Abstract
Objective:
The main aim of this work was to report on trabecular bone score (TBS) by dual-energy X-ray absorptiometry (DXA) of healthy Italian subjects to be used as a reference standard for future study in clinical and research settings. The secondary aim was to investigate the link between TBS and conventional parameters of bone and body composition by DXA.
Methods:
250 individuals of 5 age bands (spanning from 18 to 70 years of age, equally distributed for both age and sex) were prospectively recruited. A lumbar spine (LS) DXA scan (Lunar iDXA™; GE Healthcare, Madison, WI) was acquired for each subject and then analysed with the latest version of TBS iNsight v. 2.1 (Med-Imaps, Pessac, France) software. LS bone mineral density (LS BMD), Z-score, T-score and TBS values were collected. Pearson's test was used to investigate the correlations between TBS and LS BMD and the influence of age, body mass index (BMI) and body composition on these parameters.
Results:
A significant decrease of TBS and LS BMD was observed with ageing in both males (TBS mean values from 1.486 to 1.374; LS BMD mean values from 1.219 to 1.187) and females (TBS mean values from 1.464 to 1.306; LS BMD mean values from 1.154 to 1.116). No statistically significant difference was achieved among males and females of the same age group for both TBS and LS BMD, with the exception of the fifth age group. A significant correlation was found between LS BMD and TBS values in both sexes (r = 0.555–0.655, p < 0.0001). BMI influenced LS BMD but not TBS. TBS values were inversely correlated with some fat mass parameters, in particular with visceral adipose tissue (in males: r = −0.332, p < 0.001; in females: r = −0.348, p < 0.0001). No significant correlation was found between TBS and total lean mass, opposite to LS BMD (in males: r = 0.418; p < 0.0001; in females: r = −0.235; p < 0.001).
Conclusion:
This report is an attempt to start building a database for healthy Italian people providing age- and sex-specific reference curves for TBS. This could help clinicians to improve patient management in the detection of impaired bone mineral status and to monitor bone changes.
Advances in knowledge:
The study reports TBS values of a selectively enrolled Italian healthy population, ranging from younger to older ages and including males as a reference standard. Moreover, links between body composition and TBS are explored.
INTRODUCTION
Osteoporosis is a major public health disease affecting hundreds of millions of people worldwide. In 2010, about 22 million females and 5.5 million males were estimated to have osteoporosis in the European Union.1 Bone fractures, occurring more frequently at hip and spine, represent the main clinical consequence of the disease, and they are associated with increased mortality and morbidity, impaired quality of life for patients and also increased health costs.2 Currently, the clinical diagnosis of osteoporosis is based on the assessment of areal bone mineral density (aBMD) as measured by dual-energy X-ray absorptiometry (DXA).3,4
Although aBMD by DXA is a major determinant of bone strength and fracture risk, it does not allow the assessment of bone geometry to distinguish between neither cortical nor trabecular bone (bone quality). As a matter of fact, most individuals with a fragility fracture may have aBMD values in the osteopenic or even normal range.5
Today the technologies used to determine skeletal microarchitecture, such as histomorphometric analysis, micro-CT of the transiliac crest bone biopsy, high-resolution peripheral quantitative CT and MRI, are not routinely available.6
In the past few years, the evolution of DXA technology has allowed more advanced tools in the assessment of the bone status with the ambition to go beyond the quantification of bone tissue, trying to provide the clinicians other qualitative properties of the bone with potentially bone mineral density (BMD)-independent information. DXA hot sites of measurement for osteoporosis are two: spine and femur. In the latter, the advanced hip analysis or hip structure analysis, depending on the manufacturer, was planned and integrated to provide architectural/biomechanical features of the femur. In the former, the trabecular bone score (TBS) was proposed and developed by Pothuaud et al7 to exploit image properties and potential of DXA,5,6 with the first article published in Bone in 2008.
TBS evaluates pixel grey-level variations in DXA images of the lumbar spine (LS).8 These variations have been associated with bone microarchitecture,8 but DXA images are projectional images with very limited spatial resolution that cannot visualize trabecular architecture. Consequently, TBS is not a physical measurement but a texture index representative of trabecular bone independent of BMD. It would aim at being a surrogate of bone microarchitecture.9,10
TBS is an easy tool, available for all densitometric equipment of the main manufacturers. Unlike aBMD, TBS is not affected by osteoarthritis.11 It can be calculated retrospectively, allowing the estimation of bone texture from a previously acquired DXA scan.
Studies have begun and are being published, although TBS clinical value has only just been put under investigation. Preliminary experience gave positive results in osteoporosis and, more generally, in metabolic bone diseases: (a) discrimination and prediction of fractures, (b) impact of osteoarthritis, (c) glucocorticoids, (d) anorexia nervosa, (e) rheumatoid arthritis, (f) hyperparathyroidism, (g) effects of antiresorptive agents and (h) diabetes.8,12–18
Evidence suggests that TBS, in addition to aBMD and clinical risk factors, improves the prediction of fracture risk (vertebral and other conventional sites). Since most individuals with fragility fractures have aBMD values not in the osteoporotic range but in the osteopenic or even normal range, TBS could be especially useful in this kind of patient and should be considered in osteoporosis screening and clinical management. Nevertheless, the usefulness of TBS independent of aBMD is controversial.12,19
For clinical use, TBS data as a reference standard for different populations worldwide are required. At present, a TBS normative database has collected data only from French, Swiss, US non-Hispanic and Japanese females.19–22
The aim of our work was to start building a TBS age-specific normative database in a population of Caucasian Italian females and males, enrolled following strictly selective criteria for healthy status. We also tried to correlate TBS and the six most relevant parameters of body composition.23
METHODS AND MATERIALS
Study design and population
Volunteers from 18 to 70 years old among blood donors of Sant'Orsola-Malpighi Hospital were prospectively enrolled in order to form five age bands, equally composed by sex: A, 18–30 years old; B, 31–40 years old; C, 41–50 years old; D, 51–60 years old; E, 61–70 years old (total: 250 patients; 25 males and 25 females for each group). All participants were of normal weight or overweight [body mass index (BMI) between 18 and 30 kg m−2] “healthy” Caucasians, living in Italy. The detailed list of criteria for the selection of “healthy” people was carefully described in an article published by our team in 2012.23
Subjects with osteoporotic, fragility fractures or abnormal vertebrae, pregnant females, subjects with surgical hardware, implantable devices or foreign bodies, or those who were recently submitted to diagnostic tests using nuclides or barium or radio-opaque substance were excluded.
The study was approved by the ethics committee of our hospital and was conducted in respect of the Declaration of Helsinki.
Dual-energy X-ray absorptiometry
DXA scan was performed using a new narrow-angle fan-beam densitometer (Lunar iDXA™; GE Healthcare, Madison, WI; enCORETM 2011 software v. 13.6; GE Healthcare, Madison, WI). The scanner was calibrated daily using a standard calibration block supplied by the manufacturer. All metal items were removed before densitometry, and the patients were examined wearing only underwear and a cloth gown.
Lumbar analysis
Measurements were obtained with the subject lying in a supine position with their legs supported on a padded box to flatten the pelvis and lower spine (from the first to the fourth lumbar vertebra); the bright pointer was centred on the midpoint of the line joining the two superior iliac crests. The analysis was based upon the mean values of each vertebral BMD. L1–L4 (LS) BMD and T-score were considered.
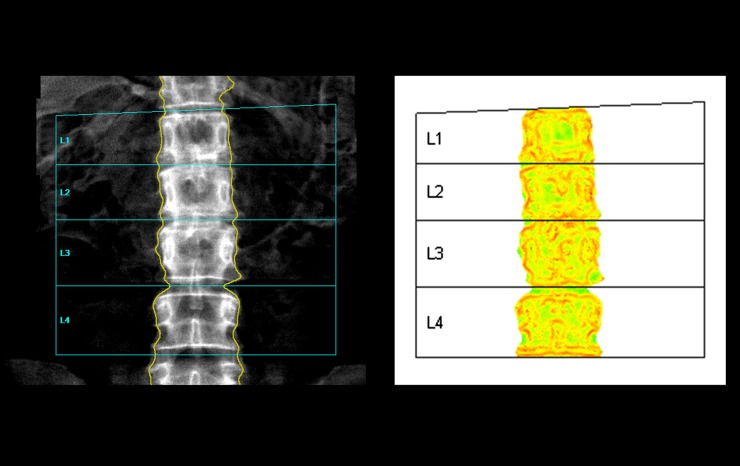
TBS was evaluated in the same region of measurement by using the latest version of TBS iNsight software v. 2.1 (Med-Imaps, Pessac, France) proved also for male (Figure 1). TBS was calculated as the mean value of the individual measurements for each vertebra and their combinations from L1 through L4.
Figure 1.
Conventional lumbar spine analysis (on the left). On the right, the reference graph for trabecular bone score. Homogeneous low-density zones (for example, the interspace between the neural arches of the two contiguous vertebrae) appear light grey, indicating a good trabecular representation. On the contrary, high-density contrast zones (for example, the cortex of the spinous processes, pedicles and vertebral plateaus) are coloured in dark grey, indicating a poor bone status.
Whole-body analysis
The subjects were placed in a supine position with arms at sides slightly separated from the trunk and correctly centred on the scanning field. Regions of interest were defined by the analytical programme including six different corporeal districts: total body, trunk, upper limbs, lower limbs, android region and gynoid. For each region, DXA scanned the weight (in grams) of total mass, fat mass (FM), non-bone lean mass (LM) and bone mineral content. Moreover, visceral fat analysis was performed by CoreScan (Lunar iDXA; enCORE 2011 software v. 13.6), a new software option for the assessment of visceral fat (mass and volume) in the android region.
Total body FM (a), total body LM (b), total body FM/LM (c), android/gynoid FM (A/G FM; d) android FM (e) and visceral adipose tissue (VAT) (f) were considered and used for the analysis as the most representative body composition markers in terms of general balance of masses (a, b and c), central/peripheral distribution of FM (d), central or VAT compartment (e and f).
Statistical analysis
The normal distribution of our sample population was tested by skewness and excess kurtosis test; normal ranges were considered for values between −2 and +2.
Data were analysed by analysis of variance and Mann–Whitney U test for differences between males and females of the same age groups. Multivariate analysis of variance was also performed to establish differences in values and trends of parameters and indexes among the different age groups. Results are reported as frequencies or mean and standard deviation (±SD).
Pearson's test was used to investigate the correlations between TBS and LS BMD and the influence of age and BMI on both bone parameters. Pearson's test was also performed to investigate correlation among body composition and bone parameters (total body FM, total body LM, total body FM/LM, android FM, A/G FM, VAT and TBS, LS BMD, respectively). The analysis was performed in the male and female populations separately.
Two-tailed p-value was considered significant for values <0.05. StatView® statistical package v. 5.0.1 (for Windows®; SAS Inc., Chicago, IL) was used for the analysis.
RESULTS
The normal distribution of the population was proved for all parameters.
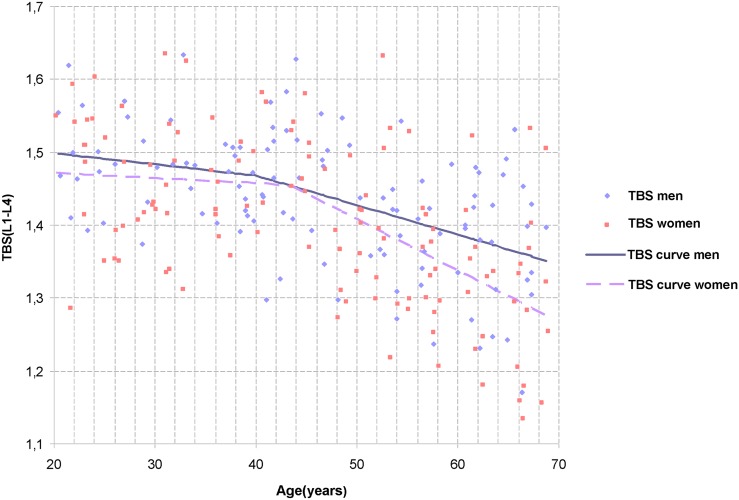
Among subjects under 50 years old, none presented a Z-score value under the range of normality (−2.0). Among subjects over 50 years old, 54 (54.0%; mean age: 60.05 ± 5.4 years) presented a T-score value in the range of normality, 38 (38.0%; mean age, 59.5 ± 5.2 years) had osteopenia and 8 (8.0%; mean age, 62.65 ± 5.7 years) were affected by osteoporosis. Features and descriptive statistics of the population are detailed in Table 1, and the TBS normative data curves, in males and females, are shown in Figure 2. A significant decrease of TBS was observed with ageing in both males and females (p < 0.0001). LS BMD also significantly decreased with ageing in both sexes (p = 0.04, in males; p < 0.0001, in females). No statistically significant difference was achieved among males and females of the same age group for TBS, with the exception of the fifth age group (p = 0.031); similar results were also observed for LS BMD (p = 0.0001).
Table 1.
Descriptive statistics of the cohort (mean ± standard deviation)
| Age (group, years) | Body mass index (kg m−2) | Trabecular bone score | Lumbar spine bone mineral density (g cm−2) | Z-score | T-score |
|---|---|---|---|---|---|
| 18–30 (A) | |||||
| M | 23.9 ± 2.2 | 1.486 ± 0.068 | 1.219 ± 0.114 | 0.1 ± 1.0 | 0.0 ± 1.0 |
| F | 22.1 ± 2.5 | 1.464 ± 0.085 | 1.154 ± 0.099 | 0.0 ± 0.8 | −0.2 ± 0.8 |
| p-value (sex) | 0.009 | NS | NS | NS | NS |
| 31–40 (B) | |||||
| M | 24.3 ± 2.7 | 1.467 ± 0.055 | 1.226 ± 0.132 | 0.0 ± 1.0 | 0.1 ± 1.1 |
| F | 23.8 ± 3.5 | 1.461 ± 0.088 | 1.181 ± 0.119 | 0.1 ± 1.0 | 0.0 ± 1.0 |
| p-value (sex) | NS | NS | NS | NS | NS |
| 41–50 (C) | |||||
| M | 24.7 ± 2.8 | 1.471 ± 0.090 | 1.210 ± 0.128 | 0.0 ± 1.0 | −0.1 ± 1.1 |
| F | 23.7 ± 2.8 | 1.433 ± 0.086 | 1.175 ± 0.115 | 0.3 ± 0.8 | 0.0 ± 1.0 |
| p-value (sex) | NS | NS | NS | NS | NS |
| 51–60 (D) | |||||
| M | 25.0 ± 3.1 | 1.386 ± 0.069 | 1.120 ± 0.119 | −0.5 ± 0.9 | −0.8 ± 1.0 |
| F | 23.5 ± 2.4 | 1.364 ± 0.103 | 1.066 ± 0.147 | 0.0 ± 1.2 | −0.9 ± 1.2 |
| p-value (sex) | NS | NS | 0.035 | NS | NS |
| 61–70 (E) | |||||
| M | 25.8 ± 2.7 | 1.374 ± 0.097 | 1.168 ± 0.150 | 0.0 ± 1.2 | −0.4 ± 1.3 |
| F | 25.7 ± 3.7 | 1.306 ± 0.112 | 1.009 ± 0.125 | 0.0 ± 0.1 | −1.4 ± 1.1 |
| p-value (sex) | NS | 0.031 | 0.0001 | NS | 0.006 |
| Total | |||||
| M | 24.8 ± 2.7 | 1.435 ± 0.090 | 1.187 ± 0.134 | −0.1 ± 1.0 | −0.2 ± 1.1 |
| F | 23.7 ± 3.2 | 1.405 ± 0.112 | 1.116 ± 0.138 | 0.1 ± 1.0 | −0.5 ± 1.2 |
| p-value (sex) | 0.001 | NS | 0.0001 | NS | NS |
|
p-value (age) | |||||
| M | NS | <0.0001 | 0.04 | <0.0001 | 0.04 |
| F | <0.001 | <0.0001 | <0.0001 | NS | <0.0001 |
F, female; M, male; NS, not statistically significant.
Figure 2.
Trabecular bone score (TBS) age-related changes in the lumbar spine L1–L4. The black line represents the male normative TBS curves for age. The grey dashed line represents the female normative TBS curves for age.
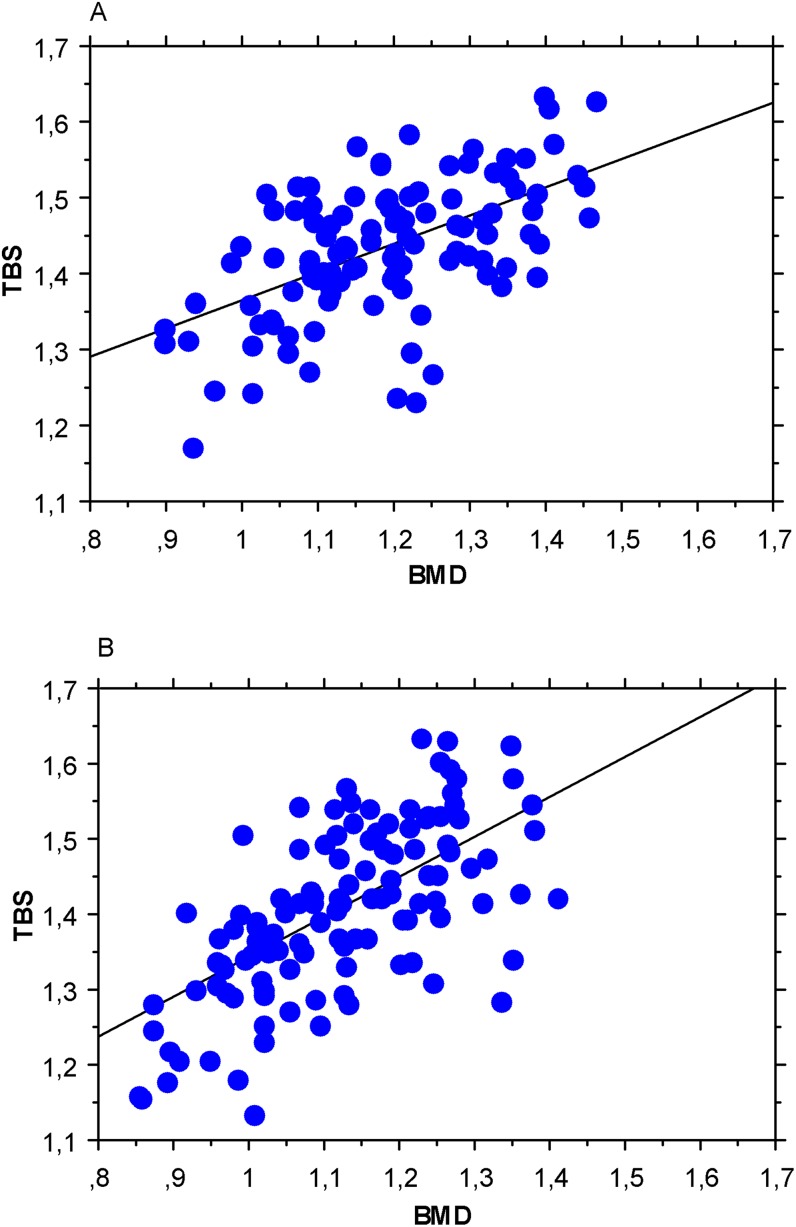
In both sexes, TBS values showed a significant positive correlation with LS BMD (Figure 3; Table 2) (r = 0.480–0.700; p < 0.0001).
Figure 3.
Pearson's test between lumbar spine bone mineral density (LS BMD) and trabecular bone score (TBS) in males (a) and in females (b).
Table 2.
Pearson's correlation test among bone and body composition parameters by dual-energy X-ray absorptiometry
| Body mass index | LS BMD | T-score | Total body FM | Total body LM | Total body FM/LM | Android/gynoid FM | Android FM | Visceral adipose tissue | |
| LS BMD | |||||||||
| M | 0.202a | – | 0.999b | NS | 0.418b | NS | NS | NS | NS |
| F | 0.208a | – | 0.999b | NS | 0.235c | NS | NS | NS | NS |
| Trabecular bone score | |||||||||
| M | NS | 0.555b | 0.552b | NS | NS | 0.216a | 0.231a | 0.257d | 0.332c |
| F | NS | 0.655b | 0.657b | NS | NS | 0.246d | 0.215a | 0.227a | 0.348b |
F, female; FM, fat mass; LM, non-bone lean mass; LS BMD, lumbar spine bone mineral density; M, male; NS, not statistically significant.
p < 0.05.
p < 0.0001.
p < 0.001.
p < 0.01.
LS BMD was poorly influenced by BMI in both males and females (r = 0.202–0.418; p < 0.05), while TBS was independent of BMI in both sexes (p > 0.05) (Table 2).
Concerning correlations among bone and body composition parameters, several inverse weak relationships were found among TBS and total body FM/total body LM, android FM, A/G FM and VAT (r = −0.216 to −0.348; p < 0.05 and <0.0001, respectively).
No significant correlations were documented between LS BMD and examined body composition parameters with the exception of total body LM (r = 0.235–0.418; p < 0.001 and <0.0001, respectively) (Table 2).
DISCUSSION
TBS is a pure texture measurement, which has been shown to be helpful for clinicians to identify patients affected by osteoporosis providing additional information to BMD.8 Nevertheless, the validation of TBS is still limited because the correlations between this method and three-dimensional trabecular microarchitecture parameters (trabecular thickness, trabecular number, trabecular spacing, connectivity density) were proven in only in vitro or ex vivo models.9,10
New recent advancements in DXA technology provided improved image resolution. Today, iDXA presents a greater number of detectors and shows the best resolution (1.05 mm longitudinally, 0.6 mm laterally) and image quality. The improved resolution guarantees a more precise bone edge detection and, consequently, the development of superior algorithms of measurements.
A recent article demonstrated that TBS reproducibility, compared with BMD reproducibility, was significantly lower.24 On the other hand, TBS and BMD precision on iDXA instrument were found comparable, without significant differences between males and females (% coefficient of variation of L1–L4 BMD and TBS, 1.9% and 1.4%, respectively).10
Accumulating evidence suggests that TBS used in addition to aBMD improves the prediction of osteoporotic fracture risk,13,15,19,25,26 especially in patients with aBMD in the range of normality or osteopenia.
However, the role of TBS is still under investigation and, at present, opinions in literature12,19,27 are controversial. Several studies suggested that LS aBMD and TBS are equally able to predict fractures;12,27 other studies supported TBS as a stronger parameter than aBMD in predicting fractures.19
In this work, we generated age-specific reference values for spinal TBS of healthy Caucasian Italian males and females between the ages of 18 and 70 years. These data may support clinicians in the interpretation as well as the comparison of TBS results in both clinical and research settings.
The salient features of this study are (a) reporting of a selectively enrolled healthy population for TBS, (b) inclusion of males, (c) patients ranging from younger to older ages, (d) Italian population and (e) links between body composition and TBS.
Only a few authors collected subjects to get normal or control references.19–22 To our knowledge, the only studies that provided TBS in ageing are limited to the recent articles of Dufour et al20 (5942 French females from 45 to 85 years old), Del Rio et al22 (102 Spanish females from 50 to 91 years old), Lamy et al21 (411 Swiss females from 50 to 80 years old) and Iki et al19 (2571 Japanese females from 15 to 79 years old).
In our population, TBS and LS BMD showed a general decrease from 18 to 60 years old in males; in the fifth age group, TBS kept decreasing, while LS BMD presented a slight increase. In females, the trends of TBS and LS BMD were substantially superimposed during ageing.
In agreement with Dufour et al,20 Del Rio et al22 and Lamy et al,21 a similar reduction in TBS and LS BMD was revealed in those aged 45–70 years (shared age range), although mean TBS values were lower than found in our population. This could be also due to the selective inclusion criteria used during the enrolment to define the healthy status. A minor rate of decrease in TBS compared with Japanese females19 was documented in our population.
The correlation between LS BMD and TBS (r = 0.655) of our female population was higher than that previously demonstrated by Dufour et al20 and the Manitoba study28 (r = 0.311–0.320), whereas overlapping results have been reported in the OFELY study12 (r = 0.580). However, it is important to highlight that BMD showed a weaker correlation with TBS in males rather than in females (r = 0.555 vs 0.655) and that this correlation decreased with age (r = 0.644 for Group A; r = 0.483 for Group E—p < 0.05) in both sexes.
Evidence of the effects of body composition on bone status is still controversial. Nowadays, adipose tissue, especially visceral compartment, is considered one of the most important active endocrine organs of the human body, in addition to its role of excess energy storage.29 Adipose tissue releases a wide variety of protein, called adipokines, which are known to be involved in the complex regulation of bone physiology.30–32 On the other hand, both muscle and bone have recently emerged as endocrine organs with potential effects on adipose tissue and glucose homeostasis, through the release of a multitude of molecules (osteokines and myokines, respectively).33,34
Although the protective effect of overweight and obesity on bone mineral status seems clear, there is still controversy as to which compartment, FM or lean mass, is more important in determining BMD.35,36 A study by Hu et al37 found that lean mass, but not FM, is associated with LS BMD in both sexes, in accordance with previous studies. On the other hand, any correlation among TBS and FM was observed. These relationships suggest that lean mass could not have an influence on the LS microarchitecture, whereas it could have a role in strengthening of the bone density, perhaps owing to its traction effect.
An inverse significant correlation among TBS and several FM abdominal parameters was found in both females and males, although the correlations were weak. Specifically, VAT was the body composition parameter with the highest correlation, particularly in females. This result could be owing to the endocrinological role of VAT and to its damaging effect on bone microarchitecture.
The relatively low number of enrolled subjects could represent a major limitation for the study, but these should be considered in light of the very selective inclusion criteria used to collect an effective “healthy” population.
The cross-sectional fashion remains another limitation for the analysis to draw conclusions about the longitudinal evolution of TBS values in healthy people; for this purpose, the inclusion of the old population would also be recommended, although recruitment criteria assumed in this study would be hardly respected. Results of this study are not truly representative of the entire Italian population because the enrolment was centred in Emilia Romagna.
A further limitation is the lack of a radiographic assessment of osteoarthritis. In a previous study,11 degenerative changes of the spine and their severity had no significant effects on TBS, but did affect BMD measurements.
In conclusion, this report provides TBS values of healthy Italian subjects in their adulthood; these data might be used to help clinicians in the assessment of patients in the detection and monitoring of impaired bone mineral status, as well as a reference for future investigations on pathological human conditions and differences between countries.
Contributor Information
A Bazzocchi, Email: abazzo@inwind.it.
F Ponti, Email: pontifederico@libero.it.
D Diano, Email: dani84.dd@libero.it.
U Albisinni, Email: ugo.albisinni@ior.it.
G Battista, Email: g.battista@unibo.it.
G Guglielmi, Email: g.guglielmi@unifg.it.
REFERENCES
- 1.Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 2013; 8: 137. doi: 10.1007/s11657-013-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper C, O'Neill T, Silman A. The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group. Bone 1993; 14(Suppl. 1): S89–97. [DOI] [PubMed] [Google Scholar]
- 3.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 2007; 83: 509–17. doi: 10.1136/pgmj.2007.057505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazzocchi A, Diano D. Dual-energy x-ray absorptiometry in obesity. CMAJ 2014; 186: 48. doi: 10.1503/cmaj.120149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazakia GJ, Majumdar S. New imaging technologies in the diagnosis of osteoporosis. Rev Endocr Metab Disord 2006; 7: 67–74. doi: 10.1007/s11154-006-9004-2 [DOI] [PubMed] [Google Scholar]
- 6.Silva BC, Bilezikian JP. Trabecular bone score: perspectives of an imaging technology coming of age. Arq Bras Endocrinol Metabol 2014; 58: 493–503. doi: 10.1590/0004-2730000003456 [DOI] [PubMed] [Google Scholar]
- 7.Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 2008; 42: 775–87. doi: 10.1016/j.bone.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 8.Bousson V, Bergot C, Sutter B, Levitz P, Cortet B; Scientific Committee of the Groupe de Recherche et d’Information sur les Ostéoporoses. Trabecular bone score (TBS): available knowledge, clinical relevance, and future prospects. Osteoporos Int 2012; 23: 1489–501. doi: 10.1007/s00198-011-1824-6 [DOI] [PubMed] [Google Scholar]
- 9.Roux JP, Wegrzyn J, Boutroy D. Relationship between trabecular bone score (TBS), bone mass and microarchitecture in human vertebrae: an ex vivo study. Osteoporos Int 2012; 23(Suppl. 2): S327. P597. [Google Scholar]
- 10.Krueger D, Libber J, Binkley N. Spine trabecular bone score precision, a comparison between GE lunar standard and high-resolution densitometers. J Clin Densitom 2015; 18: 226–32. doi: 10.1016/j.jocd.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Kolta S, Briot K, Fechtenbaum J, Paternotte S, Armbrecht G, Felsenberg D, et al. TBS result is not affected by lumbar spine osteoarthritis. Osteoporos Int 2014; 25: 1759–64. doi: 10.1007/s00198-014-2685-6 [DOI] [PubMed] [Google Scholar]
- 12.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 2013; 24: 77–85. doi: 10.1007/s00198-012-2188-2 [DOI] [PubMed] [Google Scholar]
- 13.Krieg MA, Aubry-Rozier B, Hans D, Leslie WD; Manitoba Bone Density Program. Effects of anti-resorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int 2013; 24: 1073–8. doi: 10.1007/s00198-012-2155-y [DOI] [PubMed] [Google Scholar]
- 14.Bréban S, Briot K, Kolta S, Paternotte S, Ghazi M, Fechtenbaum J, et al. Identification of rheumatoid arthritis patients with vertebral fractures using bone mineral density and trabecular bone score. J Clin Densitom 2012; 15: 260–6. doi: 10.1016/j.jocd.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 15.Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, et al. “Trabecular bone score” (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone 2013; 53: 154–9. doi: 10.1016/j.bone.2012.11.041 [DOI] [PubMed] [Google Scholar]
- 16.Kamali MS, Mavalwala J. Diversity of topological palmar patterns in Iranian populations. Anthropol Anz 1990; 48: 85–97. [PubMed] [Google Scholar]
- 17.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 2011; 26: 2762–9. doi: 10.1002/jbmr.499 [DOI] [PubMed] [Google Scholar]
- 18.Popp AW, Guler S, Lamy O, Senn C, Buffat H, Perrelet R, et al. Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: a three-year study. J Bone Miner Res 2013; 28: 449–54. doi: 10.1002/jbmr.1775 [DOI] [PubMed] [Google Scholar]
- 19.Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese population-based osteoporosis (JPOS) cohort study. J Bone Miner Res 2014; 29: 399–407. doi: 10.1002/jbmr.2048 [DOI] [PubMed] [Google Scholar]
- 20.Dufour R, Winzenrieth R, Heraud A, Hans D, Mehsen N. Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women. Osteoporos Int 2013; 24: 2837–46. doi: 10.1007/s00198-013-2384-8 [DOI] [PubMed] [Google Scholar]
- 21.Lamy O, Metzger M, Krieg MA, Aubry-Rozier B, Stoll D, Hans D. OsteoLaus: prediction of osteoporotic fractures by clinical risk factors and DXA, IVA and TBS. [In French.] Rev Med Suisse 2011; 7: 2130, 2132–4, 2136. [PubMed] [Google Scholar]
- 22.Del Rio LM, Winzenrieth R, Cormier C, Di Gregorio S. Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture? A Spanish case-control study. Osteoporos Int 2013; 24: 991–8. doi: 10.1007/s00198-012-2008-8 [DOI] [PubMed] [Google Scholar]
- 23.Bazzocchi A, Diano D, Ponti F, Andreone A, Sassi C, Albisinni U, et al. Health and ageing: a cross-sectional study of body composition. Clin Nutr 2012; 32: 569–78. doi: 10.1016/j.clnu.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 24.Bandirali M, Poloni A, Sconfienza LM, Messina C, Papini GD, Petrini M, et al. Short-term precision assessment of trabecular bone score and bone mineral density using dual-energy X-ray absorptiometry with different scan modes: an in vivo study. Eur Radiol 2015; 25: 2194–8. doi: 10.1007/s00330-015-3606-6 [DOI] [PubMed] [Google Scholar]
- 25.Leslie WD, Weiler HA, Nyomba BL. Ethnic differences in adiposity and body composition: the First Nations bone health study. Appl Physiol Nutr Metab 2007; 32: 1065–72. doi: 10.1139/H07-068 [DOI] [PubMed] [Google Scholar]
- 26.Kim T, Sung J, Song YM, Lee K, Cho SI. Sex difference between body composition and weight-bearing bone mineral density in Korean adult twins: healthy twin study. Calcif Tissue Int 2011; 88: 495–502. doi: 10.1007/s00223-011-9483-3 [DOI] [PubMed] [Google Scholar]
- 27.Leslie WD, Aubry-Rozier B, Lix LM, Morin SN, Majumdar SR, Hans D. Spine bone texture assessed by trabecular bone score (TBS) predicts osteoporotic fractures in men: the Manitoba Bone Density Program. Bone 2014; 67: 10–4. doi: 10.1016/j.bone.2014.06.034 [DOI] [PubMed] [Google Scholar]
- 28.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 2011; 14: 302–12. doi: 10.1016/j.jocd.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 29.Campos RM, Lazaretti-Castro M, Mello MT, Tock L, Silva PL, Corgosinho FC, et al. Influence of visceral and subcutaneous fat in bone mineral density of obese adolescents. Arq Bras Endocrinol Metabol 2012; 56: 12–18. [DOI] [PubMed] [Google Scholar]
- 30.Reid IR. Relationships between fat and bone. Osteoporos Int 2008; 19: 595–606. doi: 10.1007/s00198-007-0492-z [DOI] [PubMed] [Google Scholar]
- 31.Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab 2007; 92: 2046–52. doi: 10.1210/jc.2006-2855 [DOI] [PubMed] [Google Scholar]
- 32.Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta 2008; 387: 31–5. doi: 10.1016/j.cca.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 33.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007; 130: 456–69. doi: 10.1016/j.cell.2007.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cianferotti L, Brandi ML. Muscle-bone interactions: basic and clinical aspects. Endocrine 2014; 45: 165–77. doi: 10.1007/s12020-013-0026-8 [DOI] [PubMed] [Google Scholar]
- 35.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2006; 2: 35–43. doi: 10.1038/ncprheum0070 [DOI] [PubMed] [Google Scholar]
- 36.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007; 92: 1640–6. doi: 10.1210/jc.2006-0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu WW, Zhang H, Wang C, Gu JM, Yue H, Ke YH, et al. Lean mass predicts hip geometry and bone mineral density in chinese men and women and age comparisons of body composition. J Clin Densitom 2012; 15: 434–42. doi: 10.1016/j.jocd.2012.02.004 [DOI] [PubMed] [Google Scholar]