Abstract
The study aim was to investigate the relationship of chronic ethanol-induced inflammation leading to vascular endothelial injury and elevation of blood pressure (BP) in a rat model. Male Fisher rats were divided into two groups of six animals each and treated as follows: (1) Control (5% sucrose, orally) daily for 12 weeks and (2) 20% ethanol (4 g kg −1, orally) daily for 12 weeks. The mean arterial blood pressure was recorded every week. The animals were anesthetized with pentobarbital after 12 weeks; thoracic aorta were isolated and analyzed for aortic reactivity response, inflammatory mediators, oxidant/antioxidant enzyme protein expression and endothelial nitric oxide-generating system. The results show that the mean BP was significantly elevated 12 weeks after ethanol ingestion. The increased BP was related to increased aortic inflammation (tumor necrosis factor [TNF]-α; nitric oxide synthase [iNOS], COX-2 and MCP-1 protein expression) and elevated angiotensin II levels in alcohol-treated group compared to control. Aortic Nicotinamide adenine dinucleotide phosphate reduced (NADPH) oxidase activity, membrane and cytosolic subunits p22phox and p47phox expression and Mn-SOD activity and protein expression significantly increased, whereas nitric oxide (NO), endothelial NO synthase (eNOS), vascular endothelial growth factor (VEGF)-A and CuZn-SOD activity and protein expression significantly decreased in alcohol-treated group compared to control. The acetylcholine-mediated vasorelaxation response was depressed in the aorta of ethanol-treated rats compared to control. In conclusion, chronic ethanol-induced elevation in BP is related to increased aortic inflammation, elevated angiotensin II levels, induction of NADPH oxidase causing endothelial injury, depletion of CuZn-SOD, down-regulation of endothelial NO generating system and impaired vascular relaxation in rats.
Keywords: cardiovascular toxicity, inflammation, oxidative stress, alcohol, hypertension
Introduction
An alcoholic beverage (ethanol) is consumed regularly by most of the human societies in the world. However, its abuse is a major public health problem in the United States, affecting more than 20 million individuals, leading to loss of 100,000 lives annually.1,2 Chronic high dose ethanol consumption most commonly causes hepatic, gastrointestinal, nervous and cardiovascular injuries leading to physiological dysfunctions.3 Recent epidemiological and clinical studies have demonstrated that chronic ethanol consumption (more than three drinks per day) is associated with an increased incidence of hypertension and an increased risk of cardiovascular diseases.4-7 However, the molecular mechanisms and possible mediators through which alcohol causes vascular injury and raises blood pressure remain elusive. Ethanol is extensively metabolized to acetaldehyde by the enzyme alcohol dehydrogenase and acetaldehyde is further oxidized to acetate by acetaldehyde dehydrogenase/oxidase in the liver,8,9 which may lead to the generation of reactive oxygen species (ROS). On the other hand, chronic high-dose ethanol ingestion induces hepatic microsomal cytochrome P450 II E1, leading to generation of 1-hydroxy ethyl radical.9,10 These ROS oxidize cellular DNA, RNA and proteins and initiate membrane lipid peroxidation leading to production of inflammatory mediators and the depletion of the antioxidant defense system causing cellular oxidative stress.
The balance of both oxidant and antioxidant factors in the vascular system has an important role in protecting the blood vessels thus allowing normal contractile function and blood pressure. Reactive oxygen and nitrogen species as well as inflammatory cytokines/chemokines such as tumor necrosis factor alpha (TNF-α) and monocyte chemotactic protein 1 (MCP-1) and inducible enzymes cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) may be the key factors contributing to cardiac vessel wall endothelial cell homeostasis and vascular tone in alcohol-induced hypertension.11 Moreover, imbalance of specific endogenous vasoconstrictor such as angiotensin II, endothelin-1 and norepinephrine and vasodilator nitric oxide (NO) may also play a role in alcohol-induced vascular injury and hypertension. Angiotensin II stimulates superoxide production via AT1 receptor, by activating NADPH oxidase in the vascular wall.12,13 Superoxide productions through NADPH oxidase activation (p22phox expression) has been demonstrated in rats made hypertensive with angiotensin II infusion.14 The production of NO in the endothelium is critically dependent on the function of endothelial NO synthase (eNOS), which is regulated by vascular endothelial growth factor (VEGF).15-17 In the endothelium, NO reacts with superoxide anion to form toxic peroxynitrite radical leading to endothelial injury and impairment.18 However, the endothelium also has an elaborate antioxidant defense system including antioxidant enzymes such as superoxide dismutase (SOD), catalase and glutathione peroxidase and tripeptide glutathione (GSH) to protect the vascular endothelium against harmful ROS.19,20 Copper/Zinc (CuZn-SOD) and manganese (Mn-SOD) are the first line of antioxidant defense in cytosolic and mitochondrial compartments of the cell to scavenge superoxide anion.21 Our earlier studies have shown that chronic alcohol-induced hypertension and oxidative injury to the vascular system was related to the depletion of the antioxidant defense system and enhanced lipid peroxidation.22-24 However, the molecular mechanism of chronic ethanol-induced hypertension in response to inflammatory and oxidative stress signaling in the aortic endothelium is unclear. Therefore, this study was designed in order to investigate the relationship of chronic ethanol-induced inflammatory/oxidative stress signaling in the vascular endothelium and elevation of blood pressure (BP) in a rat model.
Methods
Chemicals
Chemicals such as NADPH, lucigenin, phenylephrine, acetylcholine, o-phenanthroline, p-hydroxymercuribenzoic acid, pepstatin A and peroxidase conjugated antibody were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). Mono and polyclonal antibodies of TNF-α, eNOS, iNOS, COX-2, MCP-1, CuZn-SOD, Mn-SOD, VEGF-A, p22phox and p47phox and β-actin were purchased from Santa Cruz Biotechnology, Santa Cruz, California, USA and Abcam, Cambridge, Massachusetts, USA. BCA protein assay reagent was purchased from Pierce Company (Rockford, Illinois, USA). Angiotensin II ELISA kit was purchased from SPI BIO (Cayman Chemical, Ann Arbor, Michigan, USA).
Animals
Male Fisher rats (7 weeks old, 200–220 g) were obtained from Charles River (Wilmington, Massachusetts) and kept in the school's animal facility for 1 week for quarantine. The rats were fed ad libitum with Rodent Laboratory Chow (Ralston Purina Company, Indianapolis, Indiana, USA). Feed consisted of protein (23.4%), fat (4.5%) and balanced with carbohydrates, fibers, vitamins and minerals. The rats were maintained on a 12:12 h light-dark photoperiod. They were randomly divided into two groups and treated as follows:
Group I (Control): Rats were administered 5% sucrose (1 mL/kg) via oral gavage tube once a day in the morning (9–11 a.m.) for 12 weeks for equivalent caloric intake (n = 6).
Group II (Ethanol): Rats were administered 20% ethanol (4g/kg) via oral gavage tube once a day in the morning (9–11 a.m.) for 12 weeks (n = 6).
The dose of the ethanol and sucrose treatment in rats has been adapted to previous reports.22-24 The mean blood pressure was measured through tail-cuff method weekly three times in the afternoon (2–4 p.m.) using non-invasive BP monitor model NIBP-8 (Columbus Instruments, Ohio, USA). Animals were anesthetized with pentobarbital (40 mg/kg, i. p.) 24 h after the last treatments. Thoracic aortas were isolated and used for tissue bath reactivity experiments and remaining aortas were immersed in liquid nitrogen and stored at −80°C until further biochemical analysis could be completed.
In vitro tissue bath technique for recording tension in aortic rings
After anesthesia, thorax was opened and the descending thoracic aorta isolated carefully and cleaned of surrounding tissue under a dissecting microscope.22-24 The aortic ring segments (2–3 mm) were mounted horizontally on stainless steel wire hooks in isolated organ bath containing 10 mL of Krebs buffer at 37°C (Myobath-2, WPI, Sarasota, Florida, USA). The steel wire is connected to a force displacement transducer for isometric recording of changes in force. The signals were recorded and analyzed via computer using Biopack Systems Inc. (Santa Barbra, California, USA). The composition of the Krebs solution was (mM): NaCl, 96.87; KCl, 5.16; MgSO4, 1.22; NaHCO3, 25.56; CaCl2, 1.33; L-ascorbic acid, 0.11; ethylenediaminetetraacetic acid (EDTA), 0.34; and dextrose, 1.01. The Krebs bicarbonate solution was equilibrated with 95% O2 and 5% CO2. The aortic rings were first challenged with 125 mM KCl in Krebs solution and the maximum contraction response recorded. The contractile agonist phenylephrine was added at increasing concentration to the tissue chamber to induce 70%–80% of the established maximum contraction. The aortic segments were allowed to equilibrate for 1 hour at an initial tension of 1g. After equilibration, concentration-response curve to phenylephrine was recorded. In ring segments pre-contracted with 5 × 10−7 M phenylephrine, concentration-response curve to acetylcholine was generated.
NADPH oxidase assay
NADPH activity was assayed based on superoxide-induced lucigenin photoemission as described by Cui and Douglas25 and reported earlier.22-24
Superoxide scavenging enzyme assay
SOD activity was determined according to the method of Misra and Fridovich26 at room temperature. Activity was expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50%, which is equal to 1 unit. A total of 20 mM NaCN was used to inhibit CuZn-SOD activity to get the Mn-SOD activity. CuZn-SOD activity was calculated by subtracting Mn-SOD activity from total SOD activity.
Western blot analysis of protein expression
Frozen aortic tissues were thawed on ice in cold Phosphate buffered Saline (PBS) (pH 7) then lysed in mammalian-protein extraction reagent (M-PER) lysis buffer (Pierce) containing EDTA and protease inhibitor cocktail. Protein concentration was determined using BCA reagents (Pierce) according to the manufacturer's instructions. Extracted proteins (30 μg) were resolved on 12.5% SDS polyacrylamide gel (SDS PAGE) running gel and a 5% stacking gel. Proteins were then electrotransferred onto nitrocellulose membranes. After blocking in 5% nonfat powdered milk for 1 hour, the membranes were washed and then treated with antibodies to TNF-α, eNOS, iNOS, COX-2, MCP-1, CuZn-SOD, Mn-SOD, VEGF-A, p22phox and p47phox and β-actin (1:1000 and 1:5000) for overnight at 4°C (Santa Cruz Biotechnology; Abcam). After washing, the blot was incubated with horseradish peroxidase-conjugated secondary antibody IgG (1:5000 and 1:10000) for 1 hour at room temperature. The washed blot was then treated with SuperSignal West Pico chemiluminescent substrate (Pierce) for positive antibody reaction. Membranes were exposed to X-ray film (KODAK) for visualization and densitometric quantization of protein bands using AlphaEaseFC software (Alpha Innotech).
NO assay
The NO levels in the aortic tissues were determined by NO assay kit (Cayman Chemical) as described previously.22-24 The concentration was expressed as n moles per mg protein.
Determination of angiotensin II
The level of angiotensin II was analyzed in aortic tissue extracts prepared in PBS containing inhibitor cocktail (25 mM EDTA, 0.44 mM o-phenanthroline, 1 mM p-hydroxymercuribenzoic acid and 0.12 mM pepstatin A) using specific ELISA kit from SPI BIO (Cayman Chemical) as described previously.24
Protein assay
Protein concentration in tissues was estimated using BCA reagent kit (Pierce) and bovine serum albumin (BSA) was used as a standard.
Statistical analysis
The data were expressed as mean ± SEM. The data for biochemical and physiological parameters were analyzed statistically using two-way analysis of variance (ANOVA) followed by Duncan's multiple range test using the SAS statistical software package (SAS Institute, Cary, North Carolina, USA) for comparison of treated groups with control group. The relationship of changes in BP and biochemical variables was performed by multiple linear regression analyses. The 0.05 level of probability was used for statistical significance.
Results
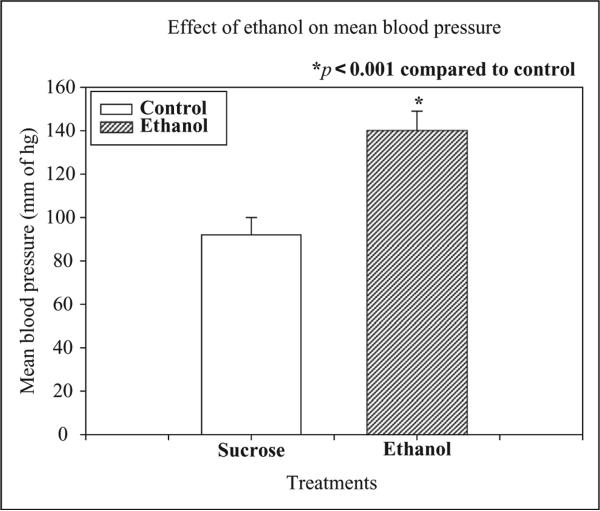
The effect of chronic ethanol administration on changes in blood pressure (BP) is depicted in Figure 1. Chronic ethanol administration significantly (p < 0.001) increased mean BP compared to control after 12 weeks treatment.
Figure 1.
Effects of chronic ethanol (20%, v/v) ingestion (4 g/kg, orally) for 12 weeks on the mean blood pressure (MBP) in rats. The control animals received 5% sucrose (1 mL/kg, orally) daily for 12 weeks. Chronic ethanol ingestion significantly increased mean BP after 12 weeks in rats as compared to control (n = 6, *p < 0.001).
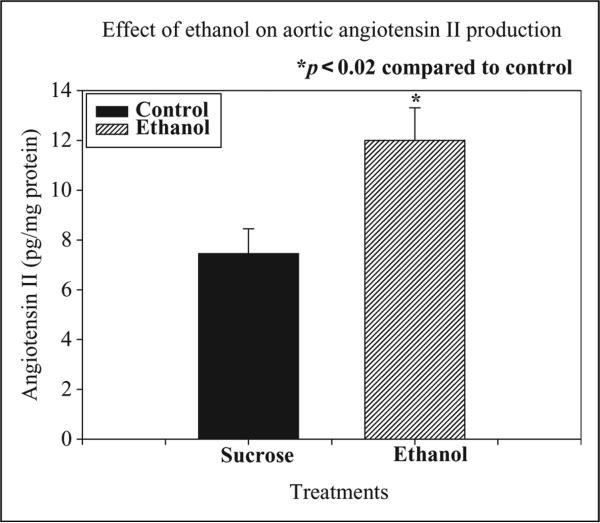
The effect of chronic ethanol administration on aortic angiotensin II levels is depicted in Figure 2. Chronic ethanol treatment significantly increased aortic angiotensin II levels (179% of control, p < 0.02) after 12 weeks indicating the up-regulation of angiotensin II production in the blood vessels of the rats. The increase in aortic angiotensin II level was well correlated with elevation in BP (r 0.88).
Figure 2.
Effects of chronic ethanol (20%, v/v) ingestion (4 g/kg, orally) daily for 12 weeks on aortic angiotensin II levels in rats. The control animals received 5% sucrose (1 mL/kg, orally) daily for 12 weeks. Chronic ethanol ingestion significantly increased aortic angiotensin II levels after 12 weeks in rats as compared to control (n = 6; *p < 0.02).
The effect of chronic ethanol administration on aortic inflammatory mediators TNF-α, COX-2 and MCP-1 protein expression is depicted in Figure 3. TNF-α protein expression was profoundly increased along with induction of COX-2 enzyme and MCP-1 expression (4.5, 3 and 2 folds, respectively) in ethanol-treated rats compared to control group, indicating an induction of inflammatory response in the blood vessels of the rats.
Figure 3.
Effects of chronic ethanol (20%, v/v) ingestion (4 g/kg, orally) for 12 weeks on aortic inflammatory mediators tumor necrosis factor alpha (TNF-α); cyclooxygenase-2 (COX-2) and monocyte chemotactic protein 1 (MCP-1) protein expression in rats. The control animals received 5% sucrose (1 mL/kg, orally) daily for 12 weeks. Western blot analysis showed increased TNF-α, COX-2 and MCP-1 expressions (4.5, 3 and 2 folds, respectively) in the aorta of ethanol-treated rats compared to control group.
The effect of chronic ethanol administration on superoxide generating enzyme NADPH oxidase activity, subunits p22phox and p47phox protein expressions as well as superoxide scavenging enzyme CuZn-SOD and Mn-SOD activities and protein expressions in the aorta of rats is depicted in Table 1 and Figure 4. Chronic ethanol treatment significantly increased aortic NADPH oxidase activity (300% of control, p < 0.001) compared to control (Table 1) after 12 weeks, indicating the enhanced superoxide production in the blood vessels of the rats. The enzyme activation was corresponded with induction of membrane and cytosolic subunits (p22phox and p47phox) protein expressions (twofold) in the aorta of ethanol-treated rats compared to controls (Figure 4). The increase in aortic NADPH oxidase activity was well correlated with elevation in mean BP (r 0.89). Chronic ethanol ingestion significantly increased mitochondrial Mn-SOD activity (210% of control, p < 0.001; Table 1) and Mn-SOD protein expression (twofold) compared to control (Figure 4) in the aorta of the rats. Chronic ethanol ingestion significantly inhibited cytosolic CuZn-SOD activity (33% of control, p < 0.001) in the aorta of the rats compared to control (Table 1), indicating the oxidative stress response of ethanol on the vascular system. The enzyme activity inhibition was corresponded to the down-regulation of CuZn-SOD protein expression (0.5-fold) in the aorta of ethanol-treated rats compared to control (Figure 4). The inhibition of aortic CuZn-SOD activity was well correlated with elevation in mean BP (r = 0.78).
Table 1.
Effect of chronic ethanol ingestion on aortic NADPH oxidase, CuZn-SOD and Mn-SOD activities and nitric oxide (NO) levels in ratsa
| Groups | NADPH oxidase activity (units/mg protein) | CuZn-SOD activity (units/mg protein) | Mn-SOD activity (units/mg protein) | Nitric oxide (NO; n moles/mg protein) |
|---|---|---|---|---|
| 1. Control (n = 6) | 0.66 ± 0.12 | 25.86 ± 1.94 | 6.54 ± 0.43 | 23.34 ± 1.89 |
| 2. Ethanol (n = 6) | 1.98 ± 0.21b | 8.57 ± 0.64b | 13.75 ± 1.47b | 12.23 ± 1.23b |
Abbreviations: CuZn: Copper/Zinc, SOD: superoxide dismutase,
Rats were 20% ethanol at a dose of 4 g/kg, orally daily for 12 weeks. Control rats received 5% sucrose (1 mL/kg, orally) daily for 12 weeks. All values are expressed as mean ± SE. NADPH oxidase activity is expressed as change in photoemission units/min/mg protein; SOD activities are expressed as change in 50% inhibition of autooxidation of epinephrine/min/mg protein.
p < 0.001 compared to control group 1.
Figure 4.
Effects of chronic ethanol (20%, v/v) ingestion (4 g/kg, orally) daily for 12 weeks on superoxide generating enzyme NADPH oxidase subunits p22phox and p47phox protein expressions and superoxide scavenging enzyme CuZn-SOD and Mn-SOD protein expressions in the aorta of the rats. The control animals received 5% sucrose (1 mL/kg, orally) daily for 12 weeks. Western blot analysis showed increased membrane and cytoslolic subunits (p22phox and p47phox) of NADPH oxidase and mitochondrial Mn-SOD protein expressions (twofold) in the aorta of rats treated with ethanol compared to control. However cytosolic CuZn-SOD protein expression was decreased (0.5-fold) in the aorta of rats treated with ethanol compared to control.
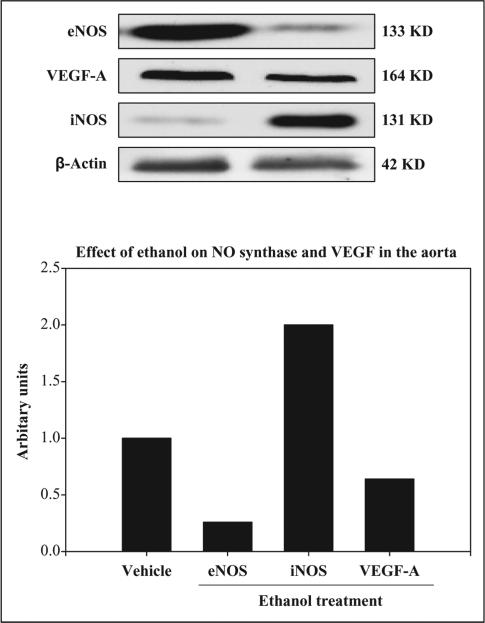
The effect of chronic ethanol administration on aortic NO levels and its generating system eNOS, iNOS and VEGF-A protein expressions is depicted in Table 1 and Figure 5. Chronic ethanol treatment significantly decreased aortic NO level (52% of control, p < 0.001) indicating decreased NO bioavailability in the vascular endothelium of the rats (Table 1). The decrease in aortic NO levels was correlated with increase in mean BP (r = 0.69). Chronic ethanol treatment decreased aortic eNOS and VEGF-A protein levels (0.25 and 0.75 fold, respectively) compared to control (Figure 5) indicating the NO generation is impaired in the vascular endothelium of the rats. The decrease in aortic eNOS protein levels was well correlated with increase in mean BP (r 0.73). The decrease in aortic VEGF-A protein levels was correlated with increase in mean BP (r = 0.67). Chronic ethanol treatment induced aortic iNOS protein expression (twofold) compared to control (Figure 5), indicating an induction of inflammatory/oxidative stress response of chronic ethanol in the blood vessels of the rats.
Figure 5.
Effects of chronic ethanol (20%, v/v) ingestion (4 g/kg, orally) for 12 weeks on nitric oxide-generating systems endothelial NO synthase (eNOS), nitric oxide synthase (iNOS) and vascular endothelial growth factor (VEGF)-A protein expressions in the aorta of rats. The control animals received 5% sucrose (1 mL/kg, orally) daily for 12 weeks. Western blot analysis showed the aortic eNOS and VEGF-A protein expressions decreased (0.25 and 0.75 fold, respectively) in rats treated with ethanol as compared to control. However iNOS protein expression was increased (twofold) in the aorta of rats treated with ethanol compared to control.
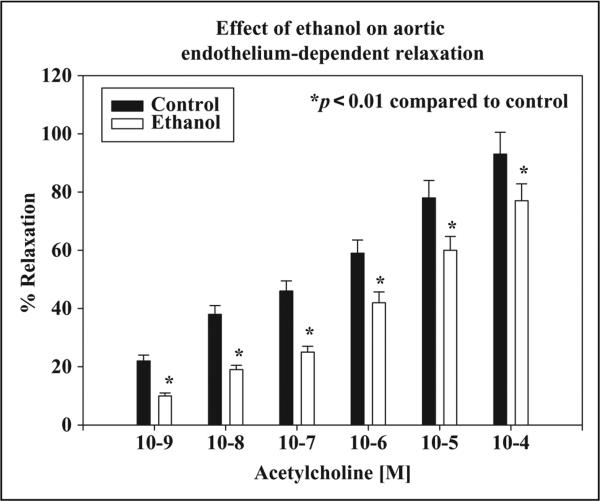
The effects of chronic ethanol ingestion on the endothelium-dependent relaxation produced by acetylcholine in rat thoracic aortic rings are depicted in Figure 6. Chronic ethanol significantly decreased the endothelium-dependent relaxation of the aorta compared to control (p < 0.01), indicating vascular endothelial dysfunction in ethanol-treated hypertensive rats.
Figure 6.
Effects of chronic ethanol (20%, v/v) ingestion (4 g/kg, orally) daily for 12 weeks on aortic endothelium-dependent relaxation elicited by acetylcholine (ACh) in rats. The control animals received 5% sucrose (1 mL/kg, orally) daily for 12 weeks. Chronic ethanol ingestion significantly decreased aortic endothelium-dependent relaxation response elicited by acetylcholine in rats as compared to control (n = 6; *p < 0.01).
The aortic longitudinal sections stained with hematoxylin and eosin depicted monocyte and lymphocyte infiltration and macrophage invasion in ethanol-treated group compared to controls (unpublished observation, data not shown), indicating an inflammatory response of ethanol in the aorta. Moreover, our earlier studies also demonstrated an increased blood and aortic level of angiotensin II, TNF-α and interleukine-6 in ethanol-treated rats compared to controls further demonstrates aortic inflammation in chronic alcohol ingestion.9,11,22,24
Discussion
The aim of the present study was to investigate the relationship of chronic ethanol-induced inflammation leading to vascular endothelial injury and elevation of BP in a rat model. The data indicate that administration of chronic oral dose of 20% ethanol (4 g/kg) for 12 weeks, which corresponds to more than 3 standard alcoholic drinks per day in humans,7,27-29 significantly increased mean BP in rats. This dose is also considered as moderate to heavy dose but not considered as binge drinking. The blood alcohol level was 203 mg/dL in ethanol-fed rats compared to controls (1.5 mg/dL), which is above the limit of blood alcohol levels (100 mg/dL) for those individuals driving vehicles. The increase in BP was well correlated with enhanced production of inflammatory mediators such as TNF-α, COX-2 and MCP-1 in the aorta of chronic ethanol-fed rats compared to control. Proinflammatory cytokines (TNF-α, MCP-1) and COX-2 enzyme have been shown to be elevated in alcoholic patients as well as in chronically ethanol-fed experimental animals.11,30-34 It is likely that these inflammatory mediators enhanced ROS production causing oxidative injury to the aortic endothelium leading to impaired vascular relaxation and hypertension in chronic ethanol-fed rats. Inflammation and oxidative stress are intertwined and the pro-inflammatory cytokine are capable of inducing ROS production in the vascular system.35 Beside inflammatory mediator's production and ROS generation, chronic ethanol ingestion may induce the production and release of other vascular factors responsible for hypertension. The increase in BP was well correlated with enhanced production of angiotensin II in the aorta (r = 0.88) of ethanol-fed rat compared to control. Angiotensin II, a powerful vasoconstrictor, has been demonstrated to cause the vasoconstriction by increasing the production of superoxides via induction of NADPH oxidase in the vascular wall.12-14 Our data demonstrates a significant increase in NADPH oxidase activity and its subunits protein expression in chronic alcohol-treated hypertensive rats, indicating that superoxide production through angiotensin II-mediated activation of NADPH oxidase is implicated in alcohol-induced vascular injury and hypertension. Clinical studies in human also suggested the role of superoxide anions in hypertension.36,37 The increase in BP was well correlated with activation of aortic NADPH oxidase activity (r = 0.79) in the aorta of ethanol-fed rat compared to control. Therefore, it is suggested that angiotensin converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs) as well as superoxide scavenger (Tempol) are likely to be clinically useful for the intervention of chronic ethanol-induced hypertension.
Chronic ethanol administration to rats for 12 weeks resulted in a significant depletion of superoxide scavenging enzyme CuZn-SOD activity as well as protein expression compared to control indicating oxidative injury to the vascular system. Superoxide dismutase is considered the first line of defense against the deleterious effects of oxygen radicals in the cells and it scavenges superoxide by catalyzing the dismutation of superoxide to H2O2 and O2. In mammals, three isozymes of superoxide dismutase exist in cells viz. CuZn-SOD, located primarily in the cytosol, Mn-SOD located in the mitochondrial matrix and ecSOD located in extra cellular spaces.38,39 CuZn-SOD activities are known to be susceptible to inactivation by ROS.39 Therefore, chronic ethanol ingestion resulted in excess generation of ROS causing oxidative inactivation of CuZn-SOD enzyme protein in the aorta of rats. On the other hand, data further indicate that aortic mitochondrial Mn-SOD activity and protein expression in chronic ethanol-treated rats significantly increased suggesting an adaptive response to get rid of excess superoxides generated by NADPH oxidase activation. Moreover, the fluxes in SOD activity in either direction (up or down regulation) may relate to the presence of excess ROS.40 Mn-SOD is induced by inflammatory cytokines such as TNF-α which is profoundly increased in the aorta of chronic alcohol-treated rats.41 The increase in aortic NADPH oxidase activity and depletion of CuZn-SOD activity in the present study further suggest the role of oxidants/antioxidant enzymes in blood pressure regulation. The data further show that chronic ethanol administration depleted the aortic NO levels, a potent endothelium-derived vasodilator. The biological activity of NO is limited by intracellular formation of superoxide anion. NO reacts instantly with superoxide anion to form peroxynitrite radical, which when protonated can give rise to a strong oxidant similar to hydroxyl radical.18 Clinical and experimental studies have also shown that chronic ethanol consumption either interferes with NO production or release of NO from endothelial cells.42,43 It is most conceivable that ethanol-induced superoxide generation in the aortic endothelium as evidenced by profound NADPH oxidase activation and inhibition of superoxide scavenging enzyme CuZn-SOD which reacts with NO to form peroxynitrite radicals is most likely implicated in diminishing NO bioavailability leading to hypertension.
In the vascular endothelium, NO is synthesized by constitutive endothelial NO synthase (eNOS) enzyme, whereas inducible NO synthase (iNOS) also produces NO under inflammatory conditions.44 The data shows that chronic alcohol treatment down-regulated the aortic eNOS protein expression but up-regulated the iNOS protein expression, but the overall aortic NO levels depleted suggesting that most of the NO reacted with excess superoxides to form peroxynitrite. The production of NO by the endothelium is critically dependent on the function of eNOS. The activity of eNOS is specifically targeted by various regulatory factors within the caveolae system that control NO levels.44 It is likely that chronic alcohol ingestion alters the endothelial membrane lipids and proteins as well as cofactor tetrahydrobiopterin, resulting in the inhibition of eNOS activity causing depressed NO bioavailability leading to endothelial dysfunction and hypertension. Chronic alcohol administration to rats has been shown to increase binding of caveolin-1 protein with eNOS in the liver causing diminished enzyme activity.45 The production of NO by eNOS is also regulated by VEGF in the endothelium.15 Reports also suggest a crucial role of VEGF in the regulation of endothelial NO production, maintenance and repair of the luminal endothelium as well as endothelium-dependent vascular relaxation.46,47 Chronic ethanol ingestion decreased aortic VEGF protein expressions, hence vascular endothelial function is also impaired by chronic high dose of ethanol in rats. Other clinical studies have shown the down-regulation of NO-generating system in the tissues and induction of hypertension in chronic alcoholic subjects.16,27 NO mediates endothelium-dependent vascular relaxation elicited by acetylcholine, which plays an important role in the regulation of blood pressure.48 The data of the present study demonstrate a significant loss of endothelium-dependent vascular relaxation response in rats chronically fed ethanol compared to control. Taken together, these data suggest that chronic ethanol ingestion suppresses the endothelium-dependent vascular relaxation due to the down-regulation of NO synthesis, leading to rise in blood pressure of rats.
In summary, chronic alcohol ingestion causes an increase in aortic inflammation, oxidative endothelial injury and down regulates the aortic endothelial NO-generating system leading to loss of vascular relaxation response and hypertension in rats.
Acknowledgments
Funding
This work was supported in part by RCMI NIH grant # 2G12RR03050-19.
References
- 1.Li TK, Hewitt BG, Grant BF. Alcohol use disorders and mood disorders: a National Institute on Alcohol Abuse and Alcoholism perspective. Biol Psychiat. 2004;56:718–720. doi: 10.1016/j.biopsych.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.McGinnis JM, Foege WH. Actual causes of death in the United States. J Am Med Assoc. 1993;70:2207–2212. [PubMed] [Google Scholar]
- 3.Lieber CS. Hepatic and other medical disorders of alcoholism: from pathogenesis to treatment. J Stud Alcohol. 1998;59:9–25. doi: 10.15288/jsa.1998.59.9. [DOI] [PubMed] [Google Scholar]
- 4.Beilin LJ, Puddey IB. Alcohol and hypertension: an update. Hypertension. 2006;47:1035–1038. doi: 10.1161/01.HYP.0000218586.21932.3c. [DOI] [PubMed] [Google Scholar]
- 5.Estruch R, Coca A, Rodicio J. High blood pressure, alcohol and cardiovascular risk. J Hypertens. 2005;23:226–229. doi: 10.1097/00004872-200501000-00039. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan NM. Alcohol and hypertension. Lancet. 1995;345:1588–1589. doi: 10.1016/s0140-6736(95)90110-8. [DOI] [PubMed] [Google Scholar]
- 7.Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension. 2008;51:1080–1087. doi: 10.1161/HYPERTENSIONAHA.107.104968. [DOI] [PubMed] [Google Scholar]
- 8.Mira L, Maia L, Barreira L, Manso CF. Evidence for free radical generation due to NADH oxidation by aldehyde oxidase during ethanol metabolism. Arch Biochem Biophys. 1995;318:53–58. doi: 10.1006/abbi.1995.1203. [DOI] [PubMed] [Google Scholar]
- 9.Scott RB, Reddy SK, Husain K, Somani SM. Time course response of ethanol on hepatic antioxidant system and cytochrome P-450 II E1 in rat. Environ Nutr Interact. 1999;3:217–231. [Google Scholar]
- 10.Anandatheerthavarada HK, Shanker SK, Bhamre S, Boyd MR, Song BJ, Ravindranath V. Induction of brain cytochrome P450IIE1 by chronic ethanol treatment. Brain Res. 1993;601:279–285. doi: 10.1016/0006-8993(93)91721-4. [DOI] [PubMed] [Google Scholar]
- 11.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Cir Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 13.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Novel role of NADH/NADPH oxidase-derived hydrogen peroxide in angiotensin II induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 14.Fukui T, Ishizaka N, Rajgopalan S, Laursen JB, Capers Q, Taylor WR, et al. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Cir Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999;41:509–510. doi: 10.1016/s0008-6363(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 16.Isner JM. Myocardial gene therapy. Nature. 2002;415:234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Zee R, Murohara T, Luio Z, Zollmann F, Passeri J, Lekutat C. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 18.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxinitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Nat Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meilhac O, Ramachandran S, Chiang K, Santanam N, Parthasarathy S. Role of arterial wall antioxidant defense in benificial effects of exercise on atherosclerosis in mice. Arterios Thromb Vasc Biol. 2001;21:1681–1688. doi: 10.1161/hq1001.097106. [DOI] [PubMed] [Google Scholar]
- 20.Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 21.Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann N Y Acad Sci. 1994;738:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- 22.Husain K. Vascular endothelial oxidative stress in alcohol-induced hypertension. Cell Mol Biol. 2007;53:70–77. [PubMed] [Google Scholar]
- 23.Husain K, Vazquez-Ortiz M, Lalla J. Down regulation of aortic nitric oxide and antioxidant systems in chronic alcohol-induced hypertension in rats. Hum Exp Toxicol. 2007;26:427–434. doi: 10.1177/0960327106072993. [DOI] [PubMed] [Google Scholar]
- 24.Husain K, Vazquez-Ortiz M, Ansari RA, Malafa MP, Lalla J. Chronic alcohol-induced oxidative endothelial injury relates to angiotensin II levels in the rat. Mol Cell Biochem. 2008;307:51–58. doi: 10.1007/s11010-007-9583-6. [DOI] [PubMed] [Google Scholar]
- 25.Cui X, Douglas JG. Arachidonic acid activates c-jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc Natl Acad Sci U S A. 1997;94:3771–3776. doi: 10.1073/pnas.94.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide-dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 27.Mukama KJ, Ascherio A, Mittleman MA, Conigrave KM, Camargo CA, Kawachi I, et al. Alcohol and risk of ischemic stroke in men: the role of drinking patterns and usual beverage. Ann Intern Med. 2005;142:11–19. doi: 10.7326/0003-4819-142-1-200501040-00007. [DOI] [PubMed] [Google Scholar]
- 28.Puddey I, Rakic V, Dimmitt SB, Beilin LJ. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors-a review. Addiction. 1999;94:649–663. doi: 10.1046/j.1360-0443.1999.9456493.x. [DOI] [PubMed] [Google Scholar]
- 29.Thakker KD. An overview of health risks and benefits of alcohol consumption. Alcohol Clin Exp Res. 1998;22:285S–295S. doi: 10.1097/00000374-199807001-00003. [DOI] [PubMed] [Google Scholar]
- 30.Augustyńska B, Araszkiewicz A, Odroważ-Sypniewska G, Grodzki L, Sobczyk J. Monocyte chemotactic protein-1 (MCP-1) concentration in alcohol dependent women. Eur Psychiat. 2009;24:S406. [Google Scholar]
- 31.Deaciuc IV, Alappat JM, McDonough KH, D'Souza NB. Effect of chronic alcohol consumption by rat on tumor necrosis factor alpha and interleukin 6 clearances in vivo by the isolated, perfused liver. Biochem Pharmacol. 1996;52:89–99. doi: 10.1016/0006-2952(96)00416-9. [DOI] [PubMed] [Google Scholar]
- 32.Simonyi A, Woods D, Sun AY, Sun GY. Grape polyphenols inhibit chronic ethanol-induced COX-2 mRNA expression in rat brain. Alcohol Clin Exp Res. 2002;26:352–357. [PubMed] [Google Scholar]
- 33.Yin MM, Wheeler M, Kono H, Thurman RG. Essential role of TNF in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Bagby GJ, Stoltz D, Oliver P, Schwarzenberger PO, Kolls JK. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate induced tumor necrosis factor-alpha production in human monocytic cells. Alcohol Clin Exp Res. 2001;25:444–449. [PubMed] [Google Scholar]
- 35.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Ann Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 36.Digies V, Fiorillo C, Cosmi L, Rossetti M, Lenuzza M, Guidi D, et al. Reactive oxygen species and antioxidant status in essential arterial hypertension during therapy with dihydropyridine calcium channel antagonists. Clin Ther. 2000;151:15–18. [PubMed] [Google Scholar]
- 37.Jun T, Ke-yan F, Catalano M. Increased superoxide anion production in humans: a possible mechanism for the pathogenesis of hypertension. J Hum Hypertens. 1996;10:305–309. [PubMed] [Google Scholar]
- 38.Marklund SL. Regulation of cytokines of extracellular superoxide dismutase and other superoxide dismutase isozyme in fibroblasts. J Biol Chem. 1992;267:6696–6701. [PubMed] [Google Scholar]
- 39.Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJ. Superoxide dismutase undergo proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919–11927. [PubMed] [Google Scholar]
- 40.Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–241. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- 41.Guo Z, Boekhoudt GH, Boss JM. Role of the intronic enhancer in tumor necrosis factor-mediated induction of manganese superoxide dismutase. J Biol Chem. 2003;278:23570–23578. doi: 10.1074/jbc.M303431200. [DOI] [PubMed] [Google Scholar]
- 42.Puddey I, Zilkens R, Croft K, Beilin LJ. Alcohol and endothelial function: a brief review. Clin Exp Pharmacol Physiol. 2001;28:1020–1024. doi: 10.1046/j.1440-1681.2001.03572.x. [DOI] [PubMed] [Google Scholar]
- 43.Slomiany BL, Piotrowski J, Slomiany A. Alterations in buccal mucosal endothelin-1 and nitric oxide synthase with chronic alcohol ingestion. Biochem Mol Biol Int. 1998;45:681–688. doi: 10.1080/15216549800203082. [DOI] [PubMed] [Google Scholar]
- 44.Michel T, Feron O. Nitric oxide synthases: which, where, how and why? Lab Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Abdel-Rahman AA. Effect of chronic ethanol administration on hepatic eNOS activity and its association with caveolin-1 and calmodulin in female rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:579–585. doi: 10.1152/ajpgi.00282.2004. [DOI] [PubMed] [Google Scholar]
- 46.Liu MH, Jin H, Floten HS, Ren Z. Vascular endothelial growth factor–mediated, endothelium-dependent relaxation in human internal mammary artery. Ann Thorac Surg. 2002;73:819–824. doi: 10.1016/s0003-4975(01)03404-x. [DOI] [PubMed] [Google Scholar]
- 47.Seipelt RG, Backer CL, Mavroudis C, Stellmach Vl. Topical VEGF enhanceshealingofthoracic aortic anastomosis for coarctation in a rabbit model. Circulation. 2003;108:150–154. doi: 10.1161/01.cir.0000087388.15066.1f. [DOI] [PubMed] [Google Scholar]
- 48.Furchgott RF, Zawadski JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]