Abstract
Background
Adenosine serves many functions within the CNS, including inhibitory and excitatory control of neurotransmission. The understanding of adenosine dynamics in the brain is of fundamental importance. The goal of the present study was to explore subsecond adenosine fluctuations in the rat brain in vivo.
Method
Long Evans rats were anesthetized and a carbon fiber electrode was positioned in the motor cortex or dorsal striatum. Real time electrochemical recordings were made at the carbon fiber electrodes every 100 ms by applying a triangular waveform (−0.4 to +1.5 V, 400 V/s). Adenosine spikes were identified by the background-subtracted cyclic voltammogram.
Results
The frequency of detected adenosine spikes was relatively stable in both tested regions, and the time intervals between spikes were regular and lasted from 1 to 5 seconds within an animal. Spike frequency ranged from 0.5 to 1.5 Hz in both the motor cortex and the dorsal striatum. Average spike amplitudes were 85 ± 11 and 66 ± 7 nM for the motor cortex and the dorsal striatum, respectively.
Comparison with Existing Methods
The current study established that adenosine signaling can operate on a fast time scale (within seconds) to modulate brain functions.
Conclusions
This finding suggests that spontaneous adenosine release may play a fast, dynamic role in regulating an organism’s response to external events. Therefore, adenosine transmission in the brain may have characteristics similar to those of classical neurotransmitters, such as dopamine and norepinephrine
1. Introduction
Adenosine, initially discovered as a potent vasodilator,1 is an important signaling molecule that is now known to be involved in the regulation of many physiological functions and pathological conditions.2 For example, adenosine plays an important role in regulating sleep as both the duration and depth of sleep are profoundly modulated by elevated concentrations of this neuromodulator in the basal forebrain.3 Increases in extracellular adenosine in the brain triggered by ischemia4 and seizures5,6 provide evidence for its role as an endogenous neuroprotective mechanism. Moreover, augmentation of adenosinergic transmission appears to be an efficient strategy to suppress pharmacoresistant seizures.5
Adenosine is preferentially released from neurons, while glial cells may also secrete this purine7. It exerts its neuromodulatory actions through the interaction with four G-protein coupled receptors (i.e. A1, A2A, A2B and A3) in the brain and peripheral tissues.8,9,10 A1 and A2B are highly expressed in the cortex and striatum.8,11,12 A1 receptors are intensely involved in the regulation of adenosine signaling in the striatum.13,14
Since adenosine is one of the most important neuromodulators in the brain, there is an enormous interest in understanding the dynamics of adenosine signaling in the extracellular space. However, a complete picture of how (amount, speed) adenosine is released, how fast it is taken back inside of cells, and what are the functional consequences of these changes requires sensitive methods to detect adenosine in real time.
The Venton group successfully developed fast-scan cyclic voltammetry (FSCV) approaches using carbon-fiber microelectrodes15 to directly detect electrically-evoked adenosine release in vivo14,16 and in vitro.17,18 Other groups have begun effectively using this approach to quantify extracellular adenosine concentrations in the CNS of different species, including humans.19,20 The Zylka group, for example used this approach to demonstrate that spontaneous, transient adenosine release (not evoked by electrical, chemical or mechanical stimulation) could be detected in the mouse spinal cord in vitro. 21,22 Recent work from the Venton group revealed that spontaneous adenosine can be rapidly released and cleared in the dorsal striatum and prefrontal cortex (PFC) of anesthetized rats.13 Together, these recent findings suggest that adenosine may have a rapid neuromodulatory role in addition to its previously characterized function as a tonic modulator of neurotransmission.
The present study was designed to further characterize adenosine signaling in the striatum and motor cortex using FSCV, and explore how spontaneous adenosine release may be affected by external stimuli. Despite a number of observed similarities, our results demonstrate some intriguing differences from those reported in earlier work.13 We also report, for the first time, that spontaneous adenosine release can be evoked in response to external stimuli.
2. Methods
2.1. Animals
Male Long Evans rats (350–470 g) were purchased from Harlan (Indianapolis, IN) and housed under a 12:12h light/dark cycle with food and water available ad libitum in standard polypropylene cages. All experiments were performed according to the guidelines of the National Institute of Health and were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine. All efforts were made to avoid or minimize suffering.
2.2. Electrode Preparation
Carbon fiber electrodes were prepared by aspirating a single carbon fiber (T-650, 7 μm diameter) into a 1.2mm × 0.68mm glass tube (A-M Systems, Inc. Sequim, WA). The glass tube was tapered at 53.9°C to form sealed microelectrodes using a pipette puller (Narishige; Tokyo, Japan). The outspreading carbon fiber was cut beyond the glass fiber to about 65–75 μm. An electrical connection was made with the carbon fiber within the glass tube by applying silver paint onto a lead wire (SQUIRES Electronic, Cornelius, OR) and inserting it into the tube. The electrode was attached to a stereotaxic arm and lowered into the motor cortex or dorsal striatum. Reference electrodes (Ag/AgCl) were prepared from silver wires (A-M Systems Inc., Carlsborg, WA) by dipping the wire in HCl (1 M) and running electrical current through it for 30 seconds.
2.3. Chemicals and drugs
All reagents were purchased from Fisher Scientific unless otherwise stated. Adenosine (Sigma-Aldrich, St. Louis, MO) was prepared as a 10 mM stock solution in 0.1 M perchloric acid and diluted to the desired concentration (0.5, 1.0 and 5 μM) in a tris buffer on the day of electrode calibration. The buffer solution at pH 7.4 contained 15 mM Tris, 140 mM NaCl, 3.25 mM KCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, 1.2 mM MgCl2 and 2.0 mM Na2SO4 in double distilled water (Mega Pure System, Corning Glasswork, Corning, NY). Urethane (Sigma-Aldrich) was dissolved in saline and administered at 1.3 g/kg. Injected volumes were 2 ml/kg (i.p.).
2.4. FSCV recordings
Rats were anesthetized with urethane and placed in a stereotaxic frame. A hole for carbon fiber electrode insertion was drilled (from bregma: anterior-posterior, 0.4 mm; lateral, 2.4 mm). An Ag/AgCl reference electrode was implanted in the contralateral hemisphere and a carbon fiber electrode was positioned in the motor cortex (dorsal-ventral 2.0 mm) or dorsal striatum (dorsal-ventral, 4.5 – 5.0 mm). The reference and carbon fiber electrodes were connected to a voltammetric amplifier (UNC Electronics Design Facility, Chapel Hill, NC) and voltammetric recordings were made at the carbon fiber electrodes every 100 ms by applying a triangular waveform (−0.4 to +1.5 V, 400 V/s). Data were digitized (National Instruments, Austin, TX) and stored on a computer. Adenosine spikes were identified by the background-subtracted cyclic voltammogram. Carbon fiber microelectrodes were calibrated in a flow cell with known concentrations of adenosine (0.5 – 1.0 μM) after experiments (postcalibration).
2.5. Experimental procedure
An electrode was placed in the brain region of interest and a triangular waveform was applied. An electrode was allowed to equilibrate for at least 30 min before any recordings were taken. Adenosine spikes were detected every 3 minutes for at least 30 min using Tar Heel CV, written in LabVIEW (National Instruments; Austin, TX).23 At the end of data collection sessions, rats were subjected to a 3 s tail pinch, where recordings were taken before and after the stimulus for a total duration of at least 15 s. Tail pinches were performed as previously described24 with soft rubber gloves to avoid any tissue damage and generation of electrical noise artifacts. The rat tail was pressed with the thumb and the index finger with a pressure of approximately 3.0 MPa.
2.6. Data analysis
Data were analyzed using GraphPad Prism 6.1 (GraphPad Software Inc. Lajolla, CA). The relationship between cyclic voltammograms obtained during calibration of adenosine in vitro and its detection in vivo in the rat brain was determined using Pearson’s correlation. The stability of the frequency and amplitude of spikes generated over 30 min periods in the motor cortex and striatum were compared using repeated measure ANOVA. The event detection function of Clampfit 10.4 (Molecular Devices, Sunnyvale, CA) was used to measure the amplitude, interevent interval, and duration of adenosine transients. OriginPro 7.0 (OriginLab, Northampton, MA) was used to determine cumulative distribution functions. Statistical analysis of adenosine transient distribution properties were conducted with the non-parametric, Kolmogorov-Smirnov test. Effects of tail pinches on the amplitude of adenosine efflux were analyzed with one-way ANOVA. The evaluation of adenosine concentrations before and during tail pinches was performed by calculating the area under the curve (definite integral) in Matlab. Student’s t-test was used for comparison of means. Data are presented as mean ± SEM. All results were considered significant at the 95% confidence level.
3. Results and discussion
3.1. Identification of Spontaneous Adenosine Transients Detected in Vivo
In our experiments, spontaneous adenosine transients were measured at the carbon fiber electrode by applying a triangular waveform from −0.4 to +1.5 V and back versus an Ag/AgCl reference electrode, at a rate of 400 V/s. The same waveform and scanning rate were used to detect electrically-evoked adenosine changes in slice preparations18,25 and in vivo recordings, including measurements in the brain of anesthetized animals14,16 and humans.19,20 Identification of adenosine spikes was based on an analysis of voltammograms, which provide electrochemical information on the analyte. Figure 1 demonstrates that, as observed with the voltammogram obtained during the detection of 1.0 μM adenosine injected into a flow cell (Fig. 1A), voltammograms for spontaneous spikes measured in the rat brain (Fig. 1B,C) had a maximal oxidation current at 1.4 V. Statistical analysis revealed a significant positive correlation between the flow cell and brain-derived voltammograms (r=0.88, R2=0.74, P<0.0001). The same features for adenosine identification were demonstrated in previous voltammetric studies.14,18,20
Figure 1. Identification of adenosine in vitro and in vivo using fast scan cyclic voltammetry.
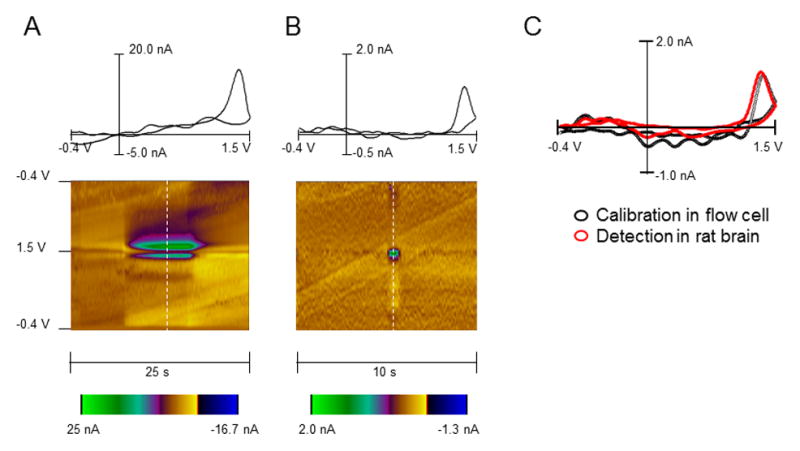
(A) In vitro calibration of the carbon fiber electrode to adenosine at a concentration of 1.0 μM in the flow cell. A 2D color plot (bottom) shows time on the abscissa, potential on the ordinate and current in false color. The dashed white line on the color plot indicates where the background subtracted voltammogram (on top of color plot) was obtained. (B) In vivo spontaneous adenosine release detected in the rat striatum. The cyclic voltammogram and 2-D color plot match the in vitro calibration. The maximal oxidation for each case occurs at 1.4 V. (C) Cyclic voltammograms obtained for adenosine during an electrode calibration in a flow cell (black circles) and during recordings in vivo (red circles). The voltammograms with the same maximal current at 1.4 V were selected to demonstrate identical electrochemical features of signals.
It should be noted that the occurrence of an additional oxidation peak was reported by several groups.13,21,22 However, in the case of spontaneous spike recordings, this characteristic only became evident when the cyclic voltammogram was taken at a specific time point, half a second following the first peak.13 We could not see a secondary peak at this or any timepoint under our experimental conditions, both in vitro (flow cell calibration) and in vivo. The reason for this discrepancy is unclear at the present time. Therefore, a traditional voltammogram with the single oxidation event at ≈ 1.4 V was used for the identification of spontaneous adenosine spikes. Furthermore, the first report on spontaneous adenosine transients has clearly demonstrated that the frequency of these spikes can be effectively modulated through A1 receptors in the striatum of anesthetized rats.13 Since several other analytes, including hydrogen peroxide and ATP, provide cyclic voltammograms with peaks around the same potential as adenosine,17, 26 this demonstration can be considered as an important additional pharmacological verification for adenosine. Using the same pharmacological tool, cyclopentyladenosine (CPA), we explored how the activation of A1 receptors affects the frequency and amplitude of striatal transients (Fig. 2). Specifically, after stable baseline data were collected for at least for 30 minutes, the drug was injected at 2 mg/kg, i.p. and recordings were made for 30–40 minutes. No changes in the oxidation/reduction patterns of the cyclic voltammograms were observed after the drug administration (data not shown), suggesting that, as previously shown13, CPA did not affect the chemical being detected. Notably, CPA had no effect on the amplitude of detected spikes (P= 0.518, n=4) (Fig. 2A, B) but markedly reduced the frequency of these events, to 30% of baseline (P=0.007, n=4) (Fig 2A, C). In contrast, vehicle injection had no significant effect on either spike amplitude or frequency (P=0.234 and P=0.638, respectively, n=4) (Fig. 2B, C). These data are in good agreement with a previous study13, where A1 receptor activation with CPA decreased the frequency of spontaneous adenosine transients, while the amplitude was not altered. Therefore, based on both electrochemical and pharmacological criteria, the detected chemical was identified as adenosine.
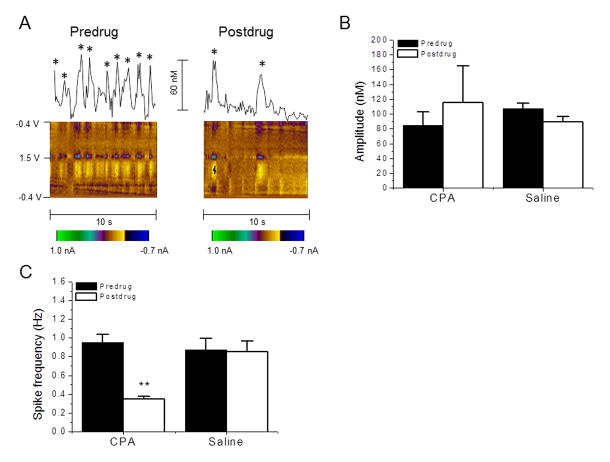
Figure 2. Effect of the A1 agonist, cyclopentyladenosine (CPA) on adenosine release.
(A) Representative traces and 2D color plots depicting the detection of adenosine in the dorsal striatum before (left) and after (right) CPA (2 mg/kg, i.p.) administration. Stars on top of spikes indicate that the obtained cyclic voltammogram has characteristics of adenosine detection. (B) The average amplitude was obtained from all spikes detected per rat for 10 s across four rats before and 30 min after the drug. Neither CPA nor saline (vehicle control) administration affect the amplitude of striatal adenosine transients. (C) CPA administration results in a significant reduction in adenosine spike frequency. **P < 0.01, paired t-test. Data are means ± SEM of four rats per group.
3.2. Characterization of Adenosine Spikes in the Motor Cortex and Dorsal Striatum
In a subset of six animals, the stability of spontaneous adenosine spikes was monitored in the motor cortex and dorsal striatum of anesthetized rats. Recordings were made with three minute intervals over 30 minute periods. The three dimensional color plots topographically represent voltammetric data collected in two representative animals (Fig. 3A, B). The average adenosine concentration obtained from all spikes, which were identified during the ten second recording periods, was used to determine the amplitude of spontaneous effluxes. We found that the frequency was relatively stable in both tested regions across the 30 minute recording period [F (9, 90) = 0.7907; P=0.626] (Fig. 3C) and this parameter was similar in the motor cortex and striatum [n=6 rats, F (1, 10) = 0.06, P=0.817] (Fig. 3C). On average, the number of spikes per second ranged from 0.6 ± 0.1 to 1.0 ± 0.1 in both areas. This parameter was quite stable within a single animal, while it varied from rat to rat with a minimum of 0.5 and maximum of 1.5. Similar to the frequency of spikes, their amplitudes were relatively stable over time in both brain areas [n=6 rats, F (9, 90) = 1.297; P=0.250] (Fig. 3D). The average amplitude over the 30 min recording period fluctuated from 75 ± 10 to 94 ± 18 nM in the motor cortex and from 123 ± 29 to 150 ± 43 nM in the dorsal striatum. Noticeably, larger error bars for striatal values indicated a higher variability in the amplitude of adenosine spikes in this area in comparison with the motor cortex.
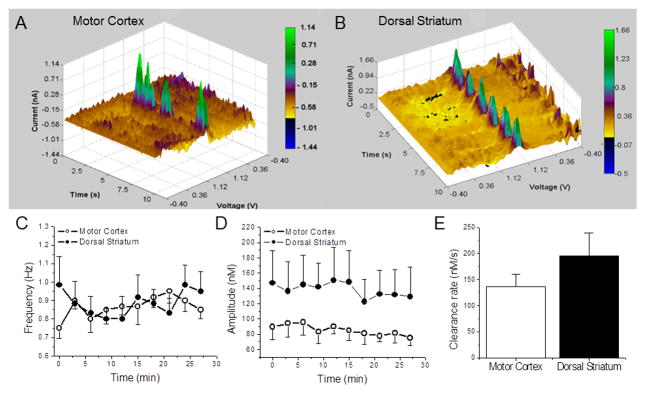
Figure 3. Detection and stability of adenosine transients in vivo.
3D plots showing adenosine oxidation currents as functions of time and voltage in the motor cortex (A) and the dorsal striatum (B) of anesthetized rats. Green spikes indicate adenosine transients that were identified by cyclic voltammograms. (C) Spike frequency (on y-axis) in the motor cortex and dorsal striatum were plotted as a function of the 30 minute recording time (on x-axis). Each time point is an average per rat detected across 6 rats, within 10 s intervals. There was no significant difference in the frequency of spikes between the two regions. (D) Average amplitude was plotted as a function of time. This parameter was relatively stable in both tested regions across the 30 minute recording period (P > 0.05; n=6). (E) The clearance rate was calculated by dividing the maximal amplitude with the amount of time it takes adenosine to disappear. The average clearance rate was plotted as a function of area. There was no significant difference in the rate between the two regions (P > 0.05; n = 7).
The clearance rate was determined in μM/s from the peak amplitude of adenosine spikes to the baseline value. Since adenosine transients from each rat were approximately the same size, five spikes were randomly chosen per rat (n=7 rats) over the 30 min recording time to compare the clearance rates between motor cortex and dorsal striatum. We found that the clearance rates in the motor cortex (137 ± 23 nM/s, n=7) and dorsal striatum (196 ± 44 nM/s) were not significantly different [unpaired t-test, P=0.264] (Fig. 3E).
To more rigorously characterize adenosine release events, additional criteria for the identification of adenosine spikes were created to avoid any inaccuracies in the calculations of spike parameters. We employed an unbiased threshold detection method to identify and characterize adenosine events from recorded traces. Analysis parameters were optimized to minimize inclusion of baseline noise and the threshold trigger for event detection was adjusted to identify peaks at least two times greater than the noise level. Event amplitude, duration, interevent interval, and frequency were calculated for each detected spike. Frequency was calculated by dividing the number of peaks detected by the length of each recording. Detected events were then visually inspected to exclude spurious events. The peaks detected from this analysis matched events in 2D color plots and cyclic voltammograms having characteristics of adenosine. As listed in Table 1, 88 spikes from motor cortex recordings (n = 11 rats) and 110 peaks from dorsal striatum recordings (n = 12 rats) were identified by this analysis. Table 1 also shows that average spike amplitudes, durations, interevent intervals, and frequencies were not significantly different between motor cortex and dorsal striatum.
Table 1.
Averages (±SEM) of adenosine transients detected in the motor cortex and dorsal striatum.
| Motor Cortex | Dorsal Striatum | |
|---|---|---|
| # of recordings | 11 | 12 |
| # of spikes detected | 88 | 110 |
| Amplitude (nM) | 85.15 ±10.90 | 65.35 ±7.13 |
| Duration (s) | 0.72 ±0.07 | 0.69 ±0.10 |
| Interevent interval (s) | 1.08 ±0.08 | 1.02 ±0.11 |
| Frequency (Hz) | 0.77 ±0.05 | 0.92 ±0.11 |
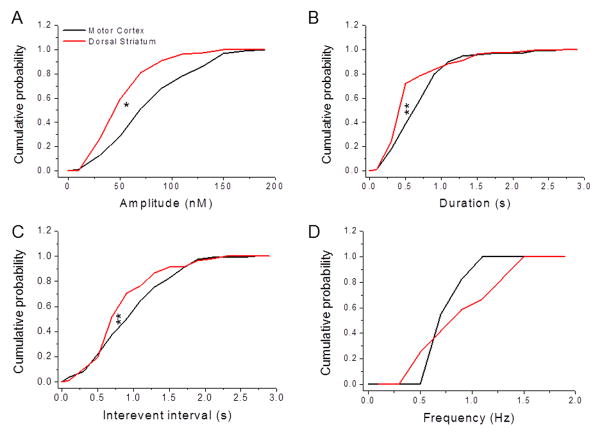
We further evaluated the identified adenosine spikes by analyzing the underlying distributions of events in motor cortex and dorsal striatum (Fig. 4). This analysis revealed that the spike amplitudes in motor cortex were skewed towards larger amplitudes compared to dorsal striatum (Kolmogorov-Smirnov test, D = 0.66, P = 0.04) (Fig. 4A). Likewise, adenosine spike durations (D = 0.68, P = 0.003) (Fig. 4B) and interevent intervals (D = 0.65, P = 0.001) (Fig. 4C) were skewed towards longer times in motor cortex compared to dorsal striatum. The distributions of spike frequencies, however, were not different between the two brain regions (D = 0.5, P = 0.67) (Fig. 4D).
Figure 4. Characteristics of adenosine transients recorded from the motor cortex and dorsal striatum.
Cumulative distribution functions of various parameters of adenosine spikes recorded from motor cortex (11 rats) and dorsal striatum (12 rats) regions were plotted as cumulative probability curves and compared. (A) Adenosine spike amplitudes recorded from the motor cortex were larger than those recorded in the dorsal striatum (Kolmogorov-Smirnov test, D = 0.66, P < 0.05). (B) The durations of adenosine spikes were longer in the motor cortex compared to the dorsal striatum (D = 0.68, P < 0.01). (C) The interevent intervals between adenosine spikes were longer in the motor cortex than the dorsal striatum (D = 0.65, P < 0.01). (D) Adenosine spike frequencies were not different between the motor cortex and the dorsal striatum (D = 0.5, P = 0.67).
It should be noted that the average concentration of adenosine released was in the same range (slightly lower) than that reported in the first study on spontaneous adenosine release (180 nM) in the rat brain13 and is in line with its baseline concentration (50 to 200 nM) determined with other techniques.7 However, our results indicate that adenosine can be released on a much shorter time scale (seconds versus minutes). Earlier, adenosine release was detected on average once every 3–4 minutes and importantly, it was not a periodic event.13 In our experiments, spontaneous adenosine spikes were observed within seconds with relatively regular intervals. Based on this observation, it could be speculated that adenosine transients can be caused by pacemaker firing. Thus, adenosine can operate in a faster signaling mode than previously assumed. In fact, this is a better fit with its role as a neuromodulator.
There are a number of factors which can account for the difference between the current study and earlier published results.13 This may include some variance in the scanning potentials used (1.5 V vs. 1.45 V), the type of electrode employed (uncoated vs. Nafion-coated, connection with silver paint vs. connection with 1 M KCl), the pretreatment of electrodes (electrodes were soaked in 2-propanol in the previous study), recording schedules, the strain (Long Evans vs Sprague-Dawley) and age of rats (animals were older in our experiments) and the level of anesthesia. However, the main cause for the discrepancy is likely the analytic methods used to quantify adenosine transients. In fact, any transient signal without a secondary peak was not counted in the previous work, whereas all data in this study were analyzed using principal component regression.13 As we indicated above, the second oxidation peak is not a rigorous criterion for the identification of spontaneous adenosine.
Based on the results of both studies, we can conclude that adenosine is available for signaling in physiologically relevant concentrations for seconds and then it is quickly cleared from the extracellular space. However, it is important to highlight that, in both cases, recordings were performed on anesthetized animals. The information on how anesthesia affects spontaneous adenosine release is not available. Therefore, we cannot exclude a dramatic change in adenosine signaling under this condition. Experiments on freely moving animals are necessary to explore adenosine transmission under physiological conditions.
3.3. Effect of an external stimulus on spontaneous adenosine release
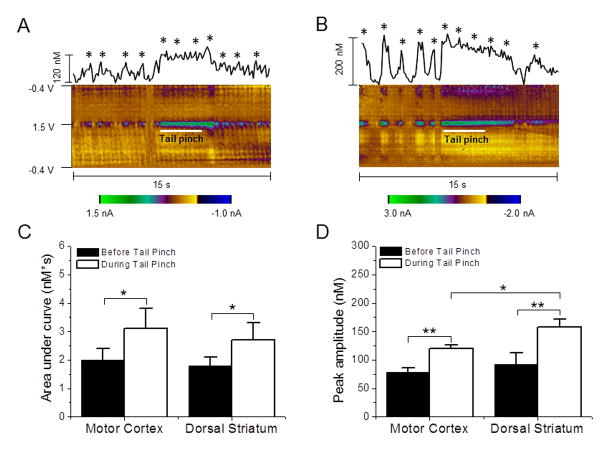
All recordings were performed under the condition of urethane anesthesia during the entire study. In contrast to experiments on freely moving animals, anesthetized preparations limit the possibility of exploring neurochemical responses to different external stimuli. However, the effects of aversive stimuli, such as a tail pinch, on brain neurotransmission can be observed even in anesthetized animals.27,28 According to a previous study, a tail pinch triggers subsecond dopamine release in several striatal regions including the dorsal striatum.24 It is very well known that adenosine is a powerful modulator of dopaminergic neurotransmission.29,30,31 The latest study by the Venton group revealed that momentary adenosine changes can transiently modulate phasic dopamine release via the A1 receptor on the scale of seconds.17 Therefore, a fast escalation in spontaneous adenosine efflux in some brain regions can be expected under the experimental conditions such as when the rat is exposed to noxious stimuli, which induces brief dopamine release. Figure 5 shows changes in spontaneous adenosine concentrations in the rat brain observed after administration of brief, 3 s tail pinches. The total amount of the released neuromodulator was evaluated by calculating the area under the curve during an aversive stimulus, and during the 3 seconds preceding the stimulus (Fig. 5A, B). Administration of a tail pinch significantly increased this parameter in the motor cortex from 2.0 ± 0.4 to 3.1 ± 0.7 [paired t-test, P= 0.017] and in the dorsal striatum from 1.8 ± 0.3 to 2.7 ± 0.6 [paired t-test, P= 0.025] (Fig. 5C). In both regions, this parameter increased by a nearly identical amount (55% in MC vs. 50% in DS). Importantly, averaged magnitudes of adenosine spikes in tested brain areas before a tail pinch were not significantly different [unpaired t-test; P =0.907], while they were significantly higher in the striatum than in the cortex when rats were exposed to the stimulus [unpaired t-test, P=0.034] (Fig. 5D). This difference in adenosine response can reflect a stronger dopamine innervation in the dorsal striatum in comparison with the cortical region. Thus, these transient adenosine changes are more likely to be involved in neuromodulatory function and serve to control the release of dopamine, while their modulatory role for other neurotransmitters cannot be ruled out at the present time.
Figure 5. Adenosine changes detected in response to tail pinches in the motor cortex and dorsal striatum.
Representative concentration-time plots of adenosine release and standard 2D color plots are obtained before, during, and after a tail pinch in the motor cortex (A) and dorsal striatum (B). A tail pinch exposure is indicated by a white line in color plots. A star on the top of spikes indicates that the cyclic voltammogram fits with adenosine detection. (C) Adenosine release was augmented during the tail pinch period compared to the period before the stimulus in both the motor cortex and dorsal striatum. (D) Peak magnitudes of adenosine transients were also larger during the tail pinch compared to the period preceding the stimulus in both the motor cortex and dorsal striatum. The tail pinch response was significantly larger in the dorsal striatum compared to the motor cortex. *P < 0.05, **P < 0.01, paired t-test. Data are means ± SEM of 7 rats per group.
4. Conclusions
The present work confirms previous findings that revealed spontaneous, transient adenosine release in the brain of anesthetized rats using fast scan cyclic voltammetry.13 Despite a number of observed similarities in the characterization of adenosine spikes, some important new features were revealed in the current study. First, we found that the frequency of spontaneous adenosine events was significantly higher and intervals between spikes were more constant under our experimental conditions. These data suggest that adenosine signaling can operate on a faster time scale to modulate brain functions. Second, our analysis of spike distributions revealed that spontaneous adenosine events can vary by brain region. A number of factors, such as a dissimilar basal concentration of adenosine, and/or the level of adenosine receptor expression and synthesis rate, could be responsible for this difference. Finally, we observed marked adenosine effluxes in the motor cortex and dorsal striatum in response to an external noxious stimulus, a brief tail pinch. This finding suggests that spontaneous adenosine release may play a fast, dynamic role in regulating an organism’s response to external events. Therefore, adenosine transmission in the brain may have characteristics similar to those of classical neurotransmitters, such as dopamine and norepinephrine.
Highlights.
Spontaneous adenosine events can vary by brain region
Adenosine signaling can operate on a much faster time scale (seconds vs minutes)
Subsecond adenosine is released in response to noxious stimuli
Acknowledgments
This work was supported by NIH grants AA022449, AA7565, GM102773, and AA17531 and by the Russian Science Foundation grant N14-15-00131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68(3):213–37. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014;7(6):581–91. doi: 10.1016/j.jcin.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Wylen DG, Park TS, Rubio R, Berne RM. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986;6(5):522–8. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- 5.Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11(1):25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- 6.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32(5):618–24. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 7.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79(3):463–84. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 8.Wei CJ, Li W, Chen J-F. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2011;1808(5):1358–1379. doi: 10.1016/j.bbamem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Dalziel HH, Westfall DP. Receptors for adenine nucleotides and nucleosides: subclassification, distribution, and molecular characterization. Pharmacol Rev. 1994;46(4):449–66. [PubMed] [Google Scholar]
- 10.Fredholm BB, IJzerman AP, Jacobson KA, Klotz K-N, Linden J International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacological Reviews. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 11.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59(4):355–96. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 12.Latini S, Pazzagli M, Pepeu G, Pedata F. A2 adenosine receptors: their presence and neuromodulatory role in the central nervous system. General Pharmacology: The Vascular System. 1996;27(6):925–933. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One. 2014;9(1):e87165. doi: 10.1371/journal.pone.0087165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cechova S, Elsobky AM, Venton BJ. A1 receptors self-regulate adenosine release in the striatum: evidence of autoreceptor characteristics. Neuroscience. 2010;171(4):1006–15. doi: 10.1016/j.neuroscience.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swamy BEK, Venton BJ. Subsecond Detection of Physiological Adenosine Concentrations Using Fast-Scan Cyclic Voltammetry. Analytical Chemistry. 2006;79(2):744–750. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- 16.Cechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008;105(4):1253–63. doi: 10.1111/j.1471-4159.2008.05223.x. [DOI] [PubMed] [Google Scholar]
- 17.Ross AE, Nguyen MD, Privman E, Venton BJ. Mechanical stimulation evokes rapid increases in extracellular adenosine concentration in the prefrontal cortex. Journal of Neurochemistry. 2014;130(1):50–60. doi: 10.1111/jnc.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pajski ML, Venton BJ. Adenosine Release Evoked by Short Electrical Stimulations in Striatal Brain Slices is Primarily Activity Dependent. ACS Chem Neurosci. 2010;1(12):775–787. doi: 10.1021/cn100037d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Gompel JJ, Bower MR, Worrell GA, Stead M, Chang SY, Goerss SJ, Kim I, Bennet KE, Meyer FB, Marsh WR, Blaha CD, Lee KH. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia. 2014;55(2):233–44. doi: 10.1111/epi.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SY, Kim I, Marsh MP, Jang DP, Hwang SC, Van Gompel JJ, Goerss SJ, Kimble CJ, Bennet KE, Garris PA, Blaha CD, Lee KH. Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clin Proc. 2012;87(8):760–5. doi: 10.1016/j.mayocp.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Street SE, Kramer NJ, Walsh PL, Taylor-Blake B, Yadav MC, King IF, Vihko P, Wightman RM, Millan JL, Zylka MJ. Tissue-nonspecific alkaline phosphatase acts redundantly with PAP and NT5E to generate adenosine in the dorsal spinal cord. J Neurosci. 2013;33(27):11314–22. doi: 10.1523/JNEUROSCI.0133-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Street SE, Walsh PL, Sowa NA, Taylor-Blake B, Guillot TS, Vihko P, Wightman RM, Zylka MJ. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol Pain. 2011;7:80. doi: 10.1186/1744-8069-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst. 2003;128(12):1413–9. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 24.Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience. 2012;201:331–7. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pajski M, Venton BJ. The mechanism of electrically stimulated adenosine release varies by brain region. Purinergic Signalling. 2013;9(2):167–174. doi: 10.1007/s11302-012-9343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AE, Venton BJ. Sawhorse waveform voltammetry for selective detection of adenosine, ATP, and hydrogen peroxide. Anal Chem. 2014;86(15):7486–93. doi: 10.1021/ac501229c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106(12):4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(5666):2040–2. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 29.Okada M, Mizuno K, Kaneko S. Adenosine A1 and A2 receptors modulate extracellular dopamine levels in rat striatum. Neurosci Lett. 1996;212(1):53–6. doi: 10.1016/0304-3940(96)12780-4. [DOI] [PubMed] [Google Scholar]
- 30.Quarta D, Borycz J, Solinas M, Patkar K, Hockemeyer J, Ciruela F, Lluis C, Franco R, Woods AS, Goldberg SR, Ferre S. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem. 2004;91(4):873–80. doi: 10.1111/j.1471-4159.2004.02761.x. [DOI] [PubMed] [Google Scholar]
- 31.Quarta D, Ferre S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004;88(5):1151–8. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]