEW, ASC, and CD caused 3.4, 5.1, and 3.6 mean log reductions in E. coli O157:H7 (Table 3), the numbers of log reductions caused in L. monocytogenes and Salmonella were 6.9, 8.0, and 7.0 and 5.1, 5.4, and 7.0, respectively (Tables 4 and 5). Among these three sanitizers, ASC and CD were more effective against L. monocytogenes and Salmonella, while SAEW was the least effective against E. coli O157:H7 (Tables 3, 4, and 5).
TABLE 3.
Parameter estimates of the meta-analysis model predicting the mean log reduction of E. coli O157:H7 in fresh produce as a function of sanitizer, sanitizer concentration, and washing time and temperature
| Parameter | Meana | SE | Pr > |t| | AIC/BIC |
|---|---|---|---|---|
| Predictors of R | ||||
| Sanitizer | ||||
| AA | 2.094 B | 0.535 | <0.0001 | 742/816 |
| ASC | 5.103 G | 0.419 | <0.0001 | |
| CA | 2.362 C | 0.440 | <0.0001 | |
| CH | 3.192 E | 0.526 | <0.0001 | |
| CD | 3.555 F | 0.615 | <0.0001 | |
| HP | 1.987 B | 0.507 | <0.0001 | |
| LA | 2.537 D | 0.425 | <0.0001 | |
| MA | 2.429 CD | 0.445 | <0.0001 | |
| OW | 2.060 B | 0.436 | <0.0001 | |
| Oz | 1.628 A | 0.490 | 0.001 | |
| PAA | 3.167 E | 0.441 | <0.0001 | |
| SAEW | 3.455 F | 0.485 | <0.0001 | |
| SH | 2.677 D | 0.393 | <0.0001 | |
| TA | 2.369 C | 0.535 | <0.0001 | |
| Tsunami 100 | 2.976 E | 0.472 | <0.0001 | |
| Log concn | 0.367 | 0.053 | <0.0001 | |
| Temp | 0.019 | 0.009 | 0.049 | |
| Time | 0.071 | 0.007 | <0.0001 | |
| Variances | ||||
| s2u (intercept) | 0.689 | I2 = 64% | ||
| s2 (residual) | 0.392 |
Different letters indicate that sanitizers have significantly different effects.
TABLE 4.
Parameter estimates of the meta-analysis model predicting the mean log reduction of L. monocytogenes in fresh produce as a function of sanitizer, sanitizer concentration, and washing time and temperature
| Parameter | Meana | SE | Pr > |t| | AIC/BIC |
|---|---|---|---|---|
| Predictors of R | ||||
| Sanitizer | ||||
| ASC | 5.442 F | 0.493 | <0.0001 | 327/361 |
| CA | 3.282 A | 0.696 | <0.0001 | |
| CD | 7.058 G | 0.857 | <0.0001 | |
| LA | 3.620 B | 0.711 | <0.0001 | |
| MA | 4.218 D | 0.711 | <0.0001 | |
| PAA | 3.980 C | 0.622 | <0.0001 | |
| SAEW | 5.147 E | 0.678 | <0.0001 | |
| SH | 3.594 B | 0.502 | <0.0001 | |
| Log concn | 0.617 | 0.091 | <0.0001 | |
| Temp | ND | |||
| Time | 0.081 | 0.008 | <0.0001 | |
| Variances | ||||
| s2u (intercept) | 0.613 | I2 = 60% | ||
| s2 (residual) | 0.397 |
Different letters indicate that sanitizers have significantly different effects. ND, not determined. Temperature effect could not be estimated, as log reduction values for L. monocytogenes were mostly obtained at ambient temperature (mean = 21.0°C).
TABLE 5.
Parameter estimates of the meta-analysis model predicting the mean log reduction of Salmonella spp. in fresh produce as a function of sanitizer, sanitizer concentration, and washing time and temperature
| Parameter | Meana | SE | Pr > |t| | AIC/BIC |
|---|---|---|---|---|
| Predictors of R | ||||
| Sanitizer | ||||
| AA | 3.622 A | 0.504 | <0.0001 | 850/908 |
| ASC | 8.095 G | 0.517 | <0.0001 | |
| CA | 4.010 B | 0.500 | <0.0001 | |
| CD | 8.225 G | 0.672 | <0.0001 | |
| HP | 4.451 C | 0.553 | <0.0001 | |
| LA | 3.797 A | 0.515 | <0.0001 | |
| MA | 4.000 B | 0.532 | <0.0001 | |
| PAA | 6.675 EF | 0.504 | <0.0001 | |
| SAEW | 6.901 F | 0.755 | <0.0001 | |
| SDS | 3.779 A | 0.582 | <0.0001 | |
| SH | 5.980 D | 0.436 | <0.0001 | |
| TSP | 6.315 E | 0.535 | <0.0001 | |
| Log concn | 0.771 | 0.070 | <0.0001 | |
| Temp | ND | |||
| Time | 0.049 | 0.007 | <0.0001 | |
| Variances | ||||
| s2u (intercept) | 1.269 | I2 = 80% | ||
| s2 (residual) | 0.313 |
Different letters indicate that sanitizers have significantly-different effects. ND, not determined. Temperature effect could not be estimated, as log reduction values for Salmonella spp. were mostly obtained at ambient temperature (mean = 22.5°C).
A way to visualize the effect of type of sanitizer on microbial log reduction is through the construction of forest plots. To this end, the fitted meta-analysis models from Tables 3, 4, and 5 were solved for a hypothetical treatment with sanitizer concentration of 0.001 g/100 ml and exposure time of 3 min at ambient temperature in order to predict the mean log reductions of E. coli O157:H7, L. monocytogenes, and Salmonella. These predicted values are illustrated as forest plots in Fig. 6, 7, and 8. Under the hypothetical treatment conditions, it was found that Oz and ASC resulted in the lowest (0.14 [−0.50, 0.78]) and highest (3.86 [3.24, 4.49]) log reductions of E. coli O157:H7, respectively (Fig. 6). For L. monocytogenes, the lowest and highest log reductions would be obtained with the use of CA (0.37 [−0.77, 1.50]) and ASC (2.47 [1.41, 3.53]) as sanitizers (Fig. 7), while for Salmonella, AA and ASC led to the lowest (0.49 [−0.58, 1.57]) and highest (4.40 [3.40, 5.40)] log reductions, respectively (Fig. 8).
FIG 6.
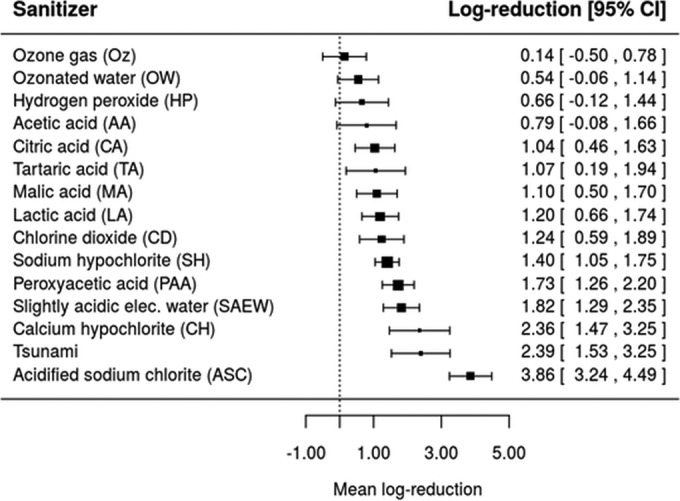
Forest plot of the overall mean log reduction of E. coli O157:H7 on a population of fresh fruits and vegetables that would be achieved by different sanitizers using a common hypothetical treatment at a concentration of 0.001 g/100 ml and a washing or exposure time and temperature of 3 min and 25°C.
FIG 7.
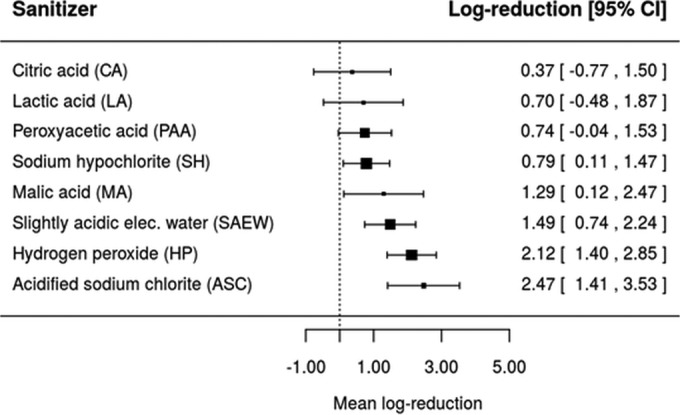
Forest plot of the overall mean log reduction of L. monocytogenes on a population of fresh fruits and vegetables that would be achieved by different sanitizers using a common hypothetical treatment at a concentration of 0.001 g/100 ml and a washing or exposure time and temperature of 3 min and 21.0°C.
FIG 8.
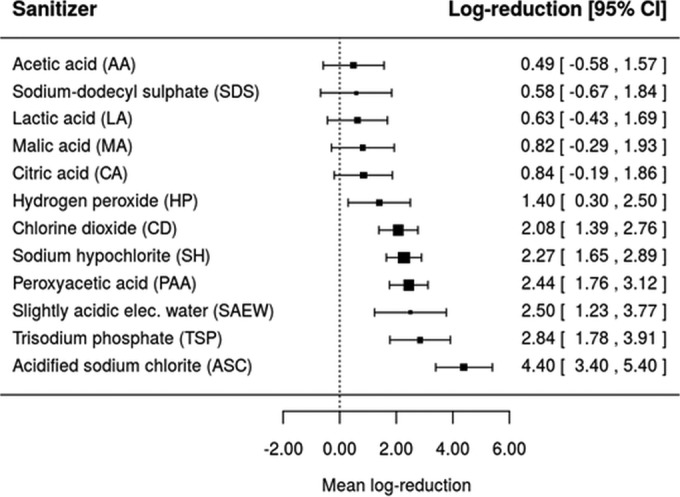
Forest plot of the overall mean log reduction of Salmonella spp. on a population of fresh fruits and vegetables that would be achieved by different sanitizers using a common hypothetical treatment at a concentration of 0.001 g/100 ml and a washing or exposure time and temperature of 3 min and 22.5°C.
Cluster analysis of sanitizers.
Through a hierarchical clustering analysis, the sanitizers were grouped in four clusters according to their bactericidal efficacies. The meta-analytical dendrogram of sanitizers is shown in Fig. 9. Through this approach it was possible to find four groups: waters (blanks) and groups with low bactericidal efficacy, medium bactericidal efficacy, and high bactericidal efficacy.
FIG 9.
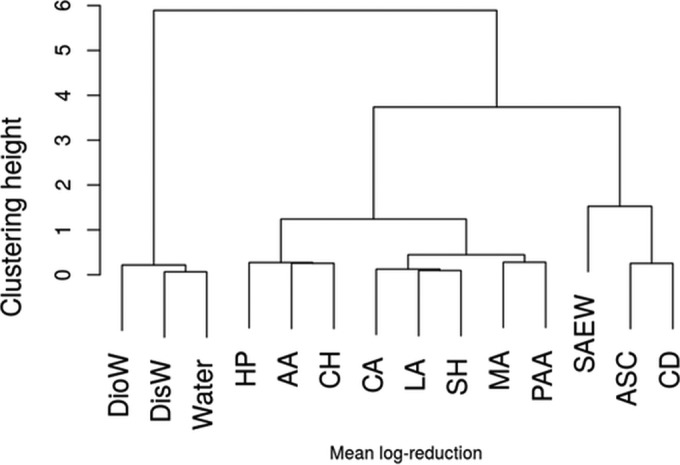
Dendrogram of sanitizers clustered hierarchically showing four main groups according to bactericidal efficacy.
DISCUSSION
Microbial safety is a major concern for fresh produce industry because of the recurrent implication of fruits and vegetables in foodborne disease outbreaks (3, 5, 6). The use of sanitizers during the washing step comprises the main measure aiming to safeguard the safety of fruits and vegetables at postharvest steps. It is recognized that the effectiveness of washing procedures applied during processing of ready-to-eat fruits and vegetables is affected by several factors, such as washing conditions (temperature, time, water circulation, etc.), type of produce (whole, pieces, leafy, etc.) and sanitizers (chemical principle, concentration, etc.) (15). In view of this, models predicting the global effectiveness of sanitizers used in washing treatments of minimally processed vegetables are not available in the literature. In order to contribute to the field, in this study we applied a meta-analysis approach to assess E. coli O157:H7, L. monocytogenes, and Salmonella inactivation by sanitizers during washing of fresh produce. We were able to collect data on the microbial log reduction achieved by the sanitizers used during washing treatment of minimally processed fruits and vegetables from 55 primary studies. This first resulted in data on inactivation of E. coli O157:H7, L. monocytogenes, and Salmonella by 27 sanitizers. These records were further refined, leading to data for 18 sanitizers, 30 types of fruits and vegetables, and 1,025 microbial log reduction values (Table 1).
Meta-analysis models by sanitizer.
Our first approach in this study was to construct a meta-analysis model by sanitizer, which allowed us to compare the effectivenesses of treatments among pathogens and fruits and vegetables (Table 2). From Table 2 it can be seen that log reduction for pathogens was affected by the sanitizer concentration, washing water temperature, and increase in time. This is particularly true for treatments with AA, CA, water, ASC, and HP, but the opposite was found for treatments with SH and CD (Table 2). The higher sensitivity of chlorine-based solutions to the increase of washing water temperature is well known and is deemed one of the major limitations for a wider application of these compounds during sanitation of fresh produce (14, 15, 67).
A major finding of the meta-analytical model by sanitizer (equation 3) is that we found the susceptibility of the three pathogens studied to the treatments (Table 2). The pathogens tend to be more resistant when lower intercepts are obtained in a specific treatment, while when higher intercepts are attained the pathogens tend to be less resistant. The fact that E. coli O157:H7 and L. monocytogenes presented the lower intercepts for treatments such AA, CA, MA, SAEW, PAA, SH, CD, ASC, and HP indicates that these pathogens are the most resistant to sanitizing treatments applied during washing of fruits and vegetables (Table 2). The higher global resistance of E. coli O157:H7 to sanitizers widely used by the fresh-produce industry, such as SH and CD, can provide further insights on the reasons why this pathogen is commonly involved in fresh-produce disease outbreaks (68). Despite the fact that L. monocytogenes presented similar resistances to almost all the sanitizers deemed also ineffective against E. coli O157:H7 (Table 2), it is known that the former pathogen is more susceptible to inhibition in vegetables (69, 70). Salmonella was found to be more resistant than E. coli O157:H7 and L. monocytogenes only when water was assessed as a washing treatment (Table 2). Nonetheless, as we used water (tap water [W], distilled water [DisW], and deionized water [DioW]) as blanks (concentration of 0 g/100 ml), the inactivation effect, even low, is due to factors such as temperature and time. If one considers the application of water at increasing time and temperature (Table 1), the inactivation of Salmonella and other pathogens will be higher. SAEW seemed to be the most effective sanitizer for the inactivation of the three pathogens studied (Table 2). The fact that temperature and concentration covariates were not included in the meta-analysis model for some sanitizing treatments indicates the need for these data to be generated. This will further allow the improvement of meta-analysis predictions (equation 3) (Table 2).
Despite the fact that data were gathered from different primary studies (Table 1), scatter plots show that there is still a reasonable agreement between observed and predicted values for each of the sanitizers (Fig. 1). The fitted versus observed plots highlight how good the meta-analysis models represented the data. This is a great accomplishment of this study, as we were able to combine data from 40 primary studies (Table 1).
Further, when the impact of a realistic sanitizing treatment (equal sanitizer concentration and washing time) was assessed, we found that the same sanitizing treatment would achieve a different log reduction, which is dependent upon the type of produce (Fig. 2 to 5). The fact that sanitizing treatments applied to leafy greens seemed to result in lower log reductions than for other types of produce (for instance, carrot, tomato, and cantaloupe) might be related to the physicochemical nature of leafy green surfaces (71). Besides, when leafy vegetables are diced, chopped, or shredded, plant tissue damage takes place, which may result in their increased attachment (72, 73) and even growth in vegetable tissues (74). Another possible reason for the differences obtained between the inactivation of pathogens during washing of leafy greens and other produce, such as carrot, tomato, and cantaloupe, could be related to the different mechanics during washing (brushing can be applied) or even the physicochemical nature of plant surfaces (71, 75, 76).
Meta-analysis models by pathogen.
Data partitioned by pathogen were used to make comparisons among the bactericidal efficacy of sanitizers (Tables 3 to 5). It should be highlighted that sanitizers listed in these tables are not the same for the three pathogens considering these data were not available. Thus, because of data sparseness, we fitted the model to data available. The fact that more sanitizing treatment data (n = 15) on the inactivation of E. coli O157:H7 during fresh-produce washing were available, followed by their effects on Salmonella (n = 12) and L. monocytogenes (n = 8), can reflect the relative concern of commodity-pathogen combinations. It is noteworthy that E. coli O157:H7 and Salmonella are the pathogens most frequently associated with foodborne disease outbreaks linked to fresh produce (3, 5, 6, 68). Therefore, one would expect to find more sanitizer options to be applied during washing aiming to ensure effective disinfection of fresh produce and safeguard public health.
From Tables 3 to 5, it can be seen that weak organic acids, such as CA, AA, and LA, presented the lowest effect on microbial log reduction. The antimicrobial efficiency of weak organic acids is highly dependent on the pH of the final solution applied for fresh produce disinfection, as pH affects the concentration of undissociated acid formed (77, 78). Moreover, it is known that the antimicrobial activity of organic acids is highly dependent on the type of acid (77, 79). This limitation, plus the fact that depending on the organic acid, there might be impact on food taste and flavor and that high biochemical oxygen demand (BOD) and chemical oxygen demand (COD) values may be found in wastewater, will certainly limit their application in washing water of fresh produce industry (78).
SH was another class of sanitizers that appeared among those compounds with the lowest effects on microbial log reduction (Tables 4 and 5). SH is a highly used chemical principle for sanitization of fresh produce, given its high cost benefit (14, 15), despite the fact that SH solutions are highly affected by the organic matter concentration and pH of the washing water (14, 15, 78). Another weakness of SH application as a sanitizer for fresh produce is the concern with the formation of compounds with potentially carcinogenic or mutagenic effects, such as chloramines and trihalomethanes (14, 80, 81). Because of these risks, the use of SH for fresh-produce sanitation has been prohibited in some parts of the world, such as Europe (15, 77, 78). From the data presented in Tables 3 to 5, it becomes clear that SH presented a higher effect on microbial log reductions only for E. coli O157:H7 (Table 3). Nonetheless, this should be carefully interpreted, as the mean effects of all sanitizers tested against E. coli O157:H7 were lower than those found for Salmonella and L. monocytogenes (Tables 4 and 5). This may reinforce the hypothesis that E. coli O157:H7 presents an intrinsic higher resistance to sanitizing agents commonly used for fresh-produce sanitation.
In contrast to SH and organic acid sanitizers, SAEW, ASC, and PAA were found to be the mostly highly efficient chemicals in reducing microbial contamination during washing of fresh produce (Tables 3 to 5). PAA is a chemical successfully used in sanitation of equipment used in food industry (82, 83). PAA can be applied in a wide range of temperature and water physicochemical parameters (including pH and calcium and magnesium contents), presence of organic matter (15, 77, 78). On the other hand, SAEW is deemed highly effective in sanitizing and less inexpensive, with ease of application and of handling (84). Nonetheless, SAEW has some limitations concerning equipment corrosion and low stability of the antimicrobial solution (15, 77, 78, 85, 86). ASC was approved for application in fresh produce sanitation 15 years ago (87). It has been proved to be a highly efficient antimicrobial treatment when applied in the range of 0.5 to 1.2 g liter−1 (50, 88). Nonetheless, ASC has been found to cause physiological damages in fresh produce even when used at concentrations to 1.2 g liter−1, allowed by the FDA (31, 89).
Although data presented in Tables 3 to 5 already suggest the range of efficiencies of the assessed sanitizing treatments over the three pathogens studied, we further established a hypothetical treatment (0.001 g/100 ml; washing time and temperature of 3 min and 25°C, respectively) to be able to visualize, through forest plots, the log reductions caused by each sanitizer for each pathogen. As seen in Fig. 6 to 8, Oz, CA, and AA would cause the lowest log reductions on E. coli O157:H7, L. monocytogenes, and Salmonella, respectively. Oz effects on the microbial log reductions for E. coli O157:H7 were found to be lower than those of the other sanitizers (Table 3). The antimicrobial efficacy of Oz is known to be highly influenced by the level of O3 soluble in the washing water, contact time, water agitation, water pH, and organic matter content (78, 90–92). Although Oz has been reported as a highly antimicrobial agent for fresh-produce washing applications (15), its application in high concentrations (>1 ppm) is not feasible because of likely damages prone to be caused in fresh produce as well as the corrosion potential of equipment (77, 78). This can reinforce that the antimicrobial effectiveness of these compounds seems to be highly dependent upon factors such as time and temperature (78).
On the other hand, ASC was consistently the most effective sanitizer for the three pathogens studied (Fig. 6 to 8). Nonetheless, these findings should be interpreted with care because the rankings given in Fig. 6 to 8 were created for a constant sanitizer concentration (0.001 g/100 ml; washing time and temperature of 3 min and 25°C), when in fact each sanitizer operates at a recommended and proper concentration. These rankings are useful to illustrate the power of sanitizers, but in practice, the use of some of these sanitizers may require specific time and temperature conditions and specific concentrations. For example, it is known that chlorine-based sanitizers have a range of increased antimicrobial activities and that above a certain pH, the increase in chlorine concentration will not result in any further gain from the antimicrobial point of view (14).
Cluster analysis of sanitizers.
A further assessment of the meta-analysis models was the use of cluster analysis to group the sanitizers according to bactericidal efficacy by means of hierarchical clustering analysis (Fig. 9). This is a better approach than the previous forest plots (Fig. 6, 7, and 8), because the clustering method instead takes into account the slope of the sanitizer concentration (equation 6) and implicitly considers the specific range of concentrations at which each sanitizer operates (viz., the sanitizer concentration is not assumed to be constant for all the chemicals). Moreover, the clustering method combines the log reduction data for all three pathogens. Because the clustering distance (Fig. 9) is calculated from the variables β0, β1, β2, and β3, which characterize the disinfectant capacity of the sanitizers, the elements will be grouped together based on similar antimicrobial activity. From Fig. 9, four clusters can be identified. All the waters (DioW, DisW, and W) have been grouped together, indicating that their bactericidal power is comparable and the lowest of all. A second group with slightly higher bactericidal efficacy is that formed by HP, AA, and CH (for the concentrations recommended for fresh produce washing), and we can label the group as having low bactericidal efficacy. A second category,medium bactericidal efficacy, is given by the organic acids CA, LA, MA, and PAA and the inorganic SH. Although SH apparently should have a stronger bactericidal effect, it is grouped with the organic acids because for the low concentrations allowed for produce washing, its effect is comparable to that of the organic acids. The fourth group can be labeled as having high bactericidal efficacy and includes SAEW, ASC and the gaseous CD. While ASC and CD have comparable bactericidal effects, SAEW has the highest effect of all (Fig. 9).
Conclusions.
Through a meta-analysis approach, we were able to assess more than 1,000 data on log reduction of the three main bacterial pathogens impairing the safety of fresh produce. We were able to build predictive models by sanitizer and by pathogen. The study is the first to gather data from a great number of papers (n = 40) and packed in such a way that the outputs could be compared. The difficulties in doing such a comparison have been cited as one of the major limitations of work in this field (15). Furthermore, through the hierarchical clustering analysis performed, we were able to classify sanitizers by their bactericidal efficacies.
The findings of this study can be seen as an achievement of very practical relevance, as they can serve regulators to rank sanitizers based on their antimicrobial efficiencies. For example, depending on pathogen of greatest concern in a specific produce item, a sanitizer with the highest bactericidal power could be suggested as preferential for use. Altogether, the outcomes of the present study can serve as scientific information for decision-making (risk-benefit analysis). Regulations can be further harmonized and developed taking into account the findings reported herein.
Supplementary Material
ACKNOWLEDGMENTS
A. S. Sant'Ana (funded by process CNPq 302763/2014-7 and process FAPESP 2015/20223-0), A. C. B. Rezende (funded by process FAPESP 13/19520-4), and L. Prado-Silva are grateful to Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support of projects undertaken at Laboratory of Quantitative Food Microbiology, University of Campinas. U. Gonzales-Barron acknowledges financial support provided by the Portuguese Foundation for Science and Technology (FCT) through the award of a 5-year Investigator Fellowship (IF) in the mode of Development Grants (IF/00570).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02216-15.
REFERENCES
- 1.Rekhy R, McConchie R. 2014. Promoting consumption of fruit and vegetables for better health. Have campaigns delivered on the goals? Appetite 79:113–123. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. 2014. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callejón RM, Rodríguez-Naranjo MI, Ubeda C, Hornedo-Ortega R, Garcia-Parrilla MC, Troncoso AM. 2015. Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne Pathog Dis 12:32–38. doi: 10.1089/fpd.2014.1821. [DOI] [PubMed] [Google Scholar]
- 4.Lynch MF, Tauxe RV, Hedberg CW. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol Infect 137:307–315. doi: 10.1017/S0950268808001969. [DOI] [PubMed] [Google Scholar]
- 5.Kozak GK, MacDonald D, Landry LFJ. 2013. Foodborne outbreaks in Canada linked to produce. J Food Prot 74:173–183. [DOI] [PubMed] [Google Scholar]
- 6.Walsh KA, Bennett SD, Mahovic M, Gould LH. 2014. Outbreaks associated with cantaloupe, watermelon, and honeydew in the United States, 1973–2011. Foodborne Pathog Dis 11:945–952. doi: 10.1089/fpd.2014.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, McLaughlin SF, Henkhaus JK, Leopold B, Bielaszewska M, Prager R, Brzoska PM, Moore RL, Guenther S, Rothberg JM, Karch H. 2011. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One 6(7):e22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooley MB, Quiñones B, Oryang D, Mandrell RE, Gorski L. 2014. Prevalence of shiga toxin producing Escherichia coli, Salmonella enterica, and Listeria monocytogenes at public access watershed sites in a California Central Coast agricultural region. Front Cell Infect Microbiol 4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strawn LK, Danyluk MD, Worobo RW, Wiedmann M. 2014. Distribution of Salmonella subtypes differs between two US produce growing regions. Appl Environ Microbiol 80:3982–3991. doi: 10.1128/AEM.00348-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEgan R, Chandler JC, Goodridge LD, Danyluk MD. 2014. Diversity of Salmonella isolates from central Florida surface waters. Appl Environ Microbiol 80:6819–6827. doi: 10.1128/AEM.02191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stea EC, Purdue LM, Jamieson RC, Yost CK, Truelstrup Hansen L. 2015. Comparison of the prevalences and diversities of Listeria species and Listeria monocytogenes in an urban and a rural agricultural watershed. Appl Environ Microbiol 81:3812–3822. doi: 10.1128/AEM.00416-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linke K, Rückerl I, Brugger K, Karpiskova R, Walland J, Muri-Klinger S, Tichy A, Wagner M, Stessl B. 2014. Reservoirs of Listeria species in three environmental ecosystems. Appl Environ Microbiol 80:5583–5592. doi: 10.1128/AEM.01018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapin TK, Nightingale KK, Worobo RW, Wiedmann M, Strawn LK. 2014. Geographical and meteorological factors associated with isolation of Listeria species in New York State produce production and natural environments. J Food Prot 77:1919–1928. doi: 10.4315/0362-028X.JFP-14-132. [DOI] [PubMed] [Google Scholar]
- 14.Suslow T. 1997. Postharvest chlorination: basic properties and key points for effective disinfection, p 1–8. University of California, Division of Agriculture and Natural Resources, Davis, CA. [Google Scholar]
- 15.Gil MI, Selma MV, López-Gálvez F, Allende A. 2009. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int J Food Microbiol 134:37–45. doi: 10.1016/j.ijfoodmicro.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Pérez Rodríguez F, Campos D, Ryser ET, Buchholz AL, Posada-Izquierdo GD, Marks BP, Zurera G, Todd E. 2011. A mathematical risk model for Escherichia coli O157:H7 cross-contamination of lettuce during processing. Food Microbiol 28:694–701. doi: 10.1016/j.fm.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. 2007. FDA finalizes report on 2006 spinach outbreak. US Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 18.Danyluk MD, Schaffner DW. 2011. Quantitative assessment of the microbial risk of leafy greens from farm to consumption: preliminary framework, data, and risk estimates. J Food Prot 74:700–708. doi: 10.4315/0362-028X.JFP-10-373. [DOI] [PubMed] [Google Scholar]
- 19.Al-Nabulsi AA, Osaili TM, Obaidat HM, Shaker RR, Awaisheh SS, Holley RA. 2014. Inactivation of stressed Escherichia coli O157:H7 cells on the surfaces of rocket salad leaves by chlorine and peroxyacetic acid. J Food Prot 77:32–39. doi: 10.4315/0362-028X.JFP-13-019. [DOI] [PubMed] [Google Scholar]
- 20.Chang AS, Schneider KR. 2012. Evaluation of overhead spray-applied sanitizers for the reduction of Salmonella on tomato surfaces. J Food Sci 77:65–69. [DOI] [PubMed] [Google Scholar]
- 21.Ijabadeniyi OA, Ngcobo NS. 2013. Bacterial pathogens isolated from vegetables and application of sanitizers for control of L. monocytogenes ATCC 7644 in cucumber. Food Agric Environ 11:15–19. [Google Scholar]
- 22.Inatsu Y, Kitagawa T, Bari ML, Nei D, Juneja V, Kawamoto S. 2010. Effectiveness of acidified sodium chlorite and other sanitizers to control Escherichia coli O157:H7 on tomato surfaces. Foodborne Pathog Dis 7:629–635. doi: 10.1089/fpd.2009.0429. [DOI] [PubMed] [Google Scholar]
- 23.Inatsu Y, Kitagawa T, Nakamura N, Kawasaki S, Nei D, Bari ML, Kawamoto S. 2013. Effectiveness of stable ozone microbubble containing water on reducing bacteria load on selected leafy vegetables. Acta Hortic 989:161–166. [Google Scholar]
- 24.Neal JA, Marquez-Gonzalez M, Cabrera-Diaz E, Lucia LM, O'Bryan CA, Crandall PG, Ricke SC, Castillo A. 2012. Comparison of multiple chemical sanitizers for reducing Salmonella and Escherichia coli O157:H7 on spinach (Spinacia oleracea) leaves. Food Res Int 45:1123–1128. doi: 10.1016/j.foodres.2011.04.011. [DOI] [Google Scholar]
- 25.Neo SY, Lim PY, Phua LK, Khoo GH, Kim SJ, Lee SC, Yuk HG. 2013. Efficacy of chlorine and peroxyacetic acid on reduction of natural microflora, Escherichia coli O157: H7, Listeria monocyotgenes and Salmonella spp. on mung bean sprouts Food Microbiol 36:475–480. [DOI] [PubMed] [Google Scholar]
- 26.Palma-Salgado S, Pearlstein AJ, Luo Y, Park HK, Feng H. 2014. Whole-head washing, prior to cutting, provides sanitization advantages for fresh-cut Iceberg lettuce (Latuca sativa L.). Int J Food Microbiol 179:18–23. doi: 10.1016/j.ijfoodmicro.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Gonzales-Barron U, Cadavez V, Sheridan JJ, Butler F. 2013. Modelling the effect of chilling on the occurrence of Salmonella on pig carcasses at study, abattoir and batch levels by meta-analysis. Int J Food Microbiol 163:101–113. doi: 10.1016/j.ijfoodmicro.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Sutton AJ, Abrams KR, Jones DR. 2001. An illustrated guide to the methods of meta-analysis. J Eval Clin Pract 7:135–148. doi: 10.1046/j.1365-2753.2001.00281.x. [DOI] [PubMed] [Google Scholar]
- 30.Alexandre EMC, Brandão TRS, Silva CLM. 2013. Impact of non-thermal technologies and sanitizer solutions on microbial load reduction and quality factor retention of frozen red bell peppers. Innov Food Sci Emerg Technol 17:99–105. doi: 10.1016/j.ifset.2012.11.009. [DOI] [Google Scholar]
- 31.Allende A, McEvoy J, Tao Y, Luo Y. 2009. Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control 20:230–234. doi: 10.1016/j.foodcont.2008.05.009. [DOI] [Google Scholar]
- 32.Bari ML, Ukuku DO, Kawasaki T, Inatsu Y, Isshiki K, Kawamoto S. 2005. Combined efficacy of nisin and pediocin with sodium lactate, citric acid, phytic acid, and potassium sorbate and EDTA in reducing the Listeria monocytogenes population of inoculated fresh-cut produce. J Food Prot 68:1381–1387. [DOI] [PubMed] [Google Scholar]
- 33.Behrsing J, Winkler S, Franz P, Premier R. 2000. Efficacy of chlorine for inactivation of Escherichia coli on vegetables. Postharvest Biol Technol 19:187–192. doi: 10.1016/S0925-5214(00)00092-2. [DOI] [Google Scholar]
- 34.Chun HH, Song KB. 2013. The combined effects of aqueous chlorine dioxide, fumaric acid, and ultraviolet-C with modified atmosphere packaging enriched in CO2 for inactivating preexisting microorganisms and Escherichia coli O157: H7 and Salmonella Typhimurium inoculated on buckwheat sprouts. Postharvest Biol Technol 86:118–124. doi: 10.1016/j.postharvbio.2013.06.031. [DOI] [Google Scholar]
- 35.Forghani F, Oh DH. 2013. Hurdle enhancement of slightly acidic electrolyzed water antimicrobial efficacy on Chinese cabbage, lettuce, sesame leaf and spinach using ultrasonication and water wash. Food Microbiol 36:40–45. doi: 10.1016/j.fm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Ge C, Bohrerova Z, Lee J. 2013. Inactivation of internalized Salmonella Typhimurium in lettuce and green onion using ultraviolet C irradiation and chemical sanitizers. J Appl Microbiol 114:1415–1424. doi: 10.1111/jam.12154. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Chen H. 2011. Effect of organic acids, hydrogen peroxide and mild heat on inactivation of Escherichia coli O157:H7 on baby spinach. Food Control 22:1178–1183. doi: 10.1016/j.foodcont.2011.01.012. [DOI] [Google Scholar]
- 38.Issa-Zacharia A, Kamitani Y, Miwa N, Muhimbula H, Iwasaki K. 2011. Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for decontamination of fresh ready-to-eat vegetables and sprouts. Food Control 22:601–607. doi: 10.1016/j.foodcont.2010.10.011. [DOI] [Google Scholar]
- 39.Lee S-Y, Costello M, Kang D-H. 2004. Efficacy of chlorine dioxide gas as a sanitizer of lettuce leaves. J Food Prot 67:1371–1376. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Wu C. 2013. Enhanced inactivation of Salmonella Typhimurium from blueberries by combinations of sodium dodecyl sulfate with organic acids or hydrogen peroxide. Food Res Int 54:1553–1559. doi: 10.1016/j.foodres.2013.09.012. [DOI] [Google Scholar]
- 41.López-Gálvez F, Allende A, Selma MV, Gil MI. 2009. Prevention of Escherichia coli cross-contamination by different commercial sanitizers during washing of fresh-cut lettuce. Int J Food Microbiol 133:167–171. doi: 10.1016/j.ijfoodmicro.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Keeratipibul S, Phewpan A, Lursinsap C. 2011. Prediction of coliforms and Escherichia coli on tomato fruits and lettuce leaves after sanitizing by using artificial neural networks. LWT Food Sci Technol 44:130–138. doi: 10.1016/j.lwt.2010.05.015. [DOI] [Google Scholar]
- 43.Kenney SJ, Beuchat LR. 2002. Comparison of aqueous commercial cleaners for effectiveness in removing Escherichia coli O157:H7 and Salmonella Muenchen from the surface of apples. Int J Food Microbiol 74:47–55. doi: 10.1016/S0168-1605(01)00716-4. [DOI] [PubMed] [Google Scholar]
- 44.Kenney SJ, Beuchat LR. 2002. Survival of Escherichia coli O157:H7 and Salmonella Muenchen on apples as affected by application of commercial fruit waxes. Int J Food Microbiol 77:223–231. doi: 10.1016/S0168-1605(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 45.Keskinen LA, Annous BA. 2011. Efficacy of adding detergents to sanitizer solutions for inactivation of Escherichia coli O157:H7 on Romaine lettuce. Int J Food Microbiol 147:157–161. doi: 10.1016/j.ijfoodmicro.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Kwon KY, Kang KA, Yoon KS. 2011. Effects of sodium hypochlorite and acidified sodium chlorite on the morphological, microbiological, and sensory qualities of selected vegetables. Food Sci Biotechnol 20:759–766. doi: 10.1007/s10068-011-0106-6. [DOI] [Google Scholar]
- 47.Mahmoud BSM, Bhagat AR, Linton RH. 2007. Inactivation kinetics of inoculated Escherichia coli O157:H7, Listeria monocytogenes and Salmonella enterica on strawberries by chlorine dioxide gas. Food Microbiol 24:736–744. doi: 10.1016/j.fm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Mahmoud BSM, Vaidya NA, Corvalan CM, Linton RH. 2008. Inactivation kinetics of inoculated Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Poona on whole cantaloupe by chlorine dioxide gas. Food Microbiol 25:857–865. doi: 10.1016/j.fm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Rahman SME, Ding T, Oh DH. 2010. Inactivation effect of newly developed low concentration electrolyzed water and other sanitizers against microorganisms on spinach. Food Control 21:1383–1387. doi: 10.1016/j.foodcont.2010.03.011. [DOI] [Google Scholar]
- 50.Ruiz-Cruz S, Acedo-Félix E, Díaz-Cinco M, Islas-Osuna MA, González-Aguilar GA. 2007. Efficacy of sanitizers in reducing Escherichia coli O157:H7, Salmonella spp. and Listeria monocytogenes populations on fresh-cut carrots. Food Control 18:1383–1390. doi: 10.1016/j.foodcont.2006.09.008. [DOI] [Google Scholar]
- 51.Sagong HG, Lee SY, Chang PS, Heu S, Ryu S, Choi YJ, Kang DH. 2011. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int J Food Microbiol 145:287–292. doi: 10.1016/j.ijfoodmicro.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Sengun IY, Karapinar M. 2005. Effectiveness of household natural sanitizers in the elimination of Salmonella typhimurium on rocket (Eruca sativa Miller) and spring onion (Allium cepa L.). Int J Food Microbiol 98:319–323. doi: 10.1016/j.ijfoodmicro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Su X, D'Souza DH. 2012. Reduction of Salmonella Typhimurium and Listeria monocytogenes on produce by trisodium phosphate. LWT Food Sci Technol 45:221–225. doi: 10.1016/j.lwt.2011.08.010. [DOI] [Google Scholar]
- 54.Sy KV, Murray MB, Harrison MD, Beuchat LR. 2005. Evaluation of gaseous chlorine dioxide as a sanitizer for killing Salmonella, Escherichia coli O157:H7, Listeria monocytogenes, and yeasts and molds on fresh and fresh-cut produce. J Food Prot 68:1176–1187. [DOI] [PubMed] [Google Scholar]
- 55.Tian JQ, Bae YM, Lee SY. 2013. Survival of foodborne pathogens at different relative humidities and temperatures and the effect of sanitizers on apples with different surface conditions. Food Microbiol 35:21–26. doi: 10.1016/j.fm.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Tomás-Callejas A, López-Velasco G, Artés F, Artés-Hernández F. 2012. Acidified sodium chlorite optimisation assessment to improve quality of fresh-cut tatsoi baby leaves. J Sci Food Agric 92:877–885. doi: 10.1002/jsfa.4664. [DOI] [PubMed] [Google Scholar]
- 57.Trinetta V, Morgan MT, Linton RH. 2010. Use of high-concentration-short-time chlorine dioxide gas treatments for the inactivation of Salmonella enterica spp. inoculated onto Roma tomatoes. Food Microbiol 27:1009–1015. doi: 10.1016/j.fm.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Ukuku DO. 2004. Effect of hydrogen peroxide treatment on microbial quality and appearance of whole and fresh-cut melons contaminated with Salmonella spp. Int J Food Microbiol 95:137–146. doi: 10.1016/j.ijfoodmicro.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Velázquez LDC, Barbini NB, Escudero ME, Estrada CL, Guzmán AMS. 2009. Evaluation of chlorine, benzalkonium chloride and lactic acid as sanitizers for reducing Escherichia coli O157:H7 and Yersinia enterocolitica on fresh vegetables. Food Control 20:262–268. doi: 10.1016/j.foodcont.2008.05.012. [DOI] [Google Scholar]
- 60.Xu W, Qu W, Huang K, Guo F, Yang J, Zhao H, Luo Y. 2007. Antibacterial effect of grapefruit seed extract on food-borne pathogens and its application in the preservation of minimally processed vegetables. Postharvest Biol Technol 45:126–133. doi: 10.1016/j.postharvbio.2006.11.019. [DOI] [Google Scholar]
- 61.Yuk H-G, Bartz JA, Schneider KR. 2005. Effectiveness of individual or combined sanitizer treatments for inactivating Salmonella spp. on smooth surface, stem scar, and wounds of tomatoes. J Food Sci 70:M409–M414. [Google Scholar]
- 62.Zhang C, Lu Z, Li Y, Shang Y, Zhang G, Cao W. 2011. Reduction of Escherichia coli O157:H7 and Salmonella enteritidis on mung bean seeds and sprouts by slightly acidic electrolyzed water. Food Control 22:792–796. doi: 10.1016/j.foodcont.2010.11.018. [DOI] [Google Scholar]
- 63.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to meta-analysis. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 64.Pinheiro J, Bates D, Debroy S, Sarkar D. 2013. Nlme: linear and nonlinear mixed effects models. R package version 3.1-111. The R Development Core Team, Vienna, Austria. [Google Scholar]
- 65.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48. [Google Scholar]
- 66.Kaufman L, Rousseeuw PJ. 2005. Finding groups in data. John Wiley & Sons, Somerset, NJ. [Google Scholar]
- 67.López-Velasco G, Tomás-Callejas A, Sbodio A, Artés-Hernández F, Suslow TV. 2012. Chlorine dioxide dose, water quality and temperature affect the oxidative status of tomato processing water and its ability to inactivate Salmonella. Food Control 26:28–35. doi: 10.1016/j.foodcont.2011.12.016. [DOI] [Google Scholar]
- 68.Sivapalasingam S, Friedman CR, Cohen L, Tauxe RV. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J Food Prot 67:2342–2353. [DOI] [PubMed] [Google Scholar]
- 69.Hanning IB, Johnson MG, Ricke SC. 2008. Precut prepackaged lettuce: a risk for listeriosis? Foodborne Pathog Dis 5:731–746. doi: 10.1089/fpd.2008.0142. [DOI] [PubMed] [Google Scholar]
- 70.Sant'Ana AS, Igarashi MC, Landgraf M, Destro MT, Franco BDG. 2012. Prevalence, populations and pheno- and genotypic characteristics of Listeria monocytogenes isolated from ready-to-eat vegetables marketed in. Int J Food Microbiol 155:1–9. doi: 10.1016/j.ijfoodmicro.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 71.Brandl MT. 2006. Fitness of human enteric pathogens on plants and implications for food safety. Annu Rev Phytopathol 44:367–392. doi: 10.1146/annurev.phyto.44.070505.143359. [DOI] [PubMed] [Google Scholar]
- 72.Aruscavage D, Miller SA, Ivey MLL, Lee K, LeJeune JT. 2008. Survival and dissemination of Escherichia coli O157:H7 on physically and biologically damaged lettuce plants. J Food Prot 71:2384–2388. [DOI] [PubMed] [Google Scholar]
- 73.Patel J, Sharma M. 2010. Differences in attachment of Salmonella enterica serovars to cabbage and lettuce leaves. Int J Food Microbiol 139:41–47. doi: 10.1016/j.ijfoodmicro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Brandl MT. 2008. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl Environ Microbiol 74:5285–5289. doi: 10.1128/AEM.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D, Sela S. 2009. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol 75:6076–6086. doi: 10.1128/AEM.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yaron S, Römling U. 2014. Biofilm formation by enteric pathogens and its role in plant colonization and persistence. Microb Biotechnol 7:496–516. doi: 10.1111/1751-7915.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Artés F, Gómez P, Aguayo E, Escalona V, Artés-Hernández F. 2009. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharvest Biol Technol 51:287–296. doi: 10.1016/j.postharvbio.2008.10.003. [DOI] [Google Scholar]
- 78.Ölmez H, Kretzschmar U. 2009. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci Technol 42:686–693. doi: 10.1016/j.lwt.2008.08.001. [DOI] [Google Scholar]
- 79.Park SH, Choi MR, Park JW, Park KH, Chung MS, Ryu S, Kang DH. 2011. Use of organic acids to inactivate Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. J Food Sci 76:M293–M298. doi: 10.1111/j.1750-3841.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- 80.Nieuwenhuijsen MJ, Toledano MB, Elliott P. Uptake of chlorination disinfection by-products; a review and a discussion of its implications for exposure assessment in epidemiological studies. J Expo Sci Environ Epidemiol 10:586–599. [DOI] [PubMed] [Google Scholar]
- 81.Hua G, Reckhow DA. 2007. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res 41:1667–1678. doi: 10.1016/j.watres.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 82.Rossoni EMM, Gaylarde CC. 2000. Comparison of sodium hypochlorite and peracetic acid as sanitising agents for stainless steel food processing surfaces using epifluorescence microscopy. Int J Food Microbiol 61:81–85. doi: 10.1016/S0168-1605(00)00369-X. [DOI] [PubMed] [Google Scholar]
- 83.Bagge-Ravn D, Ng Y, Hjelm M, Christiansen JN, Johansen C, Gram L. 2003. The microbial ecology of processing equipment in different fish industries–analysis of the microflora during processing and following cleaning and disinfection. Int J Food Microbiol 87:239–250. doi: 10.1016/S0168-1605(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 84.Huang YR, Hung YC, Hsu SY, Huang YW, Hwang DF. 2008. Application of electrolyzed water in the food industry. Food Control 19:329–345. doi: 10.1016/j.foodcont.2007.08.012. [DOI] [Google Scholar]
- 85.Tanaka N, Fujisawa T, Daimon T, Fujiwara K, Tanaka N, Yamamoto M, Abe T. 1999. The effect of electrolyzed strong acid aqueous solution on hemodialysis equipment. Artif Organs 23:1055–1062. doi: 10.1046/j.1525-1594.1999.06224.x. [DOI] [PubMed] [Google Scholar]
- 86.Kiura H, Sano K, Morimatsu S, Nakano T, Morita C, Yamaguchi M, Maeda T, Katsuoka Y. 2002. Bactericidal activity of electrolyzed acid water from solution containing sodium chloride at low concentration, in comparison with that at high concentration. J Microbiol Methods 49:285–293. doi: 10.1016/S0167-7012(01)00385-2. [DOI] [PubMed] [Google Scholar]
- 87.Code of Federal Regulations. 2000. Title 21, part 173.325. Secondary direct food additives permitted in food for human consumption: acidified sodium chlorite solutions. US Government Publishing Office, Washington, DC: (http://www.access.gpo.gov/nara/cfr/waisidx_00/21cfr173_00.html). [Google Scholar]
- 88.Gonzalez RJ, Luo Y, Ruiz-Cruz S, McEvoy JL. 2004. Efficacy of sanitizers to inactivate Escherichia coli O157:H7 on fresh-cut carrot shreds under simulated process water conditions. J Food Prot 67:2375–2380. [DOI] [PubMed] [Google Scholar]
- 89.Bosilevac JM, Wheeler TL, Rivera-Betancourt M, Nou X, Arthur TM, Shackelford SD, Kent MP, Jaroni D, Osborn MS, Rossman M, Reagan JO, Koohmaraie M. 2004. Protocol for evaluating the efficacy of cetylpyridinium chloride as a beef hide intervention. J Food Prot 67:303–309. [DOI] [PubMed] [Google Scholar]
- 90.Bablon G, Bellany WD, Bourbigot M-M, Daniel FB, Doré M, Erb F, Gordon G, Lauglais B, Laplanche A, Legube B, Martin G, Massechelein WJ, Pacey G, Reckhow DA, Ventresque C. 1991. Fundamental aspects. p 11–132. In Langlais B, Reckhow DA, Brink DR (ed), Ozone in water treatment: application and engineering. American Water Works Association Research Foundation, Denver, CO. [Google Scholar]
- 91.Kim JG, Yousef AE, Dave S. 1999. Application of ozone for enhancing the microbiological safety and quality of foods: a review. J Food Prot 62:1071–1087. [DOI] [PubMed] [Google Scholar]
- 92.Achen M. 2000. Efficacy of aqueous ozone in inactivating Escherichia coli O157:H7 in pure cell suspensions and on apples. M.S. thesis The Ohio State University, Columbus, OH. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.