Abstract
Background
Myocardial infarction (MI) is an ischemic wound that recruits millions of leukocytes. MI-associated blood leukocytosis correlates inversely with patient survival, yet the signals driving heightened leukocyte production after MI remain incompletely understood.
Methods and Results
Using parabiosis surgery, this study shows that soluble danger signals, among them interleukin-1 beta (IL-1β), increase bone marrow hematopoietic stem cell (HSC) proliferation after MI. Data obtained in bone marrow reconstitution experiments reveal that IL-1β enhances HSC proliferation by both direct actions on hematopoietic cells and through modulating the bone marrow’s hematopoietic microenvironment. An antibody that neutralizes IL-1β suppresses these effects. Anti-IL-1β treatment dampens the post-MI increase in HSC proliferation. Consequently decreased leukocyte numbers in the blood and infarct reduce inflammation and diminish post-MI heart failure in ApoE−/− mice with atherosclerosis.
Conclusions
The presented insight into post-MI bone marrow activation identifies a mechanistic target for muting inflammation in the ischemically damaged heart.
Keywords: myocardial infarction, bone marrow hematopoiesis, hematopoietic stem cells, interleukin-1β
Myocardial infarction (MI) inflicts a sterile cardiac wound that, within minutes, recruits leukocytes from circulation at a rate of several hundred thousand cells per day. This demand depletes blood pool leukocytes quickly and requires continuous resupply over the next several days. In mice, the spleen initially serves as a leukocyte reservoir1 contributing ~50% of myeloid cells to the infarct in the early hours after coronary ligation. Thereafter, emergency hematopoiesis fuels the increased demand for myeloid cells2–4. Sympathetic nervous system activity triggers hematopoietic progenitor migration to the spleen, initiating extramedullary myelopoiesis3.
The mechanisms of increased hematopoietic system activity after ischemic injury are incompletely understood5. The sympathetic nervous system activates the bone marrow after MI3 and in mice exposed to chronic psychosocial stress6. This activation increases hematopoietic stem and progenitor cell (HSPC) proliferation and migration via chemokine C-X-C motif ligand 12 (CXCL12)/C-X-C chemokine receptor type 4 (CXCR4) signaling7. Soluble factors released from ischemic myocardium into the blood may also signal to the bone marrow to drive hematopoietic stem cells (HSC) proliferation remotely. These post-MI stimuli could act on either HSC directly or niche cells that regulate the bone marrow microenvironment. Data obtained from mice with infection or injected systemic stimuli suggest that circulating danger signals may activate hematopoiesis8, 9.
The pro-inflammatory cytokine interleukin-1 beta (IL-1β) may provide one such hematopoiesis activation signal. Increased bone marrow progenitor proliferation after injection of the chemical compound alum, which is used as a vaccination adjuvant, was attenuated in IL-1 receptor deficient mice10. IL-1β also stimulates myelopoiesis in obesity11. IL-1β is first synthesized as its cytosolic precursor pro-IL-1β and then gives rise to its active form via caspase-1, an enzyme in turn regulated by the NLRP3 inflammasome12, 13. IL-1β and IL-1α both signal using the receptor IL-1R114, 15. IL-1R2, the other IL-1 receptor, functions as a decoy for IL-116, 17. Further, IL-1β instigates inflammation in atherosclerotic plaque18 and ischemic myocardium13. IL-1β rises in patient serum after acute MI19, and both preclinical and clinical pilot data suggest anti-IL-1 therapy can provide benefit after MI20–22 and in atherosclerosis23–25.
This study shows that after MI, soluble factors that reach the bone marrow via the circulation significantly accelerate hematopoiesis. Parabiosis experiments revealed that IL-1β stimulates systemic leukocyte production by a) directly acting on hematopoietic stem cells and b) modulating the stem cell microenvironment in the bone marrow. Administration of an anti-mouse IL-1β reduced post-MI emergency hematopoiesis and attenuated leukocytosis. In ApoE−/− mice with atherosclerosis, anti-IL-1β therapy moderated leukocyte overproduction, supported resolution of infarct inflammation and ameliorated post-MI heart failure.
METHODS
A detailed methods description is provided in the online supplement.
Experimental animals
We used female C57BL/6J (WT, n = 162), B6.129S7-Il1r1tm1Imx/J (IL1R1−/−, n = 28) and apolipoprotein E–deficient (ApoE−/−; B6.129P2-Apoetm1Unc/J, n = 24) mice aged 8–12 weeks (The Jackson Laboratories, Bar Harbor, ME, USA) for our studies. We also used transgenic mice expressing green fluorescent protein (GFP) under the Nestin-promoter (Nestin-GFP, n = 10)26, 27. Nestin-GFP mice were a gift from Dr. Grigori Enikolopov (Cold Spring Harbor Laboratory, NY, USA). Age-matched mice were randomly allocated either to control or treatment groups. The study was approved by the Subcommittee on Animal Research Care at Massachusetts General Hospital (Boston, MA).
Myocardial infarction surgery
Myocardial infarction was induced by permanent ligation of the left anterior descending coronary artery as described previously3. Ischemia Reperfusion Injury was induced and assessed as described previously28. Please also see the online supplement.
Neutralizing IL-1β
The IL-1β neutralizing antibody was a donation from Novartis (Basel, Switzerland). The antibody selectively binds IL-1β, thus blocking the interaction of the cytokine with its receptors. We used a monoclonal, mouse anti-mouse IL-1β IgG2a/k antibody derived from an IgG1/k antibody as described by Geiger et al.29. The in vitro potency IC50 is about 25 pM, and its affinity to murine IL-1β is about 300 pM. The t1/2 in mice is 14 days30. We initiated treatment 2 h after induction of MI with subcutaneously injecting 10 mg/kg bodyweight of the IL-1β antibody (or a mouse monoclonal IgG2a antibody raised against cyclosporine A as isotype control). We repeated injections once weekly over the study period.
BrdU experiments
To assess proliferation, we used FITC/APC BrdU (bromodeoxyuridine) flow kits (BD Bioscience). For these BrdU pulse experiments, one mg BrdU was injected intraperitoneally 24h prior to euthanization and subsequent organ harvest. After surface staining, intracellular BrdU staining was performed according to the manufacturer’s protocol. Flow cytometry and cell sorting procedures are described in the online supplement.
Bone marrow reconstitution
We lethally irradiated WT or IL1R1−/− mice with 950 cGy. We then reconstituted mice with 2x106 whole bone marrow cells from either WT or IL1R1−/− mice. Two to four months later mice received coronary ligation. Please also see the online supplement.
Administration of recombinant mouse IL-1β
We injected 2.5 μg of recombinant mouse IL-1β (R&D Systems, MN, USA) i.p. daily over two days as described elsewhere31 and then harvested the bone marrow 48h after the first administration.
Statistics
Statistical analyses were carried out using GraphPad Prism software version 6 (GraphPad Software, Inc.). Results are displayed as mean ± standard deviation (SD). First, values were tested for Gaussian distribution (D’Agostino-Pearson omnibus normality test). For two-group comparisons, an unpaired t-test was applied to normally distributed variables, a Mann-Whitney test to non-normally distributed variables. For comparing more than two groups, a one-way ANOVA test, followed by a Sidak’s test for multiple comparisons, was applied. P values of < 0.05 indicated statistical significance.
RESULTS
IL-1β released after MI activates bone marrow hematopoiesis
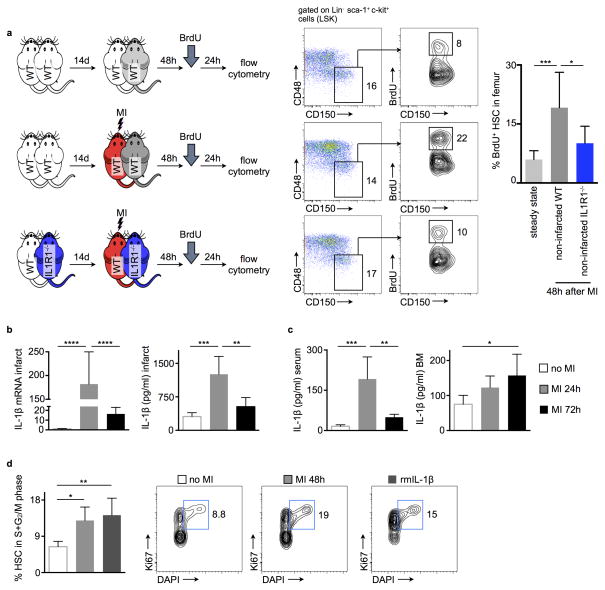
It is unclear how the hematopoietic system, which responds to acute myocardial ischemia with increased proliferation, receives signals to increase leukocyte production. We asked whether HSC activation relies on a circulating factor, released after MI, that reaches the bone marrow via the blood. To test this hypothesis, we joined the circulation of two wild type mice in parabiosis. After 2 weeks, a period necessary to establish shared circulation, one parabiont underwent coronary ligation while the other did not. Two days later, we assessed HSC proliferation in the bone marrow of the non-infarcted parabiont by BrdU incorporation (Fig. 1a). Lin− Sca-1+ c-Kit+ CD48− CD150+ HSC proliferation in non-infarcted parabionts increased significantly (Fig. 1a), indicating that factor(s) circulating in blood contribute to hematopoietic activation after MI. In humans, IL-1β levels rise in the blood after MI19. Accordingly, we found IL-1β mRNA and protein levels increased in the infarcted heart (Fig. 1b), in circulation and in the bone marrow (Fig. 1c.) These data indicate this cytokine transfers signals from the site of ischemic injury to the hematopoietic system. Injecting recombinant mouse IL-1β (rmIL-1β) into naive mice triggered HSC proliferation similar to that observed after MI (Fig. 1d). We next joined wild type and interleukin-1 receptor 1 knockout mice (IL1R1−/−) in parabiosis. Two days after coronary artery ligation in one parabiont, increased HSC proliferation observed in the non-ischemic wild type parabionts significantly diminished in IL1R1−/− mice (Fig. 1a). Taken together, these data indicate that IL-1β crossed from the circulation of the infarcted parabiont to that of the non-infarcted parabiont and instigated increased HSC proliferation in the bone marrow.
Figure 1.
IL-1β is released after MI and promotes HSC proliferation. (a) Schematic of the experimental set-up. Flow cytometric gating and quantification of proliferation rates (BrdU incorporation, bromodeoxyuridine) of bone marrow HSC (hematopoietic stem cells) of parabionts in the steady state (upper row), of the non-infarcted wild type (WT) parabiont 48h after infarcting the partner (middle row) and of the non-infarcted interleukin-1 receptor 1 knockout (IL1R1−/−) parabiont 48h after infarcting the partner (lower row). Numbers next to gates indicate population frequencies (%) (n = 7–8 per group). (b) Quantification of interleukin-1 beta (IL-1β) mRNA and protein levels in the heart, blood and bone marrow (c) in the steady state and after acute myocardial infarction (MI) (n = 5 per group). (d) Flow cytometric gating and quantification of proliferation rates (cell cycle analysis) of bone marrow HSCs in the steady state, 48h after MI and after administration of recombinant mouse IL-1β (rm IL-1β) (n = 5 per group, mean ± SD, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, one-way ANOVA).
IL-1β effector cells
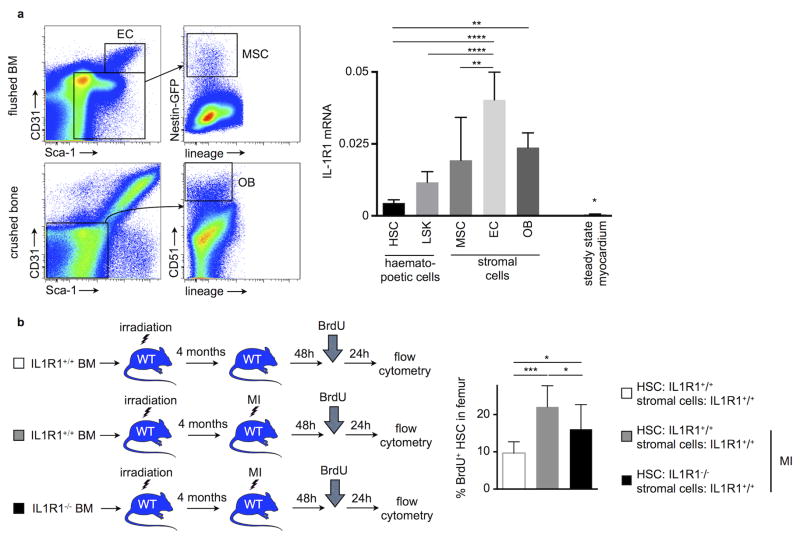
Bone marrow stromal cells form a microenvironment that harbors hematopoietic stem and progenitor cells (HSPC)7. These niche cells maintain hematopoiesis by producing factors that signal to HSPC and regulate their dormancy, retention and lineage bias. Therefore, our next question addressed how IL-1β stimulates HSC proliferation: does IL-1β affect hematopoietic cells directly or act on niche cells regulating HSC activity? To address this issue, we investigated which bone marrow cells can sense IL-1β. Using qRT-PCR in FACS-isolated bone marrow cells (for gating; please see Fig. 2a), we found that HSC, Lin− Sca-1+ c-Kit+ (LSK), as well as all stromal cells regulating HSPC activity, including mesenchymal stromal cells, endothelial cells and osteoblasts, express the interleukin-1 receptor 1 (Fig. 2a), with higher expression in the non-hematopoietic niche cells. We then reconstituted lethally irradiated wild type mice with whole bone marrow from either wild type (recipients’ bone marrow HSC and bone marrow stromal cells are IL1R1+/+) or from IL1R1−/− mice (recipients’ bone marrow HSC lack IL1R1 while the bone marrow stromal cell are IL1R1 competent). Four months later, mice underwent coronary ligation. Subsequent analysis showed significantly decreased HSC proliferation in mice reconstituted with IL1R1−/− bone marrow (Fig. 2b). In these mice, in which hematopoietic but not niche cells were unresponsive to IL-1β, BrdU incorporation in HSC was still markedly above chimeras without MI (Fig. 2b). The data indicate that IL-1β’s hematopoietic effect involves direct as well as indirect action on hematopoietic cells. To compare IL-1β effects on HSC with indirect effects on hematopoiesis via niche cells, we transplanted wild type bone marrow into lethally irradiated IL1R1−/− recipients (Suppl. Fig. 1a). In this scenario, bone marrow hematopoietic cells are responsive to IL-1β while the marrow’s stromal cells are not. In a second group, lethally irradiated WT mice received IL1R1−/− bone marrow, resulting in the opposite situation. We then investigated the relevance of these two scenarios for post MI recovery (Suppl. Fig. 1b and c). Cardiac function by MRI was similar in both groups 21 days after coronary ligation, and blood leukocyte numbers were also comparable (Suppl. Fig. 1d–1f) indicating that IL-1β’s direct action on HSPC and indirect action via stromal cells are equally important for the post MI increase in leukocyte production.
Figure 2.
IL-1β drives HSC proliferation directly. (a) Flow cytometric gating for different bone marrow (BM) stromal cell populations (EC, bone marrow endothelial cells; MSC, bone marrow mesenchymal stromal cells; OB, bone osteoblastic lineage cells). Quantification of interleukin-1 receptor 1 (IL-1R1) expression on hematopoietic (HSC and Lin− sca-1+ c-kit+ cells (LSK)) and stromal (EC, MSC and OB) bone marrow cells by qRT-PCR (n = 4–5 per group). (b) Lethally irradiated wild type (WT) mice were reconstituted with either wild type or IL1R1−/− bone marrow. Four months later, mice received MI and HSC proliferation was quantified 48h later (n = 8–9 per group, mean ± SD, *p<0.05, **p<0.01, ****p<0.0001, one-way ANOVA).
Neutralizing IL-1β reduces post-MI bone marrow HSPC proliferation and leukocytosis
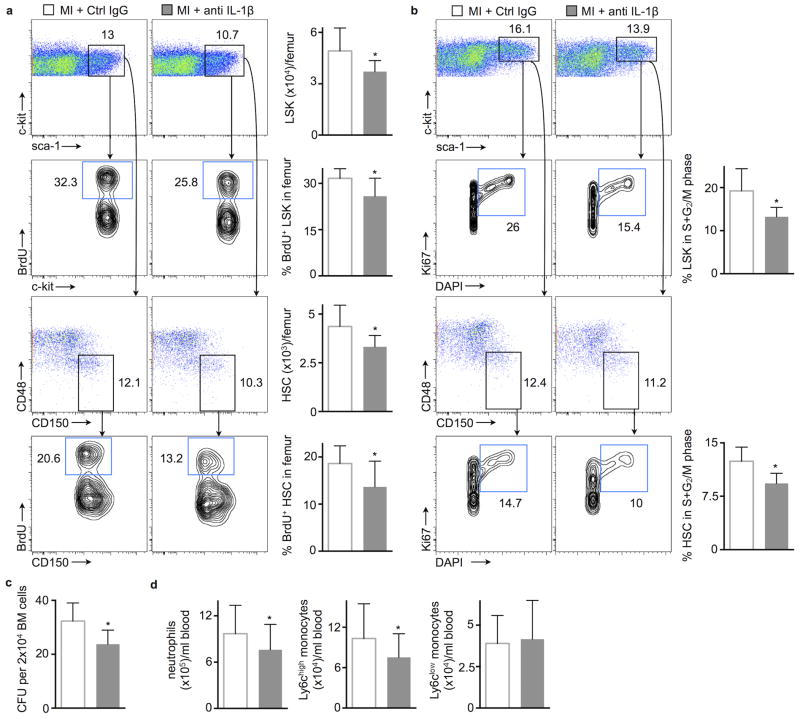
Thus far, these data show IL-1β involvement in triggering post-MI emergency hematopoiesis. This activated heart-bone marrow axis may represent a therapeutic target for reducing leukocyte overproduction after MI, a process that may lead to heart failure and secondary ischemic events5. To test how IL-1β neutralization changes leukocyte production, we chose the clinically relevant murine version of canakinumab. Treatment began two hours after coronary ligation, and we assessed bone marrow HSPC proliferation by measuring BrdU incorporation 48 hours later. HSC and LSK proliferation were markedly lower in treated mice compared to a control IgG treated cohort that also underwent coronary artery ligation (Fig. 3a). The treatment effects were somewhat lower than the decrease in HSC proliferation observed in IL-1R−/− mice, possibly because IL-1R1−/− mice lack both, IL-1α as well as the IL-1β signaling. Nevertheless, antibody treatment significantly reduced frequencies of HSC and LSK in the active S+G2/M phase of the cell cycle, as determined by Ki67 and DAPI staining for cell cycle analysis (Fig. 3b). A third method of measuring hematopoietic progenitor proliferation detected a lower number of colony forming units derived from the bone marrow of treated mice (Fig. 3c). Reduced hematopoietic progenitor activity led to fewer circulating neutrophils on day 3 and decreased neutrophil and Ly6Chigh monocyte levels on day 7 after MI (Suppl. Fig. 2a and Fig. 3d).
Figure 3.
Neutralizing IL-1β lowers HSC proliferation after MI. (a) Flow cytometric gating and quantification of proliferation rates with BrdU incorporation in bone marrow LSK and HSC, 48h after MI. Numbers next to gates indicate population frequencies (%) (n = 10–11 per group). (b) Cell cycle analysis in bone marrow LSK and HSC 48h after MI (n = 5–6 per group). (c) Bone marrow colony forming unit (CFU) assay 48h after MI (n = 6 per group). (d) Blood neutrophils and monocytes 7 days after MI (n = 14 per group, mean ± SD, *p<0.05, Mann-Whitney test).
Neutralizing IL-1β reduces extramedullary hematopoiesis
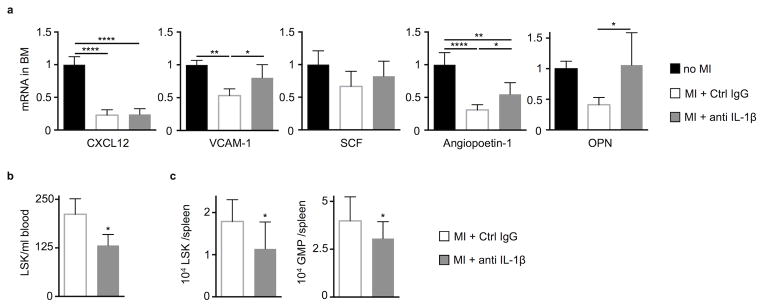
The bone marrow chimera data indicate that IL-1β may act on bone marrow niche cells. Indeed, anti-IL-1β treatment preserved mRNA levels of VCAM-1, angiopoietin-1 and osteopontin after coronary ligation (Fig. 4a). Cell-specific treatment effects are shown in Suppl. Fig. 3. These effects likely contributed to the treatment-induced decrease in myeloid cell production, as angiopoietin-1, osteopontin and CXCL12 regulate hematopoietic progenitor quiescence32, 33. VCAM-1 retains HSPC in the hematopoietic niche. When VCAM-1 expression falls after MI, HSPC release into circulation and seed the spleen3. Indeed, HSPC release from the bone marrow into the blood decreased in mice treated with the anti-IL-1β antibody (Fig. 4b). Consequently, spleens of mice treated with anti-IL-1β antibody contained fewer LSK and GMP (granulocyte-macrophage progenitors) than controls (Fig. 4c).
Figure 4.
Neutralizing IL-1β changes the bone marrow microenvironment and dampens progenitor release after MI. (a) mRNA quantification of HSC retention factors (CXCL12, chemokine (C-X-C motif) ligand 12, VCAM-1, vascular cell adhesion molecule 1; SCF, stem cell factor and OPN, osteopontin) in flushed bone marrow with qRT-PCR 48h after MI (n = 6 per group, one-way ANOVA). (b) LSK numbers in the blood 4d after MI (n = 5–6 per group). (c) Splenic LSK and GMP (granulocyte-macrophage progenitors), 7d after MI (n = 9–11 per group, mean ± SD, *p<0.05, **p<0.01, Mann-Whitney test).
Neutralizing IL-1β reduces inflammation in myocardial infarcts
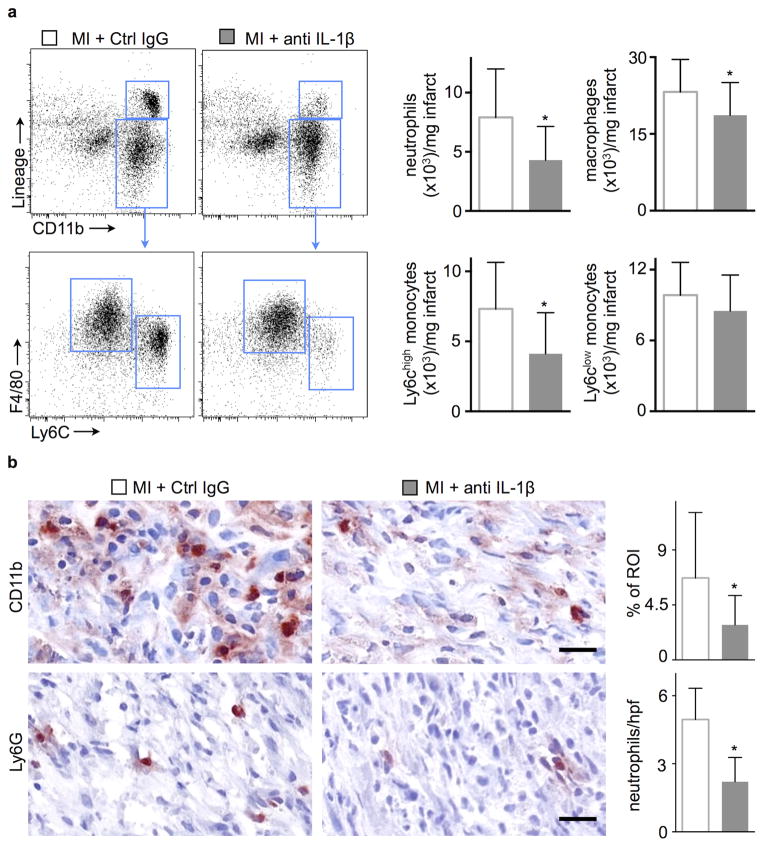
Leukocytes mobilize rapidly to the infarct after onset of ischemia, and, due to their fast turnover, the extent of their accumulation depends on their supply from the circulation5, 34. We consequently tested whether lowering blood leukocyte numbers in the anti-IL-1β treatment group would reduce leukocyte recruitment to the ischemic heart. Indeed, we detected fewer recruited myeloid cells on day 3 and 7 after coronary ligation by flow cytometry and immunohistochemistry (Fig. 5 and Suppl. Fig. 2b). These data corroborate previous reports by the Frangogiannis group35, 36.
Figure 5.
Neutralizing IL-1β attenuates inflammation in infarcted hearts. (a) Flow cytometric gating and quantification of neutrophils, macrophages and monocytes in infarcted hearts 7d after coronary ligation (n=15 per group). (b) Immunohistochemical evaluation of 7d old infarcts for myeloid cells (CD11b) and neutrophils (Ly6G). Bar graphs show percentage of positive staining per region of interest (ROI) or number of cells per high power field (hpf). Scale bar indicates 50 μm (n = 6 per group, mean ± SD, *p<0.05, **p<0.01, Mann-Whitney test).
Therapeutically targeting IL-1β signaling improves infarct healing and reduces post-MI remodeling
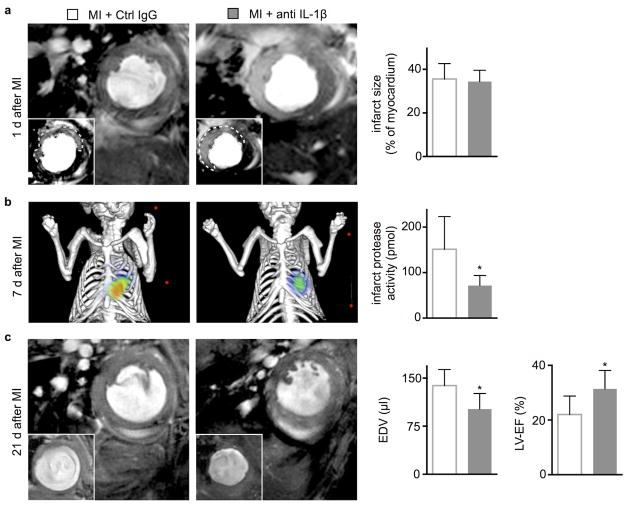
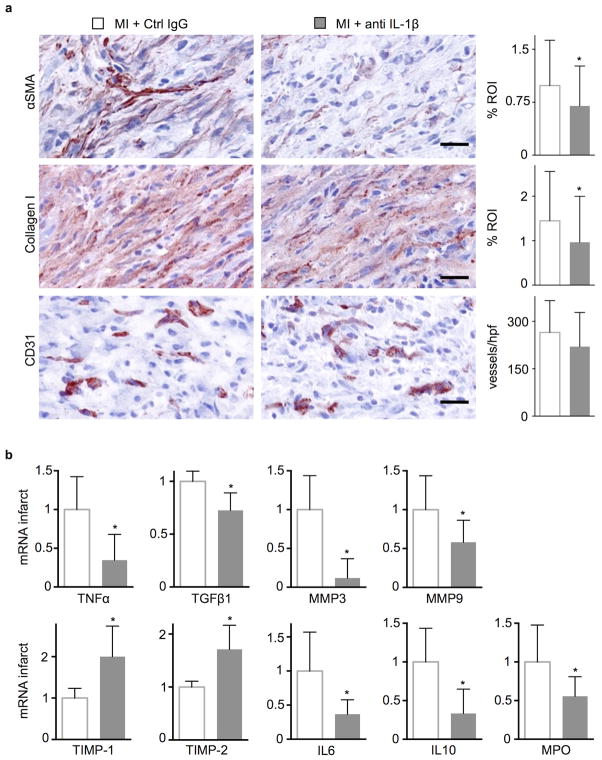
We next evaluated the impact of antibody-mediated IL-1β signaling blockade on infarct healing and post-MI heart failure in ApoE−/− mice on high-fat diet. We chose to study dyslipidemic mice because they mimic two aspects of clinical myocardial infarction: an atherogenic milieu and chronically activated innate immunity37. Even without MI ApoE−/− mice have augmented myelopoiesis due to increased progenitor cycling in the bone marrow38 and the spleen39. In a serial imaging trial, delayed enhancement MRI determined comparable infarct size in both cohorts on day 1 after permanent occlusion of the coronary artery (Fig. 6a). In a separate cohort, anti-IL-1β treatment did not change ischemia reperfusion injury (Suppl. Fig. 4). On day 7 after MI, mice underwent FMT/CT imaging to determine infarct protease activity with a molecular imaging agent (pan-cathepsin reporter)40. At this time point, this molecular imaging signal reports on the resolution of cardiac inflammation. FMT/CT revealed that anti-IL-1β treatment significantly reduced protease activity in the infarct (Fig. 6b) which we located using the hybrid CT modality. Mice were then re-imaged by MRI 3 weeks after MI. In comparison to controls, anti-IL-1β treated ApoE−/− mice exhibited smaller end-diastolic volumes and a better preservation of the left ventricular ejection fraction (Fig. 6c). Histological analysis of wound healing biomarkers in a cohort of WT mice sacrificed on day 7 after coronary ligation showed that anti-IL-1β treatment reduced staining for the myofibroblast marker α smooth muscle actin (αSMA) and collagen-1, while the density of sprouting capillaries in the granulation tissue did not change (Fig. 7a). In accord with the histological data, TGF-β1 and MMP-3 mRNA fell and TIMP-1 and TIMP-2 mRNA increased in the infarct and borderzone of the treatment group 7 days after coronary ligation (Fig. 7b). In addition, mRNA levels for the pro-inflammatory genes tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6) fell, concordant with the observed reduction of inflammatory myeloid cells at this time point.
Figure 6.
In vivo imaging of anti-IL-1β treatment effects on infarct protease activity and post-MI remodeling. (a) Evaluation of post-MI remodeling in ApoE−/− mice by cardiac MRI. Each panel shows the short axis of the mid left-ventricular segment at the end of diastole. Infarct size was measured as gadolinium enhanced hypokinetic area on day 1 after MI. (b) Imaging infarct protease activity 7d after MI in ApoE−/− mice by Fluorescence Molecular Tomography-Computed Tomography (FMT/CT) (n = 8 per group). (c) End-diastolic volumes (EDV) and left-ventricular ejection fraction (LV-EF) were examined serially on day 1 and 21 and are displayed as absolute values on day 21 (n = 6 per group, mean ± SD, *p<0.05, Mann-Whitney test).
Figure 7.
Neutralizing IL-1β reduces cardiac fibrosis. (a) Immunohistochemical evaluation of 7d old infarcts for myofibroblasts (alpha-smooth muscle actin, αSMA), collagen deposition (collagen-1) and neovessels (CD31). Bar graphs show percentage of positive staining per region of interest (ROI) or number of vessels per high power field (hpf). Scale bar indicates 50 μm (n = 6 per group). (b) qRT-PCR of infarct tissue, values relative to GAPDH expression with the “MI+Ctrl IgG” group set as 1 (n = 6 per group, mean ± SD, *p<0.05, Mann-Whitney test).
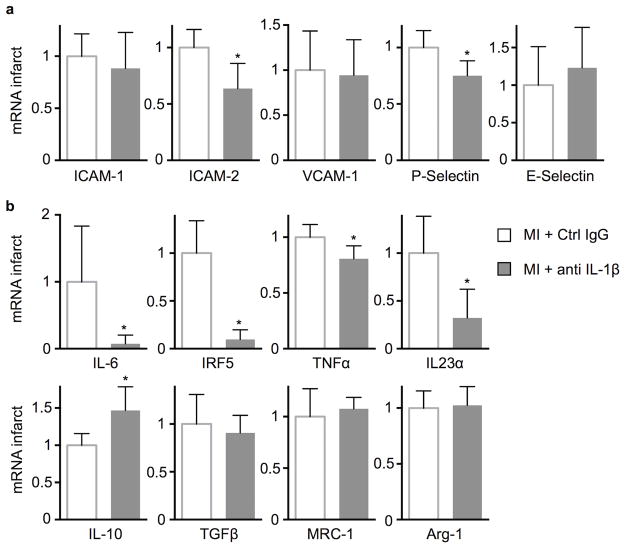
Anti-IL-1β treatment reduces adhesion molecule expression and shifts macrophage phenotype
To explore how local mechanisms may contribute to the observed salient effects of anti-IL-1β treatment, we examined the mRNA levels of adhesion molecules in infarct tissue. IL-1β neutralization reduced ICAM-2 and P-selectin expression, while ICAM-1, VCAM-1 and E-selectin expression were unchanged on day 7 after coronary artery ligation (Fig. 8a), indicating that reduced IL-1β signaling dampens leukocyte recruitment. We also investigated how IL-1β influences the infarct macrophage phenotype. We isolated CD45high CD11bhigh F4/80high macrophages by flow sorting from 4 day old infarcts and measured mRNA levels of several M1/M2 macrophage markers (Fig. 8b). Anti-IL-1β treatment shifted macrophage polarization away from the inflammatory M1 phenotype, indicating that anti-IL-1β therapy augments resolution of inflammation in acute infarcts.
Figure 8.
Anti-IL-1β treatment reduces leukocyte adhesion molecule expression in infarcts and changes macrophage polarization. (a) qRT-PCR for leukocyte adhesion molecule expression in infarct tissue 7d after MI, values relative to GAPDH expression with the “MI+Ctrl IgG” group set as 1 (n = 6 per group). (b) Quantified macrophage polarization markers in FACS-sorted infarct macrophages 4d after coronary ligation. qRT-PCR values are relative to GAPDH expression with the “MI+Ctrl IgG” group set as 1 (n = 6 per group, mean ± SD, *p<0.05, Mann-Whitney test).
DISCUSSION
Recent experiments link atherosclerosis and MI, its dreaded clinical complication, with bone marrow activation5. Yet whether soluble systemic factors mediate this crosstalk between organs remained unknown. This study provides evidence that circulating, blood-borne factors contribute to bone marrow activation during MI. After joining the circulation of two mice in parabiosis, one mouse underwent coronary ligation. The observed increase in HSC proliferation in the bone marrow of the non-infarcted parabiont must result from signaling by soluble mediators that crossed from the circulation of the infarcted mouse to the non-infarcted mouse. Here we report that IL-1β participates in the process and contributes to bone marrow activation after MI via direct effects on hematopoietic cells and indirect effects that modulate the hematopoietic bone marrow microenvironment. Finally, we found that neutralizing IL-1β with the murine version of an antibody currently under investigation in a large-scale human trial41 can limit bone marrow activation. Anti-IL-1β treatment lowered the post-MI increase in HSC proliferation, reducing leukocytes in the blood and ischemic heart. Inhibiting this driver of inflammation favored infarct healing and attenuated left ventricular remodeling in mice with atherosclerosis, a harbinger of the development of heart failure post MI, as in previous reports on non-atherosclerosis-prone mice35, 42.
Although the bone marrow generates billions of cells every day, leukocyte numbers in the steady state follow circadian rhythms within tightly regulated boundaries. Only a minor fraction of HSC actively cycle, while the niche cells forming the hematopoietic microenvironment use signals such as CXCL12 to keep stem cells quiescent7. In response to infection and other stressors, a variety of signals, including interferons and Toll-like receptor ligands, increase leukocyte output to meet the heightened demand for these innate immune cells during host defense against invading pathogens. These danger signals may act directly on hematopoietic stem cells that express receptors to sense danger-associated molecular patterns. Alternatively, alarmins may modulate the activity of niche cells, including endothelial cells and mesenchymal stromal cells, among others7, 43. In the setting of cardiovascular disease, blood leukocytosis correlates with mortality5; however, the signals that increase leukocyte production are incompletely understood. In mice with atherosclerosis, insufficient cholesterol efflux from cells may induce increased HSPC cycling in the bone marrow38 and spleen39, thereby leading to hyperlipidemia-associated monocytosis44, heightening macrophage recruitment into the vessel wall and progressing inflammation in the plaque. In this regard, a high macrophage burden characterizes plaques that have given rise to MI in humans45.
During MI, increased sympathetic nervous system activity may trigger an additional burst of hematopoiesis by reducing bone marrow levels of CXCL12 and SCF, important HSC retention factors that induce quiescence3. We hypothesized that circulating factors also contribute to activating hematopoiesis, as seen after infection or other systemic injury. We specifically tested IL-1β because previous studies have implicated this cytokine in regulating hematopoiesis after injection of the chemical compound alum10 and because IL-1β levels increase after myocardial infarction in rats46 and humans19. Our data indicate that post-MI, IL-1β signaling in the bone marrow acts through two mechanisms: IL-1β directly binds IL-1R1 expressed by HSC and indirectly affects leukocyte production through signaling to the niche cell compartment. In this microenvironment, antagonizing IL-1β preserved several HSC retention factors and inhibited the HSC and LSK release after MI, reducing their migration to the spleen and attenuating extramedullary hematopoiesis.
Systemically neutralizing IL-1β lowers blood levels of inflammatory monocytes and neutrophils. These myeloid cells drive plaque inflammation, and myeloid cell supply in blood likely influences rates of recruitment into the arterial wall and ischemic myocardium. In atherosclerotic plaque, lower leukocyte numbers may lead to smaller lesion size and reduced vulnerability18, 47–49. In the MI, reducing blood leukocytosis supports resolution of inflammation and mitigates subsequent left ventricular dilation. The ejection fraction was preserved 3 weeks after coronary ligation, likely also due to altered macrophage polarization that enhanced myocardial tissue repair50. Our observations of IL-1β’s system-wide action correlate well with previous reports on its local effects in the infarcted heart35, 36, 42, 46, 51, 52.
Our results have direct clinical relevance, as these experiments used a species-appropriate antibody to neutralize IL-1β in mice with MI equivalent to the clinically-approved drug for syndromes associated with function gain of the NALP3 inflammasome, which generates active IL-1β. The large-scale trial CANTOS is testing whether the FDA-approved anti-human IL-1β antibody can limit recurrent events in stable post-MI patients who have residual inflammation, despite full standard-of-care treatments, as gauged by a highly sensitive C-reactive protein concentration in blood above median41. The data presented here provide novel mechanistic insight on this drug’s actions. Additionally, our results suggest patients who might have recurrent acute MI while receiving anti-IL-1β antibody would not be at greater risk for enhanced post-MI heart failure. Indeed, these observations support a trial in acute MI, a proposition tested in the MRC trial53 with a much shorter acting IL-1 receptor antagonist that inhibits both the alpha and beta isoforms of this multipotent mediator.
Supplementary Material
Acknowledgments
We thank the CSB Mouse Imaging Program (Jessica Truelove and Derrick Jeon) for assistance with imaging. We thank Meredith Weglarz, Kathryn Folz-Donahue and Laura Prickett-Rice from the Flow Cytometry Core Facility (Massachusetts General Hospital, Center for Regenerative Medicine and Harvard Stem Cell Institute) for assistance with cell sorting.
Funding Sources: This work was funded in part by grants from Novartis (to P.L., F.S., M.N.) and the National Institute of Health (HL117829, HL225454 and HL096576 to M.N. and HL080472 to P.L.). Hendrik B. Sager and Timo Heidt were supported by the Deutsche Forschungsgemeinschaft (SA1668/2-1 and HE-6382/1-1).
Footnotes
Disclosures: The authors received research grant support and study medication from Novartis. Dr. Libby is a member of the scientific advisory board, receives research support, and is involved in clinical trial leadership for Novartis. Dr. Libby is a member of the scientific advisory board for Interleukin Genetics.
References
- 1.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, Wojtkiewicz G, Feruglio PF, King K, Baker JN, van der Laan AM, Borodovsky A, Fitzgerald K, Hulsmans M, Hoyer F, Iwamoto Y, Vinegoni C, Brown D, Di Carli M, Libby P, Hiebert SW, Scadden DT, Swirski FK, Weissleder R, Nahrendorf M. Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell Stem Cell. 2015;16:477–487. doi: 10.1016/j.stem.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 10.Ueda Y, Cain DW, Kuraoka M, Kondo M, Kelsoe G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J Immunol. 2009;182:6477–6484. doi: 10.4049/jimmunol.0803961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 13.Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 16.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (Warsz) 2009;57:165–176. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheedy FJ, Moore KJ. IL-1 signaling in atherosclerosis: sibling rivalry. Nat Immunol. 2013;14:1030–1032. [Google Scholar]
- 19.Guillen I, Blanes M, Gomez-Lechon MJ, Castell JV. Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6. Am J Physiol. 1995;269:R229–R235. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 20.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ, Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011;216:313–320. doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Devlin CM, Kuriakose G, Hirsch E, Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc Natl Acad Sci U S A. 2002;99:6280–6285. doi: 10.1073/pnas.092324399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 26.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 28.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geiger T, Towbin H, Cosenti-Vargas A, Zingel O, Arnold J, Rordorf C, Glatt M, Vosbeck K. Neutralization of interleukin-1 beta activity in vivo with a monoclonal antibody alleviates collagen-induced arthritis in DBA/1 mice and prevents the associated acute-phase response. Clin Exp Rheumatol. 1993;11:515–522. [PubMed] [Google Scholar]
- 30.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine. 2008;44:141–148. doi: 10.1016/j.cyto.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kfoury Y, Mercier F, Scadden DT. SnapShot: The hematopoietic stem cell niche. Cell. 2014;158:228–228. e1. doi: 10.1016/j.cell.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112:1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol. 2013;191:4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E, Figueiredo JL, Pittet MJ, Weissleder R. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100:1218–1225. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, Salloum FN, Smithson L, Dinarello CA. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- 43.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 44.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 46.Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55:329–340. doi: 10.1016/s0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 47.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377. e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 52.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MAJ. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crossman DC, Morton AC, Gunn JP, Greenwood JP, Hall AS, Fox KA, Lucking AJ, Flather MD, Lees B, Foley CE. Investigation of the effect of Interleukin-1 receptor antagonist (IL-1ra) on markers of inflammation in non-ST elevation acute coronary syndromes (The MRC-ILA-HEART Study) Trials. 2008;9:8. doi: 10.1186/1745-6215-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.